Abstract
Background.
Kidney transplant candidates may be incompatible with their intended living donors because of the presence of antibodies against HLA and/or ABO. To increase the possibility of finding an acceptable kidney donor for these patients, the Scandiatransplant Exchange Program (STEP) program within Scandiatransplant was launched in 2019.
Methods.
This is a retrospective review of our experiences from the first 4 y of the STEP program, including details about the match runs, performed transplantations, and recipient outcomes within the program.
Results.
During 2019–2022, 11 match runs and 4 reruns were performed. In total, 114 pairs and 6 anonymous donors participated in these match runs. Fifty-one pairs (45%) participated in 1 match run, 31 pairs (27%) participated in 2 match runs, and 32 pairs (29%) participated in ≥3 match runs. Seventy-two individuals (63%) participated because of HLA incompatibility, 19 (17%) because of ABO incompatibility, and 7 (6%) because of both HLA and ABO incompatibility.
Forty percent of the patients enrolled in the program underwent transplantation. In total, 49 transplantations have so far been performed within the program, and 46 (94%) of the recipients had a functioning kidney graft at follow-up in February 2023.
Conclusions.
The STEP program offers sensitized patients an enlarged pool of living donors and a chance of a compatible international living donor, resulting in an increased number of total transplantations. Currently, STEP is one of the largest transnational kidney exchange programs and has improved the situation for patients waiting for kidney transplantation in Scandiatransplant.
Transplant outcomes, such as rejection rates and more importantly, graft survival, are better with living donor transplantation than with deceased donor transplantation.1 Unfortunately, almost 1 of 3 potential living kidney donors is deemed incompatible with their intended recipient owing to DSAs against HLA and/or ABO.2 To augment the living donor pool for these recipients, Kidney Exchange Programs (KEP) have been established in many countries.3,4
Scandiatransplant is an organ allocation organization for all transplantation units in Denmark, Estonia, Finland, Iceland, Norway, and Sweden, covering a population of approximately 28.9 million inhabitants. The organization was established in 1969, and since then, organ exchange from deceased donors has occurred between the member hospitals.
Many initiatives have been undertaken within Scandiatransplant to increase the possibility of finding suitable kidney grafts for sensitized recipients. The Scandiatransplant Acceptable Mismatch Program was introduced in 2009.5 In 2022, kidneys from 269 living donors were transplanted (9.18 per million population) within Scandiatransplant.
In this article, we describe the newly established Scandiatransplant Exchange Program (STEP), in which kidneys from living donors are exchanged between participating transplant hospitals. This was preceded by the Swedish Transplant Exchange Program, formed in 2018,6 in which 7 exchanges were performed. In 2019, the Swedish pilot program was replaced by STEP, with participating member hospitals in Sweden and Denmark.7 Finland joined the program in 2020, followed by Norway in 2022, and Iceland joined STEP in 2023.
The main reason for extending a national KEP such as the Swedish Transplant Exchange Program beyond its national borders is to increase the size of the donor pool. This increases the probability of participating patients finding a feasible match, thereby reducing the risk of accumulating highly sensitized patients awaiting transplantation.
Participating Scandiatransplant centers developed joint routines and checklists for all steps in the process to ensure standardized criteria, smooth cooperation, high quality, and safety for the participating couples. Furthermore, to handle and maximize the benefits of KEP, a data management system was developed. This resulted in an extension of the existing Scandiatransplant software, including a dedicated matching and optimization algorithm. The collected data are similar to what has been described as the common core of information that needs to be collected in a KEP to make relevant assessments.8
At present, match runs are conducted 3 to 4 times per year, followed by reruns when broken cycles are identified early. A steering committee was established with representatives from each country, including experts in data management, organ allocation, matching theory, immunology, transplant surgery, and nephrology.
This article presents our experiences and the clinical outcomes of all patients transplanted during the first 4 y of the program.
MATERIAL AND METHODS
Recipient and Donor Management
Patients who are eligible for living donor kidney transplantation at a transplant center within Scandiatransplant can join the program. Each patient had at least 1 medically acceptable living donor. Both recipients and donors must provide informed consent for participation in STEP. The evaluation of living donors followed the national guidelines of each center. Acceptance criteria for living donors are similar between countries, but the local recipient center must approve the proposed living donor before accepting the exchange. All preoperative and postoperative investigations, surgical procedures, and clinical follow-up were performed locally at the transplantation units.
Accepted donor–recipient pairs can choose to be included in ≥1 match runs. Recipients can simultaneously be on the waiting list for kidney transplantation from a deceased donor. During the match runs, the recipients are temporarily deactivated on the waiting list.
Anonymous, Nondirected Donors
Anonymous, nondirected, living donors can join STEP and initiate kidney exchange chains. In the case of anonymous donation, the chain is ended by transplantation to a patient on the waitlist at the center in charge of the anonymous donor workup.
ABO Blood Group
All ABO blood groups are, by default, considered acceptable to the recipient. If any are not acceptable because of high isohemagglutinin titers, selected blood groups can be set as unacceptable for individual patients. ABO-incompatible (ABOi) transplantations were performed according to local routines.
HLA Typing and Antibody Identification
For patients and donors who are eligible for STEP, second-field resolution HLA genotyping is mandatory for the following loci: HLA-A, -B, -C, -DRB1, -DRB3/4/5, -DQB1, -DQA1, -DPA1, and -DPB1.
Furthermore, it is mandatory to test recipient sera with Labscreen Single Antigen (One Lambda, Inc., 22801 Roscoe Blvd, West Hill, CA) and to transfer the raw data directly to the Scandiatransplant web application. By default, an antibody reactivity of >2000 mean fluorescence intensity is considered unacceptable. However, it is possible to perform individual modifications to each specific test bead to determine which antigens are acceptable and which are not acceptable for a recipient.
Genomic HLA typing, HLA antibody identification, and determination of unacceptable HLA antigens were performed decentralized in the local EFI-accredited tissue typing laboratories.
Quality control of data was performed before and after each run, both manually and with incorporated functionalities in the application. The calculated panel-reactive antibody (cPRA) level was computed in the Scandiatransplant web application and was based on all (including historical) HLA antibodies.
When a match was found and the first evaluation phase had passed, the exchange of blood samples was arranged, and virtual crossmatch and donor HLA typing were confirmed at the recipient center.
The Algorithm/Optimization
The current STEP matching algorithm used by the Scandiatransplant web application consists of 2 parts: an initial immunological match followed by solving an optimization problem.
The algorithm for the initial immunological match compares information on acceptable ABO blood groups and discloses bead reactivity toward potential donor HLA alleles to determine whether recipients have any HLA DSAs against potential donors in the program.
The optimization problem is formulated as an integer linear program and identifies the matching for a given pool of patient–donor pairs based on the following hierarchical matching objectives:
Maximize the number of transplanted patients.
Short cycles (ie, maximizing the number of selected cycles to reduce logistical problems associated with long exchange cycles).
Prioritize patients according to a low matching probability (ie, the number of recipients who are difficult to transplant because of HLA sensitization should be maximized).
Compatible blood groups (ie, the number of ABOi transplants should be minimized).
Because the matching objectives may conflict with each other, the algorithm first searches for all matchings that maximize the number of transplanted patients (hierarchy 1), and, within all such matchings, chooses those that prioritize short cycles (hierarchy 2). In the unlikely event that this procedure does not result in unique matching, all matchings that satisfy the matching objectives criteria are regarded as equally good from the viewpoint of hierarchical objectives, and any of them may be selected. This hierarchical optimization technique is ideally suited for situations with potential trade-offs between matching objectives, which also explains why it is commonly adopted in KEPs worldwide.3 Because the algorithm searches in a strict hierarchical order, there is no weighting of the matching objectives.
The maximum setting for cycle length was 3-way exchanges, and for chains, it was 2 pairs + 1 anonymous donor + 1 waiting list recipient.
Statistical Methods
Statistics were made on data extracted from the Scandiatransplant web application in March 2023. Data entry in the Scandiatransplant database is dynamic; therefore, data are subject to changes based on prospective data submissions and/or corrections. Mean, median, and interquartile range (IQR) values were calculated using Microsoft Excel 365 version 2303. Kaplan-Meier graft survival analysis (censored for death) was performed using the statistical tool R version 4.1.0.
RESULTS
Match Runs
Between 2019 and 2022, 114 pairs and 6 anonymous donors participated in STEP.
Seventy-two recipients (63%) participated because of HLA incompatibility, 19 (17%) because of ABO incompatibility, 7 (6%) because of both HLA and ABO incompatibility, and 16 (14%) with other indications.
Eleven matching runs were performed. Moreover, because some cycles were broken in the early evaluation process, 4 match runs were followed by reruns. In these reruns, previously detected incompatible pairs were blocked.
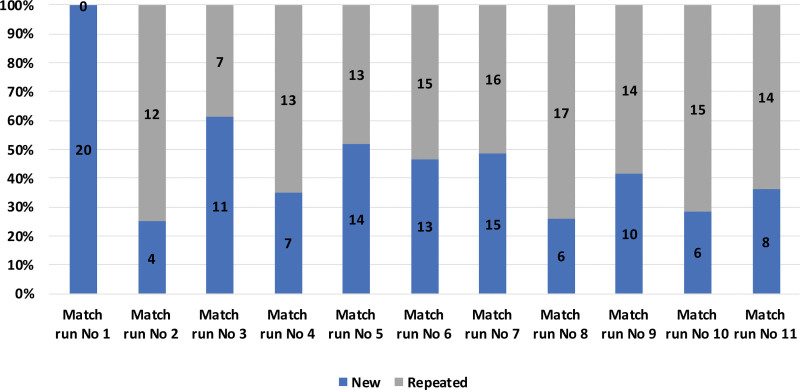
On average, 22 donor–recipient pairs were included in each match run, the largest number being in match run 7, in which 31 pairs and one anonymous donor participated. On average, 9 possible transplantations were identified in each run. Match run 7 identified 23 possible transplantations, which is the highest number to date. Most of the donor–recipient pairs included in the STEP participate in several match runs. Excluding data from the first match run, 59% (mean) of the pairs in each match run have participated in the previous run (Figure 1).
FIGURE 1.
Distribution in each match run of new pairs (new) and pairs included in several match runs (repeated).
Out of the 114 pairs, altogether 51 pairs (45%) participated in 1 match run, 31 pairs (27%) participated in 2 match runs, and 32 pairs (29%) participated in 3 or more match runs (Table 1). The number of match runs correlates with the level of HLA sensitization of the recipients, because the median cPRA level increases with the number of participations.
TABLE 1.
Match run participations divided by all the pairs that have participated (N = 114), median cPRA of all participating recipients, and the number of transplantations (N = 49)
| No. pairs | Median recipient cPRA, % | Transplanted pairs, n (%) | |
|---|---|---|---|
| Participated in 1 match run | 51 | 66 | 28 (54) |
| Participated in 2 match runs | 31 | 82 | 12 (39) |
| Participated in 3 match runs | 12 | 86 | 3 (25) |
| Participated in 4 or more match runs | 20 | 97.5 | 2 (10) |
cPRA, calculated panel-reactive antibody.
Table 1 indicates that it is difficult to find a suitable kidney graft for recipients who have participated in numerous match runs without undergoing transplantation.
In total, 11 match runs identified 56 cycles/chain, of which 35 (62.5%) did not proceed to transplantation. Most commonly, 21 cycles or chains were broken because of immunological incompatibility: 16 because of unacceptable DSA and/or repeated HLA mismatch, 4 recipients had unacceptably high ABO titers, and 1 patient had a positive crossmatch. Nonimmunological reasons for canceled cycles were lack of communication (n = 1), registration errors (n = 2), withdrawal of consent, changed clinical decisions, or acute illness (n = 11).
Thirty-four of the 114 pairs (31%) came out in ≥1 cycles/chain that were broken and did not proceed to transplantation through STEP. Thirty-five of 114 pairs (30%) with a median participation of 2 match runs (range, 1–8; IQR, 1–3.5) have so far not obtained a match; 77% of these patients had a cPRA >80%.
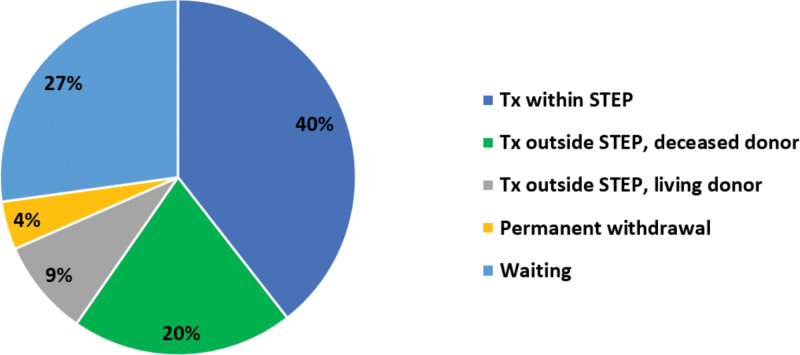
The status at the end of the year 2022 of all 114 donor–recipient pairs enrolled in STEP is depicted in Figure 2.
FIGURE 2.
Status at the end of the year 2022 of all donor–recipient pairs included in STEP. STEP, Scandiatransplant Exchange Program; Tx, transplant.
Transplantations
In total, 21 cycles/chains led to 49 transplantations, which means that 40% of the pairs enrolled in STEP were transplanted through the program (Figure 2).
Among the 21 cycles and chains, 12 were 2-way exchanges (57%), 5 were 3-way exchanges (24%), and 4 were chains initiated by an anonymous, nondirected living kidney donor. Fifty-four percent (54%) of the 2-way exchanges that were identified resulted in transplantation, whereas only 20% of the 3-way exchanges and 44% of the chains led to transplantation.
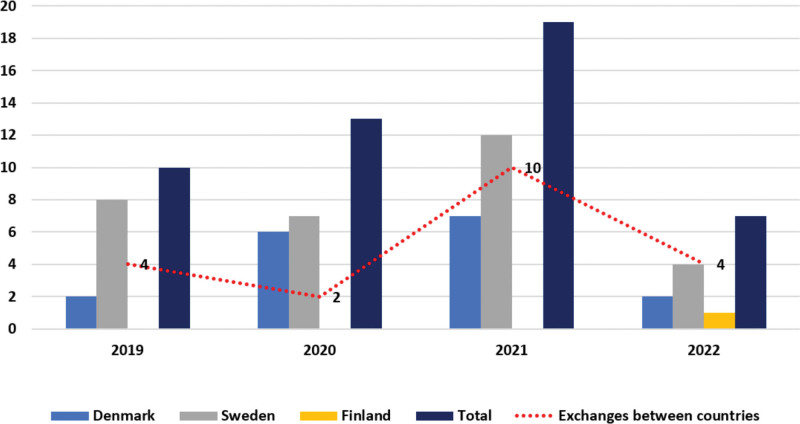
Denmark and Sweden had the most participants in the STEP during the first 4 y (Figure 3). Forty-one percent of the transplantations have been performed with a kidney imported from another Scandiatransplant country.
FIGURE 3.
The distribution of transplantations per year and country and out of these the number of kidneys exchanged between countries.
Twenty-six of the 49 transplantations (53%) were ABO-incompatible compared with 20% of the living kidney donor transplantations performed outside STEP in Scandiatransplant during the same period.
Demographics
The demographics of the 49 transplantations performed in STEP during the period 2019–2022 are listed in Table 2. The median recipient age was 50 y (range, 3–74) and the median donor age was 47 y (range, 26–70). The largest age difference between donor and transplant recipient was 43 y. Sixty-five percent of the recipients and 47% of the donors were female individuals, with gender mismatch in 59% of the transplantations. The median recipient body mass index was 24.3 (range, 13.5–31.9) and the median donor body mass index was 24.8 (range, 20.6–33.6).
TABLE 2.
Demographics of recipients, living donors, and transplantations (N = 49)
| Recipients | |
|---|---|
| Median age at transplantation, y | 50 (range, 3–74; IQR, 38–59) |
| ABO blood group, % | |
| A | 29 |
| B | 10 |
| AB | 6 |
| O | 55 |
| Median BMI, kg/m2 | 24.5 (range, 13.5–31.9; IQR, 22.1–27.5) |
| Transplant number, % | |
| 1 | 65 |
| 2 | 29 |
| 3 | 4 |
| ≥4 | 2 |
| cPRA, % | |
| 80–100 | 33 |
| 20–79 | 49 |
| 0–19 | 18 |
| Female, % | 65 |
| Diagnosis, % | |
| Polycystic kidney disease | 21 |
| Chronic glomerulonephritis | 8 |
| IgA nephropathy | 14 |
| Renal vascular disease | 12 |
| Diabetes | 2 |
| Congenital renal hypoplasia | 6 |
| Chronic renal failure, cause uncertain | 16 |
| Miscellaneous | 21 |
| Induction therapy, % | |
| Basiliximab | 86 |
| None | 14 |
| Immunosuppression, % | |
| MMF, tacrolimus, steroids | 88 |
| MMF, tacrolimus | 8 |
| Everolimus, tacrolimus, steroids | 4 |
| Median waiting time before Tx, total, mo | 12.5 (range, 0–109; IQR, 8.8–23.3) |
| Dialysis before Tx, % | |
| Preemptive | 49 |
| 0–12 mo | 14 |
| 13–24 mo | 8 |
| 25–36 mo | 14 |
| ≥37 mo | 15 |
| Living donors | |
| Median age at donation, y | 47 (range, 26–70; IQR, 41–58) |
| ABO blood group, % | |
| A | 53 |
| B | 10 |
| AB | 4 |
| O | 33 |
| Median BMI, kg/m2 | 24.8 (range, 20.5–33.6; IQR, 23.9–28.0) |
| Female, % | 47 |
| Relation, % | |
| Spouse | 42 |
| Sibling | 12 |
| Friend | 12 |
| Son/daughter | 10 |
| Parent | 8 |
| Other relation | 8 |
| Anonymous donor | 8 |
| Transplantations | |
| Median CIT, min | 329 (range, 60–615; IQR, 223–429) |
| Broad HLA mismatches, % | |
| 0 HLA-A mismatches | 10 |
| 0 HLA-B mismatches | 2 |
| 0 HLA-DRB1 mismatches | 14 |
| 0 HLA-DQB1 mismatches | 53 |
BMI, body mass index; CIT, cold ischemia time; cPRA, calculated panel-reactive antibody; IgA, immunoglobulin A; IQR, interquartile range; MMF, mycophenolate mofetil; Tx, transplant.
Approximately half of the recipients had been waiting for a deceased donor before STEP was initiated, and the waiting time from entry on the general waiting list to the actual transplant through STEP ranged from 0 to 109 mo (IQR, 8.8–23.3). The median waiting time from match identification to transplantation was 4.5 mo (range, 1.7–8.3).
In 49% of patients, the transplantations were done before the patient had started dialysis.
Patients with cPRA of 0% had a mean waiting time of 9 mo, patients with cPRA between 1% and 79% waited 16 mo, and highly sensitized patients with cPRA of ≥80% waited 27 mo.
The median cPRA of transplanted recipients was 68%, and 33% of the transplanted were highly sensitized (cPRA ≥80%). In the group still waiting for transplantation (Figure 2), the median cPRA was 98%, with 68% highly sensitized. Of all transplanted patients, 17 patients (35%) were retransplanted and in the group of patients still not transplanted, 52% were waiting for a retransplantation.
Prioritization of HLA identity between recipients and donors is currently not part of the STEP matching algorithm. The median number of HLA-A, -B, DRB1, and DQB1 broad-level mismatches was 4 (range, 1–8).
Recipients with blood groups AB and A had shorter mean waiting times (9 and 15 mo, respectively) than those with blood groups O and B (20 and 24 mo, respectively). The ABO blood group distribution of the transplanted recipients in STEP was 55% blood group O, 29% blood group A, 10% blood group B, and 6% blood group AB, which is like the patients still waiting for a match in STEP (58%, 26%, 10%, and 6%, respectively).
The median cold ischemia time (CIT) for the transplants was 329 min (range, 60–615). In 8 occasions, the exchange occurred within the transplant center, whereas in 41 occasions, the kidney was shipped between centers. In 47 of 49 transplanted recipients (96%), immediate graft function, defined as lowering of creatinine within the first postoperative day, was observed, and 2 patients experienced delayed graft function (DGF), with a duration of 2 and 4 d. The CITs for these recipients were 2.8 and 6.3 h, respectively, compared with the mean CIT of 5.5 h for all patients, and no correlation was observed between CIT and DGF.
All 49 recipients were alive, and 46 (94%) had a functioning kidney and 3 individuals (6%) had returned to dialysis.
Within the first year after transplantation, 8 recipients (16%) experienced biopsy-proven acute rejection; of these, 2 had antibody-mediated rejection, 4 had T cell–mediated rejection, and 2 presented borderline changes. These 8 recipients were all transplanted with an ABO-compatible kidney, and the median cPRA of the recipients was 65% (range, 0%–77%).
Of the 3 graft failures, 1 kidney did not recover its function after combined acute T cell–mediated rejection and antibody-mediated rejection, 1 patient experienced several episodes of T cell–mediated rejection and lost the graft 1 y and 7 mo after transplantation, and 1 patient experienced severe side effects from immunosuppression (diarrhea), had poor kidney function after rejection, and the graft was removed at the patient’s request within the first year.
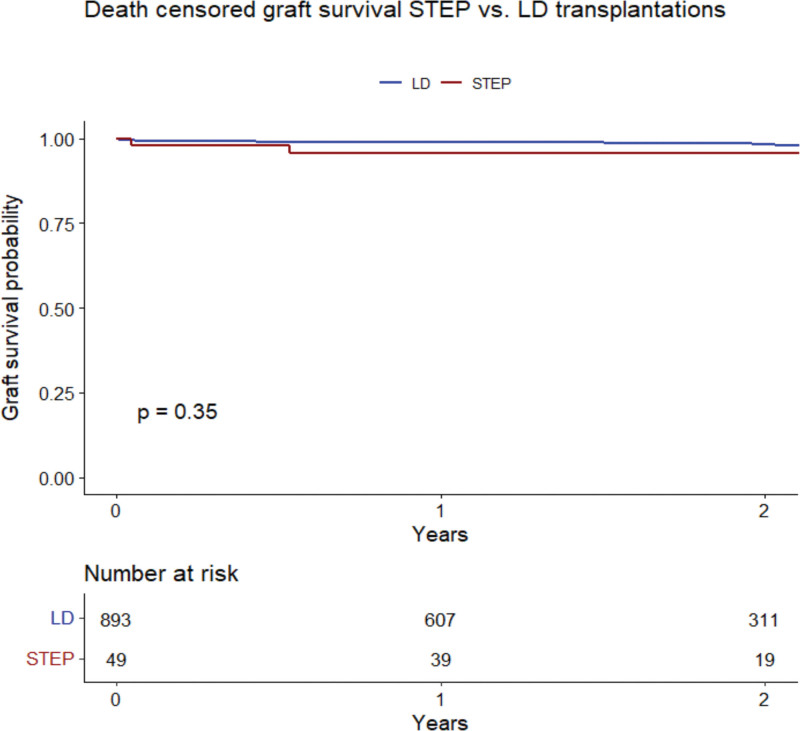
After living donor kidney transplantation in STEP, 2-y allograft survival was 95.8% (95% confidence interval, 90.2-100.0; Figure 4). In living donor kidney transplantation performed in Scandiatransplant outside STEP (n = 893), 2-y graft survival was 98.2% (95% confidence interval, 97.2-99.3) during the same period. There was no significant difference in graft survival between the STEP and living donor groups (P = 0.35).
FIGURE 4.
Kaplan-Meier curve comparing graft survival of recipients transplanted with kidneys from LDs through STEP and outside STEP (LD). LD, living donor; STEP, Scandiatransplant Exchange Program.
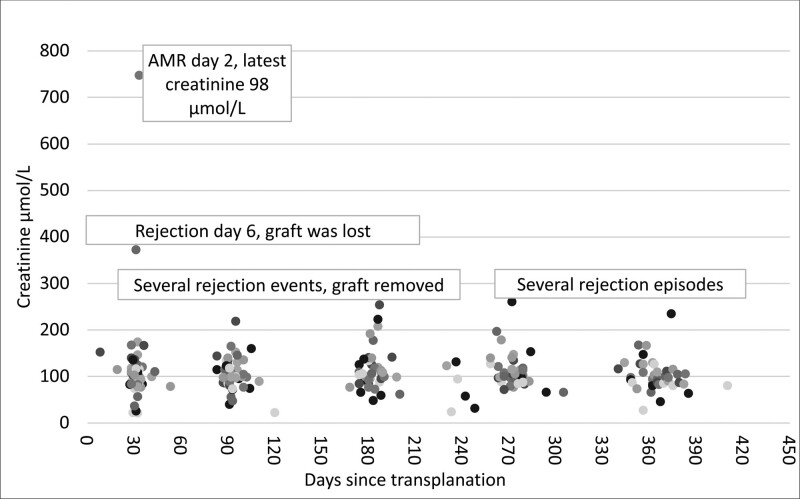
For patients with functioning grafts transplanted in STEP (n = 46), the median creatinine 1 y postoperatively (or latest available value) was 98 µmol/L (range, 28–235 µmol/L; Figure 5).
FIGURE 5.
Recipient creatinine values posttransplantation. AMR, antibody-mediated rejection.
DISCUSSION
To our knowledge, the first international kidney exchange was conducted in 1999 between an incompatible donor–recipient pair from Switzerland and a pair from Germany.9 However, this exchange was ad hoc in the sense that it was not a result of a structured clinical program. In 2016, the first binational program within Europe was launched with harmonized strategies between Austria and the Czech Republic, which led to the first European cross-border kidney exchange based on cooperation agreements.10
Currently, there are 4 established transnational KEPs: Australian and New Zealand Paired Kidney Exchange (Australia and New Zealand), CAI (Czech Republic, Austria, and Israel), KEP South Transplant Alliance (Spain, Italy, and Portugal), and STEP.8
Some of the key features of these 4 multinational programs are listed in Table 3. An important difference between transnational KEPs is whether they have a merged or sequential patient–donor pool. The former means that participating countries merge their national pools of patient–donor pairs into 1 joint transnational pool, and there are no parallel national KEPs. In contrast, in a sequential pool, only patient–donor pairs that have not found a match in their national KEPs are transferred to the transnational KEP. Another important difference is whether all national transplant centers in the participating countries are part of the transnational KEP. STEP stands out because it is the only transnational KEP with a merged donor–recipient pool that includes all national transplant centers in the participating countries and where no national program exists in parallel.
TABLE 3.
Summary of the key features of the 4 existing transnational KEPs
| Program | ANZKX | CAI | KEPSAT | STEP |
|---|---|---|---|---|
| Participating countries | Australia and New Zealand | Czech Republic, Austria and Israel | Spain, Italy, and Portugal | Denmark, Finland, Norway, and Sweden |
| First transplant | 2019 | 2016 | 2018 | 2019 |
| Pool type | Merged pool | Merged pool | Sequential pool | Merged pool |
| Participation by all national transplant centers? | Yes | No | No | Yes |
| Participating transplant centers | Australia (22 doing transplants, 13 doing retrieval) and New Zealand (3) | Austria (1), Czech Republic (1), and Israel (1) | Spain (10), Italy (7), and Portugal (1) | Denmark (3),Finland (1), Norway (1), and Sweden (4) |
ANZKX, Australian and New Zealand Paired Kidney Exchange; CAI, Czech Republic, Austria and Israel; KEPSAT, Kidney Exchange Program South Transplant Alliance; STEP, Scandiatransplant Exchange Program.
In STEP, 40% of the included pairs eventually participated in a kidney exchange, which is like the matching probability in the well-established Dutch National KEP.11 Also, for 30% of the donor–recipient pairs, a possible exchange has been identified that did not lead to transplantation, and for 30% of the pairs, no possible exchange has been found. Highly sensitized patients accumulate in the pool, as is illustrated both by the median cPRA of 68% of the transplanted compared with those waiting with a median cPRA of 98% and by the fact that the number of match run participation correlates with the degree of HLA sensitization.
In STEP, it is possible for ABOi pairs to participate voluntarily. This differs from praxis in the Dutch program, in which participation in the exchange program is mandatory for at least 1 match run before ABOi transplantation is performed.
The STEP outcomes of transplantations performed in the period 2019–2022 were excellent, almost half of the transplantations were done preemptively, and 1-y graft survival was 96%. In comparison, in the UK Living Kidney Sharing Scheme 1-y graft survival was 96%,12 and in the US National Kidney Registry, 1-y graft survival was 98%.13
The possible negative impact of prolonged CIT is a concern when shipping kidneys from living donors across borders. Out of 49 transplantations, 23 patients (47%) had a CIT of >6 h, and one of these patients (4%) experienced DGF, with a duration of 4 d. In comparison, in the UK Living Kidney Sharing Scheme program, the incidence of DGF was significantly higher in the group with CIT of >340 min (3.5% versus 2%).12
Out of the 49 transplantations, 3 (6%) failed during the observation period. All 3 grafts experienced acute rejection, which is considered the main cause of graft failure. The rate of biopsy-proven acute rejection (16%) was similar to what has been reported previously in KEPs (8%–18%).14
When preparing for STEP, HLA matching on 11 loci on second-field resolution and unique matching directly on mean fluorescence intensity values from the solid bead assay were initiated to make it possible to broadly include HLA-sensitized recipients and avoid positive pretransplant crossmatches. During the 11 match runs, only 1 pretransplant positive crossmatch occurred, resulting in 1 broken chain. However, we still have not overcome the immunological challenges in this setting, because 60% of the broken cycles/chains are related to immunological reasons. There is a learning curve, which indicates that some issues occur less frequently over time. Furthermore, adjustments have been made in the Scandiatransplant software to solve some of the issues; for example, it has become possible to set alleles as unacceptable at the serological split level.
The immunological preparation and matching approach in STEP differ from other deceased donor-matching programs within Scandiatransplant. Default settings are less restrictive when it comes to both HLA and ABO, trying to broaden the matching options, which is possible with living donors because you do not have the same time pressure in the evaluation process as you have with deceased donors. A risk-based approach and thorough case-to-case evaluation in a controlled environment may provide important knowledge that can be used in other allocation settings in the future. However, this strategy probably also plays a key role in the relatively high number of cycles broken for immunological reasons.
In the current algorithm, we prioritize the maximum number of transplantations. This priority was decided because we wanted to achieve an acceptable number of transplanted patients. Obviously, there is a risk that this priority could increase the number of HLA mismatches and ABOi transplantations, resulting in an increased need for apheresis treatment and an increased risk of HLA immunization.
Furthermore, there is also the risk of primarily finding donors for patients who are weakly or not HLA-sensitized, thereby accumulating the difficult-to-transplant patients in the pool.
Possible solutions could be prioritize to recipients with a low matching probability and prioritize 3-way exchanges with nested 2-way exchanges because the results show that 2-way exchanges are more likely to result in transplantation.
In the ongoing development of STEP, Scandiatransplant has had cooperation, information, and software sharing with the European Network for Collaboration on KEPs. In this process, the Scandiatransplant software was compared and validated up against the European Network for Collaboration on KEPs simulation tool in relation to the programming of the optimization criteria and simulation outcome.15
Simulations were conducted to evaluate modifications in the STEP matching algorithm and different priorities. The simulations were performed using 50 randomly selected pairs having participated in STEP, reflecting the “real life” pool size and securing acceptable data quality for the initial immunological match.
In these simulations, it was decided to evaluate:
High priority for difficult-to-transplant patients, as reflected by a low transplantability score (ie, low match probability).
Higher priority for patients who have participated in several match runs.
Higher priority 3-way exchanges that have nested 2 ways.
Based on these considerations, 6 different combinations of optimization criteria were created, and simulations were conducted. The results were compared between the present algorithm used in STEP and optimized algorithms.
In summary, no large differences were observed, demonstrating that it is possible to make small changes without compromising the number of transplantations and that the current algorithm works well. The sequential match runs and alternative optimization criteria are being discussed for additional simulation projects in the future. However, based on the outcomes of the simulations, the decision was made to keep the algorithm unchanged.
With many years of experience in deceased donor allocation within Scandiatransplant, it could also be an interesting challenge to consider the linkage between living and deceased donor programs. This can be achieved by the initiation of chains using a deceased donor.16 This option can be explored when all participating countries reach their expected full potential.
Another approach to help more immunologically challenging patients, which is continuously discussed within the Scandiatransplant, is to cooperate with other KEPs in Europe by participating in a sequential pool.
By doing so, the participating donor–recipient pairs in the pool will be more heterogenous than in the Scandiatransplant setting, which would potentially increase the chances of identifying possible exchanges. However, there are many practical, legal, and regulatory challenges to overcome in such a new constellation, remembering that the transplant hospitals within the Scandiatransplant have established agreements and a fundamental trust throughout >50 y of cooperation.
In summary, since 2019, STEP has become one of the largest multinational KEPs. STEP has increased the possibility of sensitized patients to be transplanted with a kidney from a living donor and, indirectly, has also increased the number of kidneys from deceased donors that are available to patients on the kidney waiting list. Additionally, anonymous, nondirected donors, and ABOi pairs were included in the program. The medical outcomes have been excellent, like those of other living donor kidney transplantations.
Footnotes
The study was supported by Scandiatransplant, Aarhus, Denmark, and Sahlgrenska University Hospital, Gothenburg, Sweden.
The authors declare no conflicts of interest.
I.D.W. collected and analyzed the data, contributed to the design of the study, and revised the article. P.L. drafted the article and contributed to the study design. T.A. drafted parts of the article and revised it. A.B., C.B., I.H., K.S., S.S.S., and L.W. provided data and revised the article. J.P.L., H.B., J.L., K.K., L.B., M.B., and M.B.A. revised the article.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Contributor Information
Ilse Duus Weinreich, Email: ilse.duus.weinreich@scandiatransplant.org.
Tommy Andersson, Email: tommy.andersson@nek.lu.se.
Margrét Birna Andrésdóttir, Email: mband@landspitali.is.
Mats Bengtsson, Email: mats.bengtsson@igp.uu.se.
Alireza Biglarnia, Email: alireza.biglarnia@skane.se.
Claus Bistrup, Email: claus.bistrup@rsyd.dk.
Line Boulland, Email: lboullan@ous-hf.no.
Helle Bruunsgaard, Email: helle.bruunsgaard@regionh.dk.
Ilkka Helanterä, Email: ilkka.helantera@hus.fi.
Kulli Kölvald, Email: kulli.kolvald@kliinikum.ee.
Jouni Lauronen, Email: jouni.lauronen@bts.redcross.fi.
Jørn Petter Lindahl, Email: linjor@ous-hf.no.
Karin Skov, Email: karin.skov@auh.rm.dk.
Lars Wennberg, Email: lars.wennberg@regionstockholm.se.
REFERENCES
- 1.Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. N Engl J Med. 2021;385:729–743. [DOI] [PubMed] [Google Scholar]
- 2.Segev DL, Gentry SE, Warren DS, et al. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293:1883–1890. [DOI] [PubMed] [Google Scholar]
- 3.Biro P, Haase-Kromwijk B, Andersson T, et al. ; ENCKEP COST Action. Building kidney exchange programmes in Europe—an overview of exchange practice and activities. Transplantation. 2019;103:1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari P, Weimar W, Johnson RJ, et al. Kidney paired donation: principles, protocols and programs. Nephrol Dial Transplant. 2015;30:1276–1285. [DOI] [PubMed] [Google Scholar]
- 5.Weinreich I, Bengtsson M, Lauronen J, et al. Scandiatransplant acceptable mismatch program—10 years with an effective strategy for transplanting highly sensitized patients. Am J Transplant. 2022;22:2869–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wennberg L, Lindner P, Linders J, et al. First donations and transplantations performed in Swedish kidney exchange program [article in Swedish]. Lakartidningen. 2019;116:FL4A. [PubMed] [Google Scholar]
- 7.Skov K, Weinreich ID, Bruunsgaard H, et al. The Nordic kidneyexchangeprogramme. Article in Danish. Ugeskr Laeger. 2020;182:V04200209. [PubMed] [Google Scholar]
- 8.Smeulders B, Pettersson W, Viana A, et al. Data and optimisation requirements for kidney exchange programs. Health Informatics J. 2021;27:14604582211009918. [DOI] [PubMed] [Google Scholar]
- 9.Thiel G, Vogelbach P, Gurke L, et al. Crossover renal transplantation: hurdles to be cleared!. Transplant Proc. 2001;33:811–816. [DOI] [PubMed] [Google Scholar]
- 10.Bohmig GA, Fronek J, Slavcev A, et al. Czech-Austrian kidney paired donation: first European cross-border living donor kidney exchange. Transpl Int. 2017;30:638–639. [DOI] [PubMed] [Google Scholar]
- 11.de Klerk M, Kal-van Gestel JA, Haase-Kromwijk BJ, et al. ; Living Donor Kidney Exchange Program. Eight years of outcomes of the Dutch Living Donor Kidney Exchange Program. Clin Transpl. 2011;287–290. [PubMed] [Google Scholar]
- 12.van de Laar SC, Robb ML, Hogg R, et al. The impact of cold ischaemia time on outcomes of living donor kidney transplantation in the UK Living Kidney Sharing Scheme. Ann Surg. 2021;274:859–865. [DOI] [PubMed] [Google Scholar]
- 13.Leeser DB, Thomas AG, Shaffer AA, et al. Patient and kidney allograft survival with national kidney paired donation. Clin J Am Soc Nephrol. 2020;15:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kher V, Jha PK. Paired kidney exchange transplantation—pushing the boundaries. Transpl Int. 2020;33:975–984. [DOI] [PubMed] [Google Scholar]
- 15.Klimentova X, Audry B, Andersson T, et al. WG3-WG4 Handbook of the COST Action CA15210. European Network for Collaboration on Kidney Exchange Programmes; 2021. [Google Scholar]
- 16.Wang W, Leichtman AB, Rees MA, et al. Kidney paired donation chains initiated by deceased donors. Kidney Int Rep. 2022;7:1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]