Abstract
Introduction
Efficacy and safety of the attachment inhibitor fostemsavir + optimized background therapy (OBT) were evaluated through 48 and 96 weeks in the phase 3 BRIGHTE trial in heavily treatment-experienced (HTE) adults failing their current antiretroviral regimen. Here, we report 240-week efficacy and safety of fostemsavir + OBT in adults with multidrug-resistant human immunodeficiency virus (HIV)-1 in BRIGHTE.
Methods
Heavily treatment-experienced adults failing their current regimen entered the randomized cohort (RC; 1–2 fully active antiretrovirals available) or non-randomized cohort (NRC; no fully active antiretrovirals available) and received open-label fostemsavir + OBT (starting Day 8 in RC and Day 1 in NRC). Endpoints included proportion with virologic response (HIV-1 RNA < 40 copies/mL, Snapshot), immunologic efficacy, and safety.
Results
At Week 240, 45% and 22% of the RC and NRC, respectively, had virologic response (Snapshot); 7% of the RC and 5% of the NRC had missing data due to coronavirus disease 2019 (COVID-19)-impacted visits. In the observed analysis, 82% of the RC and 66% of the NRC had virologic response. At Week 240, mean change from baseline in CD4+ T-cell count was 296 cells/mm3 (RC) and 240 cells/mm3 (NRC); mean CD4+/CD8+ ratio increased between Weeks 96 and 240 (RC 0.44 to 0.60; NRC 0.23 to 0.32). Between Weeks 96 and 240, four participants discontinued for adverse events, one additional participant experienced a drug-related serious adverse event, and six deaths occurred (median last available CD4+ T-cell count, 3 cells/mm3). COVID-19-related events occurred in 25 out of 371 participants; all resolved without incident.
Conclusion
Through ~5 years, fostemsavir + OBT demonstrated durable virologic and immunologic responses with no new safety concerns between Weeks 96 and 240, supporting this regimen as a key therapeutic option for HTE people with multidrug-resistant HIV-1.
Trial registration
ClinicalTrials.gov, NCT02362503.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00870-6.
Keywords: Attachment inhibitor, Advanced HIV disease, CD4+/CD8+ ratio, CD4+ T-cell count, Virologic response
Key Summary Points
| Why carry out this study? |
| Heavily treatment-experienced (HTE) individuals with HIV-1 have limited therapeutic options, and new antiretroviral drug classes are needed for this population. |
| Efficacy and safety of fostemsavir + optimized background therapy (OBT) have previously been evaluated through 96 weeks; here, we report long-term efficacy and safety of fostemsavir + OBT in HTE adults with multidrug-resistant HIV-1 from the phase 3 BRIGHTE trial. |
| What was learned from this study? |
| A population with advanced disease and multidrug-resistant HIV-1 was able to achieve durable virologic responses and clinically meaningful improvements in CD4+ T-cell count and CD4+/CD8+ ratio on treatment with fostemsavir + OBT. |
| The safety and tolerability profile of fostemsavir + OBT remained consistent with prior observations through 96 weeks, with no new safety trends. |
| Long-term results provide further support for fostemsavir as a key therapeutic option for HTE people with multidrug-resistant HIV-1. |
Introduction
Heavily treatment-experienced (HTE) individuals with human immunodeficiency virus (HIV)-1 have limited therapeutic options due to multidrug resistance, drug intolerance/toxicity, and comorbidities [1–3]. Constructing suppressive antiretroviral (ARV) regimens for HTE individuals is difficult [4], leading to advanced disease and higher mortality rates [1]. New ARV classes that have mechanisms of action distinct from existing therapies, lack cross-resistance and drug–drug interactions with other ARVs, and are well tolerated are needed for this population [3].
Fostemsavir is a prodrug of temsavir, a first-in-class gp120-directed attachment inhibitor approved in combination with other ARVs for adults with multidrug-resistant HIV-1 who are otherwise unable to construct suppressive regimens due to resistance, prior intolerance, or safety concerns [5, 6]. Temsavir binds directly to HIV-1 gp120 and locks it in a closed conformation [7], allosterically interfering with the ability of gp120 to attach to CD4 on target cells. Temsavir is effective against CCR5-, CXCR4-, and dual-tropic strains [8, 9], and no in vitro or clinical evidence of cross-resistance to the CCR5 antagonist maraviroc has been reported [10, 11].
Efficacy and safety of fostemsavir + optimized background therapy (OBT) were evaluated through 48 and 96 weeks in the phase 3 BRIGHTE trial in HTE adults failing their current ARV regimen [12, 13]. At Week 96, 60% of randomized cohort (RC) participants and 37% of non-randomized cohort (NRC) participants had virologic response (HIV-1 RNA < 40 copies/mL by Snapshot). Clinically relevant increases in mean [standard deviation (SD)] CD4+ T-cell count were observed in both cohorts [RC 205 (191); NRC 119 (202) cells/mm3] [13]. Fostemsavir + OBT was well tolerated, with no new safety concerns identified between Weeks 48 and 96.
BRIGHTE was planned to continue beyond Week 96 until participants could access fostemsavir through other means. The trial extended into the COVID-19 pandemic, impacting participant ability to attend study visits [14]. Here, we report efficacy and safety of fostemsavir + OBT in HTE adults with multidrug-resistant HIV-1 through Week 240 of BRIGHTE.
Methods
Study Population
BRIGHTE (ClinicalTrials.gov, NCT02362503) included 113 investigational sites in 22 countries across Africa, Asia–Pacific, Europe, North America, and South America. Participants were HTE adults (aged ≥ 18 years) with HIV-1 failing their current ARV regimen (screening HIV-1 RNA ≥ 400 copies/mL). Participants had ≤ 2 fully active and available ARVs remaining. Fully active was based on susceptibility (determined by historical or baseline resistance testing; see Supplementary Methods), and availability was based on whether the participant was tolerant of, eligible for, and willing to take (in the case of enfuvirtide only) the ARV. There were no screening temsavir susceptibility criteria.
BRIGHTE was performed in accordance with the Declaration of Helsinki. Study protocols, amendments, and other required documents were reviewed and approved by national, regional, and/or institutional review boards or ethics committees (see Supplementary Material for list of ethics committees). All participants provided written informed consent before study initiation.
Study Design
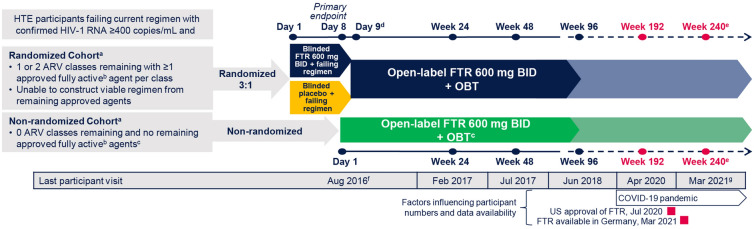
After screening, participants were assigned to the RC or NRC based on number of fully active and available ARVs (Fig. 1). Those with 1 or 2 available ARVs entered the RC and were randomly assigned 3:1 to receive oral fostemsavir 600 mg twice daily or placebo + current failing regimen for 8 days, after which all participants received open-label fostemsavir + OBT. Participants with no available ARVs were assigned to the NRC and received open-label fostemsavir + OBT starting on Day 1; NRC participants could include other investigational ARVs in their OBT. Participants received their first fostemsavir dose between February 2015 and August 2016 and continued fostemsavir while on study regardless of virologic response. BRIGHTE remains ongoing until all participants can access fostemsavir by other means; the last Week 240 visit occurred in March 2021. A total of 80 participants gained access to commercial fostemsavir and completed the study before the 240-week data cutoff; 68 had their Week 240 visit before study completion and were included in the 240-week analysis.
Fig. 1.
Study design. ARV antiretroviral, BID twice daily, FTR fostemsavir, HTE heavily treatment-experienced, OBT optimized background therapy. aThere were no screening temsavir susceptibility criteria. bFully active is based on susceptibility (current or historical resistance measures) and availability [the participant is tolerant of, eligible for, and willing to take (in the case of enfuvirtide only) the ARV]. cUse of investigational agents as part of OBT was permitted in the non-randomized cohort only. dSubsequent time points were measured from the start of open-label FTR 600 mg BID + OBT. eThe study is expected to be conducted until participants can access FTR through other means (e.g., marketing approval). fLast study participant first dose. gDatabase lock June 2021
Outcomes
The Week 240 interim analysis was conducted to evaluate efficacy and safety of fostemsavir + OBT beyond Week 96 in BRIGHTE participants who remained on study. Briefly, we evaluated the proportion of participants with HIV-1 RNA < 40 copies/mL using the US Food and Drug Administration (FDA) Snapshot and observed analyses. Additional endpoints included change from baseline in log10 HIV-1 RNA, CD4+ T-cell count, and CD4+/CD8+ ratio. Safety and tolerability were assessed as frequency of any serious adverse events (SAEs), adverse events (AEs) leading to discontinuation, grade 3–4 laboratory abnormalities, occurrence of new acquired immunodeficiency syndrome (AIDS)-defining events, and death. Before Week 24, protocol-defined virologic failure (PDVF) was defined as confirmed (or last available before discontinuation) plasma HIV-1 RNA ≥ 400 copies/mL after prior confirmed suppression to < 400 copies/mL or > 1 log10 copies/mL increase in HIV-1 RNA at any time above nadir, where nadir is ≥ 40 copies/mL. At and after Week 24, PDVF was defined as confirmed (or last available before discontinuation) HIV-1 RNA ≥ 400 copies/mL. Emergence of genotypic and phenotypic resistance to temsavir among participants with PDVF was also evaluated.
Statistical Analysis
The intention-to-treat-exposed (ITT-E) population, which included all participants who received ≥ 1 dose of study treatment, was used for efficacy and safety analyses. Virologic response (HIV-1 RNA < 40 copies/mL) was assessed in the ITT-E population using the FDA Snapshot algorithm, with missing virologic data or change in OBT considered treatment failure. Participants who completed BRIGHTE before their Week 240 visit were not included in the Week 240 Snapshot analysis. Observed analyses were also conducted for virologic and immunologic outcomes through Week 240 using participants from the ITT-E population with data in the analysis window (participants who discontinued the study, were on study but missing virologic data, or completed the study were not included in observed analyses). Safety data were summarized by cohort, randomized treatment, and total group for the RC and total study population. Subgroup analyses evaluated efficacy by baseline disease characteristics and OBT composition.
Results
Participant Disposition
Overall, 371 participants were enrolled in BRIGHTE, 272 in the RC and 99 in the NRC. Baseline disease characteristics were indicative of advanced disease in both cohorts but to a greater extent in the NRC (Table S1). Median baseline viral load was 4.66 and 4.31 log10 copies/mL in the RC and NRC, respectively. Median baseline CD4+ T-cell count was 99.5 and 41.0 cells/mm3 in the RC and NRC, respectively, with a similar proportion of participants with CD4+ T-cell count < 200 cells/mm3 (RC 73%; NRC 80%).
At the Week 240 data cutoff, 156 participants remained on study, 80 had completed and transitioned to commercially available fostemsavir (12 before completing their Week 240 visit), and 135 had withdrawn (Figure S1).
Virologic Efficacy
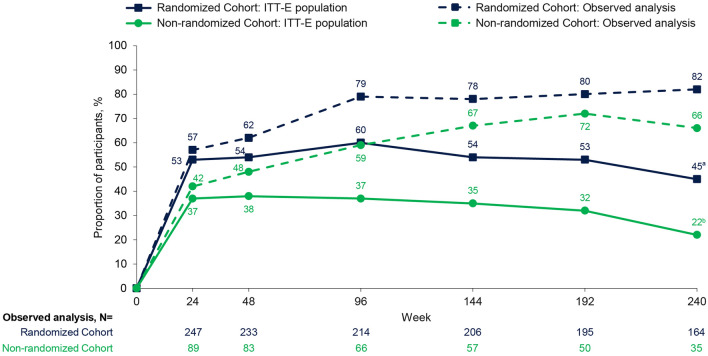
In the ITT-E population, virologic response rates in the RC and NRC (FDA Snapshot) were consistent between Weeks 48 and 192, ranging from 53% to 60% and 32% to 38%, respectively (Fig. 2). Virologic response rates declined to 45% (RC) and 22% (NRC) at Week 240; however, Snapshot virologic non-responders included 24 participants (RC, n = 19; NRC, n = 5) who had no virologic data due to COVID-19 pandemic-related disruptions in access to care (Table 1). Virologic response rates generally decreased with worsening baseline disease (Table S2).
Fig. 2.
Virologic response (HIV-1 RNA < 40 copies/mL) through Week 240 by Snapshot analysis (ITT-E) and observed analysis. ITT-E participants without an HIV-1 RNA value at the relevant time point or those who changed OBT due to lack of efficacy up to each time point counted as failures. ITT-E intention-to-treat exposed, OBT optimized background therapy. aITT-E population, N = 267. bITT-E population, N = 92
Table 1.
Virologic outcomes by Snapshot (ITT-E) and observed analyses and protocol-defined virologic failure through week 240
| Outcome, n (%) | Randomized cohort | Non-randomized cohort | ||||
|---|---|---|---|---|---|---|
| Week 96 | Week 192a | Week 240b | Week 96 | Week 192a | Week 240b | |
| Snapshot analysis, ITT-E population | ||||||
| Number of participants | 272 | 272 | 267 | 99 | 99 | 92 |
| HIV-1 RNA < 40 copies/mL | 164 (60) | 145 (53) | 120 (45) | 37 (37) | 32 (32) | 20 (22) |
| HIV-1 RNA ≥ 40 copies/mL | 80 (29) | 90 (33) | 89 (33) | 43 (43) | 43 (43) | 43 (47) |
| Data in window not below threshold | 32 (12) | 27 (10) | 20 (7) | 15 (15) | 5 (5) | 5 (5) |
| D/C for lack of efficacy | 9 (3) | 12 (4) | 14 (5) | 3 (3) | 6 (6) | 6 (7) |
| D/C for other reason while not below threshold | 17 (6) | 21 (8) | 24 (9) | 6 (6) | 10 (10) | 10 (11) |
| Change in background ART | 22 (8) | 30 (11) | 31 (12)c | 19 (19) | 22 (22) | 22 (24)d |
| No virologic data | 28 (10) | 37 (14) | 58 (22) | 19 (19) | 24 (24) | 29 (32) |
| D/C study due to adverse event or death | 15 (6) | 16 (6) | 17 (6) | 14 (14) | 18 (8) | 18 (20) |
| D/C study for other reasons | 8 (3) | 15 (6) | 19 (7) | 4 (4) | 4 (4) | 4 (4) |
| Missing data during window but on study | ||||||
| Not COVID-19 related | 5 (2) | 2 (< 1) | 3 (1) | 1 (1) | 0 | 2 (2) |
| COVID-19 relatede | – | 4 (1) | 19 (7) | – | 2 (2) | 5 (5) |
| Observed analysis | ||||||
| Number of participants | 214 | 195 | 164 | 66 | 50 | 35 |
| HIV-1 RNA < 40 copies/mL | 170 (79) | 156 (80) | 135 (82) | 39 (59) | 36 (72) | 23 (66) |
| Protocol-defined virologic failuref | 63 (23) | 75 (28) | 80 (29) | 49 (49) | 52 (53) | 53 (54) |
ART antiretroviral therapy, D/C discontinued, ITT-E intention-to-treat exposed
aWeek 192 was the last study time point that included all participants from the original ITT-E population (no participants had completed the study)
bAt Week 240, 12 participants had completed the study by transitioning to locally approved fostemsavir (the first fostemsavir approval was in the USA in July 2020)
cWeek 240 HIV-1 RNA was < 40 copies/mL for 17 of these 31 participants
dWeek 240 HIV-1 RNA was < 40 copies/mL for 4 of these 22 participants
eCOVID-19-related missing data were the result of missed visits, partial visits where only drug was dispensed, and late visits that were outside the analysis window
fProtocol-defined virologic failure was defined as the following: before Week 24, confirmed (or last available before discontinuation) HIV-1 RNA ≥ 400 copies/mL after confirmed suppression to < 400 copies/mL or confirmed > 1 log10 copies/mL increase in HIV-1 RNA above nadir where nadir is ≥ 40 copies/mL; at or after Week 24, confirmed (or last available before discontinuation) HIV-1 RNA ≥ 400 copies/mL
Virologic non-response was due to change in OBT in 22 out of 92 (24%) and 31 out of 267 (12%) NRC and RC participants, respectively. A total of 4 NRC and 17 RC participants who changed OBT had HIV-1 RNA < 40 copies/mL at Week 240. In the observed analysis at Week 240, HIV-1 RNA was < 40 copies/mL for 135 out of 164 (82%) RC participants and 23 out of 35 (66%) NRC participants and < 400 copies/mL for 155 out of 164 (95%) RC participants and 28 out of 35 (80%) NRC participants.
After Week 96, 17 and 4 additional participants met PDVF criteria in the RC and NRC, respectively (Table 1), bringing the total number of PDVFs to 80 (29%) in the RC and 53 (54%) in the NRC. Protocol-defined virologic failure was generally more frequent among participants with worse baseline disease in both cohorts (Table S3). Of the 133 participants who met PDVF, 58 (RC, n = 42; NRC, n = 16) had nadir HIV-1 RNA < 400 copies/mL after PDVF while on fostemsavir through Week 240. Of participants with nadir HIV-1 RNA < 400 copies/mL after PDVF, 22 (RC, n = 19; NRC, n = 3) maintained HIV-1 RNA < 400 copies/mL through Week 240. Notably, 32 out of 42 and 11 out of 16 participants in the RC and NRC, respectively, had no changes to their OBT between PDVF and suppression.
Virologic response rate by Snapshot was 8% (1 of 13) at Week 240 for NRC participants who included ibalizumab in their initial OBT (Figure S2). After 2018 regulatory approval [15], six RC participants added ibalizumab to their OBT; at Week 240, 67% (4 of 6) had HIV-1 RNA < 40 copies/mL (Table S4).
Virology
A total of 71 out of 80 RC participants and 51 out of 53 NRC participants with PDVF had genotypic and phenotypic resistance data available at Week 240. Resistance-associated pre-specified substitutions of interest (S375H/I/M/N/T, M426L, M434I/K, and M475I [5, 16–19]) were emergent in 30 out of 71 (42%) RC participants and 33 out of 51 (65%) NRC participants. S375N or M426L were the most frequent substitutions (Table S5). In vitro, 89% (RC 26 of 30; NRC 30 of 33) of successfully tested gp160 variants with emergent pre-specified substitutions were less susceptible to temsavir than their respective baseline sequences (> threefold change from baseline). In total, 35 out of 80 (44%) RC and 40 out of 53 (75%) NRC participants had treatment-emergent substitutions or > threefold change in temsavir susceptibility at PDVF. Of those, 9 out of 35 (26%) RC and 6 out of 40 (15%) NRC participants remained on study and had HIV-1 RNA < 40 copies/mL after PDVF. Most variants without emergent substitutions were similarly susceptible to temsavir as before treatment initiation, with 83% (RC 34 out of 38; NRC 11 out of 16) of tested variants having a threefold change or less from baseline to time of on-treatment testing. Notably, by Week 240, five participants (RC, n = 2; NRC, n = 3) had a > 100-fold change in susceptibility without any emergent substitutions of interest.
Immunologic Efficacy
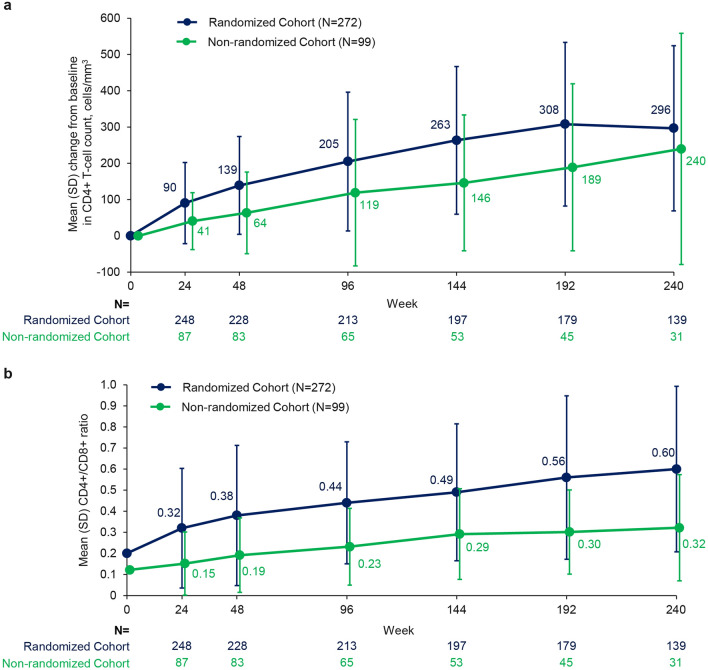
In RC participants, mean CD4+ T-cell count increased from baseline through Week 192 and stabilized thereafter (observed analysis; Fig. 3A). CD4+ T-cell count also improved in NRC participants over time (mean change from baseline, 240 cells/mm3 at Week 240; observed analysis). Substantial improvements in CD4+ T-cell count were evident in all baseline CD4+ T-cell count subgroups but numerically greatest in those with < 20 cells/mm3 (mean [SD], 338 [250] cells/mm3) and 20 to < 50 cells/mm3 at baseline (332 [88] cells/mm3; Figure S3). By Week 240, 67% (22 of 33) of remaining participants with baseline CD4+ T-cell count < 20 cells/mm3 and 92% (12 of 13) with baseline CD4+ T-cell count 20 to < 50 cells/mm3 had CD4+ T-cell count ≥ 200 cells/mm3. Participants with baseline HIV-1 RNA ≥ 100,000 copies/mL and those with Week 240 HIV-1 RNA ≥ 40 copies/mL also demonstrated substantial mean increases (353 and 251 cells/mm3, respectively).
Fig. 3.
a Change in CD4+ T-cell count from baseline to Week 240 and b CD4+/CD8+ ratio through Week 240 (observed analysis). Baseline mean (SD): CD4+ T-cell count, RC 152.5 (182.0) and NRC 99.4 (130.8) cells/mm3; CD4+/CD8+ ratio, RC 0.20 (0.24) and 0.12 (0.12). Error bars represent standard deviation
In the RC, mean (SD) CD4+/CD8+ ratio improved continuously, reaching 0.60 (0.39) at Week 240 (Fig. 3B). Of the RC participants, 60% (83 of 139) achieved CD4+/CD8+ ratio ≥ 0.45, including 47% (49 of 104 with available data) with baseline ratio < 0.3. CD4+/CD8+ ratio also continuously improved in the NRC, reaching a mean (SD) of 0.32 (0.25) by Week 240. Overall, 29% (9 of 31) of the NRC achieved CD4+/CD8+ ratio ≥ 0.45, including 16% (4 of 25 with available data) with baseline ratio < 0.3.
Safety
Proportion of participants with any AE was comparable between cohorts from Weeks 96–240, though NRC participants experienced more grade 3–4 AEs, SAEs, AEs leading to discontinuation, and deaths (Table 2). There were four new discontinuations due to AEs (RC, n = 3; NRC, n = 1). Infections (not COVID-19-related) were the most common reason for AE-related discontinuations through 96 weeks and accounted for two of the withdrawals after Week 96 (pneumonia, cytomegaloviral pneumonia); the other two were due to polyneuropathy and rash. Drug-related grade 2–4 AEs occurring in ≥ 2% of participants were nausea (n = 17), diarrhea (n = 8), headache (n = 7), and immune reconstitution inflammatory syndrome (n = 7), all reported before the Week 96 data cutoff except for three events of nausea. Serious AEs occurring in ≥ 2% of participants were pneumonia (n = 25), cellulitis (n = 10), acute myocardial infarction (n = 8), acute kidney injury (n = 8, all with identified reversible causes not related to study drug), COVID-19 (n = 7), sepsis (n = 6), and coronary artery disease (n = 6). Of the 16 drug-related SAEs that occurred in 13 participants, only 1 occurred after Week 96 (supraventricular tachycardia, which resolved without interruption to fostemsavir treatment).
Table 2.
Cumulative summary of safety
| Parameter, n (%) | Randomized cohort (N = 272) | Non-randomized cohort (N = 99) | Total (N = 371) | |||
|---|---|---|---|---|---|---|
| Week 96 | Week 240 | Week 96 | Week 240 | Week 96 | Week 240 | |
| Any AE | 249 (92) | 259 (95) | 98 (99) | 98 (99) | 347 (94) | 357 (96) |
| Any grade 2–4 AE | 216 (79) | 242 (89) | 87 (88) | 94 (95) | 303 (82) | 336 (91) |
| Drug-related grade 2–4 AEs | 57 (21) | 65 (24) | 22 (22) | 23 (23) | 79 (21) | 88 (24) |
| Drug-related grade 2–4 AEs occurring in ≥ 2% of participants in either cohort | ||||||
| Nausea | 9 (3) | 12 (4) | 5 (5) | 5 (5) | 14 (4) | 17 (5) |
| Diarrhea | 6 (2) | 5 (2) | 3 (3) | 3 (3) | 9 (2) | 8 (2) |
| Headache | 6 (2) | 6 (2) | 1 (1) | 1 (1) | 7 (2) | 7 (2) |
| IRIS | 6 (2) | 6 (2) | 1 (1) | 1 (1) | 7 (2) | 7 (2) |
| Vomiting | 4 (1) | 4 (1) | 2 (2) | 2 (2) | 6 (2) | 6 (2) |
| Fatigue | 3 (1) | 3 (1) | 2 (2) | 2 (2) | 5 (1) | 5 (1) |
| Asthenia | 2 (< 1) | 2 (< 1) | 2 (2) | 2 (2) | 4 (1) | 4 (1) |
| Any grade 3–4 AE | 78 (29) | 110 (40) | 49 (49) | 60 (61) | 127 (34) | 170 (46) |
| Any SAEa | 92 (34) | 122 (45) | 48 (48) | 55 (56) | 140 (38) | 177 (48) |
| Drug-related SAEsb | 9 (3) | 10 (4) | 3 (3) | 3 (3) | 12 (3) | 13 (4) |
| AEs leading to D/Cc | 14 (5) | 17 (6) | 12 (12) | 13 (13) | 26 (7) | 30 (8) |
| CDC class C events | 23 (8) | 25 (9) | 15 (15) | 19 (19) | 38 (10) | 44 (12) |
| Deathsd | 12 (4) | 15 (6) | 17 (17) | 20 (20) | 29 (8) | 35 (9) |
AE adverse event, CDC Centers for Disease Control and Prevention, D/C discontinuation, IRIS immune reconstitution inflammatory syndrome, SAE serious AE
aSAEs occurring in ≥ 2% of participants were pneumonia (n = 25), cellulitis (n = 10), acute myocardial infarction (n = 8), acute kidney injury (n = 8, all with identified reversible causes not related to study drug), COVID-19 (n = 7), sepsis (n = 6), and coronary artery disease (n = 6)
bDrug-related SAEs (16 events in 13 participants) included IRIS (n = 3); nephrolithiasis (n = 2); and 1 each of acute kidney injury, hyperglycemia, hyperkalemia, loss of consciousness, myocarditis, hepatocellular cytolysis, rhabdomyolysis, fetal growth restriction, disorientation, and rash through the Week 96 data cutoff and supraventricular tachycardia (n = 1) after the Week 96 data cutoff
cThe most common AEs leading to discontinuation were related to infections (n = 12); four participants discontinued because of an AE after the Week 96 cutoff (one each for pneumonia, cytomegaloviral pneumonia, polyneuropathy, and rash)
dOf the 35 deaths, 6 occurred since Week 96; 12 deaths were AIDS-related (5 since Week 96), 12 were acute infections (1 since Week 96), 6 were non-AIDS-related malignancies, and the remaining 5 were related to other conditions. Six deaths occurred after the participant withdrew from the study. One death occurred on the day of study withdrawal for AEs
Through Week 240, there were 35 deaths (RC, n = 15; NRC, n = 20), 6 of which occurred after study withdrawal and 1 on the day of withdrawal. Six deaths (three per cohort) occurred after Week 96: five were AIDS-related (rectal cancer, pulmonary septic shock, dyspnea, HIV wasting syndrome, and progression of AIDS disease) and the sixth was due to acute infection (pneumonia). CD4+ T-cell counts in these six participants ranged from 1 to 98 cells/mm3 at baseline and from 0 to 171 cells/mm3 at last observation. Among all deaths, median last recorded CD4+ T-cell count was 10 cells/mm3, and last recorded CD4+ T-cell count was < 200 cells/mm3 in 32 of the 35 deaths (Table S6).
Through the 240-week data cutoff, six participants became pregnant, including two between Weeks 96 and 240. Per protocol and after a benefit–risk assessment by the investigator, participants were permitted to continue fostemsavir. Three pregnancies led to normal births of healthy infants with no complications, two had complications (one fetal growth restriction and one premature birth) but led to otherwise normal births of infants with no congenital abnormalities, and one ended in an elective abortion.
COVID-19 Events
There were 18 confirmed COVID-19 diagnoses and 10 related events among 25 participants, including 7 SAEs due to hospitalization. The most recent CD4+ T-cell counts of participants who were hospitalized ranged from 164 to 1641 cells/mm3, and 5 out of 7 participants were virologically suppressed. Median event duration was 19 days (range 15–43). Treatment for COVID-19 often included prophylactic anticoagulants and supplemental oxygen, and no changes were made to any ARV regimen. All events resolved without further complication or interruption to study treatment, and no COVID-19-related deaths occurred.
Discussion
We evaluated 240-week efficacy and safety of fostemsavir + OBT in HTE participants with advanced disease (112 of 371 [30%] participants had a baseline CD4+ T-cell count < 20 cells/mm3) and limited treatment options (≤ 2 non-investigational ARVs available). Between Weeks 96 and 240, virologic suppression rates were generally stable. Slightly lower virologic response rates by Snapshot at Week 240 were driven partly by missing data due to COVID-19-related visit restrictions. In the observed analysis, proportions of participants with HIV-1 RNA < 40 copies/mL were generally stable through Week 240 in each cohort. Overall, fostemsavir + OBT was a durable effective treatment option for this HTE population with multidrug-resistant HIV-1.
The incidence of PDVF in BRIGHTE was consistent with observations from prior studies in similar patient populations [20–23]. Most PDVFs occurred during the first 2 years [13]; after Week 96, PDVF occurred in an additional 6% of RC participants and 4% of NRC participants by the Week 240 data cutoff, consistent with observations over 5 years in the BENCHMRK-1 and -2 trials [20].
In participants with PDVF, pre-specified emergent substitutions of interest, primarily S375N or M426L, were observed in fewer than half of evaluable RC participants and approximately two-thirds of evaluable NRC participants; most of the examined envelopes containing these substitutions had > threefold decreases from baseline in susceptibility to temsavir. Most participants without emergent pre-specified substitutions had envelopes similarly susceptible to temsavir versus baseline; however, five had a substantial change (> 100-fold) without any known emergent substitutions of interest. One participant had S375S/M mixture at baseline that became a pure substitution at PDVF, and it is possible that the remaining individuals may have substitutions not yet identified as important to the interaction between temsavir and the HIV-1 envelope. Some participants with treatment-emergent substitutions or > threefold decreases in susceptibility to temsavir who remained on study had HIV-1 RNA < 40 copies/mL after PDVF (RC, 9 of 35; NRC, 6 of 40). Notably, considerable improvements in CD4+ T-cell count occurred even among participants with HIV-1 RNA ≥ 40 copies/mL at Week 240, supporting the benefit–risk profile of fostemsavir even in participants without virologic suppression. When choosing an ARV regimen for HTE individuals, including those with apparent resistance to temsavir, healthcare providers should consider factors such as virologic response, change in CD4+ T-cell count, changes in susceptibility to OBT, and availability of other ARVs.
In the absence of emergent genotypic or phenotypic changes, other factors may contribute to PDVF, such as incomplete adherence to the treatment regimen. Adherence was not measured in BRIGHTE, and there are known challenges to optimal ARV adherence among HTE individuals, such as pill burden and treatment interruptions due to toxicity or comorbidities [1]. Indeed, a substantial proportion of participants had virologic re-suppression after meeting PDVF criteria without any change in treatment regimen, which may be related to improved adherence.
Steady increases in CD4+ T-cell counts were seen through 240 weeks of fostemsavir-based treatment in both cohorts, with participants who were the most immune-suppressed at baseline achieving the largest increases in CD4+ T-cell count: 74% of RC participants with baseline CD4+ T-cell count < 50 cells/mm3 had CD4+ T-cell count ≥ 200 cells/mm3 at Week 240; the corresponding value in the NRC was 46%. Indeed, mean change from baseline in CD4+ T-cell count in the NRC (240 cells/mm3) was close to that in the RC (296 cells/mm3), which is previously unseen in a population with advanced disease and suggests that the observed immunologic effects primarily reflect the action of fostemsavir rather than OBT.
In virologically suppressed individuals, a CD4+/CD8+ ratio < 0.3 is associated with a significantly higher risk of non-AIDS-defining events or death compared with a ratio > 0.45 [24]. Steady increases in CD4+/CD8+ ratio were observed throughout the study, with 60% of RC participants and 29% of NRC participants achieving CD4+/CD8+ ratio ≥ 0.45 by Week 240. The CD4+ T-cell count and CD4+/CD8+ ratio responses seen in fostemsavir-treated individuals, even those who were viremic, might be directly related to its unique mechanism of action. Temsavir may prevent interactions between gp120 and receptors on uninfected CD4+ T cells and monocytes that can activate these cells [25–27] and/or induce CD4+ T-cell depletion via antibody-dependent cellular cytotoxicity [28], formation of syncytium, or initiation of apoptosis in bystander CD4+ T cells [29].
Fostemsavir + OBT was generally well tolerated. Discontinuations due to AEs were infrequent (overall, 8%); most occurred within the first 24 weeks (21 of 30). Through Week 240, infections and HIV disease progression accounted for most serious safety events. Cumulative rates of AEs reflected the advanced disease of the study population. Overall, there were no changes in the safety and tolerability profile of fostemsavir-based ARV regimens since the 96-week assessment.
Through Week 240, 25 participants were diagnosed with 28 COVID-19-related events. Of the 18 confirmed diagnoses, 11 were managed as outpatients while 7 required hospitalization. No deaths due to COVID-19 occurred. Most of the participants who were hospitalized had CD4+ T-cell counts > 200 cells/mm3 and were virologically suppressed, which likely contributed to positive outcomes. No COVID-19 events resulted in treatment interruption or OBT change. Overall, these results were reassuring since comorbidities common to people with HIV-1 and/or immunocompromised status are associated with poorer COVID-19 outcomes [30–33].
While fostemsavir is not considered to be teratogenic, genotoxic, or fetotoxic, contraception and pregnancy testing for individuals of childbearing potential were mandated in BRIGHTE. However, continuation of fostemsavir during pregnancy was allowed if the investigator considered it to be in the participant’s best interest. All five pregnancies with fostemsavir exposure that were carried to term resulted in live births of infants with no congenital abnormalities. Although additional data are needed, these results are reassuring for HTE women of childbearing potential who may benefit from fostemsavir treatment.
A total of 6 of the 35 deaths occurred after Week 96. Five were AIDS-related, and the sixth was due to pneumonia. The 9% mortality rate reflects the advanced disease of the study population. Median (IQR) baseline CD4+ T-cell count for the overall population was 80 (11–202) cells/mm3, and median baseline CD4+ T-cell count was even lower among participants who died (11 cells/mm3). Moreover, 32 of the 35 participants who died had CD4+ T-cell count < 200 cells/mm3 at last observation.
Limitations of BRIGHTE include the lack of a comparator group beyond the initial 8-day blinded period and use of highly individualized OBT [34]. Although virologic response rates (Snapshot analysis) were consistent through Week 192, beyond this time point, results were confounded by missing data due to COVID-19-related disruptions in access to care. Adherence, which was not measured in BRIGHTE, affects virologic response rates and other outcomes.
Conclusion
Heavily treatment-experienced participants with multidrug-resistant HIV-1 who completed ~5 years of treatment with fostemsavir-based regimens experienced durable virologic responses and clinically meaningful improvements in CD4+ T-cell count and CD4+/CD8+ ratio. The safety and tolerability profile of fostemsavir-based ARV regimens remained consistent with previous observations through 96 weeks, and no new safety trends emerged. Together, these findings provide further support for fostemsavir as a key therapeutic option for HTE people with multidrug-resistant HIV-1.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all BRIGHTE clinical trial participants and their families and all BRIGHTE investigators. Data included in this manuscript have previously been presented in part at the 24th International AIDS Conference; 29 July–2 August, 2022; Virtual and Montreal, Canada; Poster EPB160.
Medical Writing and Editorial Assistance
Editorial assistance was provided under the direction of the authors by Marc Potempa, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Author Contributions
Margaret Gartland, Amy Pierce, and Max Lataillade contributed to the conception and design of the study. Judith A. Aberg, Jose V. Madruga, Fernando Mendo Urbina, Christine Katlama, Shannon Schrader, Joseph J. Eron, Princy N. Kumar, and Eduardo Sprinz contributed to the acquisition of data. Bronagh Shepherd, Marcia Wang, Margaret Gartland, Shiven Chabria, and Amy Pierce contributed to the analysis of data. Judith A. Aberg, Bronagh Shepherd, Marcia Wang, Jose V. Madruga, Fernando Mendo Urbina, Christine Katlama, Shannon Schrader, Joseph J. Eron, Princy N. Kumar, Eduardo Sprinz, Margaret Gartland, Shiven Chabria, Andrew Clark, Amy Pierce, and Max Lataillade contributed to the interpretation of data. Bronagh Shepherd, Margaret Gartland, Shiven Chabria, Andrew Clark, Amy Pierce, and Allan R. Tenorio contributed to drafting the manuscript. All authors contributed to critically revising the manuscript for important intellectual content and approved the manuscript for publication.
Funding
This study and the journal’s Rapid Service Fee were funded by ViiV Healthcare, Durham, NC, USA.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to privacy reasons. Anonymized individual participant data and study documents can be requested for further research from https://www.clinicalstudydatarequest.com.
Declarations
Conflict of Interest
Judith A. Aberg has received grants from Emergent BioSolutions, Frontier Technologies, Gilead, GSK, Janssen, Merck, Pfizer, Regeneron, and ViiV Healthcare, which were paid to her institution, and participated in scientific advisory boards for GSK, Merck, and ViiV Healthcare. Bronagh Shepherd and Marcia Wang are employees of and may own stock in GSK. Jose V. Madruga has received grants for participation in scientific advisory boards from Gilead, GSK, Janssen, MSD, and ViiV Healthcare; sponsorship to attend international congresses from Gilead and Janssen; personal fees for lectures from Gilead, GSK, Janssen, MSD, Pfizer, and ViiV Healthcare; and has served as an investigator in clinical trials sponsored by Gilead, GSK, Janssen, MSD, Pfizer, Sanofi, and ViiV Healthcare. Fernando Mendo Urbina has received honoraria from Johnson and Johnson. Christine Katlama has received grants and/or personal fees from ViiV Healthcare, Gilead, and MSD. Shannon Schrader has received honoraria from Gilead and Janssen. Joseph J. Eron has received grants from Gilead, Janssen, and ViiV Healthcare, which were paid to his institution, and consultant fees from Gilead, Merck, and ViiV Healthcare. Princy N. Kumar has received grants from Eli Lilly, GSK, Merck, Gilead, Regeneron, American Gene Technologies, and BioHaven, which were paid to her institution; participated in data safety monitoring/advisory boards for Johnson & Johnson, ViiV Healthcare, Gilead, TheraTechnologies, and Merck; and holds stock/stock options in Merck, Johnson & Johnson, GSK, Gilead, Pfizer, and Moderna. Eduardo Sprinz has received grants for participation in scientific advisory boards from Abbott, Gilead, GSK, Janssen, MSD, Roche, and ViiV Healthcare. Margaret Gartland, Shiven Chabria, Andrew Clark, Amy Pierce, Max Lataillade, and Allan R. Tenorio are employees of ViiV Healthcare and may own stock in GSK.
Ethical Approval
BRIGHTE was performed in accordance with the Declaration of Helsinki. Study protocols, amendments, and other required documents were reviewed and approved by national, regional, and/or institutional review boards or ethics committees (see Supplementary Material for list of ethics committees). All participants provided written informed consent before study initiation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Priest J, Hulbert E, Gilliam BL, Burton T. Characterization of heavily treatment-experienced people with HIV and impact on health care resource utilization in US commercial and Medicare Advantage health plans. Open Forum Infect Dis. 2021;8:ofab562. doi: 10.1093/ofid/ofab562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puertas MC, Ploumidis G, Ploumidis M, et al. Pan-resistant HIV-1 emergence in the era of integrase strand-transfer inhibitors: a case report. Lancet Microbe. 2020;1:e130–e135. doi: 10.1016/S2666-5247(20)30006-9. [DOI] [PubMed] [Google Scholar]
- 3.Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–818. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenia D, Di Carlo D, Flandre P, et al. HIV MDR is still a relevant issue despite its dramatic drop over the years. J Antimicrob Chemother. 2020;75:1301–1310. doi: 10.1093/jac/dkz554. [DOI] [PubMed] [Google Scholar]
- 5.Rukobia [prescribing information]. Durham, NC: ViiV Healthcare; 2022.
- 6.Rukobia [summary of product characteristics]. Amersfoort, Netherlands: ViiV Healthcare BV; 2022.
- 7.Pancera M, Lai Y-T, Bylund T, et al. Crystal structures of trimeric HIV envelope with entry inhibitors BMS-378806 and BMS-626529. Nat Chem Biol. 2017;13:1115–1122. doi: 10.1038/nchembio.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowicka-Sans B, Gong Y-F, McAuliffe B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56:3498–3507. doi: 10.1128/AAC.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartland M, Arnoult E, Foley BT, et al. Prevalence of gp160 polymorphisms known to be related to decreased susceptibility to temsavir in different subtypes of HIV-1 in the Los Alamos National Laboratory HIV Sequence Database. J Antimicrob Chemother. 2021;76:2958–2964. doi: 10.1093/jac/dkab257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Zhou N, Sun Y, et al. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother. 2013;57:4172–4180. doi: 10.1128/AAC.00513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose R, Gartland M, Li Z, et al. Clinical evidence for a lack of cross-resistance between temsavir and ibalizumab or maraviroc. AIDS. 2022;36:11–18. doi: 10.1097/QAD.0000000000003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozal M, Aberg J, Pialoux G, et al. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med. 2020;382:1232–1243. doi: 10.1056/NEJMoa1902493. [DOI] [PubMed] [Google Scholar]
- 13.Lataillade M, Lalezari JP, Kozal M, et al. Safety and efficacy of the HIV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced individuals: week 96 results of the phase 3 BRIGHTE study. Lancet HIV. 2020;7:e740–e751. doi: 10.1016/S2352-3018(20)30240-X. [DOI] [PubMed] [Google Scholar]
- 14.Chabria S, De Wit S, Pierce A, et al. Characterization of heavily treatment-experienced HIV-1–infected clinical trial participants infected with SARS-CoV-2 COVID-19: fostemsavir BRIGHTE phase 3 clinical trial [Abstract] Open Forum Infect Dis. 2021;8(Suppl 1):S509. doi: 10.1093/ofid/ofab466.1030. [DOI] [Google Scholar]
- 15.Trogarzo [prescribing information]. Montréal, Québec, Canada: Theratechnologies Inc; 2022.
- 16.Ray N, Hwang C, Healy MD, et al. Prediction of virological response and assessment of resistance emergence to the HIV-1 attachment inhibitor BMS-626529 during 8-day monotherapy with its prodrug BMS-663068. J Acquir Immune Defic Syndr. 2013;64:7–15. doi: 10.1097/QAI.0b013e31829726f3. [DOI] [PubMed] [Google Scholar]
- 17.Zhou N, Nowicka-Sans B, McAuliffe B, et al. Genotypic correlates of susceptibility to HIV-1 attachment inhibitor BMS-626529, the active agent of the prodrug BMS-663068. J Antimicrob Chemother. 2014;69:573–581. doi: 10.1093/jac/dkt412. [DOI] [PubMed] [Google Scholar]
- 18.Zhou N, Nowicka-Sans B, Zhang S, et al. In vivo patterns of resistance to the HIV attachment inhibitor BMS-488043. Antimicrob Agents Chemother. 2011;55:729–737. doi: 10.1128/AAC.01173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lataillade M, Zhou N, Joshi SR, et al. Viral drug resistance through 48 weeks, in a phase 2b, randomized, controlled trial of the HIV-1 attachment inhibitor prodrug, fostemsavir. J Acquir Immune Defic Syndr. 2018;77:299–307. doi: 10.1097/QAI.0000000000001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eron JJ, Cooper DA, Steigbigel RT, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13:587–596. doi: 10.1016/S1473-3099(13)70093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379:645–654. doi: 10.1056/NEJMoa1711460. [DOI] [PubMed] [Google Scholar]
- 22.Akil B, Blick G, Hagins DP, et al. Dolutegravir versus placebo in subjects harbouring HIV-1 with integrase inhibitor resistance associated substitutions: 48-week results from VIKING-4, a randomized study. Antivir Ther. 2015;20:343–348. doi: 10.3851/IMP2878. [DOI] [PubMed] [Google Scholar]
- 23.Katlama C, Clotet B, Mills A, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther. 2010;15:1045–1052. doi: 10.3851/IMP1662. [DOI] [PubMed] [Google Scholar]
- 24.Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015;2:e98–106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 25.Levast B, Barblu L, Coutu M, et al. HIV-1 gp120 envelope glycoprotein determinants for cytokine burst in human monocytes. PLoS One. 2017;12:e0174550. doi: 10.1371/journal.pone.0174550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Cornò M, Donninelli G, Varano B, Da Sacco L, Masotti A, Gessani S. HIV-1 gp120 activates the STAT3/interleukin-6 axis in primary human monocyte-derived dendritic cells. J Virol. 2014;88:11045–11055. doi: 10.1128/JVI.00307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nazli A, Kafka JK, Ferreira VH, et al. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol. 2013;191:4246–4258. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- 28.Prévost J, Richard J, Medjahed H, et al. Temsavir protects bystander cells from ADCC and blocks cytokine burst by monocytes [Poster 217]. Presented at: Conference on Retroviruses and Opportunistic Infections; February 12–16, 2022; Virtual.
- 29.Février M, Dorgham K, Rebollo A. CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses. 2011;3:586–612. doi: 10.3390/v3050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinelli MA, Jones BLH, Gandhi M. COVID-19 outcomes and risk factors among people living with HIV. Curr HIV/AIDS Rep. 2022;19:425–432. doi: 10.1007/s11904-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertagnolio S, Thwin SS, Silva R, Diaz J. Clinical features and prognostic factors of COVID-19 in people living with HIV hospitalized with suspected or confirmed SARS-CoV-2 infection. July 15, 2021. World Health Organization. https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-HIV-2021.1. Accessed 29 June 2023.
- 32.Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11:6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesoriero JM, Swain C-AE, Pierce JL, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York state. JAMA Netw Open. 2021;4:e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment. Guidance for industry. November 2015. Food and Drug Administration. https://www.fda.gov/files/drugs/published/Human-Immunodeficiency-Virus-1-Infection--Developing-Antiretroviral-Drugs-for-Treatment.pdf. Accessed 29 June 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available due to privacy reasons. Anonymized individual participant data and study documents can be requested for further research from https://www.clinicalstudydatarequest.com.