Abstract
PURPOSE
With the recent approval of the KRAS G12C inhibitor sotorasib for patients with advanced KRAS G12C-mutant non–small cell lung cancer (NSCLC), there is a new need to identify factors associated with activity and toxicity among patients treated in routine practice.
MATERIALS AND METHODS
We conducted a multicenter retrospective study of patients treated with sotorasib outside of clinical trials to identify factors associated with real-world progression free survival (rwPFS), overall survival (OS), and toxicity.
RESULTS
Among 105 patients with advanced KRAS G12C-mutant NSCLC treated with sotorasib, treatment led to a 5.3-month median rwPFS, 12.6-month median OS, and 28% real-world response rate. KEAP1 comutations were associated with shorter rwPFS and OS (rwPFS hazard ratio [HR], 3.19; P = .004; OS HR, 4.10; P = .003); no significant differences in rwPFS or OS were observed across TP53 (rwPFS HR, 1.10; P = .731; OS HR, 1.19; P = .631) or STK11 (rwPFS HR, 1.66; P = .098; OS HR, 1.73; P = .168) comutation status. Notably, almost all patients who developed grade 3 or higher treatment-related adverse events (G3+ TRAEs) had previously been treated with anti–PD-(L)1 therapy. Among these patients, anti–PD-(L)1 therapy exposure within 12 weeks of sotorasib was strongly associated with G3+ TRAEs (P < .001) and TRAE-related sotorasib discontinuation (P = .014). Twenty-eight percent of patients with recent anti–PD-(L)1 therapy exposure experienced G3+ TRAEs, most commonly hepatotoxicity.
CONCLUSION
Among patients treated with sotorasib in routine practice, KEAP1 comutations were associated with resistance and recent anti–PD-(L)1 therapy exposure was associated with toxicity. These observations may help guide use of sotorasib in the clinic and may help inform the next generation of KRAS G12C-targeted clinical trials.
Real-world analysis by @rohit_thum @RielyMD @KCArbourMD identifies KEAP1 mutations associated with resistance and recent anti–PD-(L)1 therapy exposure associated with toxicity among patients receiving sotorasib for advanced KRAS G12C+ NSCLC.
INTRODUCTION
KRAS is the most frequently mutated oncogene in patients with non–small cell lung cancer (NSCLC), with activating mutations in KRAS found in up to 30% of patients with nonsquamous NSCLC.1,2 KRAS G12C is the most common subtype, present in approximately 40% of patients with KRAS-mutant lung cancer.3 For decades, KRAS had been considered an undruggable target.4,5 However, recent efforts aimed at developing inactive conformation-specific KRAS inhibitors6 have led to the first KRAS-directed therapies with meaningful clinical activity.
CONTEXT
Key Objective
Given recent approval of the KRAS G12C inhibitor sotorasib for patients with advanced KRAS G12C-mutant non–small cell lung cancer, there is a new need to identify features associated with activity and toxicity in the real-world setting. This multicenter retrospective study examined clinical and genomic features associated with progression-free survival (PFS), overall survival (OS), and clinically significant toxicity among patients treated with sotorasib in routine practice.
Knowledge Generated
In this real-world analysis, patients with concurrent mutations in KEAP1 experienced shorter PFS and OS with sotorasib, while there were no significant associations between concurrent TP53 or STK11 mutations and activity. In addition, high-grade toxicities, particularly hepatotoxicity, were almost exclusively associated with recent anti–PD-(L)1 therapy exposure before sotorasib.
Relevance
Careful consideration of patient genomics and recent systemic therapy exposures are required for management of sotorasib in the clinic. These results may also help inform ongoing KRAS G12C-directed clinical trials.
Sotorasib is a small molecule that covalently modifies the mutant cysteine in the KRAS G12C protein, irreversibly locking KRAS G12C in an inactive conformation and blocking interaction with downstream effectors.7 Sotorasib was granted accelerated approval by the US Food and Drug Administration (FDA) in 2021 on the basis of a multicenter, single-arm, phase II study that demonstrated a 37% objective response rate, 6.8-month median progression-free survival (PFS), and 12.5-month median overall survival (OS) for sotorasib among patients with advanced KRAS G12C-mutant advanced NSCLC with disease progression on previous systemic therapy.8 In addition to sotorasib, multiple other G12C-directed agents are currently under clinical development, including adagrasib,9 which was recently granted accelerated approval by the US FDA, as well as GDC-6036,10 LY3537982,11 and JDQ443.12
Despite the activity of sotorasib demonstrated in clinical trials, there are currently no real-world data describing clinical activity and toxicities observed among patients treated with sotorasib as part of routine care to help guide adoption in the clinic. In addition, although patterns of acquired resistance to sotorasib have been identified, including development of subclonal secondary RAS alterations and bypass pathway alterations,13 our understanding of the determinants of primary response or resistance to sotorasib remains limited. Concurrent inactivating STK11 and KEAP1 mutations have been associated with worse outcomes to anti–PD-(L)1 therapy in NSCLC,14,15 and concurrent KEAP1 mutations have been associated with shorter responses to both platinum-based chemotherapy and anti–PD-(L)1 therapy.3 However, responses to sotorasib and adagrasib have been observed across STK11/KEAP1 mutation status in clinical trials,8,9 and the impact of comutation status on durability of response or OS has not been reported.
To identify clinical and genomic features associated with outcomes, we conducted a retrospective analysis of patients treated with sotorasib outside of the clinical trial setting. We also sought to explore the rate of clinically significant toxicities in a real-world population, and explore associations of pretreatment clinical characteristics with development of toxicity. Our results may help inform future clinical trials, provide insights into the optimal sequencing of sotorasib in the clinic, and may have implications for other KRAS G12C-directed agents currently under clinical development.
MATERIALS AND METHODS
Study Population
We retrospectively identified patients with advanced KRAS G12C-mutant NSCLC who initiated sotorasib outside of clinical trials between June 2021 and August 2022 at three institutions: Memorial Sloan Kettering Cancer Center (MSKCC), New York Presbyterian/Columbia University Irving Medical Center (Columbia), and NYU Langone Perlmutter Cancer Center (NYU), all in New York, NY. The study was approved as a retrospective research protocol by the MSKCC Institutional Review Board/Privacy Board.
Clinical Data and Immunogenomic Analyses
Clinical data, including demographics, clinicopathologic features, targeted next-generation sequencing (NGS) results, clinical and radiographic outcomes, and descriptions of toxicities, were abstracted. Genomic data describing the presence or absence of concurrent TP53, STK11, and KEAP1 comutations were identified by the tissue NGS and plasma circulating tumor DNA platforms described in the Data Supplement (Supplementary Table S1). Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) tissue NGS samples (v5, v6, and v7) were analyzed for tumor mutational burden. Normalized mutation burden was calculated as the absolute mutation burden (number of nonsynonymous mutations/sample) divided by the genomic coverage for that sample as previously described.16 When available, PD-L1 tumor cell immunohistochemistry results obtained before sotorasib initiation were included.
Clinical Outcomes
The clinical outcomes under investigation were real-world PFS (rwPFS), time on drug, OS, and real-world response rate by investigator assessment (rwRR). rwPFS was defined as the amount of time between sotorasib initiation and either radiographic worsening or clinical progression of disease as documented in the treating physician's notes. Patients who did not have documented clinical progression but in whom sotorasib was discontinued or changed for other reasons, including toxicity or physician/patient preferences, were censored at the date of last contact or the start date of the next treatment regimen. Time on drug was defined as the amount of time between the first date and last date of sotorasib treatment, and OS was defined as the amount of time between sotorasib initiation and death from any cause. To assess real-world response, the last computed tomography scan of the chest, abdomen, and/or pelvis and/or brain magnetic resonance imaging documenting, all known sites of disease before sotorasib initiation, was reviewed and compared with the results of the first corresponding scans obtained at least 6 weeks after sotorasib initiation. Scan comparisons for assessment of response rate and rwPFS were completed manually by investigators by adapting the PRISSMM framework,17 a structured framework to assess changes in cancer status over time. On the basis of manual review of the impression section of radiology reports, we defined categories of rwRR to include improvement, defined by cancer improving/responding, stability, defined by stability/no change or mixed changes, or worsening, defined by cancer progressing/worsening/enlarging. Real-world response (response) was defined as radiographic improvement, and real-world disease control was defined as either radiographic improvement or stability. Assessment of changes in size of brain metastases were not included in assessment of rwRR, but were included as evidence of progression of disease if a new brain lesion was identified after initiation of treatment.
Toxicity
Medical records and results of routine laboratory testing were reviewed to identify treatment-related adverse events (TRAEs) and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.18 Medical records were also reviewed to identify the incidence of sotorasib dose reduction because of TRAEs, and the incidence of sotorasib discontinuation because of TRAEs, as documented in the treating physician's notes.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism v9 and the survival package in R version 4.2.2. For all proportions, 95% CIs were calculated by the Wilson/Brown method. rwPFS, time on drug, and OS were estimated using Kaplan-Meier methodology, with 95% CIs for median survival times reported. 95% CIs for odds ratios (ORs) were calculated using the Baptista-Pike method. Univariate group comparisons between Kaplan-Meier curves were performed using log-rank tests, and the Mantel-Haenszel method was used to determine hazard ratios (HRs) between groups. Fisher's exact test was used to compare proportions between univariate groups. All reported P values are two-sided, and significance level was set at P = .05 for all analyses.
RESULTS
Baseline Clinical and Pathologic Characteristics
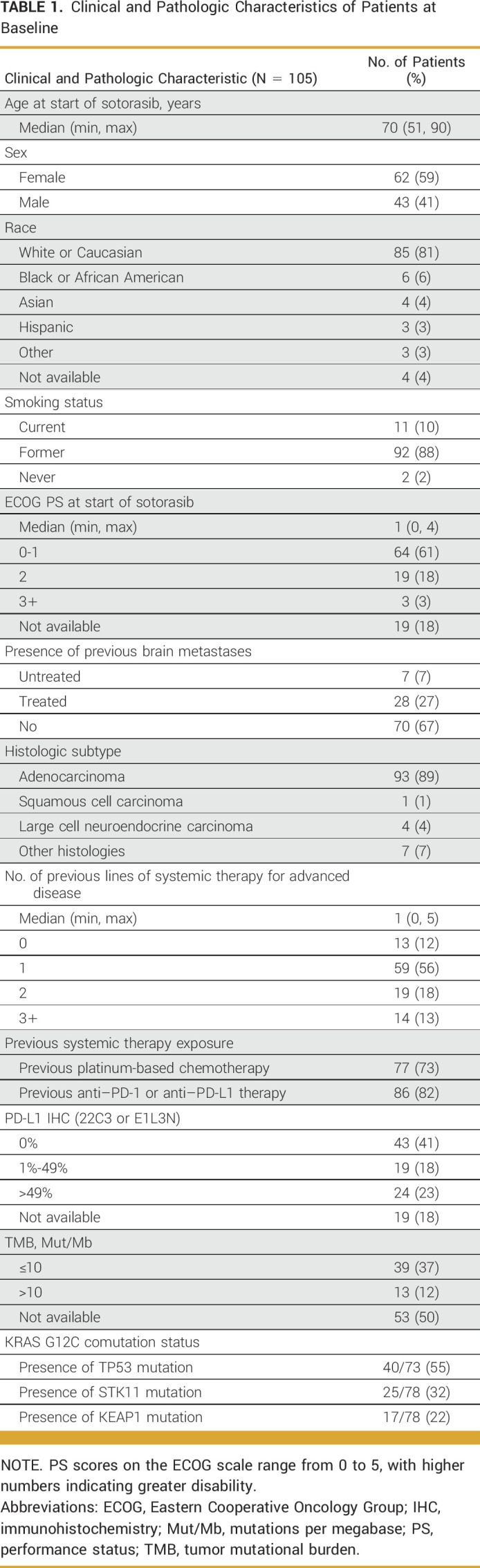
A total of 105 patients with advanced KRAS G12C-mutant NSCLC treated with standard-of-care sotorasib were identified and included in the analysis (Table 1). The data cutoff date was January 15, 2023, and the median duration of follow-up was 13.1 months.
TABLE 1.
Clinical and Pathologic Characteristics of Patients at Baseline
Clinical Activity of Standard-of-Care Sotorasib
Among patients in our cohort, the median rwPFS was 5.3 months (95% CI, 3.6 to 6.6 months; Fig 1A), the median time on drug was 7.2 months (95% CI, 4.6 to 10.4 months; Data Supplement [Supplementary Fig S1]), and the median OS was 12.6 months (95% CI, 8.3 months to could not be evaluated [NA]; Fig 1B). Among 102 of 105 patients evaluable for response assessment, the rwRR was 28% (95% CI, 20 to 37) and the real-world disease control rate was 74% (95% CI, 64 to 81). Notably, among 13 patients who were treatment-naïve in the advanced setting (median age of 60 years and median Eastern Cooperative Oncology Group performance status [ECOG PS] of 1 at initiation), median rwPFS was 11.0 months (95% CI, 7.3 months to NA) and median OS was not reached with a median duration of follow-up of 14.1 months.
FIG 1.
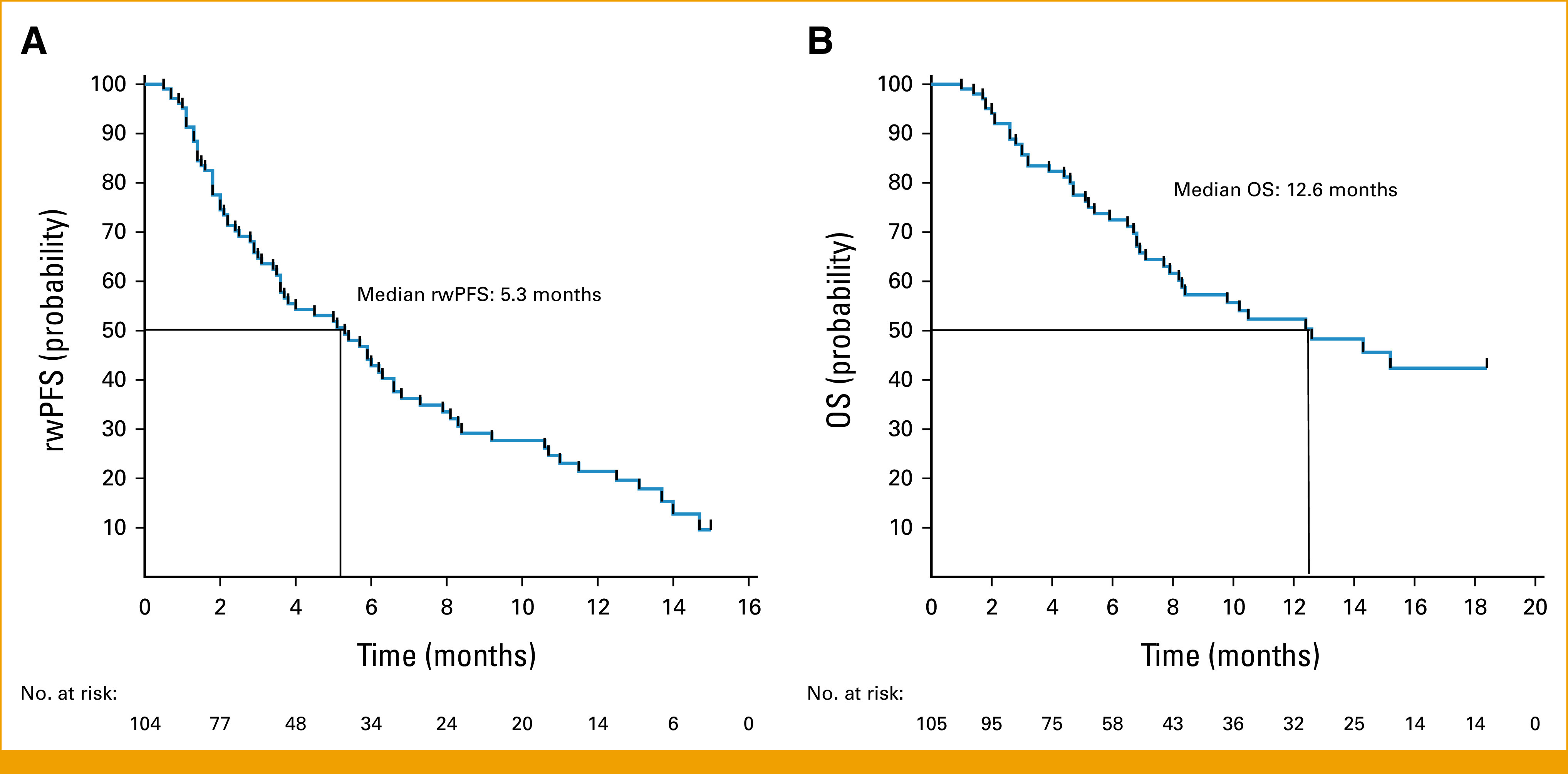
rwPFS and OS among patients who received sotorasib for advanced KRAS G12C-mutant Non–Small Cell lung cancer. Kaplan-Meier curve of (A) rwPFS and (B) OS among evaluable patients. Tick marks indicate censored data. OS, overall survival; rwPFS, real-world progression-free survival.
Pathologic Features and Clinical Activity of Sotorasib
We next evaluated pathologic features associated with sotorasib activity in our cohort. Most notably, KEAP1 comutations were associated with shorter rwPFS (HR for progression or death, 3.19; 95% CI, 1.46 to 6.95; P = .004; Data Supplement [Supplementary Fig S2]) and OS (HR, 4.10; 95% CI, 1.64 to 10.3; P = .003) to sotorasib (Fig 2), with a median rwPFS of 2.0 months (95% CI, 1.4 months to NA) and median OS of 5.2 months (95% CI, 3.0 months to NA) in the KEAP1 comutation subgroup (N = 17). Among patients with and without STK11 comutations, no significant differences in rwPFS (HR, 1.66; 95% CI, 0.91 to 3.04; P = .098) or OS (HR, 1.73; 95% CI, 0.79 to 3.77; P = .168) were observed. We observed no significant association of TP53, STK11, or KEAP1 mutations with real-world response (Data Supplement [Supplementary Table S2]). In addition, we observed no significant differences in OS (HR, 1.01; 95% CI, 0.53 to 1.95; P = .973) or real-world response (OR, 0.76; 95% CI, 0.28 to 1.97; P > .634) among PD-L1–negative tumors compared with PD-L1–positive tumors, and no significant differences in OS (HR, 0.96; 95% CI, 0.41 to 2.32; P = .944) or real-world response (OR, 0.92; 95% CI, 0.23 to 3.14; P > .999) among tumor mutational burden (TMB) ≤ 10 tumors compared with TMB > 10 tumors (Data Supplement [Supplementary Fig S3]).
FIG 2.
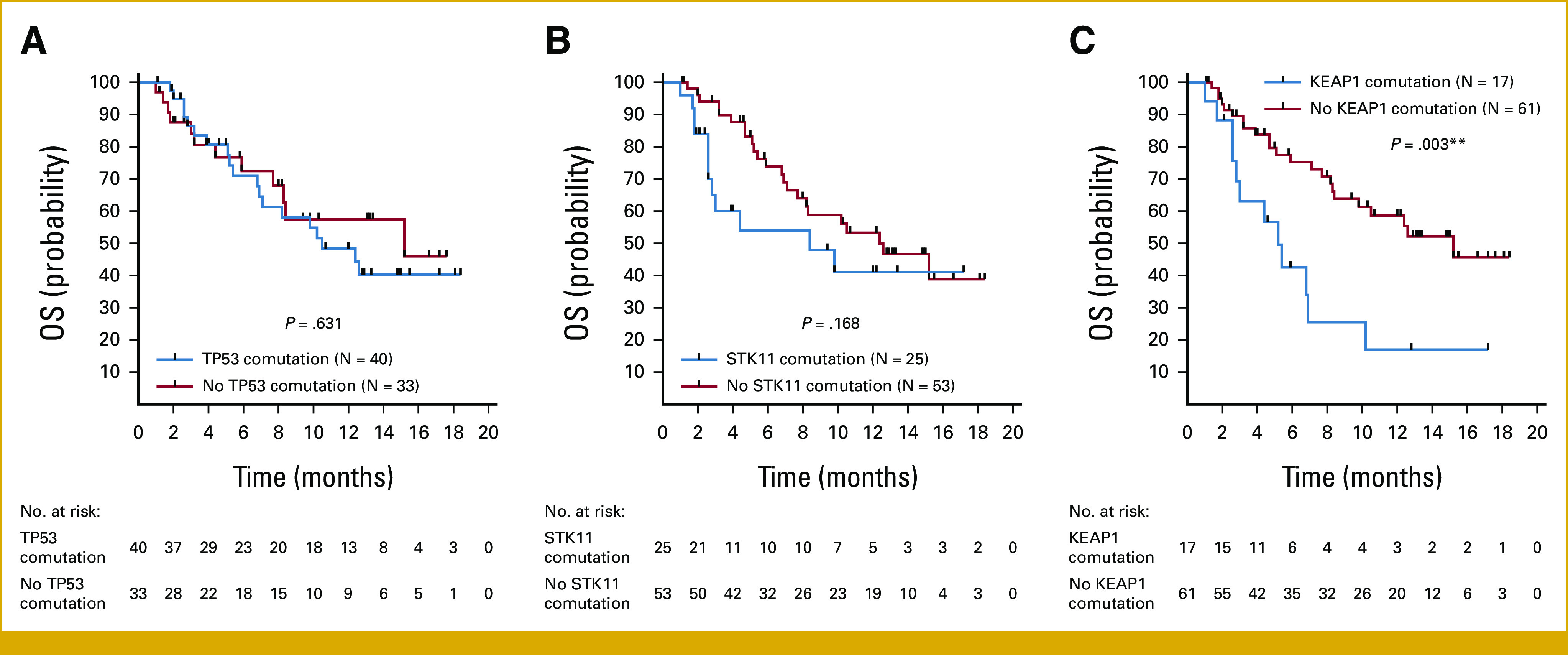
Influence of concurrent TP53, STK11, and KEAP1 mutations on OS to sotorasib. Kaplan-Meier curves of OS among patients with KRAS G12C-mutant Non–Small Cell lung cancer with or without concurrent mutations in (A) TP53, (B) STK11, and (C) KEAP1. Tick marks indicate censored data. **P < .01 for log-rank test between curves. OS, overall survival.
Sotorasib Toxicity and Associations With Recent Previous Lines of Therapy
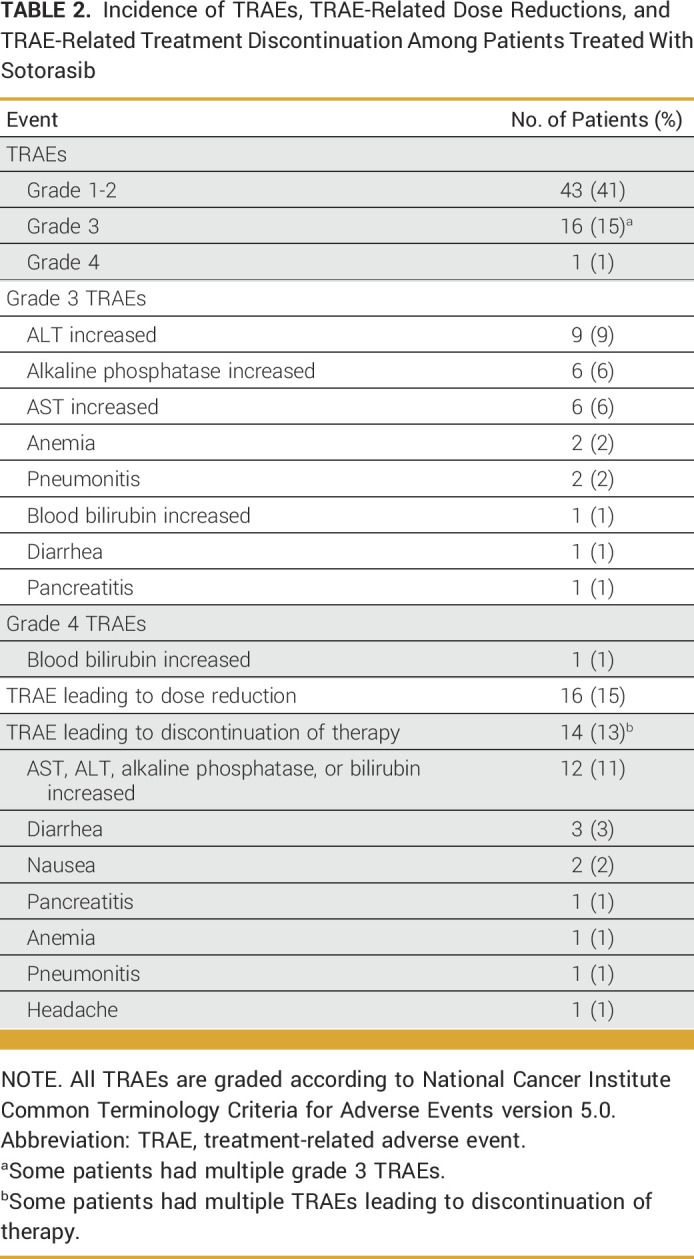
We next evaluated the incidence of, and features associated with, clinically significant toxicity from sotorasib administration. Almost all patients in our cohort initiated sotorasib at a dose of 960 mg once daily (102 of 105 patients; 97%). The most common grade 3 (G3) or higher TRAEs were elevated liver function tests (Table 2), and the most common reasons for TRAE-related discontinuation of therapy were elevated liver function tests (observed in 86% of discontinuations) and diarrhea (21% of discontinuations).
TABLE 2.
Incidence of TRAEs, TRAE-Related Dose Reductions, and TRAE-Related Treatment Discontinuation Among Patients Treated With Sotorasib
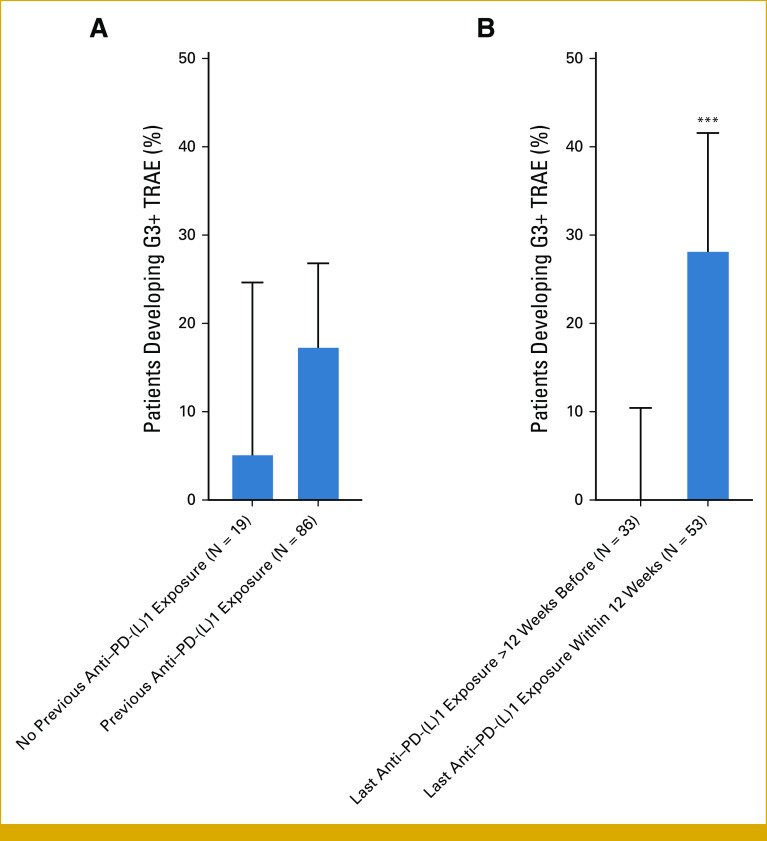
We first aimed to explore clinical features of patients who were predisposed to development of sotorasib toxicity. In particular, we explored whether previous exposure to anti–PD-(L)1 therapy was associated with G3 or higher (G3+) TRAEs to sotorasib and/or TRAE-related discontinuation of treatment. Notably, 15 of 16 cases of G3+ sotorasib-related TRAEs in our cohort were observed in patients with previous anti–PD-(L)1 therapy exposure, with 15 of 86 (17%) of patients with previous anti–PD-(L)1 therapy exposure experiencing G3+ sotorasib TRAEs. Of the 19 anti–PD-(L)1 therapy-naïve patients, only one experienced a G3+ sotorasib TRAE (Fig 3A). In addition, all 14 patients who experienced TRAE-related sotorasib discontinuation had previously been treated with anti–PD-(L)1 agents. By contrast, previous platinum-based chemotherapy did not appear to be associated with development of G3+ sotorasib TRAEs (no previous platinum exposure: five of 28 patients with G3+ TRAEs [18%], previous platinum exposure: 11 of 77 patients with G3+ TRAEs [14%]; OR, 0.77; P = .760), or TRAE-related sotorasib discontinuation (no previous platinum exposure: five of 28 patients with TRAE-related sotorasib discontinuation [18%], previous platinum exposure: nine of 77 patients with TRAE-related sotorasib discontinuation [12%]; OR, 0.61; P = .517).
FIG 3.
Influence of previous anti–PD-(L)1 therapy exposure on incidence of severe sotorasib-related adverse events. (A) Incidence of G3+ sotorasib-related adverse events among patients with no previous anti–PD-(L)1 exposure (1/19; 5%) and previous exposure (15/86; 17%). (B) Among patients with previous anti–PD-(L)1 exposure (N = 86 total), incidence of G3+ sotorasib-related TRAEs among patients with last anti–PD-(L)1 exposure more than 12 weeks before initiation of sotorasib (0/33; 0%) and among patients with last exposure within 12 weeks of initiation of sotorasib (15/53; 28%). ***P < .001 for Fisher's exact test. G3+, grade 3 or higher; TRAE, treatment-related adverse event.
We then sought to identify the aspects of previous anti–PD-(L)1 therapy exposure that predispose patients to toxicity from sotorasib. Among the 86 patients with previous anti–PD-(L)1 therapy exposure, 25 (29%) had previously experienced an immune-related adverse event (IRAE) as defined by ASCO guidelines.19 Previous IRAEs were not associated with development of G3+ sotorasib-related adverse events (no previous IRAEs: 10 of 61 with G3+ sotorasib TRAEs [16%], previous IRAEs: five of 25 with G3+ sotorasib TRAEs [20%]; OR, 1.28; P = .757). We did, however, observe a striking association between the proximity of the last dose of anti–PD-(L)1 therapy to sotorasib initiation and development of toxicity from sotorasib; 15 of 16 cases of G3+ sotorasib TRAEs and 13 of 14 cases of sotorasib TRAE-related treatment discontinuation were observed among patients with previous anti–PD-(L)1 exposure within 12 weeks of sotorasib initiation. Among the 53 patients with anti–PD-(L)1 therapy exposure within 12 weeks of initiation of sotorasib, 15 (28%) experienced G3+ sotorasib-related TRAEs, whereas there were no cases of G3+ sotorasib AEs among the 33 patients with last anti–PD-(L)1 exposure more than 12 weeks before initiation of sotorasib (Fig 3B).
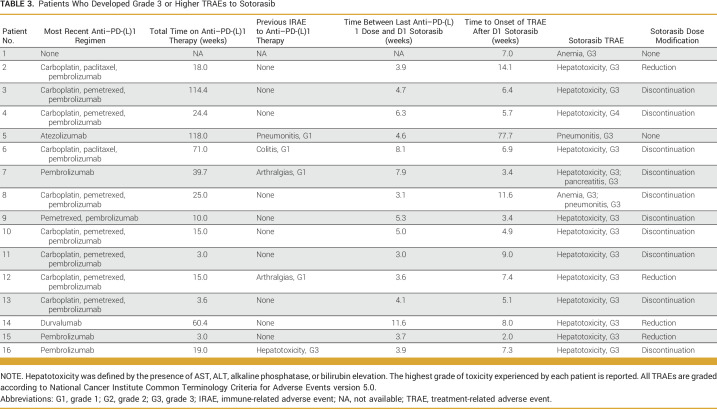
Among the 15 of 16 patients who experienced a G3+ TRAE after previous anti–PD-(L)1 therapy exposure (Table 3), patients received their last dose of anti–PD-(L)1 therapy a median of 4.6 weeks (range, 3.0 to 11.6 weeks) before sotorasib initiation, with only five of 15 patients having experienced a previous IRAE. Thirteen of these 15 patients experienced G3+ hepatotoxicity, with a median time to onset of hepatotoxicity of 6.4 weeks (range, 2.0 to 14.1 weeks). Among these 13 patients, five were treated with systemic steroids, with four experiencing clinical improvement, defined as improvement to grade 1 hepatotoxicity or better within 12 weeks of steroid initiation.
TABLE 3.
Patients Who Developed Grade 3 or Higher TRAEs to Sotorasib
DISCUSSION
The accelerated approval of sotorasib for patients with advanced KRAS G12C-mutant NSCLC represented a meaningful advance in the emerging field of KRAS-directed targeted therapy. This approval has also provided an opportunity to evaluate the optimal sequencing of sotorasib in the context of other lines of therapy, and how sotorasib may be safely and effectively combined with other treatments, including platinum-based chemotherapy and/or anti–PD-(L)1 immunotherapy. To begin to address these questions, we performed a multicenter retrospective analysis of patients treated with sotorasib outside of the clinical trial setting.
Clinicians appeared to have prioritized sotorasib early in the course of care, with a median of one previous line of therapy among patients. In addition, patients in our study appeared relatively fit, with a majority of patients with an ECOG PS of 0-1 at initiation of sotorasib. Our results roughly correspond to the results of the recently presented randomized phase III study of sotorasib versus docetaxel in the second-line or later setting, in which sotorasib treatment in patients with a median of one previous line of therapy exhibited a RECIST objective response rate of 28% (95% CI, 22 to 35), median PFS of 5.6 months (95% CI, 4.3 to 7.8 months), and median OS of 10.6 months (95% CI, 8.9 to 14.0 months).20
Co-occurring genomic alterations underlie significant heterogeneity in disease biology and response to systemic therapy in patients with KRAS-mutant NSCLC, with TP53, STK11, and KEAP1 the most commonly comutated genes in patients with KRAS G12C-mutant disease.21 STK11 and KEAP1 comutations have previously been established as markers of resistance to anti–PD-(L)1 therapy in KRAS-mutant NSCLC,14,15 and KEAP1 comutations are associated with shorter responses to platinum-based chemotherapy in these patients.3 However, the influence of these markers on clinical activity of KRAS-directed targeted therapies has remained unclear. In this cohort, KEAP1 mutations were associated with shorter rwPFS and OS to sotorasib, whereas STK11 mutations were not associated with statistically significant differences in rwPFS and OS. These results should continue to be evaluated in larger prospective studies and potentially be incorporated as stratification factors in future randomized clinical trials.
Most notably, our study sheds significant light on patients who are uniquely at risk for development of toxicity related to sotorasib. Recent anecdotal evidence and case reports22 have suggested links between previous anti–PD-(L)1 therapy exposure and development of toxicity with sotorasib administration. We found clear associations between previous anti–PD-(L)1 therapy exposure, specifically within 12 weeks of initiation of sotorasib, and both G3+ TRAEs to sotorasib and TRAE-related sotorasib discontinuation, with treatment-related hepatotoxicity the most common G3+ TRAE. Our results appear analogous to previous observations of severe adverse events seen with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors administered shortly after anti–PD-(L)1 therapy,23 and help further contextualize the increased toxicity signals recently observed with combination sotorasib and pembrolizumab or atezolizumab.24 Awareness of these findings may assist clinicians with optimal timing and sequencing of these therapies in the clinic and devising appropriate follow-up plans after sotorasib initiation among patients who are started shortly after exposure to anti–PD-(L)1 therapies. In addition, independent of the effects of dose reduction or discontinuation, whether hepatotoxicity encountered shortly after anti–PD-(L)1 therapy exposure is responsive to steroid administration is an important future consideration. Finally, whether these phenomena are observed with other KRAS G12C inhibitors, including the newly approved adagrasib, remains unclear.
Although this work is limited by the retrospective nature of the analysis and modest sample size, it reflects outcomes from real-world management of patients from multiple institutions. In this report, retrospective response assessment was completed manually by investigators using the PRISSMM framework17 rather than RECIST version 1.1 measurements. Nevertheless, we observed relative concordance between rwPFS and time on drug in our study, and assessments linked to treatment duration in lung cancer have been identified as reasonable proxies for RECIST-defined PFS.25 An additional challenge of real-world outcome analyses can be uniform collection of toxicity data. Although the frequency of TRAE-related sotorasib dose reductions and discontinuations identified in our study was similar to frequencies observed in the CodeBreaK100 study,8 the documented frequency of grade 1-2 and G3 TRAEs was lower in our study, which may reflect differences in documentation of toxicities between clinical trial and real-world settings.
Our study suggests that sotorasib is associated with modest single-agent activity in the real-world setting, and that assessment of KRAS comutation status and recent exposure to anti–PD-(L)1 therapy are important considerations before sotorasib administration in the clinic. Further exploration of best strategies to mitigate toxicity is crucial to optimize targeted therapies for patients with advanced NSCLC harboring KRAS G12C mutations.
Benjamin Herzberg
Honoraria: Eisai
Consulting or Advisory Role: Amgen
Research Funding: Repare Therapeutics (Inst), IDEAYA Biosciences (Inst), Amgen (Inst), Revolution Medicines (Inst), Astellas Pharma (Inst)
Bob T. Li
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Amgen (Inst), Lilly (Inst), MORE Health (Inst), Bolt Biotherapeutics (Inst), Ambrx (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst), Karger Publishers—Book royalty, Shanghai Jiao Tong University Press—Book royalty
Travel, Accommodations, Expenses: MORE Health, Jiangsu Hengrui Medicine
Uncompensated Relationships: Amgen, AstraZeneca, Genentech, Lilly, Boehringer Ingelheim, Daiichi Sankyo
Isabel Preeshagul
Consulting or Advisory Role: OncLive, Dava Oncology, G1 Therapeutics, LabCorp, Pfizer, Novartis, Novartis, Genentech, Jazz Pharmaceuticals, Intellisphere, AstraZeneca, Boehringer Ingelheim
Fernando C. Santini
Speakers' Bureau: AstraZeneca
Marc Ladanyi
Consulting or Advisory Role: AstraZeneca, ADC Therapeutics, Paige.AI, Merck, Bayer
Research Funding: Merus NV (Inst), Elevation Oncology (Inst), Rain Therapeutics
Soo-Ryum Yang
Honoraria: Prime Education
Consulting or Advisory Role: InVitae
Piro Lito
Leadership: Frontier Medicines, BioTheryX
Stock and Other Ownership Interests: Frontier Medicines, Biotheryx
Consulting or Advisory Role: Black Diamond Therapeutics, Repare Therapeutics, AmMax Bio, Revolution Medicines, OrbiMed, Frontier Medicines, PAQ-Tx, BioTheryX
Speakers' Bureau: Boehringer Ingelheim, Ikena Oncology
Research Funding: Mirati Therapeutics (Inst), Revolution Medicines (Inst), Amgen (Inst), Boehringer Ingelheim (Inst), Virtec pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat BRAF mutant cancers (Inst), I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat KRAS mutant cancers (Inst)
Gregory J. Riely
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Mirati Therapeutics (Inst), Merck (Inst), Takeda (Inst), Lilly (Inst), Pfizer (Inst), Rain Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Bayer, Merck
Other Relationship: Pfizer, Roche/Genentech, Takeda, Mirati Therapeutics
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Kathryn C. Arbour
Consulting or Advisory Role: Sanofi, Genzyme, Novartis
Research Funding: Mirati Therapeutics (Inst), Genentech (Inst), Revolution Medicines (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a poster and abstract the Targeted Therapies of Lung Cancer Meeting, Santa Monica, CA, February 23, 2023, and at the ASCO Annual Meeting, Chicago, IL, June 4, 2023.
SUPPORT
Supported by National Institute of Health grants (cancer center Grant Nos. P30CA008748 to MSKCC, T32-CA009207 to R.T.) and philanthropy (grants from John and Georgia DallePezze to MSKCC, The Ning Zhao & Ge Li Family Initiative for Lung Cancer Research and New Therapies, and the James A. Fieber Lung Cancer Research Fund). K.C.A. is supported by the LUNGevity Foundation.
DATA SHARING STATEMENT
Deidentified individual participant data, including clinical and genomic data, can be made available upon request.
AUTHOR CONTRIBUTIONS
Conception and design: Rohit Thummalapalli, Afsheen Iqbal, Soo-Ryum Yang, Gregory J. Riely, Kathryn C. Arbour
Financial support: Gregory J. Riely
Administrative support: Bob T. Li, Gregory J. Riely, Joshua K. Sabari
Provision of study materials or patients: Bob T. Li, Fernando C. Santini, Gregory J. Riely, Joshua K. Sabari
Collection and assembly of data: Ezra Bernstein, Benjamin Herzberg, Bob T. Li, Juliana Eng, Marc Ladanyi, Soo-Ryum Yang, Joshua K. Sabari
Data analysis and interpretation: Benjamin Herzberg, Bob T. Li, Isabel Preeshagul, Fernando C. Santini, Marc Ladanyi, Soo-Ryum Yang, Ronglai Shen, Piro Lito, Joshua K. Sabari, Kathryn C. Arbour
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benjamin Herzberg
Honoraria: Eisai
Consulting or Advisory Role: Amgen
Research Funding: Repare Therapeutics (Inst), IDEAYA Biosciences (Inst), Amgen (Inst), Revolution Medicines (Inst), Astellas Pharma (Inst)
Bob T. Li
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Amgen (Inst), Lilly (Inst), MORE Health (Inst), Bolt Biotherapeutics (Inst), Ambrx (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst), Karger Publishers—Book royalty, Shanghai Jiao Tong University Press—Book royalty
Travel, Accommodations, Expenses: MORE Health, Jiangsu Hengrui Medicine
Uncompensated Relationships: Amgen, AstraZeneca, Genentech, Lilly, Boehringer Ingelheim, Daiichi Sankyo
Isabel Preeshagul
Consulting or Advisory Role: OncLive, Dava Oncology, G1 Therapeutics, LabCorp, Pfizer, Novartis, Novartis, Genentech, Jazz Pharmaceuticals, Intellisphere, AstraZeneca, Boehringer Ingelheim
Fernando C. Santini
Speakers' Bureau: AstraZeneca
Marc Ladanyi
Consulting or Advisory Role: AstraZeneca, ADC Therapeutics, Paige.AI, Merck, Bayer
Research Funding: Merus NV (Inst), Elevation Oncology (Inst), Rain Therapeutics
Soo-Ryum Yang
Honoraria: Prime Education
Consulting or Advisory Role: InVitae
Piro Lito
Leadership: Frontier Medicines, BioTheryX
Stock and Other Ownership Interests: Frontier Medicines, Biotheryx
Consulting or Advisory Role: Black Diamond Therapeutics, Repare Therapeutics, AmMax Bio, Revolution Medicines, OrbiMed, Frontier Medicines, PAQ-Tx, BioTheryX
Speakers' Bureau: Boehringer Ingelheim, Ikena Oncology
Research Funding: Mirati Therapeutics (Inst), Revolution Medicines (Inst), Amgen (Inst), Boehringer Ingelheim (Inst), Virtec pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat BRAF mutant cancers (Inst), I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat KRAS mutant cancers (Inst)
Gregory J. Riely
Research Funding: Novartis (Inst), Roche/Genentech (Inst), Mirati Therapeutics (Inst), Merck (Inst), Takeda (Inst), Lilly (Inst), Pfizer (Inst), Rain Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Bayer, Merck
Other Relationship: Pfizer, Roche/Genentech, Takeda, Mirati Therapeutics
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Kathryn C. Arbour
Consulting or Advisory Role: Sanofi, Genzyme, Novartis
Research Funding: Mirati Therapeutics (Inst), Genentech (Inst), Revolution Medicines (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14:5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boch C, Kollmeier J, Roth A, et al. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): Routine screening data for central Europe from a cohort study. BMJ Open. 2013;3:e002560. doi: 10.1136/bmjopen-2013-002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arbour KC, Jordan E, Kim HR, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24:334–340. doi: 10.1158/1078-0432.CCR-17-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cox AD, Fesik SW, Kimmelman AC, et al. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lito P, Solomon M, Li L-S, et al. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351:604–608. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 8. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non–Small Cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 10. Sacher A, Patel MR, Miller WH., Jr Phase Ia study to evaluate GDC-6036 monotherapy in patients with non–small cell lung cancer (NSCLC) with KRAS G12C mutation. J Thorac Oncol. 2022;17:S8–S9. [Google Scholar]
- 11. Ammakkanavar NR, Call J, Shimizu T, et al. Abstract CT202: A first-in-human phase 1 study of LY3537982, a novel, highly selective and potent KRAS G12C inhibitor in patients with KRAS G12C mutant advanced solid tumors (trial in progress) Cancer Res. 2022;82(12 suppl):CT202. [Google Scholar]
- 12. Weiss A, Lorthiois E, Barys L, et al. Discovery, preclinical characterization, and early clinical activity of JDQ443, a structurally novel, potent, and selective covalent oral inhibitor of KRASG12C. Cancer Discov. 2022;12:1500–1517. doi: 10.1158/2159-8290.CD-22-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, Murciano-Goroff YR, Xue JY, et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature. 2021;599:679–683. doi: 10.1038/s41586-021-04065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ricciuti B, Arbour KC, Lin JJ, et al. Diminished efficacy of programmed death-(ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol. 2022;17:399–410. doi: 10.1016/j.jtho.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kehl KL, Elmarakeby H, Nishino M, et al. Assessment of deep natural language processing in ascertaining oncologic outcomes from radiology reports. JAMA Oncol. 2019;5:1421–1429. doi: 10.1001/jamaoncol.2019.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf [Google Scholar]
- 19. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 20. Johnson M. Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study. Ann Oncol. 2022;33(suppl 7):S808–S869. [Google Scholar]
- 21. Arbour KC, Rizvi H, Plodkowski AJ, et al. Treatment outcomes and clinical characteristics of patients with KRAS-G12C-mutant non-small cell lung cancer. Clin Cancer Res. 2021;27:2209–2215. doi: 10.1158/1078-0432.CCR-20-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Begum P, Goldin RD, Possamai LA, et al. Severe immune checkpoint inhibitor hepatitis in KRAS G12C-mutant NSCLC potentially triggered by sotorasib: Case report. JTO Clin Res Rep. 2021;2:100213. doi: 10.1016/j.jtocrr.2021.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schoenfeld AJ, Arbour K, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30:839–844. doi: 10.1093/annonc/mdz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li BT, Falchook G, Durm GA, et al. CodeBreaK 100/101: First report of safety/efficacy of sotorasib in combination with pembrolizumab or atezolizumab in advanced KRAS p.G12C NSCLC. IASLC World Conference on Lung Cancer, Vienna, Austria, August. 2022:6–9. [Google Scholar]
- 25. Blumenthal GM, Gong Y, Kehl K, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small cell lung cancer. Ann Oncol. 2019;30:830–838. doi: 10.1093/annonc/mdz060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified individual participant data, including clinical and genomic data, can be made available upon request.