Abstract
Probiotics confer many beneficial effects on several illnesses, ranging from microbial diarrhea to inflammatory diseases. This study was conducted on whether Levilactobacillus brevis KU15147 obtained from kimchi has anti-inflammatory effects in RAW 264.7 cells stimulated with lipopolysaccharide (LPS) and antioxidant potential. L. brevis KU15147 reduced nitric oxide and prostaglandin E2 levels with decreasing the activation of inducible nitric oxide synthase and cyclooxygenase-2 without cell cytotoxicity. In addition, L. brevis KU15147 attenuated proinflammatory cytokine production including tumor necrosis factor-α, interleukin (IL)-1β, and IL-6 in RAW 264.7 cells stimulated with LPS. Additionally, L. brevis KU15147 reduced the activity of nuclear factor-κB, activator protein-1, and mitogen-activated protein kinase signaling pathways. Furthermore, L. brevis KU15147 downregulated the production of reactive oxygen species. Therefore, L. brevis KU15147 was concluded that had an inhibition effect on LPS-induced inflammatory responses and can be used in functional foods to suppress inflammatory diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01318-w.
Keywords: Anti-inflammatory, Levilactobacillus brevis, NF-κB signaling, AP-1 signaling, MAPK signaling
Introduction
Lipopolysaccharide (LPS), the endotoxin of gram-negative bacteria, can provoke inflammatory responses (Gasparrini et al., 2021). Although inflammation is a necessary immune response for the host, excess inflammation has a damaging effect by overgeneration of nitric oxide (NO), prostaglandin E2 (PGE2), and proinflammatory cytokines and may cause severe diseases like fever, atherosclerosis, and septic shock (Yang et al., 2018). Additionally, the production of NO and PGE2 is controlled by inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, individually (Song et al., 2022).
Macrophage, a type of white blood cell, secretes innumerable inflammatory cytokines and mediators against bacterial products (Cohen and Mosser, 2013; Lee et al., 2016). Through the inflammatory response, macrophages generate proinflammatory cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 (Monmai et al., 2021). When macrophages are stimulated, intracellular signaling like nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) signaling are activated (Yu et al., 2019). The MAPK signaling consists of three protein kinases; c-jun N-terminal kinase (JNK), P38, and extracellular signal-regulated kinase (ERK) 1/2, which consequentially elevate the revelation of inflammation associated genes (Kang and Hyun, 2020; Kim et al., 2022b). c-Jun is included in activator protein (AP)-1 signaling and is related to synthetic processes in chondrocytes (Park et al., 2018). Loss of chondrocytes can be the primary cause of diseases such as osteoarthritis and degenerative arthritis (Ge et al., 2017). Moreover, iNOS and PGE2 production is controlled by signaling pathways like AP-1 and NF-κB (Kim et al., 2020).
Levilactobacillus brevis is abundant in sauerkraut and pickle, and is an important lactic acid bacteria in the kimchi during fermentation process (Kim et al., 2017). Probiotics have various positive effects like antioxidant, immunostimulatory, anti-tumor, and anti-inflammatory activities (Lee et al., 2015). In addition, antioxidant responses are associated with cellular oxidative stress and inflammatory damage (Ren et al., 2016). Levilactobacillus brevis KU15147 has been described to have immune stimulating and antioxidant effects but its anti-inflammatory effect was not investigated (Kim et al., 2021).
This study was performed to investigate the anti-inflammatory effects of L. brevis KU15147 thorugh the production of proinflammatory mediators and cytokines, like NO, PGE2, and proinflammatory cytokines. As well the important inflammatory signaling pathways like NF-κB, AP-1, and MAPK signaling pathways in RAW 264.7 cells stimulated with LPS were studied. Additionally, the impacts of L. brevis KU15147 on the production of reactive oxygen species (ROS) in RAW 264.7 cells were examined.
Materials and methods
Probiotic strains and sample preparation
Lacticaseibacillus rhamnosus GG (LGG, KCTC 5033) was acquired from the Korean Collection for Type Cultures (Jeollabuk-do, Korea). It was utilized for relative analysis as a typical commercial probiotic strain. Levilactobacillus brevis (KU15147 and KU15154) were separated from the fermented Korean kimchi (Kim et al., 2021). To estimate the anti-inflammatory effects of probiotic strains, LGG, L. brevis strains (KU15147 and KU15154) were cultured at 37 °C overnight in MRS broth (KisanBio Co., Ltd., Seoul, Korea). And then, the cultures were centrifuged at 4 °C at 12,000 × g for 15 min. The cells were cleaned with phosphate buffered saline (PBS) (HyClone, Logan, UT, USA) and resuspended in DMEM as ten-fold to 7 log CFU/mL for the bacterial sample by adjusting the absorbance at 600 nm (L. brevis strains having 0.5–0.6; LGG strain having about 0.9).
Cell culture condition
The RAW 264.7 cell line (KCLB 40071) was acquired from the Korean Cell Line Bank (Seoul, Korea), was maintained and incubated in Dulbecco’s modified Eagle medium (DMEM; ThermoFisher, Carlsbad, CA, USA) augmented by 10% fetal bovine serum (ThermoFisher) and 1% antibiotics (ThermoFisher) at 37 °C in 5% CO2 circumstances.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability of bacterial samples or LPS was confirmed using MTT assay in RAW 264.7 cells (Yu et al., 2019). In brief, RAW 264.7 cells were placed to each well by 2 × 105 cells/well in 96-well plates and precultured for 4 h, after that 50 µL of bacterial samples were treated for 2 h. After, the cells were induced with 1 µg/mL LPS for 22 h. And then, 100 µL of the supernatant was used for NO assays, and then 0.5 mg/mL MTT solution in PBS was treated per well and the cells were reacted for 1 h. Following discarding the solution, 200 µL of dimethyl sulfoxide (DMSO) was treated per well for dissolving formazan deposits. The optical density of each well was evaluated at 570 nm. Cell viability was calculated on the proportion of optical density measured to the LPS positive groups.
Production of NO, PGE2, and proinflammatory cytokines
NO production was evaluated utilizing Griess assay in RAW 264.7 cells stimulated with LPS (Kim et al., 2022b). One hundred microliter of supernatant from the MTT assay was transferred to another 96-well plate. Supernatants from each well were accompanied by 100 µL of Griess reagent and reacted for 15 min. The optical density was determined at 540 nm, and then nitrite levels of supernatant were determined utilizing a standard curve (NaNO2 in DMEM).
To determine the production of PGE2 and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in RAW 264.7 cells stimulated with LPS, an ELISA kit was used. Cells (5 × 105 cells/well) were seeded in 12-well plate and cultured for 4 h. Bacterial samples were added to cells for 2 h, and cells were induced with 1 µg/mL LPS for 22 h. After incubation, supernatants of media were gathered and diluted in DMEM. The production of PGE2 and proinflammatory cytokines were defined consistent with the instruction of manufacturer.
Reverse transcription-polymerase chain reaction (RT-PCR)
The mRNA expression of iNOS, COX-2, and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in RAW 264.7 cells stimulated with LPS were determined utilizing RT-PCR, as the methods of previous study (Yu et al., 2020). The cells (1 × 106 cells/well) were pre-incubated in 6-well plates for 24 h. Bacterial samples were added for 2 h and then induced with 1 µg/mL LPS for another 22 h. The cells were rinsed two times as well as ice-cold PBS. Total RNA was obtained using commercial RNA isolation kit (Qiagen, Hilden, Germany). The cDNA synthesis kit (Bio-line, London, UK) was used for synthesize cDNA, following manufacturer’s manuals. One hundred microliter of PCR mixture contained PCR master mix, cDNA, and specific primers. The mixture was then reacted as 95 °C for 2 min for polymerase initiation, subsequently 40 cycles of 95 °C for 5 s, and 65 °C for 30 s. The Ct value was controlled utilizing the β-actin and the result assessed with the 2−△△Ct method. The sequences of each primer are represented in Table 1S.
ROS staining
ROS production in RAW 264.7 cells was defined as previously depicted (Kwon et al., 2019). Briefly, cells (5 × 105 cells/well) were pre-cultivated in 12-well plate for 4 h, bacterial samples were added for 2 h, and the cells were induced with 1 µg/mL LPS for another 22 h. Later washing two times with PBS, the cells were hatched with 20 µM of 2′7′-dichlorofluorescin diacetate (DCF-DA) in the dark for 30 min. The cells were cleaned using PBS and fluorescence intensity was determined using a fluorescence microscope (Nikon Co. Ltd., Tokyo, Japan).
Western blotting
The expression of proteins that are related to inflammatory responses was investigated utilizing western blotting, as previously described (Kim et al., 2022a), with minor adjustments. In brief, RAW 264.7 cells (1 × 106 cells/well) were planted in cell dishes (60 mm) and pre-cultured for 22 h. Following incubation, the cells were exposed to bacterial samples for 2 h, after which were treated with 1 µg/mL of LPS for 22 h (for iNOS and COX-2 production), 15 min (for NF-κB and AP-1 pathway, ERK 1/2, and JNK), and 5 min (for p38) respectively. The lysis buffer (iNtRON Biotechnology, Gyeonggi-do, Korea) including cocktails with protease and phosphatase inhibitor (Thermo Scientific Pierce, Rockford, IL, USA) were employed to prepare cell lysates. Bio-Rad protein assay kit (Contra Costa, CA, USA) was used to quantify protein, and equal amounts (20 µg) of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). A 10% gel was transferred to a polyvinyl difluoride membrane (Merck Millipore, Danvers, MA, USA), which was blocked with 5% skim milk for 1 h and probed with primary antibodies against iNOS, COX-2 (Thermo Scientific Pierce), ERK 1/2, JNK, and p38 at 4 °C overnight. The other primary antibodies were used from Cell Signaling Technology Inc. (Danvers, MA, USA). After rinsing with Tris-buffered saline including Tween 20 (TBS-T), the membrane was simmered with secondary antibodies (Cell Signaling Technology Inc.) for 2 h at 23 °C. After washing with TBS-T, protein bands were revealed using an enhanced chemiluminescence detection kit (Bio-Rad) and X-ray blue film. The bands were digitized via ImageJ software of National Institutes of Health (Bethesda, MD, USA).
Statistical data analysis
Each experiment was repeated in triplicate. The experimental results are presented as the mean ± standard deviation (SD) by triplicate. Statistical data analysis was implemented using one-way analysis of variance (ANOVA) subsequently Duncan’s multiple range test in IBM SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). Data with p < 0.05 were judged statistically significant.
Results and discussion
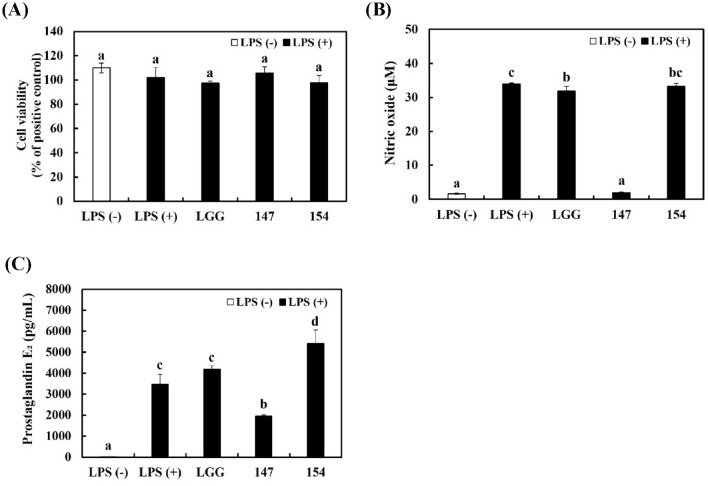
Downregulation of NO and PGE2 production in RAW 264.7 cells stimulated with LPS without cytotoxicity
It has been reported that probiotics can offer defense against environmental contaminants and diseases related to the gut microbiota (Kang et al., 2022). The gastrointestinal tract microbiota affect human health through modulating host nutrition and immune function, and probiotics influence the human gastrointestinal tract and microbiota (Gorreja and Walker, 2022). Additionally, gut macrophages control inflammatory responses against external threats such as harmful pathogens, bacteria, and antigens (Smith et al., 2011). LPS induce macrophages to generate proinflammatory cytokines, therefore, macrophages play a critical role in the progress of chronic inflammatory disorders (Zhou et al., 2022). The production of NO and PGE2 is representative of the inflammatory reaction in macrophages.
To ascertain the effects of bacterial samples in RAW 264.7 cells stimulated with LPS, the concentrations of NO and PGE2 were determined. As shown in Fig. 1A, bacterial samples had no harmful effects on RAW 264.7 cells (> 97% cell viability). Treatment with LPS distinctly induced the production of NO (Fig. 1B) and PGE2 (Fig. 1C) in RAW 264.7 cells. Although LGG and L. brevis KU15154 had no impact on the production of NO and PGE2, L. brevis KU15147 showed significant inhibition of NO and PGE2. These results imply that L. brevis KU15147 has anti-inflammatory potential in LPS-stimulated RAW 264.7 cells without cytotoxicity.
Fig. 1.
Cell viability and inflammatory mediator production in RAW 264.7 cells stimulated with LPS. (A) Cell viability, (B) NO production, and (C) PGE2 concentration. LGG, Lacticaseibacillus rhamnosus GG; 147, Levilactobacillus brevis KU15147; 154, Levilactobacillus brevis KU15154. Cells were treated with the bacterial sample for 2 h and then stimulated with 1 μg/mL LPS for 22 h. Different letters show significant differences in the group of samples (p < 0.05)
Inhibition of proinflammatory cytokine expression in RAW 264.7 cells stimulated with LPS
The expression of key inflammation mediators such as NO and PGE2 and proinflammatory cytokines are mediated by macrophages and is raised by LPS stimulation (Rashid et al., 2019). TNF-α, IL-1β, and IL-6 are the prominent proinflammatory cytokines that are produced during inflammatory responses (Monmai et al., 2021).
To estimate the anti-inflammatory effects of the bacterial samples, the production of proinflammatory cytokines was quantified in RAW 264.7 cells. Figure 2A–C showed that 1 μg/mL LPS treatment significantly induced TNF-α, IL-1β, and IL-6 production in RAW 264.7 cells. In contrast, pretreatment of L. brevis KU15147 before LPS stimulation substantially inhibited the production of proinflammatory cytokines in comparison with pretreatment with LGG and L. brevis KU15154. Similar to the ELISA results, transcriptional expression levels of proinflammatory cytokines were significantly increased following stimulation with LPS (Fig. 2D–F). In contrast, L. brevis KU15147 treatment notably suppressed the production of TNF-α (0.38 ± 0.02-fold), IL-1β (0.09 ± 0.01-fold), and IL-6 (0.40 ± 0.03-fold) in LPS-stimulated RAW 264.7 cells, which is significantly increased than that obtained with other bacterial samples. L. brevis KU15147 may have inhibited the following cellular signaling pathways effectively by reducing the expression of IL-6 (Fig. 2B, E), because IL-6 performs an crucial role in the severe response as a stimulus (Gabay, 2006).
Fig. 2.
Production of pro-inflammatory cytokines in RAW 264.7 cells stimulated with LPS. ELISA results of (A) TNF-α, (B) IL-1β, and (C) IL-6. Relative mRNA expression levels of (D) TNF-α, (E) IL-1β, and (F) IL-6. LGG, Lacticaseibacillus rhamnosus GG; 147, Levilactobacillus brevis KU15147; 154, Levilactobacillus brevis KU15154. Cells were treated with the bacterial sample for 2 h and then stimulated with 1 μg/mL LPS for 22 h. Different letters show significant differences in the group of samples (p < 0.05)
Decrease of iNOS and COX-2 expression in RAW 264.7 cells stimulated with LPS
NO is synthesized by iNOS, which stimulates inflammatory responses against external stimuli, such as bacterial LPS and cytokines (Park et al., 2018). Therefore, the production of NO and iNOS is regarded as a crucial target to examine LPS-induced inflammatory reaction (Lee et al., 2007). COX-2 is responsible for expressing PGE2 that modulates inflammatory response by producing TNF-α, IL-1β, and IL-6 (Castejon et al., 2019).
The mRNA and protein expression of iNOS and COX-2, were measured with RT-PCR and western blot analysis. 1 μg/mL LPS treatment increased the expression of iNOS and COX-2 in RAW 264.7 macrophages (Fig. 3). However, treatment of L. brevis KU15147 distinctly lessened the protein levels of iNOS and COX-2 in RAW 264.7 cells stimulated with LPS (Fig. 3A–C). Similarly, iNOS (0.44 ± 0.04-fold) and COX-2 (0.12 ± 0.01-fold) mRNA levels were most effectively reduced by the treatment with L. brevis KU15147 (Fig. 3D–E). These results showed that L. brevis KU15147 blocked the production of NO and PGE2 by reducing the iNOS and COX-2, respectively.
Fig. 3.
Production of iNOS and COX-2 in RAW 264.7 cells stimulated with LPS. (A) Western blot analysis of iNOS and COX-2. Protein bands of (B) iNOS/β-actin and (C) COX-2/β-actin using image J. Relative mRNA expression levels of (D) iNOS and (E) COX-2. LGG, Lacticaseibacillus rhamnosus GG; 147, Levilactobacillus brevis KU15147; 154, Levilactobacillus brevis KU15154. Cells were treated with the bacterial sample for 2 h and then stimulated with 1 μg/mL LPS for 22 h. Different letters show significant differences in the group of samples (p < 0.05)
Suppression of NF-κB and AP-1 activation in RAW 264.7 cells stimulated with LPS
LPS stimulates the macrophages to induce inflammatory responses through activation of cellular signaling pathways (Liu et al., 2020). Cellular signaling pathways mediated by toll-like receptor (TLR)-4 lead to NF-κB-mediated increase in the expression of inflammatory cascade effector enzyme and cytokines in RAW 264.7 cells stimulated with LPS (Lee et al., 2016). Phosphorylation of p65 NF-κB and degradation of IκB-α by inflammation stimuli induce NF-κB translocation to the nucleus (Park et al., 2021). AP-1, another transcription factor, regulates inflammatory genes in RAW 264.7 macrophages (Kang et al., 2019). iNOS, COX-2, and proinflammatory cytokines are controlled by AP-1 signaling pathways via their promoters on their DNA-binding sites (Shin et al., 2020). In addition, MAPK signaling pathways stimulate downstream transcription factors to elevate the expression of AP-1 complexes (Lee et al., 2017). NF-κB and AP-1 signaling pathways upregulate iNOS and COX-2 after LPS treatment in macrophages (Lee et al., 2014).
To define whether L. brevis KU15147 have anti-inflammatory potential associated with cellular signaling pathways, the expression of NF-κB and AP-1 activation were examined. 1 μg/mL LPS treatment induced the expression of p-p65 and degradation of IκB-α in RAW 264.7 cells but treatment with bacterial samples reduced the activation of NF-κB, which was more pronounced after pretreatment with L. brevis KU15147 (Fig. 4A–C). These results imply that L. brevis KU15147 attenuates the stimulation of NF-κB signaling pathways by inhibiting the degradation of NF-κB and Iκ-Bα complexes. Treatment with L. brevis KU15147 also downregulated the expression of c-Jun and p-c-Jun AP-1, compared to treatment with LPS-only positive control groups (Fig. 4D–F). According to these results, L. brevis KU15147 effectively suppressed AP-1 signaling pathways by mitigating phosphorylation and translocation of c-Jun.
Fig. 4.
NF-κB and AP-1 activation in RAW 264.7 cells stimulated with LPS. (A) Western blot analysis of NF-κB, (B) protein levels of p-p65/p65, (C) protein levels of IκB-α/β-actin. (D) western blot analysis of AP-1 signaling, (E) protein levels of c-Jun/α-tubulin, and (F) protein levels of p–c-Jun/α-tubulin using Image J. LGG, Lacticaseibacillus rhamnosus GG; 147, Levilactobacillus brevis KU15147; 154, Levilactobacillus brevis KU15154. Cells were treated with the bacterial sample for 2 h and then stimulated with 1 μg/mL LPS for 15 min for NF-κB, and 60 min for c-Jun AP-1 signaling pathway. Different letters show significant differences in the group of samples (p < 0.05)
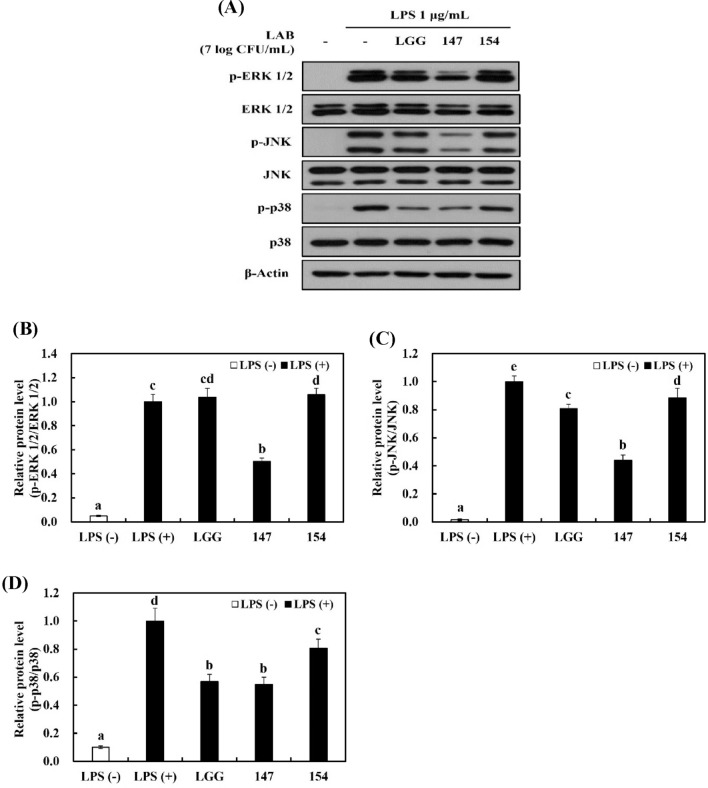
Inhibition of MAPKs activation in RAW 264.7 cells stimulated with LPS
The MAPK signaling pathway performs a critical role in cell activities, such as apoptosis, survival, and proliferation. In inflammatory responses, LPS treatment markedly increases the phosphorylation of MAPKs to translocate into the nucleus and generate inflammatory mediators (Qiu et al., 2021; Venkatesan et al., 2018).
Western blot analysis was implemented to verify the influence of bacterial samples on MAPK signaling pathways in RAW 264.7 cells stimulated with LPS. Figure 5A showed that LPS stimulation strongly led to the phosphorylation of ERK 1/2, JNK, and p38 in comparison with LPS-negative group. LGG and L. brevis KU15154 had an repressing effect on the expression of p-p38 and p-JNK, whereas L. brevis KU15147 effectively downregulated both p-p38 (Fig. 5A, D) and p-JNK (Fig. 5A, C). Furthermore, L. brevis KU15147 notably suppressed the expression of p-ERK compared to the other bacterial samples (Fig. 5A–B). LGG displayed a similar effect as L. brevis KU15147 on p-p38 expression; it may be associated with the antioxidant effect of LGG (Yang et al., 2019). In fact, p38 MAPK signaling pathways are closely associated with oxidative stress (Kim et al., 2004). Therefore, the results suggest that L. brevis KU15147 constrains LPS-stimulated inflammation by controlling the MAPK signaling pathway.
Fig. 5.
MAPK activation in RAW 264.7 cells stimulated with LPS. (A) Western blot analysis of MAPK-related factors. Protein bands of (B) p-ERK 1/2/ERK 1/2, (C) p-JNK/JNK, and (D) p-p38/p38 using Image J. LGG, Lacticaseibacillus rhamnosus GG; 147, Levilactobacillus brevis KU15147; 154, Levilactobacillus brevis KU15154. Cells were treated with the bacterial sample for 2 h and then induced with 1 μg/mL LPS for 5 min for p38 MAPKs and 15 min for ERK, JNK, and MAPK. Different letters show significant differences in the group of samples (p < 0.05)
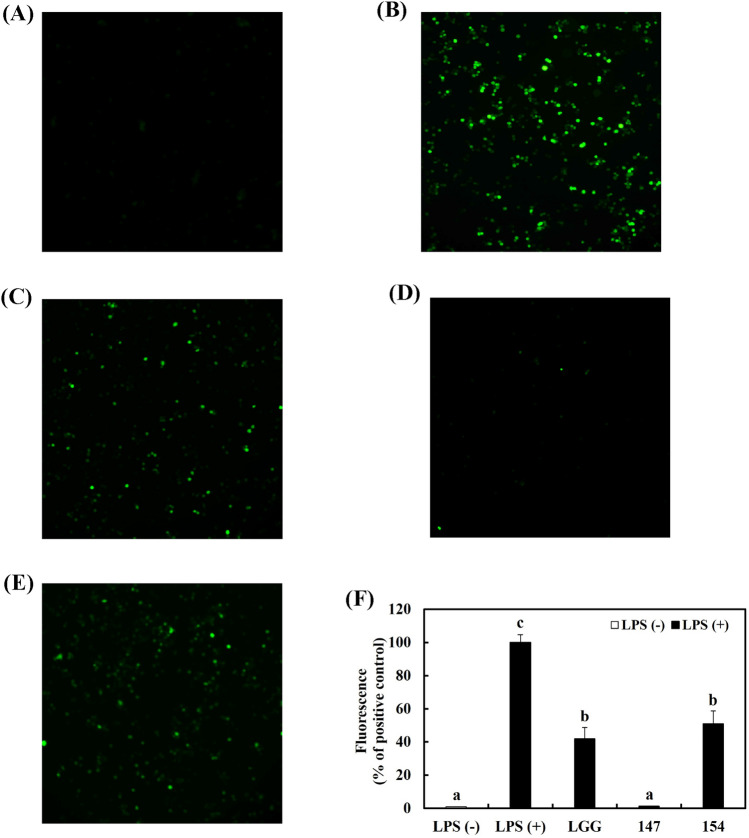
Inhibition of ROS production in RAW 264.7 cells
In RAW 264.7 cells, the occurrence of NO and inflammatory cytokines increase ROS production. Oxidative stress induced by the generation of ⋅OH causes several disorders like atherosclerosis and chronic pulmonary disease (Forman and Zhang, 2021; Lee et al., 2014). ROS production in RAW 264.7 cells was assessed because cellular inflammation responses are related to oxidative stress.
Figure 6A, B showed that treatment with 1 μg/mL LPS raised the generation of ROS in macrophages in comparison with the LPS-negative group. In contrast, pretreatment with bacterial samples significantly reduced ROS generation, and among them, L. brevis KU15147 exhibited the strongest effect similar to LPS-negative group (Fig. 6C–F).
Fig. 6.
Production of ROS by L. brevis KU15147 in RAW 264.7 cells stimulated with LPS. (A) LPS-negative control group, (B) LPS-positive control group, (C) LGG and LPS-treated group, (D) L. brevis KU15147 and LPS-treated group, (E) L. brevis KU15154 and LPS treated group, (F) fluorescence intensity of ROS production. LGG, Lacticaseibacillus rhamnosus GG; 147, Levilactobacillus brevis KU15147; 154, Levilactobacillus brevis KU15154. Different letters show significant differences in the group of samples (p < 0.05)
In conclusion, L. brevis KU15147 exhibited anti-inflammatory potential in RAW 264.7 cells stimulated with LPS. As expected, LPS stimulation generated oxidative stress and inflammation responses in RAW 264.7 cells. L. brevis KU15147 mitigated NO and PGE2 production by controlling the expression of iNOS and COX-2. The production of proinflammatory cytokines were downregulated. In addition, L. brevis KU15147 affected several signaling pathways. The stimulation of NF-κB, AP-1, and MAPKs was inhibited by this bacterial strain. ROS in RAW 264.7 cells stimulated with LPS was also alleviated by L. brevis KU15147. Because of the importance of intestinal health, the search for novel lactic acid bacteria and discovering its health functionality are an active area of research. In consequence of the anti-inflammatory effect of L. brevis KU15147 investigated in this study, this bacterial strain may be applied in functional foods for inflammatory disorders.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2023.
Declarations
Conflict of interest
All authors confirmed that they have no conflict of interest in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun-Hyun Hyun, Email: jhhyun25@naver.com.
Hyung-Seok Yu, Email: hyungseok_yu@naver.com.
Im-Kyung Woo, Email: aimee425@naver.com.
Gil-Woong Lee, Email: woong9299@naver.com.
Na-Kyoung Lee, Email: lnk11@konkuk.ac.kr.
Hyun-Dong Paik, Email: hdpaik@konkuk.ac.kr.
References
- Castejon ML, Sánchez-Hidalgo M, Aparicio-Soto M, González-Benjumea A, Fernández-Bolaños JG, Alarcón-de-la-Lastra C. Olive secoiridoid oleuropein and its semisynthetic acetyl-derivatives reduce LPS-induced inflammatory response in murine peritoneal macrophages via JAK-STAT and MAPKs signaling pathways. Journal of Functional Foods. 2019;58:95–104. doi: 10.1016/j.jff.2019.04.033. [DOI] [Google Scholar]
- Cohen HB, Mosser DM. Extrinsic and intrinsic control of macrophage inflammatory responses. Journal of Leukocyte Biology. 2013;94:913–919. doi: 10.1189/jlb.0413236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nature Reviews Drug Discovery. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Research & Therapy. 2006;8:1–6. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini M, Forbes-Hernande TY, Cianciosi D, Quiles JL, Mezzetti B, Xiao J, Giampieri F, Battino M. The efficacy of berries against lipopolysaccharide-induced inflammation: A review. Trends in Food Science & Technology. 2021;117:74–91. doi: 10.1016/j.tifs.2021.01.015. [DOI] [Google Scholar]
- Ge HX, Zou FM, Li Y, Liu AM, Tu M. JNK pathway in osteoarthritis: pathological and therapeutic aspects. Journal of Receptors and Signal Transduction. 2017;37:431–436. doi: 10.1080/10799893.2017.1360353. [DOI] [PubMed] [Google Scholar]
- Gorreja F, Walker WA. The potential role of adherence factors in probiotic function in the gastrointestinal tract of adults and pediatrics: a narrative review of experimental and human studies. Gut Microbes. 2022;14:149214. doi: 10.1080/19490976.2022.2149214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HK, Hyun CG. Anti-inflammatory effect of d-(+)-cycloserine through inhibition of NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Natural Product Communications. 2020;15:1–11. doi: 10.1177/1934578X20920481. [DOI] [Google Scholar]
- Kang SJ, Yang J, Lee NY, Lee CH, Park IB, Park SW, Lee HJ, Park HW, Yun HS, Chun T. Monitoring cellular immune responses after consumption of selected probiotics in immunocompromised mice. Food Science of Animal Resources. 2022;42:903–914. doi: 10.5851/kosfa.2022.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Shin JS, Kim SY, Noh YS, Lee SJ, Hwang H, Deyou T, Jang YP, Lee KY. Caffeoyloxy-5,6-dihydro-4-methyl-(2H)-pyran-2-one isolated from the leaves of Olinia usambarensis attenuates LPS-induced inflammatory mediators by inactivating AP-1 and NF-κB. Chemico-Biological Interactions. 2019;309:108718. doi: 10.1016/j.cbi.2019.06.031. [DOI] [PubMed] [Google Scholar]
- Kim JG, Kim MJ, Lee JS, Sydara K, Lee S, Byun S, Jung SK. Smilax guianensis vitman extract prevents LPS-induced inflammation by inhibiting the NF-κB pathway in RAW 264.7 cells. Journal of Microbiology and Biotechnology. 2020;30:822–829. doi: 10.4014/jmb.1911.11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KT, Yang SJ, Paik HD. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Science and Biotechnology. 2021;30:257–265. doi: 10.1007/s10068-020-00853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Johnson VJ, Shin TY, Sharma RP. Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-κB signaling pathways. Experimental Biology and Medicine. 2004;229:203–213. doi: 10.1177/153537020422900209. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang KH, Kim SH, Lee S, Lee SH, Ha ES, Sung NJ, Kim JG, Chung MJ. Lactic acid bacteria directly degrade N-nitrosodimethylamine and increase the nitrite-scavenging ability in kimchi. Food Control. 2017;71:101–109. doi: 10.1016/j.foodcont.2016.06.039. [DOI] [Google Scholar]
- Kim WJ, Hyun JH, Lee NK, Paik HD. Protective effects of a novel Lactobacillus brevis strain with probiotic characteristics against Staphylococcus aureus lipoteichoic acid-induced intestinal inflammatory response. Journal of Microbiology and Biotechnology. 2022;32:205–211. doi: 10.4014/jmb.2110.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Yu HS, Lee NK, Paik HD. Levilactobacillus brevis KU15151 inhibits Staphylococcus aureus lipoteichoic acid–induced inflammation in RAW 264.7 macrophages. Probiotics and Antimicrobial Proteins. 2022;14:767–777. doi: 10.1007/s12602-022-09949-x. [DOI] [PubMed] [Google Scholar]
- Kwon HC, Bae H, Seo HG, Han SG. Chia seed extract enhances physiochemical and antioxidant properties of yogurt. Journal of Dairy Science. 2019;102:4870–4876. doi: 10.3168/jds.2018-16129. [DOI] [PubMed] [Google Scholar]
- Lee AS, Jung YJ, Kim D, Nguyen-Thanh T, Kang KP, Lee S, Park SW, Kim W. SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages. Biochemical and Biophysical Research Communications. 2014;450:1363–1369. doi: 10.1016/j.bbrc.2014.06.135. [DOI] [PubMed] [Google Scholar]
- Lee MH, Lee JM, Jun SH, Lee SH, Kim NW, Lee JH, Ko NY, Mun SH, Kim BK, Lim BO, Choi DK, Choi WS. The anti-inflammatory effects of Pyrolae herba extract through the inhibition of the expression of inducible nitric oxide synthase (iNOS) and NO production. Journal of Ethnopharmacology. 2007;112:49–54. doi: 10.1016/j.jep.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Science and Technology. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- Lee SB, Lee WS, Shin JS, Jang DS, Lee KT. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. International Immunopharmacology. 2017;49:21–29. doi: 10.1016/j.intimp.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Lee WS, Shin JS, Jang DS, Lee KT. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. International Immunopharmacology. 2016;40:146–155. doi: 10.1016/j.intimp.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Liu S, Yang T, Ming TW, Gaun TKW, Zhou T, Wang S, Ye B. Isosteroid alkaloids with different chemical structures from Fritillariae cirrhosae bulbus alleviate LPS-induced inflammatory response in RAW 264.7 cells by MAPK signaling pathway. International Immunopharmacology. 2020;78:106047. doi: 10.1016/j.intimp.2019.106047. [DOI] [PubMed] [Google Scholar]
- Monmai C, Nam JH, Lim JH, Rod-In W, Lee TH, Park WJ. Anti-inflammatory activities of the mixture of strawberry and rice powder as materials of fermented rice cake on RAW264.7 macrophage cells and mouse models. Food Science and Biotechnology. 2021;30:1409–1416. doi: 10.1007/s10068-021-00929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Cha HJ, Lee H, Kim GY, Choi YH. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Archives of Biochemistry and Biophysics. 2021;706:108926. doi: 10.1016/j.abb.2021.108926. [DOI] [PubMed] [Google Scholar]
- Park J, Kwak CH, Ha SH, Kwon KM, Abekura F, Cho SH, Chang YC, Lee YC, Ha KT, Chung TW, Kim CH. Ganglioside GM3 suppresses lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophage cells through NF-κB, AP-1, and MAPKs signaling. Journal of Cellular Biochemistry. 2018;119:1173–1182. doi: 10.1002/jcb.26287. [DOI] [PubMed] [Google Scholar]
- Qiu T, Sun Y, Wang X, Zheng L, Zhang H, Jiang L, Zhu X, Xiong H. Drum drying-and extrusion-black rice anthocyanins exert anti-inflammatory effects via suppression of the NF-κB/MAPKs signaling pathways in LPS-induced RAW 264.7 cells. Food Bioscience. 2021;41:100841. doi: 10.1016/j.fbio.2020.100841. [DOI] [Google Scholar]
- Rashid HU, Xu Y, Ahmad N, Muhammad Y, Wang L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorganic Chemistry. 2019;87:335–365. doi: 10.1016/j.bioorg.2019.03.033. [DOI] [PubMed] [Google Scholar]
- Ren H, Hao J, Liu T, Zhang D, Lv H, Song E, Zhu C. Hesperetin suppresses inflammatory responses in lipopolysaccharide-induced RAW 264.7 cells via the inhibition of NF-κB and activation of Nrf2/HO-1 pathways. Inflammation. 2016;39:964–973. doi: 10.1007/s10753-016-0311-9. [DOI] [PubMed] [Google Scholar]
- Shin JS, Kang SY, Lee HH, Kim SY, Lee DH, Jang DS, Lee YT. Patriscabrin F from the roots of Patrinia scabra attenuates LPS-induced inflammation by downregulating NF-κB, AP-1, IRF3, and STAT1/3 activation in RAW 264.7 macrophages. Phytomedicine. 2020;68:153167. doi: 10.1016/j.phymed.2019.153167. [DOI] [PubMed] [Google Scholar]
- Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunology. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MW, Park JY, Kim WJ, Kim KT, Paik HD. Fermentative effects by probiotic Lactobacillus brevis B7 on antioxidant and anti-inflammatory properties of hydroponic ginseng. Food Science and Biotechnology. 2022;32:169–180. doi: 10.1007/s10068-022-01183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan T, Choi YW, Lee J, Kim YK. Falcarindiol inhibits LPS-induced inflammation via attenuating MAPK and JAK-STAT signaling pathways in murine macrophage RAW 264.7 cells. Molecular and Cellular Biochemistry. 2018;445:169–178. doi: 10.1007/s11010-017-3262-z. [DOI] [PubMed] [Google Scholar]
- Yang SH, Le B, Androutsopoulos VP, Tsukamoto C, Shin TS, Tsatsakis AM, Chung G. Anti-inflammatory effects of soyasapogenol I-αa via downregulation of the MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. Food and Chemical Toxicology. 2018;113:211–217. doi: 10.1016/j.fct.2018.01.050. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Lee JE, Lim SM, Kim YJ, Lee NK, Paik HD. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Science and Biotechnology. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Kim WJ, Bae WY, Lee NK, Paik HD. Inula britannica inhibits adipogenesis of 3T3-L1 preadipocytes via modulation of mitotic clonal expansion involving ERK 1/2 and Akt signaling pathways. Nutrients. 2020;12:3037. doi: 10.3390/nu12103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Lee NK, Choi AJ, Choe JS, Bae CH, Paik HD. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 isolated from kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells. Journal of Microbiology and Biotechnology. 2019;29:1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- Zhou J, Que Y, Pan L, Li X, Zhu C, Jin L, Li S. Supervillin contributes to LPS-induced inflammatory response in THP-1 cell-derived macrophages. Inflammation. 2022;45:356–371. doi: 10.1007/s10753-021-01551-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.