Abstract
Purpose
To evaluate whether stone dust can be obtained from all prevailing stone composition types using the novel pulsed thulium:YAG (p-Tm:YAG), including analysis of stone particle size after lithotripsy.
Methods
Human urinary stones of 7 different compositions were subjected to in vitro lithotripsy using a p-Tm:YAG laser with 270 µm silica core fibers (Thulio®, Dornier MedTech GmbH®, Wessling, Germany). A cumulative energy of 1000 J was applied to each stone using one of three laser settings: 0.1 J × 100 Hz, 0.4 J × 25 Hz and 2.0 J × 5 Hz (average power 10 W). After lithotripsy, larger remnant fragments were separated from stone dust using a previously described method depending on the floating ability of dust particles. Fragments and dust samples were then passed through laboratory sieves to evaluate stone particle count according to a semiquantitative analysis relying on a previous definition of stone dust (i.e., stone particles ≤ 250 µm).
Results
The p-Tm:YAG laser was able to produce stone dust from lithotripsy up to measured smallest mesh size of 63 µm in all seven stone composition types. Notably, all dust samples from all seven stone types and with all three laser settings had high counts of particles in the size range agreeing with the definition stone dust, i.e., ≤ 250 µm.
Conclusion
This is the first study in the literature proving the p-Tm:YAG laser capable of dusting all prevailing human urinary stone compositions, with production of dust particles ≤ 250 µm. These findings are pivotal for the broader future implementation of the p-Tm:YAG in clinical routine.
Keywords: Laser, Pulsed thulium:YAG, Kidney stones, Stone dust, Endourology, Ureteroscopy
Introduction
Urinary stone disease has become increasingly prevalent worldwide [1–5]. For more than three decades, the laser has been established as the mainstay of treatment when it comes to endourological surgery and lithotripsy [6]. Particularly over the last decade, dusting properties of lasers used for lithotripsy have become increasingly recognized as an important factor affecting major outcomes of stone surgery [7–9].
Currently, the holmium:yttrium–aluminum–garnet (Ho:YAG) and the more recent thulium fiber laser (TFL) are widely used in endourological procedures [10–12]. Additionally, novel pulsed thulium:yttrium–aluminum–garnet (p-Tm:YAG) lasers have recently been introduced to the market for clinical use. Based on a limited series of in vitro evaluations, the p-Tm:YAG laser seems to come with promising stone dusting properties [13, 14]. It is important to note that these preliminary reports were based on lithotripsy models using artificial Bego Stones—and not human urinary stones—with the primary outcome being stone ablation efficiency rather than evaluation of stone dust per se. Two recent in vivo studies evaluated the clinical efficacy and safety of the p-Tm:YAG on case series of patients undergoing retrograde intrarenal surgery [15] and mini-percutaneous nephrolithotomy [16]. Both studies conclude that the p-Tm:YAG seems very promising, with patients included having a range of stone densities, although no information regarding exact differing stone compositions was provided. In the mini-percutaneous nephrolithotomy study, the authors specifically state not investigating stone composition as a study limitation. Considering the above, a study is warranted to verify whether the new p-Tm:YAG laser is capable of dusting prevailing human urinary stone types. For this purpose, the present study includes an established lithotripsy and stone dust collection model, with analysis of stone particles size after lithotripsy of the seven most common human urinary stone types using the new p-Tm:YAG laser.
Material and methods
Human urinary stones were retrieved from a large stone biobank from Tenon Hospital, Paris encompassing the following stone composition types: calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), uric acid (UA), carbapatite (CA), struvite (STR), brushite (BR) and cystine (CYS). Stones were selected to match a volume of approximately 100 to 200 mm^3, were extracted without laser lithotripsy and had a > 90% degree of purity based on infrared spectroscopy. To simulate in vivo settings, all stones were submerged in saline for 24 h prior to experiments, as kidney stones are of a crystalline structure primarily, but grow in a biological environment with complex intercrystalline spaces [17] likely filled with urine.
Each stone was separately subjected to laser lithotripsy using the Dornier® Thulio® p-Tm:YAG with its 270-µm-core-diameter Dornier® Thulio® Performance reusable laser fiber (Dornier MedTech GmbH®, Wessling, Germany). The characteristics of the novel p-Tm:YAG laser are summarized in Table 1.
Table 1.
Pulsed thulium:YAG (p-Tm:YAG) laser characteristicsa
| Characteristic | Description | |||
|---|---|---|---|---|
| Laser energy source | Solid-state, diode-pumped, thulium-doped YAG crystal | |||
| Wavelength | 2013 nm | |||
| Laser settings | Operating mode | Pulsed only | ||
| Output power | 100W | |||
| Peak power | Max 3.7 kW | |||
| Pulse energy range | 0.1–2.5 J | |||
| Pulse frequency range | 5–300 Hz | |||
| Pulse length range | 150–1200 μs | |||
| Laser fibers | Laser fibers dimensions | Manufacturer label | Core diameter | Outer diameter |
| 270 Micron Slim | 272 ± 5 µm | 400 ± 30 µm | ||
| 400 Micron | 365 ± 10 µm | 550 ± 30 µm | ||
| 600 Micron | 550 ± 12 µm | 750 ± 30 µm | ||
| 1000 Micron | 940 ± 15 µm | 1400 ± 50 µm | ||
| Length of laser fiber | 3 m | |||
| Reusability of laser fiber | Single use and reusable (10x) available in same sizes | |||
| Machine | Machine weight | 97 kg (laser device) | ||
| Machine dimensions |
(W) 42 × (D) 62 × (H) 139 cm (Including monitor) |
|||
| Operating noise emission | ≤ 65 dB | |||
| Power source requirement | 115/208–240 VAC, single phase, max. 15 A, 50/60 Hz | |||
| Cooling system | Internal closed-loop water cooling system | |||
| Pedal characteristics | Two-pedal footswitch with two additional buttons—(Standby/Ready and changing of laser settings). Wireless and wired options | |||
aAccording to manufacturer
We used three laser settings for lithotripsy: 0.1 J × 100 Hz, 0.4 J × 25 Hz and 2.0 J × 5 Hz, with each sample from the same stone type being treated with a different laser setting and reaching a cumulative applied energy of 1000 J.
All settings resulted in an average power of 10W. For the first setting, we chose the lowest pulse energy available (0.1 J) on the graphical user interface (GUI) touchscreen in “Dusting” mode with an according frequency (100 Hz) to reach 10W. For the second setting, we chose a pulse energy that is generally accepted as an adequate dusting setting (0.4 J × 25 Hz) for Ho:YAG and TFL lithotripsy (“Dusting mode” on the GUI) [9, 18]. For the third setting, 2.0 J × 5 Hz was chosen as a typical fragmenting setting, meeting the aforementioned 10 W agreement (“Standard Fragmenting” mode on the GUI). Since pulse duration could not be changed and was not displayed in the “Dusting” and “Standard Fragmenting” modes, we did not explore this laser setting. For each stone sample, as the average power used (10 W) was the same to reach a common cumulative energy (1000 J), this means the lasing time was the same throughout all experiments (1000 J/10 W = 100 s).
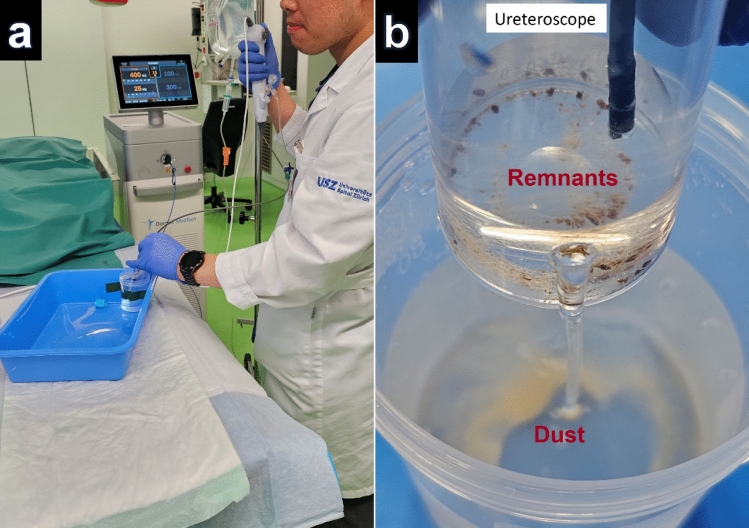
The OTU WiScope (OTU Medical Inc, CA, USA) flexible ureteroscope was used for laser lithotripsy under direct endoscopic vision in a 10-ml glass cuvette, with 0.9% sodium chloride under gravity irrigation pressure of 40 cmH20 at room temperature (21 °C) (Fig. 1a). The irrigation overflow was collected in a 100 ml plastic container. Lithotripsy was performed freehand with painting movements of the laser fiber tip over stone samples for the dusting settings and pinpoint movements for the fragmenting laser setting. The laser fiber tip was cut through the blue protective jacket with regular metallic surgical scissors before lithotripsy for each sample. All experiments were performed using the same reusable laser fiber.
Fig. 1.
Experimental setup for lithotripsy and separation process. a Lithotripsy setup with ureteroscope inserted into a glass cuvette, b post-lithotripsy separation process in a “remnants” 60 ml container with a hole at 1 cm from bottom of container and overflow into a “dust” 100 ml container
Considering that there is currently no generally accepted definition of stone dust, stone dust was separated from larger remnant fragments after laser lithotripsy using a previously described method employed in prior studies [19, 20] that depends on the floating ability of dust particles. This was done by evacuation of spontaneously floating stone dust over a 5 mm hole located 1 cm above the bottom of a 60 ml plastic container upon constant irrigation over the flexible ureteroscope (40 cmH2O, empty working channel) (Fig. 1b). The resultant irrigation overflow was collected in the aforementioned 100 ml plastic container, thus merging all stone dust together in a “dust” sample. The remaining stone fragments in the 60 ml container are referred to as “remnants” sample from here on.
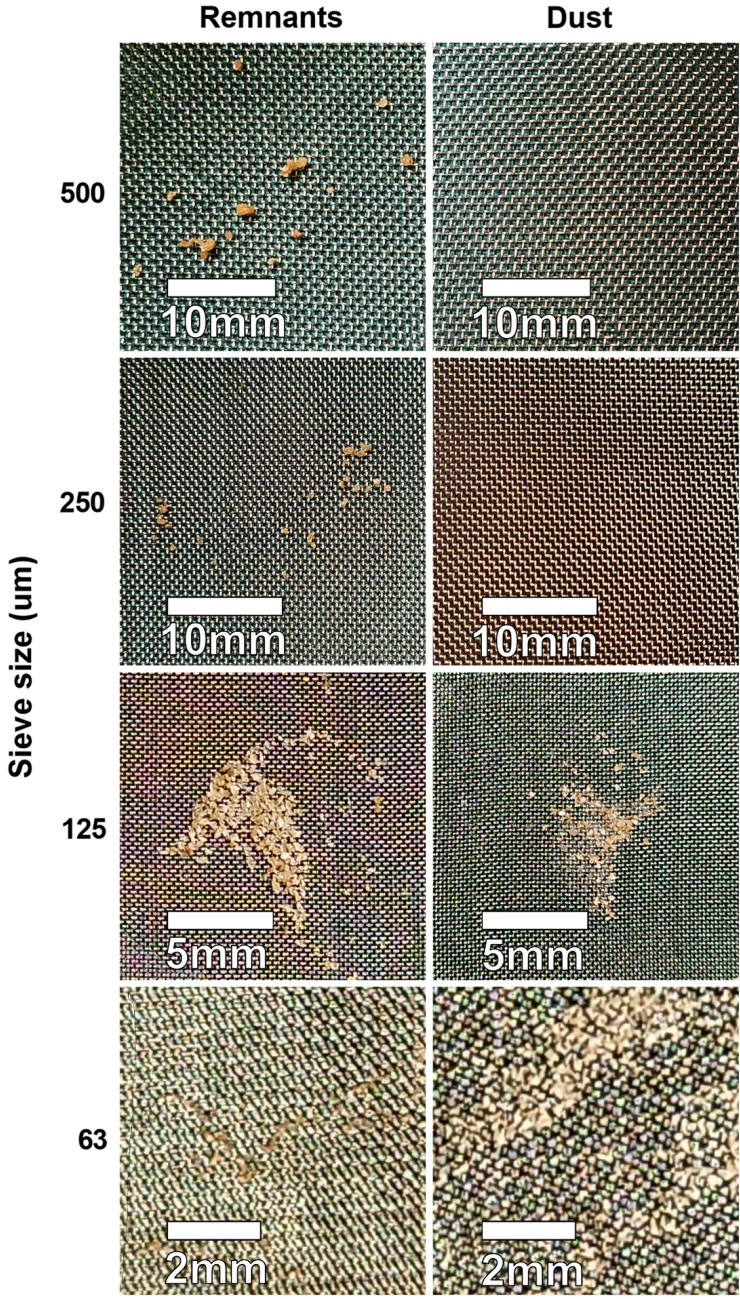
For stone size analyses, each sample of remnants and dust was then separately further processed by passing through stacked laboratory sieves (Eisco sorting sieves, Eisco Scientific LLC, NY, USA) for semiquantitative analysis (Fig. 2). Mesh size openings of 500 µm, 250 µm, 125 µm and 63 µm were used to separate the stone particles into different size categories. A total of 500 ml of saline was poured over the stacked sieves to ensure full sedimentation of particles over the whole range of sieves. After this, the sieves of each sample were photographed separately for analysis of the stone particle count. To ensure that the whole sieve area was consistently included in the photographs, we used a holder to fix the camera. A reference marker of 1 cm placed on each sieve sample was used to calibrate the scale for Fig. 2 using the software ImageJ (version 1.53tRRID:SCR_003070) [21]. A previously proposed stone size limit of ≤ 250 µm was used for the definition of stone dust [7].
Fig. 2.
Examples of stone particles gathered on sieves’ surfaces. Images here are cropped parts of the sieve photographs. Particles are from sieving process of a cystine stone subjected to lithotripsy with the p-Tm:YAG laser @ 2.0Jx5Hz = 10W. The semiquantitative analysis of this sample is found in Fig. 3
Statistical analysis
The number of stone particles for each sample and each entire sieve surface was evaluated and categorized into particle counts of 1–10 (low), 11–50 (moderate) and > 50 (high) by two authors (JK and EXK), based on the corresponding sieves’ photographs. Discrepancies were resolved by consensus between these two authors. A Spearman’s correlation analysis was performed to compare overall sieve size and respective stone particle count categories. A two-sided p value < 0.05 was considered statistically significant. All descriptive and statistical analyses were performed with GraphPad Prism 9.5.1 (GraphPad Software, La Jolla CA, USA).
Results
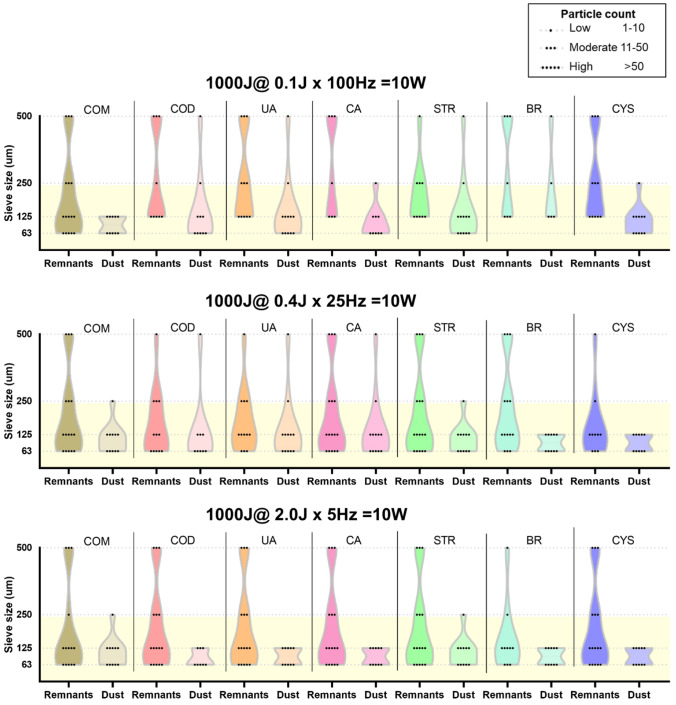
The p-Tm:YAG laser was able to produce dust from lithotripsy up to the smallest measured mesh size of 63 µm in all seven urinary stone composition types (Fig. 3).
Fig. 3.
Stone particle size distribution after lithotripsy with p-Tm:YAG. Particle size distribution represented by particle category counts (low, moderate and high) on sieve surfaces of various mesh sizes for three laser settings. Stone compositions: calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), uric acid (UA), carbapatite (CA), struvite (STR), brushite (BR) and cystine (CYS). Yellow area denotes the size range from a prior definition of stone dust [7]
Particularly, all dust samples from all seven stone types and with all three laser settings were found to have high counts of particles in the size range agreeing with the definition of stone dust, i.e., ≤ 250 µm. A few isolated dust samples showed low count of particles > 250 µm.
In the remnants samples, a low to moderate count of larger stone fragments (> 250 µm) was found in addition to a moderate to high count of stone dust particles (≤ 250 µm) for all seven stone types and with all three laser settings.
When considering comparisons between the different stone types, no obvious pattern of differing stone particle count distribution either for dust or for remnants samples was found within each laser setting. The same was valid when considering comparisons between the different laser settings for each stone type. The only general observation was that the particle count on smaller sieve sizes was consistently higher than on larger sized sieves, resulting in a significant negative correlation when comparing sieve size with stone particle count (rS = − 0.77, p = 0.01).
Discussion
To the best of our knowledge, this is the first study in the literature to reveal the p-Tm:YAG laser being capable of dusting human urinary stone composition types commonly found in clinical routine. Additionally, this study confirms a size limit of ≤ 250 µm applicable to all stone types for the definition of stone dust, according to a separation process based on the spontaneous floating and evacuation of stone dust [7].
The findings of this study are pivotal for the broader future implementation of the p-Tm:YAG in clinical routine, considering that studies found in the literature so far have presented data based on non-human stone lithotripsy models (BegoStone, plaster of paris, gypsum/glass) [13, 14, 22], or if conducted in humans did not investigate laser effects on different human urinary stone compositions [16]. It is of particular interest to note that the p-Tm:YAG was amenable to lithotripsy of cystine stones, akin to the Ho:YAG and TFL [23]. One may recall the frequency-doubled double-pulse neodymium:YAG (FREDDY) laser which was originally proposed as a potential technology for lithotripsy in the 1980s, until it was found ineffective at fragmenting cystine stones[24, 25]. That shortcoming eventually allowed for the holmium:YAG laser to become the gold standard for lithotripsy in those days. To that extent, the present study falls into the legacy of fundamental research approving newer technology for lithotripsy [20].
Providing a semiquantitative analysis of stone dust is of high importance, considering that stone dusting has become a broadly adopted technique for laser lithotripsy, while the exact definition of stone dust is still a matter a debate. To date, the most comprehensive definition of stone dust relies on a trifecta based on laboratory testing [7]: (1) spontaneous floating with 40 cm H2O irrigation pressure; (2) mean sedimentation time of more than 2 s through 10 cm saline solution; and (3) fully able to be aspirated through a 3.6 F ureteroscope’s working channel. This definition has been applied to in vivo clinical laser studies [26, 27], however clinical relevance has not been fully demonstrated yet.
Small stone particles ≤ 250 µm originating from p-Tm:YAG lithotripsy were found to spontaneously evacuate upon irrigation in the present experimental setup, as prior proved possible with Ho:YAG [19] and TFL studies [20]. These small particles were considered as stone dust per prior stone dust definition [7].
Overall, the smaller the sieve size, the higher the particle count, and vice versa. This significant correlation is arguably explained by the smaller particles being the result of innumerable stone breakdown cycles and the exponential nature of the lithotripsy process (e.g., 1 fragment becomes 16 particles after 4 fragmentation iterations, etc.). The presence of dust particles in the remnants samples is likely due to the fact that the irrigation flow during the separation process was limited to < 100 ml—until the 100 ml container was full. If the separation irrigation process was longer, there would likely be much lower counts of dust in the remnants sample, akin to the ureteroscopic time needed for a retrograde intrarenal surgery procedure or over days post-procedure with the kidney producing urine.
Of interest, we noted the presence of stone dust particles ≤ 250 µm in the 2.0 J × 5 Hz fragmenting setting. This fragmentation setting may in fact translate to a pop-dusting technique, particularly toward the later pulse counts, due to the duration required to apply a total energy of 1000 J in order to maintain consistency of energy applied across all three laser settings in a small glass cuvette [28]. Comparatively, fragmenting lithotripsy techniques would rather rely on a much lower pulse count limited to the breakdown of the initial stone in few fragments for basket extraction. It is still intriguing to see that this high pulse energy setting led to dust in this study, which is an undesirable property for fragmentation purposes, arguably due to potential impairment of vision from produced dust or leading to inefficiency in achieving multiple fragments in as short a possible time. Considering the above, it would be interesting to evaluate which of the current laser technologies is most adequate for fragmentation in future studies.
The authors of the present study want to emphasize that the results found in this study may be inferable to other p-Tm:YAG generators operating at a similar wavelength and with comparable peak powers and pulse durations—although p-Tm:YAG laser generators from differing manufacturers may differ in their properties and shall therefore be evaluated in separate studies. Conversely, the results are not transferable to the TFL, a different laser technology [29] for which a distinct evaluation has been published before [20].
The study has several potential limitations. The present study is an in vitro attempt to assess the p-Tm:YAG laser lithotripsy dusting characteristics that may impact on in vivo surgery. The interpretation of the data must therefore be taken with care since environmental factors arguably may impact on clinical translation of the findings of this study. The size of the initial stones submitted to lithotripsy was rather small and was not standardized; therefore, conclusions on the efficacy of different laser settings cannot be drawn from this study. Rather, the presence and relative number of particles within each stone setting and laser setting was reviewed. Accordingly, stone particle samples were not weighed, but particle numbers counted, and quantified with count categories of different particle sizes, which allowed a semiquantitative analysis of the results. Finally, we noted that there were few large particles of > 250 µm in some dust samples. These few larger particles may have been inadvertently collected as a result of mechanisms such as pop-corning or pop-dusting dynamics during laser lithotripsy and may therefore be understood as sample contaminants that do not impact on the conclusions drawn from this study: The p-Tm:YAG is capable of producing moderate to high amounts of stone dust particles ≤ 250 µm.
Areas for exploration in future studies would include evaluation of stone composition changes with the novel p-Tm:YAG, and investigating mechanisms of relevance for stone dusting that are not perfectly understood yet. It is indeed desirable to have fine stone dust in laser lithotripsy and to refine ways to achieve it.
Conclusions
The novel p-Tm: YAG laser is capable of dusting all seven common human urinary stone compositions, with the production of dust particles ≤ 250 µm, in keeping with prior definition of stone dust. These findings are pivotal for the broader future implementation of the p-Tm:YAG in clinical routine.
Author contributions
JK was responsible for protocol/project development, data collection or management, data analysis and manuscript writing/editing. EV, VDC, MC, AS, FP and FP were involved in protocol/project development, data analysis and manuscript writing/editing. FS, MH, CP and DE contributed to data analysis and manuscript writing/editing. MD took part in data collection or management, data analysis and manuscript writing/editing. OT participated in research concept, data analysis and manuscript writing/editing. EXK assisted with research concept, protocol/project development, data collection or management, data analysis and manuscript writing/editing.
Funding
Open access funding provided by University of Zurich. No funding was received for conducting this study.
Data availability
On request to corresponding author for raw data on the experimental setup.
Declarations
Conflict of interest
Etienne Xavier Keller is a speaker and/or consultant for Coloplast, Olympus, Boston Scientific, Recordati, Debiopharm and Alnylam, and has no specific conflicts of interest relevant to this work. All other authors have no relevant financial or non-financial interests to disclose. The Thulio® p-Tm:YAG laser generator was provided by Dornier MedTech Gmbh® for a short time period specifically dedicated to the present study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geraghty RM, Jones P, Somani BK. Worldwide trends of urinary stone disease treatment over the last two decades: a systematic review. J Endourol. 2017;31(6):547–556. doi: 10.1089/end.2016.0895. [DOI] [PubMed] [Google Scholar]
- 2.Edvardsson VO, Indridason OS, Haraldsson G, Kjartansson O, Palsson R. Temporal trends in the incidence of kidney stone disease. Kidney Int. 2013;83(1):146–152. doi: 10.1038/ki.2012.320. [DOI] [PubMed] [Google Scholar]
- 3.Huang WY, Chen YF, Carter S, Chang HC, Lan CF, Huang KH. Epidemiology of upper urinary tract stone disease in a Taiwanese population: a nationwide, population based study. J Urol. 2013;189(6):2158–2163. doi: 10.1016/j.juro.2012.12.105. [DOI] [PubMed] [Google Scholar]
- 4.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2–3):e86–e96. [PMC free article] [PubMed] [Google Scholar]
- 5.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Jr, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63(5):1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg P, Traxer O. Update on lasers in urology 2014: current assessment on holmium:yttrium–aluminum–garnet (Ho:YAG) laser lithotripter settings and laser fibers. World J Urol. 2015;33(4):463–469. doi: 10.1007/s00345-014-1395-1. [DOI] [PubMed] [Google Scholar]
- 7.Keller EX, De Coninck V, Doizi S, Daudon M, Traxer O. What is the exact definition of stone dust? An in vitro evaluation. World J Urol. 2021;39(1):187–194. doi: 10.1007/s00345-020-03178-z. [DOI] [PubMed] [Google Scholar]
- 8.Matlaga BR, Chew B, Eisner B, Humphreys M, Knudsen B, Krambeck A, et al. Ureteroscopic laser lithotripsy: a review of dusting vs fragmentation with extraction. J Endourol. 2018;32(1):1–6. doi: 10.1089/end.2017.0641. [DOI] [PubMed] [Google Scholar]
- 9.Doizi S, Keller EX, De Coninck V, Traxer O. Dusting technique for lithotripsy: what does it mean? Nat Rev Urol. 2018;15(11):653–654. doi: 10.1038/s41585-018-0042-9. [DOI] [PubMed] [Google Scholar]
- 10.Giusti G, Pupulin M, Proietti S. Which is the best laser for lithotripsy? The referee point of view. Eur Urol Open Sci. 2022;44:20–22. doi: 10.1016/j.euros.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traxer O, Sierra A, Corrales M. Which is the best laser for lithotripsy? Thulium fiber laser. Eur Urol Open Sci. 2022;44:15–17. doi: 10.1016/j.euros.2022.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Ghani KR. Which is the best laser for lithotripsy? Holmium laser. Eur Urol Open Sci. 2022;44:27–29. doi: 10.1016/j.euros.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petzold R, Miernik A, Suarez-Ibarrola R. In vitro dusting performance of a new solid state thulium laser compared to holmium laser lithotripsy. J Endourol. 2021;35(2):221–225. doi: 10.1089/end.2020.0525. [DOI] [PubMed] [Google Scholar]
- 14.Kraft L, Yilmaz M, Petzold R, Gratzke C, Suarez-Ibarrola R, Miernik A. Dusting efficiency of a novel pulsed thulium: yttrium aluminum garnet laser vs a thulium fiber laser. J Endourol. 2022;36(2):259–265. doi: 10.1089/end.2021.0441. [DOI] [PubMed] [Google Scholar]
- 15.Panthier F, Solano C, Chicaud M, Kutchukian S, Candela L, Doizi S, et al. Initial clinical experience with the pulsed solid-state thulium YAG laser from Dornier during RIRS: first 25 cases. World J Urol. 2023 doi: 10.1007/s00345-023-04501-0. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann J, Rosenbaum CM, Netsch C, Gross AJ, Becker B. First clinical experience of a novel pulsed solid-state thulium:YAG laser during percutaneous nephrolithotomy. J Clin Med. 2023 doi: 10.3390/jcm12072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan SR, Hackett RL. Role of organic matrix in urinary stone formation: an ultrastructural study of crystal matrix interface of calcium oxalate monohydrate stones. J Urol. 1993;150(1):239–245. doi: 10.1016/S0022-5347(17)35454-X. [DOI] [PubMed] [Google Scholar]
- 18.Sierra A, Corrales M, Piñero A, Traxer O. Thulium fiber laser pre-settings during ureterorenoscopy: Twitter’s experts’ recommendations. World J Urol. 2022;40(6):1529–1535. doi: 10.1007/s00345-022-03966-9. [DOI] [PubMed] [Google Scholar]
- 19.Keller EX, de Coninck V, Audouin M, Doizi S, Bazin D, Daudon M, et al. Fragments and dust after Holmium laser lithotripsy with or without “Moses technology”: how are they different? J Biophotonics. 2019 doi: 10.1002/jbio.201800227. [DOI] [PubMed] [Google Scholar]
- 20.Keller EX, De Coninck V, Doizi S, Daudon M, Traxer O. Thulium fiber laser: ready to dust all urinary stone composition types? World J Urol. 2020 doi: 10.1007/s00345-020-03217-9. [DOI] [PubMed] [Google Scholar]
- 21.Rasband WS. U. S. National Institutes of Health, Bethesda, Maryland, USA1997–2018. p. ImageJ
- 22.Kraft L, Petzold R, Suarez-Ibarrola R, Miernik A. In vitro fragmentation performance of a novel, pulsed Thulium solid-state laser compared to a Thulium fibre laser and standard Ho:YAG laser. Lasers Med Sci. 2022;37(3):2071–2078. doi: 10.1007/s10103-021-03495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark CS, Gnanappiragasam S, Thomas K, Bultitude M. Cystinuria: an overview of challenges and surgical management. Front Surg. 2022 doi: 10.3389/fsurg.2022.812226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubosq F, Pasqui F, Girard F, Beley S, Lesaux N, Gattegno B, et al. Endoscopic lithotripsy and the FREDDY laser: initial experience. J Endourol. 2006;20(5):296–299. doi: 10.1089/end.2006.20.296. [DOI] [PubMed] [Google Scholar]
- 25.Yates J, Zabbo A, Pareek G. A comparison of the FREDDY and holmium lasers during ureteroscopic lithotripsy. Lasers Surg Med. 2007;39(8):637–640. doi: 10.1002/lsm.20545. [DOI] [PubMed] [Google Scholar]
- 26.Corrales M, Traxer O. Initial clinical experience with the new thulium fiber laser: first 50 cases. World J Urol. 2021;39(10):3945–3950. doi: 10.1007/s00345-021-03616-6. [DOI] [PubMed] [Google Scholar]
- 27.Sierra A, Corrales M, Kolvatzis M, Traxer O. Initial clinical experience with the thulium fiber laser from Quanta System: first 50 reported cases. World J Urol. 2022;40(10):2549–2553. doi: 10.1007/s00345-022-04096-y. [DOI] [PubMed] [Google Scholar]
- 28.Pietropaolo A, Jones P, Whitehurst L, Somani BK. Role of ‘dusting and pop-dusting’ using a high-powered (100 W) laser machine in the treatment of large stones (≥ 15 mm): prospective outcomes over 16 months. Urolithiasis. 2019;47(4):391–394. doi: 10.1007/s00240-018-1076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traxer O, Keller EX. Thulium fiber laser: the new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J Urol. 2020;38(8):1883–1894. doi: 10.1007/s00345-019-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On request to corresponding author for raw data on the experimental setup.