Clonal hematopoiesis is associated with 8.6-fold higher risk of doxorubicin-induced cardiotoxicity.
Abstract
PURPOSE
The main dose-limiting toxicity of anthracyclines is cardiotoxicity. Clonal hematopoiesis (CH), somatic mutations in hematopoietic stem or progenitor cells in patients without hematologic malignancy, is also associated with risk for adverse cardiovascular events and worse outcomes overall. We hypothesize that CH increases risk for doxorubicin-induced cardiotoxicity (DIC).
METHODS
We conducted a retrospective cohort study in patients treated with doxorubicin for cancer (N = 100). Patients (n = 25) had incident symptomatic heart failure, decline in left ventricular ejection fraction, or arrhythmia. CH was identified using paired peripheral blood and tumor DNA.
RESULTS
After adjusting for age at doxorubicin initiation, diabetes, dyslipidemia, and chest radiation, high cumulative dose of doxorubicin (>240 mg/m2; odds ratio [OR], 7.00; 95% CI, 1.77 to 27.74; P = .0056), CH (OR, 8.58; 95% CI, 2.05 to 35.99; P = .0033), and history of smoking (OR, 3.15; 95% CI, 1.00 to 9.93; P = .0495) were associated with DIC.
CONCLUSION
This study provides preliminary evidence for CH as a predictive risk factor for DIC, which, with further investigation, could serve as an important precision medicine biomarker for the large number of patients with cancer who have CH.
INTRODUCTION
Anthracyclines (eg, doxorubicin, daunorubicin, and epirubicin) are among the most widely used chemotherapeutic agents, serving as the base of treatment for both solid cancers and hematologic malignancies; however, risk for cardiotoxicity is a treatment-limiting factor. Presenting as reduced left ventricular ejection fraction (LVEF), arrhythmias, or heart failure, anthracycline-based cardiotoxicities are not uniformly predicted by cumulative dose, posing a clinical challenge for oncologists and patient outcomes.1 We hypothesize that clonal hematopoiesis (CH) increases patients' risk for anthracycline-induced cardiotoxicity.
CONTEXT
Key Objective
The key objective of this study was to investigate clonal hematopoiesis (CH) as an independent molecular risk factor for doxorubicin-induced cardiotoxicity (DIC). To our knowledge, this is the first-ever evaluation of CH as a predictor of DIC across patients with diverse cancer types.
Knowledge Generated
This was a retrospective cohort study with a sample size of 100 patients, a DIC rate of 25%, and a CH rate of 13%. We observed a statistically significant association between CH and DIC, with patients with CH having 8.6-fold higher risk of DIC compared with patients without CH after adjusting for known risk factors, including cumulative doxorubicin dose.
Relevance
The major limiting factor for doxorubicin use is the risk of cardiotoxicity. Biomarkers to predict risk for these toxicities, such as CH, have the potential to inform precision management for a wide range of patients, including cancer patients who have CH.
CH is defined by somatically mutated populations of hematopoietic cells in individuals without other hematologic abnormalities.2 In addition to increased risk for hematologic malignancies, CH has been recently described in the literature as a potential causal cardiovascular risk factor.3,4 CH is associated with decreased survival in part due to a risk for coronary heart disease, atherosclerosis, and heart failure. To our knowledge, there are no data evaluating CH as a potential risk factor for adverse cardiac events across patients with cancer who receive doxorubicin-based chemotherapies. This study investigates CH as a risk factor for doxorubicin-induced cardiotoxicity (DIC).
METHODS
This was a retrospective cohort study that included patients treated with doxorubicin who were consented to Moffitt Cancer Center's institutional biorepository, the Total Cancer Care Protocol5 (MCC#14690; Advarra institutional review board [IRB] Pro00014441); use of genetic data for this study was approved under a release protocol (MCC#19772, Advarra IRB Pro00029764). For inclusion, patients were required to have baseline and follow-up (ie, after doxorubicin treatment) cardiac evaluation with multigated acquisition scan, echocardiogram, electrocardiogram, Holter monitor, or a combination of these modalities. All patients who met the inclusion criteria between 2003 and 2019 were included in the study. Doxorubicin dose was stratified as high (defined as cumulative dose >240 mg/m2) versus low (cumulative dose ≤240 mg/m2) on the basis of conventional clinical dosing. For all patients, total cumulative dose of doxorubicin administered during the study was the same as lifetime cumulative dose of doxorubicin as none of the patients had a history of previous use of doxorubicin or other anthracyclines. Cases experienced DIC, which was defined as new symptomatic heart failure, a decline of LVEF of 15% (or 10% if baseline LVEF was ≤55%), or arrhythmia after doxorubicin initiation; controls did not have any of these. Clinical and cardiotoxicity parameters were obtained through manual review of the patients' electronic health records. Patients' sex, race, and ethnicity were also collected from electronic medical records.
CH was identified using paired peripheral blood and tumor DNA. Whole-exome sequencing reads were aligned to the human genome (GRCh38) using BWA-MEM.6 Variant calling was performed using Genome Analysis ToolKit best practices and MuTect2.7,8 Downstream filtering was conducted to identify CH following the premise previously described.9,10 Briefly, variants in 76 CH-related genes were considered for filtering. A coverage ≥20× with >1 variant read on each strand was required. Likely germline mutations were removed by excluding variants with variant allele frequencies (VAFs) >35% in paired blood and tumor and mutations common (>0.005) in population-based databases.11,12 Variants with ≥2× VAF in tumor compared with blood were excluded as likely tumor-derived. Likely pathogenic mutations were selected by excluding synonymous and nonsynonymous variants, which were recovered if they were previously reported in CH or cancer.13,14 Remaining blood-derived variants at a VAF of ≥2% were classified as CH.
Descriptive statistics for demographic and clinical characteristics were compared between cases and controls. Univariable and multivariable logistic regressions were used to determine predictors associated with DIC. The selection for multivariable model variables was based on clinical rationale and statistical significance from univariable models. Univariable and multivariable Cox proportional hazards regressions were used to investigate the relationship between risk factors and survival time. Survival time was defined as the number of months elapsed between the date of diagnosis and the date of last encounter or death. A Kaplan-Meier curve and a log-rank test were used to compare overall survival between patients with or without DIC. A P value of <.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS, Cary, NC).
RESULTS
A total of 100 patients treated with doxorubicin were included. Average follow-ups for cases (6.2 years; range, 0.03-15.9) and controls (6.3 years; 0.03-15.9) were similar. Breast cancer was the most common malignancy (44 of 100) followed by sarcoma (40 of 100) and multiple myeloma (12 of 100). Cardiac evaluations occurred frequently during doxorubicin treatment (median, 3.1 months; IQR, 2.1-4.8) and less frequently during post-treatment follow-up (median, 10.6 months; IQR, 8.0-12.1). Incident DIC occurred in 25% (25 of 100) of patients. The average time to DIC was 4.4 years (IQR, 1.2-7.3). DIC events included incident heart failure (n = 5), decline of LVEF (n = 12), and arrhythmia (n = 13); all heart failure cases also had reduced LVEF. The median time between doxorubicin initiation and blood collection was 0.14 years (IQR, –0.02 to 1.14 years). There was no difference in the proportion of samples collected before doxorubicin initiation in cases versus controls (12 of 25 v 24 of 75; P = .16).
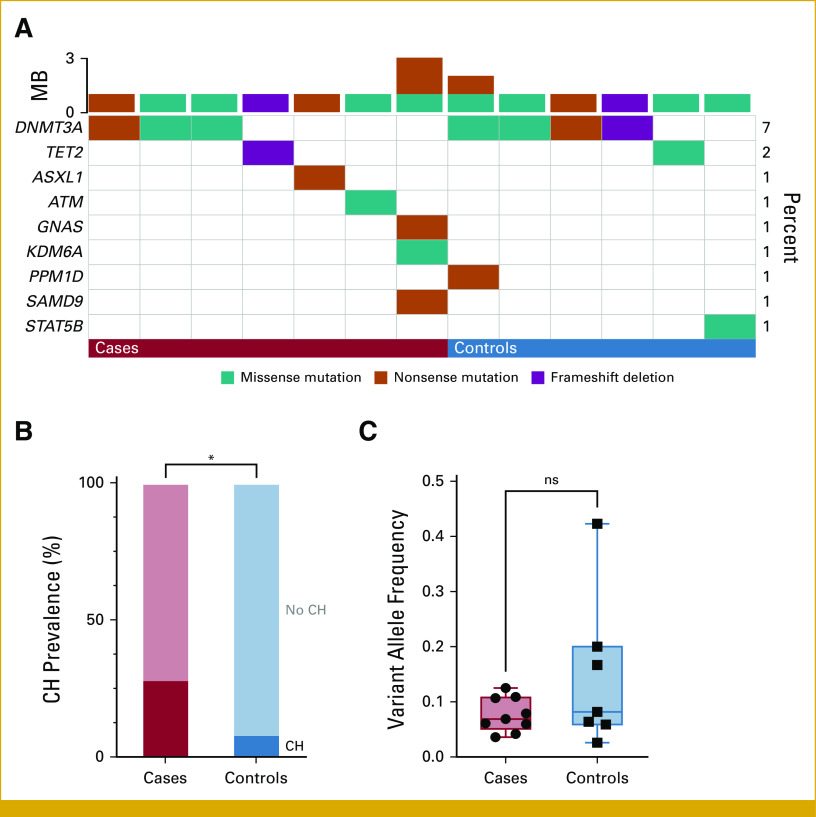
A total of 16 CH mutations were detected in 13% of patients (13 of 100; Fig 1A). The most frequently mutated gene was DNMT3A (7 of 16 mutations), followed by TET2 (2 of 16); other genes (ATM, ASXL1, GNAS, KDM6A, PPM1D, SAMD9, STAT5B) each had one mutation (Fig 1A). The median VAF was 7.4% (range, 2.6%-42.3%) and was not different between cases and controls (6.9% v 8.2%, respectively; Mann-Whitney P = .47; Figs 1B and 1C). A total of 13 patients (13%) had a history of chemotherapy, radiation, or a combination of both before assessment for CH status. Of those, two were positive for CH—one patient with a history of chemotherapy 2 months before blood sample collection and one patient with a history of radiation 8 years before blood sample collection. There was no statistically significant difference in the prevalence of CH between patients with or without a history of chemotherapy and/or radiation (15.4% v 12.6%, respectively; P = .68).
FIG 1.
(A) Oncoplot of CH mutations in cases (n = 7 of 25) and controls (n = 6 of 75). Each column represents a patient, and each row represents a gene of interest. Colored cells indicate the presence of a mutation in that gene and patient. Types of mutations are classified according to colors as shown in the legend on the bottom. (B) Prevalence of CH in cases and controls. (C) Boxplot of variant allele frequency of CH mutations in cases and controls, depicted as median and range. *P < .05. CH, clonal hematopoiesis; MB, mutation burden; ns, not significant.
CH and high cumulative dose of doxorubicin were associated with cardiotoxicity in univariable analysis (Table 1). After adjusting for age at doxorubicin initiation, diabetes, dyslipidemia, and chest radiation in the multivariable model, high cumulative dose of doxorubicin (odds ratio [OR], 7.00; 95% CI, 1.77 to 27.7; P = .0056), CH (OR, 8.58; 95% CI, 2.05 to 36.0; P = .0033), and history of smoking (OR, 3.15; 95% CI, 1.00 to 9.93; P = .0495) were associated with DIC (Table 1; Fig 2). The association of DIC with high cumulative dose of doxorubicin (OR, 4.91; 95% CI, 1.62 to 14.89; P = .0048) and CH (OR, 6.51; 95% CI, 1.76 to 24.06; P = .005) also persisted when only those variables plus age were included in a multivariable model.
TABLE 1.
Patient Characteristics and Their Associations With Cardiotoxicity
FIG 2.
Forest plot for associations with doxorubicin-induced cardiotoxicity in the multivariable logistic regression model. Values represent odds ratios with 95% CIs. Statistically significant variables are shown in red. OR, odds ratio.
The proportional hazards assumption was supported for all variables. In survival analysis, cancer type (breast cancer v non–breast cancer; hazard ratio [HR], 0.29; 95% CI, 0.13 to 0.62; P = .0016), hormonal treatment (HR, 0.36; 95% CI, 0.14 to 0.93; P = .034), high cumulative dose of doxorubicin (HR, 2.34; 95% CI, 1.19 to 4.58; P = .013), and chest radiation (HR, 0.44; 95% CI, 0.22 to 0.90; P = .025) were significant predictors in univariable Cox proportional hazard regression; however, these statistical associations did not persist in the multivariable Cox model. The mean follow-up time for patients who died was 5.24 years (IQR, 1.75-7.99), which infers sufficient time to see the effect of selected variables on DIC. Patients with DIC had shorter survival time than patients without DIC (log-rank P = .017; Fig 3).
FIG 3.
Kaplan-Meier curve for overall survival. The graph shows the overall survival in months in cases who had DIC (red) and controls who did not (blue). DIC, doxorubicin-induced cardiotoxicity; OS, overall survival.
DISCUSSION
CH is associated with a chronic proinflammatory state and confers an independent two-fold increase in cardiovascular risk in healthy individuals3; whether this risk translates to patients with cancer treated with anthracyclines is unknown. To our knowledge, this study provides some of the first evidence that CH may be an independent risk factor for DIC. One retrospective analysis showed that TET2 CH mutations, specifically, increase risk for anthracycline-induced cardiotoxicity in patients with lymphoma.15 Furthermore, functional studies using mouse models of TP53-CH (ie, Trp53-mutated) showed that doxorubicin led to disproportional expansion of Trp53-mutated versus wild-type cells and the Tp53-CH models had greater cardiac functional impairment and ventricular wall thinning than wild-type mice.16 Thus, the current literature is limited to specific CH mutations in patients with lymphoma and noncancer mouse models. Our study shows that nonspecific CH mutations are associated with DIC in patients with breast cancer, sarcoma, and multiple myeloma. CH mutations may constitute a precision medicine tool for DIC risk stratification.
Anthracyclines are used for treatment of many malignancies, including breast cancers, acute leukemia, lymphomas, and childhood solid tumors. Because of their wide use, a large number of patients are affected by anthracycline-associated cardiotoxicity. In fact, cardiovascular mortality is a leading cause of death in patients with cancer who develop cardiac symptoms after anthracycline administration.17 The most common cardiac toxicity of anthracyclines is cardiomyopathy, which has a manifestation ranging from the asymptomatic decline of LVEF to the development of symptomatic heart failure. The incidence of cardiomyopathy is dose-dependent and ranges between 5% and 8% at a cumulative dose of 450 mg/m2.18 When accounting for asymptomatic decline in LVEF as an adverse cardiac event, the incidence is approximately 65% at the cumulative dose of 550 mg/m2, approximately 35% at the cumulative dose of 350 mg/m2, and 7% at the cumulative dose of 150 mg/m2.19 Arrhythmogenic effects of doxorubicin have been described in early and late periods of treatment and can cause nonbenign arrhythmias, including atrial fibrillation in over 10% of patients.18 The risks of anthracycline-induced cardiotoxicities also depend on other factors, including age, female sex, race, and pre-existing cardiac conditions. In our study, which included 23 of 100 patients who received cumulative doses of doxorubicin >240 mg/m2, the incidence of DIC was 25%. This result is consistent with rates of adverse cardiac events described in the literature when accounting for inclusion of asymptomatic decline in LVEF and new-onset arrhythmias as criteria for identifying cardiotoxic cases.
The timeline of anthracycline-induced cardiotoxicity used to be grouped into acute, early-onset chronic, and late-onset chronic categories; however, a recent approach recognizes that anthracycline-induced cardiotoxicity is a continuous phenomenon that starts with the first exposure and may progress to overt heart failure over years.20 A study of 2,625 patients found that most anthracycline-induced cardiotoxicities can be detected within the first year.21 In that study, the median time elapsed from the final dose of anthracycline to the development of cardiotoxicity was 3.5 months. In our study, the median time elapsed from the final dose of doxorubicin to cardiotoxicity was 5.1 months and 84% of cases were detected within the first year. These results are in line with expectations on the basis of the published literature.
Epidemiologic and mechanistic biologic data provide a clear link between CH and cardiovascular disease risk. In fact, the CH-associated risk for cardiovascular disease in population-based cohorts is on a par with classic Framingham risk factors.2 The similarities in the mechanism of CH-mediated adverse effects to anthracycline-induced cardiotoxicity also draw a compelling parallel to hypothesize a link between CH and DIC. First, a primary mechanism by which anthracyclines induce cardiotoxicity is through generation of reactive oxygen species, leading to increased oxidative stress and cardiac tissue inflammation. Similarly, mouse models of Tet2-CH increase inflammasome activation, leading to enhanced interleukin-1β secretion and cardiac dysfunction.22 Second, anthracyclines also cause enhanced trafficking of neutrophils to the myocardium to increase toxicity, which has been shown to be exacerbated in mouse models of CH.23,24 Consistent with previous studies of CH and cardiovascular disease, the two most frequently mutated genes in our cohort were DNMT3A and TET2.2 Both types of CH mutations drive a proinflammatory state through mechanisms that increase expression of proinflammatory cytokines in macrophages.25,26 In our study, DNMT3A was the most common mutation and almost half (3 of 7) of patients with DNMT3A-CH experienced DIC. Taken together, these data suggest that the mechanism driving the observed association between CH and DIC may be induction of proinflammatory pathways.
The potential impact of CH on cardiotoxicity risk is amplified in patients with cancer because of the higher prevalence of CH in this population. In population-based cohorts, the CH prevalence is generally around 10% to 15% for individuals older than 65 years; however, in patients with cancer, this prevalence is higher across all age groups, corresponding to an overall prevalence of approximately 25%.27-29 We observed a prevalence of 13% in this study population. The lower-than-reported prevalence may be explained by multiple factors. First, whole-exome sequencing was used to identify CH mutations. Because of lower overall sequencing coverage, the ability to detect low-frequency variants (eg, <5% VAF) is hindered; therefore, we might have missed low-VAF CH mutations in some patients. However, the consistently stronger effects of high VAF (eg, >10%) CH mutations across studies provide evidence that misclassification of low-VAF CH might have a limited impact on the ability to detect clinically meaningful associations.2,30,31 Another factor that can affect reported prevalence of CH is the filtering criteria used to classify CH. In this study, we focused on a subset of CH- and hematologic malignancy–associated genes and variants likely to affect protein function (eg, missense, stop gains, etc). As such, the prevalence of CH reported in our population corresponds more closely CH in potential drivers’ prevalences that have been reported previously. As such, the prevalence of CH reported in our study is more in line with prevalences reported that specifically focus on myeloid driver mutations (eg, potential driver-CH).27
This is a proof-of-concept study because of the limited sample size and lack of replication. Effect size and power for CH, especially gene-specific effects, may be further limited by our relatively young patient population (average age 52 years) since CH is strongly skewed toward increased ages. The univariable analysis finding of improved survival for patients with breast cancer may be confounded by early-stage breast cancers since those patients are more likely to receive chest wall radiation and hormone therapy and have better prognosis compared with other cancers included.
In summary, in this study, the odds of DIC were estimated to be 8.6-fold higher in patients with CH than those without CH after adjusting for other known DIC risk factors. Additional studies are needed to confirm the suggested association of CH with DIC.
ACKNOWLEDGMENT
This research was made possible through the Total Cancer Care Protocol at the H. Lee Moffitt Cancer Center & Research Institute and supported, in part, by the Collaborative Data Services Core Facility, Tissue Core Facility, Molecular Genomics Core Facility, and Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292).
SUPPORT
The whole-exome sequencing data included in this work were obtained through the ORIEN Avatar Project, which was managed and funded, in part, by Aster Insights (formerly M2Gen), a for-profit company. Aster Insights received funding from third-party partners to partially support data generated in the Avatar Project. These funders had no role in the design of this study, analysis, interpretation of the data, or preparation of the manuscript for publication. Funding for this work was also provided by Research Innovation & Scholarly Endeavors (RISE) 2021, Summer Scholarly Award Experience, University of South Florida.
DATA SHARING STATEMENT
The data that support the findings of this study are included in the Data Supplement.
AUTHOR CONTRIBUTIONS
Conception and design: Jamila Mammadova, Shridar Ganesan, Roohi Ismail-Khan, Nancy Gillis
Financial support: Nancy Gillis
Provision of study materials or patients: Roohi Ismail-Khan
Collection and assembly of data: Jamila Mammadova, Christelle Colin-Leitzinger, Nancy Gillis
Data analysis and interpretation: Jamila Mammadova, Christelle Colin-Leitzinger, Diep Nguyen, Rahul Mhaskar, Shridar Ganesan, Yi-Han Tang, Mingxiang Teng, Nancy Gillis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shridar Ganesan
This author is an Associate Editor for JCO Precision Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Merck
Stock and Other Ownership Interests: Ibris, Inspirata, Merck, Silagene
Consulting or Advisory Role: Inspirata, Novartis, Roche, Foghorn Therapeutics, Foundation Medicine, Merck Sharp & Dohme, Silagene, EQRx, EMD Serono, KayoThera, Ipsen
Research Funding: M2Gen (Inst), Gandeeva Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: I hold two patents for digital imaging that may be licensed to Ibris, Inc and Inspirata, Inc
Other Relationship: NIH/NCI, NIH/NCI
Yi-Han Tang
Employment: Moffitt Cancer Center
Roohi Ismail-Khan
Employment: Eli Lilly/Loxo Oncology
Stock and Other Ownership Interests: Lilly
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1. Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–325. doi: 10.3322/caac.21341. [DOI] [PubMed] [Google Scholar]
- 2. Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105. doi: 10.1016/j.yjmcc.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pascual-Figal DA, Bayes-Genis A, Díez-Díez M, et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2021;77:1747–1759. doi: 10.1016/j.jacc.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 5. Fenstermacher DA, Wenham RM, Rollison DE, et al. Implementing personalized medicine in a cancer center. Cancer J. 2011;17:528–536. doi: 10.1097/PPO.0b013e318238216e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H.https://arxiv.org/abs/1303.3997 Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv. 2013:1303.3997.
- 7. DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin D, Sato T, Cibulskis K, et al. https://www.biorxiv.org/content/early/2019/12/02/861054 Calling somatic SNVs and indels with Mutect2. bioRxiv, Cold Spring Harbor Laboratory, 2019.
- 9. Peres LC, Colin-Leitzinger CM, Teng M, et al. Racial and ethnic differences in clonal hematopoiesis, tumor markers, and outcomes of patients with multiple myeloma. Blood Adv. 2022;6:3767–3778. doi: 10.1182/bloodadvances.2021006652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coombs CC, Gillis NK, Tan X, et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res. 2018;24:5918–5924. doi: 10.1158/1078-0432.CCR-18-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. 1000 Genomes Project Consortium. Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bick AG, Weinstock JS, Nandakumar SK, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586:763–768. doi: 10.1038/s41586-020-2819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tate JG, Bamford S, Jubb HC, et al. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatakeyama K, Hieda M, Semba Y, et al. TET2 clonal hematopoiesis is associated with anthracycline-induced cardiotoxicity in patients with lymphoma. JACC CardioOncol. 2022;4:141–143. doi: 10.1016/j.jaccao.2022.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sano S, Wang Y, Ogawa H, et al. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight. 2021;6:e146076. doi: 10.1172/jci.insight.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: Addressing the unresolved issues. Circulation. 2012;126:2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buza V, Rajagopalan B, Curtis AB. Cancer treatment-induced arrhythmias: Focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol. 2017;10:e005443. doi: 10.1161/CIRCEP.117.005443. [DOI] [PubMed] [Google Scholar]
- 19. Henriksen PA. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart. 2018;104:971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 20. Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 21. Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 22. Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sano S, Wang Y, Ogawa H. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight. 2021;6:e146076. doi: 10.1172/jci.insight.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huerga Encabo H, Aramburu IV, Garcia-Albornoz M, et al. Loss of TET2 in human hematopoietic stem cells alters the development and function of neutrophils. Cell Stem Cell. 2023;30:781–799.e9. doi: 10.1016/j.stem.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 25. Hormaechea-Agulla D, Matatall KA, Le DT, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell. 2021;28:1428–1442.e6. doi: 10.1016/j.stem.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cull AH, Snetsinger B, Buckstein R, et al. Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol. 2017;55:56–70.e13. doi: 10.1016/j.exphem.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 27. Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382.e4. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abelson S, Collord G, Ng SWK, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. doi: 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52:1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are included in the Data Supplement.