Abstract
PURPOSE
Increased awareness of the distinct tumor biology for adolescents and young adults (AYAs) with cancer has led to improvement in outcomes for this population. However, in cholangiocarcinoma (CCA), a paucity of data exist on the AYA population. To our knowledge, we present the largest study to date on AYA disease biology, treatment patterns, and survival outcomes in CCA.
METHODS
A multi-institutional cohort of patients with CCA diagnosed with intrahepatic cholangiocarcinoma (ICC) or extrahepatic cholangiocarcinoma (ECC) was used for analysis. Retrospective chart review was conducted on patients who were 50 years old and younger (young; n = 124) and older than 50 years (older; n = 723).
RESULTS
Among 1,039 patients screened, 847 patients met eligibility (72% ICC, 28% ECC). Young patients had a larger median tumor size at resection compared with older patients (4.2 v 3.6 cm; P = .048), more commonly had N1 disease (65% v 43%; P = .040), and were more likely to receive adjuvant therapy (odds ratio, 4.0; 95% CI, 1.64 to 9.74). Tumors of young patients were more likely to harbor an FGFR2 fusion, BRAF mutation, or ATM mutation (P < .05 for each). Young patients were more likely to receive palliative systemic therapy (96% v 69%; P < .001), targeted therapy (23% v 8%; P < .001), and treatment on a clinical trial (31% v 19%; P = .004). Among patients who presented with advanced disease, young patients had a higher median overall survival compared with their older counterparts (17.7 v 13.5 months; 95% CI, 12.6 to 22.6 v 11.4 to 14.8; P = .049).
CONCLUSION
Young patients with CCA had more advanced disease at resection, more commonly received both adjuvant and palliative therapies, and demonstrated improved survival compared with older patients. Given the low clinical trial enrollment and poor outcomes among some AYA cancer populations, data to the contrary in CCA are highly encouraging.
CCF continues to fund studies that offer insights to the entire Cholangiocarcinoma community
INTRODUCTION
Cholangiocarcinoma (CCA) is an aggressive malignancy of the biliary duct epithelium with a rising incidence and mortality globally.1,2 Although the median age at diagnosis of intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) is 67 and 72 years, respectively,1 young patients are increasingly affected by this disease. A recent study showed that people younger than 50 years had a higher rate of annual percentage growth in incidence of biliary tract cancers compared with those older than 50 years.3 In addition, death rates because of intrahepatic biliary tract malignancies in the young adult population have been increasing since 1980.4 Most young adult patients have no identifiable risk factor, and relatively little is known about their disease biology, treatment choices, and outcomes.
CONTEXT
Key Objective
Cholangiocarcinoma (CCA) is an uncommon malignancy of the biliary tract with a poor prognosis, and a paucity of studies exist on the adolescent and young adult (AYA) patient population with this disease. To our knowledge, the current multi-institutional study of 847 patients represents the largest analysis of disease biology, treatment patterns, and survival outcomes in AYAs compared with older adults with CCA.
Knowledge Generated
Young adults (50 years and younger) with CCA more commonly had larger tumors and node-positive disease at resection. Their tumors more frequently harbored alterations such as FGFR2 fusions, BRAF mutations, and ATM mutations. Young adults also more commonly received adjuvant and/or palliative systemic therapy, and they more commonly participated in a clinical trial. In our cohort, young patients who presented with advanced disease had a higher median survival than their older counterparts.
Relevance
Young patients with CCA appear to harbor distinct biology with multiple actionable alterations and receive a greater amount of treatment than older patients. Further prospective studies of AYA patients with CCA will be critical for tailoring treatment algorithms for these patients.
Adolescent and young adults (AYAs) with cancer have gained increasing attention as a distinct entity in oncology in recent years. The precise definition of this population has varied across studies, with the National Cancer Institute Progress Review Group on AYA in 2006 as patients aged 15 to 39 years old and other studies adopting upper limits of age at 45 or 50 years.5-7 AYAs with cancer have experienced a relatively lower rate of improvement in survival outcomes compared with their older and younger counterparts.8 Although AYAs tend to demonstrate better survival than older patients in most malignancies, notable exceptions include colorectal and breast cancers.9,10 In colon cancer, where early onset disease is on the rise,11,12 young patients present more frequently with advanced-stage disease and poor prognostic pathologic features compared with older patients. In line with this, multiple studies have shown poorer survival in young patients with colon cancer13-15 although the data on prognosis are mixed with some recent studies showing similar or improved survival compared with older patients.16-19 In breast cancer, studies have similarly shown an association between young age at diagnosis and poor prognostic features such as hormone receptor–negative disease, increased tumor grade, and lymphovascular involvement.20-23 Given the above, increasing recognition has been given to the fact that young patients with cancer often have differences in tumor biology and treatment utilization compared with older patients with the same type of cancer.
In CCA, a rare malignancy, limited data exist regarding differences in disease biology and outcomes in the AYA population compared with other age groups. One study, in which age 45 years was used as a cutoff for AYA patients, showed no difference between AYAs and older patients in overall survival (OS) across all stages, but it did show a lower OS in AYAs with stage IV disease as compared with older patients.7 The challenges of rare cancer research are reflected in the fact that this study involved 18 AYA patients from three hepatobiliary surgery centers in China and 32 AYA patients included from multiple public databases. Given the increasing overall incidence of CCA and the disproportionately increasing incidence of some biliary malignancies in younger patients, a detailed understanding of the biology and management patterns that differentiate young and older patients is paramount for improving clinical outcomes. Our multi-institutional Cholangiocarcinoma in the Young (CITY) Study examines the differences in clinical presentation, histologic and molecular pathology, patterns of treatment utilization, clinical trial enrollment rates, and survival outcomes in young versus older patients with CCA.
METHODS
Data Collection
Patients with CCA were identified from institutional tumor registries using the International Classification of Diseases (ICD) codes for CCA (ICD-9-155.1, 156.9, or 156.1; ICD-10 C22.1, 24.9, 24.8, 24.0) and from institutional biliary tract cancer databases. Eligibility criteria for the study included (1) age 18 years or greater, (2) histologic confirmation of CCA, and (3) diagnosis after June 1, 2009. A cutoff of June 1, 2009, was used to align with the date that gemcitabine and cisplatin became the standard-of-care treatment for advanced biliary tract cancer.24 Participating institutions included Mass General Cancer Center, University of California San Francisco, MD Anderson Cancer Center, Mayo Clinic, Vanderbilt University, University of Virginia, Beth Israel Deaconess Medical Center, and St Vincent's Medical Center. This study was performed on a protocol approved by the Institutional Review Boards of each participating institution.
A set of prespecified definitions were used across institutions for uniform data collection as per Appendix Table A1. Data were extracted by medical trainees or trained research assistants, and attending medical oncologists adjudicated ambiguous cases.
Molecular Analysis
Results of tumor molecular profiling performed as a routine part of clinical care were obtained from retrospective chart review. Institutional platforms comprised MGH SNaPshot,25 MSK IMPACT,26 UCSF500 Cancer Gene Panel,27 Columbia University Combined Cancer Panel,28 Mayo Clinic's CANCP, Dana Farber Cancer Institute's Oncopanel,29 Jackson Laboratory's Cancer Treatment Profile,30 and University of Wisconsin's Oncoplex.29 Commercial panels used included FoundationOne.31
Statistics
Chi-square, Fisher's exact, and unpaired t-tests and Wilcoxon rank-sum tests were used to compare independent variables in young and older patients. Graph Pad Prism version 9 and MedCalc version 19.7 were used for statistical analysis (MedCalc Software Ltd, Ostend, Belgium; MedCalc32; 2020). STATA 1733 was used in multivariate analyses. Multivariate analysis models for OS analyses included all variables that had a population representation of over 10% and resulted as significant on univariate analysis. FGFR2 fusions and BRAF mutations were also added to the models, given their clinical significance in CCA. A P value of <.05 was considered significant. Progression-free survival (PFS), recurrence-free survival (RFS), and OS were calculated by Kaplan-Meier analysis, with log rank-sum tests being used to calculate P values between groups. Patients were censored at the time of last follow-up if they did not meet the specified end point.
RESULTS
Study Population
Among 1,039 patients evaluated for the study, 847 met eligibility criteria (Fig 1A). The most common reason for exclusion was diagnosis of gallbladder adenocarcinoma (n = 129), followed by the date of diagnosis of CCA being before June 1, 2009 (n = 60). Most patients had ICC (72%), and the remainder had ECC (28%). The median follow-up for the study was 14.7 months.
FIG 1.
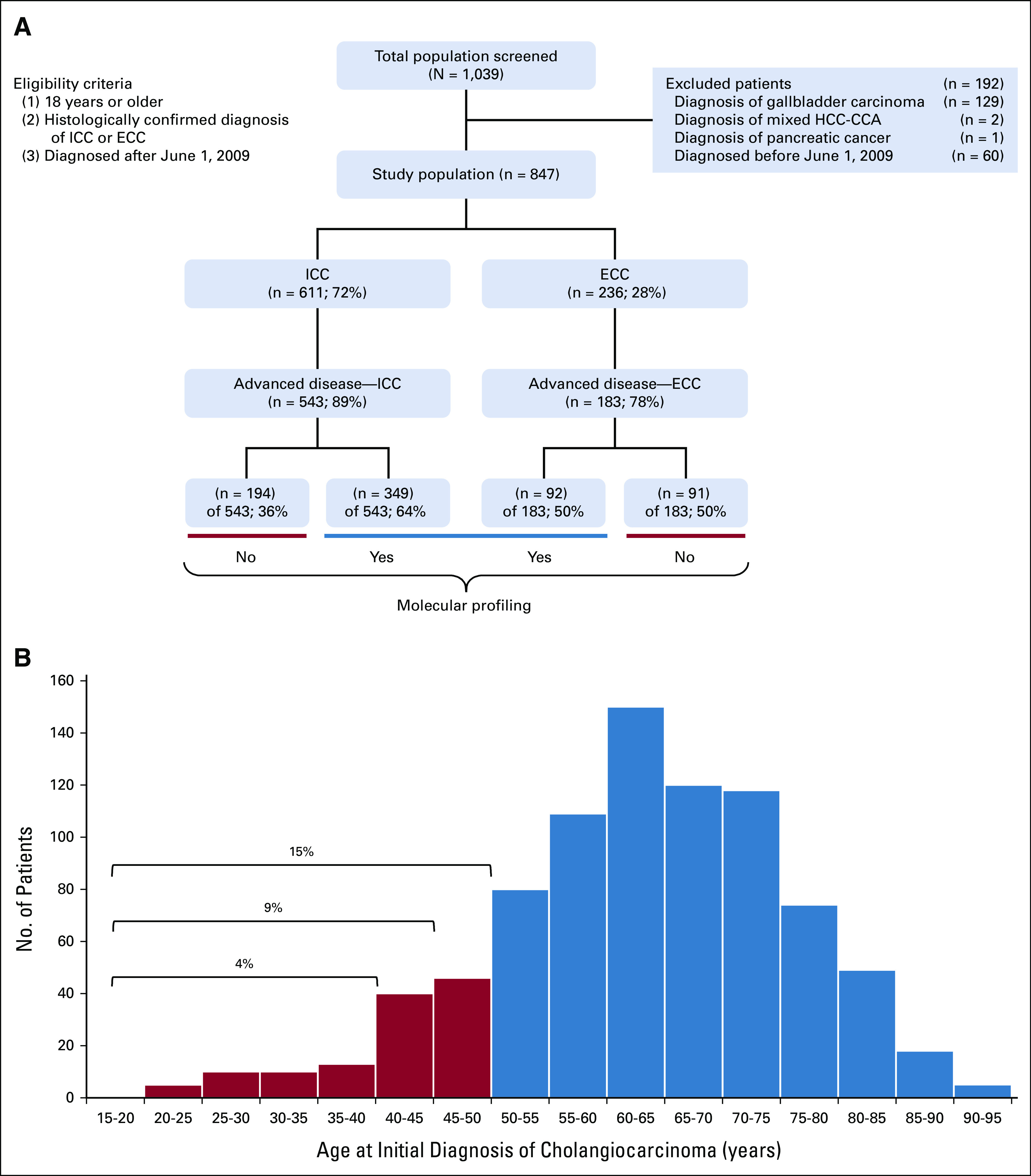
Baseline characteristics of the study population. (A) CONSORT diagram of the study depicting eligibility and exclusion criteria, the frequency of patients with ICC and ECC, and the frequency of molecular profiling among patients with advanced disease. (B) Histogram of patients stratified by age of initial diagnosis. The 847-patient cohort was subdivided into groups on the basis of age of initial CCA diagnosis, in increments of 5 years. Patients 50 years and younger represented 15% of the cohort. CCA, cholangiocarcinoma; ECC, extrahepatic cholangiocarcinoma; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
In defining the AYA population for this study, we assessed the percentage of patients in each age group in increments of 5 years. In a cohort of 847 patients, the 40 years and younger population, the 45 years and younger population, and the 50 years and younger population included 38 (4%), 78 (9%), and 124 (15%) patients, respectively. Therefore, we selected age 50 years as a cutoff to allow for sufficient statistical power in our analyses (Fig 1B).
Demographics, Risk Factors, and Disease Presentation
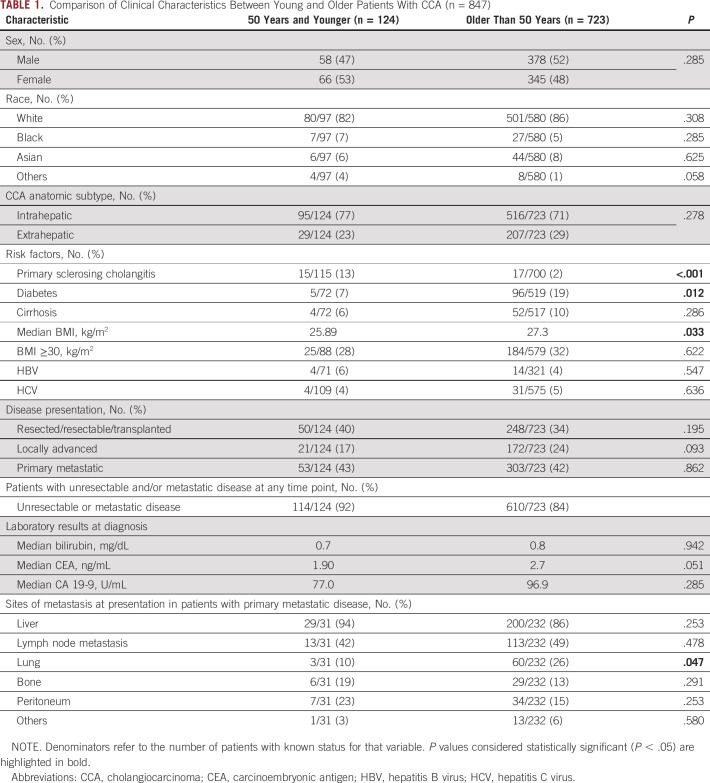
Patient demographics, anatomic subtype of CCA (ICC v ECC), and risk factors were compared in the young and older populations. No significant differences were detected in sex, race, or anatomic subtype, but differences were detected in the frequencies of some known risk factors (Table 1). Younger patients were more likely to have primary sclerosing cholangitis (13% v 2%; P < .001), as has been previously observed. Older patients were more likely to have diabetes (19% v 7%; P = .012) and a higher median BMI (27.3 v 25.9 kg/m2; P = .033).
TABLE 1.
Comparison of Clinical Characteristics Between Young and Older Patients With CCA (n = 847)
Disease presentation characteristics including extent of disease, patterns of metastases, tumor markers, and bilirubin were also examined by age group (Table 1). In both young and older patients, approximately 40% of patients presented with resectable disease, 20% with locally advanced disease, and 40% with primary metastatic disease. In evaluation of metastasis patterns among patients who presented with primary metastatic disease, older patients were more likely to have lung metastases (26% v 10%; P = .047).
Pathologic Differences From Surgical Resection and Tumor Molecular Profiling
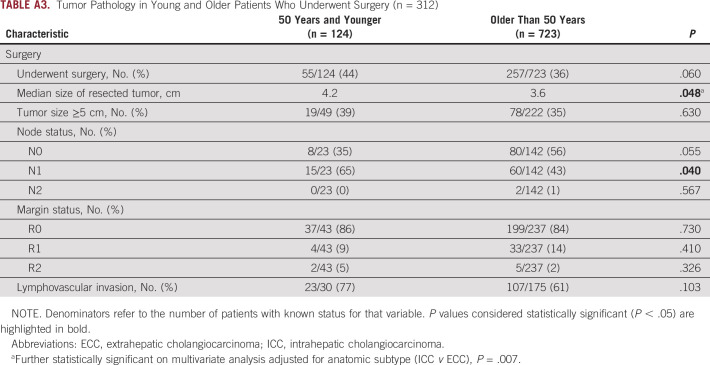
Among patients who underwent surgical resection, tumor pathology was compared in young versus older patients (Appendix Table A3). In the overall population, 37% of patients underwent surgical resection, including 44% of young patients and 36% of older patients. In this population, young patients had a larger median tumor size on pathology (4.2 v 3.6 cm; P = .048). This observation held on multivariate analysis adjusted for anatomic subtype (ICC v ECC), with the median tumor size being 1.02 cm greater in young patients (P = .007). Young patients also more commonly had N1 disease compared with older patients (65% v 43% respectively; P = .040). Rates of margin positivity, lymphovascular invasion, and perineural invasion did not vary significantly by age. In patients who had a surgical resection or biopsy, there was no significant difference in tumor grade (Appendix Table A4).
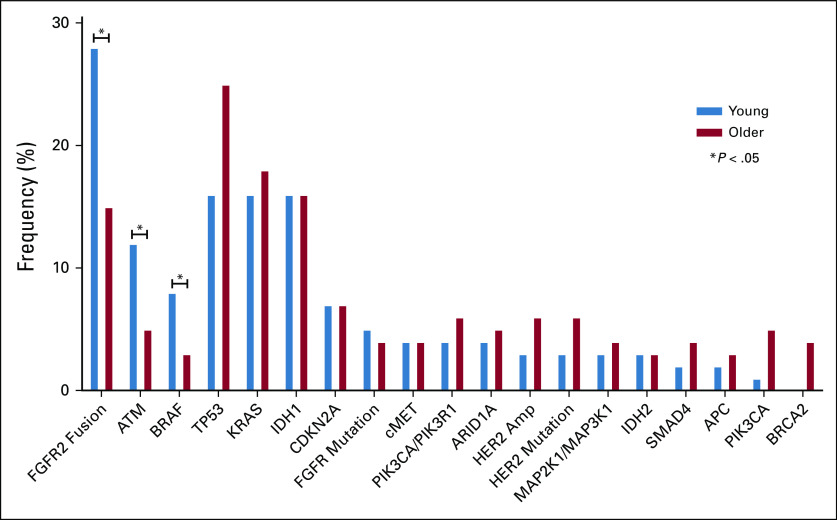
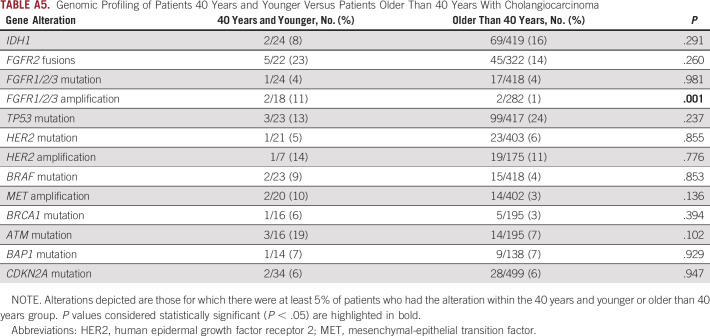
Genomic profiling of tumors was also compared in the young and older populations. Molecular profiling data were available for 441 of 726 patients with advanced disease (61%), including 349 (64%) advanced patients with ICC and 92 (50%) patients with ECC (Fig 1A). The majority of patients underwent tumor profiling using MGH SNaPshot (n = 290) or Foundation One (n = 146) platforms. Among patients with advanced disease, young patients more often underwent genetic profiling when compared with older patients (74% v 58%; P = .002). Among older patients with advanced disease, profiling was more common in those with ICC compared with those with ECC (62% v 48%; P = .003); however, there was no significant difference in profiling frequency among young patients with ICC and ECC (76% v 64%; P = .213). Analysis of profiled tumors revealed that young individuals were more likely than older individuals to have an FGFR2 fusion (28%, 14 of 50 v 15%, 34 of 234; P = .021) but not an FGFR mutation (5%, 4 of 77 v 4%, 13 of 363; P = .505). Young patients were also more likely to have a mutation in ATM (12%, 6 of 49 v 5%, 11 of 233; P = .044) or BRAF (8%, 6 of 76 v 3%, 11 of 363; P = .046) albeit numbers were relatively small. No other aberrations were found to differ significantly by age (Fig 2). When we applied a cutoff age of 40 years for the genomic analysis of our cohort, no differences in the landscape of genomic alterations were identified other than FGFR1/2/3 amplifications, which were more frequently found in younger patients (11%, 2 of 18 v 1%, 2 of 282; P = .001). However, these data should be interpreted with caution given the small number of patients younger than 40 years in our cohort (Appendix Table A5).
FIG 2.
Genomic profiling of young (50 years and younger) versus older (older than 50 years old) patients with CCA. In an analysis of 459 patients who underwent genomic profiling for CCA, FGFR2 fusions, ATM mutations, and BRAF mutations were more commonly seen in younger patients. Starred columns highlight genes with statistically significant differences in rates of alterations present between young and older patients (all P < .05). All gene labels indicate point mutations at the gene with the following exceptions, which include copy number alterations: ATM, CDKN2A, cMET, PIK3CA/PIK3R1, ARID1A, PIK3CA, and SMAD4. CCA, cholangiocarcinoma.
Differences in Treatment Patterns
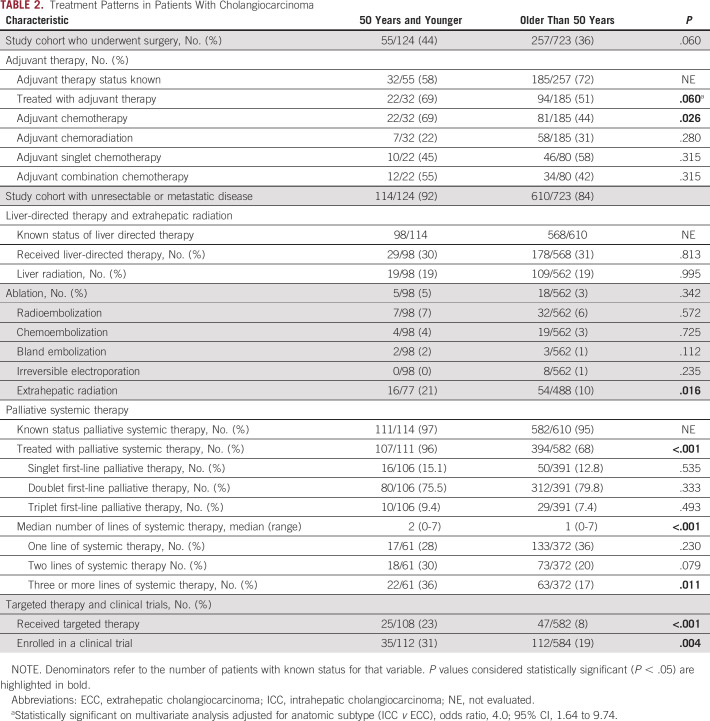
Treatment patterns were evaluated in the adjuvant and palliative setting for young and older patients (Table 2). Among those who underwent surgical resection, a trend was seen toward increased use of adjuvant therapy in the young compared with older population (69% v 51%; P = .060); when reanalyzed by multivariate analysis and adjusted for anatomic subtype of disease (ICC v ECC), this trend became significant with young patients more likely to receive adjuvant therapy (odds ratio, 4.0; 95% CI, 1.64 to 9.74). Notably, younger patients were more likely to receive adjuvant chemotherapy (69% v 44%; P = .026). In addition, among patients who received adjuvant chemotherapy, rates of use of doublet or triplet regimens were not significantly different between young and older patients, at 55% and 42%, respectively (P = .315).
TABLE 2.
Treatment Patterns in Patients With Cholangiocarcinoma
We next studied patterns of usage of liver-directed therapy and extrahepatic radiation in our cohort. Young patients were more likely to receive extrahepatic radiation, mainly for bone metastases, as compared with their older counterparts (21% v 10%, respectively; P = .016). No statistically significant difference was found between young and older patients for receipt of liver-directed therapies.
Patterns of receipt of palliative systemic therapy and enrollment in clinical trials were studied in patients with unresectable or metastatic disease, which included patients with locally advanced, primary metastatic, or recurrent metastatic disease (Table 2). We found that young patients were more likely to receive palliative systemic therapy compared with older patients (96% v 68%; P < .001). Among patients for whom the number of lines of therapy received was known, young patients received a median of two lines of systemic chemotherapy compared to one line in older patients (P < .001). Young patients were also more likely to receive targeted therapy (23% v 8%; P < .001) and enroll in a clinical trial (31% v 19%; P = .004). To assess if enrichment of FGFR2 fusions in the younger populations accounted for the differences in targeted therapy receipt and clinical trial enrollment, we repeated the analysis excluding patients with FGFR2 fusions. In this analysis, young patients still had a significantly higher frequency of receipt of targeted therapy (23% v 8%; P < .001), but the difference in rate of clinical trial enrollment became nonsignificant (23% v 16%; P = .076). The spectrum of targeted therapies used in young and older patients was similar, with FGFR2 fusions, IDH1 mutations, BRAF mutations, and MET amplifications being the most commonly targeted alterations (Fig 3). Most of these targeted therapies were administered via clinical trial. Encouragingly, we observed that the frequency of receipt of different targeted therapies was proportionate to the frequency with which the targetable alterations were found in our genomic data in the young and older cohorts (Figs 2 and 3).
FIG 3.
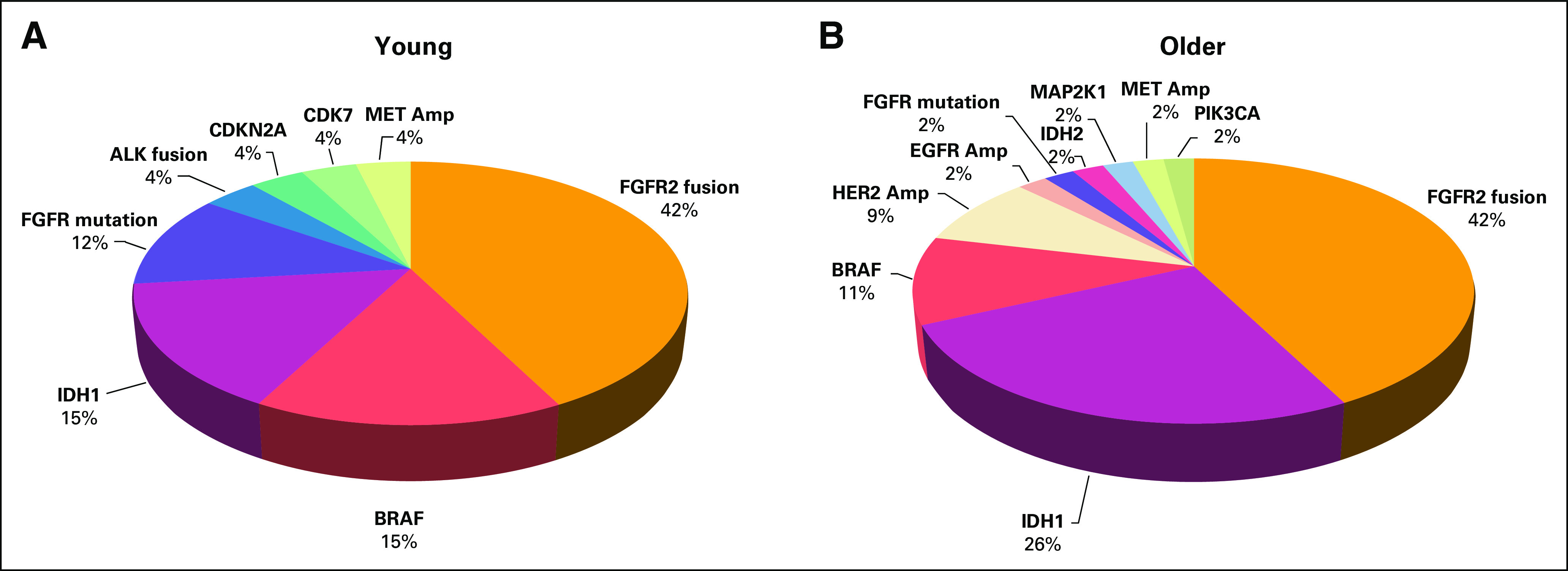
Spectrum of gene alterations targeted with targeted therapies in (A) young (50 years and younger) and (B) older (older than 50 years) patients in our cohort. Among patients who received targeted therapy, the majority in both age groups received targeted therapy directed at FGFR2 fusions or IDH1 mutations. Gene names without the type of alteration indicated refer to mutations of those genes. ALK, anaplastic lymphoma kinase; Amp, amplification; HER2, human epidermal growth factor receptor 2; MET, mesenchymal-epithelial transition factor.
Differences in Outcomes Between Young and Older Patients
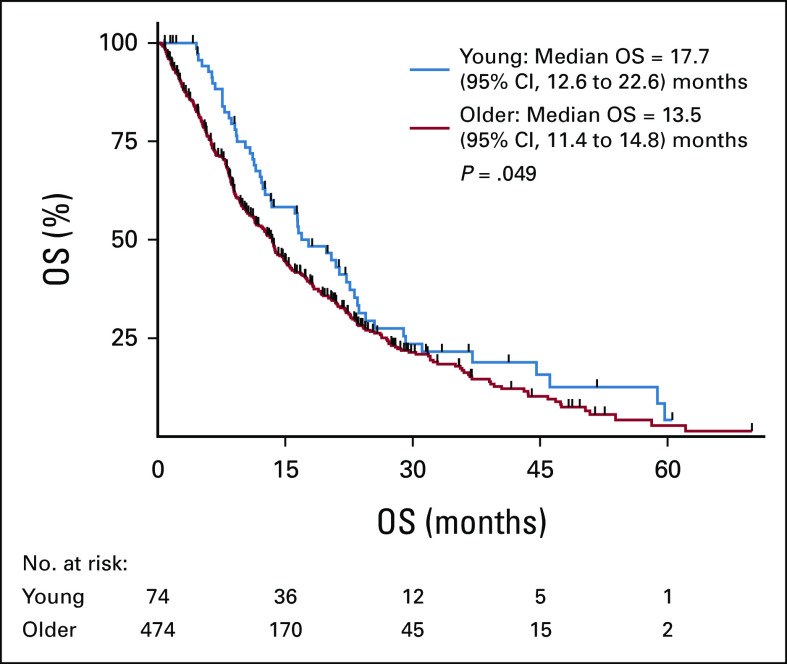
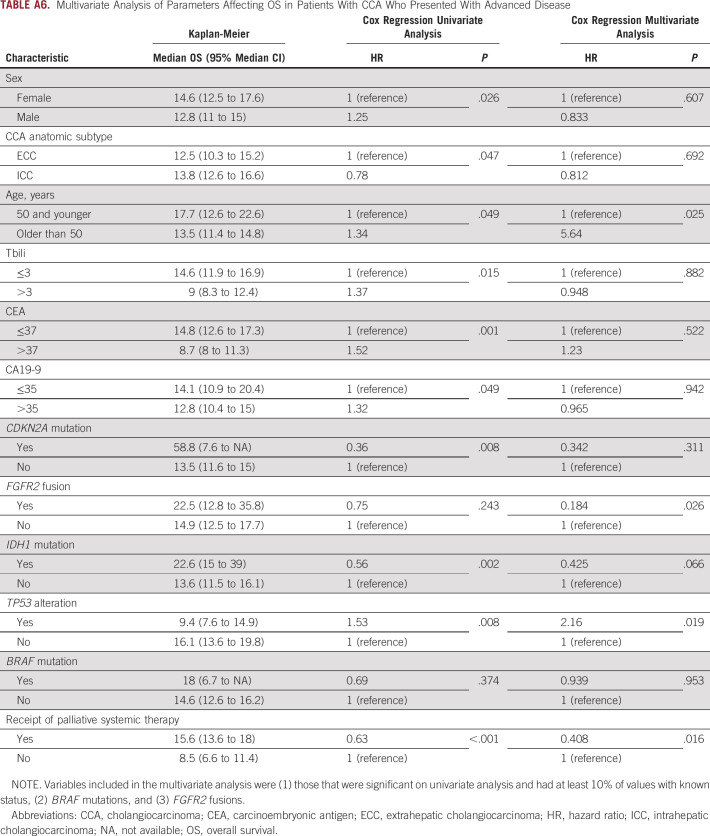
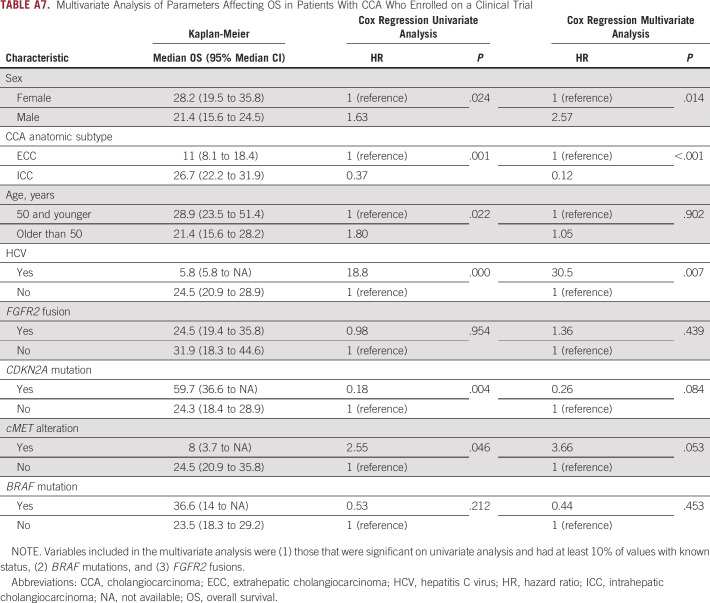
Differences in OS were analyzed to examine whether young patients with CCA experienced different survival outcomes compared with older patients. Young patients who presented with unresectable or metastatic disease were found to have a higher median overall survival (mOS) from the date of diagnosis compared with their older counterparts (17.7 v 13.5 months; 95% CI, 12.6 to 22.6 v 11.4 to 14.8; P = .049; Fig 4). This difference held in a multivariate analysis model (Appendix Table A6). The mOS from initial diagnosis in the overall population, including those who presented with early-stage disease and underwent resection, was similar in younger versus older patients (23.5 v 19.2 months; 95% CI, 19.2 to 29.2 v 16.9 to 21, respectively; P = .166). RFS in patients who underwent resection was also similar for young and older patients (9.3 v 10.1 months; 95% CI, 7.5 to 14.2 v 8.9 to 69.4; P = .857). Among patients who received at least one line of palliative systemic therapy for advanced disease, young patients had a similar mOS compared with older patients (19.8 v 14.9 months, respectively; 95% CI, 13.6 to 23.5 v 13.4 to 16.9; P = .134). Among patients treated on a clinical trial, younger patients had a higher mOS from the date of diagnosis of their advanced disease (young v older: 28.9 v 21.4 months; 95% CI, 23.5 to 51.4 v 15.6 to 28.2; P = .022). This result held when patients with FGFR2 fusions were removed from the analysis (44.6 v 20.4 months; 95% CI, 21.4 to 58.8 v 14 to 28.2; P = .019); however, it did not hold in a multivariate analysis model (Appendix Table A7). No difference was seen in median PFS on frontline gemcitabine/cisplatin or second-line FOLFOX in young versus older patients.
FIG 4.
Differences in median overall survival between young (50 years and younger) and older (older than 50 years) patients who presented with advanced disease. Compared with older patients, young patients who presented with advanced disease had higher median OS. OS, overall survival.
DISCUSSION
To our knowledge, the CITY Study represents the largest multi-institutional analysis of AYAs with CCA and the first to compare clinical trial enrollment and treatment patterns in young and older patients with this disease. A key reason for paucity of data on AYAs with cancer is that cancer prevalence in this age group is low, and this population has only recently been recognized as a distinct entity warranting focused study.34,35 Research in this population is additionally challenging given that the number of new cases of bile duct cancer in the United States is also fortunately low, with approximately 8,000 cases diagnosed annually, as compared with 284,000 for breast cancer and 150,000 for colorectal cancer.36 Thus, multi-institutional collaboration is essential to drive understanding and progress in rare subpopulations of rare cancers, and indeed, the findings of this study are based on contributions from eight cancer centers with a commitment to improving outcomes for patients with CCA.
A key finding of the study was that FGFR2 fusions, BRAF mutations, and ATM mutations are enriched in young patients. Recognition of the clinical phenotype of patients with FGFR2 fusions and BRAF mutations, most of which were V600E, is particularly relevant given the therapeutic implications of these alterations. The combination of trametinib and dabrafenib in patients with treatment-refractory BRAF V600E–mutant biliary malignancies showed an overall response rate of 47%.37 Three selective ATP-competitive FGFR inhibitors, pemigatinib, infigratinib, and futibatinib, gained conditional regulatory approval in 2020, 2021, and 2022, respectively, on the basis of objective response rates of 23%-42% in patients with advanced, refractory FGFR2 fusion or rearrangement-positive CCA. Multiple studies have demonstrated an enrichment of FGFR2 fusions in younger patients,38-40 and while all patients with CCA should undergo tumor molecular profiling given the high rate of actionable alterations in this disease, knowledge of this genotype-phenotype association can prompt patients and clinicians to pursue tumor profiling in young patients early and also maintain persistence if initial attempts at profiling fail because of insufficient tissue.
Across multiple cancer types, the AYA literature has consistently demonstrated that younger patients undergo surgery for more advanced-stage tumors and receive more lines of palliative therapy than older patients, and our findings stand consistent with this pattern of practice. The relatively improved outcomes of young patients after surgery have been attributed to higher rates of receipt of adjuvant therapy, fewer comorbidities leading to more radical surgical procedures, and increased lymph node excisions intraoperatively.16,41 Similarly, in our study, young patients received adjuvant chemotherapy more often than older patients, and notably, the median RFS after surgery in both age groups was similar despite young patients having higher rates of N1 disease. Studies in different cancers including colon cancer42 and breast cancer43,44 indicate that young patients receive more lines of therapy in the palliative setting for advanced disease, and again, our study in CCA stood consistent with this. The decision algorithms that lead to resection of later-stage disease, increased adjuvant and palliative therapy use across multiple AYA cancers, and the consequent clinical outcomes of these practices warrant further exploration to appropriately balance the risks and benefits of this practice.
Our study findings diverged from the AYA literature in the category of clinical trial enrollment. AYA patients with cancer have been shown to have a lower rate of accrual to clinical trials than pediatric and older patients.8,35 However, we were encouraged to find that in patients with CCA, rates of clinical trial enrollment and receipt of targeted therapy did not significantly differ between young and older patients. Furthermore, young and older patients who enrolled on clinical trials had similar survival outcomes. The CITY Study encouragingly highlights that AYAs with CCA, unlike AYAs with some other cancers, are indeed accessing and benefiting from clinical trials, but it also raises awareness that a fair proportion of all patients still do not receive treatment on a trial.
Young patients with colorectal carcinoma and breast cancer present with more advanced disease stages and can often experience worse survival outcomes than older patients according to several studies.45,46 By contrast, our study found that young patients with CCA who present with advanced stages of the disease live longer and may potentially harbor more favorable disease biology when compared with their peers with other malignancies.
A limitation of our study was that our patient population was derived mainly from people presenting to tertiary care cancer centers. This may potentially lead to selection bias as the population of patients referred to these institutions may be enriched for those who underwent tumor profiling and were found to have actionable tumor mutations conferring eligibility for clinical trial participation. Another limitation of our retrospective study is the issue of incomplete data for some variables; this was addressed by using a large data set to have sufficient power for analyses and only studying variables with sufficient datapoints to achieve statistical power.
In conclusion, the CITY Study highlights the power of multi-institutional collaboration in generating real-world data to gain insights into AYA populations with cancer. Epidemiology and outcomes among AYAs with cancer vary by cancer type,47 and therefore, an in-depth study of AYA populations in each individual malignancy is needed. This study provides another example of a malignancy where AYAs receive more aggressive treatment, and understanding the risks and benefits of these choices is critical for optimizing both longevity and quality of life for this population. Further studies that stand to improve outcomes for AYAs include those focused on closing the equity gap as AYAs with poorer sociodemographic characteristics have been shown to have higher mortality rates.48 Overall, although prioritization has historically been given to pediatric and adult populations with cancer, increasing recognition of AYAs as a unique entity allows for tailored interventions to support this previously under-recognized population of patients with cancer.
ACKNOWLEDGMENT
We would like to acknowledge support from The Cholangiocarcinoma Foundation (CCF), the International Cholangiocarcinoma Research Network (ICRN), and Jacqui Lewis and the Rare Initiative, and Joe and Katie Comeau.
APPENDIX
TABLE A1.
Definitions of Key Terms Used Across Institutions
TABLE A2.
Sites of Metastases at Most Recent Scan in Patients With Advanced Disease
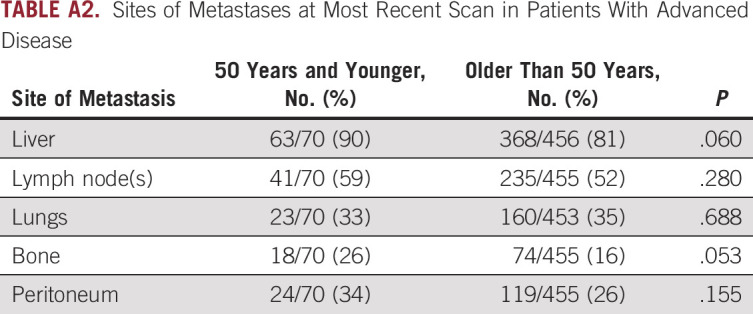
TABLE A3.
Tumor Pathology in Young and Older Patients Who Underwent Surgery (n = 312)
TABLE A4.
Tumor Differentiation Grade in Young and Older Patients Who Had a Biopsy or Surgery
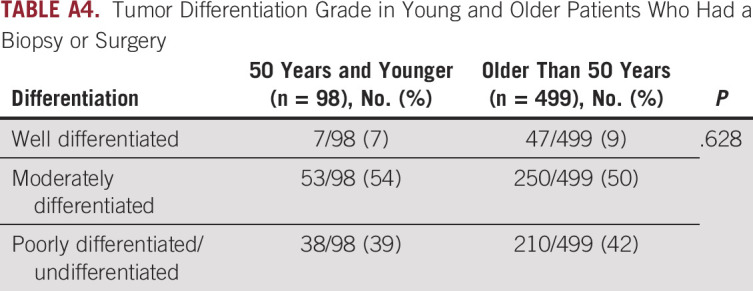
TABLE A5.
Genomic Profiling of Patients 40 Years and Younger Versus Patients Older Than 40 Years With Cholangiocarcinoma
TABLE A6.
Multivariate Analysis of Parameters Affecting OS in Patients With CCA Who Presented With Advanced Disease
TABLE A7.
Multivariate Analysis of Parameters Affecting OS in Patients With CCA Who Enrolled on a Clinical Trial
Leontios Pappas
Stock and Other Ownership Interests: Moderna Therapeutics, Eli Lilly
Kristen Spencer
Consulting or Advisory Role: QED Therapeutics, Helsinn Therapeutics, Lynx Group, Caris Life Sciences
Osama E. Rahma
Employment: Outcomes4me, AstraZeneca/MedImmune
Leadership: Outcomes4me, AstraZeneca/MedImmune
Stock and Other Ownership Interests: Outcomes4Me, AstraZeneca/MedImmune
Honoraria: Merck, Clinical Care Options, MI Bioresearch, PRMA Consulting, Leerink, Alaunus Global
Consulting or Advisory Role: Celgene, Alcimed, GfK, Merck, Five Prime Therapeutics, Putnam Associates, Defined Health, PureTech, Leerink, Genentech, Imvax, GlaxoSmithKline, Maverick Therapeutics, Bayer, Sobi
Research Funding: Amgen (Inst), Merck
Patents, Royalties, Other Intellectual Property: Pending patent (DFCI 2386.010) (Inst), PD-1/PD-L1 (Inst)
Travel, Accommodations, Expenses: Merck, Clinical Care Options, PureTech, PRMA Consulting, Genentech
Marc Roth
Employment: HCA Midwest Health
Mary Linton B. Peters
Stock and Other Ownership Interests: Eli Lilly, Medtronic, Procter & Gamble, Merck, Abbott Laboratories, Amgen, Johnson & Johnson, Pfizer, Agios
Consulting or Advisory Role: Agios
Research Funding: Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Exelixis (Inst), BeiGene (Inst), Berg Pharma (Inst), Merck (Inst), Bayer (Inst), Nucana (Inst), Eli Lilly (Inst), Helsinn Therapeutics (Inst)
Andrew X. Zhu
Employment: I-Mab
Leadership: I-Mab
Stock and Other Ownership Interests: I-Mab
Consulting or Advisory Role: Eisai, Eli Lilly, Merck, Roche/Genentech, Sanofi, Bayer, Exelixis
Kylie Boyhen
Stock and Other Ownership Interests: Editas Medicine
Christine VanCott
Employment: HCA Healthcare
Leadership: HCA Healthcare
Stock and Other Ownership Interests: HCA Healthcare
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1369301/summary
Tushar Patel
Stock and Other Ownership Interests: GE H
Consulting or Advisory Role: Moderna Therapeutics
Lewis R. Roberts
Honoraria: Focal Medical Communications, Science First Communications Private Limited, Mumbai, India, Roche Kenya Limited, Nairobi, Kenya, Kolkata Liver Meeting, Kolkata, India—Liver Foundation, West Bengal, India
Consulting or Advisory Role: Bayer (Inst), Grail (Inst), RedHill Biopharma (Inst), Tavec (Inst), Exact Sciences (Inst), Hepion Pharmaceuticals (Inst), Novartis Venture Fund (Inst), Medscape (Inst), Roche (Inst), Global Life Sciences (Inst), Clinical Care Options (Inst), Lynx Group (Inst), AstraZeneca (Inst), Genentech (Inst), Pontifax (Inst), MedEd Design LLC (Inst), Roche Africa (Inst)
Speakers' Bureau: Bayer
Research Funding: ARIAD (Inst), BTG (Inst), Exact Sciences (Inst), Gilead Sciences (Inst), Wako Diagnostics (Inst), Glycotest (Inst), RedHill Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No. 9,469,877: Materials and Methods for Diagnosis, Prognosis, Monitoring of Recurrence, and Assessment of Therapeutic/Prophylactic Treatment of Pancreaticobiliary Cancer (Inst), Five Prime Therapeutics. Royalties, Five Prime Therapeutics. Royalties (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
Stacie Lindsey
Leadership: Cholangiocarcinoma Foundation
Travel, Accommodations, Expenses: Incyte
Nora Horick
Employment: Northwest Biotherapeutics
Stock and Other Ownership Interests: Northwest Biotherapeutics
Patents, Royalties, Other Intellectual Property: An immediate family member holds patents, has patents pending, and receives royalties from a technology related to health or medicine
A. John Iafrate
Consulting or Advisory Role: Repare Therapeutics, Kinnate Biopharma, Oncoclinicas Brasil, PAIGE.AI
Patents, Royalties, Other Intellectual Property: ArcherDX exclusive license to AMP technology
Laura Williams Goff
Consulting or Advisory Role: QED Therapeutics, Genentech, Merck, AstraZeneca, Exelixis, Boehringer Ingelheim, Cardinal Health, Atheneum Consulting, Relay Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Agios (Inst), ASLAN Pharmaceuticals (Inst), BeiGene (Inst), Basilea (Inst), Merck (Inst)
Kabir Mody
Employment: IMV
Stock and Other Ownership Interests: CytoDyn, ONCOtherapeutics, IMV
Consulting or Advisory Role: Celgene, Genentech/Roche, Merrimack, Eisai, AstraZeneca, Vicus Therapeutics, Ipsen, Boston Scientific, BTG, Exelixis, Incyte (Inst), QED Therapeutics
Research Funding: FibroGen (Inst), Senhwa Biosciences (Inst), ARIAD (Inst), TRACON Pharma (Inst), MedImmune (Inst), Agios (Inst), ArQule (Inst), Taiho Pharmaceutical (Inst), Gritstone Bio (Inst), Incyte (Inst), Merck (Inst), Vyriad (Inst), Turnstone Bio (Inst), AstraZeneca (Inst), Basilea (Inst)
Mitesh J. Borad
Stock and Other Ownership Interests: Gilead Sciences, AVEO, Intercept Pharmaceuticals, Spectrum Pharmaceuticals
Consulting or Advisory Role: G1 Therapeutics, Fujifilm (Inst), Agios (Inst), Insys Therapeutics (Inst), Novartis (Inst), ArQule (Inst), Celgene (Inst), Inspyr Therapeutics, Halozyme (Inst), Pieris Pharmaceuticals (Inst), Taiho Pharmaceutical (Inst), Immunovative Therapies, Exelixis, Lynx Group, Genentech, Western Oncolytics, KLUS Pharma, De Novo Pharmaceuticals, Merck, Imvax
Research Funding: Boston Biomedical (Inst), miRNA Therapeutics (Inst), Senhwa Biosciences (Inst), MedImmune (Inst), BioLineRx (Inst), Agios (Inst), Halozyme (Inst), Celgene (Inst), Threshold Pharmaceuticals (Inst), Toray Industries (Inst), Dicerna (Inst), SillaJen (Inst), Eisai (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Isis Pharmaceuticals (Inst), Incyte (Inst), Sun Biopharma (Inst), ARIAD (Inst), ImClone Systems (Inst), QED Therapeutics (Inst), Incyte (Inst), Puma Biotechnology (Inst), Adaptimmune (Inst), Merck Serono (Inst), RedHill Biopharma (Inst), Basilea (Inst), AstraZeneca (Inst), ZielBio (Inst)
Travel, Accommodations, Expenses: ArQule, Celgene, AstraZeneca
Rachna T. Shroff
Consulting or Advisory Role: Exelixis, Merck, QED Therapeutics, Incyte, AstraZeneca, Taiho Pharmaceutical, Boehringer Ingelheim, Servier, Genentech, Basilea
Research Funding: Pieris Pharmaceuticals, Taiho Pharmaceutical, Merck, Exelixis, QED Therapeutics, Rafael Pharmaceuticals, Bristol Myers Squibb, Bayer, Immunovaccine, Seagen, Novocure, Nucana, Loxo/Eli Lilly, Faeth Therapeutics, NGM Biopharmaceuticals
Milind M. Javle
Honoraria: QED Therapeutics, Incyte, TransThera Biosciences, Merck, EMD Serono/Merck, AstraZeneca/MedImmune
Consulting or Advisory Role: QED Therapeutics, OncoSil, Incyte, Mundipharma, AstraZeneca, Merck, EMD Serono, Basilea Pharmaceutical
Other Relationship: Rafael Pharmaceuticals, Incyte, Pieris Pharmaceuticals, Merck, Merck Serono, Novartis, Seagen, BeiGene, QED Therapeutics, Bayer
R. Kate Kelley
Consulting or Advisory Role: Agios (Inst), AstraZeneca (Inst), Merck (Inst), Kinnate Biopharma, Exelixis/Ipsen (Inst), Regeneron, Tyra Biosciences, Compass Therapeutics
Research Funding: Eli Lilly (Inst), Exelixis (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), MedImmune (Inst), Merck Sharp & Dohme (Inst), Agios (Inst), AstraZeneca (Inst), Adaptimmune (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), QED Therapeutics (Inst), EMD Serono (Inst), Partner Therapeutics (Inst), Genentech/Roche (Inst), Surface Oncology (Inst), Relay Therapeutics (Inst), Loxo/Eli Lilly (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Lipika Goyal
Consulting or Advisory Role: Alentis Therapeutics, QED Therapeutics, AstraZeneca, Taiho Pharmaceutical, Incyte, Sirtex Medical, Genentech, Exelixis, TransThera Biosciences, Merck, Black Diamond Therapeutics, Synthekine, Eisai/H3 Biomedicine, Tyra Biosciences, Kinnate Biopharma, Compass Therapeutics, Blueprint Medicines, Servier
Uncompensated Relationships: Agios, Debiopharm Group, Taiho Pharmaceutical, Merck, Boehringer Ingelheim
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2017 Gastrointestinal Cancers Symposium (ASCO GI), San Francisco, California, January 19-21, 2017.
SUPPORT
M.L.B.P. reports funding from the National Cancer Institute (K08CA248473). J.K.L. reports funding from the National Institutes of Health (R37 CA225655). L.G. receives funding from the American Cancer Society Clinical Scientist Development Grant 134013‐CSDG‐19‐163‐01‐TBG, the NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003, V Foundation for Cancer Research Translational Grant, and the Cholangiocarcinoma Foundation Andrea Marie Fuquay Research Fellowship.
L.P. and I.B. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Leontios Pappas, Kristen Spencer, Andrew X. Zhu, Tushar Patel, Stacie Lindsey, Laura Williams Goff, Milind M. Javle, Lipika Goyal
Financial support: Stacie Lindsey, A. John Iafrate
Administrative support: Jen Stanton, Stacie Lindsey, Jochen K. Lennerz, A. John Iafrate
Provision of study materials or patients: Stephanie Reyes, Mary Linton B. Peters, Andrew X. Zhu, Lewis R. Roberts, Jochen K. Lennerz, A. John Iafrate, Laura Williams Goff, R. Katie Kelley
Collection and assembly of data: Leontios Pappas, Islam Baiev, Stephanie Reyes, Andrea Grace Bocobo, Apurva Jain, Tri Minh Le, Osama E. Rahma, Jordan Maurer, Jen Stanton, Karen Zhang, Anaemy Danner De Armas, Thomas T. Deleon, Marc Roth, Mary Linton B. Peters, Andrew X. Zhu, Kylie Boyhen, Christine VanCott, Lewis R. Roberts, Stacie Lindsey, Jochen K. Lennerz, A. John Iafrate, Laura Williams Goff, Kabir Mody, Mitesh J. Borad, Rachna T. Shroff, Milind M. Javle, R. Katie Kelley, Lipika Goyal
Data analysis and interpretation: Leontios Pappas, Islam Baiev, Jordan Maurer, Andrew X. Zhu, Lewis R. Roberts, Stacie Lindsey, Nora Horick, Jochen K. Lennerz, A. John Iafrate, Laura Williams Goff, Kabir Mody, Rachna T. Shroff, Milind M. Javle, R. Katie Kelley, Lipika Goyal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Leontios Pappas
Stock and Other Ownership Interests: Moderna Therapeutics, Eli Lilly
Kristen Spencer
Consulting or Advisory Role: QED Therapeutics, Helsinn Therapeutics, Lynx Group, Caris Life Sciences
Osama E. Rahma
Employment: Outcomes4me, AstraZeneca/MedImmune
Leadership: Outcomes4me, AstraZeneca/MedImmune
Stock and Other Ownership Interests: Outcomes4Me, AstraZeneca/MedImmune
Honoraria: Merck, Clinical Care Options, MI Bioresearch, PRMA Consulting, Leerink, Alaunus Global
Consulting or Advisory Role: Celgene, Alcimed, GfK, Merck, Five Prime Therapeutics, Putnam Associates, Defined Health, PureTech, Leerink, Genentech, Imvax, GlaxoSmithKline, Maverick Therapeutics, Bayer, Sobi
Research Funding: Amgen (Inst), Merck
Patents, Royalties, Other Intellectual Property: Pending patent (DFCI 2386.010) (Inst), PD-1/PD-L1 (Inst)
Travel, Accommodations, Expenses: Merck, Clinical Care Options, PureTech, PRMA Consulting, Genentech
Marc Roth
Employment: HCA Midwest Health
Mary Linton B. Peters
Stock and Other Ownership Interests: Eli Lilly, Medtronic, Procter & Gamble, Merck, Abbott Laboratories, Amgen, Johnson & Johnson, Pfizer, Agios
Consulting or Advisory Role: Agios
Research Funding: Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Exelixis (Inst), BeiGene (Inst), Berg Pharma (Inst), Merck (Inst), Bayer (Inst), Nucana (Inst), Eli Lilly (Inst), Helsinn Therapeutics (Inst)
Andrew X. Zhu
Employment: I-Mab
Leadership: I-Mab
Stock and Other Ownership Interests: I-Mab
Consulting or Advisory Role: Eisai, Eli Lilly, Merck, Roche/Genentech, Sanofi, Bayer, Exelixis
Kylie Boyhen
Stock and Other Ownership Interests: Editas Medicine
Christine VanCott
Employment: HCA Healthcare
Leadership: HCA Healthcare
Stock and Other Ownership Interests: HCA Healthcare
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1369301/summary
Tushar Patel
Stock and Other Ownership Interests: GE H
Consulting or Advisory Role: Moderna Therapeutics
Lewis R. Roberts
Honoraria: Focal Medical Communications, Science First Communications Private Limited, Mumbai, India, Roche Kenya Limited, Nairobi, Kenya, Kolkata Liver Meeting, Kolkata, India—Liver Foundation, West Bengal, India
Consulting or Advisory Role: Bayer (Inst), Grail (Inst), RedHill Biopharma (Inst), Tavec (Inst), Exact Sciences (Inst), Hepion Pharmaceuticals (Inst), Novartis Venture Fund (Inst), Medscape (Inst), Roche (Inst), Global Life Sciences (Inst), Clinical Care Options (Inst), Lynx Group (Inst), AstraZeneca (Inst), Genentech (Inst), Pontifax (Inst), MedEd Design LLC (Inst), Roche Africa (Inst)
Speakers' Bureau: Bayer
Research Funding: ARIAD (Inst), BTG (Inst), Exact Sciences (Inst), Gilead Sciences (Inst), Wako Diagnostics (Inst), Glycotest (Inst), RedHill Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No. 9,469,877: Materials and Methods for Diagnosis, Prognosis, Monitoring of Recurrence, and Assessment of Therapeutic/Prophylactic Treatment of Pancreaticobiliary Cancer (Inst), Five Prime Therapeutics. Royalties, Five Prime Therapeutics. Royalties (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
Stacie Lindsey
Leadership: Cholangiocarcinoma Foundation
Travel, Accommodations, Expenses: Incyte
Nora Horick
Employment: Northwest Biotherapeutics
Stock and Other Ownership Interests: Northwest Biotherapeutics
Patents, Royalties, Other Intellectual Property: An immediate family member holds patents, has patents pending, and receives royalties from a technology related to health or medicine
A. John Iafrate
Consulting or Advisory Role: Repare Therapeutics, Kinnate Biopharma, Oncoclinicas Brasil, PAIGE.AI
Patents, Royalties, Other Intellectual Property: ArcherDX exclusive license to AMP technology
Laura Williams Goff
Consulting or Advisory Role: QED Therapeutics, Genentech, Merck, AstraZeneca, Exelixis, Boehringer Ingelheim, Cardinal Health, Atheneum Consulting, Relay Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Agios (Inst), ASLAN Pharmaceuticals (Inst), BeiGene (Inst), Basilea (Inst), Merck (Inst)
Kabir Mody
Employment: IMV
Stock and Other Ownership Interests: CytoDyn, ONCOtherapeutics, IMV
Consulting or Advisory Role: Celgene, Genentech/Roche, Merrimack, Eisai, AstraZeneca, Vicus Therapeutics, Ipsen, Boston Scientific, BTG, Exelixis, Incyte (Inst), QED Therapeutics
Research Funding: FibroGen (Inst), Senhwa Biosciences (Inst), ARIAD (Inst), TRACON Pharma (Inst), MedImmune (Inst), Agios (Inst), ArQule (Inst), Taiho Pharmaceutical (Inst), Gritstone Bio (Inst), Incyte (Inst), Merck (Inst), Vyriad (Inst), Turnstone Bio (Inst), AstraZeneca (Inst), Basilea (Inst)
Mitesh J. Borad
Stock and Other Ownership Interests: Gilead Sciences, AVEO, Intercept Pharmaceuticals, Spectrum Pharmaceuticals
Consulting or Advisory Role: G1 Therapeutics, Fujifilm (Inst), Agios (Inst), Insys Therapeutics (Inst), Novartis (Inst), ArQule (Inst), Celgene (Inst), Inspyr Therapeutics, Halozyme (Inst), Pieris Pharmaceuticals (Inst), Taiho Pharmaceutical (Inst), Immunovative Therapies, Exelixis, Lynx Group, Genentech, Western Oncolytics, KLUS Pharma, De Novo Pharmaceuticals, Merck, Imvax
Research Funding: Boston Biomedical (Inst), miRNA Therapeutics (Inst), Senhwa Biosciences (Inst), MedImmune (Inst), BioLineRx (Inst), Agios (Inst), Halozyme (Inst), Celgene (Inst), Threshold Pharmaceuticals (Inst), Toray Industries (Inst), Dicerna (Inst), SillaJen (Inst), Eisai (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Isis Pharmaceuticals (Inst), Incyte (Inst), Sun Biopharma (Inst), ARIAD (Inst), ImClone Systems (Inst), QED Therapeutics (Inst), Incyte (Inst), Puma Biotechnology (Inst), Adaptimmune (Inst), Merck Serono (Inst), RedHill Biopharma (Inst), Basilea (Inst), AstraZeneca (Inst), ZielBio (Inst)
Travel, Accommodations, Expenses: ArQule, Celgene, AstraZeneca
Rachna T. Shroff
Consulting or Advisory Role: Exelixis, Merck, QED Therapeutics, Incyte, AstraZeneca, Taiho Pharmaceutical, Boehringer Ingelheim, Servier, Genentech, Basilea
Research Funding: Pieris Pharmaceuticals, Taiho Pharmaceutical, Merck, Exelixis, QED Therapeutics, Rafael Pharmaceuticals, Bristol Myers Squibb, Bayer, Immunovaccine, Seagen, Novocure, Nucana, Loxo/Eli Lilly, Faeth Therapeutics, NGM Biopharmaceuticals
Milind M. Javle
Honoraria: QED Therapeutics, Incyte, TransThera Biosciences, Merck, EMD Serono/Merck, AstraZeneca/MedImmune
Consulting or Advisory Role: QED Therapeutics, OncoSil, Incyte, Mundipharma, AstraZeneca, Merck, EMD Serono, Basilea Pharmaceutical
Other Relationship: Rafael Pharmaceuticals, Incyte, Pieris Pharmaceuticals, Merck, Merck Serono, Novartis, Seagen, BeiGene, QED Therapeutics, Bayer
R. Kate Kelley
Consulting or Advisory Role: Agios (Inst), AstraZeneca (Inst), Merck (Inst), Kinnate Biopharma, Exelixis/Ipsen (Inst), Regeneron, Tyra Biosciences, Compass Therapeutics
Research Funding: Eli Lilly (Inst), Exelixis (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), MedImmune (Inst), Merck Sharp & Dohme (Inst), Agios (Inst), AstraZeneca (Inst), Adaptimmune (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), QED Therapeutics (Inst), EMD Serono (Inst), Partner Therapeutics (Inst), Genentech/Roche (Inst), Surface Oncology (Inst), Relay Therapeutics (Inst), Loxo/Eli Lilly (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Lipika Goyal
Consulting or Advisory Role: Alentis Therapeutics, QED Therapeutics, AstraZeneca, Taiho Pharmaceutical, Incyte, Sirtex Medical, Genentech, Exelixis, TransThera Biosciences, Merck, Black Diamond Therapeutics, Synthekine, Eisai/H3 Biomedicine, Tyra Biosciences, Kinnate Biopharma, Compass Therapeutics, Blueprint Medicines, Servier
Uncompensated Relationships: Agios, Debiopharm Group, Taiho Pharmaceutical, Merck, Boehringer Ingelheim
No other potential conflicts of interest were reported.
REFERENCES
- 1. Saha SK, Zhu AX, Fuchs CS, et al. Forty-year trends in cholangiocarcinoma incidence in the U.S.: Intrahepatic disease on the rise. Oncologist. 2016;21:594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: An analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16:117. doi: 10.1186/s12876-016-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health. 2019;4:e137–e147. doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 4.Bleyer A, Barr R, Ries L, et al. Cancer in Adolescents and Young Adults. Cham, Springer International Publishing AG; 2017. [Google Scholar]
- 5. Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125:2002–2010. doi: 10.1002/cncr.31994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: A SEER-based analysis with comparison to other young-onset cancers. J Invest Med. 2017;65:311–315. doi: 10.1136/jim-2016-000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng H, Tong H, Yan J, et al. Genomic features and clinical characteristics of adolescents and young adults with cholangiocarcinoma. Front Oncol. 2019;9:1439. doi: 10.3389/fonc.2019.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albritton K, Caligiuri M, Anderson B, et al. Closing the gap: Research and care imperatives for adolescents and young adults with cancer. Presented at National Cancer Advisory Board Meeting, Bethesda, MD, September 6, 2006. https://deainfo.nci.nih.gov/advisory/ncab/archive/139_0906/presentations/AYAO.pdf.
- 9. Johnson RH, Anders CK, Litton JK, et al. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. 2018;65:e27397. doi: 10.1002/pbc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan SA, Morris M, Idrees K, et al. Colorectal cancer in the very young: A comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg. 2016;51:1812–1817. doi: 10.1016/j.jpedsurg.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 12. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109:djw322. doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: A call to action. Mayo Clin Proc. 2014;89:216–224. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 14. Tricoli JV, Boardman LA, Patidar R, et al. A mutational comparison of adult and adolescent and young adult (AYA) colon cancer. Cancer. 2018;124:1070–1082. doi: 10.1002/cncr.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang JT, Huang KC, Cheng AL, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. 2003;90:205–214. doi: 10.1002/bjs.4015. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez L, Brennan K, Karim S, et al. Disease characteristics, clinical management, and outcomes of young patients with colon cancer: A population-based study. Clin Colorectal Cancer. 2018;17:e651–e661. doi: 10.1016/j.clcc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 17. Cheng E, Blackburn HN, Ng K, et al. Analysis of survival among adults with early-onset colorectal cancer in the National Cancer Database. JAMA Netw Open. 2021;4:e2112539. doi: 10.1001/jamanetworkopen.2021.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cercek A, Chatila WK, Yaeger R, et al. A comprehensive comparison of early-onset and average-onset colorectal cancers. J Natl Cancer Inst. 2021;113:1683–1692. doi: 10.1093/jnci/djab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipsyc-Sharf M, Zhang S, Ou FS, et al. Survival in young-onset metastatic colorectal cancer: Findings from cancer and leukemia group B (alliance)/SWOG 80405. J Natl Cancer Inst. 2022;114:427–435. doi: 10.1093/jnci/djab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. J Am Med Assoc. 2006;295:2492. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 21. Carvalho FM, Bacchi LM, Santos PPC, et al. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics. 2010;65:1033–1036. doi: 10.1590/S1807-59322010001000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: Features of disease at presentation. Ann Oncol. 2002;13:273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 23. Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 25. Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: A clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sireci AN, Aggarwal VS, Turk AT, et al. Clinical genomic profiling of a diverse array of oncology specimens at a large academic cancer center: Identification of targetable variants and experience with reimbursement. J Mol Diagn. 2017;19:277–287. doi: 10.1016/j.jmoldx.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 29. Garcia EP, Minkovsky A, Jia Y, et al. Validation of oncopanel a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–758. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 30. Ananda G, Mockus S, Lundquist M, et al. Development and validation of the JAX Cancer Treatment ProfileTM for detection of clinically actionable mutations in solid tumors. Exp Mol Pathol. 2015;98:106–112. doi: 10.1016/j.yexmp.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MedCalc Software BV: MedCalc Statistical Software version 19.2.6, Ostend, Belgium, 2020. https://www.medcalc.org.
- 33. StataCorp: Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, 2021.
- 34. Coccia PF. Overview of adolescent and young adult oncology. JCO Oncol Pract. 2019;15:235–237. doi: 10.1200/JOP.19.00075. [DOI] [PubMed] [Google Scholar]
- 35. Bleyer A, Tai E, Siegel S. Role of clinical trials in survival progress of American adolescents and young adults with cancer-and lack thereof. Pediatr Blood Cancer. 2018;65:e27074. doi: 10.1002/pbc.27074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Cancer Society 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf Cancer facts & figures 2021.
- 37. Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 38. Pu X, Ye Q, Cai J, et al. Typing FGFR2 translocation determines the response to targeted therapy of intrahepatic cholangiocarcinomas. Cell Death Dis. 2021;12:256. doi: 10.1038/s41419-021-03548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kongpetch S, Jusakul A, Lim JQ, et al. Lack of targetable FGFR2 fusions in endemic fluke-associated cholangiocarcinoma JCO Glob Oncol 10.1200/GO.20.00030, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain A, Borad MJ, Kelley RK, et al. Cholangiocarcinoma with FGFR genetic aberrations: A unique clinical phenotype JCO Precis Oncol 10.1200/PO.17.00080, 2018 [DOI] [PubMed] [Google Scholar]
- 41. Quah HM, Joseph R, Schrag D, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol. 2007;14:2759–2765. doi: 10.1245/s10434-007-9465-x. [DOI] [PubMed] [Google Scholar]
- 42. Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg. 2017;152:452. doi: 10.1001/jamasurg.2016.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy BL, Day CN, Hoskin TL, et al. Adolescents and young adults with breast cancer have more aggressive disease and treatment than patients in their forties. Ann Surg Oncol. 2019;26:3920–3930. doi: 10.1245/s10434-019-07653-9. [DOI] [PubMed] [Google Scholar]
- 44. Frank S, Carton M, Dubot C, et al. Impact of age at diagnosis of metastatic breast cancer on overall survival in the real-life ESME metastatic breast cancer cohort. Breast. 2020;52:50–57. doi: 10.1016/j.breast.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. You YN, Xing Y, Feig BW, et al. Young-onset colorectal cancer: Is it time to pay attention? Arch Intern Med. 2012;172:287. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 46. Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 47. Miller KD, Fidler‐Benaoudia M, Keegan TH, et al. Cancer statistics for adolescents and young adults. CA Cancer J Clin. 2020;70:443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 48. Alvarez EM, Force LM, Xu R, et al. The global burden of adolescent and young adult cancer in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022;23:27–52. doi: 10.1016/S1470-2045(21)00581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]