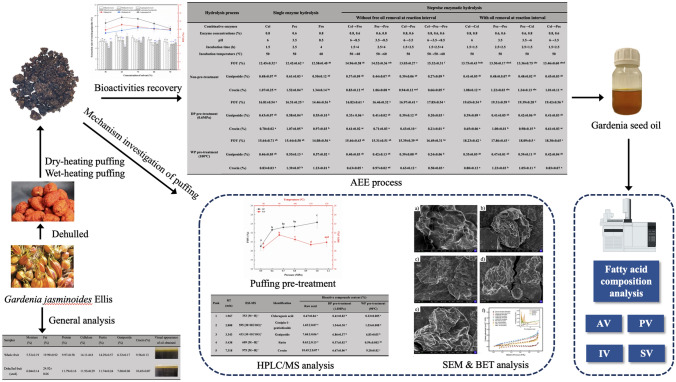
Abstract
Gardenia jasminoides Ellis, a representative for “homology of medicine and food”, can be used to produce pigment and edible oil. Here, aqueous enzymatic extraction (AEE) combined with puffing pre-treatment was explored to prepare oil from gardenia seeds. Both wet-heating puffing (WP) at 90 °C and dry-heating puffing (DP) at 1.0 MPa facilitated the release of free oil by AEE, resulting in the highest free oil yields (FOY) of 21.8% and 23.2% within 3 h, much higher than that of un-puffed group. Additionally, active crocin and geniposide were also completely released. The FOY obtained was much higher than mechanical pressing method (10.44%), and close to solvent extraction (25.45%). Microstructure analysis indicated that gardenia seeds expanded by dry-heating puffing (1.0 MPa) had a larger, rougher surface and porous structure than other groups. Overall, AEE coupled with puffing pre-treatment developed is an eco-friendly extraction technology with high efficiency that can be employed to oil preparation.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01319-9.
Keywords: Gardenia seed oil, Puffing pre-treatment, Aqueous enzymatic extraction, Free oil yield, Crocin, Geniposide
Introduction
Gardenia jasminoides Ellis (GjE), an evergreen shrub widely distributed in Asian countries, is always used for making tea, cooking porridge and coloring dishes, whose ripe fruit attain a reddish yellow color (Li et al., 2020; Wang et al., 2022). The desiccative ripe fruits of GjE have been applied in China for centuries as both a food and medicinal substance (Chen et al., 2020). Up to present, several useful chemical constituents have been isolated from GjE and characterized, mainly including geniposide, geniposidic acid, genipin, crocins and their derivatives. Various physiological function has been reported, including anti-inflammatory, antioxidant properties, anti-hypertension, anti-hyperglycemia, anti-cancer, anti-hyperlipidemia, neuroprotection and hepatoprotection. Furthermore, the crude fat content takes up nearly 20% of the dried fruit, and its oil level is equivalent to that of soybean, which can be applied to oil production (Chyau et al., 2022; Yin and Liu, 2018). According to previous study, gardenia oil are rich in unsaturated fatty acids, particularly palmitic acid and linoleic acid, which exhibit pharmacological effects on regulating blood pressure, body fat metabolism, and reducing serum cholesterol (Cai et al., 2015). Tao et al. (2014) stated that the gardenia oil may contain effective constituents to be used for depression therapy. Thus, gardenia oil can be regarded as a kind of oil with excellent quality and health benefits. Nowadays, plant oils (especially nut oils) have been applied by the food industry to develop nutraceuticals due to their healthy fatty acid composition, richness in sterols, fat-soluble vitamins, phenolic compounds and distinct flavors (Ferreira et al., 2022). However, a large amount of gardenia meals is discarded as waste after extraction of gardenia yellow pigment. Therefore, preparation of valued oil from discarded gardenia meals is essential, not only for increasing the value of GjE, but also for meeting the growing demand for natural healthy plant oils.
Various methods have been used for extracting oil from gardenia fruit, including cold pressing extraction (CPE), solvent extraction (SE), supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE) were commonly used methods, each with its own advantages and limitations (Xiao et al., 2017). CPE can greatly preserve the unique flavor of oils, but has low yield and high labor intensity, resulting in higher production cost (Xu et al., 2021). Three conventional extraction methods including cold pressing, petroleum ether extraction and ultrasound-assisted extraction (UAE) methods were compared by Cai et al. (2015). The oil extraction yields of 8.59%, 7.4%, 5.6% and 10.89% were obtained for UAE, petroleum ether extraction, cold pressing and Soxhlet extraction, indicated the high extraction efficiency of UAE. Nevertheless, SE is generally applied for on large-scale oil extraction. Extensive refining can result in the loss of active compounds and presence of residual solvent, which limited the use of SE methods in minor oilseeds with high value. He et al. (2010) and Tao et al. (2014) explored supercritical carbon dioxide (SC-CO2) extraction of oil from whole gardenia fruits; the extraction pressure, temperature and CO2 flow rate were optimized and the highest oil yields of 9.77% and 11.48% was obtained. However, high investment costs and small extraction capacity may hinder its widely use (Jha and Sit, 2022). Recently, AEE has emerged as a promising plant oil extraction technology, which is eco-friendly, cost-saving, and mild in reaction conditions,while preserving the health benefits of plant oils. AEE has been applied in the extraction of sunflower seeds, camellia seeds, rice bran, peanuts (De Aquino et al., 2022; Jiang et al., 2010; Meng et al., 2018; Xu et al., 2021). Gardenia fruit composition was unique compared with these oilseeds. However, limited literature is available on the extraction of gardenia oil by AEE, so it is necessary to explore this method for oil preparation from gardenia fruit.
For AEE process, the degree of disruption of the oleaginous materials cell walls by enzymes is an important factor in term of oil yield and extraction efficiency. Puffing pre-treatment on gardenia seeds before hydrolysis, may be helpful in improving extraction efficiency. Puffing involves subjecting the oleaginous material to a sudden decline in pressure at high temperature and pressure, resulting in a porous texture of food matrix (Kim et al., 2018). High pressure can be achieved by heating vaporization, superheated steam, mechanical extrusion, compressed air treatment in a closed cavity. However, the specific effects of each puffing method and their impact on yield and quality of oil by AEE are not clear.
In this work, gardenia seeds are first pre-treated using various puffing methods, followed by recovery of bioactive crocin and geniposide using 60% isopropanol which will decrease their involvement into the emulsion. The obtained gardenia meals were used to prepare oil by AEE, and puffing conditions and hydrolysis parameters were optimized in term of FOY and retention of active components. Furthermore, we evaluate the microstructure, specific surface area, pore volume, and pore size of puffed gardenia seeds to investigate the degree of cell wall damage. The quality of the gardenia seed oil was compared with oil obtained through traditional methods in terms of nutritional composition and quality.
Materials and methods
Plant materials and chemicals
Gardenia jasminoides Ellis fruits were purchased from the local market (Hangzhou, China). Cellulase (EC 3.2.1.4 from Aspergillus niger, enzyme activity, 10,000 U/g) and pectinase (EC 3.2.1.15 from Aspergillus niger, enzyme activity, 30,000 U/g) were obtained from Aladdin Bio-Chem Technology, Shanghai, China. Alcalase (alkaline serine endopeptidase from Bacillus licheniformis, enzyme activity, 280,800 U/g) was purchased from Novozyme (TianJin, China). Geniposide (≥ 98%), crocin (≥ 95%, composition: crocin-I, 74.9%; crocin-II, 15.5%; crocin-III, 9.65%) and chlorogenic acid (≥ 98%) were obtained from Sigma-Aldrich Co. (Shanghai, China). Rutin (≥ 98%) and genipin-1-gentiobioside (≥ 98%) were obtained from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China). All other chemicals and solvents used were of analytical and chromatographic grade.
Nutritional composition
The moisture (AOAC 934.06), protein (AOAC 950.48), fat (AOAC 963.15) of the whole and the dehulled gardenia fruits were determined according to the AOAC (1990) methods. Carbohydrate was analyzed according to method reported by Rehman et al. (2001). The pectin and cellulose were determined by colorimetric hydroxyl-phenyl-phenol method and anthrone colorimetry method as described by Wang et al. (2021).
Puffing pre-treatment
Dry-heating puffing (DP)
The dehulled samples were first adjusted the moisture to 12%, then placed into puffing tank (B-H, Hangzhou Chengyuan Trading Co., LTD, Hangzhou). The puffing tank is heated by charcoal fire while rotating and stirring until the gauge inside the tank rises to 0.5, 0.6, 0.7, 0.8, 1.0 MPa, and then the pressure is instantly released to atmospheric pressure. The puffing process finishes.
Wet-heating puffing (WP)
The dehulled samples were soaked in deionized water to rehydrate to 12%, and then sent it into the puffing tank. The puffing tank was pressurized by air compressor, and steam was injected into the puffing tank. When the temperature rose to 80, 90, 100, 110, 120 °C, the pressure was adjusted to 1.0 MPa and kept for 5 min. Vacuum valve connecting vacuum tank and puffing tank was opened, the puffing pre-treatment was completed at this moment. Then the puffed sample was dried at 80 °C for 2–3 h until the desired moisture content (4–5%) was reached (Zhang et al., 2021a).
Aqueous enzymatic extraction process
Dried gardenia seeds were pulverized by using a Moulinex AR1044 grinder and screened through a 40-mesh sieve. Before hydrolysis, the gardenia powder was first extracted with methanol, ethanol or isopropanol at a ratio of 1:5 (w/v) assisted by ultrasound (100 W for 30 min) to remove bioactivities, which may facilitate the emulsification during AEE. The residual pomace was mixed with distilled water at a ratio of 1:7 (w/v), and subjected to heat treatment of 90 °C for 10 min. When the mixture was cooled down to desired temperature, various enzyme was added and enzymatic hydrolysis processes was performed at optimal condition of each enzyme. The specific enzyme hydrolysis conditions are presented in Table 2.
Table 2.
Free oil yield and its geniposide and crocin content under different enzymatic hydrolysis process and different pre-treatment
| Hydrolysis process | Single enzyme hydrolysis | Stepwise enzymatic hydrolysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without free oil removal at reaction interval | With oil removal at reaction interval | ||||||||||
| Combinative enzymes | Cel | Pec | Pro | Cel → Pro | Pec → Pro | Cel → Pec | Cel → Pec → Pro | Cel → Cel | Pec → Pec | Pec → Cel | Cel → Pec |
| Enzyme concentrations (%) | 0.8 | 0.6 | 0.8 | 0.8, 0.6 | 0.6, 0.8 | 0.8, 0.6 | 0.8, 0.6, 0.6 | 0.8, 0.8 | 0.6, 0.6 | 0.6, 0.8 | 0.8, 0.6 |
| pH | 6 | 3.5 | 8.5 | 6 → 8.5 | 3.5 → 8.5 | 6 → 3.5 | 6 → 3.5 → 8.5 | 6 | 3.5 | 3.5 → 6 | 6 → 3.5 |
| Incubation time (h) | 1.5 | 2.5 | 4 | 1.5 + 4 | 2.5 + 4 | 1.5 + 2.5 | 1.5 + 2.5 + 4 | 1.5 + 1.5 | 2.5 + 2.5 | 2.5 + 1.5 | 1.5 + 2.5 |
| Incubation temperature (°C) | 50 | 50 | 60 | 50 → 60 | 50 → 60 | 50 | 50 → 50 → 60 | 50 | 50 | 50 | 50 |
| Non-pre-treatment | |||||||||||
| FOY (%) | 12.45 ± 0.32a | 12.42 ± 0.62a | 12.58 ± 0.40ab | 14.96 ± 0.58de | 14.52 ± 0.36cde | 13.03 ± 0.27e | 15.32 ± 0.31f | 13.75 ± 0.43bcde | 13.50 ± 0.17abcd | 13.36 ± 0.75abc | 13.46 ± 0.60abcd |
| *Geniposide (%) | 0.48 ± 0.07ab | 0.61 ± 0.03a | 0.50 ± 0.12ab | 0.37 ± 0.09ab | 0.44 ± 0.07ab | 0.39 ± 0.06ab | 0.27 ± 0.09b | 0.41 ± 0.05ab | 0.48 ± 0.0.07ab | 0.48 ± 0.02ab | 0.45 ± 0.03ab |
| *Crocin (%) | 1.07 ± 0.25ac | 1.52 ± 0.04b | 1.34 ± 0.14bc | 0.83 ± 0.12ad | 1.06 ± 0.08ac | 0.94 ± 0.12acd | 0.66 ± 0.05d | 1.08 ± 0.12ac | 1.22 ± 0.03abc | 1.24 ± 0.13abc | 1.01 ± 0.11ac |
| DP pre-treatment (0.6 MPa) | |||||||||||
| FOY (%) | 16.81 ± 0.54a | 16.51 ± 0.25a | 14.46 ± 0.36b | 16.82 ± 0.61a | 16.46 ± 0.32a | 16.97 ± 0.41a | 17.85 ± 0.54c | 19.65 ± 0.34d | 19.31 ± 0.59d | 19.39 ± 0.28d | 19.42 ± 0.56d |
| *Geniposide (%) | 0.43 ± 0.07ab | 0.58 ± 0.04b | 0.55 ± 0.10b | 0.33 ± 0.06a | 0.41 ± 0.02ab | 0.39 ± 0.12ab | 0.20 ± 0.03c | 0.39 ± 0.09a | 0.41 ± 0.05ab | 0.42 ± 0.06ab | 0.41 ± 0.03ab |
| *Crocin (%) | 0.70 ± 0.02a | 1.07 ± 0.05b | 0.97 ± 0.03b | 0.61 ± 0.02ac | 0.71 ± 0.03a | 0.43 ± 0.10c | 0.21 ± 0.01d | 0.65 ± 0.06a | 1.00 ± 0.01b | 0.98 ± 0.15b | 0.61 ± 0.03ac |
| WP pre-treatment (100 °C) | |||||||||||
| FOY (%) | 15.64 ± 0.71ab | 15.44 ± 0.50ab | 14.08 ± 0.56a | 15.46 ± 0.43ab | 15.31 ± 0.51ab | 15.39 ± 0.39ab | 16.69 ± 0.31bc | 18.23 ± 0.42c | 17.86 ± 0.43c | 18.09 ± 0.5c | 18.30 ± 0.65c |
| *Geniposide (%) | 0.46 ± 0.05ab | 0.55 ± 0.13a | 0.57 ± 0.02a | 0.40 ± 0.03ab | 0.42 ± 0.13ab | 0.39 ± 0.08ab | 0.24 ± 0.06b | 0.35 ± 0.05ab | 0.47 ± 0.01ab | 0.39 ± 0.11ab | 0.42 ± 0.04ab |
| *Crocin (%) | 0.83 ± 0.03a | 1.39 ± 0.07b | 1.23 ± 0.01b | 0.63 ± 0.05c | 0.97 ± 0.02ad | 0.63 ± 0.12c | 0.50 ± 0.03c | 0.80 ± 0.12a | 1.22 ± 0.03b | 1.05 ± 0.11d | 0.83 ± 0.07a |
Values were the mean ± SD of three gardenia seed oils which was analyzed in triplicate. Significant differences between the two processes in the same column without the same letter (p > 0.05). *Crocin, geniposide (%) means mass percentage relative to hydrolysate obtained by AEE
Single enzymatic hydrolysis
Single enzymatic hydrolysis means only one kind of enzyme was used throughout the hydrolysis process. The enzyme was added after adjusting the pH of hydrolysis solution to the optimal pH for each enzyme (using 0.1 M NaOH or HCl aqueous solutions). The enzyme hydrolysis was carried out with constant horizontal shaking at rate of 150 rpm under optimal temperature (Table 2). After the reaction, the mixture was heated to 100 °C for 10 min to deactivate the enzyme. The enzymatic hydrolysate was centrifuged at 8000×g for 20 min at 4 °C. The upper free oil was extracted three times using 15 ml of n-hexane. The extraction was subjected to rotary evaporation to remove the hexane, and free oil yield was calculated (Díaz-Suárez et al., 2021).
Stepwise enzymatic hydrolysis
Stepwise enzymatic hydrolysis means that the enzymatic hydrolysis was performed step by step, one kind of enzyme was used each step and multi enzymes were involved this process. After hydrolysis by the one enzyme finish, the resulting oil may or may not be removed, then the mixture was adjusted to the optimal pH and temperature for another enzyme, the next stage enzymatic hydrolysis by another enzyme was proceeded. Last, the free oil was recovered and calculated the free oil yield. The optimal conditions including solid to liquid ratio (w/w), pH, temperature and extraction time of the hydrolysis were obtained through single-factor experiments. Free oil yield (FOY) was calculated as the mass of free oil extracted (g) divided by the mass of the oil seeds used (g) and then multiplied by 100.
Cold pressing extraction and Soxhlet extraction process
Cold pressing extraction (CPE) and solvent extraction (SE) process were proceeded according to the previous publication with minor modifications (Polmann et al., 2019; Tang et al., 2021). Gardenia fruits was placed into a CA 59G KOMET press (IBG Monforts GmbH & Co., Germany) at the speed of 25 r/min, the whole process is allowed to proceed under 70 °C. The oil was recovered by centrifugation (4500 r/min, 10 min). For Soxhlet extraction, 5 g gardenia powder and 150 mL petroleum ether were added into a Soxhlet apparatus, extraction was performed under 80 °C for 8 h. After extraction, the extract was collected and evaporated under reduced pressure with a rotary evaporator Model RE-2000A (Yarong Biochemical Instrument Factory, Shanghai, China) at 45 °C. The remained oil was dried under nitrogen flow, weight and calculate the oil yields. The oil obtained was kept at 4 °C until use.
Scanning electron micrographs (SEM)
The morphology of raw gardenia seed powder or puffed gardenia powder was investigated with the field emission scanning electron microscope (SEM, Zeiss Gemini 500, Germany). Firstly, samples were fixed on the aluminum plate with double-sided carbon tape, then sprayed with a thin layer of gold–palladium at 5 kV. Finally, samples were observed at an accelerating voltage of 5 kV with a magnification factor of ×2500 (Hu et al., 2019).
Brunauer–Emmett–Teller (BET) specific surface area, pore volume and pore size
The BET specific surface area, the volume and size distribution of pores in puffed gardenia seeds were determined by an automatic rapid surface area and microporous analyzer (ASAP 2010, Micromeritics, USA). N2 was selected as the adsorption and desorption gas. Puffed gardenia seeds were placed in the measuring cell to degas water by heating at 150 °C for 12 h at least. Afterwards, the measuring cell was placed in an insulated tank filled with liquid nitrogen to keep the sample at − 196 °C throughout the measurement. The specific surface area was determined by the BET isotherm multipoint method in the relative pressure (P/P0) range of 0–1.0. Pore volume and pore size distribution were obtained from the desorption isotherms by Barrett–Joyner–Halenda method (Beluns et al., 2021).
HPLC and HPLC–MS analysis for bioactive compounds
Geniposide, crocin and other bioactive compounds in raw and puffed gardenia seeds were extracted with 60% ethanol under reflux in triplicate according to our previous research (Meng et al., 2020). The extraction was used for analysis of bioactive compounds by HPLC and HPLC–MS.
Geniposide and crocin in extraction were determined by a HPLC (Waters E2695) coupled with a Agilent SB-C18 (4.6 250 mm, 5 μm) column. For hydrolysate from AEE, it was first subjected to vacuum freeze drying, then extraction and determination as the same method above. Acetonitrile:water = 50:50 was used as mobile phase for isometric elution. The flow rate was 1.0 mL/min, and the injection volume was 10 μL. Peak area at 238 nm and 440 nm were measured respectively for quantitation of geniposide and crocin according to each calibration.
Bioactive compounds in extraction including chlorogenic acid, genipin-1-gentiobioside and rutin were subjected to HPLC–MS analysis. HPLC conditions followed the same method above. MS conditions were as follows: negative ion mode, ESI capillary voltage: 3.0 kV, capillary temperature: 325 °C, nebulizing gas N2: 10 L/min, drying gas N2: 10 L/min, scan range between 100 and 1200 m/z (Feng et al., 2023).
Evaluation of gardenia seed oil properties
Physicochemical features
Samples were characterized according to the official methods of AOCS (1997) as follows: acid value (Cd 3d-63), peroxide value (Cd 8-53), iodine value (Cd 1d-92). Saponification value was determined based on the protocol issued by the Ministry of Agriculture (China) of GB/T 5534-2008 (Gai et al., 2013).
Fatty acid composition analysis
The fatty acid composition was determined according to the IUPAC method 2.301, after fatty acid methyl esters (FAMEs) of gardenia seed oil (Nguyen et al., 2020). GC–MS analysis was performed on a Thermo 1300 gas chromatography/mass spectrometer (Varian, Santa Clara, CA, USA), equipped with an DB-Wax silica capillary column (60 m × 0.32 mm × 0.25 u). The mass spectrometer was operated in positive ion mode with an ionization energy of 70 eV. The GC–MS analysis conditions were as follows: initial capillary operated temperature of 90 °C (held for 5 min), raised to 200 °C at 10 °C/min (kept for 10 min), increased to 220 °C at 0.5 °C/min (kept for 5 min), raised to 240 °C at 5 °C/min, and maintained for 10 min.The temperatures for injector, detector and ion source were 230, 250 and 230 °C, respectively. Oxygen-free nitrogen was used as carrier gas with a split ratio of 1:40. Mass units were monitored from m/z 40 to 500. The components were designated by comparison to the NIST library mass spectra. The quantity of fatty acids was calculated by the peak area normalization law and represented as the relative percent of each fatty acid to the total fatty acids (Liu et al., 2019).
Statistical analysis
All the samples (each sample with replicates data) were analyzed and the average value for each sample was performed using the tool available in OriginLab 2022b (MA, USA). The results are expressed as the mean ± standard error. The difference between data groups were tested by one way ANOVA and Duncan’s multiple range tests (SPSS for Windows software release 18; SPSS Inc., New York, USA). The chosen level was considered significant when p < 0.05.
Results and discussion
Nutritional composition analysis for gardenia fruits and gardenia seeds
Table 1 shows the proximate composition of gardenia fruits including geniposide, and crocin. The moisture content of the whole gardenia fruit contained moisture was 5.53 ± 0.19%, the crude fat was 19.99 ± 0.92%, crude protein 9.97 ± 0.58%, cellulose 14.11 ± 0.57%, pectin 14.29 ± 0.57%, geniposide 6.32 ± 0.17% and crocin content was 9.56 ± 0.13%. The nutritional composition was similar to those reported by Chyau et al. (2022). After the removal of shell, the contents of moisture, fat, protein, geniposide and crocin in gardenia seed were increased to 6.04 ± 0.14%, 24.92 ± 0.06%, 11.79 ± 0.16%, 7.68 ± 0.06%, and 10.45 ± 0.07%, while cellulose and pectin decreased to 11.93 ± 0.29% and 11.74 ± 0.24%, indicating that the lipids, geniposide and crocin tend to be enriched in the gardenia seeds, while the peel is rich in cellulose and pectin. It is worth noting that the crude fat content account for nearly 25% of the gardenia seeds, equivalent to the oil content of soybean, which showed a tremendous potential of gardenia seeds to be used as valuable oil crop. The full gardenia fruit and dehulled gardenia seed were hydrolyzed with cellulase with a pH of 6.0 at 50 °C for 1.5 h. The pictures for the obtained products were shown in Table 1. Apparently, severe emulsification was found for the full gardenia hydrolysate so as to no free oil was extracted. This may due to too much polysaccharide (especially pectin) was released from peel, being involved into the emulsion. For gardenia seeds hydrolysate, clear oil–water interface was found, and free oil yield of 9.07 ± 0.22% was obtained. Based on these finding, gardenia seeds were used to prepare oil by AEE in subsequent experiments.
Table 1.
Proximate composition, geniposide and crocin content in the gardenia fruit
| Samples | Moisture (%) | Fat (%) | Protein (%) | Cellulose (%) | Pectin (%) | Geniposide (%) | Crocin (%) | Visual appearance of oil obtained |
|---|---|---|---|---|---|---|---|---|
| Whole fruit | 5.53 ± 0.19 | 19.99 ± 0.92 | 9.97 ± 0.58 | 14.11 ± 0.8 | 14.29 ± 0.57 | 6.32 ± 0.17 | 9.56 ± 0.13 |  |
| Dehulled fruit (seed) | 6.04 ± 0.14 | 24.92 ± 0.06 | 11.79 ± 0.16 | 11.93 ± 0.29 | 11.74 ± 0.24 | 7.68 ± 0.06 | 10.45 ± 0.07 |  |
Aqueous enzymatic extraction for gardenia seed oil
Bioactive compounds recovery by solvent extraction and its impact on free oil yield by AEE
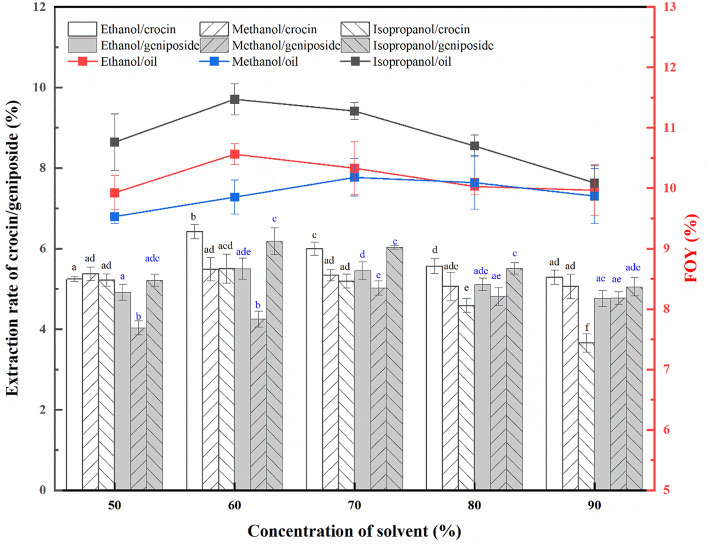
Geniposide and crocin are the major bioactive components in gardenia fruit, with a wide range of pharmacological properties such as hepatoprotective, choleretic, neuroprotective, anti-inflammatory, antioxidant, antitumor, anti-apoptotic and anti-diabetic activities (Tian et al., 2022). Hence, before AEE process, recovering geniposide and crocin by solvent was essential for value-added full utilization of gardenia crop. The solvent used should be polar enough to extract geniposide and crocin, but against hydrophobic oil. So, methanol, ethanol and isopropanol solutions at different concentration were used to extract geniposide and crocin, the residual meal were hydrolyzed by cellulase to prepare gardenia seed oil. Yields for geniposide, crocin and FOY were shown in Fig. 1. Result indicated that 60% ethanol extraction resulted in the optimum crocin yield of 6.42 ± 0.17%, while 60% isopropanol gave the best geniposide yield of 6.18 ± 0.34% and the highest free oil yield of 11.46 ± 0.30%. The higher removal for amphiphile geniposide and crocin by 60% isopropanol may decreased their involvement into emulsion, produced a higher FOY. Thus, the removal of geniposide and crocin with 60% isopropanol was desired before performing AEE for gardenia seeds.
Fig. 1.
The extraction rate of geniposide, crocin from gardenia seeds by various solvent at different concentration and corresponding oil yields followed by aqueous cellulase hydrolysis. Different letters (a–e) over bars represent significant differences among extraction rate of geniposide/crocin with various solvents and concentration (p < 0.05)
Gardenia seed oil preparation by various aqueous enzymatic extraction processes
In the oil crop cells, oils are usually present in lipid cells surrounded by components such as lignin, pectin, hemicellulose, cellulose and protein in form of a complex that binds other macromolecules (Li et al., 2011). In view of characteristics composition, embodied by high level of cellulose, pectin and low level of protein and carbohydrate, for gardenia seeds, cellulase (Cel), pectinase (Pec), protease (Pro) or their combination was used to disrupt the cell wall and lipid complex. Under each optimal conditions including pH, temperature, and enzyme load, AEE processes were performed. The FOY and geniposide, crocin level in hydrolysate were determined and compared, as shown in Table 2. For single enzymatic hydrolysis, regardless of using Cel, Pec or Pro, FOYs were all between 12.42 and 12.58%, no significant difference was found among groups. However, the optimal hydrolysis time for Cel is the shortest with value of 1.5 h compared with 2.5 h for Pec and 4 h for Pro.
To enhance the oil preparation performance, sequential combined hydrolysis by multiple enzymes was performed. Data suggested that Cel → Pec increase the FOY to 13.03%, but did not reach our expected level. Pec → Pro and Cel → Pro further improve the FOY to 14.52% and 14.96% within 6.5 and 5.5 h, respectively. Moreover, Cel → Pec → Pro produced the highest FOY of 15.32% in 8 h. This results was in line with those reported by Liu et al. (2019) and Wei et al. (2022) reported when preparing S. mukorossi seed kernels oil and Cinnamomum camphora seeds oil by using AEE method. Commonly, the choice of enzyme depends on the cell composition of the oil-bearing material. Cellulose, hemicellulose and pectin are the three main phytochemical components that constitute the primary wall, secondary wall and middle lamella of the cell of oil crops (Hu et al., 2020). In addition, the protein network of the lipid-based membranes surrounding the lipid corpuscles need to be effectively dissolved and hydrolyzed to release oil from the cell. Nevertheless, complex hydrolysis process and long hydrolysis time as 8 h for Cel → Pec → Pro group were not desired industrially. During stepwise hydrolysis, oil product removal between stage-hydrolysis did not further improve the FOY for Cel → Cel, Pec → Pec, Pec → Cel and Cel → Pec combinations, with values between 13.36% to 13.75%.
Oil preparation by AEE from puffed gardenia seeds under various conditions
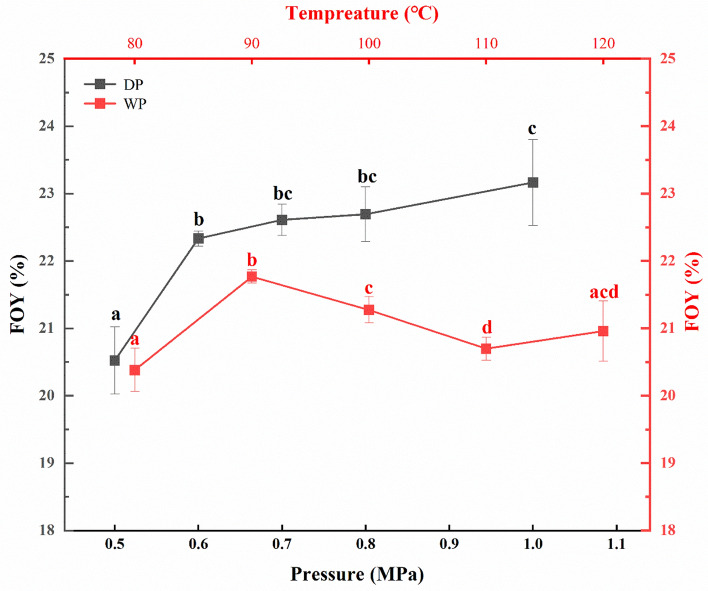
In view of high crude fat content of 25% for gardenia seeds, however only 15% oil was extracted. Even after eliminating product inhibition, sequential enzymatic hydrolysis still could not reach a satisfied FOY. These indicated that oilseed structure was not destroyed completely by AEE process resulted in inadequate release for bound oil. Puffing was reported to be an effective method to create a porous structure so as to expand the apparent volume of oilseed. Before AEE, the dry-heat puffing (0.6 MPa) and wet-heat puffing (100 °C) were performed for gardenia seed, and the FOYs were compared with those from un-puffed seeds and listed in Table 2. The data suggested that the effect of puffing is obvious, and dry-heat puffing is superior to wet-heat puffing in term of oil release by AEE. For single enzymatic hydrolysis process, the FOYs increased from about 12% for control to 14.08–15.64% for wet-heat puffing at 100 °C and 14.46–16.81% for dry-heating puffing at 0.6 MPa. While for stepwise enzymatic hydrolysis process, the FOYs were further increased to 15.31–18.30% and 16.46–19.65%, respectively. What should be particular noted is when dry-heating gardenia seeds puffed at 0.6 MPa was hydrolyzed by Cel → Cel produced the highest FOY of 19.65% within 3 h. Thus, Cel → Cel two-stage hydrolysis was used to compare impacts of puffing at various temperatures (80–120 °C) and pressures (0.5–1.0 MPa) on FOY. In addition, to prevent emulsion involved by hydrolysate, calcium iron was added to precipitate polysaccharide according to our previous findings (Meng et al., 2018; Peng et al., 2019). The selection for range of puffing parameter (temperature and pressure) referred to those reported by Yang et al. (2022) and Zhang et al. (2021a) with minor modifications. The results were showed in Fig. 2, for dry-heating puffing, FOYs increased from 20.52 to 23.16% as puffing pressure increased from 0.5 to1.0 MPa. In view of higher free oil recovery of 93.0% (yields of 23.16%) by puffing at 1.0 MPa, no further increased pressure was considered, otherwise which would result in much loss of active compound in gardenia seeds (refer to Table 3). When wet-heat puffing was performed with pressure was set at 1.0 MPa, the puffing temperature point is between 80–120 °C, and corresponding FOYs fall into range of 20.38–21.76%. The optimal temperature for WP was 90 °C, the corresponding FOY is 21.76%, which is slightly lower than that for dry-heating puffing. Both are higher than that of non-pre-treatment group with value of 68.44% and 58.25% higher, respectively. With respect to this obvious enhancing effects on oil yields during AEE by puffing pretreatment, the possible mechanism was elucidated as following: The rapid evaporation of water and air inside the seed resulted from a sudden pressure drop by puffing (both DP and WP) would produce a strong tearing force, which broke down the microstructure of oilseeds, expanded its volume, produced rough and porous structures, and enable large ratio surface area exposed to enzyme and solvent. One aspect, high destroy degree for cell structure of gardenia seeds facilitate the enzymatic hydrolysis performances, resulted in higher oil release from seeds. Another aspect, larger surface area exposed to isopropanol may enhance the extraction of amphiphilic crocin and geniposide as well as polysaccharides, decreasing their involvement into emulsification during AEE. The amphiphilic substance would result in emulsification embedding large amount of oil. The polysaccharides may help stabilizing the emulsion formed (Meng et al., 2018). Both aspects all accounted for the enhanced oil yields during AEE process acted by puffing.
Fig. 2.
The yields of free oil from gardenia seeds puffed at different conditions obtained by using AEE method. Different letters (a–c) over bars represent significant differences among FOY with various pressures/temperatures (p < 0.05)
Table 3.
Effects of different pre-treatments on the content of bioactive compounds in gardenia seeds
| Peak | RT (min) | ESI–MS | Identification | Bioactive compounds content (%) | ||
|---|---|---|---|---|---|---|
| Raw seed | DP pre-treatment (1.0 MPa) | WP pre-treatment (90 °C) | ||||
| 1 | 1.967 | 353 [M−H]− | Chlorogenic acid | 0.47 ± 0.04a | 0.41 ± 0.02b | 0.23 ± 0.005c |
| 2 | 2.808 | 595 [M+HCOO]− | Genipin-1-gentiobioside | 1.65 ± 0.07a | 1.54 ± 0.30a | 1.53 ± 0.008a |
| 3 | 3.343 | 433 [M+HCOO]− | Geniposide | 7.68 ± 0.06a | 6.86 ± 0.37b | 6.83 ± 0.05b |
| 4 | 5.438 | 609 [M−H]− | Rutin | 0.63 ± 0.13a | 0.57 ± 0.02b | 0.59 ± 0.002ab |
| 5 | 7.318 | 975 [M−H]− | Crocin | 10.45 ± 0.07a | 6.67 ± 0.06b | 9.20 ± 0.02c |
Values were the mean ± SD of three gardenia seed oils which was analyzed in triplicate. The average values in the same row followed by the same superior letters were not significantly different (p > 0.05)
Besides of puffing, microwave (Gai et al., 2013; Liu et al., 2022), ultrasonic (Liu et al., 2019; Liu et al., 2022), extrusion (Jung et al., 2009), high pressure (Zhang et al., 2021b) are also commonly used pretreatment method for oil preparation by AEE. It was reported that these methods enhanced oil extraction, with increases in oil yield varied from 8.3 to 44.4% (Ferreira et al., 2022). This highlighted the advantages of puffing compared with other pretreatment methods when coupled with AEE for oil preparation. Puffing technique was mature and easy to scale up industrially. It is also suitable for samples with high moisture content, unlike other pre-treatment methods that require deeper drier material (Sun et al., 2021).
Microstructure morphology of gardenia seeds puffed under various conditions
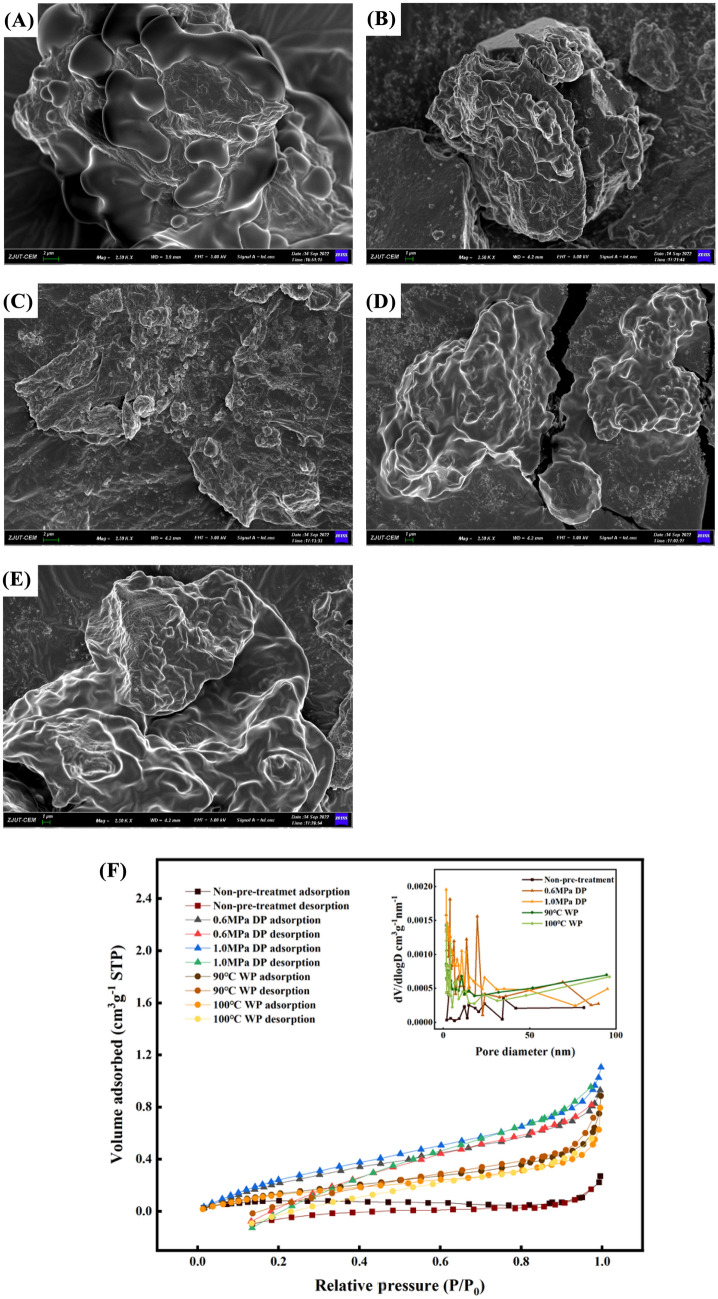
The morphology of microstructure for gardenia seeds puffed by various ways were characterized by using the field emission scanning electron microscope, and shown in Fig. 3. It was observed that electron micrographs for raw gardenia seed presents a compact structure and a smooth surface. After puffing, the microstructures for gardenia seeds became rough, full of irregular pore sizes and occasional large cavities, indicating that the rapid evaporation of water resulted from a sudden pressure drop by puffing produces a strong tearing force, which broke down the microstructure of gardenia seeds and exposed large ratio surface area to enzyme and solvent. Obviously, dry-heating puffing creates more destructive power in comparison with wet-heating puffing, and expansion degree increased as puffing pressure raised from 0.6 to 1.0 MPa. For wet-heating puffing at 1.0 MPa, less difference in microstructure for oilseeds was observed when temperature is increased between 90 and 100 °C, however it seems that puffing at 90 °C produce more rough structure which is more suitable for oil extraction by AEE (Wen et al., 2022).
Fig. 3.
Microstructure analysis of puffed gardenia seeds. (A–E) SEM images of gardenia seeds under different pre-treatment methods [A raw seeds, B DP (0.6 MPa), C DP (1.0 MPa), D WP (90 °C), E WP (100 °C)]. (F) Nitrogen adsorption isotherms and pore size distribution of gardenia seeds under different pre-treatment methods
Surface area, pore volume, and pore size of puffed gardenia seeds
The porous structure of seeds had a positive effect on the contact between enzymes and active sites during AEE process. Figure 3(F) showed the N2 adsorption–desorption isotherm for raw gardenia seeds, DP and WP treated seeds. According to the IUPAC classification, all groups of gardenia seeds exhibited characteristic type-IV hysteresis loop, related to the mesoporous structures (Wang et al., 2022). Based on the BET analysis, the results of BET surface area, pore volume, and pore size were listed in Table S1. Compared with un-puffed seeds (0.3336m2/g), seeds under 1.0 MPa DP pre-treatment (1.4219m2/g) exhibited a much larger BET surface area and similar rule was found for pore volume, indicating more porous property. This was due to the extreme explosion conditions which provided the seeds with DP pre-treatment (1.0 MPa) with excellent porosity, thus the enhanced performance of DP pre-treatment was verified from effective destroys of the cell structure of gardenia seeds, Moreover, the average pore radius of DP pre-treatment seeds was smaller than that of non-pre-treatment seeds by 1.7–7.5%, which might be explained by the dense structure of DP pre-treatment seeds at the surface (Huang et al., 2022).
HPLC–MS analysis of the heat-sensitive bioactive compounds in puffed gardenia seeds
The preparation of gardenia yellow pigment is the primary application for gardenia fruit. While the functional components like crocin in gardenia seed are thermally unstable. Higher temperature and pressure environment generated during puffing may be detrimental. Hence, its specific impact on active components should be clarified. Here, the main functional components in raw gardenia seeds and puffed gardenia seeds were extracted by 60% isopropanol, and the main products including chlorogenic acid (Rt 1.967 min), genipin-1-gentiobioside (Rt 2.808 min), geniposide (Rt 3.343 min), rutin (Rt 5.438 min), crocin (Rt 7.318 min) were identified using HPLC–ESI–MS, and quantified with HPLC coupled diode array detector by monitoring absorption at 240 nm and 440 nm for geniposide and crocin. The chromatograph and composition were shown in Fig. S1. The impact of puffing ways on their composition was showed in Table 3. Except for genipin-1-gentiobioside, all the active components including geniposide, crocin, rutin and chlorogenic acid decreased significantly through puffing pre-treatment against non-pre-treatment group. The worst loss was observed for chlorogenic acid, with values of 51.1% and 12.8% for wet-heating puffing and dry-heating puffing, indicating its sensitivity to damp heat than to dry heat. On the contrary, crocin is more prone to be destroyed upon dry heat puffing, with content decreased to 6.67% from 10.45% (control group), compared to 9.20% for wet heat puffing. Geniposide, similar with rutin, about 10% loss against control group was found through puffing pretreatment regardless of puffing ways.
In addition, the levels of geniposide and crocin in hydrolysis solution released by AEE process were determined and presented in Table 2. With geniposide and crocin in hydrolysate and those extracted into isopropanol considered, their sum of amount is equivalent to those in gardenia seeds, indicating that geniposide and crocin were completely released by AEE. The highest contents of geniposide and crocin in hydrolysis solution of 1.52 ± 0.04% and 0.61 ± 0.03% respectively, were obtained for Pec hydrolysis using raw oilseeds, the secondary is for Cel → Cel group with values of 1.08% and 0.41%. Compared to raw gardenia seeds, puffing resulted in lower levels of crocin in hydrolysate by AEE regardless of hydrolysis ways, and much low level of crocin in hydrolysate for dry-heating puffing than for wet-heating puffing (about 20% lower), which also supported that puffing resulted in observable loss of crocin. While the level of geniposide had not changed that much as crocin. These findings also verified the analytical results of HPLC to some degree.
Characterization of gardenia seed oil prepared by various methods
The yields of the gardenia seed oil by various preparation methods and their fatty composition, active components, and quality index were compared. The color of all the oil extracted by various methods presents light yellow to yellow. For all the oil preparation method, Soxhlet extraction gave the highest yield of 25.45%, CPE produced the lowest yield of 10.44%. The oil yield for AEE is much higher than pressing method, with values of 13.75%, 21.76% and 23.16% upon raw seed, WP seeds and DP seeds. The PV for oils did not show significant difference among all the extraction methods, with values between 1.75 and 2.36 meq/g. For AV of oils, pressing method, solvent extraction and AEE with raw seed showed similar AVs of 1.25, 1.94 and 1.81 mg KOH/g, while puffed seed oils with higher AVs of 3.92 and 3.83 mg KOH/g. This is disadvantageous from view of oil quality (Xu et al., 2021). Slightly high AV should arise from hydrolysis of triglyceride under high-temperature and high-pressure conditions during puffing. In addition, content of active crocin and geniposide in oils are varied dependent on oil extraction methods. Higher levels of geniposide and crocin with value of 150.76 and 247.46 μg/g were obtained for oil prepared by AEE method, compared to levels of 14.13 & 102.16 μg/g for SE and 136.60 & 190.36 μg/g and for CPE method, embodied the advantage of enriching high value-added by-products. Moreover, the composition of fatty acid in oil prepared by various methods were compared. Results showed that there is no significant difference among oils prepared by five extraction methods. Typically, gardenia seed oil mainly includes palmitic acid C16:0, oleic acid C18:1, linoleic acid C18:2, stearic acid C18:0 etc., with each content of 16.11–19.16%, 32.93–35.08%, 37.02–43.81% and 3.1–4.28%. Unsaturated fatty acids account for about 80% of total fatty acid, plus enrichment of active geniposide and crocin in oil, indicating high nutritional value of gardenia seed oil produced by AEE coupled with puffing pretreatment.
Generally, the gardenia fruit is the seed of shrubs, which is apparently different from most other oilseeds with respect to its low starch, protein content, and high cellulose content. Its seed coat is tough and resistant to enzymatic hydrolysis, hence, its FOY obtained by any combined AEEs is low as 12–14%. It is difficult to puffing for the gardenia seed, and few reports on puffing were found. Although, the puffing performances of gardenia seed for both DP and WP were not comparable to those for other oilseeds, such as Camelia seed (Peng et al., 2019), Torreya grandis seed (Wen et al., 2022), pumpkin seed (Jiao et al., 2014), the results on microstructure, pore size as well as its distribution of puffed seeds indicated that the relative higher surface exposure created by puffing meet the requirements for oil preparation by AEE. The related oil recovery is around 93%. The increased ratio surface area of seeds exposed to enzyme and solvent by puffing, resulted in higher hydrolysis degree of substrate and removal of crocin, geniposide and polysaccharides by isopropanol, leading to more oil set free and less involved into emulsification, accounted for the enhanced oil yields during AEE process by puffing.
Another aspect, WP and DP pretreatment, each has its own characteristics. DP showed slightly higher puffing performances including destruction of oilseed structure and oil yields than WP. However, its high temperature is disadvantages to retention of heat sensitive active substances. WP produced puffing effects under lower temperature, hence better protect the active components and is more energy-saving, and green. This hot and humid steam may also be helpful to break down the cellulose tissue in seed (Li et al., 2022), which facilitate the enzymatic hydrolysis. One can choose WP or DP pretreatments against practical requirements. These findings indicated that puffing pretreatment combined with combined AEE process may be a promising, high efficiency, economic and green process for oil preparation from gardenia seed by AEE.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the financial support provided by the National Natural Science Foundation of China (32272242, 32001739, 31972109), Zhejiang Province Key Research and Development Projects (2021C02013, 2020C02018, 2019C02069), Open Foundation of Key Laboratory of Marine Fishery Resources Exploitment & Utilization of Zhejiang Province (SL2022008)
Author contributions
CJ: Writing-original draft, Data curation, Validation and Experiment preparation; LW: Investigation and Software; XL: Experiment preparation; YL: Resource; NY: Formal analysis; XN: Language polishing; QY: Methodology and Writing-review & editing; XM: Writing-review & editing, Conceptualization, Supervision, Funding acquisition.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chengyu Jin, Email: jinchengyu23@163.com.
Lingyun Wang, Email: wanglingyun22@163.com.
Xiaoying Liu, Email: 1035063930@qq.com.
Yuanchao Lu, Email: yclu@zjut.edu.cn.
Ningxiang Yu, Email: ningxiangyu92@163.com.
Xiaohua Nie, Email: niexiaohua2000@zjut.edu.cn.
Qin Ye, Email: yq719@163.com.
Xianghe Meng, Email: mengxh@zjut.edu.cn.
References
- AOAC . Official Methods of Analysis. 15. Washington DC: Association of Analytical Chemists; 1990. [Google Scholar]
- AOCS . Official and Recommended Practices of the AOCS. 5. Champaign: American Oil Chemists’ Society Press; 1997. [Google Scholar]
- Beluns S, Gaidukovs S, Platnieks O, Gaidukova G, Mierina I, Grase L, Starkova O, Brazdausks P, Thakur VK. From wood and hemp biomass wastes to sustainable nanocellulose foams. Industrial Crops and Products. 2021;170:113780. doi: 10.1016/j.indcrop.2021.113780. [DOI] [Google Scholar]
- Cai X, Zhang R, Guo Y, He J, Li S, Zhu Z, Liu G, Liu Z, Yang J. Optimization of ultrasound-assisted extraction of gardenia fruit oil with bioactive components and their identification and quantification by HPLC-DAD/ESI-MS2. Food & Function. 2015;6:2194–2204. doi: 10.1039/C5FO00205B. [DOI] [PubMed] [Google Scholar]
- Chen L, Li M, Yang Z, Tao W, Wang P, Tian X, Li X, Wang W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. Journal of Ethnopharmacology. 2020;257:112829. doi: 10.1016/j.jep.2020.112829. [DOI] [PubMed] [Google Scholar]
- Chyau CC, Chiu CY, Hsieh HL, Hsieh DWC, Hsieh CR, Chang CH, Peng RY. High-purity preparation of enzyme transformed trans-crocetin reclaimed from gardenia fruit waste. Plants-Basel. 2022;11:281. doi: 10.3390/plants11030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aquino DS, Roders C, Vessoni AM, Stevanato N, Da Silva C. Assessment of obtaining sunflower oil from enzymatic aqueous extraction using protease enzymes. Grasasaceites. 2022;73:e452. doi: 10.3989/gya.0323211. [DOI] [Google Scholar]
- Díaz-Suárez P, Rosales-Quintero A, Fernandez-Lafuente R, Pola-Sánchez E, Hernández-Cruz MC, Ovando-Chacón SL, Rodrigues RC, Tacias-Pascacio VG. Aqueous enzymatic extraction of Ricinus communis seeds oil using Viscozyme L. Industrial Crops and Products. 2021;170:113811. doi: 10.1016/j.indcrop.2021.113811. [DOI] [Google Scholar]
- Feng J, Wang J, Bu T, Ge Z, Yang K, Sun P, Wu L, Cai M. Structural, in vitro digestion, and fermentation characteristics of lotus leaf flavonoids. Food Chemistry. 2023;406:135007. doi: 10.1016/j.foodchem.2022.135007. [DOI] [PubMed] [Google Scholar]
- Ferreira IJB, Alexandre EMC, Saraiva JA, Pintado M. Green emerging extraction technologies to obtain high-quality vegetable oils from nuts: A review. Innovative Food Science & Emerging Technologies. 2022;76:102931. doi: 10.1016/j.ifset.2022.102931. [DOI] [Google Scholar]
- Gai QY, Jiao J, Mu PS, Wang W, Luo M, Li CY, Zu YG, Wei FY, Fu YJ. Microwave-assisted aqueous enzymatic extraction of oil from Isatis indigotica seeds and its evaluation of physicochemical properties, fatty acid compositions and antioxidant activities. Industrial Crops and Products. 2013;45:303–311. doi: 10.1016/j.indcrop.2012.12.050. [DOI] [Google Scholar]
- He W, Gao Y, Yuan F, Bao Y, Liu F, Dong J. Optimization of supercritical carbon dioxide extraction of gardenia fruit oil and the analysis of functional components. Journal of the American Oil Chemists’ Society. 2010;87:1071–1079. doi: 10.1007/s11746-010-1592-z. [DOI] [Google Scholar]
- Hu B, Li Y, Song J, Li H, Zhou Q, Li C, Zhang Z, Liu Y, Liu A, Zhang Q, Liu S, Luo Q. Oil extraction from tiger nut (Cyperus esculentus L.) using the combination of microwave-ultrasonic assisted aqueous enzymatic method-design, optimization and quality evaluation. Journal of Chromatography A. 2020;1627:461380. doi: 10.1016/j.chroma.2020.461380. [DOI] [PubMed] [Google Scholar]
- Hu B, Wang H, He L, Li Y, Li C, Zhang Z, Liu Y, Zhou K, Zhang Q, Liu A, Liu S, Zhu Y, Luo Q. A method for extracting oil from cherry seed by ultrasonic-microwave assisted aqueous enzymatic process and evaluation of its quality. Journal of Chromatography A. 2019;1587:50–60. doi: 10.1016/j.chroma.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Huang H, Ettoumi F, Li L, Xu Y, Luo Z. Emulsification-based interfacial synthesis of citral-loaded hollow MIL-88A for the inhibition of potato tuber sprouting. Food Chemistry. 2022;393:133360. doi: 10.1016/j.foodchem.2022.133360. [DOI] [PubMed] [Google Scholar]
- Jha AK, Sit N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends in Food Science & Technology. 2022;119:579–591. doi: 10.1016/j.tifs.2021.11.019. [DOI] [Google Scholar]
- Jiang L, Hua D, Wang Z, Xu S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food and Bioproducts Processing. 2010;88:233–238. doi: 10.1016/j.fbp.2009.08.002. [DOI] [Google Scholar]
- Jiao J, Li ZG, Gai QY, Li XJ, Wei FY, Fu YJ, Ma W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chemistry. 2014;147:17–24. doi: 10.1016/j.foodchem.2013.09.079. [DOI] [PubMed] [Google Scholar]
- Jung S, Maurer D, Johnson LA. Factors affecting emulsion stability and quality of oil recovered from enzyme-assisted aqueous extraction of soybeans. Bioresource Technology. 2009;100:5340–5347. doi: 10.1016/j.biortech.2009.03.087. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim SY, Kim DO, Kim BY, Baik MY. Puffing, a novel coffee bean processing technique for the enhancement of extract yield and antioxidant capacity. Food Chemistry. 2018;240:594–600. doi: 10.1016/j.foodchem.2017.07.161. [DOI] [PubMed] [Google Scholar]
- Li H, Song C, Zhou H, Wang N, Cao D. Optimization of the aqueous enzymatic extraction of wheat germ oil using response surface methodology. Journal of the American Oil Chemists’ Society. 2011;88:809–817. doi: 10.1007/s11746-010-1731-6. [DOI] [Google Scholar]
- Li N, Fan M, Li Y, Qian H, Zhang H, Qi X, Wang L. Stability assessment of crocetin and crocetin derivatives in Gardenia yellow pigment and Gardenia fruit pomace in presence of different cooking methods. Food Chemistry. 2020;312:126031. doi: 10.1016/j.foodchem.2019.126031. [DOI] [PubMed] [Google Scholar]
- Liu N, Ren G, Faiza M, Li D, Cui J, Zhang K, Yao X, Zhao M. Comparison of conventional and green extraction methods on oil yield, physicochemical properties, and lipid compositions of pomegranate seed oil. Journal of Food Composition and Analysis. 2022;114:104747. doi: 10.1016/j.jfca.2022.104747. [DOI] [Google Scholar]
- Liu Z, Gui M, Xu T, Zhang L, Kong L, Qin L, Zou Z. Efficient aqueous enzymatic-ultrasonication extraction of oil from Sapindus mukorossi seed kernels. Industrial Crops and Products. 2019;134:124–133. doi: 10.1016/j.indcrop.2019.03.065. [DOI] [Google Scholar]
- Li W, He X, Chen Y, Lei L, Li F, Zhao J, Zeng K, Ming J. Improving antioxidant activity and modifying Tartary buckwheat bran by steam explosion treatment. LWT - Food Science and Technology. 2022;170:114106. doi: 10.1016/j.lwt.2022.114106. [DOI] [Google Scholar]
- Meng X, Ge H, Ye Q, Peng L, Wang Z, Jiang L. Efficient and response surface optimized aqueous enzymatic extraction of Camellia oleifera (Tea Seed) oil facilitated by concurrent calcium chloride addition. Journal of the American Oil Chemists’ Society. 2018;95:29–37. doi: 10.1002/aocs.12009. [DOI] [Google Scholar]
- Meng X, Liu X, Zou Y, Shao S. Gardenia oil preparation by aqueous enzymatic extarction. Journal of the Chinese Cereals and Oils Association. 35: 117–124 (2020)
- Nguyen HC, Vuong DP, Nguyen NTT, Nguyen NP, Su CH, Wang FM, Juan HY. Aqueous enzymatic extraction of polyunsaturated fatty acid–rich sacha inchi (Plukenetia volubilis L.) seed oil: An eco-friendly approach. LWT - Food Science and Technology. 2020;133:109992. doi: 10.1016/j.lwt.2020.109992. [DOI] [Google Scholar]
- Peng L, Ye Q, Liu X, Liu S, Meng X. Optimization of aqueous enzymatic method for Camellia sinensis oil extraction and reuse of enzymes in the process. Journal of Bioscience and Bioengineering. 2019;128:716–722. doi: 10.1016/j.jbiosc.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Polmann G, Badia V, Frena M, Teixeira GL, Rigo E, Block JM, Camino Feltes MM. Enzyme-assisted aqueous extraction combined with experimental designs allow the obtaining of a high-quality and yield pecan nut oil. LWT - Food Science and Technology. 2019;113:108283. doi: 10.1016/j.lwt.2019.108283. [DOI] [Google Scholar]
- Rehman Z, Salariya AM, Zafar SI. Effect of processing on available carbohydrate content and starch digestibility of kidney beans (Phaseolus vulgaris L.) Food Chemistry. 2001;73:351–355. doi: 10.1016/S0308-8146(00)00311-3. [DOI] [Google Scholar]
- Sun X, Shokri S, Wang Z, Li B, Meng X. Optimization of explosion puffing drying for browning control in Muskmelon (Cucumis melo L.) using Taguchi orthogonal arrays. LWT - Food Science and Technology. 2021;142:111021. doi: 10.1016/j.lwt.2021.111021. [DOI] [Google Scholar]
- Tang L, Liu H, Wen J, Xu Y, Tian W, Li L, Yu Y, Lin X, Fu M. Study on ultrahigh-pressure extraction technology on properties of yellow extract from gardenia fruit. Journal of Food Composition and Analysis. 2021;104:104186. doi: 10.1016/j.jfca.2021.104186. [DOI] [Google Scholar]
- Tao W, Zhang H, Xue W, Ren L, Xia B, Zhou X, Wu H, Duan J, Chen G. Optimization of supercritical fluid extraction of oil from the fruit of Gardenia jasminoides and its antidepressant activity. Molecules. 2014;19:19350–19360. doi: 10.3390/molecules191219350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Qin S, Han J, Meng J, Liang A. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi) Journal of Ethnopharmacology. 2022;289:114984. doi: 10.1016/j.jep.2022.114984. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang J, Mujumdar AS, Jin X, Liu ZL, Zhang Y, Xiao HW. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa) Food Hydrocolloids. 2021;118:106808. doi: 10.1016/j.foodhyd.2021.106808. [DOI] [Google Scholar]
- Wang J, Ye Q, Yu N, Huan W, Sun J, Nie X, Meng X. Preparation of multiresponsive hydrophilic molecularly imprinted microspheres for rapid separation of gardenia yellow and geniposide from gardenia fruit. Food Chemistry. 2022;374:131610. doi: 10.1016/j.foodchem.2021.131610. [DOI] [PubMed] [Google Scholar]
- Wei C, Xiao K, Li H, Qi Y, Zou Z, Liu Z. Optimization of ultrasound assisted aqueous enzymatic extraction of oil from Cinnamomum camphora seeds. LWT - Food Science and Technology. 2022;164:113689. doi: 10.1016/j.lwt.2022.113689. [DOI] [Google Scholar]
- Wen S, Lu Y, Yu N, Nie X, Meng X. Microwave pre-treatment aqueous enzymatic extraction (MPAEE): A case study on the Torreya grandis seed kernels oil. Journal of Food Processing and Preservation. 2022;46:e17115. doi: 10.1111/jfpp.17115. [DOI] [Google Scholar]
- Xiao W, Li S, Wang S, Ho CT. Chemistry and bioactivity of Gardenia jasminoides. Journal of Food and Drug Analysis, Dietary Natural Compounds. 2017;25:43–61. doi: 10.1016/j.jfda.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Hao J, Wang Z, Liang D, Wang J, Ma Y, Zhang M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT - Food Science and Technology. 2021;149:111817. doi: 10.1016/j.lwt.2021.111817. [DOI] [Google Scholar]
- Yang Y, Yuan B, Yu P, Jia Y, Zhou Q, Sun J. Flavor characteristics of peanut butter pretreated by radio frequency heating, explosion puffing, microwave, and oven heating. Food Chemistry. 2022;394:133487. doi: 10.1016/j.foodchem.2022.133487. [DOI] [PubMed] [Google Scholar]
- Yin F, Liu J. Research and application progress of Gardenia jasminoides. Chinese Herbal Medicines. 2018;10:362–370. doi: 10.1016/j.chmed.2018.09.001. [DOI] [Google Scholar]
- Zhang Y, Chen C, Wang N, Chen Y, Yu J, Zheng X, Li S, Chen Y. Developing a new modification technology of oat flour based on differential pressure explosion puffing. LWT - Food Science and Technology. 2021;141:110967. doi: 10.1016/j.lwt.2021.110967. [DOI] [Google Scholar]
- Zhang Y, Sun Q, Liu S, Wei S, Xia Q, Ji H, Deng C, Hao J. Extraction of fish oil from fish heads using ultra-high pressure pre-treatment prior to enzymatic hydrolysis. Innovative Food Science & Emerging Technologies. 2021;70:102670. doi: 10.1016/j.ifset.2021.102670. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.