Abstract
Purpose:
To develop a tool to predict a woman’s treatment pattern for bothersome urinary urgency and/or urinary urgency incontinence over 1 year after presenting for care at urology or urogynecology clinics.
Methods:
The Symptoms of Lower Urinary Tract Dysfunction Research Network observational cohort study enrolled adult women with bothersome urinary urgency and/or urinary urgency incontinence using the Lower Urinary Tract Symptoms (LUTS) Tool who were seeking care for lower urinary tract symptoms. Treatments for urinary urgency and/or urgency incontinence were ordered from least to most invasive. Ordinal logistic and Cox proportional hazard regression models were fit to predict the most invasive level of treatment during follow-up and overactive bladder medication discontinuation, respectively. Binary logistic regression was performed to predict sling treatment during the study follow-up. Clinical tools were then created using the models listed above to predict treatment pattern over 12 months.
Results:
Among 349 women, 281 reported urinary urgency incontinence, and 68 reported urinary urgency at baseline. The highest level of treatment during the study was as follows: 20% no treatment, 24% behavioral treatments, 23% physical therapy, 26% overactive bladder medication, 1% percutaneous tibial nerve stimulation, 3% onabotulinum toxin A, and 3% sacral neuromodulation. Slings were placed in 10% (n=36) of participants prior to baseline and in 11% (n=40) during study follow-up. Baseline factors associated with predicting the most invasive level of treatment included baseline level of treatment, hypertension, urinary urgency incontinence severity, stress urinary incontinence severity, and anticholinergic burden score. Less severe baseline depression and less severe urinary urgency incontinence were associated with overactive bladder medication discontinuation. Urinary urgency and stress urinary incontinence severity were associated with sling placement during the study period. Three tools are available to predict: 1) highest level of treatment; 2) overactive bladder medication discontinuation; and 3) sling placement.
Conclusions:
Overactive bladder treatment prediction tools developed in this study can help providers individualize treatment plans and identify not only patients at risk for treatment discontinuation but also patients who may not be escalated to potentially beneficial overactive bladder treatments, with the goal to improve clinical outcomes for patients suffering from this chronic and often debilitating condition.
Keywords: overactive bladder, urinary urgency, urgency urinary incontinence, treatment
INTRODUCTION
The lifetime prevalence of overactive bladder (OAB) in women has been estimated to be 30%.1 According to the International Continence Society, OAB is defined as urinary urgency (UU), usually with urinary frequency and nocturia, with or without urgency urinary incontinence (UUI).2,3 Patients who report OAB symptoms are often managed with multiple treatments; however, similar to other chronic conditions,4 patient adherence to treatments is poor, with discontinuation rates of medications nearing 50% by the first month of treatment.5 Improvement in clinical symptoms can be greatly impacted by patient adherence to treatment,6 since some studies have demonstrated an association between patients who continue to take their medications and improved urinary symptoms and quality of life compared with patients who discontinue therapy;7 however, these studies may reflect response to treatment bias. Prior studies exploring patient adherence to OAB treatments have reported that patients are often lost to follow-up, and few receive third-line treatments, such as intradetrusor onabotulinumtoxin A injections (BTX) or sacral neuromodulation (SNM).8
Factors inherent to treatment modality (e.g., efficacy, adverse effects, and cost [both financial and time]); patient factors (e.g., education, sex, and age); and physician factors (e.g., training and bias) influence medication adherence and treatment access.9–12 Currently, limited epidemiological data exist describing these patterns of OAB treatments over time. In order to improve care for our patients with OAB, it is essential to gain a better understanding of treatment patterns, in particular treatment escalation and de-escalation. Given that treatment continuation is key to providing effective management of patients’ symptoms, it is also important to identify patient factors associated with treatment patterns, with the goal to improve compliance or to transition patients to third-line therapies more readily. Thus, the primary objective of this study was to develop clinical tools to predict treatment patterns over 1 year for women presenting to care at urology or urogynecology clinics with UU and/or UUI using data from the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) cohort study. Specifically, the aim was to develop clinical models using participant demographic and clinical characteristics that will accurately predict OAB treatment patterns.
MATERIALS and METHODS
LURN completed a multi-center, prospective observational cohort study at six US research sites. LURN enrolled adult participants seeking specialty care for lower urinary tract symptoms (LUTS)13 from 2015–2018. Participants were eligible if they reported at least one urinary symptom in the past month based on the LUTS Tool14 and were seeking care from a LURN physician (i.e., urologist or urogynecologist) for the first time or a return visit (males only). The LUTS Tool assesses frequency and bother of 18 LUTS in the past month. All LUTS symptoms except incontinence items are rated on a 5-point Likert scale (“never,” “rarely,” “sometimes,” “often,” and “almost always”). Frequency of incontinence symptoms was rated using the following 5-point Likert scale: “less than once a month,” “a few times a month,” “a few times a week,” “daily,” or “many times a day.”14,15 Additional study inclusion and exclusion criteria, as well as power calculations, have been published previously.16 After an initial baseline visit, participants were followed every 3 months. This study was approved by the IRB at each site, and written informed consent was obtained from all participants before enrollment.
The current study includes women who reported bothersome UU of “sometimes,” “often,” or “always” on the LUTS Tool with a 1-month recall period. UU was defined as a sudden need to rush to urinate (Question [Q] 6 on the LUTS Tool) or a sudden need to rush to urinate for the fear of leaking (Q12) during the past month. These participants were further categorized as having UUI if they responded “sometimes,” “often,” or “always” on Q16b – leaked urine in connection with a sudden need to rush to urinate. Information regarding presence and severity of stress urinary incontinence (SUI) was also abstracted.
Several independent predictors were evaluated when developing the clinical models. Demographic variables included age at study enrollment, race, and education. The following baseline clinical characteristics were also evaluated: 1) body mass index (BMI); 2) comorbidities (history of hypertension, diabetes, psychiatric diagnosis, stroke or transient ischemic attack [TIA], and hyperlipidemia); 3) use of diuretics; 4) anticholinergic burden (ACB) score 15; 5) UU and UUI symptom severity questions from the LUTS Tool; 6) SUI severity (average of LUTS Tool items related to leaking urine in connection with laughing, sneezing, or coughing, and in connection with physical activities, such as exercising or lifting a heavy object); 7) Patient‐Reported Outcomes Measurement Information System (PROMIS) measures17 (gastrointestinal bowel incontinence and diarrhea, constipation, depression, anxiety, physical functioning, and sleep disturbance measures); 8) quality of life due to urinary symptoms, as measured by Q8 of the American Urological Association Symptom Index (AUA-SI);18 and 9) the Perceived Stress Scale (PSS).19 Treatments for LUTS were collected at baseline and every 3 months for 1 year. Medication start and stop dates and procedure dates were collected. Treatments of interest to this study are: 1) behavioral therapy (BT); 2) pelvic floor physical therapy (PT); 3) OAB medications; 4) percutaneous tibial nerve stimulation (PTNS); 5) BTX; and 6) SNM. These treatments were ordered from 1 to 6, respectively, from the least to the most invasive. Treatments included in BT and OAB medications are listed in Supplementary Table 1. We then grouped these six treatments into four clinically distinct levels, given the low numbers of participants receiving advanced therapies: Level 1: BT; Level 2: PT; Level 3: OAB medication; and Level 4: PTNS, BTX, and SNM. BTX was assumed to be effective for 6 months from the procedure date. Participants on SNM were assumed to continue SNM therapy unless the battery was removed. Midurethral sling surgery was analyzed as a separate procedure of interest, as it is a treatment for SUI rather than OAB. Reporting this treatment is important, as it reflects a subset of patients with mixed urinary incontinence.
Models were fit to predict three outcomes: 1) most invasive treatment over the 12-month study period; 2) time to OAB medication discontinuation for participants who presented on or started an OAB medication during the 12-month study period; and 3) midurethral sling placement over the 12-month study period. Candidate predictors were selected by expert opinion. Model selection used backward elimination for all models described above. Covariates significant at 0.10 level were included. To adjust for baseline treatments, level of treatment at baseline and prior sling placement at baseline were included in the level of treatment model as predictors, regardless of p-value.
To obtain predicted probabilities of treatment escalation and de-escalation from baseline to study follow-up, we fit a multivariable ordinal logistic regression model predicting the most invasive level of treatment during the 12-month study period using baseline characteristics. We then used this model to obtain predicted probabilities of each level of treatment. For participants who reported OAB medication use during the study, a multivariable Cox proportional hazard model was fit to obtain predicted probabilities of discontinuing OAB medication use during the study period. Lastly, binary logistic regression was performed to predict sling treatment during study follow-up.
Model performance was evaluated using Brier scores, C-statistics, and calibration curves. Brier scores capture both discrimination and calibration, while C-statistics assess only discrimination. Calibration curves were obtained using the Regression Modeling Strategies (rms) R package to visually evaluate how close model-predicted probabilities are to observed outcomes.20 All model performance metrics were internally validated using bootstrapping with 500 resamples and reported as bias-corrected, unless noted otherwise. All model performance analyses were performed using R software, version 4.0.3 (R Development Core Team, Vienna). All other statistical analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Among 545 women enrolled in LURN, 349 participants presented with bothersome UU or UUI; 281 reported bothersome UUI, and 68 reported bothersome UU alone at baseline (Table 1). Mean (± standard deviation) age was 57 (± 15) years old, and participants were mostly White (80%) and non-Hispanic (95%). Mean BMI was 32 (± 8) kg/m2. Table 2 describes the participant-reported measures, including the LUTS Tool and the PROMIS measures, at baseline. Table 3 describes the most invasive level of treatments participants reported at or prior to their baseline visit, as well as the most invasive level of treatment during the 12-month study period. Prior to or at baseline, 52% of participants reported undergoing BT, 11% reported PT, and 10% were taking OAB medications. Few participants had PTNS (n=1), BTX (n=2), or SNM surgery (n=1). During the study period, few participants (n=24) escalated to Level 4 treatments.
Table 1.
Participant characteristics at baseline
| Total (n=349) | Urinary urgency with incontinence (n=281) | Urinary urgency without incontinence (n=68) | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age | 57.1 (14.6) | 57.6 (14.0) | 54.9 (16.6) |
| Race β | |||
| African American or Black | 48 (14%) | 40 (14%) | 8 (12%) |
| Other* | 22 (6%) | 17 (6%) | 5 (7%) |
| White | 278 (80%) | 223 (80%) | 55 (81%) |
| Ethnicity | |||
| Hispanic/Latino | 12 (3%) | 9 (3%) | 3 (4%) |
| Non-Hispanic/Non-Latino | 330 (95%) | 265 (94%) | 65 (96%) |
| Unknown | 7 (2%) | 7 (2%) | 0 (0%) |
| Education β | |||
| < HS diploma/GED | 9 (3%) | 7 (3%) | 2 (3%) |
| HS diploma/GED | 33 (10%) | 29 (10%) | 4 (6%) |
| Some college/tech school – no degree | 92 (27%) | 77 (28%) | 15 (23%) |
| Associates degree | 40 (12%) | 31 (11%) | 9 (14%) |
| Bachelor’s degree | 96 (28%) | 81 (29%) | 15 (23%) |
| Graduate degree | 75 (22%) | 54 (19%) | 21 (32%) |
| Physical Exam and Clinical Information | |||
| BMI (kg/m2) β | 31.6 (8.2) | 32.0 (8.4) | 29.5 (6.6) |
| Current smoker β | 29 (8%) | 24 (9%) | 5 (7%) |
| Former smoker β | 95 (27%) | 77 (28%) | 18 (26%) |
| Number of alcoholic drinks per week γ | |||
| Has not had alcohol in the past | 69 (20%) | 55 (20%) | 14 (21%) |
| 0 to 3 drinks per week | 222 (65%) | 179 (65%) | 43 (64%) |
| 4 to 7 drinks per week | 38 (11%) | 30 (11%) | 8 (12%) |
| ≥ 8 drinks per week | 13 (4%) | 11 (4%) | 2 (3%) |
| SUI δ | 233 (67%) | 203 (73%) | 30 (44%) |
| Hypertension β | 139 (40%) | 120 (43%) | 19 (28%) |
| Diabetes | 58 (17%) | 46 (16%) | 12 (18%) |
| Psychiatric diagnosis β | 153 (44%) | 130 (46%) | 23 (34%) |
| History of stroke or TIA β | 15 (4%) | 13 (5%) | 2 (3%) |
| Hyperlipidemia β | 110 (32%) | 88 (31%) | 22 (32%) |
| On diuretics | 52 (15%) | 44 (16%) | 8 (12%) |
| IPAQ γ | |||
| Low activity | 194 (57%) | 161 (59%) | 33 (50%) |
| Moderate activity | 43 (13%) | 36 (13%) | 7 (11%) |
| High activity | 101 (30%) | 75 (28%) | 26 (39%) |
|
LUTS Treatment Prior to or at Baseline
(not mutually exclusive) |
|||
| Behavioral therapy | 240 (69%) | 195 (69%) | 45 (66%) |
| Pelvic floor physical therapy | 47 (13%) | 39 (14%) | 8 (12%) |
| OAB medication # | 36 (10%) | 29 (10%) | 7 (10%) |
| PTNS | 1 (0%) | 0 (0%) | 1 (1%) |
| BTX | 2 (1%) | 2 (1%) | 0 (0%) |
| SNM | 1 (0%) | 1 (0%) | 0 (0%) |
| Sling | 36 (10%) | 28 (10%) | 8 (12%) |
Table values are mean (standard deviation) or percent (frequency).
Missing <2%
Missing 2%−5%
Other race includes American Indian, Alaska Native, Asian/Asian American, Native Hawaiian, Pacific Islander, and “other”.
Endorsed urinary incontinence when laughing, coughing, or sneezing or with exercise at “Sometimes” or greater on the LUTS Tool.
OAB medication only included current medication use at baseline.
HS, high school; GED, General Educational Development; BMI, body mass index; TIA, transient ischemic attack; IPAQ, International Physical Activity Questionnaire; LUTS, lower urinary tract symptoms; OAB, overactive bladder; PTNS, Percutaneous Tibial Nerve Stimulation; SNM, sacral neuromodulation
Table 2.
Participant-reported measures at baseline
| Total (n=349) | Urinary urgency with incontinence (n=281) | Urinary urgency without incontinence (n=68) | |
|---|---|---|---|
|
| |||
| Overall LUTS tool a score ¥ | 47.7 (12.8) | 49.6 (12.6) | 39.9 (10.5) |
| LUTS tool frequency score γ | 55.8 (18.4) | 56.8 (17.8) | 51.8 (20.1) |
| LUTS tool post-micturition score β | 48.4 (26.0) | 48.9 (26.2) | 46.4 (25.0) |
| LUTS tool urgency score γ | 65.0 (18.0) | 68.5 (17.5) | 50.5 (12.5) |
| LUTS tool voiding difficulty score λ | 28.3 (21.1) | 28.7 (21.1) | 26.8 (21.3) |
| LUTS tool pain score γ | 16.3 (21.7) | 16.3 (21.5) | 16.2 (22.8) |
| LUTS tool UI score λ | 45.4 (19.3) | 50.1 (16.6) | 26.6 (17.6) |
| Quality of life due to urinary symptoms (0=delighted to 6=terrible) λ | 4.7 (1.1) | 4.8 (1.1) | 4.3 (1.2) |
| PROMIS b depression (T-score) γ | 50.4 (9.0) | 50.9 (9.1) | 48.2 (8.0) |
| PROMIS anxiety (T-score) γ | 51.4 (9.4) | 51.6 (9.6) | 50.4 (8.5) |
| PROMIS physical function (T-score) γ | 45.7 (10.4) | 45.0 (10.4) | 48.6 (10.2) |
| PROMIS sleep disturbance (T-score) γ | 51.9 (4.8) | 52.0 (4.9) | 51.4 (4.7) |
| PROMIS GI diarrhea (T-score) γ | 49.7 (9.6) | 50.0 (9.7) | 48.2 (9.5) |
| PROMIS GI constipation (T-score) λ | 52.2 (8.2) | 52.2 (8.1) | 51.9 (8.4) |
| Perceived stress scale λ | 13.7 (7.8) | 13.9 (7.7) | 12.8 (7.9) |
| PROMIS GI bowel incontinence (raw scale) λ | 5.6 (2.7) | 5.7 (2.8) | 5.2 (2.7) |
Table values are mean (standard deviation). The LUTS Tool scores range from 0 to 100 and were created by combining responses to related symptom severity questions from the LUTS Tool and calculating the Euclidean length of the relevant questions as a measure of overall symptom severity. PROMIS measures T-scores have a mean of 50 and a standard deviation of 10 in the US general population. The minimal clinically important differences is 3 to 5 points in T-scores across PROMIS measures.
Missing <2%
Missing 2%−5%
Missing 5%−10%
Missing 17%
LUTS, lower urinary tract symptoms; UI, urinary incontinence; PROMIS, Patient-Reported Outcomes Measurement Information System; GI, gastrointestinal
LUTS Tool14
PROMIS17
Table 3.
Most invasive level of treatment, number of participants n (%)
| Prior to or at baseline | During follow-up | |
|---|---|---|
|
| ||
| No treatment | 90 (26%) | 71 (20%) |
| Level 1: BT | 182 (52%) | 84 (24%) |
| Level 2: PT | 38 (11%) | 79 (23%) |
| Level 3: OAB medications | 35 (10%) | 91 (26%) |
| Level 4 | 4 (1.2%) | 24 (7%) |
| PTNS | 1 (0.3%) | 3 (1%) |
| BTX | 2 (0.6%) | 10 (3%) |
| SNM | 1 (0.3%) | 11 (3%) |
BT, behavioral therapy; PT, pelvic floor physical therapy; OAB, overactive bladder; PTNS, percutaneous tibial nerve stimulation; BTX, intradetrusor onabotulinumtoxinA; SNM, sacral neuromodulation
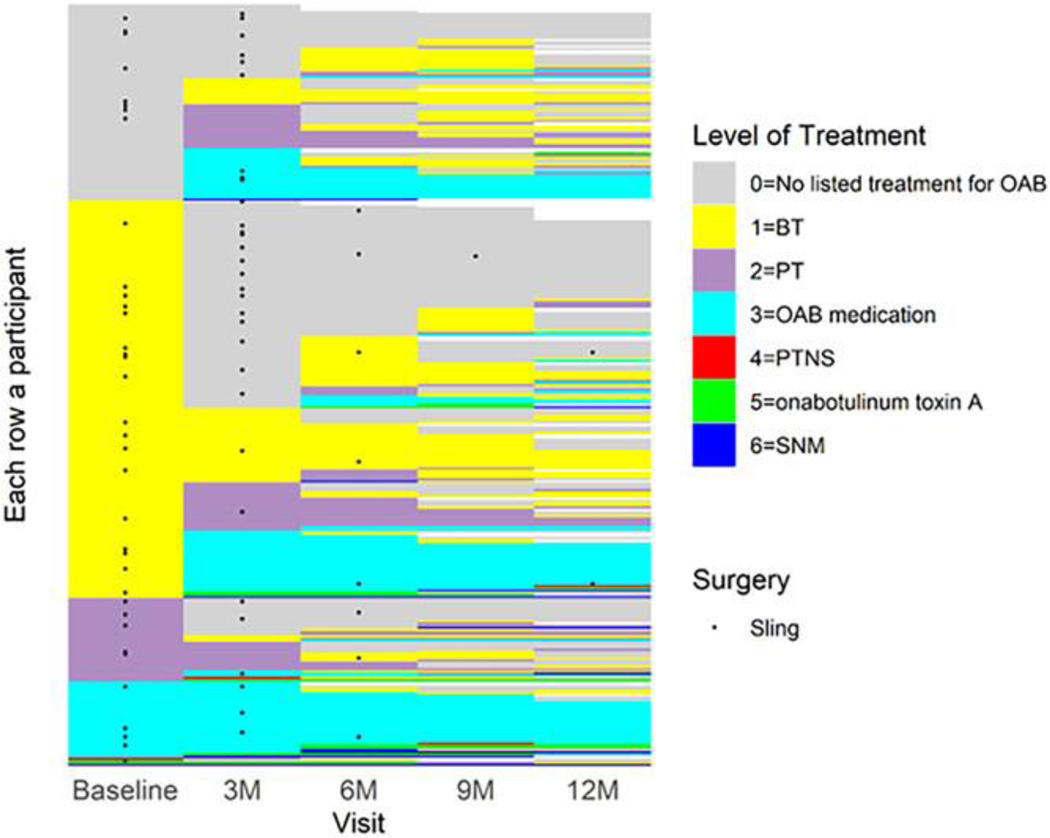
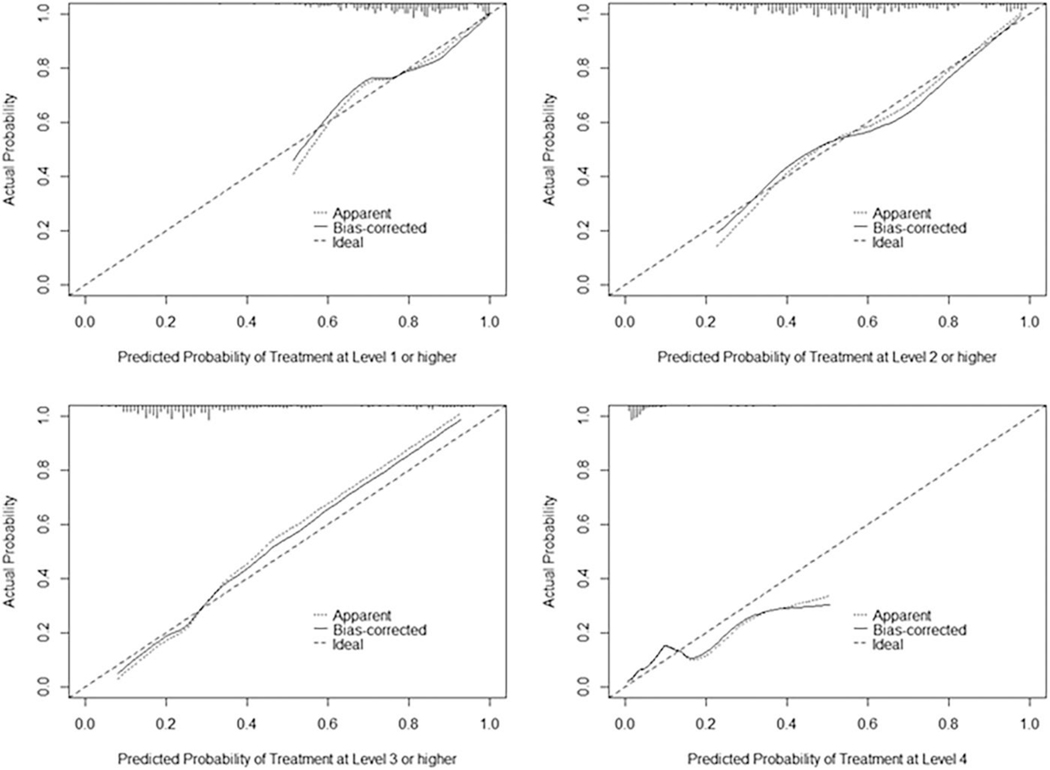
Figure 1 represents the treatment pattern of participants included in the three predictive models. All participants with bothersome UU or UUI (n=349) were included in the model predicting the most invasive level of treatment. In our complete case analysis, n=8 participants were excluded due to missing covariate values. Supplementary Table 2 shows predictors of the most invasive level of treatment during the 12-month follow-up from the ordinal logistic regression model. The model demonstrates adequate discrimination between higher and lower levels of treatment and is consistent across the different thresholds; the Brier score varies across thresholds (Table 4). The calibration curves show that the model is moderately calibrated, as most predicted probabilities are close to observed probabilities, with slight deviations from the diagonal line (Figure 2). These deviations were most notable for predicting higher levels of treatment (e.g., Levels 3 and 4), with predicted probabilities slightly underestimated at probabilities of 0.4 and higher for Level 3 or above, and overestimated at probabilities of 0.2 and higher for Level 4. A tool predicting the highest level of treatment was created using the following participant factors at baseline: 1) baseline treatment; 2) prior sling; 3) education level; 4) history of hypertension; 5) leak urine in connection with a sudden need to rush to urinate (0=never to 4=almost always); 6) SUI severity; and 7) ACB score. For example, consider a participant who presents with the following characteristics: no treatment at baseline, history of a prior sling, highest level of education is high school, history of hypertension, “sometimes” (value=2) leaks urine in connection with a sudden need to rush to urinate, no SUI severity, and whose baseline ACB score is 0. Over a 12-month period, the predicted probability that this participant would have a Level 4 treatment (PTNS, BTX, or SNM) is 2.2%; Level 3 treatment (OAB medication) or higher is 22.1%; Level 2 treatment (PT) or higher is 48.8%; and Level 1 treatment (BT) or higher is 77.5%.
Figure 1.
Lasagna plot of the highest level of treatment by visit. White space indicates missed visits. OAB, overactive bladder; BT, behavioral therapy; PT, pelvic floor physical therapy; PTNS, Percutaneous Tibial Nerve Stimulation; SNM, sacral neuromodulation.
Table 4.
Summary of model performance
| C-statistic [95% CI] (0 to 1, higher is better) | Brier Score [95% CI] (0 to 1, lower is better) | |
|---|---|---|
| Outcome: Level of Treatment | ||
| Proportional odds model | ||
| Overall | 0.686 [0.657, 0.741] | NA a |
| Level 1–4 vs. 0 | 0.686 [0.657, 0.742] | 0.154 [0.126, 0.175] |
| Level 2–4 vs. 0–1 | 0.685 [0.658, 0.741] | 0.217 [0.193, 0.226] |
| Level 3–4 vs. 0–2 | 0.686 [0.658, 0.741] | 0.162 [0.137, 0.180] |
| Level 4 vs. 0–3 | 0.684 [0.656, 0.741] | 0.063 [0.040, 0.084] |
| Separate binary logistic regression models | ||
| Level 1–4 vs. 0 | 0.663 [0.653, 0.712] | 0.158 [0.146, 0.173] |
| Level 2–4 vs. 0–1 | 0.702 [0.664, 0.779] | 0.216 [0.191, 0.226] |
| Level 3–4 vs. 0–2 | 0.796 [0.762, 0.868] | 0.159 [0.127, 0.173] |
| Level 4 vs. 0–3 | 0.673 [0.615, 0.863] | 0.065 [0.037, 0.084] |
| Outcome: Time to OAB Medication Discontinuation | ||
| Cox proportional hazard model | 0.671 [0.579, 0.766] | 0.185 [0.151, 0.247] b |
| Outcome: Sling | ||
| Binary logistic regression | 0.823 [0.829, 0.834] | 0.091 [0.089, 0.091] |
Note: Brier score captures both discrimination and calibration. It ranges between 0 and 1, with lower score indicating better model performance. C-statistic assess discrimination and ranges between 0 and 1, with higher score indicating better discrimination performance.
Brier score is inappropriate for ordinal outcome.
Brier score evaluated at 6-month since OAB medication initiation or baseline if medication was started before baseline. It is not corrected for bias.
CI, confidence interval; OAB, overactive bladder
Figure 2.
Calibration curve for proportional odds model predicting higher vs. lower level of treatment during 12-month study follow-up.
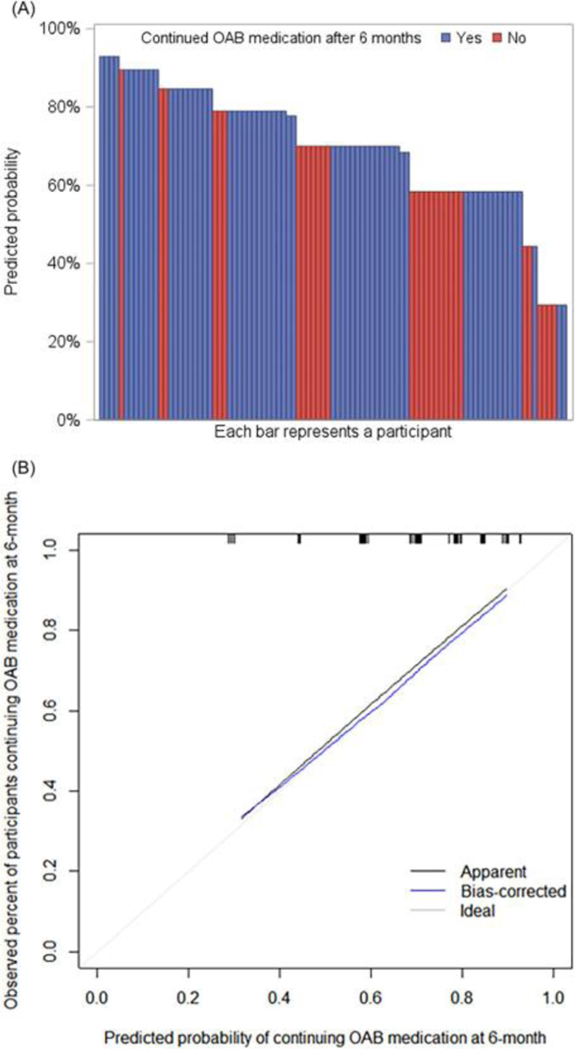
There were 105 women on OAB medications during the study, and 34 (32%) discontinued their medication during follow-up. One participant was excluded from the complete case analysis due to missing covariate values. Depression and lower UUI severity were found to be significantly associated with OAB medication discontinuation (Supplementary Table 3). The model was able to discriminate between participants with higher versus lower hazard of OAB medication discontinuation (Table 4). Among those with at least 6 months of follow-up after starting OAB medication, participants who were observed to be on OAB medication 6 months later had a higher predicted probability of continuation in the model than those who were observed to have discontinued OAB medication by 6 months, as demonstrated by the higher density of blue bars on the left and higher density of red bars on the right in Figure 3a. The model is well-calibrated with bias-corrected calibration curve close to the diagonal line, indicating predicted probabilities are close to the observed probabilities (Figure 3b). A tool predicting OAB medication discontinuation was created using PROMIS depression score and UUI severity rating. For a participant with a PROMIS depression T-score lower than 45 who “sometimes” (value=2) leaks urine in connection with a sudden need to rush to urinate, the probability they will continue their OAB medication 6 months after initiation is 84.6%.
Figure 3.
Results from Cox proportional hazard model predicting time to OAB medication discontinuation from medication initiation or from baseline if participant started medication before baseline. (a) Predicted probability of continuing OAB medication after 6 months. Participants censored before 6 months were excluded from this plot, as their true medication continuation status at 6-month was unknown. (b) Calibration curve.
The logistic regression model used to predict sling placement included 323 participants with non-missing covariate values. Among these, 39 (12%) had a sling placed during follow-up. UUI and SUI severity were found to be significantly associated with sling placement during study follow-up (Supplementary Table 4). The model demonstrated good discrimination performance (Table 4). Predicted probabilities of having a sling placed during the study follow-up were, on average, much higher for those who did have sling (Supplementary Figure 1). The model was well-calibrated when predicted probability was between 0% to 20% (Supplementary Figure 2). A prediction tool for sling placement was created using the following baseline variables: 1) history of sling; 2) feelings about condition (“If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?”); 3) leak urine with a sudden need to rush to urinate; and 4) SUI severity. For a participant at baseline with no prior sling, who feels “terrible” (value= 6) about the possibility of spending the rest of their life with their urinary condition, “almost always” (value=4) leaks urine with a sudden need to rush to urinate, and no SUI severity, the probability they will undergo a sling procedure in the next 12 months is 1.0%.
An online calculator has been created and can be accessed at the following link: https://duke-som.shinyapps.io/UUI_treatments-app/.
DISCUSSION
Principal Findings
In this study, we identified patient factors associated with several OAB treatment patterns, i.e., highest level of treatment, OAB medication discontinuation, and sling placement among women with bothersome UU and/or UUI presenting to care with a urologist or urogynecologist. Specifically, we found that baseline level of treatment, hypertension, UUI severity, SUI severity, and baseline medication ACB score predicted the highest level of treatment during the 12-month study period. Baseline depression and UUI severity were associated with OAB medication discontinuation. UUI and SUI severity were associated with sling placement during the study period.
Results in the Context of What is Known
Many factors influence whether a patient continues or discontinues a given treatment. Prior studies have associated medication adherence with patient factors, such as age, weight, sex, symptom severity, cumulative ACB score, comorbid conditions, and treatment factors, such as adverse effects, cost, and efficacy.6, 21–24 We identified a number of clinical factors that accurately predict different treatment patterns. Given that we cannot infer causality with these associations, we developed clinical tools using models designed for prediction. Using readily available patient factors, clinicians can use these tools to counsel patients with OAB about treatment options and help identify patients who have a high likelihood to discontinue treatment.
Clinical Implications
Identifying patients who are highly bothered by their UUI but are likely to discontinue treatment can help providers target those patients and individualize follow-up and future treatment plans with the goal to improve OAB symptoms. Providers can access the prediction tool online (https://duke-som.shinyapps.io/UUI_treatments-app/) and therefore can readily integrate the use of this tool into their clinical practice. In turn, the use of this prediction tool can help direct patient counseling and guide treatment planning for women with bothersome OAB.
Research Implications
Further research is needed to better understand factors not captured in this study that directly and indirectly shape patient treatment preferences and the reasons for treatment continuation or discontinuation. Also, as the pathophysiology of OAB is poorly understood, the association between hypertension and highest level of treatment should be explored further. Interestingly, the use of diuretics was not associated with highest level of treatment, but hypertension was; this association, for instance, may reflect an end-organ consequence of microvascular disease manifesting as OAB.
Future studies should longitudinally track patient-reported outcomes, such as adverse treatment effects as well as treatment response to determine how those impact how patients engage with available treatment. Also, we should investigate predictors of non-treatment, given that a large proportion (20%) of patients did not undergo any treatment during the study period. Lastly, given that urinary incontinence is a condition that is surrounded by social stigma, we should embark on qualitative studies designed to uncover the socio-cultural dynamics impacting treatment patterns.
Strengths and Limitations
To the best of our knowledge, our study is one of the first to describe treatment patterns and develop a prediction tool for women with bothersome OAB that incorporates all OAB treatment modalities. Unique to our study is the development of clinical tools which providers can use to counsel and tailor treatment plans for their patients, especially those at high risk for medication discontinuation. Other strengths of this study include the use of data from a 1-year multi-center prospective observational study of patients seeking specialty care for LUTS. The greatest strength of this study, however, is the development of clinical prediction tools built upon methods such as resampling, reporting multiple measures of performance, and taking advantage of the ordinal nature of the data for highest level of treatment. Furthermore, we combined these models together to create a single, clinically applicable tool.
The generalizability of the study’s findings is limited by the fact that the study cohort comprises well-educated, English-speaking, predominantly White participants seeking urologic or urogynecologic care at academic centers, and as a result, the findings of this study may not be generalizable to other populations. Other limitations include participant recall bias, which could impact the reporting of symptom severity and treatments. Another limitation is the small number of women who underwent a sling procedure during the study period. Also, given the nature of the data available, we were not able to comment on a number of important factors that would impact treatment patterns. For instance, treatment cost, time constraints, side effects, and treatment response could impact a patient’s ability or willingness to continue treatment, while limitations of insurance coverage, personal or group practice patterns, and medical training could influence a medical provider’s ability or willingness to prescribe a given treatment. As a recent study by Sebesta et al highlighted, there are many unseen social factors that influence patients’ symptom severity, and we must continue to investigate the role that unmet social needs play in patient access to treatment for OAB.25 Future research should study the relationship between patient treatment choices and social determinants of health.
CONCLUSIONS
Treatment patterns for UU and UUI are diverse, and even in specialty centers, treatment for these conditions most often involve no treatment or only conservative therapies. The findings from this study can help providers counsel patients with bothersome UU and UUI regarding treatments. With the OAB treatment prediction tools created from this study, providers can individualize treatment plans and identify not only patients at risk for treatment discontinuation but also patients who may not be escalated to potentially beneficial OAB treatments, with the goal to improve clinical outcomes for patients suffering from this chronic and often debilitating condition.
Supplementary Material
Supplementary Table 1. Treatments included in behavioral therapy and overactive bladder medications.
Supplementary Table 2. Predictors of the highest level of treatment during 12-month study follow-up. Results from ordinal logistic regression (proportional odds model)
Supplementary Table 3. Predictors of OAB medication discontinuation (among n=105 who used OAB medications)
Supplementary Table 4. Predictors of having sling during 12-month study follow-up
Supplementary Figure 1. Predicted probabilities of having sling during 12-month study follow-up
Supplementary Figure 2. Calibration curve for sling model predicting probability of having sling during 12-month study follow-up
ACKNOWLEDGEMENTS
Heather Van Doren, Senior Medical Editor with Arbor Research Collaborative for Health, provided editorial assistance on this manuscript.
Funding Support
This is publication number 36 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) through cooperative agreements (grant numbers DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK099879).
Research reported in this publication was supported at Northwestern University, in part, by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS; grant number UL1TR001422). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PIs: Cindy Amundsen, MD, J. Eric Jelovsek, MD; Co-Is: Kathryn Flynn, PhD, Jim Hokanson, PhD, Aaron Lentz, MD, David Page, PhD, Nazema Siddiqui, MD, Lisa Wruck, PhD, Todd Harshbarger, PhD; Study Coordinators: Paige Green, MSc, Magaly Guerrero, BSc, Stephanie Yu, Summer Granger
University of Iowa, Iowa City, IA (DK097772): PIs: Catherine S Bradley, MD, MSCE, Karl Kreder, MD, MBA; Co-Is: Bradley A. Erickson, MD, MS, Daniel Fick, MD, Vince Magnotta, PhD, Philip Polgreen, MD, MPH; Study Coordinators: Chelsea Poesch, BA, Jean Walshire, AAS, Rachel Setting, BA
Northwestern University, Chicago, IL (DK097779): PIs: James W Griffith, PhD, Kimberly Kenton, MD, MS, Brian Helfand, MD, PhD; Co-Is: Carol Bretschneider, MD, David Cella, PhD, Sarah Collins, MD, Julia Geynisman-Tan, MD, Alex Glaser, MD, Christina Lewicky-Gaupp, MD, Margaret Mueller, MD, Francesca Farina, PhD, Richard Fantus, MD, Devin Boehm, BS; Study Coordinators: Hosanna An, Andrea Villegas, Melissa Marquez, MBA, Malgorzata Antoniak, PhD, Pooja Talaty, MS, Sophia Kallas, Jessica Thomas. Dr. Helfand and Ms. Talaty are at NorthShore University HealthSystem.
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne P. Cameron, MD, Aruna Sarma, PhD, Giulia Lane, MD, Priyanka Gupta, MD, Whitney Horner, MD; Jannah Thompson, MD; Payton Schmidt, MD; Study Coordinators: Linda Drnek, CCRP, Greg Mowatt, BA, Sarah Richardson, BS, Lydia Duong
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: Anna Kirby, MD; Study Coordinators: Brenda Vicars, RN; Sreya Gutta
Washington University in St. Louis, St. Louis Missouri (DK100017): PI: H. Henry Lai, MD; Co-Is: Joshua Shimony, MD, PhD, Fuhai Li, PhD; Study Coordinators: Linda Black, RN, Vivien Gardner, BSN, Patricia Hayden, BSN, Diana Wolff, Aleksandra Klim, RN, MHS, CCRC
Arbor Research Collaborative for Health, Data Coordinating Center (DK099879): PI: Robert Merion, MD, FACS; Co-Is: Victor Andreev, PhD, DSc, Brenda Gillespie, PhD, Abigail Smith, PhD; Project Manager: Jessica Durkin, M.Ed, MBA; Clinical Monitor: Melissa Sexton, BA, CCRP; Research Analysts: Margaret Helmuth, MA, Sarah Mansfield, MS. Project Associate: Julia Nashif, BA
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urology, and Hematology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: Christopher Mullins PhD; Project Advisor: Julie Barthold, MD
Abbreviations
- LURN
Symptoms of Lower Urinary Tract Dysfunction Research Network
- NIDDK
National Institute of Diabetes & Digestive & Kidney Diseases
- NIH
National Institutes of Health
- NCATS
National Center for Advancing Translational Sciences
- IRB
Institutional Review Board
- LUTS
Lower Urinary Tract Symptoms
- OAB
Overactive Bladder
- UU
Urinary Urgency
- UUI
Urgency Urinary Incontinence
- BTX
Intradetrusor Onabotulinumtoxin A
- SNM
Sacral Neuromodulation
- Q
Question
- SUI
Stress Urinary Incontinence
- BMI
Body Mass Index
- TIA
Transient Ischemic Attack
- ACB
Anticholinergic Burden
- PROMIS
Patient-Reported Outcomes Measurement Information System
- AUA-SI
American Urological Association Symptom Index
- PSS
Perceived Stress Scale
- BT
Behavioral Therapy
- PT
Pelvic Floor Physical Therapy
- PTNS
Percutaneous Tibial Nerve Stimulation
- rms
Regression Modeling Strategies
Footnotes
Ethics / Institutional Review Board (IRB) Approval Statement: The authors confirm all relevant ethical guidelines have been followed, and all research has been conducted according to the principles expressed in the Declaration of Helsinki. IRB approval has been obtained from: Ethical and Independent Review Services (E&I) IRB, an Association for the Accreditation of Human Research Protection Programs (AAHRPP) Accredited Board, Registration #IRB 00007807.
Patient Consent Statement: Informed written consent has been obtained from participants.
Clinical Trial Registration: The LURN ClinicalTrials.gov Identifier is NCT02485808.
Conflicts of Interest
The authors declare no conflicts of interest.
Data Availability Statement
The data that support the findings of this study are openly available in the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) Central Repository at https://repository.niddk.nih.gov/; please reference the acronym “LURN”.
REFERENCES
- 1.Reynolds WS, Fowke J, Dmochowski R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. [DOI] [PubMed] [Google Scholar]
- 3.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. [DOI] [PubMed] [Google Scholar]
- 4.Brown MT, Bussell J, Dutta S, Davis K, Strong S, Mathew S. Medication adherence: Truth and consequences. Am J Med Sci. 2016;351(4):387–399. [DOI] [PubMed] [Google Scholar]
- 5.Yeowell G, Smith P, Nazir J, Hakimi Z, Siddiqui E, Fatoye F. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): a systematic literature review. BMJ Open. 2018;8(11):e021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105(9):1276–1282. [DOI] [PubMed] [Google Scholar]
- 7.Andy UU, Arya LA, Smith AL, et al. Is self-reported adherence associated with clinical outcomes in women treated with anticholinergic medication for overactive bladder? Neurourol Urodyn. 2016;35(6):738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du C, Berg WT, Siegal AR, et al. A retrospective longitudinal evaluation of new overactive bladder patients in an FPMRS urologist practice: Are patients following up and utilizing third-line therapies? Neurourol Urodyn. 2021;40(1):391–396. [DOI] [PubMed] [Google Scholar]
- 9.Kato D, Uno S, Van Schyndle J, Fan A, Kimura T. Persistence and adherence to overactive bladder medications in Japan: A large nationwide real-world analysis. Int J Urol. 2017;24(10):757–764. [DOI] [PubMed] [Google Scholar]
- 10.Illiano E, Finazzi Agrò E, Natale F, Balsamo R, Costantini E. Italian real-life clinical setting: the persistence and adherence with mirabegron in women with overactive bladder. Int Urol Nephrol. 2020;52(6):1035–1042. [DOI] [PubMed] [Google Scholar]
- 11.Kalder M, Pantazis K, Dinas K, Albert US, Heilmaier C, Kostev K. Discontinuation of treatment using anticholinergic medications in patients with urinary incontinence. Obstet Gynecol. 2014;124(4):794–800. [DOI] [PubMed] [Google Scholar]
- 12.Athanasopoulos A, Giannitsas K. An overview of the clinical use of antimuscarinics in the treatment of overactive bladder. Adv Urol. 2011;2011:820816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang CC, Weinfurt KP, Merion RM, Kirkali Z, Group LS. Symptoms of Lower Urinary Tract Dysfunction Research Network. J Urol. 2016;196(1):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne KS, Barsdorf AI, Thompson C, et al. Moving towards a comprehensive assessment of lower urinary tract symptoms (LUTS). Neurourol Urodyn. 2012;31(4):448–454. [DOI] [PubMed] [Google Scholar]
- 15.Coyne KS, Sexton CC, Kopp Z, et al. Assessing patients’ descriptions of lower urinary tract symptoms (LUTS) and perspectives on treatment outcomes: results of qualitative research. Int J Clin Pract. 2010;64(9):1260–1278. [DOI] [PubMed] [Google Scholar]
- 16.Cameron AP, Lewicky-Gaupp C, Smith AR, et al. Baseline lower urinary tract symptoms in patients enrolled in LURN: A prospective, observational cohort study. J Urol. 2018;199(4):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154(5):1770–1774. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 20.Harrell FE . rms: Regression Modeling Strategies. R package version 6.2–0. 2021. [Google Scholar]
- 21.Lua LL, Pathak P, Dandolu V. Comparing anticholinergic persistence and adherence profiles in overactive bladder patients based on gender, obesity, and major anticholinergic agents. Neurourol Urodyn. 2017;36(8):2123–2131. [DOI] [PubMed] [Google Scholar]
- 22.Soda T, Tashiro Y, Koike S, Ikeuchi R, Okada T. Overactive bladder medication: Persistence, drug switching, and reinitiation. Neurourol Urodyn. 2020;39(8):2527–2534. [DOI] [PubMed] [Google Scholar]
- 23.Ivchenko A, Bödeker RH, Neumeister C, Wiedemann A. Anticholinergic burden and comorbidities in patients attending treatment with trospium chloride for overactive bladder in a real-life setting: results of a prospective non-interventional study. BMC Urol. 2018;18(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TH, Lee KS. Persistence and compliance with medication management in the treatment of overactive bladder. Investig Clin Urol. 2016;57(2):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebesta EM, Gleicher S, Kaufman MR, Dmochowski RR, Reynolds WS. Associations between unmet social needs and overactive bladder. J Urol. 2022;208(5):1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Treatments included in behavioral therapy and overactive bladder medications.
Supplementary Table 2. Predictors of the highest level of treatment during 12-month study follow-up. Results from ordinal logistic regression (proportional odds model)
Supplementary Table 3. Predictors of OAB medication discontinuation (among n=105 who used OAB medications)
Supplementary Table 4. Predictors of having sling during 12-month study follow-up
Supplementary Figure 1. Predicted probabilities of having sling during 12-month study follow-up
Supplementary Figure 2. Calibration curve for sling model predicting probability of having sling during 12-month study follow-up
Data Availability Statement
The data that support the findings of this study are openly available in the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) Central Repository at https://repository.niddk.nih.gov/; please reference the acronym “LURN”.