Abstract
Separation of blood components is required in many diagnostic applications and blood processes. In laboratories, blood is usually fractionated by manual operation involving a bulk centrifugation equipment, which significantly increases logistic burden. Blood sample processing in the field and resource-limited settings cannot be readily implemented without the use of microfluidic technology. In this study, we developed a small footprint, rapid, and passive microfluidic channel device that relied on margination and inertial focusing effects for blood component separation. No blood dilution, lysis, or labeling step was needed as to preserve sample integrity. One main innovation of this work was the insertion of fluidic restrictors at outlet ports to divert the separation interface into designated outlet channels. Thus, separation efficiency was significantly improved in comparison to previous works. We demonstrated different operation modes ranging from platelet or plasma extraction from human whole blood to platelet concentration from platelet-rich plasma through the manipulation of outlet port fluidic resistance. Using straight microfluidic channels with a high aspect ratio rectangular cross section, we demonstrated 95.4% platelet purity extracted from human whole blood. In plasma extraction, 99.9% RBC removal rate was achieved. We also demonstrated 2.6× concentration of platelet-rich plasma solution to produce platelet concentrate. The extraction efficiency and throughput rate are scalable with continuous and clog-free recirculation operation, in contrast to other blood fractionation approaches using filtration membranes or affinity-based purification methods. Our microfluidic blood separation method is highly tunable and versatile, and easy to be integrated into multi-step blood processing and advanced sample preparation workflows.
INTRODUCTION
Blood is a complex biological fluid that contains red blood cells (RBCs) in the range of 5–8 μm, white blood cells (WBCs) in the range of 7–30 μm, and platelets (PLTs) in the range of 2–3 μm, suspended in plasma. Many disease diagnostics and clinical treatments require blood fractionation. Plasma contains important circulating biomarkers1 for various human diseases ranging from cancer,2 iron deficiency,3 sepsis4 to infectious viruses that cause Ebola,5 Zika,6 or COVID-19.7 Another example of clinical process that requires blood separation is platelet transfusion.8,9 Applications of platelet transfusion or therapy include resuscitation,10,11 wound healing,12 skin reconstruction,13 fibrosarcoma,14 and thrombocytosis.15
Centrifugation is the most common approach to fractionate blood in laboratories. Low-speed centrifugation is first used to separate platelet-rich plasma (PRP) from other blood components of RBCs and WBCs, followed by high-speed centrifugation to separate platelet-poor plasma (PPP) from platelet pellets. Microfluidic technology enables near-patient diagnostics and sample preparations with advantages of portability, reliability, high separation efficiency, and automaticity.16 Some microfluidic plasma separation methods use membrane filtration.17–19 The main drawback of membrane filtration is sample loss due to absorption and clogging. Chemical hemolysis has been coupled with a paper device for blood-based diagnostics.20 It has been reported that the influence of hemolysis and leukolysis has adverse effects on the detection of circulating biomarkers and metabolites in blood samples.1,21–23
Microfluidic devices have been developed for passive and label-free blood separation without the use of filtration membrane.24 In contrast to active microfluidics devices, which usually require built-in or integrated actuation mechanism, such as acoustophoresis and dielectrophoresis, passive microfluidic devices rely on an external pumping mechanism, such as syringe or peristaltic pump. Typical passive microfluidic methods include biomimetic cell margination, inertial focusing, viscoelastic, filtration, and deterministic lateral displacement.
Fåhraeus and Lindqvist reported that the apparent blood viscosity decreased strongly in small capillary with diameter less than 300 μm in 1931.25 They attributed that the reduced viscosity was due to RBC migration to the fast flow stream along the channel centerline under the Poiseuille flow. A cell-free layer (CFL) of plasma emerged near the channel wall. Densely packed and deformable RBCs also expelled and marginated rigid platelets and WBCs from the RBC core toward the CFL. Blood margination effect has been used in whole blood separation without dilution. Straight microfluidic channel devices have been developed to separate small bacterial pathogens,26,27 rigid malaria infected or aged RBCs28,29 and leukocytes,30,31 platelets,32 and plasma33–35 from whole blood (WB). These passive microfluidic devices were highly effective and reliable in bioparticle migration in whole blood. However, their separation efficiency was usually not being optimized due to lack of proper collection control characteristics in the outlet channels, which remarkedly degraded their separation performance.
To separate the diluted blood sample, a microfluidic radial array consisted of parallel straight channels has been demonstrated to inertially focus RBCs to side outlets and remove bacteria in the center outlet.36 An asymmetrical sheath flow has been used to segment the sample fluid for bacteria separation from diluted human blood along a microfluidic channel.37 Serpentine or wavy microfluidic channels have been used to inertially order RBCs,38 extract plasma,39 or isolate cancer cells.40 Microfluidic channels with contraction-expansion array have been demonstrated to generate vortical flow structures to isolate cancer cells from diluted blood samples.41–45 Spiral inertial sorters used curvilinear microfluidic channels to create Dean vortex drag forces to focus targeted cells or particles to single equilibrium position,46 which have been demonstrated to separate RBCs, WBCs, and plasma,47 to isolate circulating tumor cells,48,49 immune cells,50–53 proteins by affinity-conjugated beads,54 viral nuclei acids in negative selection,55 extracellular vesicles,56 and extract plasma.47,57–59 Viscoelastic flows were used in inertial microfluidic channel devices to separate RBCs and WBCs60 and extract bacteria,61–63 exosome,64 or plasma.65 Another class of passive and label-free microfluidic device used controlled incremental filtration as crossflow micro-sieves to concentrate and purify WBCs66–68 and platelets.69,70
Among different kinds of passive microfluidic devices for blood separation, the straight channel structure relies on the simple particle migration mechanism and is less subjected to fabrication variance due to simple device geometry. In-plane parallelization of microfluidic channels can be easily implemented to increase the throughput rate. Microfluidic separation method without sheath flow is more favorable since the incorporation of buffer use will dilute blood samples.
A straight microfluidic channel device with rectangular cross section has been demonstrated to inertially focus 1.9 μm microparticles in dilute solution.71 The wall lift force that pushed microparticles away from the walls to the channel centerline balanced with the shear-induced inertial lift force arisen from the laminar velocity profile, forming two symmetric focused bands of microparticles at the lateral equilibrium positions along the microfluidic channel.
Fluidic resistance has been demonstrated to manipulate stream width and center position in pressure-driven microfluidic flows.72 The main novelty of this work was to use the fluidic constrictor inserted into the microfluidic outlet ports to divert the separation interface into desired outlet channel positions. Hence, switching of blood separation modes and enhancement of separation performance were achieved using the same microfluidic cross section structure. In this paper, we applied the blood margination phenomena to separate platelets or plasma from undiluted whole blood in straight microfluidic channels. We demonstrated the switch between the platelet and plasma extraction by manipulation of fluidic resistance between collect and discard outlets. We also demonstrated the concentration of platelets in platelet-rich plasma using inertial focusing.
WORKING PRINCIPLE
Straight microfluidic channel for blood separation
The working principle of the straight microfluidic channel device for blood separation is illustrated in Fig. 1. We used the margination effect in straight microfluidic channels to extract platelet or plasma from WB [Fig. 1(a)]. When undiluted WB was injected into a straight microfluidic channel at dimension of tens of micrometers, abundant and deformable RBCs were focused by hydrodynamic lift force from sidewalls to the channel axial centerline. Smaller and rigid PLTs (2–3 μm) were marginated next to the RBC core due to the intense interaction with deformable RBCs (5–8 μm). Next to the small band of the PRP region was a CFL composing of PPP in proximity to the sidewalls. Prior works have studied the distribution profiles of platelets or platelet-size particles under blood flows in microfluidic channels.73–76 We used these experimental data, particularly the distance required for effective platelet margination and the location of peak platelet concentration in the pressure-driven blood flow profiles, as the preliminary guide of our inertial margination channel geometry and outlet channel split design. The margination effect was reported to be dependent on hematocrit (HCT), channel diameter, or width, and wall shear rate.77,78 The blood flow strength is characterized by the shear rate as
| (1) |
where is the average shear rate normalized by the average flow velocity by channel diameter or width, is the dynamic viscosity, Dr is the RBC diameter, and is the RBC membrane bending rigidity.
FIG. 1.
Working principle of blood component separation with straight microfluidic channel devices. (a) Extraction of platelets or plasma from undiluted whole blood using the margination effect and manipulation of outlet fluidic resistance and (b) concentration of platelet from platelet-rich plasma using the inertial focusing effect.
To facilitate separation between the RBC core, PRP, and CFL regions, the exit of the margination channel was gradually transitioned into an expansion section that connected the outlet channels. For platelet extraction, a low fluidic resistance (R) was imposed on the side outlet channels to divert majority of RBCs into the center outlet. The PRP portion was extracted by the side outlets. For plasma extraction, a high fluidic resistance was imposed on the side outlet channels to divert both the RBC core and PRP portions into the center outlet. The PPP portion was extracted by side outlets. Similar straight microfluidic channel devices were used to concentrate PLTs from PRP to produce platelet concentrate (PC) using the inertial focusing effect [Fig. 1(b)]. In the straight microfluidic channel, randomly dispersed PLTs were aligned to form two symmetric narrow bands at the equilibrium positions where the inertial lift force balanced with the velocity gradient force. PC was collected by the outlet channel located at the inertially focused PLT band, and the PPP portion was collected by other outlet channels. In inertial focusing, the wall lift force (FL) is given by
| (2) |
where ρ and Uf are the density and fluid velocity, respectively, and ap is the bioparticle diameter. The characteristic length (LC) was the narrowest channel dimension, which was the focusing channel width for high aspect ratio cross section. The bioparticle lateral migration velocity (UL) was given by
| (3) |
where μ is the fluid dynamic viscosity.
MATERIALS AND METHODS
We designed the microfluidic device layout in AutoCAD (Autodesk, 2010LT) and fabricated the device using polydimethylsiloxane (PDMS) soft lithography technique.
Microfluidic device design
The layout of straight microfluidic channel devices for blood separation is shown in Fig. 2. The microfluidic device was composed of a constricted channel with 20 μm width and 50 μm height, which connected a single inlet with a 30° gradual expansion section. The expansion section extrapolated the flow streamlines to facilitate precise splitting of separation species at the outlet bifurcation points. The end of the expansion section contained nine equally split outlet channels, each with 82 μm width. This outlet channel width kept a good aspect ratio to reduce fabrication failure while providing good flow streamline separation resolution and a small device footprint. For margination demonstration with fluorescently labeled platelets, a microfluidic device with 8 mm margination channel length was used [Fig. 2(a)]. For extraction of platelets and plasma, margination channel lengths of 5 mm [Fig. 2(b)] and 8 mm [Fig. 2(a)] were used, respectively. The platelet or plasma was collected at the side outlet ports (O1 and O9). To optimize for platelet or plasma extraction performance, we manipulated the side outlet fluidic resistance by inserting the fluidic restrictor, for example, PEEK tubing, small Tygon™ tubing, or other PDMS microfluidic devices, into the side outlet ports (O1 and O9). The hydrodynamic resistance in the microfluidic channel with a radial cross section is given by
| (4) |
where L and r are the tube length and radius, respectively. For high aspect ratio rectangular cross section, the hydrodynamic resistance is given by
| (5) |
where H and W are the channel height and width, respectively, under the conditions of (L > > H) and (H > W).
FIG. 2.
Layout of straight microfluidic channel devices that consisted of a constricted channel with 20 μm width and 50 μm height connecting an inlet, an expansion section, and outlets for (a) fluorescently labeled platelet margination demonstration and plasma extraction, (b) platelet extraction, and (c) platelet concentration.
To concentrate platelets from PRP, we used a microfluidic device with focusing channel length of 8 mm [Fig. 2(c)]. PC was collected from outlet ports O2 and O8. We chose nine equally split outlet channels since it has been reported that the focused particle streams were positioned between ∼0.1 and ∼0.2 of the characteristic length, which represented the focusing channel width, from the sidewalls.71 We chose margination or focusing channel length of 5 or 8 mm based on prior experimental and simulation findings.26,71,73–76 Within the flow rate range we tested, the margination and inertial focusing effects were expected to complete within the constricted channel length. The geometric design parameters of straight microfluidic channel devices for blood separation are summarized in Table I.
TABLE I.
Design parameters of straight microfluidic channel devices for blood component separation. Underline indicates outlets for collection.
| Focusing channel length | Outlets | |
|---|---|---|
| Platelet margination demo/plasma extraction | 8 mm | 1:7:1 |
| Platelet extraction | 5 mm | 1:7:1 |
| Platelet concentration | 8 mm | 1:1:5:1:1 |
Microfluidic device fabrication
The Chrome masks were manufactured by Advance Reproductions Corporation (North Andover, MA). We used the microfabrication services from the University of Michigan Lurie Nanofabrication Facility to fabricate microfluidic mold masters using SU-8 patterning. The height of the microfluidic channel structures was measured by a profilometer. Upon receipt of the SU-8 patterned silicon molds at CFD Research Corporation, the microfluidic substrates were casted with PDMS (SYLGARD™ 184, Dow Corning) with a mixing ratio of 10:1 (base:curing agent) by weight. After surface treatment with a plasma cleaner (Harrick Plasma, Ithaca, NY), the PDMS substrates and cleaned microscopic glass slides were bonded together permanently.
The following subsections described the blood sample collection and preparation process. Fluorescent labeling was used for flow visualization. Microfluidic blood separation performance was characterized by the cell count.
Human whole blood collection
Fresh human WB was acquired from volunteer donors in anticoagulant acid citrate dextrose (ACD) collection vials. Blood collection protocol was approved by the CFD Research IRB board.
Fluorescent labeling of platelets in whole blood samples
To enable visualization of PLT migration in microfluidic channel device, we purified and fluorescently labeled human PLTs in double-spin centrifugation steps as follows:79 (1) human anticoagulated WB was spun down at 1000 g for 10 min to fractionate WB into the PRP, buffy coat (WBCs), and RBC layers.80 (2) The PRP fraction was spun down at 2500 g for 15 min to collect PLTs in the pellet. The RBC fraction was stored. (3) The top supernatant composed of PPP was aspirated and stored. The PLT pellet was re-suspended in Calcein AM at 1:100 dilution in 1× PBS for 20 min at room temperature for staining. (4) Next, Calcein AM labeled PLT solution was spun down at 2500 g for 15 min to remove unbound fluorescent stain with PBS. The Calcein AM stained PLT pellet was re-suspended in original PPP and mixed with stored RBCs to replicate the initial whole blood cell counts, concentration, and condition.
Cell counting
Blood cell samples were counted under an epifluorescence microscope (Nikon TE2000-U) with a 20× optical objective with additional 1.5× magnification in a hemocytometer or Nexcelom counting chambers. The RBC portion of all samples was counted in bright-field imaging with 100× dilution in 10% ACD 1×PBS. The WBC and PLT portions were counted with 10× dilution and Calcein AM (1:100) staining in fluorescence images. Cells with diameter of >4 μm were counted as WBCs, and while cells with diameter of <4 μm were counted as PLTs.
Separation performance metrics
For platelet extraction from whole blood, we defined platelet purity, recovery, enrichment ratio, and RBC removal rate as follows:
| (6) |
| (7) |
| (8) |
where [NPLT], [NRBC], and [NWBC] are the number of PLTs, RBCs, and WBCs collected from the inlet or outlets, while [CPLT], [CRBC], and [CWBC] are the concentrations of PLTs, RBCs, and WBCs from inlet or outlets, respectively. For plasma extraction from whole blood, we added the characterization of the PLT removal rate defined as follows:
| (9) |
| (10) |
For platelet concentration from PRP, we defined the concentrating factor as follows:
| (11) |
RESULTS AND DISCUSSION
Demonstration of platelet margination
We first used a straight microfluidic channel device to demonstrate PLT margination in WB. To ensure full RBC focusing on the evaluation of different flow rates, we chose the constricted channel length to be 8 mm. The PLTs were fluorescently labeled for flow trajectory visualization (see Materials and Methods section). The microfluidic device was mounted under an epifluorescence microscope (Nikon TE2000-U). Freshly prepared human WB in 10% ACD with Calcein AM labeled PLTs was driven into the constricted channel at a volume flow rate of 80 μl/min (Re ∼13) by a syringe pump (Harvard Apparatus). The fluorescence images of the flow trajectories of Calcein AM stained PLTs at the expansion section were captured under a 4× optical objective with additional 1.5× magnification [Fig. 3(a)]. A green fluorescence protein (GFP) fluorescence filter cube was used. An exposure time of 500 ms was set on the electron multiplying charge coupled device (EMCCD) camera (Andor Technology iXon3). The flow trajectories of RBCs were captured in bright-field images with an exposure time of 20 ms. In the constricted channel, abundant and deformable RBCs exerted by the wall lift force migrated to the channel axial centerline. Fluorescently labeled PLTs were marginated next to the RBC core, leaving a thin cell-free plasma layer next to the sidewalls. In the outlet expansion section, two symmetric fluorescent PLT flow trajectories were formed next to the RBC core, which was marked in red (false color). Next to the fluorescent PRP layer was the CFL region near the sidewalls. We carefully examined the fluorescence (PLTs) and bright-field (RBCs) intensity profiles near the outlets [along the yellow broken line in Fig. 3(a)] and found that two intensity profiles crossed each other at position of −16 and 16 when we normalized the entire width of the expansion section with a scale of −17 to 17 [Fig. 3(b)]. We also examined the platelet margination effect on other flow rates ranging from 20 to 120 μl/min in ESI (Fig. S1 in the supplementary material).
FIG. 3.
Demonstration of platelet (PLT) margination in whole blood using a microfluidic device with constricted channel length of 8 mm. (a) Flow trajectories of fluorescently stained PLTs in green and red blood cells (RBCs) in red (false color using bright field) at an inlet flow rate of 80 μl/min and (b) intensity profiles along the yellow broken line near the outlets in (a).
Platelet extraction from whole blood
From flow visualization experiments for fluorescent labeled platelets, we demonstrated that platelets marginated to the sidewalls from 20 to 120 μl/min with a constricted channel length of 8 mm. We expected that platelet margination could be completed with a shorter constricted channel length. Hence, we used a straight microfluidic channel device with a 5 mm long constricted channel to characterize platelet extraction performance from WB. The collected PRP sample was compared with the discard sample in Fig. 4(a). To optimize for PLT and RBC separation between collect and discard outlets, we connected the side outlet ports (O1 and O9) with other microfluidic devices to increase fluidic resistance to alter the flow trajectories. The combined fluidic resistance ratio between the collect (O1 and O9) and discard (O2–O8) outlets was 13:1 if we estimated that the fluid viscosity of the concentrated WB collected by the discard (O2–O8) outlets was 2.2 times higher than the PRP in the collect (O1 and O9) outlets. The anticoagulated WB with 10% ACD was driven into the microfluidic device at a volume flow rate of 80 μl/min using a syringe pump (Harvard Apparatus). The RBC concentration of the inlet samples was between 3.9 and 7.0 × 106/μl, which was equivalent to 35%–63% HCT. The flow trajectories of RBCs in the expansion section and outlet channels under bright-field imaging are shown in Fig. 4(b). Due to the fluidic resistance inserted in the side outlet ports, we successfully diverted the interface between RBC and plasma into the split between collect (O1 and O9) and discard (O2–O8) outlets. The RBC-depleted and PLT-enriched PRP was collected from side outlet ports O1 and O9. PLT-depleted RBCs were collected from outlet ports O2–O8. Five independent experiments at the same flow rate were conducted with five separate sets of identical devices. On average, about 4 ml of WB was processed in one single-pass inertial microfluidic separation experiment without clogging issue. About 0.3 ml PRP samples were extracted, which gave an extraction volume ratio between collect (O1 and O9) and discard (O2–O8) outlets of 1:14. The small difference with the fluidic resistance ratio estimate was due to some fluctuation of WB sample hematocrits. The processing time was about 1 h. The collected samples were cell counted with bright-field and fluorescence imaging (see Materials and methods section). The RBCs, PLTs, and WBCs were marked in red, green, and yellow arrowheads, respectively. In single pass operation, we demonstrated that inertial separation enriched the PLT content 21.6× from an initial PLT purity of 4.7% to 95.4% [Fig. 4(d)]. On average, 24.1% of PLTs was collected and 99.95% of RBCs was removed in the collect sample.
FIG. 4.
Platelet extraction from whole blood using a constricted channel with 5 mm length at a flow rate of 80 μl/min. (a) Sample comparison between collect and discard samples. (b) Flow trajectories illustrating inertial focusing of RBCs under bright-field imaging. (c) Cell counting of RBCs by red arrowhead, PLTs by green arrowhead, and white blood cells (WBCs) by yellow arrowhead using bright-field and fluorescence microscopy. (d) PLT purity and enrichment ratio plots of inlet and collect samples.
Plasma extraction from whole blood
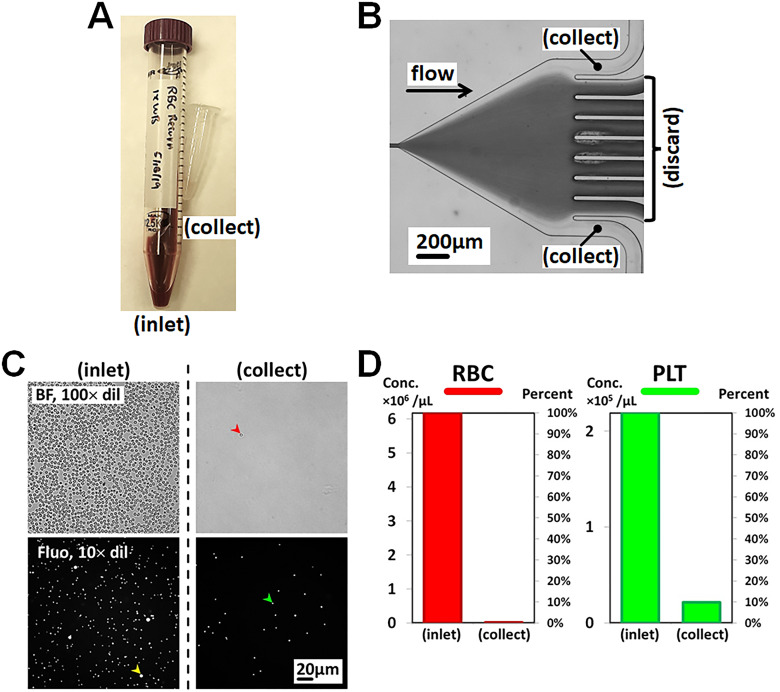
Next, a straight microfluidic channel device with 8 mm constricted channel length was used to characterize plasma extraction performance from whole blood in closed-loop operation. We chose a slightly longer constricted channel to damp out possible pulsation of the peristaltic pump. The collected PPP sample was compared with the inlet sample in Fig. 5(a). To optimize for plasma extraction, two 6 cm PEEK tubes with 63.5 μm inner diameter (ID) were inserted into side outlet ports (O1 and O9) to increase the fluidic resistance. The combined fluidic resistance ratio between the collect (O1 and O9) and discard (O2–O8) outlets was 31:1 if we estimated that the fluid viscosity of the concentrated WB collected by the discard (O2–O8) outlets was 2.2 times higher than the PPP in the collect (O1 and O9) outlets. The 1 ml anticoagulated WB with 10% ACD was driven into the microfluidic device using a peristaltic pump (P625, Instech Laboratories, Inc.) with a 0.38 mm ID tube set at 150 μl/min. We used the peristaltic pump to recirculate plasma-depleted WB collected from the center outlet ports (O2–O8) back to the inlet for continuous plasma extraction to the side outlet ports (O1 and O9) to improve plasma extraction efficiency. This recirculation operation could compensate the lower extraction volume ratio compared to platelet extraction. The experimental setup was illustrated in ESI (Fig. S2 in the supplementary material). Compared with platelet extraction in the previous subsection (Platelet extraction from whole blood), the span of the RBC flow trajectories was slightly diminished due to higher fluidic resistance in the side outlet ports. We successfully diverted the interface between RBC and plasma deeper into outlet channels O2 and O8 to ensure most platelets were collected by the recycle outlets (O2–O8). Plasma free from RBCs and PLTs was collected from side outlet ports O1 and O9 [Fig. 5(b)]. About 80 μl plasma was extracted by the inertial microfluidic device under 10 min of operation, resulting in a plasma extraction rate of 8 μl/min and extraction volume ratio between collect and discard outlets of 1:31. Sample collected from the side outlets was compared with the initial inlet sample in cell counting under optical microscopy (see Materials and Methods section) as shown in Fig. 5(c). Compared with the initial inlet sample, our closed-loop inertial microfluidic device achieved the RBC removal rate of 99.9% and the PLT removal rate of 90% in concentration measurements [Fig. 5(d)].
FIG. 5.
Plasma extraction from whole blood using a constricted channel with 8 mm length at a flow rate of 150 μl/min. (a) Sample comparison between inlet and collect samples. (b) Flow trajectories illustrating inertial focusing and diversion of RBCs into discard outlets by increasing the fluidic resistance in collect outlets. (c) Cell counting. (d) Comparison of RBC and PLT concentrations between inlet and collect samples.
Platelet concentration from platelet-rich plasma
Last, we used a straight microfluidic channel device with a constricted channel of 8 mm length to concentrate platelets from PRP sample in single pass operation. The collected PC sample was compared with the inlet sample in Fig. 6(a). To further optimize for platelet recovery, the side outlet ports of O1 and O9 were inserted with 10 cm 0.010′ Tygon™ tubing to increase fluidic resistance by 10% to divert the inertially focused platelets into outlet ports O2 and O8 for collection. The inlet sample was PRP collected from another microfluidic device for platelet extraction (see Platelet extraction from whole blood subsection). The 220 μl PRP inlet sample was driven into the inertial microfluidic device at a flow rate 120 μl/min using a syringe pump (Harvard Apparatus). The PLTs were inertially focused and formed two symmetric flow trajectories near the sidewalls in the expansion section, marked with green arrowhead, and diverted into outlet ports O2 and O8 [Fig. 6(b)]. A 27 μl concentrated platelet sample was collected in outlet ports O2 and O8, which gave an extraction ratio between collect and discard outlets of 1:7. The other outlet ports of O1, O3−O7, and O9 of the microfluidic device were grouped for discard. Collected PC sample was compared with the inlet PRP sample under optical microscopy for cell counting (see Materials and Methods section) as shown in Fig. 6(c). Our inertial microfluidic device demonstrated a platelet concentration factor of 2.6× [Fig. 6(d)]. The collected PC sample had a platelet purity of 97.8%.
FIG. 6.
Platelet concentration of platelet-rich plasma using a constricted channel with 8 mm length at a flow rate of 120 μl/min. (a) Sample comparison between inlet and collect samples. (b) Flow trajectories illustrating inertial focusing and diversion of PLTs into collect outlets. (c) Cell counting. (d) Comparison of PLT concentration between inlet and collect samples.
The operation parameters and separation performance of different blood separation modes of straight microfluidic channel devices are summarized in Tables II and III. These optimized flow rates were determined by maximum recovery of targeted species (platelet or plasma) from their flow trajectories under optical microscopy. We used different types of fluidic restrictors, dependent on each case scenario for optimized collection volume ratio between the collect and discard outlets.
TABLE II.
Inlet samples and operational parameters of straight microfluidic channel devices in platelet or plasma extractions from whole blood and platelet concentration of platelet-rich plasma.
| Inlet sample | Inlet flow rate (μl/min) | Collect flow rate (μl/min) | Volume ratio (collect:discard) | |
|---|---|---|---|---|
| Platelet extraction | Whole blood | 80 | 5.3 | 1:14 |
| Plasma extraction | Whole blood | 80 | 4.7 | 1:16 |
| Platelet concentration | Platelet-rich plasma | 120 | 15 | 1:7 |
TABLE III.
RBC, PLT, and WBC concentrations of collect samples and separation efficiency.
| Collect sample | RBC conc. (/μl) | PLT conc. (/μl) | WBC conc. (/μl) | Separation efficiency | |
|---|---|---|---|---|---|
| Platelet extraction | Platelet-rich plasma | 3.7 × 104 | 7.4 × 105 | 5.8 × 102 | PLT Purity: 95.4% PLT Extx: 24.1% RBC Remov: 99.95% |
| Plasma extraction | Platelet-poor plasma | 8.9 × 102 | 6.3 × 104 | 5.8 × 10 | RBC Remov: 99.999% PLT Remov: 97.2% WBC Remov: 99.9% |
| Platelet concentration | Platelet concentrate | 2.9 × 104 | 1.3 × 106 | 2.5 × 102 | PLT Purity: 97.8% PLT Conc: 2.6× |
CONCLUSIONS
Label-free and membrane-less blood separation has been demonstrated in straight microfluidic channel devices based on blood margination and inertial focusing effects. We demonstrated both switching of blood separation modes and optimization of separation performance using the same high aspect ratio rectangular channel structure by manipulation of outlet fluidic resistance, which was the main innovation of this paper. We have successfully demonstrated different modes of label-free, passive, and no-dilution blood separation in straight microfluidic channel devices with throughput rate ranged from 80 to 150 μl/min. The microfluidic constricted channel was made with 20 μm width and 50 μm height and 5 or 8 mm length. The microfluidic devices were fabricated using PDMS soft lithography. Separation efficiency was determined in cell count under optical microscopy. Taking advantage of blood margination phenomena, we demonstrated platelet or plasma extraction from undiluted whole blood through the manipulation of outlet fluidic resistance. In platelet extraction, high platelet purity of 95.4% and an extraction rate of 24.1% were achieved in single-pass operation. In plasma extraction, we demonstrated 99.9% RBC and 90% platelet removal rate in recirculation configuration. To improve extraction efficiency, the outlet sample was returned to the inlet by a peristaltic pump to continuously extract plasma. Using inertial focusing, we also demonstrated the concentration of platelet-rich plasma 2.6× to produce platelet concentrate with 97.8% purity.
To further enhance controllability and tunability of blood separation, on-chip flow constrictors based on PDMS deflection membrane,81 electrogate arrays,82 or liquid crystal83 can be integrated into the outlet channels to actively divert targeted species into collect outlets. The throughput of our blood separation approach is scalable by parallelization of microfluidic channels. Multi-step blood processing or complex sample preparation workflows, i.e., blood apheresis or sorting of WBC subtypes, can be implemented by connecting multiple inertial microfluidic devices with different sorting characteristics through some device interconnection efforts.84–87
SUPPLEMENTARY MATERIAL
See the supplementary material for demonstration of fluorescent labeled platelet margination at different flow rates and experimental setup of continuous plasma extraction from whole blood under a recirculation configuration.
ACKNOWLEDGMENTS
This research was sponsored by the Office of Naval Research (ONR) under Contract No. N00014-18-C-7004 and by the U.S. Army Medical Research Acquisition Activity (USAMRAA) under Contract No. W81XWH-17-C-0177. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Department of Defense. The authors acknowledge the support of the University of Michigan Lurie Nanofabrication Facility for SU-8 patterned silicon mold fabrication.
AUTHOR DECLARATIONS
Conflict of Interest
L.M.L., K.H.B., and B.P. are the authors of the pending patent PORTABLE PLATELET APHERESIS SYSTEM (USPTO Appl. No. 63/022,180).
Author Contributions
Lap Man Lee: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Visualization (equal); Writing – original draft (lead); Writing – review & editing (equal). Ketan H. Bhatt: Conceptualization (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Validation (equal); Writing – review & editing (equal). Dustin W. Haithcock: Resources (equal). Balabhaskar Prabhakarpandian: Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Supervision (equal); Writing – review & editing (equal).
DATA AVAILABILITY
The data that support the findings of this study are available within the article and its supplementary material.
REFERENCES
- 1.Mielczarek W. S., Obaje E. A., Bachmann T. T., and Kersaudy-Kerhoas M., “Microfluidic blood plasma separation for medical diagnostics: Is it worth it?,” Lab Chip 16, 3441–3448 (2016). 10.1039/C6LC00833J [DOI] [PubMed] [Google Scholar]
- 2.Cheng G., “Circulating miRNAs: Roles in cancer diagnosis, prognosis and therapy,” Adv. Drug Delivery Rev. 81, 75–93 (2015). 10.1016/j.addr.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 3.Johnson-Wimbley T. D. and Graham D. Y., “Diagnosis and management of iron deficiency anemia in the 21st century,” Ther. Adv. Gastroenterol. 4, 177–184 (2011). 10.1177/1756283X11398736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neugebauer S. et al. , “Metabolite profiles in sepsis: Developing prognostic tools based on the type of infection,” Crit. Care Med. 44, 1649–1662 (2016). 10.1097/CCM.0000000000001740 [DOI] [PubMed] [Google Scholar]
- 5.Bettini A., Lapa D., and Garbuglia A. R., “Diagnostics of Ebola virus,” Front. Public Health 11, 1123024 (2023). 10.3389/fpubh.2023.1123024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone M. et al. , “Standardized evaluation of Zika nucleic acid tests used in clinical settings and blood screening,” PLoS Negl. Trop. Dis. 17, e0011157 (2023). 10.1371/journal.pntd.0011157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H. et al. , “SARS-CoV-2 nucleocapsid plasma antigen for diagnosis and monitoring of COVID-19,” Clin. Chem. 68, 204–213 (2021). 10.1093/clinchem/hvab216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroncek D. F. and Rebulla P., “Platelet transfusions,” Lancet 370, 427–438 (2007). 10.1016/S0140-6736(07)61198-2 [DOI] [PubMed] [Google Scholar]
- 9.Freireich E. J., “Origins of platelet transfusion therapy,” Transfus. Med. Rev. 25, 252–256 (2011). 10.1016/j.tmrv.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Holcomb J. B. et al. , “Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial,” JAMA 313, 471–482 (2015). 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher A. D., Miles E. A., Cap A. P., Strandenes G., and Kane S. F., “Tactical damage control resuscitation,” Mil. Med. 180, 869–875 (2015). 10.7205/MILMED-D-14-00721 [DOI] [PubMed] [Google Scholar]
- 12.Eppley B. L., Woodell J. E., and Higgins J., “Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing,” Plast. Reconstr. Surg. 114, 1502–1508 (2004). 10.1097/01.PRS.0000138251.07040.51 [DOI] [PubMed] [Google Scholar]
- 13.Anitua E., Pino A., and Orive G., “Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity,” J. Wound Care 25, 680–687 (2016). 10.12968/jowc.2016.25.11.680 [DOI] [PubMed] [Google Scholar]
- 14.Barbieri A. et al. , “The effects of the use of platelet-rich plasma gel on local recurrence in an animal model of human fibrosarcoma,” Infect. Agents Cancer 14, 21 (2019). 10.1186/s13027-019-0237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greist A., “The role of blood component removal in essential and reactive thrombocytosis,” Ther. Apher. 6, 36–44 (2002). 10.1046/j.1526-0968.2002.00394.x [DOI] [PubMed] [Google Scholar]
- 16.Cui F., Rhee M., Singh A., and Tripathi A., “Microfluidic sample preparation for medical diagnostics,” Annu. Rev. Biomed. Eng. 17, 267–286 (2015). 10.1146/annurev-bioeng-071114-040538 [DOI] [PubMed] [Google Scholar]
- 17.Shimizu H., Kumagai M., Mori E., Mawatari K., and Kitamori T., “Whole blood analysis using microfluidic plasma separation and enzyme-linked immunosorbent assay devices,” Anal. Methods 8, 7597–7602 (2016). 10.1039/C6AY01779G [DOI] [Google Scholar]
- 18.Hauser J., Lenk G., Hansson J., Beck O., Stemme G., and Roxhed N., “High-yield passive plasma filtration from human finger prick blood,” Anal. Chem. 90, 13393–13399 (2018). 10.1021/acs.analchem.8b03175 [DOI] [PubMed] [Google Scholar]
- 19.Lopresti F., Keraite I., Ongaro A. E., Howarth N. M., La Carrubba V., and Kersaudy-Kerhoas M., “Engineered membranes for residual cell trapping on microfluidic blood plasma separation systems. A comparison between porous and nanofibrous membranes,” Membranes 11, 680 (2021). 10.3390/membranes11090680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baillargeon K. R., Bricknell J. R., and Mace C. R., “In situ hemolysis in a three-dimensional paper-based device for quantification of intraerythrocytic analytes,” Anal. Methods 12, 281–287 (2020). 10.1039/C9AY02292A [DOI] [Google Scholar]
- 21.Lippi G., Salvagno G. L., Montagnana M., Brocco G., and Guidi G. C., “Influence of hemolysis on routine clinical chemistry testing,” Clin. Chem. Lab. Med. 44, 311–316 (2006). 10.1515/CCLM.2006.054 [DOI] [PubMed] [Google Scholar]
- 22.Marques-Garcia F., “Methods for hemolysis interference study in laboratory medicine—A critical review,” EJIFCC 31, 85–97 (2020). [PMC free article] [PubMed] [Google Scholar]
- 23.Kirschner M. B. et al. , “Haemolysis during sample preparation alters microRNA content of plasma,” PLoS ONE 6, e24145 (2011). 10.1371/journal.pone.0024145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu N. et al. , “Label-free microfluidic cell sorting and detection for rapid blood analysis,” Lab Chip 23, 1226–1257 (2023). 10.1039/D2LC00904H [DOI] [PubMed] [Google Scholar]
- 25.Fåhræus R. and Lindqvist T., “The viscosity of the blood in narrow capillary tubes,” Am. J. Physiol. Legacy Content 96, 562–568 (1931). 10.1152/ajplegacy.1931.96.3.562 [DOI] [Google Scholar]
- 26.Hou H. W., Gan H. Y., Bhagat A. A. S., Li L. D., Lim C. T., and Han J., “A microfluidics approach towards high-throughput pathogen removal from blood using margination,” Biomicrofluidics 6, 024115 (2012). 10.1063/1.4710992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou H. W. et al. , “Broad spectrum immunomodulation using biomimetic blood cell margination for sepsis therapy,” Lab Chip 16, 688–699 (2016). 10.1039/C5LC01110H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou H. W. et al. , “Deformability based cell margination—A simple microfluidic design for malaria-infected erythrocyte separation,” Lab Chip 10, 2605–2613 (2010). 10.1039/c003873c [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Feng Y., Wan J., and Chen H., “Enhanced separation of aged RBCs by designing channel cross section,” Biomicrofluidics 12, 024106 (2018). 10.1063/1.5024598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shevkoplyas S. S., Yoshida T., Munn L. L., and Bitensky M. W., “Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device,” Anal. Chem. 77, 933–937 (2005). 10.1021/ac049037i [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain A. and Munn L. L., “Determinants of leukocyte margination in rectangular microchannels,” PLoS One 4, e7104 (2009). 10.1371/journal.pone.0007104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laxmi V., Tripathi S., Joshi S. S., and Agrawal A., “Separation and enrichment of platelets from whole blood using a PDMS-based passive microdevice,” Ind. Eng. Chem. Res. 59, 4792–4801 (2020). 10.1021/acs.iecr.0c00502 [DOI] [Google Scholar]
- 33.Yang S., Undar A., and Zahn J. D., “A microfluidic device for continuous, real time blood plasma separation,” Lab Chip 6, 871–880 (2006). 10.1039/B516401J [DOI] [PubMed] [Google Scholar]
- 34.Faivre M., Abkarian M., Bickraj K., and Stone H. A., “Geometrical focusing of cells in a microfluidic device: An approach to separate blood plasma,” Biorheology 43, 147–159 (2006). [PubMed] [Google Scholar]
- 35.Laxmi V., Joshi S. S., and Agrawal A., “Biophysical phenomenon-based separation of platelet-poor plasma from blood,” Ind. Eng. Chem. Res. 60, 7464–7473 (2021). 10.1021/acs.iecr.1c00659 [DOI] [Google Scholar]
- 36.Mach A. J. and Di Carlo D., “Continuous scalable blood filtration device using inertial microfluidics,” Biotechnol. Bioeng. 107, 302–311 (2010). 10.1002/bit.22833 [DOI] [PubMed] [Google Scholar]
- 37.Wu Z., Willing B., Bjerketorp J., Jansson J. K., and Hjort K., “Soft inertial microfluidics for high throughput separation of bacteria from human blood cells,” Lab Chip 9, 1193–1199 (2009). 10.1039/b817611f [DOI] [PubMed] [Google Scholar]
- 38.Di Carlo D., Irimia D., Tompkins R. G., and Toner M., “Continuous inertial focusing, ordering, and separation of particles in microchannels,” Proc. Natl. Acad. Sci. U.S.A. 104, 18892–18897 (2007). 10.1073/pnas.0704958104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., Yan S., Li W., Alici G., and Nguyen N.-T., “High throughput extraction of plasma using a secondary flow-aided inertial microfluidic device,” RSC Adv. 4, 33149–33159 (2014). 10.1039/C4RA06513A [DOI] [Google Scholar]
- 40.Zhou Y., Ma Z., and Ai Y., “Sheathless inertial cell focusing and sorting with serial reverse wavy channel structures,” Microsyst. Nanoeng. 4, 5 (2018). 10.1038/s41378-018-0005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sollier E. et al. , “Size-selective collection of circulating tumor cells using vortex technology,” Lab Chip 14, 63–77 (2014). 10.1039/C3LC50689D [DOI] [PubMed] [Google Scholar]
- 42.Dhar M. et al. , “High efficiency vortex trapping of circulating tumor cells,” Biomicrofluidics 9, 064116 (2015). 10.1063/1.4937895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Che J. et al. , “Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic vortex technology,” Oncotarget 7, 12748–12760 (2016). 10.18632/oncotarget.7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M. G., Shin J. H., Bae C. Y., Choi S., and Park J.-K., “Label-free cancer cell separation from human whole blood using inertial microfluidics at low shear stress,” Anal. Chem. 85, 6213–6218 (2013). 10.1021/ac4006149 [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Yang X., and Papautsky I., “An integrated inertial microfluidic vortex sorter for tunable sorting and purification of cells,” Technology 04, 88–97 (2016). 10.1142/S2339547816400112 [DOI] [Google Scholar]
- 46.Kuntaegowdanahalli S. S., Bhagat A. A. S., Kumarb G., and Papautsky I., “Inertial microfluidics for continuous particle separation in spiral microchannels,” Lab Chip 9, 2973–2980 (2009). 10.1039/b908271a [DOI] [PubMed] [Google Scholar]
- 47.Nivedita N. and Papautsky I., “Continuous separation of blood cells in spiral microfluidic devices,” Biomicrofluidics 7, 054101 (2013). 10.1063/1.4819275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B.-W. et al. , “Enhanced separation efficiency and purity of circulating tumor cells based on the combined effects of double sheath fluids and inertial focusing,” Front. Bioeng. Biotechnol. 9, 750444 (2021). 10.3389/fbioe.2021.750444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodríguez-Pena A. et al. , “Design and validation of a tunable inertial microfluidic system for the efficient enrichment of circulating tumor cells in blood,” Bioeng. Transl. Med. 7, e10331 (2022). 10.1002/btm2.10331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryu H., Choi K., Qu Y., Kwon T., Lee J. S., and Han J., “Patient-derived airway secretion dissociation technique to isolate and concentrate immune cells using closed-loop inertial microfluidics,” Anal. Chem. 89, 5549–5556 (2017). 10.1021/acs.analchem.7b00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tay H. M., Yeap W. H., Dalan R., Wong S. C., and Hou H. W., “Multiplexed label-free fractionation of peripheral blood mononuclear cells for identification of monocyte-platelet aggregates,” Anal. Chem. 90, 14535–14542 (2018). 10.1021/acs.analchem.8b04415 [DOI] [PubMed] [Google Scholar]
- 52.Zhu S., Wu D., Han Y., Wang C., Xiang N., and Ni Z., “Inertial microfluidic cube for automatic and fast extraction of white blood cells from whole blood,” Lab Chip 20, 244–252 (2020). 10.1039/C9LC00942F [DOI] [PubMed] [Google Scholar]
- 53.Jeon H. et al. , “Fully-automated and field-deployable blood leukocyte separation platform using multi-dimensional double spiral (MDDS) inertial microfluidics,” Lab Chip 20, 3612–3624 (2020). 10.1039/D0LC00675K [DOI] [PubMed] [Google Scholar]
- 54.Sarkar A., Hou H. W., Mahan A. E., Han J., and Alter G., “Multiplexed affinity-based separation of proteins and cells using inertial microfluidics,” Sci. Rep. 6, 23589 (2016). 10.1038/srep23589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi K. et al. , “Negative selection by spiral inertial microfluidics improves viral recovery and sequencing from blood,” Anal. Chem. 90, 4657–4662 (2018). 10.1021/acs.analchem.7b05200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tay H. M. et al. , “Direct isolation of circulating extracellular vesicles from blood for vascular risk profiling in type 2 diabetes mellitus,” Lab Chip 21, 2511–2523 (2021). 10.1039/D1LC00333J [DOI] [PubMed] [Google Scholar]
- 57.Xiang N. and Ni Z., “High-throughput blood cell focusing and plasma isolation using spiral inertial microfluidic devices,” Biomed. Microdevices 17, 110 (2015). 10.1007/s10544-015-0018-y [DOI] [PubMed] [Google Scholar]
- 58.Rafeie M., Zhang J., Asadnia M., Li W., and Warkiani M. E., “Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation,” Lab Chip 16, 2791–2802 (2016). 10.1039/C6LC00713A [DOI] [PubMed] [Google Scholar]
- 59.Robinson M., Marks H., Hinsdale T., Maitland K., and Coté G., “Rapid isolation of blood plasma using a cascaded inertial microfluidic device,” Biomicrofluidics 11, 024109 (2017). 10.1063/1.4979198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng H., Patel D., Magda J. J., Geher S., Sigala P. A., and Gale B. K., “Multiple-streams focusing-based cell separation in high viscoelasticity flow,” ACS Omega 7, 41759–41767 (2022). 10.1021/acsomega.2c06021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faridi M. A., Ramachandraiah H., Banerjee I., Ardabili S., Zelenin S., and Russom A., “Elasto-inertial microfluidics for bacteria separation from whole blood for sepsis diagnostics,” J. Nanobiotechnol. 15, 3 (2017). 10.1186/s12951-016-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iyengar S. N., Kumar T., Mårtensson G., and Russom A., “High resolution and rapid separation of bacteria from blood using elasto-inertial microfluidics,” Electrophoresis 42, 2538–2551 (2021). 10.1002/elps.202100140 [DOI] [PubMed] [Google Scholar]
- 63.Zhang T. et al. , “Microfluidic separation and enrichment of Escherichia coli by size using viscoelastic flows,” Anal. Chem. 95, 2561–2569 (2023). 10.1021/acs.analchem.2c05084 [DOI] [PubMed] [Google Scholar]
- 64.Bai J.-J. et al. , “Dean-flow-coupled elasto-inertial focusing accelerates exosome purification to facilitate single vesicle profiling,” Anal. Chem. 95, 2523–2531 (2023). 10.1021/acs.analchem.2c04898 [DOI] [PubMed] [Google Scholar]
- 65.Dai Y. et al. , “Enhanced blood plasma extraction utilising viscoelastic effects in a serpentine microchannel,” Biosensors 12, 120 (2022). 10.3390/bios12020120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sethu P., Sin A., and Toner M., “Microfluidic diffusive filter for apheresis (leukapheresis),” Lab Chip 6, 83–89 (2006). 10.1039/B512049G [DOI] [PubMed] [Google Scholar]
- 67.Lezzar D. L., Lam F. W., Huerta R., Mukhamedshin A., Lu M., and Shevkoplyas S. S., “A high-throughput microfluidic device based on controlled incremental filtration to enable centrifugation-free, low extracorporeal volume leukapheresis,” Sci. Rep. 12, 13798 (2022). 10.1038/s41598-022-16748-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abhishek K. et al. , “Red blood cell rosetting enables size-based separation of specific lymphocyte subsets from blood in a microfluidic device,” Lab Chip 23, 1804–1815 (2023). 10.1039/D2LC00817C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia H., Strachan B. C., Gifford S. C., and Shevkoplyas S. S., “A high-throughput microfluidic approach for 1000-fold leukocyte reduction of platelet-rich plasma,” Sci. Rep. 6, 35943 (2016). 10.1038/srep35943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gifford S. C. et al. , “A portable system for processing donated whole blood into high quality components without centrifugation,” PLoS ONE 13, e0190827 (2018). 10.1371/journal.pone.0190827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhagat A. A. S., Kuntaegowdanahalli S. S., and Papautsky I., “Inertial microfluidics for continuous particle filtration and extraction,” Microfluid. Nanofluid. 7, 217–226 (2009). 10.1007/s10404-008-0377-2 [DOI] [Google Scholar]
- 72.Lam E. W., Cooksey G. A., Finlayson B. A., and Folch A., “Microfluidic circuits with tunable flow resistances,” Appl. Phys. Lett. 89, 164105 (2006). 10.1063/1.2363931 [DOI] [Google Scholar]
- 73.Aarts P. A., Van den Broek S. A., Prins G. W., Kuiken G. D., Sixma J. J., and Heethaar R. M., “Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood,” Arterioscler. Off. J. Am. Heart Assoc. Inc. 8, 819–824 (1988). 10.1161/01.atv.8.6.819 [DOI] [PubMed] [Google Scholar]
- 74.Eckstein E. C., Tilles A. W., and Millero F. J. III, “Conditions for the occurrence of large near-wall excesses of small particles during blood flow,” Microvasc. Res. 36, 31–39 (1988). 10.1016/0026-2862(88)90036-2 [DOI] [PubMed] [Google Scholar]
- 75.Chang H.-Y., Yazdani A., Li X., Douglas K. A. A., Mantzoros C. S., and Karniadakis G. E., “Quantifying platelet margination in diabetic blood flow,” Biophys. J. 115, 1371–1382 (2018). 10.1016/j.bpj.2018.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugihara-Seki M. and Takinouchi N., “Margination of platelet-sized particles in the red blood cell suspension flow through square microchannels,” Micromachines 12, 1175 (2021). 10.3390/mi12101175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tilles A. W. and Eckstein E. C., “The near-wall excess of platelet-sized particles in blood flow: Its dependence on hematocrit and wall shear rate,” Microvasc. Res. 33, 211–223 (1987). 10.1016/0026-2862(87)90018-5 [DOI] [PubMed] [Google Scholar]
- 78.Müller K., Fedosov D. A., and Gompper G., “Margination of micro- and nano-particles in blood flow and its effect on drug delivery,” Sci. Rep. 4, 4871 (2014). 10.1038/srep04871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Machado E. S. et al. , “A simple double-spin closed method for preparing platelet-rich plasma,” Cureus 14, e20899 (2022). 10.7759/cureus.20899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrison T. E., Bowler J., Levins T. N., Cheng A.-L., and Reeves K. D., “Platelet yield and yield consistency for six single-spin methods of platelet rich plasma preparation,” Platelets 31, 661–666 (2020). 10.1080/09537104.2019.1663808 [DOI] [PubMed] [Google Scholar]
- 81.Lee L. M., Lee J. W., Chase D., Gebrezgiabhier D., and Liu A. P., “Development of an advanced microfluidic micropipette aspiration device for single cell mechanics studies,” Biomicrofluidics 10, 054105 (2016). 10.1063/1.4962968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salva M. L., Temiz Y., Rocca M., Arango Y. C., Niemeyer C. M., and Delamarche E., “Programmable hydraulic resistor for microfluidic chips using electrogate arrays,” Sci. Rep. 9, 17242 (2019). 10.1038/s41598-019-53885-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sengupta A., “Tuning fluidic resistance via liquid crystal microfluidics,” Int. J. Mol. Sci. 14, 22826–22844 (2013). 10.3390/ijms141122826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee L. M. et al. , “Label-free enrichment of human adipose-derived stem cells using a continuous microfluidic sorting cascade,” Lab Chip 23, 2131–2140 (2023). 10.1039/D2LC01138G [DOI] [PubMed] [Google Scholar]
- 85.Jundi B. et al. , “Leukocyte function assessed via serial microlitre sampling of peripheral blood from sepsis patients correlates with disease severity,” Nat. Biomed. Eng. 3, 961–973 (2019). 10.1038/s41551-019-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castaño N., Kim S., Martin A. M., Galli S. J., Nadeau K. C., and Tang S. K. Y., “Exponential magnetophoretic gradient for the direct isolation of basophils from whole blood in a microfluidic system,” Lab Chip 22, 1690–1701 (2022). 10.1039/D2LC00154C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fachin F. et al. , “Monolithic chip for high-throughput blood cell depletion to sort rare circulating tumor cells,” Sci. Rep. 7, 10936 (2017). 10.1038/s41598-017-11119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplementary material for demonstration of fluorescent labeled platelet margination at different flow rates and experimental setup of continuous plasma extraction from whole blood under a recirculation configuration.
Data Availability Statement
The data that support the findings of this study are available within the article and its supplementary material.