Abstract
Background
The non-interventional PROPER study generated real-world evidence on clinical outcomes following transition in routine practice from reference adalimumab to the EMA-approved SB5 biosimilar adalimumab in patients with immune-mediated inflammatory disease.
Methods
Adults with rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), Crohn’s disease (CD), or ulcerative colitis (UC) were enrolled at 63 sites across Europe. Eligible patients received ≥ 16 weeks of routine treatment with reference adalimumab before transitioning to SB5, and were followed for 48 weeks post-transition. The primary objective was to evaluate candidate predictors (clinically relevant baseline variables with incidence ≥ 15% by indication cohort) associated with persistence on SB5 at 48 weeks post-initiation. Key primary outcome measures were persistence on SB5 (estimated by Kaplan–Meier methodology) and clinical characteristics and disease activity scores at the time of transition to SB5 treatment (baseline).
Results
A total of 955 eligible patients were enrolled (RA, n = 207; axSpA, n = 127; PsA, n = 162; CD, n = 447; UC, n = 12), of whom 932 (97.6%) completed follow-up and 722 (75.6%) were still receiving SB5 at week 48. Kaplan–Meier estimates (95% confidence interval, CI) of persistence on SB5 at week 48 for RA, axSpA, PsA, and CD were 0.86 (0.80–0.90), 0.80 (0.71–0.86), 0.81 (0.74–0.86), and 0.72 (0.67–0.76), respectively. The single candidate predictor associated with probability of SB5 discontinuation before week 48 was female sex [RA, axSpA, and CD cohorts; HR (95% CI): 3.53 (1.07–11.67), 2.38 (1.11–5.14), and 2.21 (1.54–3.18), respectively]. Disease activity scores remained largely unchanged throughout the study, with proportions by cohort in remission at baseline versus week 48 being 59.2% versus 57.2%, 81.0% versus 78.0%, 94.7% versus 93.7%, and 84.0% versus 85.1% for patients with RA, axSpA, PsA, and CD, respectively. Similarly, the SB5 dosing regimen remained unchanged for the majority of patients from baseline to week 48, the most common regimen being 40 mg every 2 weeks. In total, 232 patients (24.3%) reported at least one adverse drug reaction, and most events were mild; eight patients (3.9%) in the RA cohort experienced nine serious adverse events (SAEs; two possibly related to SB5); eight patients (4.9%) in the PsA cohort experienced nine SAEs (one possibly related to SB5); 22 patients (4.9%) in the CD cohort experienced 27 SAEs (four possibly related to SB5); and no SAEs were observed in the UC cohort.
Conclusions
With the exception of female sex in RA, axSpA, and CD, none of the candidate predictors were associated with SB5 discontinuation. Persistence on SB5 was high, treatment effectiveness was maintained, and no safety signals were detected.
Trial Registration
This trial is registered with ClinicalTrials.gov: NCT04089514.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40259-023-00616-3.
Key Summary Points
| The PROPER study of patients with immune-mediated inflammatory diseases was conducted to provide real-world evidence on clinical outcomes following transition from reference adalimumab to biosimilar SB5. |
| Transition to SB5 from reference adalimumab was safe and well tolerated, with over 75% of patients remaining on SB5 at 48 weeks post-transition, and disease control was maintained. No meaningful differences in outcomes between the various immune-mediated inflammatory diseases were observed. |
| The only variable associated with SB5 discontinuation in study patients transitioning to SB5 from reference adalimumab was female sex, in the rheumatoid arthritis, axial spondyloarthritis, and Crohn’s disease cohorts. |
Introduction
Tumor necrosis factor (TNF) is a cytokine involved in the pathophysiology of immune-mediated inflammatory diseases (IMIDs) such as rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), Crohn’s disease (CD), and ulcerative colitis (UC) [1–6]. The introduction of biologic disease-modifying anti-rheumatic drugs (DMARDs) such as TNF inhibitors (e.g., adalimumab, etanercept, infliximab, golimumab, certolizumab) as therapy options for patients with IMIDs has proven invaluable, conferring rapid suppression of inflammation, delaying or preventing disease progression, maintaining remission, and improving patient quality of life [7, 8]. However, access to these DMARDs can be limited at the country level due to high cost, and at the patient level by country-specific reimbursement criteria or the requirement for high patient co-payments [9, 10]. Timely and appropriate treatment reduces the risks of developing secondary IMIDs and myocardial infarction [11–13]. Thus, untreated or inadequately treated IMIDs may increase the overall disease burden at the patient, healthcare system, and socioeconomic levels [9, 10]. The development of biosimilars as cost-effective alternatives to the reference product may mitigate some of these limitations [7]. The potentially lower cost of biosimilars compared with reference products is likely to improve patient access, thereby reducing the disease burden for the patient [9].
SB5 (Imraldi™) has been developed as a biosimilar to reference adalimumab (Humira®) [14, 15], and in phase I and III studies has demonstrated equivalent efficacy and comparable pharmacokinetic, safety, and immunogenicity profiles as the reference adalimumab [16, 17]. SB5 was granted European Medicines Agency approval in August 2017 for the same indications as reference adalimumab [18], and became available for prescription in Europe in October 2018 [19]. Transitioning from reference adalimumab to SB5 had previously been evaluated in a randomized controlled trial setting with narrow eligibility criteria in a single indication (RA) [20]; long-term, real-world evidence in representative populations is needed [21].
The PROPER study was designed to provide real-world evidence on outcomes of transition from reference adalimumab to SB5 in routine clinical practice to inform and support decision-making for physicians, patients, health-technology assessment bodies, and other stakeholders.
Methods
Study Design and Patients
PROPER was a non-interventional, single-cohort, real-world study conducted at specialist clinics across six European countries (Germany, Spain, Italy, the United Kingdom, Belgium, and Ireland) via an umbrella approach. To enable and expedite data generation across multiple indications simultaneously, a single protocol was designed for flexible implementation to capture data on real-world use of SB5 in different therapeutic areas and specialities [22]. Patients aged ≥ 18 years at SB5 initiation and with a diagnosis of RA, axSpA, PsA, CD, or UC were enrolled. All patients had initiated SB5 after 18 October 2018 as part of routine management immediately after transitioning from ≥ 16 weeks of treatment with reference adalimumab. Additional eligibility criteria were the availability of at least one disease activity score assessment at baseline (time of SB5 initiation) and provision of informed consent to participate.
Data were captured retrospectively using patient charts from 24 weeks prior to SB5 initiation, and prospectively and/or retrospectively thereafter. Clinic visits were anticipated to take place approximately every 3 months (depending on local standards of care). Individual follow-up to 48 weeks after initiation of SB5 continued regardless of SB5 continuation/discontinuation prior to week 48.
The protocol, its amendment, and the patient-informed consent form were approved by the responsible Ethics Committees. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki [23] and all applicable local regulations, and is registered with ClinicalTrials.gov (NCT04089514).
Study Objectives and Outcome Measures
Primary Objective and Outcome Measures
The primary objective was to evaluate candidate predictors of persistence on SB5 in patients diagnosed with IMIDs, where persistence was defined as the time to SB5 discontinuation from initiation (in weeks). The candidate predictors were baseline clinical characteristics (sex, relevant medical history, age at SB5 initiation, disease duration, disease activity score, and relevant concomitant therapy). Disease activity scores were those used routinely at study sites: Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP; converted DAS28-CRP (DAS28-CRPconv) was derived from DAS28 using erythrocyte sedimentation rate where C-reactive protein was unavailable [24]) for RA and at some German sites for patients with PsA; Funktionsfragebogen Hannover (FFbH) for RA; Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for axSpA; Psoriatic Arthritis Response Criteria (PsARC) swollen joint count (of 66 joints) and tender joint count (of 68 joints) for PsA; the Harvey–Bradshaw Index (HBI) for CD, and the Partial Mayo Score (PMS) for UC [see electronic supplementary material (ESM) Table S1].
Disease flares occurring on or after SB5 initiation and before or at SB5 discontinuation were defined according to the investigator’s clinical opinion. The method of diagnosis (via assessment of disease activity score, patient-reported symptoms, or secondary loss of response) and the action taken as a result of the flare were recorded for each indication.
Secondary Objectives and Outcome Measures
Secondary objectives were to describe the study population in terms of baseline clinical characteristics, SB5 utilization, biologic drug effectiveness, patient satisfaction with the SB5 administration device, and use of relevant concomitant medications, as well as to describe the immunogenicity and safety of SB5. Secondary outcome measures included patient baseline and disease characteristics; type, dose, regimen, and method of SB5 administration throughout the study [pre-filled pen (pen) or pre-filled syringe (syringe)]; indication-appropriate disease activity scores over time (ESM Table S1); patient satisfaction questionnaire (PSQ) (ESM Table S2); patient-reported outcome measures (PROMs) (ESM Table S2); use of immunosuppressant and/or use of corticosteroid and/or biologic therapy (other than SB5); anti-adalimumab antibodies (ADAs); incidence of adverse drug reactions (ADRs), defined as non-serious adverse events (AEs) considered to have a causal relationship with SB5; and all serious AEs (SAEs). All AEs were coded using the Medical Dictionary for Regulatory Activities (version 23.0) [25] and were assessed to determine whether they met the SAE criteria (ESM Table S3).
Patient satisfaction with the SB5 administration device was recorded via a PSQ (ESM Table S2), which patients completed at baseline and at routine visits corresponding with study weeks 12, 24, and 48 or the end of the study, whichever occurred first.
PROMs captured between baseline and week 48 were Health Assessment Questionnaire–Disability Index for RA, axSpA and PsA, Bath Ankylosing Spondylitis Functional Index for axSpA, and Inflammatory Bowel Disease (IBD)-Control Questionnaire 8-item subscore and Visual Analog Scale score for CD and UC (ESM Table S2).
Statistical Analysis
Sample Size Calculation
The sample size was calculated by establishing a basic clinical prediction model for the primary outcome (i.e., evaluation of candidate predictors of persistence on SB5) (ESM Section 1.3 and Table S4) [26]. All analyses were conducted using data from all eligible patients who received at least one dose of SB5.
Analysis of the Primary Outcome Measure
For the primary outcome measure, the following clinically relevant variables reported at baseline for ≥ 15% of each cohort were selected as candidate predictors: age at SB5 initiation, sex, medical history, concomitant medication, disease duration, and disease activity score. The influence of these candidate predictors on SB5 persistence was assessed by univariate Cox regression analysis (separately for each indication). The hazard ratio (HR) for each candidate predictor and the corresponding 95% confidence intervals (CIs) were calculated. Time to SB5 discontinuation was estimated by indication and for each candidate predictor using the Kaplan–Meier method.
Analysis of Secondary Outcome Measures
Secondary outcome measures were analyzed descriptively. Continuous variables were reported as mean, standard deviation, median, interquartile range, 95% CI, and minimum and maximum, as appropriate. Categorical variables were summarized as frequencies and percentages. Disease activity and PROM scores were captured as absolute values per timepoint and are presented as change in paired values from baseline to weeks 12, 24, and 48. For analysis of disease activity scores and PROMs, visit windows were applied as follows: baseline, 16 weeks prior through to 6 weeks after the first dose of SB5; week 12, 7–18 weeks post-first dose of SB5; week 24, 19–36 weeks post-first dose of SB5; week 48, 37–60 weeks post-first dose of SB5.
For the PSQ, the baseline window was SB5 initiation to 6 weeks post-first dose of SB5. For analysis of adalimumab ADAs, baseline was defined as 16 weeks prior through to 2 weeks post-first dose of SB5, and post-baseline was defined as > 2 weeks through to 60 weeks post-first dose of SB5. When multiple baseline measurements were captured, only the measurement obtained closest to SB5 initiation was included in the analysis. If multiple post-baseline measurements were captured at a particular timepoint, only the worst-case measurement was included in the analysis.
All statistical analyses were performed using SAS version 9.4 or higher.
Results
Patients—Demographics and Clinical Characteristics
Of the 1033 patients enrolled across 63 sites (Germany, n = 19; Spain, n = 14; Italy, n = 11; UK, n = 10; Belgium, n = 8; and Ireland n = 1), 955 met the eligibility criteria: 207 with RA, 127 with axSpA, 162 with PsA, 447 with CD, and 12 with UC. Among these, 932 (97.6%) completed the study (ESM Fig. S1).
Patient demographics and baseline disease characteristics are presented in Table 1. The distribution of male/female sex was balanced in the PsA and CD cohorts; the axSpA and UC cohorts were predominantly male, and the RA cohort was predominantly female.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic data are n,a n (%), or mean ± SD | Disease cohort | Total (N = 955) | ||||
|---|---|---|---|---|---|---|
| RA (n = 207) | axSpA (n = 127) | PsA (n = 162) | CD (n = 447) | UC (n = 12) | ||
| Age (years) | ||||||
| At initiation of SB5 | 60.1 ± 11.8 | 50.3 ± 13.4 | 53.3 ± 12.0 | 43.2 ± 13.8 | 43.7 ± 15.4 | 49.5 ± 14.7 |
| n a | 195 | 113 | 144 | 439 | 903 | |
| At diagnosis | 44.4 ± 12.5 | 34.6 ± 12.7 | 40.4 ± 12.5 | 30.2 ± 12.6 | 35.5 ± 12.0 | 35.5 ± 13.9 |
| Sex | ||||||
| Female | 150 (72.5) | 40 (31.5) | 73 (45.1) | 205 (45.9) | 3 (25.0) | 471 (49.3) |
| Male | 57 (27.5) | 87 (68.5) | 89 (54.9) | 242 (54.1) | 9 (75.0) | 484 (50.7) |
| BMI category, kg/m2 | ||||||
| n a | 203 | 112 | 154 | 435 | 916 | |
| < 18.5 (underweight) | 3 (1.5) | 1 (0.9) | 0 | 12 (2.8) | 0 | 16 (1.7) |
| 18.5–29.9 (normal) | 160 (78.8) | 86 (76.8) | 111 (72.1) | 371 (85.3) | 11 (91.7) | 739 (80.7) |
| ≥ 30 (obese) | 40 (19.7) | 25 (22.3) | 43 (27.9) | 52 (12.0) | 1 (8.3) | 161 (17.6) |
| Work status | ||||||
| n a | 114 | 147 | 426 | 906 | ||
| Full-time | 71 (34.3) | 62 (54.4) | 80 (54.4) | 276 (64.8) | 9 (75.0) | 498 (55.0) |
| Part-time | 23 (11.1) | 16 (14.0) | 16 (10.9) | 34 (8.0) | 0 | 89 (9.8) |
| Unemployed | 113 (54.6) | 36 (31.6) | 51 (34.7) | 116 (27.2) | 3 (25.0) | 319 (35.2) |
| Tobacco use | ||||||
| n a | 121 | 155 | 436 | 931 | ||
| Current user | 36 (17.4) | 14 (11.6) | 18 (11.6) | 122 (28.0) | 2 (16.7) | 192 (20.6) |
| Ex-user | 42 (20.3) | 38 (31.4) | 28 (18.1) | 112 (25.7) | 2 (16.7) | 222 (23.8) |
| Non-user | 129 (62.3) | 69 (57.0) | 109 (70.3) | 202 (46.3) | 8 (66.7) | 517 (55.5) |
| Disease duration (years) | ||||||
| n a | 120 | 69 | 98 | 345 | 10 | 642 |
| 13.3 ± 11.4 | 18.8 ± 13.5 | 12.2 ± 9.9 | 13.5 ± 9.4 | 11.2 ± 11.3 | 13.8 ± 10.5 | |
| Clinical status as reported by physician | ||||||
| n a | 118 | 159 | 440 | 936 | ||
| Remission | 50 (24.2) | 25 (21.2) | 50 (31.4) | 233 (53.0) | 1 (8.3) | 359 (38.4) |
| Stable | 140 (67.6) | 83 (70.3) | 98 (61.6) | 168 (38.2) | 8 (66.7) | 497 (53.1) |
| Active disease | 17 (8.2) | 10 (8.5) | 11 (6.9) | 39 (8.9) | 3 (25.0) | 80 (8.5) |
| Clinical status based on disease activity score | ||||||
| n a | 191 | 116 | 131 | 430 | ||
| Remission/inactive disease | 113 (59.2) | – | 124 (94.7) | 361 (84.0) | 7 (58.4) | |
| Low/mild activity | 40 (20.9) | 94 (81.0) | – | 44 (10.2) | 4 (33.3) | |
| Moderate activity | 35 (18.3) | – | – | 23 (5.3) | 1 (8.3) | |
| High/severe activity | 3 (1.6) | – | – | 2 (0.5) | 0 | |
| Dose regimen of reference adalimumab/SB5 at transition | ||||||
| n a | 206 | 445 | 952 | |||
| 40 mg Q2W/40 mg Q2W | 138 (67.0) | 110 (86.6) | 148 (91.4) | 315 (70.8) | 6 (50.0) | 717 (75.3) |
| 40 mg other/40 mg other | 45 (21.8) | 7 (5.5) | 8 (4.9) | 36 (8.1) | 1 (8.3) | 97 (10.2) |
| Other dosing regimens b | 23 (11.2) | 10 (7.9) | 6 (3.7) | 94 (21.1) | 5 (41.7) | 138 (14.5) |
| Concomitant therapy received by > 5% of the total patient population | ||||||
| Concomitant therapy | 153 (73.9) | 78 (61.4) | 106 (65.4) | 229 (51.2) | 10 (83.3) | 576 (60.3) |
| Methotrexate c | 106 (51.2) | 15 (11.8) | 56 (34.6) | 13 (2.9) | 1 (8.3) | 191 (20.0) |
| Folic acid d | 38 (18.4) | 10 (7.9) | 29 (17.9) | 13 (2.9) | 1 (8.3) | 91 (9.5) |
| Mesalazine | 1 (0.5) | 8 (6.3) | 0 | 51 (11.4) | 7 (58.3) | 67 (7.0) |
| Azathioprine | 1 (0.5) | 3 (2.4) | 1 (0.6) | 46 (10.3) | 3 (25.0) | 54 (5.7) |
| Cholecalciferol | 16 (7.7) | 1 (0.8) | 5 (3.1) | 27 (6.0) | 0 | 49 (5.1) |
| Received information on self-administration of SB5 | ||||||
| n a | 109 | 150 | 446 | 924 | ||
| Yes | 185 (89.4) | 95 (87.2) | 138 (92.0) | 430 (96.4) | 6 (50.0) | 854 (92.4) |
| Aware that SB5 should be removed from the refrigerator 30 min prior to injection | ||||||
| n a | 107 | 149 | 446 | 921 | ||
| Yes | 186 (89.9) | 100 (93.5) | 143 (96.0) | 437 (98.0) | 11 (91.7) | 877 (95.2) |
| Aware that SB5 can be stored unrefrigerated below 25ºC for up to 28 days | ||||||
| n a | 107 | 149 | 446 | 921 | ||
| Yes | 162 (78.3) | 62 (57.9) | 110 (73.8) | 365 (81.8) | 6 (50.0) | 705 (76.5) |
| Reason for transition from reference adalimumab to SB5 | ||||||
| n a | 206 | 445 | 952 | |||
| Mandated by health authority/payer | 2 (1.0) | 100 (78.7) | 73 (45.1) | 151 (33.9) | 2 (16.7) | 328 (34.5) |
| Cost | 137 (66.5) | 15 (11.8) | 62 (38.3) | 69 (15.5) | 10 (83.3) | 293 (30.8) |
| Adverse event | 0 | 0 | 0 | 1 (0.2) | 0 | 1 (0.1) |
| Patient decision | 5 (2.4) | 0 | 3 (1.9) | 13 (2.9) | 0 | 21 (2.2) |
| Physician decision | 58 (28.2) | 10 (7.9) | 23 (14.2) | 182 (40.9) | 0 | 273 (28.7) |
| Other | 4 (1.9) | 2 (1.6) | 1 (0.6) | 29 (6.5) | 0 | 36 (3.8) |
axSpA axial spondyloarthritis, BMI body mass index, CD Crohn’s disease, PsA psoriatic arthritis, Q2W once every 2 weeks, RA rheumatoid arthritis, SD standard deviation, UC ulcerative colitis
aPatient numbers (n) are stated where data are not reported for the total N
bIncludes any dose/frequency other than 40 mg Q2W
cIncludes methotrexate and methotrexate sodium
dIncludes folic acid (n = 87, 9.1%) and folinic acid (a folic-acid derivative; n = 4, 0.4%)
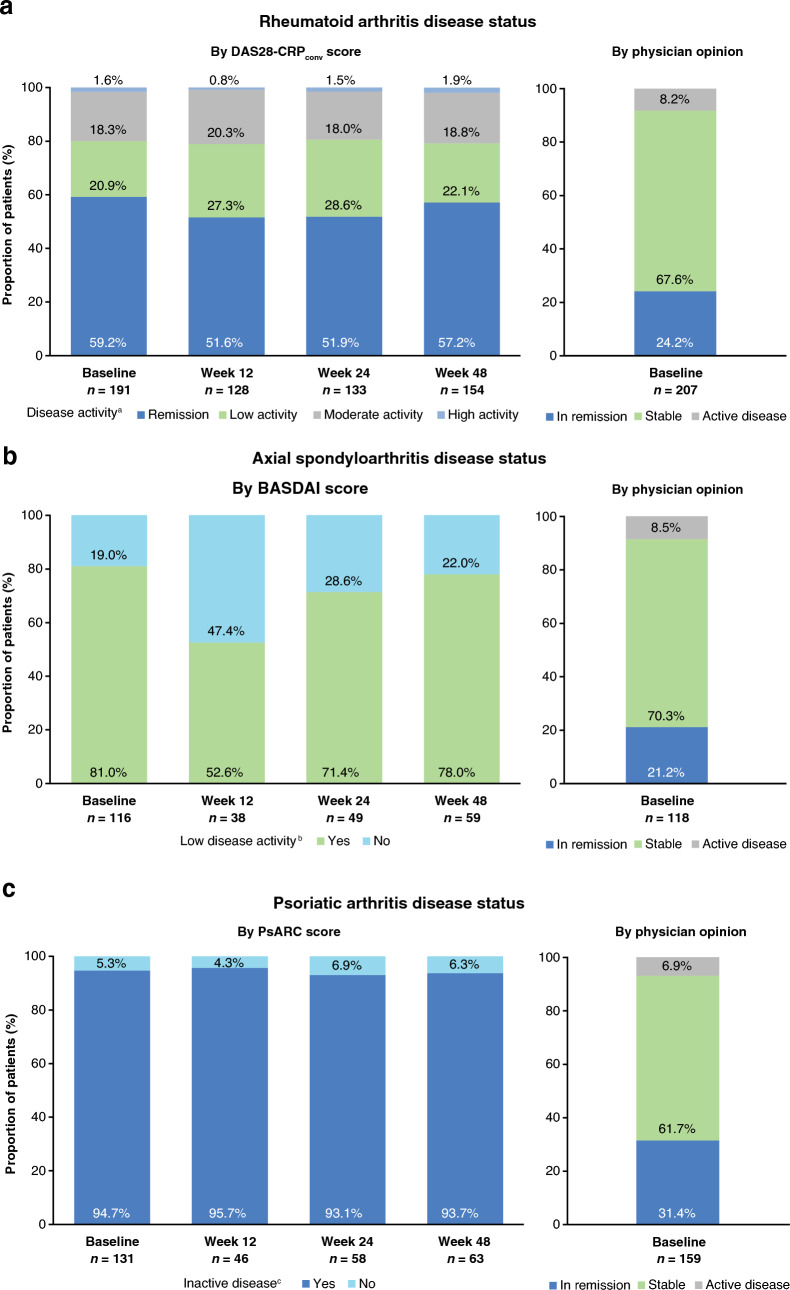
The main reasons for transition from reference adalimumab to SB5 varied by indication, and were: transition mandated by health authority/payer, cost, and physician decision. According to physician opinion, the majority of patients across all cohorts had stable disease (n = 497, 53.1%) or were in remission (n = 359, 38.4%) at baseline. Classification of disease status varied depending on whether defined by disease activity score or by physician opinion.
In the RA cohort, 59.2% of patients were in remission at baseline according to DAS28-CRPconv (score ≤ 2.4), and 24.2% and 67.6% were in remission and had stable disease, respectively, by physician opinion (Fig. 1a). In the axSpA cohort, 81.0% of patients had low disease activity scores (< 4.0) at baseline by BASDAI, and 21.2% and 70.3% were in remission and had stable disease by physician opinion, respectively (Fig. 1b). When baseline disease status was assessed using PsARC, 94.7% of patients with PsA had inactive disease (swollen joint or tender joint score < 3, with both scores available); according to physician opinion, 31.4% and 61.7% were considered to be in remission and to have stable disease, respectively (Fig. 1c). Among patients with CD, 84.0% of patients were in remission at baseline according to HBI (score < 5); according to physician opinion, 52.9% and 38.2% were considered to be in remission and to have stable disease, respectively (Fig. 1d). In the UC cohort, 58.4% of patients were in remission at baseline by PMS (score < 2), and 25.0% and 66.7% of patients were considered to be in remission and with stable disease, respectively, according to physician opinion (Fig. 1e). A summary of absolute disease activity scores and PROMs from baseline through to week 48 is provided in ESM Table S5.
Fig. 1.
Disease status at baseline by disease activity scorea,b,c,d,e and by physician opinion; a DAS28-CRPconv in patients with RA, b BASDAI in patients with axSpA, c PsARC in patients with PsA, d HBI in patients with CD, and e PMS in patients with UC. axSpA axial spondyloarthritis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CD Crohn’s disease, DAS28-CRPconv Disease Activity Score in 28 joints using C-reactive protein converted from Disease Activity Score in 28 joints using erythrocyte sedimentation rate, HBI Harvey–Bradshaw Index, PMS Partial Mayo Score, PsA psoriatic arthritis, PsARC Psoriatic Arthritis Response Criteria, RA rheumatoid arthritis, UC ulcerative colitis. aDAS28-CRPconv: remission, low activity, moderate activity, and high activity were defined by scores of ≤ 2.4, > 2.4–2.9, > 2.9–4.6, and > 4.6, respectively. bBASDAI: low disease activity was defined as a score of < 4. cPsARC: inactive disease was defined as swollen joint score < 3 or tender joint score < 3 (both scores had to be available). dHBI: remission, mild activity, moderate activity, and severe activity were defined by scores of < 5, 5–7, 8–16, and > 16, respectively. e PMS: remission, mild activity, moderate activity, and severe activity were defined by scores of < 2, 2–4, 5–7, and > 7, respectively
At baseline, the majority of patients across all cohorts received SB5 at a dosing regimen of 40 mg once every 2 weeks, remaining largely unchanged at week 48 (Table S6).
A total of 576 patients (60.3%) received at least one concomitant medication, of which the most commonly reported were methotrexate for the RA (51.2%), axSpA (11.8%), and PsA (34.6%) cohorts, and mesalazine for the CD (11.4%) and UC (58.3%) cohorts (Table 1).
Primary Objective—Evaluation of Candidate Predictors of Persistence on SB5
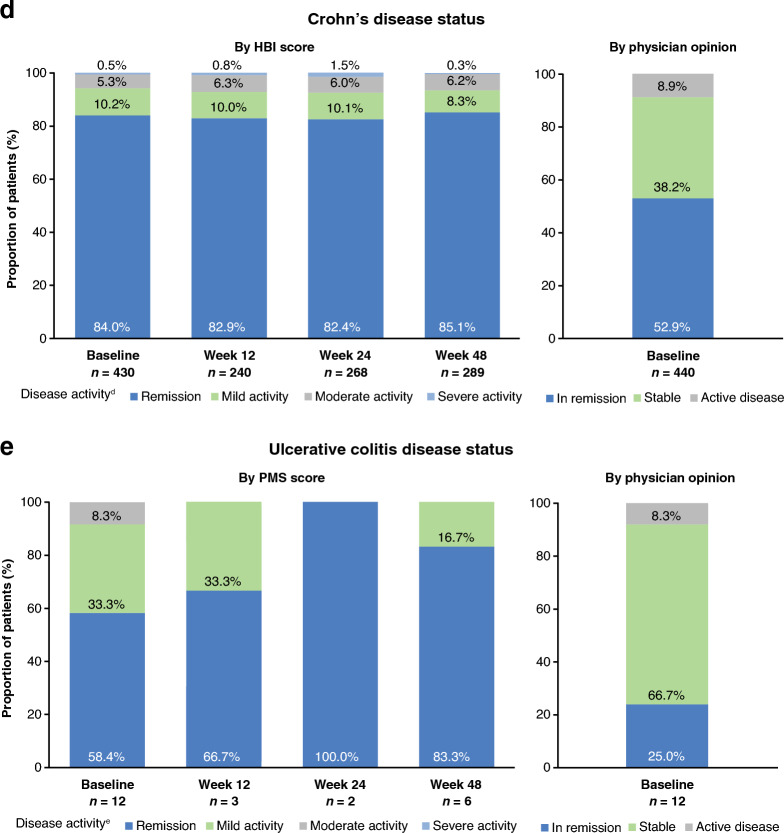
The Kaplan–Meier (95% CI) estimates of probability of persistence on SB5 at week 48 were 0.86 (0.80–0.90), 0.80 (0.71–0.86), 0.81 (0.74–0.86), and 0.72 (0.67–0.76) for the RA, axSpA, PsA, and CD cohorts, respectively (Fig. 2), and 0.50 (0.21–0.74) for the UC cohort (data not shown due to small n). Female sex was the only candidate predictor associated with likelihood of SB5 discontinuation: hazard ratios for discontinuation in females were 3.53, 2.38, and 2.21 in the RA, axSpA, and CD cohorts, respectively (Table 2).
Fig. 2.
Kaplan–Meier plot showing the probability of remaining on SB5 in patients with RA, axSpA, PsA, and CD. The number of patients with UC included in the study was too small to allow conclusions to be drawn for that cohort (data not shown). axSpA axial spondyloarthritis, CD Crohn’s disease, CI confidence interval, K–M Kaplan–Meier, PsA psoriatic arthritis, RA rheumatoid arthritis
Table 2.
Candidate predictors and hazard ratios for discontinuation of SB5 prior to week 48 post-transition
| Candidate predictor | Patients with event, n/N (%) | HR | 95% CI (Wald) |
|---|---|---|---|
| All indications | |||
| Age at time of initiation of SB5 (years) | 216/955 (22.6) | 0.99 | 0.98–1.00 |
| Female sex (reference: male) | 135/471 (28.7) | 1.85 | 1.41–2.44 |
| Duration of disease (years) | 153/642 (23.8) | 1.00 | 0.98–1.01 |
| RA | |||
| Age at time of initiation of SB5 (years) | 29/207 (14.0) | 1.01 | 0.98–1.04 |
| Female sex (reference: male) | 26/150 (17.3) | 3.53 | 1.07–11.67 |
| Duration of disease (years) | 18/120 (15.0) | 1.00 | 0.96–1.04 |
| Musculoskeletal disorders (reference: no history)a | 4/25 (16.0) | 1.14 | 0.40–3.28 |
| Vascular disorders (reference: no history)b | 1/25 (4.0) | 0.24 | 0.03–1.77 |
| Baseline medication:c other immunosuppressants (reference: not prescribed)d | 13/108 (12.0) | 0.72 | 0.35–1.50 |
| Baseline medication:c folic acid and derivatives (reference: not prescribed)e | 4/38 (10.5) | 0.68 | 0.24–1.96 |
| Baseline medication:c glucocorticoids (reference: not prescribed)f | 8/49 (16.3) | 1.22 | 0.54–2.76 |
| DAS28-CRPconv at baseline (continuous) | 28/191 (14.7) | 1.32 | 0.85–2.06 |
| axSpA | |||
| Age at time of initiation of SB5 (years) | 26/127 (20.5) | 1.01 | 0.98–1.04 |
| Female sex (reference: male) | 13/40 (32.5) | 2.38 | 1.11–5.14 |
| Duration of disease (years) | 13/69 (18.8) | 1.02 | 0.98–1.06 |
| IBD (reference: not diagnosed as a comorbidity)g | 7/26 (26.9) | 1.43 | 0.60–3.40 |
| Vascular disorders (reference: no history)b | 5/22 (22.7) | 1.22 | 0.46–3.24 |
| BASDAI at baseline (continuous) | 24/116 (20.7) | 1.18 | 0.96–1.45 |
| PsA | |||
| Age at time of initiation of SB5 (years) | 30/162 (18.5) | 0.98 | 0.95–1.00 |
| Female sex (reference: male) | 17/73 (23.3) | 1.68 | 0.81–3.45 |
| Duration of disease (years) | 21/98 (21.4) | 1.00 | 0.96–1.05 |
| Vascular disorders (reference: no history)b | 7/32 (21.9) | 1.22 | 0.52–2.85 |
| Baseline medication:c other immunosuppressants (reference: not prescribed)d | 12/54 (22.2) | 1.42 | 0.69–2.95 |
| Baseline medication:c folic acid and derivatives (reference: not prescribed)e | 7/25 (28.0) | 1.76 | 0.76–4.11 |
| PsARC disease activity score at baseline (continuous) | 1/7 (14.3) | 0.82 | 0.11–6.09 |
| CD | |||
| Age at time of initiation of SB5 (years) | 125/447 (28.0) | 0.99 | 0.98–1.01 |
| Female sex (reference: male) | 78/205 (38.0) | 2.21 | 1.54–3.18 |
| Duration of disease (years) | 96/345 (27.8) | 0.99 | 0.97–1.01 |
| Baseline medicationc: aminosalicylic acid and similar agents (reference: not prescribed)h | 10/54 (18.5) | 0.59 | 0.31–1.13 |
| Baseline medicationc: other immunosuppressants (reference: not prescribed)d | 15/57 (26.3) | 0.93 | 0.55–1.60 |
| HBI at baseline (continuous) | 118/430 (27.4) | 1.03 | 0.97–1.10 |
axSpA axial spondyloarthritis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CD Crohn’s disease, CI confidence interval, DAS28-CRPconv Disease Activity Score in 28 joints using C-reactive protein converted from the Disease Activity Score in 28 joints using erythrocyte sedimentation rate, HBI Harvey–Bradshaw Index, HR hazard ratio, IBD inflammatory bowel disease, n/N number of patients with an event/number of patients with evaluable data, PsA psoriatic arthritis, PsARC Psoriatic Arthritis Response Criteria, RA rheumatoid arthritis
aOsteoarthritis, osteopenia, osteoporosis, spondylitis, spondyloarthropathy, spondylolisthesis
bEssential hypertension, hypertension, hypotension
cBaseline medication defined as all medication with a stop date at or after SB5 initiation or a start date no later than 14 days after SB5 initiation
dAzathioprine, fumaric acid, hydroxychloroquine, hydroxychloroquine sulfate, methotrexate, methotrexate sodium
eFolic acid, folinic acid
fBudesonide, deflazacort, hydrocortisone, methylprednisolone, methylprednisolone acetate, prednisolone, prednisolone acetate
gUlcerative colitis, Crohn’s disease, inflammatory bowel disease
hMesalazine, sulfasalazine
The main reason for study withdrawal (n = 23) was loss to follow-up (n = 8, 0.8%). At week 48 (end of study), 722 patients (75.6%) were still receiving SB5 (ESM Table S7). The main reason for SB5 discontinuation was AE (n = 79, 8.3%), most commonly reported as injection site reaction (n = 66, 6.9%) (ESM Table S7).
Changes in Disease Activity Scores and PROMs Over Time
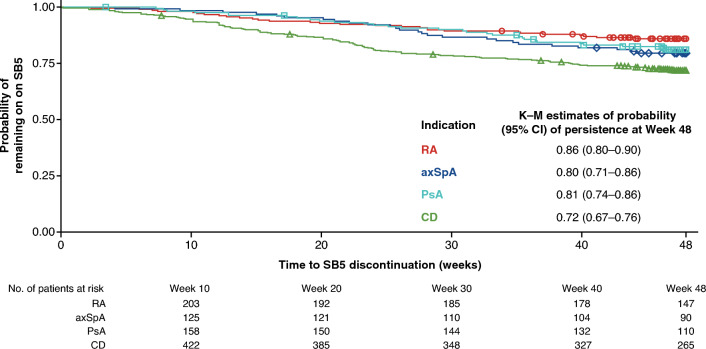
There were no meaningful changes from baseline (time of SB5 initiation) over time in disease activity scores for any of the disease cohorts [Fig. 1 (all cohorts); Fig. 3 (RA, axSpA, and CD cohorts); Table 3 (PsA cohort)], or in PROMs for the RA, axSpA, PsA, and CD cohorts (ESM Table S8). PROMs data were not analyzed for the UC cohort.
Fig. 3.
Boxplots showing change in disease activity scores over time, measured using indication-appropriate tools; a DAS28-CRPconv and b FFbH scores in patients with RA, c BASDAI in patients with axSpA, and d HBI in patients with CD. The boxes in these plots display the first and third quartiles; the median value is indicated by the horizontal line and the mean value is displayed using “+”; whiskers are drawn from the box to the most extreme point that are ≤ 1.5 times the interquartile range. Paired scores were included when values were taken at baseline and a subsequent timepoint for an individual patient; the number of patients for whom a paired score was available is shown in each plot for each timepoint. axSpA axial spondyloarthritis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CD Crohn’s disease, DAS28-CRPconv Disease Activity Score in 28 joints using C-reactive protein converted from the Disease Activity Score in 28 joints using erythrocyte sedimentation rate, FFbH Funktionsfragebogen Hannover, HBI Harvey–Bradshaw Index, RA rheumatoid arthritis
Table 3.
Paired disease activity scores over time in PsA cohort: PsARC
| Timepointa | n | Mean ± SD | 95% CI |
|---|---|---|---|
| Swollen joint score (66 joints) | |||
| Baseline | 41 | 0.7 ± 1.5 | 0.2–1.2 |
| Week 12 | 41 | 0.7 ± 1.6 | 0.2–1.2 |
| Baseline | 55 | 0.5 ± 1.2 | 0.2–0.8 |
| Week 24 | 55 | 1.0 ± 3.3 | 0.1–1.9 |
| Baseline | 54 | 0.5 ± 1.3 | 0.2–0.9 |
| Week 48 | 54 | 0.7 ± 1.8 | 0.2–1.2 |
| Tender joint score (68 joints) | |||
| Baseline | 41 | 1.9 ± 4.6 | 0.4–3.4 |
| Week 12 | 41 | 1.7 ± 3.5 | 0.5–2.8 |
| Baseline | 55 | 1.6 ± 3.0 | 0.8–2.4 |
| Week 24 | 55 | 3.2 ± 7.9 | 1.1–5.4 |
| Baseline | 54 | 1.3 ± 3.3 | 0.4–2.2 |
| Week 48 | 54 | 1.0 ± 1.9 | 0.4–1.5 |
CI confidence interval, PsA psoriatic arthritis, PsARC Psoriatic Arthritis Response Criteria, SD standard deviation
aScores derived for patients with values reported both at baseline and the subsequent timepoint
The proportion of patients in remission, having low disease activity, or having inactive disease at week 48 is shown in Fig. 1 for the RA (Fig. 1a), axSpA (Fig 1b), PsA (Fig. 1c), CD (Fig. 1d), and UC (Fig. 1e) cohorts.
Immunogenicity
ADA testing was not performed for the majority of patients either at baseline (n = 917/955, 96.0% with no test) or post-baseline (n = 880/955, 92.1% with no test). Of the 38 patients (4.0%) who were tested at baseline, two (0.2%) were ADA-positive and 36 (3.8%) were ADA-negative. Of the 75 patients (7.9%) tested post-baseline, only four (0.4%) also had a baseline test; post-baseline results were ADA-negative for 64 (6.7%) patients and ADA-positive for 11 (1.2%). Three of the four patients with both a baseline and post-baseline test were ADA-negative on both occasions, and one patient who tested negative at baseline seroconverted post-baseline.
Safety
ADRs and SAEs
In total, 232 patients (24.3%) reported at least one ADR (Table 4), which were mild in most cases (n = 134, 14.0%). The most frequent ADRs overall were injection site reactions (n = 157, 16.4%) (Table 5). Among the conditions included under the term “injection site reaction” (bruising, discomfort, extravasation, hematoma, hemorrhage, pain, pruritus, and rash), the most commonly occurring was injection site pain (n = 140, 14.7%), which was predominantly mild or moderate in severity. SAEs were reported for 40 patients (4.2%) (ESM Table S9). Eight patients (3.9%) in the RA cohort experienced nine SAEs, two of which were considered to be related to SB5: herpes zoster and pneumonia in one patient. Two patients (1.6%) in the axSpA cohort experienced a total of two SAEs, neither of which was considered to be related to SB5. Eight patients (4.9%) in the PsA cohort experienced nine SAEs; one, dyspnea, was considered to be related to SB5. Twenty-two patients (4.9%) in the CD cohort experienced a total of 27 SAEs, and the following four were considered to be related to SB5: subileus in one patient, anal fistula and perianal abscess in a second patient, and angor related to paroxysmal supraventricular tachycardia in a third patient. No SAEs were observed in the UC cohort. There were no fatal SAEs.
Table 4.
Summary of ADRs and SAEs across cohorts
| n (%) | RA (n = 207) | axSpA (n = 127) | PsA, (n = 162) | CD (n = 447) | UC (n = 12) |
|---|---|---|---|---|---|
| Patients with ≥ 1 ADR | 41 (19.8) | 38 (29.9) | 34 (21.0) | 118 (26.4) | 1 (8.3) |
| Patients with ≥ 1 SAE | 8 (3.9) | 2 (1.6) | 8 (4.9) | 22 (4.9) | 0 |
| Patients with ≥ 1 drug-related SAE | 1 (0.5) | 0 | 1 (0.6) | 3 (0.7) | 0 |
ADR adverse drug reaction, axSpA axial spondyloarthritis, CD Crohn’s disease, PsA psoriatic arthritis, RA rheumatoid arthritis, SAE serious adverse event, UC ulcerative colitis
Table 5.
Safety: most common ADRs
| Indication | ADR (high-level term) | Patients, n (%) | Events, n (%)a |
|---|---|---|---|
| RA (n = 207) | Injection site reactionsb | 30 (14.5) | 43 (71.7) |
| Application and instillation site reactions | 1 (0.5) | 3 (5.0) | |
| Pruritus NEC | 2 (1.0) | 2 (3.3) | |
| Headaches NEC | 2 (1.0) | 2 (3.3) | |
| Therapeutic and non-therapeutic responses | 2 (1.0) | 2 (3.3) | |
| axSpA (n = 127) | Injection site reactionsb | 19 (15.0) | 19 (32.2) |
| Lower respiratory tract and lung infections | 5 (3.9) | 5 (8.5) | |
| Asthenic conditions | 3 (2.4) | 3 (5.1) | |
| Bone-related signs and symptoms | 3 (2.4) | 3 (5.1) | |
| Spondyloarthropathies | 3 (2.4) | 3 (5.1) | |
| Upper respiratory tract infections | 3 (2.4) | 3 (5.1) | |
| PsA (n = 162) | Injection site reactionsb | 20 (12.3) | 23 (46.0) |
| Urinary tract infections | 3 (1.9) | 3 (6.0) | |
| Joint-related signs and symptoms | 2 (1.2) | 4 (8.0) | |
| CD (n = 447) | Injection site reactionsb | 87 (19.5) | 386 (87.9) |
| GI and abdominal pain (excluding oral and throat) | 5 (1.1) | 5 (1.1) | |
| Erythemas | 1 (0.2) | 6 (1.4) | |
| UC (n = 12) | Injection site reactionsb | 1 (8.3) | 1 (100) |
ADR adverse drug reaction, axSpA axial spondyloarthritis, CD Crohn’s disease, GI gastrointestinal, NEC not elsewhere classified, PsA psoriatic arthritis, RA rheumatoid arthritis, UC ulcerative colitis
aNumber of events as a proportion of the total number of events per indication
bIncludes the following events occurring at the injection site (n and percentage provided for the total population): bruising (n = 1, 0.1%), discomfort (n = 4, 0.4%), extravasation (n = 1, 0.1%), hematoma (n = 6, 0.6%), hemorrhage (n = 1, 0.1%), pain (n = 140, 14.7%), pruritus (n = 1, 0.1%), rash (n = 1, 0.1%), and “reaction” (n = 7, 0.7%)
Disease Flares
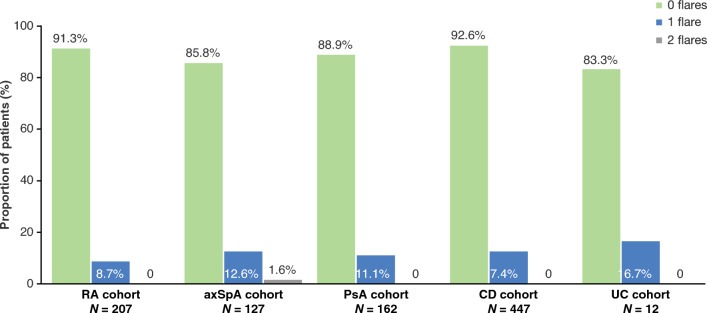
The majority of patients (866/955, 90.7%) experienced no disease flare during the study period, whilst 87 patients (9.1%) experienced a single flare episode. Only two patients (0.2%) experienced two flare episodes each; both patients were in the axSpA cohort (Fig. 4). The most common manner by which flare was detected was patient-reported symptoms (n = 88, 96.7%). Flare-related dose adjustments of biologics and non-biologics were required for 20 (22.0%) and 27 (29.7%) patients experiencing flare, respectively.
Fig. 4.
Episodes of disease flare in patients with RA, axSpA, PsA, CD, and UC. Disease flares recorded on or after initiation of SB5 and before or at discontinuation of SB5 were defined according to the investigator’s clinical opinion, who recorded the reasons underlying that decision (e.g., loss of disease control, worsening of symptoms, secondary loss of response). axSpA axial spondyloarthritis, CD Crohn’s disease, PsA psoriatic arthritis, RA rheumatoid arthritis, UC, ulcerative colitis
Patient Satisfaction
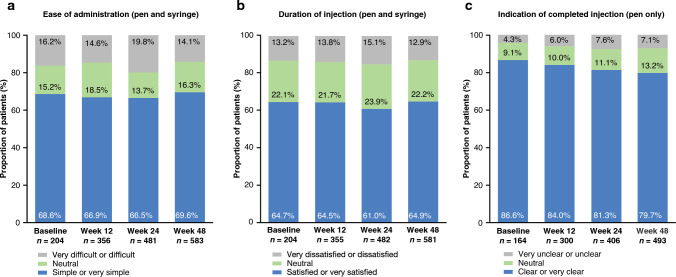
SB5 was more commonly administered via pen than by syringe both at baseline (range: 73.4–88.2%) and by week 48 (range: 72.0–88.7%) across the RA, axSpA, PsA, and CD cohorts. In the UC cohort, SB5 was administered via the pen in all 12 patients (100%) at baseline, continuing in the seven patients who were still receiving SB5 at week 48 (58.3%). There was no major shift in PSQ responses from baseline to week 48 (Fig. 5). The majority of patients reported that the injection was “simple or very simple” to administer (baseline: 55.6–83.3%; week 48: 65.9–78.8%) (Fig. 5a) and were generally “satisfied or very satisfied” with the duration of the injection (baseline: 50.0–75.0%; week 48: 63.0–69.5%) (Fig. 5b). The indication on the pen that the injection was complete was generally considered to be “clear or very clear” (baseline: 69.2–100%; week 48: 73.0–87.5%) (Fig. 5c). All PSQ responses are provided in ESM Table S10 (and exclude results for the UC cohort due to low n).
Fig. 5.
Patient satisfaction with the mode of SB5 administration (from PSQ)—all indications; a ease of administration (pre-filled pen or pre-filled syringe), b duration of injection (pre-filled pen or pre-filled syringe), and c indication of complete injection (pre-filled pen only), at each of the study measurement timepoints. PSQ Patient Satisfaction Questionnaire
Discussion
The findings of the PROPER study provide evidence of effective long-term use of SB5 in patients with IMIDs switching from reference to biosimilar adalimumab. The probability of persistence with SB5 at week 48 was high in all four cohorts analyzed (RA, axSpA, PsA, and CD), ranging from 0.72 to 0.86, with 75.6% of all patients remaining on SB5 at week 48 (range across those four cohorts, 70.7–83.6%). Of the candidate predictors of persistence evaluated in this study, only female sex was associated with increased risk for SB5 discontinuation in the RA, axSpA, and CD cohorts. These findings are comparable with data from previous adalimumab-to-SB5 treatment-switching studies [27–29]. In an analysis of two propensity score-matched cohorts of patients with CD, the probability (Kaplan–Meier estimates) of persistence with SB5 following transition from reference adalimumab at 26 weeks and 52 weeks after SB5 initiation was 0.89 (95% CI, 0.81–0.98) and 0.65 (95% CI, 0.53–0.79), respectively [27]. Similarly, in a cohort in which 95.9% of patients had RA, PsA, axSpA, or juvenile idiopathic arthritis, probabilities of persistence on SB5 were 0.95 (95% CI, 0.90–0.97) at 6 months and 0.85 (95% CI, 0.78–0.90) at 12 months after treatment initiation, respectively [28]. Rates of persistence with SB5 following transition from reference adalimumab were 84.6% and 70.8% at 26 weeks and 52 weeks post-transition, respectively, in patients with CD (89.1%), UC (9.0%), or IBD unclassified (2.0%) [29].
Long-term effectiveness was maintained at 48 weeks after switching from reference adalimumab to SB5, in line with previous findings from observational treatment-switching studies in comparable patient populations and using the same or similar definitions for clinical remission or low disease activity. In those studies, as in the present study, most patients in each cohort were in remission or had stable disease at transition, and showed no meaningful differences in disease activity measures over time post-transition (3–12 month follow-up) [27, 29, 30].
For all indication cohorts in the PROPER study there was variance between assessment of baseline clinical status determined by physician opinion and classification by disease activity scores. The proportions of patients deemed to be in remission or to have low/inactive disease were higher when determined using scored measures of disease activity than by physician opinion.
It was not possible to draw conclusions regarding the immunogenicity of SB5 (as measured by incidence of ADAs) in this study since the number of patients contributing data was small [38 patients (4.0%) at baseline and 75 patients (7.9%) post-baseline]. This reflects an apparent absence of routine ADA testing in patients receiving adalimumab, probably related to physician confidence in the low likelihood of an adverse outcome related to the immunogenic potential of SB5 [31].
No new safety signals were detected in this study; AEs were generally consistent with those reported previously in patients with rheumatic or gastroenterologic IMIDs treated with SB5 [17, 28, 29, 32]. Injection site reactions were the most frequently observed ADRs across indications, and have been reported to affect adherence to anti-TNF therapy in patients with an IMID [33, 34]. Although injection site reactions were the most common AE relating to SB5 discontinuation at week 48 in the present study, rates of persistence on SB5 remained high at week 48, and PSQ responses on the usability and experience with administration of SB5 were generally positive at both baseline and week 48. Injection site reactions were commonly observed in other studies of SB5 [17, 29]. In line with the finding that injection site pain may vary according to the type of IMID [21], the proportion of patients with injection site reactions in the present study was highest for patients with CD (19.5%), similar for patients with RA (14.5%) and axSpA (15.0%), and lowest for those with PsA (12.3%) and UC (8.3%).
The main strength of the PROPER study is that it enrolled a large study population that included rheumatology and gastroenterology IMID indications from multiple centers in six countries across Europe, and had a long follow-up period. These characteristics differentiate PROPER from other real-world observational [27, 29, 30, 32] or pivotal randomized controlled studies of biosimilars [17, 35, 36]. In addition, the PROPER study design uniquely captured patient experience on SB5 after transition from reference adalimumab by assessing both persistence and device satisfaction over 48 weeks, together with objective parameters such as effectiveness and safety. However, these findings should be considered in light of the small proportion of subjects who discontinued SB5 prior to week 48, which resulted in a similarly low incidence of potential candidate predictors, thus limiting the amount of information fitted into the Cox model for candidate predictors of discontinuation. In addition, heterogeneity in healthcare systems (e.g., with respect to reimbursement systems, access to biosimilars) between the countries represented in this population [37] may also have affected the findings. Potential site and/or regional effects on discontinuation rates were not taken into account in this analysis.
The potential impact of a “nocebo effect” should also be considered, whereby patients perceive worsening of their condition and/or experience AEs as a result of negative expectations of the treatment, even if it is essentially inert. The nocebo effect, which has been observed in other studies of biosimilars [38], may negatively affect outcomes in patients switching from reference biologics to biosimilars, particularly in a non-blinded or real-world context, potentially leading to discontinuation of the biosimilar [38, 39]. One of the known risk factors for the nocebo effect is sex, with female participants being more susceptible than male participants [40–42]. This is relevant to the findings of the present study since female sex was the only candidate predictor associated with increased risk for SB5 discontinuation. Post hoc analysis to further explore this finding is ongoing. The cost of a biosimilar relative to the reference agent is also a risk factor for the nocebo effect, as some patients associate lower cost with reduced effectiveness [40, 41]. Conversely, expense may be an important consideration in the decision to switch to a potentially more cost-effective biosimilar, as was the case in almost one-third (30.8%) of the total population and in two-thirds (66.5%) of the RA cohort in the present study. Nocebo-mitigating efforts, including communication and training, may aid informed decision-making in patients who are considering switching from a reference drug to a biosimilar [43, 44]. It is thought that implementation of a shared decision-making process may reassure and empower patients, affording them a sense of control and ownership over that process, ultimately making them less susceptible to negative nocebo effects [38]. The patients in the PROPER study were generally well informed, as reflected by physician confirmation that the majority (87.2–96.4%) had received information on self-administration of SB5 and that most patients across all disease cohorts had a good understanding of how SB5 should be stored and prepared for use.
Conclusions
The findings of this pan-European study contribute to the overall understanding of real-world usage of SB5 in a large cohort of patients with established RA, axSpA, PsA, or CD transitioning from reference adalimumab, and demonstrate that SB5 is well tolerated and effective in these patients. Persistence on SB5 was high, with three-quarters of study patients remaining on treatment long term. Of the candidate predictors identified (age, sex, comorbidities, disease duration, disease activity score, concomitant therapy), the only baseline variable associated with an increased risk of SB5 discontinuation was female sex, in patients with RA, axSpA, or CD. There were no meaningful differences in disease activity or PROM scores between baseline and week 48 post-transition (most patients in each cohort remained in remission or had stable disease), and no new safety signals were observed. These findings suggest that in the context of treatment persistence and within the baseline categories observed, there was no evidence to mitigate against transition. However, particular consideration should perhaps be given to female patients who are considering switching, since they appear to be more susceptible to discontinuation than their male counterparts. Finally, the impact of geographic region on these results is not yet known; post hoc analyses are ongoing to determine whether the data are confounded by patient geographic location.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are grateful to all patients who took part in this study. Statistical services were provided by FGK Clinical Research GmbH (Munich, Germany) and data management services were provided by Worldwide Clinical Trial (Research Triangle Park, NC, USA). The authors acknowledge Teju Chaugule, of Biogen Netherlands BV, who provided technical input and critically reviewed draft versions of this manuscript. Medical writing assistance was provided by Jacqueline Kolston, PhD and Simon Rhead, PhD, Parexel International, and was funded by Biogen International GmbH. Deepak Jadon acknowledges that his research was supported by Cambridge Arthritis Research Endeavour (CARE) and the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014).
Declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Biogen International GmbH.
Conflicts of Interest
Ulf Müller-Ladner has served as a speaker/advisor for Biogen, AbbVie, and Medac. Axel Dignass has received fees for participation in clinical trials and for review activities, such as data monitoring boards, statistical analysis, and endpoint committees from Abivax, AbbVie, Arena, Celgene/Bristol Myers Squibb, Falk, Gilead, Janssen, and Pfizer; consultancy fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene/Bristol Myers Squibb, Celltrion, Falk, Ferring, Fresenius Kabi, Galapagos, Gilead, Janssen, Eli Lilly, MSD, Pfizer, Pharmacosmos, Roche/Genentech, Sandoz/Hexal, Takeda, Tillotts, and Vifor; and payment for lectures including service on speaker bureaus from AbbVie, Amgen, Biogen, Celltrion, Falk Foundation, Ferring, Gilead/Galapagos, Janssen, Eli Lilly, MSD, Pharmacosmos, Pfizer, Takeda, Tillotts, and Vifor. Karl Gaffney has received research grants from NASS, AbbVie, UCB, Novartis, Eli Lilly, Celgene, Celltrion, Janssen, Gilead, and Biogen; consultancy fees/honoraria from AbbVie, Eli Lilly, and Novartis; and speaker/bureau fees from AbbVie, UCB, Novartis, and Eli Lilly. Deepak Jadon has received research grants, education grants, and/or consulting fees or honoraria from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Fresenius Kabi, Galapagos/Gilead, GSK, Healthcare Celltrion, Janssen, Merck, Novartis, Pfizer, Roche, and Sandoz. Marco Matucci-Cerinic has received research grants from MSD and speaker fees from Sandoz, Boehringer Ingelheim, and Biogen. Triana Lobaton has received financial research support from AbbVie, Viatris, MSD, Mundipharma, Biogen, Janssen, Pfizer, and Takeda; speaker fees from Ferring, MSD, AbbVie, Janssen, Amgen, Fresenius Kabi, Galapagos, Viatris, and Takeda; and consultancy fees from Janssen, Galapagos, Amgen, Bristol Myers Squibb, Fresenius Kabi, and Takeda. Philippe Carron has received consulting/speaker’s fees from AbbVie, Biogen, Eli Lilly, Fresenius Kabi, Galapagos, MSD, Pfizer, and UCB. Javier P. Gisbert has served as a speaker, consultant, and advisory member for, or has received research funding from, MSD, AbbVie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers Squibb, Gilead/Galapagos, Eli Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine, and Vifor Pharma. Ira Pande has received research funding, consulting, speaker fees, and sponsorship from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, MSD, Pfizer, Proctor & Gamble, and Roche. Maximilian Utzinger and Janet Addison are employees of Biogen and may hold company stock.
Availability of Data and Material
The data pertaining to this research will not be shared.
Ethics Approval
The protocol, its amendment, and the patient-informed consent form were approved by the Ethics Committees of the participating sites. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and all applicable local regulations.
Consent to Participate
Prior to enrolling a patient into the study or entering any patient data into the electronic Case Report Form, the Investigator was responsible for obtaining written informed consent from the patient through an Ethics-Committee-approved Informed Consent Form (ICF) in accordance with local practice and regulations. The patient was asked to sign the ICF only after receiving an explanation of the backgrounds, methods, procedures, and benefits and potential risks of the study, and being informed that their participation was voluntary.
Patient Consent to Publish
Not applicable.
Code Availability
Not applicable.
Author Contributions
JA developed the concept for this publication, developed the methodology, and acquired funding. Formal analysis and investigation were conducted by JA and MU who, along with UM-L, prepared the original draft of the manuscript. All authors were involved in the review and editing of the draft manuscript. KG, DJ, MM-C, TL, PC, and IP were principal investigators at their respective study sites. Supervision for this study was provided by JA, MU, UM-L, AD, DJ, TL, PC, JG, and MM-C. All authors read and approved the final version of this manuscript.
References
- 1.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mease PJ. Psoriatic arthritis: update on pathophysiology, assessment and management. Ann Rheum Dis. 2011;70(Suppl 1):i77. doi: 10.1136/ard.2010.140582. [DOI] [PubMed] [Google Scholar]
- 3.McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol. 2016;12(1):63–68. doi: 10.1038/nrrheum.2015.171. [DOI] [PubMed] [Google Scholar]
- 4.Watad A, Bridgewood C, Russell T, Marzo-Ortega H, Cuthbert R, McGonagle D. The early phases of ankylosing spondylitis: emerging insights from clinical and basic science. Front Immunol. 2018;9:2668. doi: 10.3389/fimmu.2018.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50(4):992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 2021;385(7):628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- 7.Huizinga TWJ, Torii Y, Muniz R. Adalimumab biosimilars in the treatment of rheumatoid arthritis: a systematic review of the evidence for biosimilarity. Rheumatol Ther. 2021;8(1):41–61. doi: 10.1007/s40744-020-00259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapadula G, Marchesoni A, Armuzzi A, Blandizzi C, Caporali R, Chimenti S, et al. Adalimumab in the treatment of immune-mediated diseases. Int J Immunopathol Pharmacol. 2014;27(1_suppl):33–48. doi: 10.1177/03946320140270S103. [DOI] [PubMed] [Google Scholar]
- 9.Rezk MF, Pieper B. Unlocking the value of anti-TNF biosimilars: reducing disease burden and improving outcomes in chronic immune-mediated inflammatory diseases: a narrative review. Adv Ther. 2020;37(9):3732–3745. doi: 10.1007/s12325-020-01437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kvien TK, Patel K, Strand V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum. 2022;52:151939. doi: 10.1016/j.semarthrit.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol. 2019;17(13):2704–12.e3. doi: 10.1016/j.cgh.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 12.Agca R, Smulders Y, Nurmohamed M. Cardiovascular disease risk in immune-mediated inflammatory diseases: recommendations for clinical practice. Heart. 2022;108(1):73–79. doi: 10.1136/heartjnl-2019-316378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JJ, Poon KYT, Channual JC, Shen AYJ. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244–1250. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 14.AbbVie Inc. HUMIRA® FDA Prescribing Information. United States Food and Drug Administration. 2021. https://www.rxabbvie.com/pdf/humira.pdf. Accessed 14 Jan 2023.
- 15.AbbVie Inc. HUMIRA® EMA Summary of Product Characteristics. European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. Accessed 14 Jan 2023.
- 16.Shin D, Lee Y, Kim H, Körnicke T, Fuhr R. A randomized phase I comparative pharmacokinetic study comparing SB5 with reference adalimumab in healthy volunteers. J Clin Pharm Ther. 2017;42(6):672–678. doi: 10.1111/jcpt.12583. [DOI] [PubMed] [Google Scholar]
- 17.Weinblatt ME, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Jaworski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70(1):40–48. doi: 10.1002/art.40336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biogen Inc. IMRALDI® EMA Summary of Product Characteristics. European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/imraldi-epar-product-information_en.pdf. Accessed 14 Jan 2023.
- 19.Coghlan J, He H, Schwendeman AS. Overview of Humira® biosimilars: current European landscape and future implications. J Pharm Sci. 2021;110(4):1572–1582. doi: 10.1016/j.xphs.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinblatt ME, Baranauskaite A, Dokoupilova E, Zielinska A, Jaworski J, Racewicz A, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70(6):832–840. doi: 10.1002/art.40444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gisbert JP, Gaffney K, Young D, Ebbers HC, Girolomoni G. Current evidence on the use of the adalimumab biosimilar SB5 (ImraldiTM): a multidisciplinary perspective. Expert Opin Biol Ther. 2022;22(2):109–121. doi: 10.1080/14712598.2022.2012146. [DOI] [PubMed] [Google Scholar]
- 22.Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 24.Leong KP, Tan JWL, Gao X, Koh ET, Group TRAS Conversion among the 28-joint count activity indices for rheumatoid arthritis. Eur J Rheumatol. 2020;7(3):105–111. doi: 10.5152/eurjrheum.2020.19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MedDRA Maintenance and Support Services Organization. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 23.0. McLean, Virginia [updated 2020 March]. https://admin.meddra.org/sites/default/files/guidance/file/SMQ_intguide_23_0_English.pdf. Accessed 14 Jan 2023.
- 26.Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. New York: Springer; 2009. [Google Scholar]
- 27.Lukas M, Kolar M, Reissigova J, Duricova D, Machkova N, Hruba V, et al. A switch from originator-adalimumab to the biosimilar SB5 in patients with Crohn’s disease: an analysis of two propensity score-matched cohorts. Scand J Gastroenterol. 2022;57(7):814–824. doi: 10.1080/00365521.2022.2041082. [DOI] [PubMed] [Google Scholar]
- 28.Bruni C, Gentileschi S, Pacini G, Bardelli M, Tofani L, Bartoli F, et al. Switching from originator adalimumab to biosimilar SB5 in a rheumatology cohort: persistence on treatment, predictors of drug interruption and safety analysis. Ther Adv Musculoskelet Dis. 2021;13:1759720X211033679. doi: 10.1177/1759720X211033679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derikx LAAP, Dolby HW, Plevris N, Lucaciu L, Rees CS, Lyons M, et al. Effectiveness and safety of adalimumab biosimilar SB5 in Inflammatory bowel disease: outcomes in originator to SB5 switch, double biosimilar switch and bio-naïve SB5 observational cohorts. J Crohns Colitis. 2021;15(12):2011–2021. doi: 10.1093/ecco-jcc/jjab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Adrichem RCS, Voorneveld HJE, Waverijn GJ, Kok MR, Bisoendial RJ. The non-medical switch from reference adalimumab to biosimilar adalimumab is highly successful in a large cohort of patients with stable inflammatory rheumatic joint diseases: a real-life observational study. Rheumatol Ther. 2022;9(4):1109–1118. doi: 10.1007/s40744-022-00465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency. European Public Assessment Report (EPAR) for Imraldi. EMA/CHMP/106922/2017. 2017. https://www.ema.europa.eu/en/documents/assessment-report/imraldi-epar-public-assessment-report_en.pdf. Accessed 13 Feb 2023.
- 32.Bruni C, Bitti R, Nacci F, Cometi L, Tofani L, Bartoli F, et al. Efficacy and safety of switching from reference adalimumab to SB5 in a real-life cohort of inflammatory rheumatic joint diseases. Clin Rheumatol. 2021;40(1):85–91. doi: 10.1007/s10067-020-05199-w. [DOI] [PubMed] [Google Scholar]
- 33.Gely C, Marín L, Gordillo J, Mañosa M, Bertoletti F, Cañete F, et al. Impact of pain associated with the subcutaneous administration of adalimumab. Gastroenterol Hepatol (N Y). 2020;43(1):9–13. doi: 10.1016/j.gastrohep.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Salaffi F, Di Carlo M, Farah S, Carotti M. Adherence to subcutaneous anti-TNFα agents in patients with rheumatoid arthritis is largely influenced by pain and skin sensations at the injection site. Int J Rheum Dis. 2020;23(4):480–487. doi: 10.1111/1756-185X.13803. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Genovese MC, Choy E, Perez-Ruiz F, Matsumoto A, Pavelka K, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017;76(10):1679. doi: 10.1136/annrheumdis-2016-210459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanauer S, Liedert B, Balser S, Brockstedt E, Moschetti V, Schreiber S. Safety and efficacy of BI 695501 versus adalimumab reference product in patients with advanced Crohn's disease (VOLTAIRE-CD): a multicentre, randomised, double-blind, phase 3 trial. Lancet Gstroenterol Hepatol. 2021;6(10):816–825. doi: 10.1016/S2468-1253(21)00252-1. [DOI] [PubMed] [Google Scholar]
- 37.Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PLoS ONE. 2017;12(12):e0190147. doi: 10.1371/journal.pone.0190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;10:1372. doi: 10.3389/fphar.2019.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Somer T, Deprez N, Baert D, Deceuninck M, Huys I, Mattens V, et al. P396 Acceptance of switch, patient satisfaction and adverse events after switch from adalimumab originator to biosimilar SB5 in patients with Inflammatory Bowel Disease in a real-life setting. J Crohns Colitis. 2021;15(Supplement_1):S406-S. doi: 10.1093/ecco-jcc/jjab076.520. [DOI] [Google Scholar]
- 40.Planès S, Villier C, Mallaret M. The nocebo effect of drugs. Pharmacol Res Perspect. 2016;4(2):e00208. doi: 10.1002/prp2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kravvariti E, Kitas GD, Mitsikostas DD, Sfikakis PP. Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat Rev Rheumatol. 2018;14(12):727–740. doi: 10.1038/s41584-018-0110-9. [DOI] [PubMed] [Google Scholar]
- 42.Curtis JR, Hobar C, Hansbrough K. Injection-site burning and stinging in patients with rheumatoid arthritis using injectable biologics. Curr Med Res Opin. 2011;27(1):71–78. doi: 10.1185/03007995.2010.534959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young D, Latter S, Harvey J, Addison J, Freudensprung U, Cummings F. P585 IBD Reference and Biosimilar adalimumab CroSS over Study (iBaSS): a mixed methods clinical trial of patients transitioning between originator and biosimilar adalimumab. Qualitative findings from an interim analysis presenting the patient perspective. J Crohns Colitis. 2021;15(Supplement 1):S537–S538. doi: 10.1093/ecco-jcc/jjab076.706. [DOI] [Google Scholar]
- 44.Kaneko K, Prieto-Alhambra D, Jacklin C, Bosworth A, Dickinson S, Berry S, et al. Influence of information provided prior to switching from Humira to biosimilar adalimumab on UK patients’ satisfaction: a cross-sectional survey by patient organisations. BMJ Open. 2022;12(2):e050949. doi: 10.1136/bmjopen-2021-050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.