Abstract
PURPOSE
The COVID-19 pandemic has profoundly affected cancer care worldwide, including radiation therapy (RT) for breast cancer (BC), because of risk-based resource allocation. We report the evolution of international breast RT practices during the beginning of the pandemic, focusing on differences in treatment recommendations between countries.
MATERIALS AND METHODS
Between July and November 2020, a 58-question survey was distributed to radiation oncologists (ROs) through international professional societies. Changes in RT decision making during the first surge of the pandemic were evaluated across six hypothetical scenarios, including the management of ductal carcinoma in situ (DCIS), early-stage, locally advanced, and metastatic BC. The significance of changes in responses before and during the pandemic was examined using chi-square and McNemar-Bowker tests.
RESULTS
One thousand one hundred three ROs from 54 countries completed the survey. Incomplete responses (254) were excluded from the analysis. Most respondents were from the United States (285), Japan (117), Italy (63), Canada (58), and Brazil (56). Twenty-one percent (230) of respondents reported treating at least one patient with BC who was COVID-19–positive. Approximately 60% of respondents reported no change in treatment recommendation during the pandemic, except for patients with metastatic disease, for which 57.7% (636/1,103; P < .0005) changed their palliative practice. Among respondents who noted a change in their recommendation during the first surge of the pandemic, omitting, delaying, and adopting short-course RT were the most frequent changes, with most transitioning to moderate hypofractionation for DCIS and early-stage BC.
CONCLUSION
Early in the COVID-19 pandemic, significant changes in global RT practice patterns for BC were introduced. The impact of published results from the FAST FORWARD trial supporting ultrahypofractionation likely confounded the interpretation of the pandemic's independent influence on RT delivery.
A global survey of 1,103 breast radiation oncologists across 54 nations during the initial surge of the COVID-19 pandemic revealed changes in practices including omission, delay of RT because of resource allocation, and notably, adoption of shorter RT courses for DCIS, early-stage breast cancer, postmastectomy with and without reconstruction, regional nodes, and metastatic disease. Publications of notable clinical trials supporting moderate and ultrahypofractionation reported during the pandemic may have further accelerated this adoption, particularly in early-stage and postmastectomy settings. Time will tell if the adoption of shorter courses of breast radiation therapy has become the standard of care globally.
INTRODUCTION
The COVID-19 pandemic affected radiation therapy (RT) for breast cancer (BC) delivery worldwide. To maximize clinical resources and minimize COVID-19 transmission, radiation oncologists (ROs) modified BC treatments as international professional societies established guidelines.1-5 These guidelines reflected patterns encouraging delayed RT for low-risk BC patients6-9 (less so for advanced-stage BC),7,10 abbreviating treatment regimens,6,8,11 and decreasing systemic therapy compared with surgery.6 Oncologists also reduced patient visitation, recommending initial surgery over preoperative chemotherapy,6,8 and delayed reconstructive surgery after mastectomy.8,10 While institutions,12-14 nations,8,11,15-17 and regions6,7,10,18,19 reported treatment modifications during COVID-19's peak, the global impact on BC treatment modification has not been collectively assessed. Our unique study is the only case-based global survey evaluating changes to RT recommendations for BC during the pandemic's first surge, which varied by country.
CONTEXT
Key Objective
To examine the international evolution of breast radiation therapy (RT) practices during the early stages of the COVID-19 pandemic and identify differences in treatment recommendations between countries.
Knowledge Generated
A survey conducted between July and November 2020 involving 1,103 radiation oncologists (ROs) from 54 countries found that approximately 60% of respondents reported no change in their treatment recommendations during the pandemic. The most frequent changes included omitting, delaying, or adopting short-course RT, with many transitioning to moderate hypofractionation.
Relevance
The pandemic significantly influenced RT delivery for breast cancer, as ROs worldwide swiftly embraced shorter fractionation courses. Alongside the publication of relevant clinical trials during the pandemic and ongoing studies, the trend toward widespread adoption of hypofractionation appears increasingly likely.
MATERIALS AND METHODS
The BC radiation oncology team at Massachusetts General Hospital and Dana Farber Cancer Institute (Boston, MA) initiated an international collaboration of ROs in developing a case-based survey evaluating BC RT decision-making changes during the pandemic's surge across six scenarios, meeting regularly through teleconference to develop it.
Consisting of 6 cases and 58 questions (Data Supplement), the survey was approved by Dana Farber/Harvard Cancer Center's institutional review board and was distributed to ROs who self-identified as having treated at least one patient with BC annually, with an international network of radiation oncology professional societies augmenting distribution (Table 1). It contained the following scenarios: (1) low-grade ductal carcinoma in situ (DCIS), (2) low-risk invasive BC after breast-conserving surgery, (3) early-stage invasive BC after mastectomy with immediate reconstruction, (4) invasive BC after neoadjuvant chemotherapy (NAC) and mastectomy without reconstruction, (5) invasive BC after mastectomy without reconstruction and with adjuvant chemotherapy, and (6) metastatic BC with an enlarging and bleeding breast mass. Respondents provided recommendations for two scenarios: (1) prepandemic and (2) during the pandemic's surge. Conventional fractionation was defined as 1.8-2.3 Gy per fraction, moderate hypofractionation as 2.31-3.0 Gy, and ultrahypofractionation as >5 Gy.
TABLE 1.
Participating Radiation Oncology Professional Societies/Group
The survey was translated into Spanish, Russian, and Mandarin, and distributed through REDCap on July 17, 2020, closing on November 8, 2020. Anonymous responses were compiled into a secure central database (incomplete responses were excluded [n = 254]). Categorical variables were described as counts and percentages, with chi-square and McNemar-Bowker tests used to examine the significance of changes between prepandemic and surge. P values are reported with statistical significance defined as <0.05. Statistical analysis was performed with R Studio, v. 2021.09.0 + 351 (Posit PBC, Boston, MA), and Excel 365, v. 2021 (Microsoft, Redmond, WA). This study was approved by Partners IRB (Protocol no.: 2020P001416) and nonverbal informed consent was obtained from participants before taking the survey by attesting on the webpage.
RESULTS
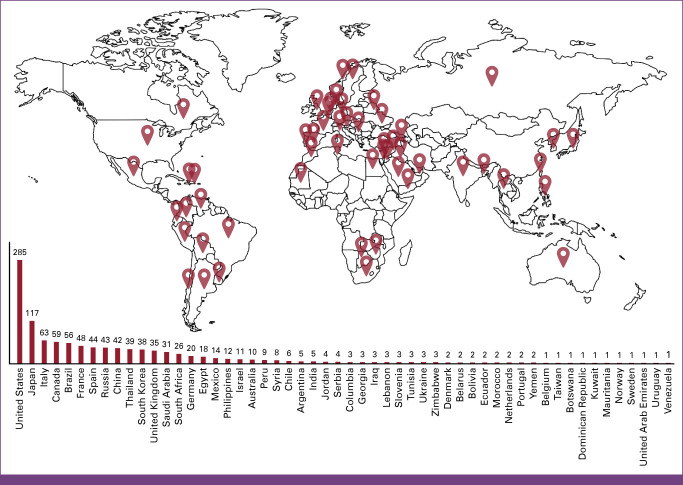
Overall, 1,103 ROs from 54 countries completed the survey (Fig 1), with the most respondents from 13 countries: United States (n = 285), Japan (n = 117), Italy (n = 63), Canada (n = 58), Brazil (n = 56), France (n = 48), Spain (n = 44), Russia (n = 43), China (n = 42), Thailand (n = 38), South Korea (n = 38), United Kingdom (n = 35), and Saudi Arabia (n = 31). ROs practiced in urban (69.8%; n = 770), suburban (19.4%; n = 214), rural (9.6%; n = 106), and other settings (1.2%; n = 13). Additionally, 49.8% (n = 549) practiced in university-affiliated hospitals, 25.7% (n = 283) in private practice, 21.1% (n = 233) in government hospitals, and 3.4% (n = 38) in other centers. Most (74.4%; n = 821) reported treating <200 patients with BC annually, while 45.6% (n = 503) reported >500 patients. In addition, 311 (28.2%) reported ≥1 patient with BC who was COVID-19–positive between November 1, 2019, and July 1, 2020. Herein, we describe treatment recommendation changes during the pandemic's surge as analyzed within six clinical cases.
FIG 1.
Total responses across 54 participating countries.
Case 1
DCIS
A 52-year-old woman was diagnosed with 1.5-cm grade 2 ER+/PR+ DCIS and treated with left lumpectomy with final surgical margins >2 mm. Adjuvant endocrine therapy was initiated (Fig 2A).
FIG 2.
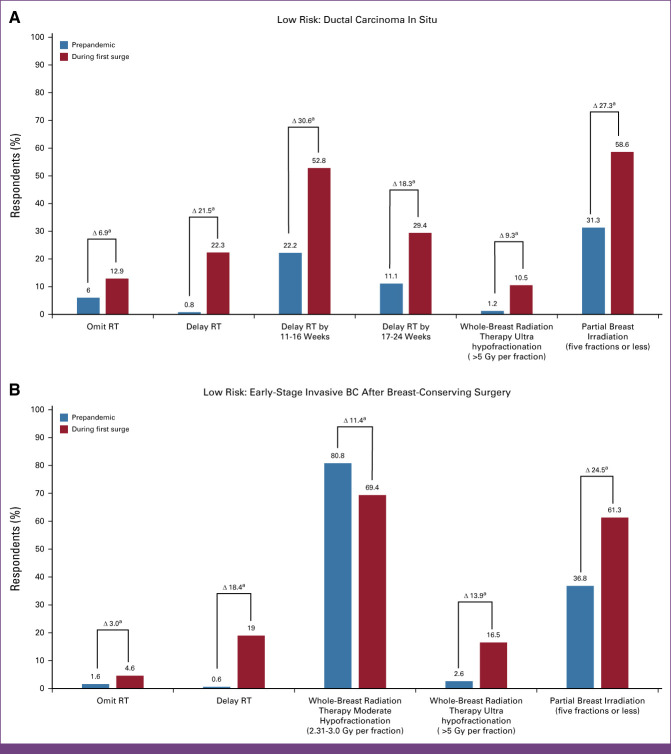
(A) Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding a DCIS case 1. A 52-year-old woman was diagnosed with 1.5-cm grade 2, ER+/PR+ DCIS and treated with a left lumpectomy with final surgical margins >2 mm. Adjuvant endocrine therapy was initiated. (B) Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding an early-stage invasive BC after breast-conserving surgery case 2. A 61-year-old female underwent a right lumpectomy revealing a 2-cm grade 2, ER+, PR+, HER2– invasive lobular carcinoma with no evidence of lymphovascular invasion. Neither of two sentinel nodes contained malignancy. The Oncotype Dx recurrence score was 8. Endocrine therapy was initiated. aResponses regarding treatment recommendation are shown. BC, breast cancer; DCIS, ductal carcinoma in situ; ER+, estrogen receptor–positive; HER2–, human epidermal growth factor receptor 2–negative; PR+, progesterone receptor–positive; RT, radiation therapy.
Prepandemic, 80.8% of respondents recommended adjuvant whole-breast RT (WBRT), 12.4% (n = 137) partial breast irradiation (PBI), 6.0% (n = 66) RT omission, and 0.8% (n = 9) delayed RT. In comparison, during the pandemic's surge, significantly more recommended delaying (22.3%, n = 246; P < .005) or omitting RT (12.9%, n = 142; P < .005). ROs from United States (40.7%), Saudi Arabia (25.8%), Canada (25.4%), and Brazil (23.2%) were most likely to delay RT during the surge, while ROs in Russia (39.5%) and Thailand (20.5%) would omit RT. Among those recommending delayed RT, most recommended an 11- to 16-week delay (22.2%, 2/9, prepandemic v 52.8%, 131/248, during surge; P < .005), while others a 17- to 24-week delay (11.1%, 1/9, prepandemic v 29.4%, 73/248, during surge; P < .005).
Of those recommending WBRT prepandemic (n = 891), 77.1% (n = 687) chose moderate hypofractionation, and 67.9% (n = 605) omitted a lumpectomy site boost. During the surge, significantly more recommended ultrahypofractionation (1.2%, 11/891, prepandemic to 10.5%, 61/581, during the surge; P < .005). Changes in fractionation varied widely, with ROs in United Kingdom (90.5%), Canada (38.9%), Spain (32.0%), and Saudi Arabia (16.7%) reporting the highest ultrahypofractionated breast RT rates during the surge. By contrast, ultrahypofractionation for DCIS was infrequent in China (6.7%), United States (3.8%), and Italy (2.3%). No respondents from Japan, France, Russia, South Korea, or South Africa recommended ultrahypofractionation for DCIS.
The most common PBI modality recommended for DCIS was external-beam RT (72.2%, 99/137), with 31.3% (31/99) favoring 30 Gy in five fractions over 2 weeks (Florence schedule), while 47.5% (47/99) favored >10 fraction regimen. This proportion shifted during the surge, with 31.3% (31/99 prepandemic) versus 58.6% (58/99 during surge; P < .005) recommending a five-fraction regimen.
Case 2
Early-Stage Invasive BC After Breast-Conserving Surgery
A 61-year-old woman underwent a right lumpectomy revealing a 2-cm grade 2 ER+/PR+/HER2– invasive lobular carcinoma with no evidence of lymphovascular invasion. Out of two sentinel nodes, zero contained malignancy. Her Oncotype Dx recurrence score was 8. Endocrine therapy was initiated (Fig 2B).
Prepandemic, 83% (n = 915) recommended WBRT, 14.7% (n = 162) PBI, 1.6% (n = 18) RT omission, and 0.6% (n = 7) RT delay. A significant increase recommended delayed RT during the surge (19.0%, n = 210; P < .005). Respondents in United States, Thailand, Canada, Saudi Arabia, and Japan reported the highest delayed-RT rates (35.0%, 20.5%, 20.3%, 19.4%, and 17.9%, respectively). There was a slight increase in omitting RT across all countries during the surge (4.6%, n = 51; P < .005); however, ROs in Russia (18.6%), Saudi Arabia (9.7%), United Kingdom (8.9%), Thailand (7.7%), and Brazil (7.1%) favored omitting RT.
Moderate hypofractionation was the most popular WBRT regimen, with a significant change between prepandemic and surge (80.8%, 739/915, and 69.4%, 459/661; P < .005, respectively); during the surge, there was a significant increase in ultrahypofractionation (2.6%, 24/915, prepandemic v 16.5%, 109/661, during surge; P < .005). Respondents in United Kingdom (89.3%), Spain (58.6%), Canada (51.5%), and Saudi Arabia (45.0%) recommended ultrahypofractionated WBRT during the surge, while those in South Africa (11.1%), Italy (10.8%), China (5.9%), United States (3.8%), and South Korea (3.4%) infrequently recommended it (no respondents in Japan, France, or Russia recommended it). For those recommending PBI, there was an increase in ≤5 fractions during the surge compared with prepandemic (61.3%, 84/137, v 36.8%, 43/117, respectively; P < .005).
Case 3
Invasive BC After Mastectomy With Immediate Reconstruction
A 54-year-old woman underwent a total simple mastectomy with immediate tissue expander reconstruction revealing a 3.4-cm grade 2 ER+/PR+/HER2– invasive ductal carcinoma and no lymphovascular invasion. Of three sentinel lymph nodes, one was positive (8-mm focus) without extranodal extension. The Oncotype Dx recurrence score was 4. Adjuvant chemotherapy was not recommended. An adjuvant aromatase inhibitor was planned (Fig 3).
FIG 3.
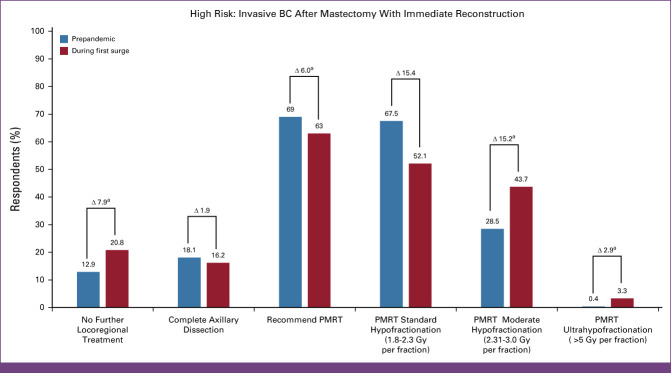
Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding an invasive BC after mastectomy with immediate reconstruction case 3. A 54-year-old woman underwent a total simple mastectomy with immediate tissue expander reconstruction. Pathology revealed a 3.4-cm grade 2 ER+/PR+/HER2– invasive ductal carcinoma and no lymphovascular invasion. Of three sentinel lymph nodes, one was positive (8-mm focus) without extranodal extension. Oncotype Dx recurrence score was 4. Adjuvant chemotherapy was not recommended. An adjuvant aromatase inhibitor was planned. aResponses regarding treatment recommendation are shown. BC, breast cancer; ER+, estrogen receptor–positive; HER2–, human epidermal growth factor receptor 2–negative; PMRT, postmastectomy radiation therapy; PR+, progesterone receptor–positive.
Prepandemic, most (69.0%, 761) recommended postmastectomy RT (PMRT), whereas a minority favored complete axillary dissection (18.1%, 200) or no further local-regional treatment (12.9%; n = 142). ROs in Spain (81.8%), Canada (81.4%), Brazil (80.4%), Thailand (79.5%), and South Korea (78.9%) favored PMRT, while those in Italy (44.4%) and Russia (41.9%) favored complete axillary dissection. During the surge, there was an increase in no further local-regional treatment (20.8%, n = 229; P < .005). However, PMRT (63.0%, n = 695; P < .005) and complete axillary dissection (16.2%, n = 179; P = .106) recommendations decreased, but only the former was statistically significant. ROs in Japan (35.0%), Italy (33.3%), Russia (32.5%), and China (31.0%) most recommended no further local-regional treatment.
Most ROs recommending PMRT chose conventional fractionation, regardless of prepandemic or surge (67.5%, 534/791, and 52.1%, 362/695, respectively). However, during the surge, recommendations significantly increased for moderate hypofractionation (from 28.5%, 217/761, to 43.7%, 304/695; P < .005) and ultrahypofractionation (from 0.4%, 3/761, to 3.3%, 23/695; P < .005). ROs in Canada, Spain, Brazil, United Kingdom, and Saudi Arabia most recommended moderate hypofractionation prepandemic (39.6%, 55.6%, 46.7%, 87.0%, and 60.9%, respectively) and during surge (70.8%, 66.7%, 63.6%, 63.6%, and 57.1%, respectively). Overall, ROs in United Kingdom (36.4%), Spain (12.1%), and Saudi Arabia (48.0%) had the highest rate of recommending an ultrahypofractionation regimen for PMRT during the surge.
Case 4
Invasive BC After NAC and Mastectomy
A 55-year-old woman with cT2N1 grade 3 triple-negative BC underwent NAC with doxorubicin, cyclophosphamide, and paclitaxel, followed by total mastectomy and sentinel lymph node biopsy. Reconstruction was not performed. A pathologic complete response was achieved, with no residual disease seen in the breast and three sentinel nodes (ypT0N0; Fig 4).
FIG 4.
Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding an invasive BC after NAC and mastectomy without reconstruction case 4. A 55-year-old woman with cT2N1, grade 3 triple-negative BC underwent NAC with doxorubicin, cyclophosphamide, and paclitaxel, followed by a total mastectomy and sentinel lymph node biopsy. Reconstruction was not performed. A pathologic complete response was achieved with no residual disease seen in the breast and three sentinel nodes (ypT0N0). aResponses regarding treatment recommendation are shown. BC, breast cancer; NAC, neoadjuvant chemotherapy; PMRT, postmastectomy radiation therapy.
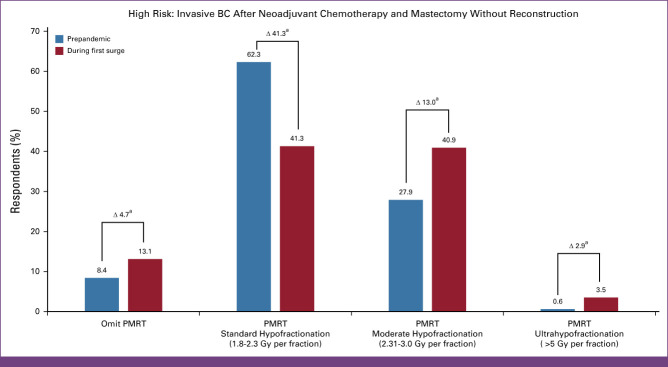
Prepandemic, most recommended PMRT using conventional fractionation (62.3%, n = 687) compared with moderate hypofractionation (27.9%, n = 308), 3.1-5.0 Gy (0.7%, 8), ultrahypofractionation (0.6%, n = 7), or no PMRT (8.4%, 93). However, during the surge, moderate hypofractionation (40.9%, n = 451; P < .005), no PMRT (13.1%, n = 144; P < .005), and ultrahypofractionated PMRT (3.5%, n = 39; P < .005) were recommended. During the surge, respondents from Canada (86.5%), Saudi Arabia (77.8%), Spain (77.5%), Brazil (69.8%), and Russia (63.6%) mostly recommended moderate hypofractionation, while those in China (23.4%), Japan (21.9%), and Saudi Arabia (14.8%) mostly omitted PMRT. In this scenario, ROs in United Kingdom reported the highest ultrahypofractionation use (66.7%).
Case 5
Invasive BC After Mastectomy Without Reconstruction and Adjuvant Chemotherapy
A 45-year-old woman underwent a left modified radical mastectomy without immediate reconstruction. Pathology revealed a 5-cm, grade 2 ER+/PR+/HER– invasive ductal carcinoma with evidence of lymphovascular invasion and five out of 15 positive axillary nodes. She completed adjuvant dose-dense doxorubicin, cyclophosphamide, and paclitaxel. An aromatase inhibitor was planned (Fig 5A).
FIG 5.
(A) Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding an invasive BC after mastectomy without reconstruction and adjuvant chemotherapy case 5. A 45-year-old woman underwent a left modified radical mastectomy without immediate reconstruction. Pathology revealed a 5-cm grade 2 ER+, PR+, HER– invasive ductal carcinoma with evidence of lymphovascular invasion and five out of 15 positive axillary nodes. She completed adjuvant dose-dense doxorubicin, cyclophosphamide, and paclitaxel. An aromatase inhibitor was planned. (B) Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding an invasive BC after mastectomy without reconstruction and adjuvant chemotherapy case 5. A 45-year-old woman underwent a left modified radical mastectomy without immediate reconstruction. Pathology revealed a 5-cm grade 2 ER+, PR+, HER– invasive ductal carcinoma with evidence of lymphovascular invasion and five out of 15 positive axillary nodes. She completed adjuvant dose-dense doxorubicin, cyclophosphamide, and paclitaxel. An aromatase inhibitor was planned. aResponses regarding treatment recommendation are shown. BC, breast cancer; ER+, estrogen receptor–positive; HER–, human epidermal growth factor receptor–negative; IMNs, internal mammary nodes; PMRT, postmastectomy RT; PR+, progesterone receptor–positive; RT, radiation therapy; SCV, supraclavicular.
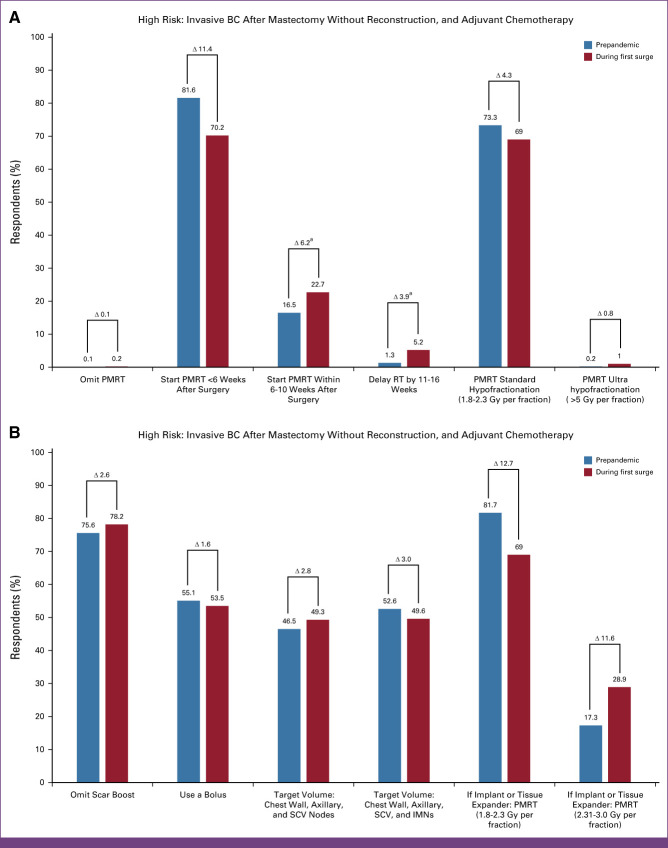
Prepandemic, most ROs (81.6%, 900) preferred to begin PMRT ≤6 weeks after surgery, while 16.5% (n = 182) would initiate PMRT >6-10 weeks after surgery. During the surge, recommendations increased for PMRT to start within 6-10 weeks after surgery (23.5%, n = 259; P < .005) or delay by 11-16 weeks after (5.2%, n = 57; P < .005). Most did not change their recommendation to delay RT during the surge, preferring to start <6 weeks (70.2%, n = 774). The surge did not change bolus or boost fractionation or use. Most recommended conventional fractionation (73.3%, n = 808 and 69.0%, n = 761), using a bolus (55.1%, n = 608, and 53.5%, n = 590), and preferring not to boost the mastectomy scar (75.6%, n = 834, and 78.2%, n = 863). Recommended target volume(s) included the chest wall, axillary nodes, and supraclavicular nodes (46.5%, n = 512), with 52.6% (n = 580) also including the internal mammary nodes, which remained relatively consistent during the surge (49.3%, n = 543, and 49.6%, n = 547, respectively). When the same hypothetical patient underwent immediate breast reconstruction with an implant or tissue expander, most recommended conventional fractionation (81.7%, n = 901, and 69.0%, n = 761) compared with moderate hypofractionation (17.3%, n = 191, and 28.9%, n = 319), prepandemic and surge, respectively.
Case 6
Metastatic BC With an Enlarging Breast Mass
A 75-year-old woman with metastatic ER+/PR+/HER2– invasive ductal carcinoma resistant to several lines of systemic therapy presents with an enlarging and bleeding 6-cm right breast mass. Karnofsky performance status is 80. Surgical resection is not planned because of the presence of multiple lung metastases (Fig 6).
FIG 6.
Distribution of responses on treatment guidelines followed before and during the COVID surge among 1,103 respondents regarding a metastatic BC with an enlarging breast mass case 6. A 75-year-old woman with metastatic ER+/PR+/HER2– invasive ductal carcinoma resistant to several lines of systemic therapy presented with an enlarging and bleeding 6-cm right breast mass. Her Karnofsky performance status was 80. Surgical resection was not planned because of the presence of multiple lung metastases. aResponses regarding treatment recommendation are shown. BC, breast cancer; ER+, estrogen receptor–positive; HER2–, human epidermal growth factor receptor 2–negative; PR+, progesterone receptor–positive.
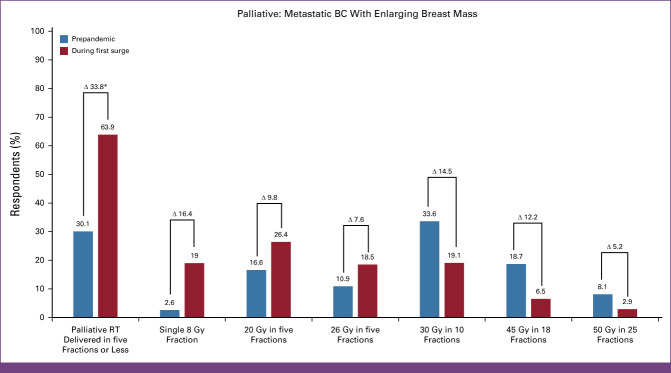
Most (60.3%, n = 665) recommended palliative RT delivered in at least 10 fractions prepandemic, specifically, 50 Gy in 25 fractions (8.1%, n = 89), 45 Gy in 18 fractions (18.7%, n = 206), and 30 Gy in 10 fractions (33.6%, n = 370). However, during the surge, most recommended palliative RT delivered in ≤5 fractions (63.9%, n = 705; P =< 0.0005): 26 Gy in five fractions (18.5%, 204/1,103), 20 Gy in five fractions (26.4%, 291/1,103), and 8 Gy in one fraction (19.0%, 210/1,103).
DISCUSSION
Our study is unique in its diverse representation and strong global collaboration between experts reporting treatment recommendations concerning the pandemic's first surge in their respective nations. It aims to determine whether the pandemic acutely affected practice patterns for patients with BC receiving RT relative to prepandemic times. Participation was robust, with ROs from 54 countries fully completing the survey, demonstrating wide variations in international BC treatment recommendations prepandemic and surge.
In cases 1 and 2, minimal change was observed, with many recommending WBRT delivered with moderate hypofractionation and no boost, prepandemic and surge. Most recommendation changes during the surge indicated delaying, omitting, or abbreviating RT fractionation. This aligned with the HYPO trial publication and published treatment guidelines for physicians prescribing RT during the pandemic,1,20-25 although the distribution was not uniform. ROs in United States, Saudi Arabia, Canada, and Brazil most recommended delayed RT, while most recommended omitting RT in Russia and Thailand. Similarly, most ultrahypofractionated RT recommendations during the surge were in the United Kingdom, Canada, Spain, and Saudi Arabia. By contrast, respondents in China, United States, South Korea, and Italy infrequently recommended ultrahypofractionation, and some countries did not recommend ultrahypofractionation (Japan, France, and Russia).
Ultrahypofractionated RT for low-risk BC recommendations by ROs in United Kingdom is informed by the FAST trial's 10-year outcomes and FAST FORWARD trial's 5-year outcomes publications,26 reported during the surge. These showed noninferior outcomes compared with standard fractionation (FAST) or moderate hypofractionation (FAST FORWARD). In United States, where practice patterns can vary significantly by geography and practice type,27 a notable increase in recommendations for ultrahypofractionated RT for early-stage BC was reported (although lower than in United Kingdom, where practice is uniform with the same dose/fractionation).28 ROs recommending PBI for early-stage BC favored increasing to ≤5 fractions during the first surge compared with prepandemic. This change toward accelerated PBI is attributed to the Florence Trial,29 which published 10-year outcomes during the initial surge and survey period. Its findings demonstrated favorable cosmetic outcomes, similar local recurrence, and similar survival compared with WBRT.
In the first high-risk BC scenario of a patient undergoing mastectomy and sentinel lymph node biopsy with reconstruction for pT2N1 hormone receptor–positive disease, most favored PMRT prepandemic. ROs in Russia and Italy recommended complete axillary dissection prepandemic (a controversial approach since ACOSOG Z11's publication, which provides evidence against such).30 For this scenario, during the first surge, a notable increase was observed in recommendations for no further local-regional treatment in a pathologically node-positive mastectomy setting. Those favoring complete axillary dissection prepandemic most recommended no further local-regional treatment.
The second high-risk BC scenario of a patient with complete pathologic response in the breast and nodes to NAC highlights ROs' comfort with moderate fractionation and ultrahypofractionation in the mastectomy setting during the surge. Notably, the willingness to omit postmastectomy radiation in the pathologic complete response setting was observed among 8.4% of respondents prepandemic and increased to 13.1% during the surge.
Although most ROs recommended PMRT delivered in conventional fractionation before and during the surge for patients with high-risk BC, our survey observed a rapid uptake in moderate hypofractionation. Respondents in Canada, Saudi Arabia, Spain, Brazil, and Russia favored moderate hypofractionation, while ROs in United Kingdom mostly recommended ultrahypofractionation. The latter is likely because of United Kingdom oncologists' ongoing experience with patients enrolled in FAST FORWARD's nodal planning study and coordinated breast RT consensus process.26,28 This comfort with moderate hypofractionation is also likely influenced by a large randomized trial conducted in China31 comparing conventional fractionation to hypofractionation in the nonreconstructive postmastectomy setting. The long-term results from this trial and findings from similar US clinical trials32,33 evaluating moderate hypofractionation in the mastectomy setting (including reconstruction) will likely influence widespread global adoption of shorter course treatments. Our survey also revealed that during the surge, recommendations to start PMRT 6-10 weeks after surgery (up to 11-16 weeks) slightly increased compared with typical time frames (within 6 weeks). Notably, for the highest-risk patient with pT3N2 hormone receptor–positive left invasive BC after mastectomy and adjuvant chemotherapy, only half recommended target volumes inclusive of internal mammary nodes (prepandemic and surge), suggesting no worldwide consensus.
In the prepandemic palliative scenario, most recommended palliative RT prescribed in ≥10 fractions. However, during the surge, most recommended ≤5 fractions, reflecting a significant change influenced by the pandemic, likely in response to protecting patients from COVID-19 exposure and mindful of their quality of life. Although we cannot assess the economic impact of this by country, it highlights physicians' willingness to recommend shorter treatment courses for terminal BC patients and raises questions about routine practice deficits in nonpandemic periods.
We must acknowledge several limitations in this study. Recall bias and well-documented survey limitations may have affected answers about prepandemic recommendations (such questions referenced practices 7-11 months before survey distribution). Recall bias may also apply to treatment recommendations for the country-specific surge scenario, which varied among nations and may not have been reached during survey distribution. Additionally, updated treatment guidelines were published during survey distribution, which may have influenced answers. Thus, for the questions related to treatment recommendations during the surge, respondents may have been unable to separate their choice from guideline recommendations. This is salient for hypofractionated RT recommendations during the pandemic's height, as several clinical trials26,31 validated it, thus making it challenging to attribute recommendation increases to COVID-19 alone.
Another limiting factor is the over-representation of countries with high response rates. Many countries (n = 40) had fewer than 25 ROs complete surveys, and 31 had ≤5 respondents. Additionally, ROs in United States were over-represented (25.8% of respondents), while ROs in Africa were under-represented. We also cannot overlook selection bias because of the unequal response rate, with only 81.3% completing the entire survey. To minimize this, our analysis of country-specific recommendations is limited to countries with >25 respondents. Finally, each country's culture and its impact on treatment heterogeneity were impossible to factor in.
It is unclear if these recommendations represent lasting changes in BC management 2 years into the pandemic. Nevertheless, as the first of its kind in breast radiation oncology during an unprecedented global health emergency, this survey has numerous strengths, including manifold responses and robust international participation. Historically there have been worldwide differences concerning volume and dose fractionation for BC radiotherapy.34-36 Our study uniquely provides a snapshot of case-specific treatment recommendations and builds upon published COVID-19–related surveys and experiences.37-43 Specifically, it demonstrates how the pandemic affected treatment, providing insights into how management varies greatly globally. Lessons gained from this experience will inform consensus guidelines for breast RT and preparedness against future pandemics. Longitudinal surveillance will reveal whether the patterns observed persist after the pandemic and, more importantly, how these changes affect outcomes.
Oluwadamilola T. Oladeru
Research Funding: ASCO/Pfizer Grant, Bristol Myers Squibb Foundation
Jee Suk Chang
Stock and Other Ownership Interests: Oncosoft
Honoraria: Accuray
Consulting or Advisory Role: Oncosoft, Accuray
Icro Meattini
Honoraria: Lilly, Novartis, Pfizer, Seagen, Accuray
Duvern Ramiah
Speakers' Bureau: Astellas Pharma
Anna Kirby
Stock and Other Ownership Interests: Aspen Healthcare
Travel, Accommodations, Expenses: Elekta
Tarek Hijal
Leadership: Multiwave Technologies
Consulting or Advisory Role: L’Oreal Canada, Merck
Speakers' Bureau: Pfizer, L’Oreal Canada
Research Funding: AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: L’Oreal Canada
Philip Poortmans
Consulting or Advisory Role: Sordina IORT Technologies
Tamer Refaat
Employment: Loyola University Medical Center, Northwestern University
Honoraria: ViewRay
Consulting or Advisory Role: ICON Clinical Research
Yazid Belkacemi
Honoraria: Saint Paul de vence
Consulting or Advisory Role: MSD
Travel, Accommodations, Expenses: Elekta Frace
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, Zai Lab, GenomiCare, Yuhan, Roche, Menarini, InventisBio Co, Ltd
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co (Inst)
Julia S. Wong
Honoraria: Wolters Kluwer
Reshma Jagsi
Employment: University of Michigan, Emory University
Stock and Other Ownership Interests: Equity Quotient
Research Funding: Genentech (Inst)
Expert Testimony: Baptist Health/Dressman Benziger Lavalle Law, Kleinbard, Sherinian & Hasso Law Firm
Other Relationship: JAMA Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/373670/summary
Alphonse Taghian
Honoraria: UpToDate
Patents, Royalties, Other Intellectual Property: There are previsionary patents submitted
Expert Testimony: Law firm in RI
Jennifer R. Bellon
Honoraria: UpToDate, Grupo Oncoclinicas, MJH Life Sciences, Varian Medical Systems
Alice Y. Ho
Honoraria: Seagen, La Roche-Posay, Merck
Consulting or Advisory Role: La Roche Posa
Research Funding: Merck (Inst), Tesaro (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: La Roche Posay
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 63rd Annual American Society for Radiation Oncology (ASTRO) Meeting, Chicago, IL, October 25, 2021.
DATA SHARING STATEMENT
The authors agree to share anonymized data upon reasonable request by researchers.
AUTHOR CONTRIBUTIONS
Conception and design: Oluwadamilola T. Oladeru, Charlotte E. Coles, Orit Kaidar-Person, Icro Meattini, Duvern Ramiah, Gustavo Nader Marta, Philip Poortmans, Josep Isern-Verdum, Tamer Refaat, Yazid Belkacemi, Feng Deng Luo, Dalia Larios, Daphna Y. Spiegel, Alphonse Taghian, Jennifer R. Bellon, Alice Y. Ho
Administrative support: Philip Poortmans, Feng Deng Luo, Phoebe Ryan
Provision of study materials or patients: Skye Hung-Chun Cheng, Philip Poortmans, Shun Lu, Julia S. Wong
Collection and assembly of data: Oluwadamilola T. Oladeru, Samantha A. Dunn, Chikako Yamauchi, Jee Suk Chang, Skye Hung-Chun Cheng, Icro Meattini, Duvern Ramiah, Anna Kirby, Tarek Hijal, Gustavo Nader Marta, Philip Poortmans, Josep Isern-Verdum, Birgitte Vrou Offersen, Tamer Refaat, Khaled Elsayad, Hussam Hijazi, Natalia Dengina, Colleen Griffin, Maya Collins, Phoebe Ryan, Jennifer R. Bellon, Alice Y. Ho
Data analysis and interpretation: Oluwadamilola T. Oladeru, Samantha A. Dunn, Jian Li, Jee Suk Chang, Orit Kaidar-Person, Icro Meattini, Anna Kirby, Gustavo Nader Marta, Philip Poortmans, Josep Isern-Verdum, Yvonne Zissiadis, Shun Lu, Laura E. Warren, Rinaa S. Punglia, Julia S. Wong, Daphna Y. Spiegel, Reshma Jagsi, Alphonse Taghian, Alice Y. Ho
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Oluwadamilola T. Oladeru
Research Funding: ASCO/Pfizer Grant, Bristol Myers Squibb Foundation
Jee Suk Chang
Stock and Other Ownership Interests: Oncosoft
Honoraria: Accuray
Consulting or Advisory Role: Oncosoft, Accuray
Icro Meattini
Honoraria: Lilly, Novartis, Pfizer, Seagen, Accuray
Duvern Ramiah
Speakers' Bureau: Astellas Pharma
Anna Kirby
Stock and Other Ownership Interests: Aspen Healthcare
Travel, Accommodations, Expenses: Elekta
Tarek Hijal
Leadership: Multiwave Technologies
Consulting or Advisory Role: L’Oreal Canada, Merck
Speakers' Bureau: Pfizer, L’Oreal Canada
Research Funding: AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: L’Oreal Canada
Philip Poortmans
Consulting or Advisory Role: Sordina IORT Technologies
Tamer Refaat
Employment: Loyola University Medical Center, Northwestern University
Honoraria: ViewRay
Consulting or Advisory Role: ICON Clinical Research
Yazid Belkacemi
Honoraria: Saint Paul de vence
Consulting or Advisory Role: MSD
Travel, Accommodations, Expenses: Elekta Frace
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, Zai Lab, GenomiCare, Yuhan, Roche, Menarini, InventisBio Co, Ltd
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co (Inst)
Julia S. Wong
Honoraria: Wolters Kluwer
Reshma Jagsi
Employment: University of Michigan, Emory University
Stock and Other Ownership Interests: Equity Quotient
Research Funding: Genentech (Inst)
Expert Testimony: Baptist Health/Dressman Benziger Lavalle Law, Kleinbard, Sherinian & Hasso Law Firm
Other Relationship: JAMA Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/373670/summary
Alphonse Taghian
Honoraria: UpToDate
Patents, Royalties, Other Intellectual Property: There are previsionary patents submitted
Expert Testimony: Law firm in RI
Jennifer R. Bellon
Honoraria: UpToDate, Grupo Oncoclinicas, MJH Life Sciences, Varian Medical Systems
Alice Y. Ho
Honoraria: Seagen, La Roche-Posay, Merck
Consulting or Advisory Role: La Roche Posa
Research Funding: Merck (Inst), Tesaro (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: La Roche Posay
No other potential conflicts of interest were reported.
REFERENCES
- 1. Coles CE, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 2020;32:279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Society for Medical Oncology (ESMO) https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/breast-cancer-in-the-covid-19-era ESMO management and treatment adapted recommendations in the COVID-19 era: Breast cancer guidelines. [DOI] [PMC free article] [PubMed]
- 3.American Society of Clinical Oncology (ASCO) https://www.asco.org/covid-resources/patient-care-info ASCO COVID-19 patient care information.
- 4.American College of Surgeons (ACS) https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/breast-cancer/ ACS COVID-19 guidelines for triage of breast cancer patients.
- 5.Society of Surgical Oncology (SSO) https://www.surgonc.org/wp-content/uploads/2020/03/Breast-Resource-during-COVID-19-3.30.20.pdf SSO resource for management options of breast cancer during COVID-19.
- 6. Gasparri ML, Gentilini OD, Lueftner D, et al. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: An international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) Breast. 2020;52:110–115. doi: 10.1016/j.breast.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez D, Sarria GJ, Wakefield D, et al. COVID's impact on radiation oncology: A Latin American survey study. Int J Radiat Oncol Biol Phys. 2020;108:374–378. doi: 10.1016/j.ijrobp.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murris F, Huchon C, Zilberman S, et al. Impact of the first lockdown for coronavirus 19 on breast cancer management in France: A multicentre survey. J Gynecol Obstet Hum Reprod. 2021;50:102166. doi: 10.1016/j.jogoh.2021.102166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184:249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luis Pendola G, Elizalde R, Vargas PS, et al. Management of non-invasive tumours, benign tumours and breast cancer during the COVID-19 pandemic: Recommendations based on a Latin American survey. Ecancermedicalscience. 2020;14:1115. doi: 10.3332/ecancer.2020.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Achard V, Aebersold DM, Allal AS, et al. A national survey on radiation oncology patterns of practice in Switzerland during the COVID-19 pandemic: Present changes and future perspectives. Radiother Oncol. 2020;150:1–3. doi: 10.1016/j.radonc.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das IJ, Kalapurakal JA, Strauss JB, et al. Adaptability and resilience of academic radiation oncology personnel and procedures during COVID-19 pandemic. Int J Environ Res Public Health. 2021;18:5095. doi: 10.3390/ijerph18105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Yao Y, Wang Q, et al. Dilemma and solutions of treatment delay in cancer patients during the COVID-19 pandemic: A single-center, prospective survey. Asia Pac J Clin Oncol. 2022;18:e338–e345. doi: 10.1111/ajco.13724. [DOI] [PubMed] [Google Scholar]
- 14. Lee S, Heo J. COVID-19 pandemic: A new cause of unplanned interruption of radiotherapy in breast cancer patients. Med Oncol. 2021;39:5. doi: 10.1007/s12032-021-01604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cavalcante FP, Novita GG, Millen EC, et al. Management of early breast cancer during the COVID-19 pandemic in Brazil. Breast Cancer Res Treat. 2020;184:637–647. doi: 10.1007/s10549-020-05877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koch CA, Lee G, Liu ZA, et al. Rapid adaptation of breast radiation therapy use during the coronavirus disease 2019 pandemic at a large academic cancer center in Canada. Adv Radiat Oncol. 2020;5:749–756. doi: 10.1016/j.adro.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poggio F, Tagliamento M, Di Maio M, et al. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol Pract. 2020;16:e1304–e1314. doi: 10.1200/OP.20.00297. [DOI] [PubMed] [Google Scholar]
- 18. Bernabe-Ramirez C, Velazquez AI, Olazagasti C, et al. HOLA COVID-19 study: Evaluating the impact of caring for patients with COVID-19 on cancer care delivery in Latin America. JCO Glob Oncol. 2022;8:e2100251. doi: 10.1200/GO.21.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsh-Yechezkel G, Chetrit A, Ben Avraham S, et al. Oncology treatments during the COVID-19 pandemic in Israel: The ONCOR study. Isr Med Assoc J. 2021;23:759–765. [PubMed] [Google Scholar]
- 20. Braunstein LZ, Gillespie EF, Hong L, et al. Breast radiation therapy under COVID-19 pandemic resource constraints-approaches to defer or shorten treatment from a Comprehensive Cancer Center in the United States. Adv Radiat Oncol. 2020;5:582–588. doi: 10.1016/j.adro.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Rashdan A, Roumeliotis M, Quirk S, et al. Adapting radiation therapy treatments for patients with breast cancer during the COVID-19 pandemic: Hypo-fractionation and accelerated partial breast irradiation to address World Health Organization recommendations. Adv Radiat Oncol. 2020;5:575–576. doi: 10.1016/j.adro.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Machiels M, Weytjens R, Bauwens W, et al. Accelerated adaptation of ultrahypofractionated radiation therapy for breast cancer at the time of the COVID-19 pandemic. Clin Oncol (R Coll Radiol) 2021;33:e166–e171. doi: 10.1016/j.clon.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loap P, Kirova Y, Takanen S, et al. Breast radiation therapy during COVID-19 outbreak: Practical advice. Cancer Radiother. 2020;24:196–198. doi: 10.1016/j.canrad.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brunt AM, Haviland JS, Sydenham M, et al. Ten-year results of FAST: A randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38:3261–3272. doi: 10.1200/JCO.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: The DBCG HYPO trial. J Clin Oncol. 2020;38:3615–3625. doi: 10.1200/JCO.20.01363. [DOI] [PubMed] [Google Scholar]
- 26. Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunn SA, Oladeru OT, Collins ME, et al. The impact of the COVID-19 pandemic on practice patterns in breast radiation oncology: A case-based survey study in the United States. Int J Radiat Oncol Biol Phys. 2021;111:e200. [Google Scholar]
- 28. Lewis P, Brunt AM, Coles C, et al. Moving forward fast with FAST-forward. Clin Oncol (R Coll Radiol) 2021;33:427–429. doi: 10.1016/j.clon.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 29. Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. 2020;38:4175–4183. doi: 10.1200/JCO.20.00650. [DOI] [PubMed] [Google Scholar]
- 30. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20:352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 32. Rugo HS, Barry WT, Moreno-Aspitia A, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance) J Clin Oncol. 2015;33:2361–2369. doi: 10.1200/JCO.2014.59.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grudzen CR, Richardson LD, Johnson PN, et al. Emergency department-initiated palliative care in advanced cancer: A randomized clinical trial. JAMA Oncol. 2016;2:591–598. doi: 10.1001/jamaoncol.2015.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meattini I, Becherini C, Boersma L, et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23:e21–e31. doi: 10.1016/S1470-2045(21)00539-8. [DOI] [PubMed] [Google Scholar]
- 35. Ngwa W, Addai BW, Adewole I, et al. Cancer in sub-Saharan Africa: A Lancet Oncology Commission. Lancet Oncol. 2022;23:e251–e312. doi: 10.1016/S1470-2045(21)00720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coles CE, Choudhury A, Hoskin PJ, et al. COVID-19: A catalyst for change for UK clinical oncology. Int J Radiat Oncol Biol Phys. 2020;108:462–465. doi: 10.1016/j.ijrobp.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giuliani M, Papadakos T, Papadakos J. Propelling a new era of patient education into practice—Cancer care post–COVID-19. Int J Radiat Oncol Biol Phys. 2020;108:404–406. doi: 10.1016/j.ijrobp.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oliveira HF, Yoshinari GH, Veras IM, et al. Impact of the COVID-19 pandemic on radiation oncology departments in Brazil. Adv Radiat Oncol. 2022;7:100667. doi: 10.1016/j.adro.2021.100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomson DJ, Yom SS, Saeed H, et al. Radiation fractionation schedules published during the COVID-19 pandemic: A systematic review of the quality of evidence and recommendations for future development. Int J Radiat Oncol Biol Phys. 2020;108:379–389. doi: 10.1016/j.ijrobp.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mo A, Chung J, Eichler J, et al. Breast cancer survivorship care during the COVID-19 pandemic within an urban New York Hospital System. Int J Radiat Oncol Biol Phys. 2021;111:e169–e170. doi: 10.1016/j.breast.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liao D, Singh K, Helou J, et al. Impact of the COVID-19 pandemic on radiotherapy patterns of practice for curative intent breast cancer patients. Int J Radiat Oncol Biol Phys. 2021;111:e219. [Google Scholar]
- 43. Merrell KW, DeWees TA, Osei-Bonsu EB, et al. COVID-19 in sub-Saharan Africa: A multi-institutional survey of the impact of the global pandemic on cancer care resources. Int J Radiat Oncol Biol Phys. 2021;111:e349–e350. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors agree to share anonymized data upon reasonable request by researchers.