Abstract
Clostridioides difficile infection (CDI) has become the most common healthcare-associated infection in the United States, with considerable morbidity, mortality, and healthcare costs. Assessing new preventive strategies is vital. We present a literature review of studies evaluating a strategy of screening and isolation of asymptomatic carriers in hospital settings. Asymptomatic detection of C. difficile is reported in ~ 10–20% of admitted patients. Risk factors for carriage include recent hospitalization, previous antibiotics, older age, lower functional capacity, immunosuppression, and others. Asymptomatic C. difficile carriers of toxigenic strains are at higher risk for progression to CDI. They are also shedders of C. difficile spores and may contribute to the persistence and transmission of this bacterium. Screening for asymptomatic carriers at hospital admission can theoretically reduce CDI by isolating carriers to reduce transmission, and implementing antibiotic stewardship measures targeting carriers to prevent progression to clinical illness. Several observational studies, summarized in this review, have reported implementing screening and isolation strategies, and found a reduction in CDI rates. Nevertheless, the data are still limited to a few observational studies, and this strategy is not commonly practiced. Studies supporting screening were performed in North America, coinciding with the period of dominance of the 027/BI/NAP1 strain. Additional studies evaluating screening, followed by infection control and antibiotic stewardship measures, are needed.
Keywords: Clostridioides difficile, Screening, Infection control, Contact isolation, Antibiotic stewardship
Key Summary Points
| Clostridioides difficile was recently designated by the Centers for Disease Control and Prevention (CDC) selected as one of five urgent threats to public health. |
| Toxigenic Clostridioides difficile asymptomatic carriage is common among hospitalized patients and carries a risk for progression to C. difficile infection (CDI) along with spread to other patients. |
| Screening for asymptomatic carriage may reduce CDI rates by contact isolation of carriers and limiting antibiotic use among them. |
| Further studies are needed to evaluate the effectiveness of this preventive strategy. |
Introduction
C. difficile Burden of the Disease
Clostridioides difficile (C. difficile), causing C. difficile infection (CDI), has become the most common healthcare-associated infection in the United States [1]. The prevalence of this infection is significant, with over 400,000 cases and almost 30,000 deaths annually from C. difficile-associated diarrhea in the US [2, 3]. The economic burden of C. difficile infection is also substantial, with direct healthcare costs exceeding US$5 billion annually [3]. Moreover, infection with C. difficile can cause a broad spectrum of illnesses, ranging from mild diarrhea to severe complications, including pseudomembranous colitis, toxic megacolon, bowel perforation, sepsis, and death [3]. The Centers for Disease Control and Prevention (CDC) designated CDI as one of five urgent threats to public health in its 2019 Antimicrobial Resistance Threat Report [4]. Given the high burden of C. difficile infection, its clinical impact, and economic consequences, assessing new strategies for CDI prevention, such as screening for asymptomatic carriers in hospital settings, is vital.
This review discusses the definitions, prevalence, and significance of asymptomatic C. difficile state among hospitalized patients. We provide an overview of currently available data on screening admitted patients for C. difficile and isolation of positive patients as a strategy for preventing CDI among hospitalized patients.
We performed a comprehensive PubMed search up until April 2023, utilizing the MeSH term "Clostridium difficile" in conjunction with the terms "carrier," "carriage," "colonization," "asymptomatic," and "screening." Our investigation focused on examining clinical studies that explored the screening of asymptomatic carriage or colonization with C. difficile.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Definition of Carriage
Clostridioides difficile carrier status is defined as any positive diagnostic test for the presence of C. difficile in the absence of diarrhea [5]. The optimal diagnostic tests for carriage include PCR (for toxin B (tcdB), binary toxin (cdtA), or tcdC deletion associated with the ribotype 027 strain) or toxigenic culture [5, 6]. It is essential to differentiate between carriage, which is a single detection of C. difficile that can represent only “pass-through” of spores, from colonization which is the persistent carriage that can be detected by the same restriction endonuclease analysis (REA) type of toxigenic C. difficile on two or more occasions [7, 8]. Nevertheless, these terms are used interchangeably in the literature [9]. We use the term ‘carriage’ in this review to address the presence of C. difficile in the absence of diarrhea unless stated 'colonization’ in the original study addressed.
C. difficile Carriage Pathogenesis and Gut Microbiome
C. difficile is a spore-forming, Gram-positive anaerobic bacillus that spreads through fecal–oral transmission of spores, which remain viable for long periods ex-vivo [10, 11].
Although initially considered strictly a nosocomial infection and not typically part of the gut microbiome, it is now well established that C. difficile strains can also circulate in the community, resulting both in community-acquired CDI and in asymptomatic carriage. The sources of these strains are multiple, and the epidemiology is complex. Human reservoirs in the community may include infants below the age of 2 years who are frequently colonized [12]. However, numerous environmental sources, including agricultural sources, contaminated food products, and household pets, probably all contribute to C. difficile circulation [13].
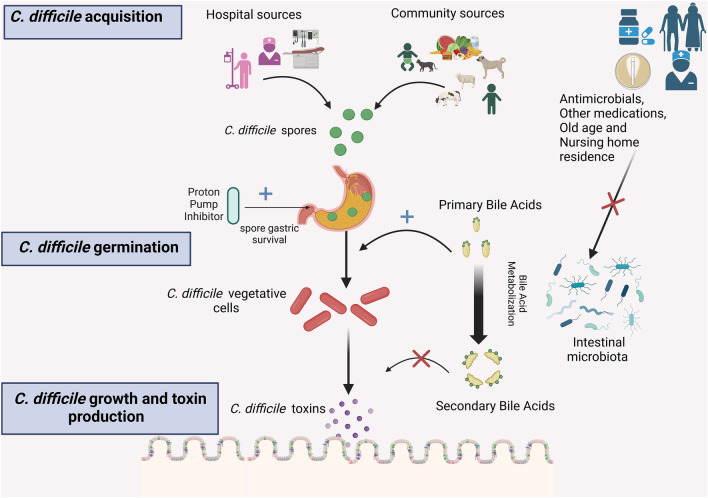
The first step in establishing C. difficile colonization is the germination of spores into toxin-producing vegetative cells, which is stimulated by primary bile acids. Secondary bile acids inhibit C. difficile growth, and members of the phylum Firmicutes can metabolize primary bile acids into secondary bile acids (see Fig. 1). A disruption in the intestinal microbiota and depletion of Firmicutes may cause an increase in primary bile acids and a decrease in secondary bile acids. This change in bile acid composition could lead to an increased risk of C. difficile colonization and infection [14].
Fig. 1.
Pathogenesis of Clostridioides difficile carriage and infection: C. difficile spores are acquired from various sources in both hospital and community settings. Hospital sources include the hospital environment, other individuals infected or colonized with C. difficile, and hospital staff. Community sources may include food products, animals, and asymptomatic carriers, particularly infants. Following the acquisition, spores may germinate into C. difficile vegetative cells, a process facilitated by primary bile acids. These vegetative cells replicate and produce toxins; the production of toxins and replication is inhibited by secondary bile acids. The composition of the intestinal microbiota, specifically the presence of certain microbes within the Phylum Firmicutes, promotes the conversion of primary bile acids into secondary bile acids. Various factors, such as antimicrobials, medications, advanced age, and residing in nursing homes, can impact the intestinal microbiota, decreasing these beneficial bacteria.
Created with BioRender.com
Our current comprehension of the progression from carriage to symptomatic CDI remains constrained. Earlier investigations have indicated that both carriers and symptomatic patients generate comparable levels of toxin [15], yet the underlying cause of why the latter group develops diarrhea remains obscure. Alterations in gut microbial composition in C. difficile carriers are less well described than in CDI patients. Still, some studies show a decreased species richness and microbial diversity in C. difficile carriers [16]. A study with antibiotic-exposed mice challenged with C. difficile spores demonstrated a shift toward Proteobacteria in animals that developed severe CDI symptoms. In contrast, the group that was only colonized by C. difficile had a microbiota dominated by Firmicutes, resembling that of mice without antibiotics [17]. Therefore, further studies are needed to understand the mechanisms of C. difficile carriage and its pathophysiology.
Risk Factors for Asymptomatic Carriage
Several epidemiological and clinical risk factors for C. difficile carriage at hospital admission have been identified. These include recent hospitalization, older age, lower functional capacity, chronic dialysis, corticosteroid or immunosuppressant use, gastric acid suppressant medication, and antibodies against toxin B [20, 27–32].
A recent study has also demonstrated a significantly increased risk for C. difficile carriage among patients with residential proximity to livestock farms [33].
In the context of C. difficile infection, the use of antibiotics is not only a well-known risk factor but is in fact an inseparable component of the pathogenesis [34]. Antibiotic treatment is also associated with the carriage of C. difficile [29, 30]. Furthermore, there is a dose–response relationship, where an increase in antibiotic use leads to a more remarkable persistence of C. difficile on the skin and in the environment [35]. A mouse model demonstrated that prolonged antibiotic treatment could also induce a “super-shedder” state, which results in the overgrowth of C. difficile and the excretion of large amounts of spores [36]. Specifically, Dubberke et al. reported cephalosporin use as a risk factor for C. difficile acquisition. This could be explained by either gut selective pressure favoring C. difficile growth, or undetected preexisting carriage, exposed following antibiotic use [29].
Prevalence of Asymptomatic C. difficile Carriage
Asymptomatic carriage rates of C. difficile among patients admitted to hospitals range from 3 to 21% [6, 18–24]. This rate can vary based on region and demographic factors. In long-term care facilities, carriage state has been reported to be as high as 50% of screened residents [25]. A recent meta-analysis reported pooled prevalence of toxigenic C. difficile carriage of 8.1% among almost 9000 hospital-admitted patients [26]. Prevalence in North American studies was 10% in this meta-analysis.
Progression from Carriage to Active Infection
While, initially, C. difficile carriage was considered protective against the future development of symptomatic CDI, and progression was considered rare [37], it is now suggested that patients asymptomatically colonized by toxigenic strains are at higher risk for progression to CDI during admission, whereas those colonized by nontoxigenic strains have no increased risk of progressing to CDI and may even be protected from developing CDI [6, 22, 26, 38–40],. The evidence of the former is limited mostly to non-adjusted data (Table 1).
Table 1.
Studies evaluating the prevalence of CDI among carriers and non-carriers
| Study ID; country | Design | Number of screened patients | Patients screened; frequency | Number of carriers | Number of CDI cases in non-carriers | Number of CDI cases in carriers | Study years |
|---|---|---|---|---|---|---|---|
| Meltzer 2018; Israel | Prospective | 2368 | Admitted; single screen | 81 | 4.6/10,000 patient days | 76.7 cases/10,000 patient days | 2017 |
| Baron 2019; USA | Prospective | 220 | Sample of admitted patients | 21 | 4/199 (2%) | 8/21 (38.1%) | 2017–2018 |
| Worley 2021;USA | Prospective | 1897 | Intensive care unit patients upon admission and weekly thereafter | 140 | 20/1757 (1.1%) | 5/140 (3.6%)a | Not described |
| Blixt 2017; Denmark | Prospective | 3565 | All patients admitted to medical wards | 213 | 80/3340 (2.4%) |
23/225 (10.2%) Adjusted ORb (95% CI) 3.92 (2.36–6.51) |
2012–2013 |
| Curry 2023; USA | Prospective | 4498 | A convenience sample of hospital and long-term care facility residents on admission | 319 | 58/4179 (1.4%) | 39/219 (12.2%) | 2016–2018 |
CDI Clostridioides difficile infection, OR odd ratio, CI confidence interval
aIn this study, 32% were carriers of non-toxigenic strains
bAdjusted to the number of admittances during the study period, age, hospitalization in the last year, sex, length of stay, and comorbidities
A large study that screened 3,605 hospital admissions found that patients carrying toxigenic strains on admission were at a significantly higher risk of developing CDI (CDI rate of 9.4% vs. 2.3% for non-toxigenic C. difficile carriers). The relative risk for developing CDI from asymptomatic carriage in this study was 9.32, while carriage of a non-toxin-encoding strain did not increase risks for CDI [38].
We have recently reported that, among 2368 patients admitted to internal medicine wards, there were 81 C. difficile carriers with an incidence of hospital onset CDI of 76.7 cases per 10,000 patient days, while among the remaining 2287 non-carriers, the incidence was 4.6 cases per 10,000 patient days [relative risk (RR) 16.6 95% CI 4.0–69.1] [32].
A meta-analysis evaluating the association between the carriage of toxigenic strains and CDI has demonstrated an almost six times higher risk for carriers [26].
The Role of Screening of Asymptomatic Carriers in the Predicting CDI
Otles et al. evaluated C. difficile screening as a predictor of hospital-onset CDI. Screening 1859 patients admitted to intensive care unit (ICU) and oncology wards, the negative predictive value (NPV) of a negative screen was high (98.5%); however, the positive predictive value (PPV) was low (9%). Nine patients out of 39 who developed CDI were detected by screening. The authors compared this prediction ability to a daily risk estimate produced by a machine-learning model. The latter had a similar NPV with a lower PPV. The model identified different patients from those identified by screening who later developed CDI. This suggests a potential advantage of a machine-learning model because screening cannot identify at-risk patients who are not already colonized [41].
Targeted Antibiotic Stewardship for C. difficile Carriers
Antimicrobial stewardship programs (ASPs) have successfully reduced CDI rates by decreasing inappropriate antimicrobial use [42–44]. Some antibiotics have been known to increase the risk of CDI [45], yet information regarding the association between specific antibiotic usage and loss or acquisition of C. difficile carriage is sparse—one study found that cephalosporin use was associated with the acquisition of C. difficile colonization while β-lactam-β-lactamase inhibitor combinations and metronidazole were associated with loss of colonization [30]. An additional study has found that piperacillin–tazobactam usage was associated with decreased rates of C. difficile carriage [46]. Nevertheless, these data are sparse, and the common knowledge is to avoid any antibiotics among carriers as much as possible.
Poirier et al. evaluated predictors of hospital-onset CDI among 513 asymptomatic carriers identified by active screening. Of these, 39 developed CDI, with exposure to multiple antibiotics and other factors, such as increased length of stay, cirrhosis, and opioids identified as predictors [47].
Since asymptomatic patients are not widely screened for CD carriage, ASPs targeted at this population have not been studied [43]. Patients with prior CDI, an easily identifiable subset of asymptomatic carriers, may represent suitable targets for focused stewardship efforts, as they are at the highest risk of developing infection [48]. One study showed a threefold increase in CDI recurrence in patients exposed to non-CDI antimicrobials after resolved CDI compared to those not exposed, regardless of the duration of treatment. However, any treatment with other antibiotics while receiving anti-CDI antibiotics was not associated with recurrent CDI [48]. After implementing an intervention to detect and isolate C. difficile carriers at hospital admission, anti-C. difficile antibiotic use was significantly decreased, although there was a small but significant increase in the global use of other antimicrobials [21].
Targeting antibiotic stewardship efforts towards CD carriers, especially those with a history of CDI, could help reduce CDI and the inappropriate use of antimicrobials.
C. difficile Shedding from Carriers
Carrier shedding of C. difficile spores is an essential factor contributing to the persistence and transmission of this bacterium. Both index patient skin and the patient’s environment have been reported to be contaminated by spores, posing a risk for healthcare-associated transmission [7, 49]. The frequency of environmental contamination with C. difficile depends on the patient’s C. difficile status (i.e., carriage or clinical CDI). A study by McFarland et al. found that fewer than 8% of rooms of culture-negative patients, 8–30% of rooms of patients with asymptomatic colonization, and 9–50% of rooms of patients with CDI were contaminated with C. difficile [50]. Moreover, we have reported in a prospective observational study that environmental shedding of toxigenic C. difficile by asymptomatic carriers is relatively frequent. The study found that 41% of rooms inhabited by C. difficile carriers had more than residual contamination, and 24% were heavily contaminated. In contrast, only one room (6%) in the control group of non-CD carriers had more than residual contamination, and none were heavily contaminated [51].
The Role of Asymptomatic Carriers in the Transmission of C. difficile and CDI Development
According to several studies, asymptomatic carriers of C. difficile play a significant role in the transmission of the bacteria. Studies using multilocus sequence typing demonstrated that only 25–55% of patients with symptomatic CDI could be linked to a previously identified CDI patient, suggesting additional pathways for transmission besides active CDI patients, such as C. difficile carriers [52–54].
Blixt et al. found that 2.6% of patients who were not exposed to C. difficile-colonized patients developed CDI, while this percentage increased to 4.6% for patients who were exposed [38]. Integrated genomic and epidemiologic analyses have identified multiple potential transmission events from asymptomatic carriers to other patients [22]. Moreover, a model of C. difficile transmission in healthcare settings confirmed that patients colonized on admission likely play a significant role in sustaining ward-based transmission [55]. Donskey et al., using genomic and epidemiological data, showed that carriers linked to a transmission had a high burden of carriage and high skin and environment shedding [7]. In a large multicenter Canadian study using whole genome sequencing, 81 (40%) of 201 CDI cases could be linked to another case in the ward, 65 (32%) of them could be linked either to a carrier or an active CDI patient, 28 (14%) could be exclusively linked to an active CDI patient, and only 12 (6%) could be solely linked to a carrier [56]. Crobach et al. [57] conducted a multicenter study, screening 2211 patients for C. difficile carriage and finding 49 patients to be colonized. The team used whole genome sequencing to analyze C. difficile from 183 CDI episodes that took place in the participating hospitals throughout the study period. Their analysis revealed that only one CDI case could potentially have resulted from transmission from a colonized patient. This study was conducted in the Netherlands, which has the lowest antibiotic consumption rate in Europe.
While it remains unknown what proportion of symptomatic infection results from transmission from asymptomatic carriers, research indicates that this does occur. And though transmission events from asymptomatic carriers might be rare, the high prevalence of asymptomatic carriage suggests that they might significantly transmit C. difficile in the hospital setting and long-term care facilities [7, 27].
The Role of Screening of Asymptomatic Carriers in Preventing Hospital-Acquired (HA) CDI
Screening policies have been suggested as an effective way to prevent C. difficile outbreaks in healthcare facilities [58, 59]. Theoretically, the identification of asymptomatic carriers can reduce the spread of C. difficile through two mechanisms: first, isolation of carriers can reduce transmission to uninfected patients, and second, antibiotic stewardship interventions targeting carriers can potentially prevent progression to symptomatic C. difficile [6].
Mathematical modeling of C. difficile transmission and simulation of screening and isolation of carriers have shown the intervention required to effectively reduce CDI rates [60–63]. This intervention was also highly cost-effective, with estimates ranging between 128 and 310$ per quality-adjusted life year, or even less if screening was targeted to high-risk populations; under some conditions, screening might lead to cost savings per case averted [64–66].
Real-life data assessing the effectiveness of carrier screening in reducing hospital-acquired CDI are sparse. Relevant studies are summarized in Table 2. Longtin et al. implemented a C. difficile carriage screening policy, along with contact isolation precautions for carriers during hospitalization, and found a significant decrease in CDI cases following the intervention. The authors estimated that the intervention could prevent ~ 60% of expected cases of healthcare-associated CDI [21]. A follow-up study from the same group showed that isolating CD carriers led to an initial increase in isolation days that was later compensated by a decrease in isolation days for CDI [67]. Cho et al. implemented universal C. dificile screening for all patients hospitalized in a hematopoietic stem cell transplantation unit; this intervention decreased the rate of hospital-acquired C. dificile from 72.5/10,000 patients days before the intervention to 14.4/10,000 patient days during the intervention period [68]. Collison et al. implemented universal inpatient C. dificile screening at an 800-bed hospital in Chicago, and the number of confirmed CDI events decreased from 13.3 events per 10,000 patient days, the year before the intervention, to 5.0 per 10,000 patient days for the 1 year after the study period [24].
Table 2.
Studies evaluating C. difficile screening
| Study ID; country | Design | Number of screened patients | Patients screened; frequency | Number of carriers | Action taken for carriers | Primary outcome | Summary of primary outcome results | Study years |
|---|---|---|---|---|---|---|---|---|
| Before-after studies evaluating screening effect on CDI rate | ||||||||
| Longtin 2016; Canada | Controlled quasi-experimental; time series analysis | 7599 | All admitted; admission only | 368 | Isolationa | Incidence of HA-CDI | Decrease of 7% of HA-CDI per 4-week period (rate ratio, 0.93; 95% CI, 0.87–0.99 per period; p = 0.02) | 2013–2015 |
| Xiao 2018; Canada (substudy of Longtin 2016) | Retrospective time series analysis | 806,357 patient-days | All admitted; admission only |
Isolation of CDI until symptom resolution: 3636 isolation-days Isolation of CDI until discharge: 6704 isolation-days Isolation of CDI + CD carriers until discharge: 10,115 isolation-days |
Isolationa | Total isolation-days |
12.9 / 26.2 / 37.8 isolation-days/1000 patient-days; 66% increase Isolation-days for CDI 11% lower (0.723–1.09) |
2008–2016 |
| Paquet-Bolduc 2018; Canada | Prospective – compared historical control periods | 114 | All in outbreak wards; single round screening on outbreak detection | 15 (13%) | Isolationa |
Incidence of HA-CDI Outbreak duration |
2 CDI (13%) of carriers; no difference in total CDI compared historical controls (median 7.0 vs 7.5 cases, p = 0.99) No difference in outbreak duration (median, 26.5 vs 34 days, p = 0.72) |
2011–2016 |
| Linsenmeyer 2018; USA | Prospective before-after | 1250 screened, 773 evaluated | Surgical wards and surgical ICU during outbreak; single screen on admission | 24 (3.1%) | Contact isolation as for CDI | Incidence of HA-CDI | Before–after intervention decrease in HA-CDI incidence per 10,000 bed days from 10.9 to 3.0 | 2016–2017 |
| Peterson 2020; USA | Prospective before–after | 63,057 admissions before, 62,760 after | Targeted screen: hospitalized within two months, past positive CD test, long-term care facility in the prior six months | NS | Contact isolation as for CDI | Incidence of HO-CDI | 5.96–4.23 cases/10,000 patient days; 2.9 in final 9 months | 2017–2018 |
| Collison 2020; USA | Prospective compared historical control periods | 47,048 | All admitted to adult inpatient units | 2010 | Contact isolation as for CDI | Incidence of lab identified C. difficile events | 13.3 cases/10,000 patient days before intervention decreases to 5.0 cases/10,000 patient days after intervention | 2015–2018 |
| Cho 2018; USA | Prospective before–after | 470 | HSCT unit admitted; single screen of stool sample | 70 | Contact isolation | Incidence of HO-CDI |
35 (50%) developed diarrhea and treated as CDI without testing HO-CDI rate in the HSCT unit decreased 72.5 cases/10,000 patient days to 14.4 in periods before and after screening |
2009–2013 |
| Non clinical studies | ||||||||
| Reagan 2023; USA | Non-clinical study mathematical model | Non-clinical | Bone-marrow unit; model for active detection and isolation (ADI) | NA | NA | Predicted CDI rates using ADI compared to testing symptomatic patients |
24.5% decrease in total cases with ADI (6.2 cases per year) 84% decrease in HA-CDI with ADI |
2014–2019 |
CDI Clostridioides difficile infection, HA-CDI health care–associated Clostridioides difficile infection, HO-CDI hospital-onset Clostridioides difficile infection, HSCT hematopoietic stem cell transplant, ICU intensive care unit
aIsolation was modified not to include the use of gowns, no private room, and no contact isolation needed on transportation
The effectiveness of screening in an outbreak setting is not conclusive, and, while a study conducted during an outbreak in a surgical ward in Boston showed that ward-based screening of all newly admitted patients and isolation of carriers averted 5 out of 10 expected hospital-acquired CDI [69], a different study that implemented a one-time unit-wide screening during CDI outbreaks and modified contact precautions for the carriers was ineffective in decreasing the number of CDI cases or the outbreak duration. In this study, unscreened patients admitted after the unit-wide screening event were probably the outbreak’s source [39].
Peterson et al. performed a non-randomized stepped-wedge initiative, during which patients previously identified as at high risk for C. difficile carriage (had a recent previous hospitalization, previous positive C. difficile test, or patients arriving from long-term care facilities) were screened for C. difficile. Those testing positive were placed into contact isolation. In this study, among 63,057 patients in the pre-intervention period, hospital-onset CDI incidence was 6 cases per 100,000 patient-days (PD), compared with 4/100,000 PD among 62,760 patients admitted during the screening period (p = 0.02). No other changes in practice or antibiotic use were reported [70].
Differences in intervention protocols may account for the variations in screening intervention results. Studies differ by the population screened (targeted high-risk population [68, 70] or universal screening [21, 24, 69]), timing of screening (single whole-ward screening [39] or screening of every new patient admitted [21, 24]), analysis of recurrent admissions (repeated admissions for the same patient included or excluded [69]), screening methods (rectal swab [21, 67] or stool sample [57, 68]); and setting (outbreak [39, 69] or endemic [21, 67, 68, 70]), as well as the contact isolation precautions implemented (modified contact isolation precautions including gloves, hand-washing with soap and water and dedicated equipment [21, 39, 67] or traditional contact isolation precautions as used for active CDI patients, including gowns and single room or cohorting [24, 69, 70]).
These studies cited above [21, 39, 67, 69, 70] were conducted in North America from 2008 to 2019, coinciding with the period of dominance of the 027/BI/NAP1 C. difficile strain in hospital outbreaks, particularly in North America [71]. Only one study [21] addressed this issue, in which the rate of the 027/BI/NAP1 strain varied throughout the different periods of follow-up, ranging between 59.2% at the beginning of follow-up to 20% near the end of the follow-up period. A sensitivity analysis that excluded the epidemic period showed similar results as the original analysis. In a study originating from the Netherlands [57], the focus was not on screening's utility for CDI prevention but rather on the onward transmission from carriers. Despite identifying 49 carriers, only a single potential case of onward transmission was reported in a European context where the 027/BI/NAP1 strain was absent. This suggests that the effectiveness of such interventions may be limited to regions with a high prevalence of endemic strains. Another possible explanation for this low onward transmission could be the specific antibiotic prescribing practices, that vary between different countries, and that are more constrictive in the Netherlands compared to North America [72].
Carrier screening remains an uncommonly used strategy; a survey among infection prevention specialists in different medical centers across the US found that only 4% of respondents indicated testing patients for asymptomatic carriage of C. difficile. Of those who reported testing patients to detect asymptomatic carriers, most reserved this policy for patients admitted to select units such as intensive care and oncology/hematopoietic cell transplant units. After detecting asymptomatic carriage of C. difficile, healthcare facilities most commonly implemented contact precautions, followed by enhanced environmental cleaning [73].
Summary and Discussion
CDI significantly burdens the healthcare system, resulting in considerable morbidity and mortality. Carriers of C. difficile probably play a role in spreading the disease through environmental shedding. Carriers of a toxigenic strain are also at higher risk of evolving into active CDI, particularly after antibiotic treatment. Screening for asymptomatic carriers could potentially reduce the incidence of CDI through improved isolation, contact precautions, and antibiotic stewardship. Most studies evaluating this strategy reported reductions in CDI rates. Nevertheless, the number of studies evaluating screening are limited, all relevant studies are observational, and the intervention applied following positive screen test differs in different studies. Moreover, practically, this strategy is not commonly practiced.
It is worth noting a limitation highlighted by Collison et al., but overlooked in other studies: providers might opt for empirical CDI treatment in carriers presenting with diarrhea. These instances might not have been correctly categorized as CDI events, possibly accounting for some of the observed decrease in CDI events following the introduction of universal screening [24].
When assessing the effectiveness of screening strategies, one must also consider their drawbacks. While the cost of screening can be substantial, a comparison with the reduced expenses from preventing CDI cases could render it cost-effective [55, 64, 66]. However, aside from financial costs, there may be additional consequences. For instance, a study revealed that 15% of carriers (9/58) among solid organ transplant recipients received unnecessary oral vancomycin treatment due to misinterpreting carriage results as active infection, leading to unwarranted antibiotic therapy [74]. Additionally, the enforcement of isolation precautions has been linked to an increased risk of adverse events, including prolonged hospital stays, elevated readmission rates, medication errors, and injuries [75, 76] It can also negatively affect patients' perception of care and satisfaction [77].
The optimal approach following positive screening for C. difficile is yet to be determined. Consideration should include type of isolation and contact precautions, duration of these measures, strategy for environmental cleaning, use of pre and/or probiotics, strategy of antibiotic stewardship, and definitions for CDI diagnosis in the presence of a positive screening (Fig. 2) In addition, screening strategies should also be more accurately defined, emphasizing the importance of screening specifically for toxigenic strains, as these are the ones posing risk for CDI.
Fig. 2.
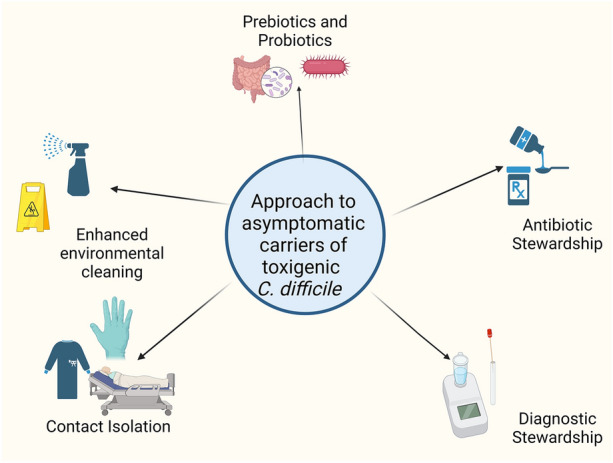
Adopting a multimodal strategy for asymptomatic carriers of C. difficile: when strategizing a screening intervention for asymptomatic toxigenic C. difficile carriers, a comprehensive multimodal approach should be deployed. Each element—including contact isolation procedures, enhanced environmental cleaning, antibiotic stewardship, diagnostic stewardship, as well as prebiotic and probiotic treatments—represents a potential intervention point or an aspect that necessitates staff education
Innovative methods for preventing CDI are emerging. Prior studies have shown that probiotics and prebiotics can be effective in preventing CDI, particularly in high-risk groups [78, 79]. Specifically, certain bacteria like Bacillus clausii and Lactobacillus reuteri are of interest because they produce substances that directly inhibit C. difficile [80, 81]. Other beneficial bacteria, such as Clostridium scindens, create secondary bile acids that increase resistance to C. difficile [82]. Moreover, non-toxigenic C. difficile can also help by competing for resources, reducing the opportunity for harmful strains to proliferate. A previous randomized controlled trial has shown that administration of non-toxigenic C. difficle spores can prevent recurrent CDI episodes [83]. Carriers of toxigenic C. difficile pose ideal candidates for such interventions due their high risk of contracting active infection.
Additional research is necessary to thoroughly evaluate the effectiveness of C. difficile screening in decreasing the burden of CDI. It is clear that any such screening intervention should constitute a multimodal effort. This includes not only enhanced contact precautions and cleaning procedures in these cases but also comprehensive staff education on screening implications for antibiotic selection and diagnostic stewardship for C. difficile testing. Moreover, healthcare-associated CDI definitions should be adjusted to encompass carriers who develop diarrhea as a distinct identity, recognizing the intricacies of this condition. Further research should assess the cost–benefit of these interventions..
Acknowledgements
Author Contribution
Mayan Gilboa and Dafna Yahav conceptualized the topic and content for the manuscript. All authors (Mayan Gilboa, Nadav Baharav, Eyal Melzer, Gili Regev-Yochay and Dafna Yahav) participated in the search and data extraction, Mayan Gilboa and Dafna Yahav wrote the initial manuscript, all authors revised and approved the final version of the manuscript.
Data availability
Not applicable for this study
Funding
No funding was received for this study or the publication of this manuscript.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Conflict of Interest
Mayan Gilboa, Nadav Baharav, Eyal Melzer, Gili Regev-Yochay and Dafna Yahav have nothing to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55 Suppl 2(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.C D C. Antibiotic Resistance Threats in the United States, 2019. mSystems.
- 5.Crobach MJT, Vernon JJ, Loo VG, Kong LY, Péchiné S, Wilcox MH. Understanding Clostridium difficile colonization. Clin Microbiol Rev. 2018;31(2):e00021-17. doi: 10.1128/CMR.00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron SW, Ostrowsky BE, Nori P, Drory DY, Levi MH, Szymczak WA, et al. Screening of Clostridioides difficile carriers in an urban academic medical center: understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41(2):149–153. doi: 10.1017/ice.2019.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donskey CJ, Sunkesula VCK, Stone ND, Gould CV, McDonald LC, Samore M, et al. Transmission of Clostridium difficile from asymptomatically colonized or infected long-term care facility residents. Infect Control Hosp Epidemiol. 2018;39(8):909–916. doi: 10.1017/ice.2018.106. [DOI] [PubMed] [Google Scholar]
- 8.Donskey CJ, Kundrapu S, Deshpande A. Colonization versus carriage of Clostridium difficile. Infect Dis Clin North Am. 2015;29(1):13–28. doi: 10.1016/j.idc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 9.FAQs for Clinicians about C. diff [Internet]. [cited 2023 May 24]. https://www.cdc.gov/cdiff/clinicians/faq.html. Accessed 23 May 2023.
- 10.Otter JA, French GL. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J Clin Microbiol. 2009;47(1):205–207. doi: 10.1128/JCM.02004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38(7):1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semon AK, Keenan O, Zackular JP. Clostridioides difficile and the microbiota early in life. J Pediatric Infect Dis Soc. 2021;10(Supplement_3):S3–7. doi: 10.1093/jpids/piab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SC, Knight DR, Riley TV. Clostridium difficile and One Health. Clin Microbiol Infect. 2020;26(7):857–863. doi: 10.1016/j.cmi.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Zhu L, Faden HS. Interactions of bile acids and the gut microbiota: learning from the differences in Clostridium difficile infection between children and adults. Physiol Genomics. 2019;51(6):218–223. doi: 10.1152/physiolgenomics.00034.2019. [DOI] [PubMed] [Google Scholar]
- 15.Pollock NR, Banz A, Chen X, Williams D, Xu H, Cuddemi CA, et al. Comparison of Clostridioides difficile stool toxin concentrations in adults with symptomatic infection and asymptomatic carriage using an ultrasensitive quantitative immunoassay. Clin Infect Dis. 2019;68(1):78–86. doi: 10.1093/cid/ciy415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Dong D, Jiang C, Li Z, Wang X, Peng Y. Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe. 2015;34:1–7. doi: 10.1016/j.anaerobe.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2(3):145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166(3):561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 19.Tschudin-Sutter S, Carroll KC, Tamma PD, Sudekum ML, Frei R, Widmer AF, et al. Impact of toxigenic Clostridium difficile colonization on the risk of subsequent C. difficile infection in intensive care unit patients. Infect Control Hosp Epidemiol. 2015;36(11):1324–1329. doi: 10.1017/ice.2015.177. [DOI] [PubMed] [Google Scholar]
- 20.Kong LY, Dendukuri N, Schiller I, Bourgault A-M, Brassard P, Poirier L, et al. Predictors of asymptomatic Clostridium difficile colonization on hospital admission. Am J Infect Control. 2015;43(3):248–253. doi: 10.1016/j.ajic.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Longtin Y, Paquet-Bolduc B, Gilca R, Garenc C, Fortin E, Longtin J, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of c difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176(6):796–804. doi: 10.1001/jamainternmed.2016.0177. [DOI] [PubMed] [Google Scholar]
- 22.Worley J, Delaney ML, Cummins CK, DuBois A, Klompas M, Bry L. Genomic determination of relative risks for Clostridioides difficile Infection from asymptomatic carriage in intensive care unit patients. Clin Infect Dis. 2021;73(7):e1727–e1736. doi: 10.1093/cid/ciaa894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth PM, Douchant K, Uyanwune Y, Larocque M, Anantharajah A, Borgundvaag E, et al. Evidence of transmission of Clostridium difficile in asymptomatic patients following admission screening in a tertiary care hospital. PLoS ONE. 2019;14(2):e0207138. doi: 10.1371/journal.pone.0207138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collison M, Murillo C, Marrs R, Bartlett A, Tesic V, Beavis KG, et al. Universal screening for Clostridioides difficile at an urban academic medical center. Infect Control Hosp Epidemiol. 2021;42(3):351–352. doi: 10.1017/ice.2020.428. [DOI] [PubMed] [Google Scholar]
- 25.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RLP, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45(8):992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 26.Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381–390. doi: 10.1038/ajg.2015.22. [DOI] [PubMed] [Google Scholar]
- 27.Eyre DW, Griffiths D, Vaughan A, Golubchik T, Acharya M, O’Connor L, et al. Asymptomatic Clostridium difficile colonisation and onward transmission. PLoS ONE. 2013;8(11):e78445. doi: 10.1371/journal.pone.0078445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leekha S, Aronhalt KC, Sloan LM, Patel R, Orenstein R. Asymptomatic Clostridium difficile colonization in a tertiary care hospital: admission prevalence and risk factors. Am J Infect Control. 2013;41(5):390–393. doi: 10.1016/j.ajic.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Loo VG, Bourgault A-M, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 30.Dubberke ER, Reske KA, Seiler S, Hink T, Kwon JH, Burnham CAD. Risk factors for acquisition and loss of Clostridium difficile colonization in hospitalized patients. Antimicrob Agents Chemother. 2015;59(8):4533–4543. doi: 10.1128/AAC.00642-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K-B, Lee M, Suh JW, Yang K-S, Chung Y, Kim JY, et al. Clinical prediction rule for identifying older patients with toxigenic Clostridioides difficile at the time of hospital admission. BMC Geriatr. 2023;23(1):127. doi: 10.1186/s12877-023-03808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meltzer E, Smollan G, Huppert A, Fluss R, Tal I, Gilboa M, et al. Universal screening for Clostridioides difficile in a tertiary hospital: risk factors for carriage and clinical disease. Clin Microbiol Infect. 2019;25(9):1127–1132. doi: 10.1016/j.cmi.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz-Price LS, Hanson R, Singh S, Nattinger AB, Penlesky A, Buchan BW, et al. Association between environmental factors and toxigenic Clostridioides difficile Carriage at hospital admission. JAMA Netw Open. 2020;3(1):e1919132. doi: 10.1001/jamanetworkopen.2019.19132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 35.Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010;31(1):21–27. doi: 10.1086/649016. [DOI] [PubMed] [Google Scholar]
- 36.Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, et al. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun. 2009;77(9):3661–3669. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351(9103):633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 38.Blixt T, Gradel KO, Homann C, Seidelin JB, Schønning K, Lester A, et al. Asymptomatic carriers contribute to nosocomial Clostridium difficile infection: a cohort study of 4508 patients. Gastroenterology. 2017;152(5):1031–1041.e2. doi: 10.1053/j.gastro.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Paquet-Bolduc B, Gervais P, Roussy J-F, Trottier S, Oughton M, Brukner I, et al. Detection and isolation of Clostridium difficile asymptomatic carriers during Clostridium difficile infection outbreaks: an exploratory study. Clin Infect Dis. 2018;67(11):1781–1783. doi: 10.1093/cid/ciy425. [DOI] [PubMed] [Google Scholar]
- 40.Curry SR, Hecker MT, O’Hagan J, Kutty PK, Alhmidi H, Ng-Wong YK, et al. Natural history of Clostridioides difficile colonization and infection following new acquisition of carriage in healthcare settings: a prospective cohort study. Clin Infect Dis. 2023;77(1):77–83. doi: 10.1093/cid/ciad142. [DOI] [PubMed] [Google Scholar]
- 41.Ötleş E, Balczewski EA, Keidan M, Oh J, Patel A, Young VB, et al. Clostridioides difficile infection surveillance in intensive care units and oncology wards using machine learning. Infect Control Hosp Epidemiol. 2023;24:1–6. doi: 10.1017/ice.2023.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzler E, Mulugeta SG, Danziger LH. The antimicrobial stewardship approach to combating Clostridium difficile. Antibiotics (Basel) 2015;4(2):198–215. doi: 10.3390/antibiotics4020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(7):1748–1754. doi: 10.1093/jac/dku046. [DOI] [PubMed] [Google Scholar]
- 44.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;30(4):CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Owens RC, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;15(46 Suppl 1):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 46.Kundrapu S, Sunkesula VCK, Jury LA, Cadnum JL, Nerandzic MM, Musuuza JS, et al. Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis. 2016;18(16):159. doi: 10.1186/s12879-016-1514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poirier D, Gervais P, Fuchs M, Roussy J-F, Paquet-Bolduc B, Trottier S, et al. Predictors of Clostridioides difficile infection among asymptomatic, colonized patients: a retrospective cohort study. Clin Infect Dis. 2020;70(10):2103–2210. doi: 10.1093/cid/ciz626. [DOI] [PubMed] [Google Scholar]
- 48.Drekonja DM, Amundson WH, Decarolis DD, Kuskowski MA, Lederle FA, Johnson JR. Antimicrobial use and risk for recurrent Clostridium difficile infection. Am J Med. 2011;124(11):1081.e1–7. doi: 10.1016/j.amjmed.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 49.Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis. 2008;46(3):447–450. doi: 10.1086/525267. [DOI] [PubMed] [Google Scholar]
- 50.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 51.Gilboa M, Houri-Levi E, Cohen C, Tal I, Rubin C, Feld-Simon O, et al. Environmental shedding of toxigenic Clostridioides difficile by asymptomatic carriers: a prospective observational study. Clin Microbiol Infect. 2020;26(8):1052–1057. doi: 10.1016/j.cmi.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Walker AS, Eyre DW, Wyllie DH, Dingle KE, Harding RM, O’Connor L, et al. Characterisation of Clostridium difficile hospital ward-based transmission using extensive epidemiological data and molecular typing. PLoS Med. 2012;9(2):e1001172. doi: 10.1371/journal.pmed.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis. 2013;57(8):1094–1102. doi: 10.1093/cid/cit475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med. 2013;369(13):1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanzas C, Dubberke ER, Lu Z, Reske KA, Gröhn YT. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect Control Hosp Epidemiol. 2011;32(6):553–561. doi: 10.1086/660013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong LY, Eyre DW, Corbeil J, Raymond F, Walker AS, Wilcox MH, et al. Clostridium difficile: investigating transmission patterns between infected and colonized patients using whole genome sequencing. Clin Infect Dis. 2019;68(2):204–209. doi: 10.1093/cid/ciy457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crobach MJT, Hornung BVH, Verduin C, Vos MC, Hopman J, Kumar N, et al. Screening for Clostridioides difficile colonization at admission to the hospital: a multi-centre study. Clin Microbiol Infect. 2023;29(7):891–896. doi: 10.1016/j.cmi.2023.02.022. [DOI] [PubMed] [Google Scholar]
- 58.Mejia-Chew C, Dubberke ER. Clostridium difficile control measures: current and future methods for prevention. Expert Rev Anti Infect Ther. 2018;16(2):121–131. doi: 10.1080/14787210.2018.1429911. [DOI] [PubMed] [Google Scholar]
- 59.Krishna A, Chopra T. Prevention of Infection due to Clostridium (Clostridioides) difficile. Infect Dis Clin N Am. 2021;35(4):995–1011. doi: 10.1016/j.idc.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Barker AK, Scaria E, Safdar N, Alagoz O. Evaluation of the cost-effectiveness of infection control strategies to reduce hospital-onset Clostridioides difficile infection. JAMA Netw Open. 2020;3(8):e2012522. doi: 10.1001/jamanetworkopen.2020.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker AK, Alagoz O, Safdar N. Interventions to reduce the incidence of hospital-onset Clostridium difficile infection: an agent-based modeling approach to evaluate clinical effectiveness in adult acute care hospitals. Clin Infect Dis. 2018;66(8):1192–1203. doi: 10.1093/cid/cix962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saab S, Alper T, Sernas E, Pruthi P, Alper MA, Sundaram V. Hospitalized Patients with cirrhosis should be screened for Clostridium difficile colitis. Dig Dis Sci. 2015;60(10):3124–3129. doi: 10.1007/s10620-015-3707-8. [DOI] [PubMed] [Google Scholar]
- 63.Reagan KA, Chan DM, Vanhoozer G, Bearman G. Estimating the effect of active detection and isolation on Clostridioides difficile infections in a bone marrow transplant unit. Infect Control Hosp Epidemiol. 2023;13:1–6. doi: 10.1017/ice.2023.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grigoras CA, Zervou FN, Zacharioudakis IM, Siettos CI, Mylonakis E. Isolation of C. difficile carriers alone and as part of a bundle approach for the prevention of Clostridium difficile Infection (CDI): a mathematical model based on clinical study data. PLoS ONE. 2016;11(6):e0156577. doi: 10.1371/journal.pone.0156577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanzas C, Dubberke ER. Effectiveness of screening hospital admissions to detect asymptomatic carriers of Clostridium difficile: a modeling evaluation. Infect Control Hosp Epidemiol. 2014;35(8):1043–1050. doi: 10.1086/677162. [DOI] [PubMed] [Google Scholar]
- 66.Bartsch SM, Curry SR, Harrison LH, Lee BY. The potential economic value of screening hospital admissions for Clostridium difficile. Eur J Clin Microbiol Infect Dis. 2012;31(11):3163–3171. doi: 10.1007/s10096-012-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao Y, Paquet-Bolduc B, Garenc C, Gervais P, Trottier S, Roussy J-F, et al. Impact of isolating Clostridium difficile carriers on the burden of isolation precautions: a time series analysis. Clin Infect Dis. 2018;66(9):1377–1382. doi: 10.1093/cid/cix1024. [DOI] [PubMed] [Google Scholar]
- 68.Cho J, Seville MT, Khanna S, Pardi DS, Sampathkumar P, Kashyap PC. Screening for Clostridium difficile colonization on admission to a hematopoietic stem cell transplant unit may reduce hospital-acquired C. difficile infection. Am J Infect Control. 2018;46(4):459–461. doi: 10.1016/j.ajic.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Linsenmeyer K, O’Brien W, Brecher SM, Strymish J, Rochman A, Itani K, et al. Clostridium difficile screening for colonization during an outbreak setting. Clin Infect Dis. 2018;67(12):1912–1914. doi: 10.1093/cid/ciy455. [DOI] [PubMed] [Google Scholar]
- 70.Peterson LR, O’Grady S, Keegan M, Fisher A, Zelencik S, Kufner B, et al. Reduced Clostridioides difficile infection in a pragmatic stepped-wedge initiative using admission surveillance to detect colonization. PLoS ONE. 2020;15(3):e0230475. doi: 10.1371/journal.pone.0230475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45(1):109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health. 2021;5(12):e893–904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kutty PK, Beekmann SE, Sinkowitz-Cochran RL, Dubberke ER, Kuhar DT, McDonald LC, et al. A national survey of testing and management of asymptomatic carriage of C. difficile. Infect Control Hosp Epidemiol. 2019;40(7):801–803. doi: 10.1017/ice.2019.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCort MN, Oehler C, Enriquez M, Landon E, Nguyen CT, Pettit NN, et al. Universal molecular Clostridioides difficile screening and overtreatment in solid organ transplant recipients. Transpl Infect Dis. 2020;22(5):e13375. doi: 10.1111/tid.13375. [DOI] [PubMed] [Google Scholar]
- 75.Tran K, Bell C, Stall N, Tomlinson G, McGeer A, Morris A, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: a multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262–268. doi: 10.1007/s11606-016-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors: a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118–1120. doi: 10.1086/673153. [DOI] [PubMed] [Google Scholar]
- 77.Mehrotra P, Croft L, Day HR, Perencevich EN, Pineles L, Harris AD, et al. Effects of contact precautions on patient perception of care and satisfaction: a prospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1087–1093. doi: 10.1086/673143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McFarland LV, Goldstein EJC, Kullar R. Microbiome-related and infection control approaches to primary and secondary prevention of Clostridioides difficile infections. Microorganisms. 2023;11(6):1534. doi: 10.3390/microorganisms11061534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mills JP, Rao K, Young VB. Probiotics for prevention of Clostridium difficile infection. Curr Opin Gastroenterol. 2018;34(1):3–10. doi: 10.1097/MOG.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ripert G, Racedo SM, Elie A-M, Jacquot C, Bressollier P, Urdaci MC. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob Agents Chemother. 2016;60(6):3445–3454. doi: 10.1128/AAC.02815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spinler JK, Auchtung J, Brown A, Boonma P, Oezguen N, Ross CL. Next-generation probiotics targeting Clostridium difficile through precursor-directed antimicrobial biosynthesis. Infect Immun. 2017;85(10):e00303-17. doi: 10.1128/IAI.00303-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weingarden AR, Dosa PI, DeWinter E, Steer CJ, Shaughnessy MK, Johnson JR, et al. Changes in colonic bile acid composition following fecal microbiota transplantation are sufficient to control Clostridium difficile germination and growth. PLoS ONE. 2016;11(1):e0147210. doi: 10.1371/journal.pone.0147210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerding DN, Meyer T, Lee C, Cohen SH, Murthy UK, Poirier A, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA. 2015;313(17):1719–1727. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable for this study