Abstract
Two 3-alkyl-substituted 2-hydroxy-1,4-naphthoquinones, NSC 113452 (NSC52) and NSC 113455 (NSC55), were evaluated for activity against Toxoplasma gondii in vitro and in murine models of acute toxoplasmosis. In vitro, both NSC52 and NSC55 significantly inhibited intracellular replication of T. gondii. In vivo, each compound was examined alone and in combination with other drugs currently used for treatment of human toxoplasmosis. Although none of the compounds protected mice against death when administered orally, both were significantly protective when administered intraperitoneally. In addition, a significant increase in survival was observed when suboptimal doses of each compound were used in combination with suboptimal doses of pyrimethamine or sulfadiazine. These results indicate that combinations of NSC52 or NSC55 with pyrimethamine or sulfadiazine have promising activity against T. gondii.
The therapy of choice for toxoplasmosis is the synergistic combination of pyrimethamine plus a sulfonamide or pyrimethamine plus clindamycin (14). This combination is effective for treatment of immunocompetent patients (7). However, it frequently fails in immunodeficient individuals who develop side effects that are of sufficient severity to require discontinuation of one or both of the drugs (13, 14). For this reason, a continued search for new therapies and/or new therapeutic approaches for treatment of human toxoplasmosis, particularly toxoplasmic encephalitis, has been a high priority in our laboratory (1–6, 11). We have previously demonstrated that the hydroxynaphthoquinone atovaquone is remarkably active against Toxoplasma gondii, both in vitro and in vivo (1, 2). It also demonstrates enhanced activity when combined with pyrimethamine or sulfadiazine (4). Hydroxynaphthoquinones containing a cyclohexyl moiety are metabolized via hydroxylation at the 4 position of the cyclohexyl ring. The secondary alcohols resulting from this process are less active against Plasmodium species. In contrast, a variety of lipophilic 4 substituents that avoided rapid metabolism demonstrated potent activity against Plasmodium falciparum, Eimeria tenella, and Theileria parva (9). Thus, we considered it of interest to examine a number of related 3-alkyl-substituted 2-hydroxy-1,4-naphthoquinones for in vitro activity against T. gondii. Two of these compounds, a 3-(4-cycloheptylphenyl)propyl-2-hydroxy-1,4-naphthoquinone termed NSC 113452 (NSC52) and a 3-(4-cyclohexylphenyl)propyl-2-hydroxy-1,4-naphthoquinone termed NSC 113455 (NSC55) (Fig. 1), significantly inhibited replication of intracellular T. gondii tachyzoites and were therefore chosen for further evaluation by using murine models of acute toxoplasmosis. Because it is unlikely that a single drug will be effective in all forms of toxoplasmosis (10) and atovaquone previously demonstrated enhanced activity in combination with pyrimethamine or sulfadiazine (4), we also examined combinations of the two new hydroxynaphthoquinones with these drugs.
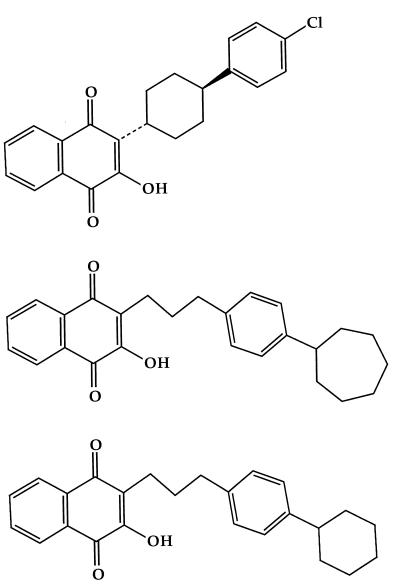
FIG. 1.
Chemical structures of atovaquone (top), NSC52 (middle), and NSC55 (bottom).
MATERIALS AND METHODS
T. gondii.
Tachyzoites of the RH strain were obtained from the peritoneal cavities of mice infected for 2 days with the parasite and were used for in vitro and in vivo evaluations as previously described (1). Tissue cysts of the C56 strain were obtained from the brains of chronically infected mice and also used to evaluate the compounds following oral infection of mice as previously described (6).
Cells.
Human foreskin fibroblasts (HFF; ATCC CRL1635) were grown in Dulbecco’s modified Eagle medium (DMEM; Gibco Bethesda Research Laboratories, Grand Island, N.Y.) containing 100 U of penicillin per ml; 1 μg of streptomycin per ml, and 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah).
Mice.
Outbred female Swiss Webster mice (Simonsen Laboratories, Gilroy, Calif.) weighing approximately 20 g at the beginning of each experiment were used. Food and water were available to the mice at all times.
Drugs.
NSC52 and NSC55 were provided by the Drug Synthesis and Chemistry Branch, National Cancer Institute, Bethesda, Md. Compounds were selected from a large pool of naphthoquinones by utilizing computerized substructure searching of the National Cancer Institute database files. Atovaquone and pyrimethamine were obtained from Burroughs Wellcome Co. (Research Triangle Park, N.C.), and sulfadiazine was obtained from City Chemical Co. (New York, N.Y.). Each drug was dissolved in a small volume of dimethyl sulfoxide (DMSO) and brought to the required volume with DMEM to prepare a 0.1 M stock solution for the in vitro assays. All dilutions were made with complete DMEM to the desired concentration. The final DMSO concentration in solution was less than 1%. For oral or intraperitoneal (i.p.) administration, both NSC52 and NSC55 were dissolved in sterile phosphate-buffered saline. Atovaquone was suspended in 0.25% carboxymethyl cellulose and sonicated for oral administration. Pyrimethamine was dissolved in 0.25% carboxymethyl cellulose for oral administration. Sulfadiazine was dissolved in double-distilled water and administered to mice in drinking water.
In vitro studies.
NSC52 and NSC55 were examined at concentrations of 0.01 to 10 μM for both activity against T. gondii and toxicity to HFF cells. Atovaquone was used at the same concentrations for comparison. In vitro activity was defined as the capacity of the drug to inhibit intracellular replication of T. gondii and was determined by using a modification of the method reported earlier (3, 11). Briefly, HFF cells were plated at 104/well in 96-well flat-bottom tissue culture microtiter plates (Costar Corp., Cambridge, Mass.) and incubated at 37°C in a 5% CO2 incubator. After reaching confluence, monolayers were infected with 4 × 104 tachyzoites/well. Four hours following infection, monolayers were washed to remove extracellular parasites and various concentrations of the test drugs were added. Triplicate wells were used for each drug concentration. Addition of the drugs to the wells marked the starting time point. [3H]uracil (1 μCi/well) was added to each well 24 h prior to harvesting of the cells at 24, 48, and 72 h following addition of the test drugs, and the plates were incubated at 37°C until the endpoint was reached. At the time of harvest, medium was removed from the wells, and 1% sodium dodecyl sulfate (Sigma Chemical Co., St. Louis, Mo.) and 100-μg/ml unlabeled uracil (Sigma) were added to each well and incubated for 30 min at room temperature. Cells were harvested following addition of 100 μl of a 10% trichloroacetic acid solution (kept at 4°C) to each well. Each well was washed with an additional 100 μl of 10% trichloroacetic acid solution three separate times and finally washed with 95% ethanol. The filters were air dried, and radioactivity was counted in a scintillation counter after addition of 5 ml of liquid scintillation cocktail (Ready Safe; Beckman Instruments, Inc., Fullerton, Calif.). Infected monolayers treated with medium that contained the respective drug diluent alone served as a negative control, and atovaquone prepared as were NSC52 and NSC55 was used as a positive control.
Drug toxicity to HFF cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide cell proliferation assay by using a Cell Titer 96 Kit (Promega Corp., Madison, Wis.). Briefly, cells were plated in triplicate wells at 103/well. Following a 4-h incubation at 37°C in a 5% CO2 incubator, various dilutions of the test drugs were added. Four hours before each time point at 24, 48, and 72 h, 14 μl of the dye indicator solution was added. Following an additional 4 h of incubation, 100 μl of the solubilization-stop solution was added to each well. Plates were kept overnight in a sealed container with a humidified atmosphere, and the A570 read was in an automatic enzyme-linked immunosorbent assay plate reader.
In vivo studies.
The activity of NSC52 and NSC55 alone or in combination with sulfadiazine or pyrimethamine was investigated by using two murine models of acute toxoplasmosis. In one model, mice were infected i.p. with 2.5 × 103 tachyzoites of the RH strain, and in the other, mice were infected orally with 10 cysts of T. gondii C56 (3). Treatment of mice infected i.p. was initiated 24 h after infection. In mice infected orally, treatment was initiated 3 days after infection. In both groups, treatment lasted for 10 days. NSC52 or NSC55 was administered i.p. or orally; atovaquone and pyrimethamine were administered orally, and sulfadiazine was administered in drinking water. Mice were observed for 30 days from the day of infection for death and time to death. Uninfected mice were treated with NSC52 and NSC55 in parallel for the same duration as infected mice and observed for signs of drug toxicity such as piloerection, lethargy, loss of weight, or death.
Statistical analysis was performed by using survival tools for StatView version 4.02 (Abacus Concepts, Berkeley, Calif.). P values were obtained by the log-rank test of the Kaplan-Meier product limited survival analysis, and a value of ≤0.05 was considered to be statistically significant.
RESULTS
In vitro results.
In two separate experiments, NSC52 and NSC55 demonstrated significant inhibition of intracellular growth of T. gondii at concentrations of 0.01 to 10 μM following 24, 48, or 72 h of exposure. The 50% inhibitory concentrations of atovaquone (as a control), NSC52, and NSC55 following 48 h of exposure were 0.13, 0.11, and 0.10 μM, respectively (Fig. 2A, C, and E). Untreated parasites in the control monolayers grew normally, as indicated by increased uptake of [3H]uracil with time. Neither NSC52 nor NSC55 showed significant toxicity to the host cells, except at the highest concentration of 10 μM (Fig. 2B, D, and F). DMSO alone at concentrations of up to 10 μM did not have any effect on intracellular toxoplasmas.
FIG. 2.
Inhibition of replication of tachyzoites of the T. gondii RH within HFF cells by exposure to atovaquone (A), NSC52 (C), or NSC55 (E). Effect of exposure to atovaquone (B), NSC52 (D), or NSC55 (F) on the growth of HFF cells. Each point represents the mean plus the standard deviation of measurements of three wells.
In vivo results obtained with drugs administered alone.
Oral treatment of mice infected with RH tachyzoites with either NSC52 at 10, 50, or 100 mg/kg/day or NSC55 at 25 or 50 mg/kg/day did not result in significant protection against death (data not shown). In contrast, when NSC52 and NSC55 were administered i.p., the results revealed significant protection against death (Fig. 3A and B). Treatment with a 50-mg/kg/day or NSC52 resulted in 30% survival (P < 0.0001) (Fig. 3A). All control mice died by day 7 of infection. A 100-mg/kg/day dose of NSC52 administered i.p. appeared to be toxic, since 50% of the treated mice died at the same time as the untreated controls and the remaining mice died a few days later. Treatment of RH-infected mice with a 25- or 50-mg/kg/day dose of NSC55 administered i.p. resulted in 20% (P < 0.01) or 10% (P = 0.4) survival, respectively (Fig. 3B). All control mice died by day 8 of infection. A dose of 100 mg/kg/day caused earlier deaths of mice, as was noted with NSC52.
FIG. 3.
Effect of treatment with NSC52 (A) or NSC55 (B) administered i.p. on survival of mice infected i.p. with tachyzoites of T. gondii RH. Rx, drug; d, day.
Treatment of mice infected orally with cysts of the C56 strain with NSC52 at 25, 50, or 100 mg/kg/day administered i.p. resulted in no effect, a 4-day prolongation of the time to death (P = 0.003), or 50% survival (P < 0.001), respectively (Fig. 4A). Treatment of similarly infected mice with NSC55 at 25 or 50 mg/kg/day also administered i.p. resulted in no effect or a 6-day prolongation of the time to death (P = 0.001), respectively (Fig. 4B). An NSC55 dose of 100 mg/kg/day produced only a 2-day prolongation of the time to death (P = 0.86). Similar results were observed in two separate experiments. A parallel experiment to determine the effect of i.p. treatment of normal mice with NSC52 revealed that an NSC52 dose of 100 mg/kg/day was slightly toxic (one mouse died at day 15 of treatment). The same dose of NSC55 was toxic and caused 100% mortality (data not shown).
FIG. 4.
Effect of treatment with NSC52 (A) or NSC55 (B) administered i.p. on survival of mice infected orally with cysts of T. gondii C56. Rx, drug; d, day.
Results of combination therapy.
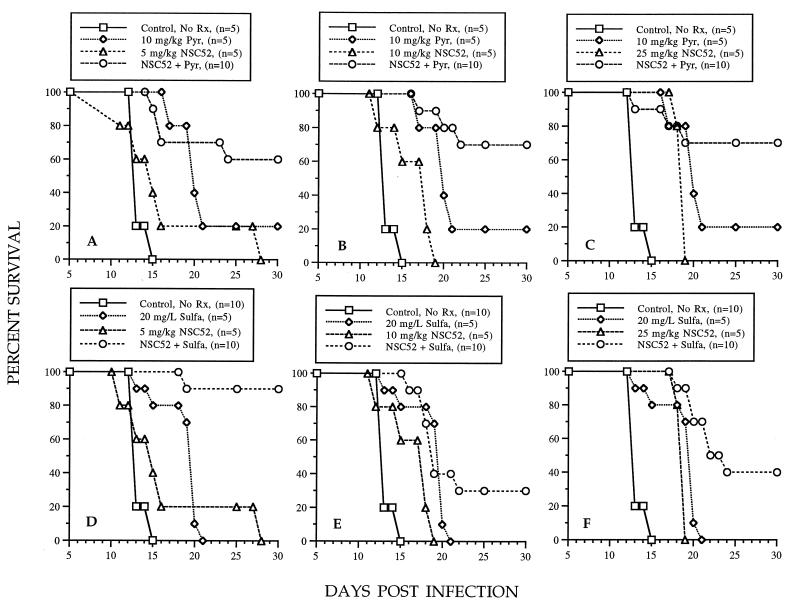
Because both NSC52 and NSC55 affected the survival of mice infected either i.p. with tachyzoites or orally with cysts similarly, we chose to evaluate drug combinations only in mice infected orally with cysts. In these experiments, a dose-response study revealed that an NSC52 dose of 5, 10, or 25 mg/kg/day caused a slight prolongation of the time to the death of mice. In contrast, at least 60% of the mice survived when any of these doses was used in combination with pyrimethamine at 10 mg/kg/day (Fig. 5A, B, and C). This dose of pyrimethamine administered alone protected only 20% of the mice. Treatment of mice with these same doses of NSC52 in combination with a sulfadiazine dose of 20 mg/liter resulted in 90, 30, and 40% survival, respectively (Fig. 5D, E, and F). Treatment with this dose of sulfadiazine administered alone resulted in a prolongation of the time to death that was not significant. In a separate experiment, uninfected mice were treated i.p. with NSC52 at 5 mg/kg/day plus sulfadiazine at 20 mg/liter in drinking water for 10 days to determine the toxicity of the combination. No signs of toxicity were noted in these mice during treatment and up to 20 days following its conclusion (data not shown).
FIG. 5.
Effect of treatment with NSC52 administered i.p. in combination with pyrimethamine (Pyr) administered orally (A, B, and C) or in combination with sulfadiazine (Sulfa) administered in drinking water (D, E, and F) on the survival of mice infected orally with cysts of T. gondii C56. P values for the combinations were 0.007, 0.0005, and 0.056, respectively, compared with respective NSC52 doses and 0.246, 0.056, and 0.167, respectively, compared with respective pyrimethamine doses (A, B, and C). P values for the combinations were 0.0001, 0.025, and 0.003, respectively, compared with respective NSC52 doses and 0.0002, 0.378, and 0.002, respectively, compared with respective sulfadiazine doses (D, E, and F). Rx, drug; d, day; L, liter.
A dose-response experiment with NSC55 also revealed that doses of 5, 10, and 25 mg/kg/day caused only a slight prolongation of the time to the death of mice. In contrast, treatment with these same doses in combination with a pyrimethamine dose of 10 mg/kg/day resulted in 70, 60, and 60% survival, respectively (Fig. 6A, B, and C). This dose of pyrimethamine administered alone protected only 20% of the mice against death. Treatment with these same doses of NSC55 in combination with a 20-mg/liter dose of sulfadiazine resulted in 50, 30, and 60% survival, respectively (Fig. 6D, E, and F). Sulfadiazine alone did not have a protective effect.
FIG. 6.
Effect of treatment with NSC55 administered i.p. in combination with pyrimethamine (Pyr) administered orally (A, B, and C) or in combination with sulfadiazine (Sulfa) administered in drinking water (D, E, and F) on the survival of mice infected orally with cysts of T. gondii C56. P values for the combinations were 0.001, 0.007, and 0.0003, respectively, compared with respective NSC55 doses and were not significant compared with respective pyrimethamine doses (A, B, and C). P values for the combinations were 0.0001, 0.078, and 0.0001, respectively, compared with respective NSC55 doses and 0.166, 0.762, and 0.0006, respectively, compared with respective sulfadiazine doses (D, E, and F). Rx, drug; d, day; L, liter.
DISCUSSION
Our results show that NSC52 and NSC55 had potent in vitro activity against T. gondii without a significant cytotoxic effect on host cells at concentrations that significantly inhibited intracellular multiplication of the parasite. Both compounds also demonstrated significant activities in protecting mice against death due to i.p. or oral infection with T. gondii. However, protection was noted only when the compounds were administered i.p.; oral administration was not effective. The reasons for this observation are not clear. Both compounds displayed toxicity to mice; an NSC52 dose of 100 mg/kg/day administered i.p. to mice infected i.p. with tachyzoites caused earlier deaths. Interestingly, this was not observed when the mice were infected orally with cysts. In contrast, an NSC55 dose of 100 mg/kg/day caused earlier deaths in mice infected i.p. with tachyzoites or orally with cysts.
Combination of doses of NSC52, NSC55, sulfadiazine, or pyrimethamine that were not protective or produced only marginal effects when administered alone were used to investigate synergistic activity. Thus, doses of NSC52 and NSC55 that were not effective and far smaller than the dose toxic for mice induced significant protection when combined with ineffective doses of pyrimethamine or sulfadiazine. Of interest was the finding that the dose of sulfadiazine needed to protect mice against death due to acute toxoplasmosis when used in combination with atovaquone was significantly lower than that which had been reported (4). Reduction of the dose of sulfadiazine may be important to eliminate the side effects that this drug causes in immunocompromised patients undergoing therapy for toxoplasmosis (14).
Drug combinations were investigated because it is highly unlikely that a single drug will be efficacious against all forms of toxoplasmosis, particularly in immunocompromised individuals. The best therapy against toxoplasmosis in these individuals is still the combination of pyrimethamine plus a sulfonamide (14). In addition, drug combinations may allow the use of lower doses with comparable or even better therapeutic results.
Drugs related to the 1,4-naphthoquinones NSC52 and NSC55, including atovaquone, have been shown to have potent activity against Theileria, Eimeria, and Plasmodium species (8). Atovaquone is remarkably active in murine toxoplasmosis and has been used to treat AIDS patients with toxoplasmic encephalitis (12). However, its relatively poor bioavailability causes variable levels in serum with consequent variability in efficacy. Thus, new formulations of atovaquone or related compounds with similar activity against T. gondii should be evaluated to find more efficient drugs for treatment of toxoplasmosis. Our results indicate that both NSC52 and NSC55 are active against T. gondii, particularly when combined with pyrimethamine or sulfadiazine. Further research with these compounds may disclose their usefulness for treatment of toxoplasmosis in humans.
ACKNOWLEDGMENTS
This work was supported by the Division of AIDS, National Institute of Allergy and Infectious Diseases, under contract N01-AI-35174.
We thank Teri Slifer, Limei Yang, Eddie Wehri, and Eric Ho for excellent technical help.
REFERENCES
- 1.Araujo F G, Huskinson J, Remington J S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo F G, Huskinson-Mark J, Gutteridge W E, Remington J S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992;36:326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo F G, Khan A A, Remington J S. Rifapentine is active in vitro and in vivo against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1335–1337. doi: 10.1128/aac.40.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo F G, Lin T, Remington J S. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J Infect Dis. 1993;167:494–497. doi: 10.1093/infdis/167.2.494. [DOI] [PubMed] [Google Scholar]
- 5.Araujo F G, Remington J S. Recent advances in the search for new drugs for treatment of toxoplasmosis. Int J Antimicrob Agents. 1992;1:153–164. doi: 10.1016/0924-8579(92)90002-9. [DOI] [PubMed] [Google Scholar]
- 6.Araujo F G, Slifer T, Remington J S. Rifabutin is active in models of murine toxoplasmosis. Antimicrob Agents Chemother. 1994;38:570–575. doi: 10.1128/aac.38.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaman M H, McCabe R E, Wong S-Y, Remington J S. Toxoplasma gondii. In: Mandel G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2455–2487. [Google Scholar]
- 8.Hudson A T, Pether M J, Randall A W. In vitro activity of 2-cycloalkyl-3-hydroxy-1,4-naphthoquinones against Theileria, Eimeria and Plasmodia species. Eur J Med Chem-Chim Ther. 1986;21:271–275. [Google Scholar]
- 9.Hudson A T, Randall A W, Fry M, Ginger C D, Hill B, Latter V S, McHardy N, Williams R B. Novel anti-malarial hydroxynaphthoquinones with potent broad-spectrum anti-protozoal activity. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- 10.Khan A A, Slifer T, Araujo F G, Polzer R J, Remington J S. Activity of trovafloxacin in combination with other drugs for treatment of acute murine toxoplasmosis. Antimicrob Agents Chemother. 1997;41:893–897. doi: 10.1128/aac.41.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A A, Slifer T, Araujo F G, Remington J S. Trovafloxacin is active against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1855–1859. doi: 10.1128/aac.40.8.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs J A. Efficacy of atovaquone in treatment of toxoplasmosis in patients with AIDS. Lancet. 1992;340:637–638. doi: 10.1016/0140-6736(92)92172-c. [DOI] [PubMed] [Google Scholar]
- 13.Wong S-Y, Israelski D M, Remington J S. AIDS associated toxoplasmosis. In: Sande M A, Volberding P, editors. The medical management of AIDS. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 460–493. [Google Scholar]
- 14.Wong S-Y, Remington J S. Toxoplasmosis in the setting of AIDS. In: Broder S, Merigan T C, Bolognesi D, editors. Textbook of AIDS medicine. Baltimore, Md: The Williams & Wilkins Co.; 1994. pp. 223–257. [Google Scholar]