Abstract
PURPOSE
Evidence suggests that neurotrophic tyrosine receptor kinase (NTRK) gene fusions in solid tumors are predictive biomarkers for targeted inhibition across a number of adult and pediatric tumor types. However, despite robust clinical response to tyrosine receptor kinase (TRK) inhibitors, the natural history and prognostic implications of NTRK fusions in solid tumors are poorly understood. It is important to evaluate their prognostic significance on survival to provide some context to the clinical effectiveness observed in clinical trials of TRK-targeted therapies.
METHODS
A systematic literature review was conducted in Medline, Embase, Cochrane, and PubMed to identify studies comparing the overall survival (OS) of patients with NTRK fusion–positive (NTRK+) versus NTRK fusion–negative (NTRK–) tumors. Five retrospective matched case-control studies published before 11 August 2022 were assessed for inclusion, and three were selected for the meta-analysis (sample size: 69 NTRK+, 444 NTRK–). Risk of bias was assessed using the Risk of Bias Assessment tool for Non-randomized Studies tool. The pooled hazard ratio (HR) was estimated using a Bayesian random-effects model.
RESULTS
In the meta-analysis, the median follow-up ranged from 2 to 14 years and the median OS was between 10.1 and 12.7 months (where reported). Comparing patients with tumors NTRK+ and NTRK–, the pooled HR estimate for OS was 1.51 (95% credible interval, 1.01 to 2.29). The patients analyzed had no previous or current exposure to TRK inhibitors.
CONCLUSION
In patients not treated with TRK inhibitor therapies, those with NTRK+ solid tumors have a 50% increased risk of mortality within 10 years from diagnosis or the start of standard therapy compared with those with NTRK– status. Although this is the most robust estimate of the comparative survival rate to date, further studies are required to reduce uncertainty.
INTRODUCTION
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions play a significant role in the development and function of the nervous system, such as the regulation of pain, thermoregulation, movement, memory, appetite, and cognition. Gene fusions involving NTRK1, 2, or 3 (encoding the neurotrophin receptors TRKA, B, and C, respectively) have been identified in several solid tumor types arising in adults and children1 and can be detected in the clinic using tumor DNA and RNA sequencing. Although rare in common solid tumors, with a frequency of <1%, NTRK gene fusions occur more frequently in some rare tumor types, for example, <5% in lung and colorectal cancers, 5%-25% in thyroid cancer, and >90% in secretory carcinoma of salivary gland.2 Laboratory evidence and clinical evidence suggest that they are oncogenic drivers of various adult and pediatric tumor types and predictive biomarkers for targeted inhibition.3
CONTEXT
Key Objective
Studies have shown that patients with neurotrophic tyrosine receptor kinase (NTRK) gene fusions in solid tumors (NTRK+) have a poorer prognosis than NTRK– patients. It would be useful to understand the prognostic significance of these genomic alterations on survival to provide additional context around the evaluation of newly emerging targeted tyrosine receptor kinase (TRK) inhibitors. The aim of this meta-analysis was to provide a more robust estimate of the prognostic value of NTRK+ status in patients in a real-world setting.
Knowledge Generated
The pooled hazard ratio from three studies suggests that NTRK+ status is associated with 50% increased risk of mortality. The patients analyzed had no previous or current exposure to TRK inhibitors.
Relevance
Robust survival estimates related to NTRK status are important for the extrapolation of findings from clinical trials to the real-world setting and providing guidance for clinical decision making around therapeutic options.
Tyrosine receptor kinase (TRK) inhibitors offer one of the latest therapeutic advancements among a range of targeted treatments on the basis of genetic alterations in malignant cells. First-generation TRK inhibitors, such as larotrectinib and entrectinib, have been shown to be effective and well-tolerated treatments in patients with NTRK+ solid tumors (NTRK+), with high response rates regardless of tumor histology.2-5 Early results from three clinical trials demonstrated that larotrectinib is effective at shrinking tumors, with durable response across a range of different cancer types, including lung, thyroid, and gastrointestinal tumors.4,6 Larotrectinib and entrectinib represent the first innovative precision therapies to receive approval from international authorization and commissioning bodies for the treatment of a specific genetic expression, regardless of the site from which the tumor originated.3 The development of second-generation TRK inhibitors is currently underway. Such targeted therapies have clear advantages over conventional cytotoxic treatments and the systemic side effects they induce.7,8
These recent developments have important implications for patients, clinicians, and genomic screening services for health care providers worldwide. However, despite robust clinical response to TRK inhibitors, the natural history and prognostic implications of NTRK fusions in solid tumors are poorly understood, largely because of the rarity of such mutations. With the recent introduction of TRK inhibitor therapies and molecular testing for NTRK gene fusions into clinical practice, it is important to evaluate the prognostic significance of NTRK gene fusions in survival to provide some context to the clinical effectiveness observed in clinical trials of NTRK-targeted therapies. In addition, although NTRK gene fusions are predictive of benefit from treatment with TRK inhibitors regardless of the tumor type, the prognostic significance of NTRK gene fusions in clinical trials in the pan-tumor setting remains unclear.
Previous studies9-13 have suggested that the prognosis for overall survival (OS) is worse in patients with NTRK+ solid tumors compared with those with NTRK fusion–negative (and/or wild-type fusions; NTRK–) solid tumors. However, these studies are based on small numbers of patients, with a large amount of uncertainty around the reported estimates. The aim of this article was to conduct a systematic review and meta-analysis of published studies comparing OS in the NTRK+ and NTRK– cohorts to increase the statistical precision of the comparative survival rates. A reliable estimate of the prognostic value of NTRK status and survival will contribute to the evidence base and provide useful insights for ongoing and future trials of therapeutic interventions.
METHODS
Search Strategy
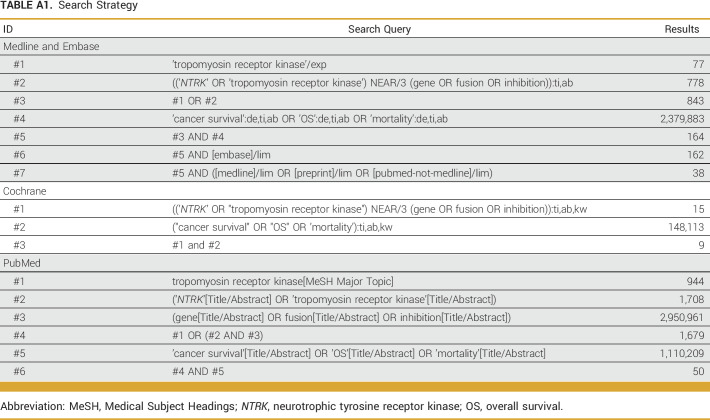
This systematic review and meta-analysis were performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.14 Studies published up to August 11, 2022, were retrieved from Medline, Embase, Cochrane, and PubMed. The full literature search strategy is presented in Appendix Table A1. No restrictions on the basis of language, time, study design, or article type were made.
Selection Criteria
Articles were included in the review if they satisfied the following eligibility criteria: (1) the population included patients with NTRK+ solid tumors, (2) OS was included as an outcome, and (3) comparative results with NTRK– tumors were provided. The results from the respective electronical databases were combined, and duplicate references were removed. Abstracts were reviewed independently by two systematic reviewers against the eligibility criteria and classified as include, exclude, or unsure. Results were compared, and where discrepancies arose between reviewers, final consensus was reached by mutual consent. If consensus could not be reached, a third reviewer casted the deciding vote. For those abstracts selected for inclusion, the double review process was repeated using the full-text articles. The primary reason for exclusion at each stage was recorded. The bibliographies of all articles included for data extraction were hand searched for further eligible articles. Only unique patient cohorts were included in the meta-analysis.
Data Extraction
The following prespecified data items were extracted from the full-text articles of included studies and associated clinical study reports (if available), otherwise, the information was acquired from conference abstracts and posters if the full text was not available: study, patient cohort, sample size, study design, data source, country, patient age and sex, practice type (academic/community), Eastern Cooperative Oncology Group (ECOG) Performance Status Scale, NTRK fusion status, index date, censoring, follow-up time, number of events, OS rate, and covariate adjustment. The data were entered into a data extraction form and independently validated. The availability of Kaplan-Meier (KM) curves and whether extraction of further data points using digitizing software and a previously published algorithm was necessary were determined.15
Risk of Bias Assessment
Risk of bias was evaluated independently by two reviewers, using the Risk of Bias Assessment tool for Non-randomized Studies.16 This is a non-numerical, domain-based scale with acceptable validity and reliability and a comprehensive coverage of important biases. It comprises six domains related to methodological quality: participant selection, confounding control, exposure measurement, outcome assessment, incomplete outcome data, and selective outcome reporting. Each domain was graded as low, high, or unclear.
Data Synthesis and Analysis
The study characteristics and availability of end point data were assessed to ascertain the feasibility of performing a quantitative evidence synthesis. First, log hazard ratios (HRs) were pooled for included studies using a fixed-effects model. Statistical heterogeneity was assessed using I2 (percentage of variability in group effect estimates because of between-study heterogeneity rather than sampling error). The fixed-effects approach assumes that all the effect estimates are evaluating a common underlying NTRK gene fusion effect, which may not be plausible in the presence of heterogeneity across studies. Ideally, given a larger number of studies, a random-effects approach would be used to incorporate any heterogeneity into the model. To address this limitation of very few studies, a Bayesian random-effects model was fitted, exploring the use of suitable prior distributions for the between-study heterogeneity parameter (including appropriate informative prior distributions).17 The random-effects models were compared using the leave-one-out information criteria (LOOIC); lower values indicate better fit. Results of the best-fitting model were presented in a forest plot, reporting the posterior mean log HR (95% credible interval [CrI]) for each study, along with the overall pooled effect (95% CrI). A funnel plot was inspected for evidence of reporting bias although a test for asymmetry was not used because of the small number of studies.18 The statistical software RStudio version 4.2.1 (meta and brms packages) was used for the analysis.
RESULTS
Search Results
The search strategy was conducted on August 11, 2022, and retrieved 265 articles (Fig 1). After the removal of duplicates, 198 abstracts were screened and 187 articles were excluded on the basis of the study eligibility criteria. The remaining 11 articles were screened on full text. Six further articles were excluded; four did not have a NTRK– comparison group, one did not report the appropriate survival outcome, and one referred to the same study reported in another article. The remaining articles were assessed in further detail for inclusion in the quantitative synthesis.9-13 The full article for the study by Bridgewater et al10 was published on August 23, 2022.19
FIG 1.
PRISMA study selection diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study and Patient Characteristics
The study and patient characteristics of the five studies assessed for inclusion in the meta-analysis are presented in Table 1. All five studies were retrospective, matched case-control studies on the basis of data from routinely collected databases from the United States, United Kingdom, and the Netherlands, and the sample sizes of NTRK+ patients ranged from 18 to 28. The proportion of male NTRK+ patients varied from 16.7% to 44.8%, and the median age was around 60 years although Bridgewater et al10,19 and Zhu et al13 included pediatric patients. ECOG status was not reported by Santi et al12 and Zhu et al,13 and there was a high percentage of missing data in the three studies where it was reported although where it was known, most patients had ECOG scores of 0-1. Matching with the comparator populations was generally reported as successful, or at least adequate, given the limitations with missing baseline data for some factors, such as ECOG score. A summary of the overlap in the different tumor types included in the NTRK+ and NTRK– groups across studies is displayed in Appendix Figure A1.
TABLE 1.
Study and Patient Characteristics of the Five Studies Assessed for Quantitative Synthesis
Table 2 summarizes the statistical methods and reporting in the five studies. The index date from which OS was measured varied between studies; two used the date of gene sequencing report, three used the start of therapy, and two used the date of diagnosis (two studies used two alternative index dates in sensitivity analyses). All studies used Cox proportional hazard models to estimate the HR for OS in patients with NTRK+ versus NTRK+ tumors; four studies provided unadjusted estimates, and two studies fitted an adjusted model. The censoring date also varied between studies; two used the date of last activity/visit in the database, two used initiation of TRK inhibitor therapy, one used the date of last death recorded in the cohort or initiation of TRK inhibitor therapy, and one study did not report this information. Median follow-up time was only reported in three studies, and it varied from 7.5 to 27.4 months. Median survival time was not estimable in the studies by Bridgewater et al10,19 and Zhu et al13 because of a lack of events, but in the three other studies, it ranged from 10 to 16.5 months.
TABLE 2.
Statistical Methods and Results of the Five Studies Assessed for Quantitative Synthesis
Studies Included in Data Synthesis
The study by Bazhenova et al9 was excluded from the data synthesis as it was based on the same database as the study by Hibar et al,11 and therefore, it was not a unique cohort providing an independent sample of data. The data in the study by Hibar et al11 were retained as it incorporated a longer more up-to-date study period (January 1, 2011-December 31, 2019, v July 31, 2018) and included a larger number of cases and controls. The study by Zhu et al13 was also excluded as a HR and KM curve were not provided because of a lack of events for OS.
Three studies, ie, the studies by Bridgewater et al10,19, Hibar et al,11 and Santi et al,12 were included in the meta-analysis, and a summary of the risk of bias assessment and funnel plot are shown in the supplementary appendix (Appendix Table A2; Appendix Fig A2). A high risk of bias was not identified in any of the domains although information was not always available, and the measurement of exposure and outcome was considered as having a low risk of bias in all three studies. There was no indication of reporting bias in the funnel plot with all three studies located in the triangular region where 95% of studies are expected to lie in the absence of bias and heterogeneity. The results related to the index date used for the primary analysis were used in the study by Hibar et al11 (advanced/metastatic/recurrent disease diagnosis), and the unadjusted results were used from the study by Santi et al12, for consistency with the two other studies.
OS Rates
The unadjusted HRs (95% CI) reported by the three studies included in the meta-analysis are displayed in Figure 2. The point estimates were very similar, indicating around a 50% increased risk of mortality among patients with NTRK+ tumors compared with patients with NTRK– tumors. This was only borderline statistically significant in the study by Hibar et al,11 which, in this meta-analysis, was the largest study with a greater number of events, but not significant in the study by Santi et al12 or Bridgewater et al.10,19 The event rates were markedly higher in the comparator groups (NTRK–) in the studies by Hibar et al11 and Santi et al12 (59%) compared with the study by Bridgewater et al10,19 (8%), despite the comparative estimates being very similar. The median OS was 10.2 months in the study by Hibar et al11 and 12.7 months in the study by Santi et al,12 but not estimable in the study by Bridgewater et al.10,19 The survival estimates for the two different index dates used in the study by Hibar et al11 were almost identical (date of advanced diagnosis: HR, 1.60 [95% CI, 1.02 to 2.50], start date last line of treatment: HR, 1.58 [95% CI, 1.01 to 2.48]). Similarly, the survival estimates for the unadjusted and adjusted analysis reported by Santi et al12 were very similar (unadjusted HR, 1.37 [95% CI, 0.78 to 2.42]; adjusted HR, 1.32 [95% CI, 0.74 to 2.35]). The survival estimate for the study by Bridgewater et al10,19 was a HR of 1.47 (95% CI, 0.39 to 5.57).
FIG 2.
Forest plot of unadjusted observed HRs (95% CIs) for overall survival. HR, hazard ratio.
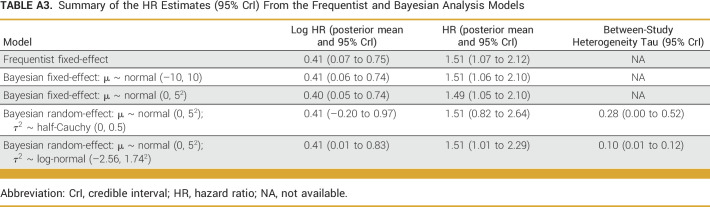
The pooled HR estimate from the fixed-effects model was 1.51 (95% CI, 1.07 to 2.12; P = .019), with I2 = 0.0% (95% CI, 0.0 to 89.6) indicating high uncertainty about the extent of between-study heterogeneity. A summary of the different models fitted is provided in Appendix Table A3. The best-fitting Bayesian random-effects model used a normal (0, 52) prior for the mean and weakly informative log-normal (–2.56, 1.742) prior for the variance of the true effect (LOOIC: model 5 [1.7] v model 4 [2.3]). The pooled HR estimate from this model was 1.51 (95% CrI, 1.01 to 2.29) with a between-study heterogeneity parameter tau of 0.10 (95% CI, 0.00 to 0.52). Figure 3 displays the posterior mean log HR for the overall pooled and study-wise estimates with 95% CrIs. In summary, NTRK+ patients have a 51% increased risk of mortality compared with NTRK– patients, and there is a 95% probability that the true HR lies between 1.01 and 2.29, given the evidence provided by the observed pooled data.
FIG 3.
Forest plot of log HRs (95% credible intervals) for overall survival from the best-fitting Bayesian random-effects model. HR, hazard ratio.
DISCUSSION
We have reported a systematic review and meta-analysis of the prognostic value of NTRK+ status for predicting OS in patients not exposed to TRK inhibitors. To our knowledge, this is the first such review conducted, providing an up-to-date synthesis of new and emerging evidence in this novel field of precision medicine using targeted TRK inhibitor therapies. Despite heterogeneity between studies, we were able to use random-effects Bayesian analysis and incorporate it into the model estimates. On the basis of the available evidence for a rare genomic alteration,20 which is now actionable, we provide a more precise and reliable estimate of the risk of mortality for patients with NTRK+ tumors in a natural setting across a range of tumor types.
Although there were few studies identified for inclusion in this review and most had a small sample size because of the rarity of the condition, the findings across studies were notably consistent. Despite there being variations across patient populations (age, patient sex, tumor type, progression) and methodology (choice of index date, matching criteria, censoring date), all studies reported the same direction of effect, that NTRK+ status increases the risk of mortality, with varying degrees of uncertainty. Furthermore, all studies included in the meta-analysis excluded patients either receiving TRK inhibitors or censored at the date of initiation of such therapies. The pooled estimate from our study suggests that this genomic alteration is associated with a 50% increased risk of mortality within the first 10 years from diagnosis or the start of standard therapy, compared with patients with NTRK– tumors. The median OS was reported to be 10.2 and 12.7 months in the two studies where it was estimable. Longer OS has been reported in recent clinical trials evaluating the efficacy of TRK inhibitors in patients with NTRK+ tumors, as would be anticipated in treated patients in a trial setting. For example, in an integrated analysis of three phase I/II clinical trials of entrectinib in 121 patients, the median OS was 33.8 months (95% CI, 23.4 to 46.4),5 and in a pooled analysis of three phase I/II clinical trials of larotrectinib in 159 patients, the median OS was 44.4 months (95% CI, 36.5 to not estimable).4
The main limitation of this review is the small number of studies included and the limited number of NTRK+ patients available in the study cohort, leading to an estimate where a high level of uncertainty remains. All the studies were retrospective case-control designs on the basis of databases collecting routine data and may not be completely representative of the wider patient population. The study populations were limited to patients who had been tested for this genetic mutation, which might have introduced selection bias. For example, testing may be more likely to be performed for certain tumor types, or in certain types of centers where testing is available, or in patients unresponsive to standard treatments. There were differences in the factors used for matching cases and controls across studies, and data were not available for all potential confounders, so bias because of confounding cannot be ruled out. However, balance was achieved by matching within study, ensuring that patient characteristics were balanced on key factors in the case and control groups. Limited information on baseline characteristics made it difficult to assess the heterogeneity of the patient populations across studies and how appropriate it was to combine them. There was limited scope to address the issue of potential heterogeneity and bias as only aggregate data summary estimates were available for three studies; however, if individual patient data became available, more sophisticated statistical methods could be used to mitigate this.
Criteria used for defining censoring times and patient inclusion criteria were not consistent across studies, and the studies used different index dates, capturing patients at mixed stages of the disease: Bridgewater et al10,19 used the first date of cancer diagnosis for the gene sequenced tumor, Hibar et al11 used the date of locally advanced/metastatic/recurrent disease diagnosis, and Santi et al12 used the date of the first postbiopsy treatment. Combining these studies in a meta-analysis assumes that the hazard function for each group is constant over the periods between different index dates. This is difficult to establish although in a sensitivity analysis, the study by Hibar et al11 obtained almost identical OS estimates when the date of the start of last available treatment line was used instead of the date of diagnosis. All three studies used Cox regression to estimate the HR; however, only the study by Bridgewater et al10,19 reported that the proportional hazard assumption was satisfied. Although it is not possible to rule out the existence of nonproportionality, the published KM curves were notably consistent and displayed a clear separation in groups after 10-12 months, with the NTRK+ group having poorer prognosis up to a follow-up of 3-4 years. The OS analyses also assumed that the prognostic effect of NTRK gene fusion is the same across a broad range of tumor types, which may not be the case.
In conclusion, we have provided a more robust estimate of the prognostic effect of NTRK+ status in a real-world setting, given the caveats outlined above and the limited window of opportunity because of the likely proliferation of NTRK-targeted therapies in the future. It would be useful if further analyses could explore how differences in the choice of index date affect estimates of OS. The clinical characteristics, treatment patterns, and outcomes in patients with NTRK+ solid tumors on standard treatments are not well characterized and are possibly highly variable across cancer types. Therefore, the study of larger cohorts to obtain tumor-specific estimates of OS would be a significant advance in knowledge. Further prognostic studies, which include additional prognostic factors to reduce residual confounding, are required to confirm the prognostic value of NTRK gene fusions and to increase precision.
APPENDIX
TABLE A1.
Search Strategy
TABLE A2.
Risk of Bias Assessment for the Three Studies Included in the Meta-Analysis Using the RoBANS
TABLE A3.
Summary of the HR Estimates (95% CrI) From the Frequentist and Bayesian Analysis Models
FIG A1.
Summary of the overlap of different tumor types included in the NTRK+ and NTRK– groups across studies. The green filled boxes indicate which tumor types were included in each study. NTRK, neurotrophic tyrosine receptor kinase.
FIG A2.
Funnel plot for the three studies included in the meta-analysis. HR, hazard ratio.
Ulrik Lassen
Honoraria: Bayer, Pfizer, Novartis
Consulting or Advisory Role: Bayer, Pfizer
Research Funding: BMS (Inst), Roche (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly (Inst)
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp Dohme, Medac, Hexal, Medupdate, I-Med Institute
Consulting or Advisory Role: Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, GSO, AOK Health Insurance, Oncology Drug Consult (ODC), Janssen-Cilag GmbH, BioNTech SE
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), SpringWorks Therapeutics (Inst), Taiho Pharmaceutical (Inst), Ipsen (Inst), Servier/Pfizer (Inst), Immatics (Inst), CPT Cellex Patient Treatment (Inst), Glycostem (Inst), BioNTech SE (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Bristol Myers Squibb, Janssen-Cilag, Daiichi Sankyo Europe GmbH
Jesus Garcia-Foncillas
Honoraria: Merck KGaA (Inst), Bayer, Sanofi, Servier (Inst), Novartis
Consulting or Advisory Role: Bayer
Speakers' Bureau: Bayer
Travel, Accommodations, Expenses: Janssen
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Gilles Vassal
Consulting or Advisory Role: Bayer, Roche/Genentech, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Hutchison MediPharma, Pyramid Biosciences
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Bayer
Noman Paracha
Employment: Bayer
Stock and Other Ownership Interests: Bayer
Marisca Marian
Employment: Bayer
Yuxian Chen
Employment: Visible Analytics Limited
Consulting or Advisory Role: Bayer
Louise Linsell
Employment: Visible Analytics Limited
Consulting or Advisory Role: Bayer
Keith Abrams
Employment: Visible Analytics Limited
Leadership: Visible Analytics Limited
Stock and Other Ownership Interests: Visible Analytics Limited
Consulting or Advisory Role: AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Medtronic
Speakers' Bureau: Association of the British Pharmaceutical Industry (ABPI)
Research Funding: Visible Analytics/Bayer (Inst)
Uncompensated Relationships: National Institute for Health & Care Excellence (NICE)
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Jesus Garcia-Foncillas, Antoine Italiano, Gilles Vassal, Noman Paracha, Marisca Marian, Keith Abrams
Administrative support: Louise Linsell, Keith Abrams
Provision of study materials or patients: Ulrik Lassen, Carsten Bokemeyer, Jesus Garcia-Foncillas
Collection and assembly of data: Antoine Italiano, Noman Paracha, Marisca Marian, Yuxian Chen, Louise Linsell
Data analysis and interpretation: Ulrik Lassen, Carsten Bokemeyer, Jesus Garcia-Foncillas, Antoine Italiano, Noman Paracha, Marisca Marian, Yuxian Chen, Louise Linsell, Keith Abrams
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ulrik Lassen
Honoraria: Bayer, Pfizer, Novartis
Consulting or Advisory Role: Bayer, Pfizer
Research Funding: BMS (Inst), Roche (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly (Inst)
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp Dohme, Medac, Hexal, Medupdate, I-Med Institute
Consulting or Advisory Role: Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, GSO, AOK Health Insurance, Oncology Drug Consult (ODC), Janssen-Cilag GmbH, BioNTech SE
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), SpringWorks Therapeutics (Inst), Taiho Pharmaceutical (Inst), Ipsen (Inst), Servier/Pfizer (Inst), Immatics (Inst), CPT Cellex Patient Treatment (Inst), Glycostem (Inst), BioNTech SE (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Bristol Myers Squibb, Janssen-Cilag, Daiichi Sankyo Europe GmbH
Jesus Garcia-Foncillas
Honoraria: Merck KGaA (Inst), Bayer, Sanofi, Servier (Inst), Novartis
Consulting or Advisory Role: Bayer
Speakers' Bureau: Bayer
Travel, Accommodations, Expenses: Janssen
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Gilles Vassal
Consulting or Advisory Role: Bayer, Roche/Genentech, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Hutchison MediPharma, Pyramid Biosciences
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Bayer
Noman Paracha
Employment: Bayer
Stock and Other Ownership Interests: Bayer
Marisca Marian
Employment: Bayer
Yuxian Chen
Employment: Visible Analytics Limited
Consulting or Advisory Role: Bayer
Louise Linsell
Employment: Visible Analytics Limited
Consulting or Advisory Role: Bayer
Keith Abrams
Employment: Visible Analytics Limited
Leadership: Visible Analytics Limited
Stock and Other Ownership Interests: Visible Analytics Limited
Consulting or Advisory Role: AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, Medtronic
Speakers' Bureau: Association of the British Pharmaceutical Industry (ABPI)
Research Funding: Visible Analytics/Bayer (Inst)
Uncompensated Relationships: National Institute for Health & Care Excellence (NICE)
No other potential conflicts of interest were reported.
REFERENCES
- 1.European Medicines Agency 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/vitrakvi Vitrakvi—EPAR—Medicines overview.
- 2. Cocco E, Scaltriti M, Drilon A. NTRK+ cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker A. Neurotrophic tyrosine kinase inhibitors: A review of implications for patients, clinicians and healthcare services. J Oncol Pharm Pract. 2020;26:2015–2019. doi: 10.1177/1078155220959428. [DOI] [PubMed] [Google Scholar]
- 4. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumors: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Demetri GD, De Braud F, Drilon A, et al. Updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors. Clin Cancer Res. 2022;28:1302–1312. doi: 10.1158/1078-0432.CCR-21-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 9. Bazhenova L, Lokker A, Snider J, et al. TRK fusion cancer: Patient characteristics and survival analysis in the real-world setting. Targ Oncol. 2021;16:389–399. doi: 10.1007/s11523-021-00815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bridgewater J, Jiao X, Parimi M, et al. Abstract 394: Prognosis and molecular characteristics of patients with TRK fusion cancer in the 100,000 Genomes Project. Cancer Res. 2021;81 suppl 13; abstr 394. [Google Scholar]
- 11. Hibar DP, Demetri GD, Peters S, et al. Real-world survival outcomes in patients with locally advanced or metastatic NTRK+ solid tumors receiving standard-of-care therapies other than targeted TRK inhibitors. PLoS One. 2022;17:e0270571. doi: 10.1371/journal.pone.0270571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santi I, Vellekoop H, Huygens S, et al. 105P prognostic value of the NTRK fusion biomarker in The Netherlands. Ann Oncol. 2021;32:S401–S402. [Google Scholar]
- 13. Zhu L, Hobbs B, Roszik J, et al. Investigating the natural history and prognostic nature of NTRK gene fusions in solid tumors. Invest New Drugs. 2022;40:157–162. doi: 10.1007/s10637-021-01157-8. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guyot P, Ades A, Ouwens MJ, et al. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 17. Turner RM, Jackson D, Wei Y, et al. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. 2015;34:984–998. doi: 10.1002/sim.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 19. Bridgewater J, Jiao X, Parimi M, et al. Prognosis and oncologic profiling of patients with tropomyosin recepter kinase fusion cancer in the 100,000 Genomes Project. Cancer Treat Res Commun. 2022;33:100623. doi: 10.1016/j.ctarc.2022.100623. [DOI] [PubMed] [Google Scholar]
- 20. Forsythe A, Zhang W, Strauss UP, et al. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol. 2020;12:1–10. doi: 10.1177/1758835920975613. [DOI] [PMC free article] [PubMed] [Google Scholar]