Abstract
Thyroid diseases, especially thyroid tumors, have a huge population in China. The postoperative patients, under China’s incomplete tertiary diagnosis and treatment system, will frequently go to tertiary hospitals for follow-up and medication adjustment, resulting in heavy burdens on both specialists and patients. To help postoperative patients recover better against the above adverse conditions, a novel mobile application ThyroidKeeper is proposed as a collaborative AI-based platform that benefits both patients and doctors. In addition to routine health records and management functions, ThyroidKeeper has achieved several innovative points. First, it can automatically adjust medication dosage for patients during their rehabilitation based on their medical history, laboratory indicators, physical health status, and current medication. Second, it can comprehensively predict the possible complications based on the patient’s health status and the health status of similar groups utilizing graph neural networks. Finally, the employing of graph neural network models can improve the efficiency of online communication between doctors and patients, help doctors obtain medical information for patients more quickly and precisely, and make more accurate diagnoses. The preliminary evaluation in both laboratory and real-world environments shows the advantages of the proposed ThyroidKeeper system.
Keywords: Health informatics, Graph neural networks, Thyroid diseases, Healthcare management
Introduction
The thyroid is a butterfly-shaped gland located at the base of the neck. As part of the endocrine system, the thyroid plays a crucial role in manufacturing hormones and regulating metabolism. Common thyroid-related disorders include hyperthyroidism, hypothyroidism, Hashimoto's thyroiditis, Graves' disease, goiter, thyroid nodules, and thyroid cancer. Treatments for thyroid diseases include medication, surgery, and chiropraxis. Thyroid disease can be a chronic condition that requires early detection and diagnosis, systematic treatment, life-long medication, follow-up supervision, routine care, and individualized management. China has the largest population in the world with over 14 hundred million people. Chinese Center for Disease Control and Prevention (CDC) reported that by the year 2014, the prevalence of thyroid diseases in China was up to 14% with over 10 million people living with hyperthyroidism, 90 million with hypothyroidism, and 100 million with thyroid nodules and thyroid cancer. However, less than 5% of them were treated.
During the COVID-19 pandemic, health resources are being reallocated, health care services are being disrupted, and the current spectrum of thyroid-related treatment is being challenged [1]. Scappaticcio et al. [2] summarized that clinical services are postponed or closed in coordination with pandemic preventative and control measures such as travel restrictions, social distancing, lockdowns, and quarantine so that people living with thyroid-related problems are having difficulties in accessing routine treatment. A multi-centered study [3] conducted in China, South Korea, Iran, and Italy discovered that the COVID-19 pandemic reduced the volume of thyroid surgeries. There were fewer outpatient surgeries in 2020 than in 2019. Fewer thyroid surgeries for advanced cancer stages were performed during the period. Hospital admissions were significantly reduced and the stay durations were dramatically shortened compared to the time without the COVID-19 pandemic. Lisco et al. [4] estimated that the COVID-19 pandemic could worsen thyroid patients' health outcomes by postponing detection and diagnosis, delaying early-stage cancer treatment, disrupting treatment, slacking follow-up supervision, decreasing operative times and hospital stays, and even increasing the rate of developing thyroid-related complications such as vocal cord paralysis.
In addition to the unfavorable factors of the COVID-19 pandemic, the imbalance of China’s medical resource allocation also worsens thyroid outcomes. In China's primary health care system, general practitioners (GPs) are prioritized to first contact people with thyroid concerns. GPs evaluate patients' conditions and make decisions on referring thyroid patients to secondary or tertiary hospitals for further treatment. After getting treated, specialists in secondary or tertiary sectors will refer thyroid patients back to the primary sector for recovery. The coordination between the primary, secondary, and the tertiary level is called dual referral, which is an ideal pathway to be further established in China's health systems [5]. In actual situations, however, thyroid patients tend to visit specialists directly in tertiary levels where high-quality health resources are centric instead of visiting GPs at the primary level. Li et al. [5] stated that the unawareness of misuse of health system make hospital overwhelmed and specialists work overload while primary health services are underused. To make it worse, because of the geographic imbalance of health resources, thyroid patients in rural areas may have less accessible or affordable health services compared to those in urban areas, which expands the health inequality of thyroid disease management [6].
To help patients with thyroid disease (especially those postoperative ones) recover better in the above-mentioned social and medical environment, we resort to mobile health (mHealth) and telemedicine.1 Telemedicine has played a dominant role in managing patients cannot obtain in-person visits. A retrospective study [7] conducted on thyroid outpatient during Covid-19 emergency found that telemedicine had the potential to avoid the potential negative impact of interruption or postponement of diagnostic and therapeutic procedures. In this paper, we apply telemedicine and mHeath technology to the life-long healthcare management of the patients with thyroid diseases, proposing a novel mobile application ThyroidKeeper, which benefits both doctors and patients within a collaborative AI-based platform.
In addition to providing common functions of health management tools (such as recording and reviewing electronic medical records, medication reminders, online doctor-patient communication, health education, etc.), ThyroidKeeper has made the following innovations according to the characteristics of the patient population:
Automated adjustment of medication dosage during rehabilitation. The system can automatically determine the dose of the next course of treatment based on the patient’s medical history, various current laboratory indicators, physical health status, and the current dose of medication.
Complications prediction and health guidance. The system can comprehensively predict the possible complications based on the patient’s own health status and the health status of similar groups utilizing graph neural networks, and prompt the patient to make health interventions in advance.
Intelligence-aided diagnosis. The system can improve the efficiency of online communication between doctors and patients, help doctors obtain medical information for patients more quickly and precisely, and make more accurate diagnoses based on graph neural networks.
The remainder of the paper is organized as follows: Sect. 2 briefly reviews the related work. Section 3 presents the requirements for implementing the three core innovations. Section 4 presents the system design and technical details. Section 5 shows our primarily evaluation on the system and further discusses its advantages and shortcomings. Section 6 concludes the paper.
Related work
There have been quite a few practical cases of utilizing information technology (including networking, human-computer interaction, machine learning, etc.) to manage thyroid diseases. Generally, the previous work can be classified into two categories: auxiliary diagnosis-oriented and heath management-oriented. For the former, the main user of the technology is doctors (although the data may be collected by patients), and the goal is simply to improve the level of diagnosis. For the latter, the goal is more to facilitate the patients’ own health interventions, although such interventions may require the participation of doctors.
Machine learning-aided diagnosis for thyroid diseases has been studied for many years. Basically, several blood test indicators, such triiodothyronine (T3), thyroxine (T4), and thyroid stimulating hormone (TSH), can reflect the health status of thyroid glands. A number of studies investigated different machine learning algorithms, such as logistic regression [8, 9], kNN [9, 10], neural networks [8, 11, 12], SVM [13, 14], etc., on these indicators to classify thyroid diseases. Besides these traditional methods and systems, mobile applications recently have been employed for collaboratively collecting and utilizing the laboratory indicators between patients and doctors, which is more relevant to our work. Thyroid-SPOT [15] is a mobile application that allows both patients and doctors to analyze thyroid function tests laboratory reports by computing the homeostatic euthyroid setpoint based on regressing thyroid function data to define the parameters that maximize the goodness-of-fit to a negative exponential model for target-centric individualized treatment. The patient version of the app is used to upload examination data, and the doctor version is used to assist doctors’ diagnoses. HypoTir [16] can make a preliminary diagnosis of thyroid diseases based on the TSH, free T4, T3 concentration, and presence of thyroid gland antibodies. It must be pointed out that through blood test indicators, it can only make judgments among normal, hypothyroidism, and hyperthyroidism. To determine the type of thyroid nodule, imaging technology (such as MRI, ultrasound, elastography, and CT) must be used [17, 18], which is out of the scope of this work.
In recent years, mobile applications (apps) have become the mainstream in building patient-centric health management systems, namely mHealth. mHealth has proven beneficial for managing chronic health conditions [19–21]. Some apps for thyroid diseases have also been proposed. Giannoula et al. [22] designed an app for differentiated thyroid cancer (DTC) patients, which offers access to useful information about thyroid cancer, diagnostic tests, and the appropriate therapies. A pilot quasiexperimental interventional trail showed that the quality of life of DTC patients was improved by self-education and self-management with the app. BOOST Thyroid App2 is a commercial system for underactive thyroid patients, which tracks over 20 symptoms related to patients’ body, mind, lifestyle, thyroid medications, and supplements. Although the system does not diagnose or provide medical advice, the quality of life of patients can still be improved by recording and discovering their own health patterns [23]. In China, WeChat app (similar to WhatsApp) has been used in the follow up of thyroid cancer patients after thyroidectomy during the COVID-19 pandemic [24]. THYROSIM [25] was a simulator of human thyroid hormone and thyrotropin regulation dynamics, which was implemented as a facile and freely accessible web-based and personal device application. THYROSIM accurately reproduced a wide range of published clinical study data reporting hormonal kinetic responses to oral hormone and was used for the visualization the effects of over-the-counter thyroid supplements. By contrast, our ThyroidKeeper is a collaborative platform between patients and doctors, where patients can obtain automated treatment and health care advice, and doctors can also improve their diagnosis efficiency.
Requirement statement
The original idea for the design and development of the ThyroidKeeper app came from one of the authors of this paper, who works in the thyroid surgery department of a large-scale tertiary hospital in China. Through telemedicine and machine learning technology, the main objectives of ThyroidKeeper are to (1) reduce the cost of medical treatment for patients after thyroid surgery and improve their quality of life, and (2) improve the efficiency of doctor-patient communication and facilitate doctors to make more accurate diagnoses during follow-up visits. Note that ThyroidKeeper does not provide any auxiliary diagnostic functions before surgery. It only serves patients and doctors during the rehabilitation period. We conducted semi-structured interviews with patients and surgeons in the author’s hospital to collect requirements for the app during September 2019 to April 2020. Finally, 67 patients and 9 surgeons participated in the interview. We summarize their main requirements and the related reasons in this section.
Requirements from patients
The outlines of the requirements from patients with thyroid disease are as follows:
Reduce the cost of follow-up visits. Postoperative patients with thyroid nodules and tumors (regardless of benign or malignant) need frequent follow-up visits within 2 years. Patients usually receive operation in tertiary hospitals in cities. They still choose to go to the same hospital for follow-up visits with the same surgeon. A follow-up visit usually requires a blood test, and it takes at least two days (it usually takes 4–6 h to get the blood test results). For those patients who live in rural or remote areas, each follow-up visit is accompanied by time, commuting, and accommodation costs. They hope that the app can help to save these costs.
Bind and communicate with different doctors. For a period of time after the operation, patients want to keep in close contact with the surgeon who performed the operation. Thus, online communication is a basic need. Thyroid disease is irreversible. Over time, these patients are likely to change doctors because they may move to other places. Thus, the app needs to bind a patient with multiple doctors. The diagnosis and recommendations of previous doctors can be used as references for the current follow-up visit.
Predict complications and provide health guidance. As part of the endocrine system, thyroid dysfunction may cause various complications, such as extraocular myositis, diabetes, arrhythmia, and so on. For those patients who already have basal metabolic diseases, these complications are more likely to occur. The patient hopes that the system will provide early warning and intervention suggestions before complications occur. Once the complications occur, the system will provide health care guidance.
Easy-to-use user interface. When inpatients are discharged from the hospital, they will receive a paper-based discharge report, which records diagnosis and treatment details. The app needs to provide photographing and upload functions to receive medical records and examination reports. The photos of these paper materials will be converted into text by an optical character recognition (OCR) module and converted into the internal electronic medical record (EMR) format by an information extraction module. In short, the app needs to minimize manually entering information as much as possible.
Track health status. As a lifelong health management tool, the app will record the process of each online consultation and the indicators of each laboratory examination. The app will provide an overview of the health of each stage (for example, a 3-month course of treatment), as well as a graph of changes in various indicators, which helps patients better understand their own health status.
Requirements from doctors
In China, doctors with advanced skilled tend to be with centric to tertiary hospitals. One of the authors of this paper states that on a weekly base he works both for outpatients and surgeries. 70% of the outpatient are postoperative whose treatment can be standardized and repetitive. Working overload and repetitively takes up their time and energy for advancing skills, causing an inefficiency on the utilization of health resources. Additionally, as public hospitals are not benefit-focused, doctors are not salary-driven. Therefore, for doctors, the basic goal of the app is to help them reduce their workload. The outlines of the requirements from doctors are as follows:
Automated medication dosage adjustment. For 80% of postoperative patients, their follow-up treatment is to adjust the medication dose. The characteristics of postoperative treatment of thyroid disease determine that the medication adjustment process can be automated. First, the medication after thyroid surgery is simple, just taking thyroxine. There are only two to three brands of thyroxine on the market, and there is almost no difference among them. Second, the dosage of each course of treatment can be calculated through a series of rules based on the type of disease, previous operation and treatment, basic health status, blood test indicators (T3, T4, TSH, etc.), and current medication dosage (refer to Sect. 4.2). Finally, the exact amount of thyroxine that the body needs to supplement is unknown. Thyroid surgery department generally has some guidelines for medication dosage adjustment. Under the guidance, even if the advice given by each doctor is slightly different, it hardly cause safety risks. We just need to constantly check and adjust.
Efficient online communication mode. In China, WeChat app (similar to WhatsApp) has been a main online communication channel between patients and doctors. However, it is usually inefficient. Since doctors’ mobile phones cannot access the electronic medical record system in the hospital, patients have to take photos of those paper-based materials and examination reports and send them to the doctors. The doctors have to reanalyze the patients’ conditions after obtaining the raw information. Such a mode is not only time-consuming and labor-intensive, but also may miss the diagnosis track (if patients have gone through multiple rounds of follow-up visits). Therefore, doctors hope that in the online communication, the relevant information of the patient can be displayed on the screen in the form of hypertext summary (rather than the original pictures), so that they can check it at any time, which reduces the transmission of original data.
Help general practitioners and interns to improve their professional capability. Quite a few postoperative patients undergo follow-up visits at general practitioners or interns. Health workers with basic skills hope that the system can provide some suggestions when they perform the online consultant, which can improve their skills. For example, when they make a diagnosis judgment, the system will provide some hints when the judgment is probably inaccurate. It will show some cases in similar patients.
Summary of core values
After requirement analysis, we summarize the core values of ThyroidKeeper as follows: (1) ThyroidKeeper provides automatic medication dosage adjustment. Postoperative patients only need to go to the community clinic for blood test and upload the test report to the system to get the medication dosage for the next course of treatment. This will significantly reduces the number of outpatient visits to specialists in tertiary hospitals, and makes the distribution of medical resources at all levels more balanced. (2) The system can predict the possible complications of patients through machine learning modeling, and propose early intervention suggestions, thereby improving their quality of life. (3) Based on the internal machine learning models, the system provides an efficient online interaction mode, which can not only help doctors obtain more accurate case information, but also help health workers with basic skills improve their professional capabilities.
System design and technical details
This section first presents the architecture of the proposed ThyroidKeeper system from the perspectives of users. To realize various requirements, the system employs both off-the-shelf and innovative techniques. Thus, after introducing the architecture, we will present some innovative methods that implement the core values of our system.
Architecture
Figure 1 illustrates the architecture of the ThyroidKeeper system. Generally, the system includes two apps (for patients and doctors, respectively) and a backend in the cloud. Apps implement a graphical user interface and instant message (IM) components. The backend implements various AI algorithms and data storage.
Fig. 1.
Architecture of ThyroidKeeper. “Mm” stands for multimedia; “IM” stands for instance message; “OCR” stands for optical character recognition; “NLP” stands for natural language processing; “KB” stand for knowledge base
User interface
ThyroidKeeper provides various user interaction modes. For those paper-based materials, the app provides photographing and upload functions. The photos will be passed to the OCR module to extract text. We use the OCR service of Baidu AI open platform,3 which has the function of specifically supporting text extraction from medical reports and bills. Although our application is limited to a single field of thyroid disease, basic natural language processing (NLP) functions are not indispensable. First, we need to identify the entities (such as various medical terms, etc.) on the patient’s electronic medical record. The same entity may have different names. In order to achieve this goal, we further improved the existing pre-training model BioBERT [26], which is based on BERT [27] but re-trained for biomedical domain. We enhanced the corpus for thyroid disease and re-trained the BioBERT model so that it is more sensitive to vocabulary in the domain. Second, basic semantics of the natural language descriptions in EMR should by analyzed, which can be archived by relation extraction. Since we have chosen the pre-training language model BERT, we utilized the exiting method [28], which only needs to simply build linear layers on top of the BERT model to obtain good results. Finally, the extracted entities and relations as (semi-)structured information will be stored in a patient database.
As we all know, under a real-world condition, the performance of OCR is affected by many factors, and the accuracy of named entity recognition and relation extraction either can hardly exceed 90%. To address this issue, after patients upload the photographing data, the app still provides a various interface components (such as text box, dialogue box, scroll bar, radio button, check box, etc.) to facilitate them to view and modify important data manually, which guarantees of the algorithms to generate correct results (refer to Sect. 4.2).
Instant message component is another important facility for the app. As mentioned in Sect. 3, once a patient decides to use the app, s/he must bind with at least one doctor, i.e., an association between a patient and a doctor is created. Hereafter, they can communicate online with each other once needed. Building an IM service involves a considerable amount of work. Fortunately, an open-source software Wildfire IM4 supports full stack development of IM service, including client APIs for Android and iOS and an IM server. The functions of Wildfire IM include instant message, image and video transmission, and audio/video telephony. Integrating Wildfire IM, ThyroidKeeper gains a powerful online communication ability. Behind the IM service, we also developed a simple interaction analysis function. Interaction analysis can extract the photographing text (patients may send a photo of examination report during conversation) and send it to OCR. It also extracts long dialogues with many medical terms and diagnostic vocabulary and send them to the NLP module, which can be viewed as formal online visits that may change the status of patients. We admit that this function is still very preliminary, and some advanced techniques such as topic detection [29] and dialogue breakdown detection [30] could be further considered.
Finally, ThyroidKeeper also reserves an interface for uploading medical image data, but the medical image processing is currently not included in the current version, because the main objective of the system is health management. In addition, the app for doctors is much simpler than that for patients, which mainly includes an IM component for online communication as well as a simple patient management function.
Internal modeling
ThyroidKeeper internally has two data sources: patient database and knowledge base (KB). The KB currently stores the static information for health guidance. The health guidance plan for each patient will be extracted and integrated from the KB based on the diagnosis result of each patient, and finally pushed to the patient. The content of KB is maintained by thyroid disease experts.
The internal models are built on the patient database whose content comes from various input and is currently implemented with Apache Accumulo database. Apache Accumulo is a distributed key-value database with cell-granular access control and ultra-high read and write performance [31], which is very suitable for the storage of security-sensitive semi-structured medical big data. Basically, we divide patient information into four categories of features, i.e., demographic, symptomatic, laboratory and diagnositic features, which will be used in modeling. ThyroidKeeper focuses on the modeling of automated medication adjustment (refer to Sect. 4.2) and symptom and diagnosis forecast (refer to Sect. 4.3). In modeling the symptom and diagnosis using graph neural networks, some estimated information might be helpful. Once patients obtains the results generated by the automatic medication adjestment model, they can optionally consult their doctors for the correctness of the results. Although the original intention of this function is to allow patients to reduce consultations, the reserved confirmation process on the one hand can help patients build trust in our system, on the other hand, it can also accumulate expert knowledge to optimize the AI modeling. The built models will be stored in the patient database.
Now, we will go to details of the two core functions, i.e., automated medication dosage adjustment and symptom and diagnosis forecast.
Automated medication dosage adjustment
Since the automated medication dosage adjustment (AMDA) algorithm involves more thyroid professional knowledge and patent intellectual property rights, this section only discloses the principle and outline of the algorithm, which is sufficient to explain the work of the algorithm.
Brief classification of thyroid diseases
ThyroidKeeper divides common diseases in thyroid surgery into six categories: (I) thyroid papillary cancer, (II) thyroid vesicular cancer, (III) medullary thyroid cancer, (IV) undifferentiated cancer, (V) benign thyroid tumor (including hyperthyroidism and postoperative hypothyroidism), and (VI) non-operation-related hypothyroidism. The first four types are malignant and usually require surgery (undifferentiated cancer (IV) may not require surgery), while the latter two are benign (benign thyroid tumor (V) may require surgery).
Because the treatment and prognosis of these types of thyroid diseases are different, the basis for medication dosage adjustment is also different. Figure 2 shows a hierarchical data requirement of the AMDA algorithm for each type of thyroid disease. At the root of the hierarchical structure is the demographic information of patients, such as gender, age, occupation, smoking state, etc., some of which may have an unignorable impact on the health of the patients. For example, in the side-effect risk classification for TSH suppression treatment, a patient whose age is between 35 and 59 is at medium risk. Another important information for all kinds of thyroid diseases is whether or not a patient is concurrent with other conditions. The concurrent conditions include cardiovascular and cerebrovascular diseases, menstruation state, osteoporosis, and pregnancy. The concurrent conditions usually have great impact on the prognosis of the diseases. For example, in the side-effect risk classification for TSH suppression treatment, a woman in her perimenopause is at mmedium risk but in her post-menopause is at high risk. In short, the information at the root of the hierarchy is important for every type of thyroid disease.
Fig. 2.
A brief classification of thyroid diseases in thyroid surgery and the data needed by the AMDA algorithm for each type of thyroid disease
The types requiring surgery
Except for non-surgical-related hypothyroidism (Type VI in Fig. 2) (such as small nodules that have not yet reached the indications for surgery, simple hypothyroidism, etc.), most thyroid tumors (regardless of malignant or benign) require surgery.5 For the benign thyroid tumor, we only need to collect the operation date and operation type (listed in Fig. 2). For the malignant thyroid tumor, there are more information should be collected, inlcuding preoperative color ultrasound (locations, sizes, and grading of nodules), preoperative laryngoscope results, pathological results of preoperative puncture, postoperative pathology type (the number of lymph node metastasis in the central area, the number of lymph node metastasis in the cervical side area), Iodine-131 treatment, distant metastasis (bone/lung/brain), and the TNM staging.6 All this information can be extracted from patient’s discharge report using OCR.
Different thyroid cancer types follow different rules for medication adjustment. For those cancers with poor prognosis (Types III and IV), since the patient is already at high risk, the objective of medication adjustment is to adjust the intake of thyroxine according to the current free T4 and TSH values so as to suppress TSH within a certain range. For those cancers with good prognosis (Types I and II), the management of TSH is based on the levels of the differentiated thyroid cancer (DTC) recurrence risk and side effect risk of TSH-suppressive therapy. In the next subsection, we will briefly explain the DTC recurrence risk and side effect risk of TSH-supperssive therapy and show how ThyroidKeeper proccesses them.
DTC recurrence risk and side effect risk of TSH-suppressive therapy
As we know, easy recurrence is one of the basic attributes of cancer. Patients with differentiated thyroid cancer (DTC) have a recurrence risk during the postoperative period. The risks of differentiated thyroid cancer (DTC) recurrence are stratified into the low, middle, or high level, based on an individual's clinical-pathological features (such as the histological subtype of the tumor, vascular invasion, distant metastasis, etc.). Due to the good prognosis of DTC, preventing recurrence is an important goal of post-operative treatment, which plays an important role in prolonging the survival of patients and even curing them completely [32].
As TSH has a direct trophic effect on the growth and differentiation of thyroid cancer cells, TSH-suppressive therapy is applied as part of the standardized treatment and long-term management for differentiated thyroid cancer. TSH-suppressive therapy is the use of exogenous L-thyroxine (T4) to lower or suppress the host thyrotropin (TSH). Side effects could be induced by TSH-suppressive therapy such as subclinical hyperthyroidism status and delayed TSH recovery [33]. Thus, the application of TSH-suppressive therapy is individualized depending on patients' health conditions (such as age, gender, comorbid conditions, menstrual status, bone mass, or smoking status, etc.) [34]. With limited research, the optimal degree of TSH reduction remains unclear and requires further exploration [35].
Figure 3 shows the DTC recurrence risks and the side effect risks of THS suppression for thyroid papillary cancer (Type-I) and their judgment criteria. For the judgment criteria, many of them can be directly obtained from the patient’s discharge reports or examination reports (such as most criteria in DTC recurrence risks), and also some of them may not be obtained from the existing electronic records (such as bone loss, risk factors for cardiovascular disease, etc.). ThyroidKeeper uses multiple UI pages to determine the risk level with patient participation. Taking DTC recurrence risk as an example, ThyroidKeeper first presents all High risk items on a page, each of which has a checkbox. If an item is satisfied based on the patient’s records, the checkbox is selected (tickled). The patients can also change the status of any checkbox if they think the automatic judgment is not true. This design is based on that many patients have sufficient knowledge of their own health conditions, especially for the underlying diseases, patients themselves may know their own conditions better than (thyroid) specialists. Therefore, on the basis that ThyroidKeeper can automatically process these judgment items, it still hands the final decision to the patient. In addition, ThyroidKeeper judges the risk from high to low. For high and medium risks, it can be determined as long as one of the criteria is satisfied. For low risk, all criteria need to be satisfied.
Fig. 3.
DTC recurrence risk and side effect risk of TSH-suppressive therapy for thyroid papillary cancer (type-I) and their judgment criteria
Having obtained the risk levels of DTC recurrence and side effects of THS suppression, ThyroidKeeper can determine the THS suppressive targets for thyroid papillary cancer (Type-I) and thyroid vesicular cancer (Type-II) at different follow-up stages, which is listed in Table 1. Our automated medication dosage adjustment algorithm is based on the THS suppression targets. It must be noticed that, for the other four kinds of thyroid diseases, also they have their own THS suppression targets (included in the algorithm). However, they do not need this double-risk analysis.
Table 1.
TSH suppression targets for types I and II patients (unit: μIU/mL)
| DTC recurrence risk | ||||
|---|---|---|---|---|
| 1 year after operation | Follow-up period | |||
| Medium & high | Low | Medium & high | Low | |
| Side effect risk of TSH suppression | ||||
| Medium & high | <0.1 | 0.5–1.0 | 0.1–0.5 | 1.0–2.0 |
| Low | <0.1 | 0.1–0.5 | <0.1 | 0.5–2.0 |
Examples of the algorithm
Our automated medication dosage adjustment (AMDA) algorithm is based on the current text results of free T4 and TSH, TSH suppression target, DTC recurrence risk, side-effect risk of TSH suppression, and current thyroxine intake. Since our algorithm has applied for patent protection, we only provide one example to illustrate the working process of our AMDA algorithm.
For example, a patient with thyroid papillary cancer (Type-I) takes a blood examination within a year after his/her last surgical operation. Table 2 shows the process of generating the output (the change of thyroxine intake) of the AMDA algorithm for this kind of patients. First, after DTC recurrence risk analysis, AMDA will has different TSH suppression targets, which eventually affects the thyroxine intakes. Then, AMDA checks the current TSH value and free T4 value obtained from the blood examination. If the free T4 value is lower than the upper bound of the normal values, the change of thyroxine is recommended according to the corresponding line (the same line indicated by the current TSH value). If the free T4 value exceeds the upper bound of the normal values, the change of thyroxine follows the recommendation of the previous line indicated by the current TSH value. For example, the DTC recurrence risk is high or medium, the free T4 is higher than the upper bound of normal values, and the current TSH value is 5.0 (in line 3), then, the change of thyroxine intake will be “increase by 25.0 (in line 2).” If the current line indicated by the TSH value is the first line of the specific TSH suppression target, AMDA will maintain the original dose (do not need to change the thyroxine intake). The AMAD algorithm does not specify the normal range of free T4 as hard values but sets the range by reading the blood examination reports, since diferent test equipments may use different values. For example, some equipment sets the normal range to pmol/L.
Table 2.
Example of the algorithm for a patient with thyroid papillary cancer within a year after the last surgical operation
| Risks | Free T4 | TSH (μIU/ml) | Change of thyroxine (μg) |
|---|---|---|---|
| DTC recurrence risk high or medium (TSH target 0–0.1) | (1) If it is lower than the upper bound of the normal value, the change of thyroxine is recommended according to the corresponding line | 0.1–0.5 | Increase by 12.5 |
| 0.5–2.0 | Increase by 25.0 | ||
| 2.0–10 | Increase by 50.0 | ||
| 10–30 | Increase by 75.0 | ||
| 30–100 | Increase by 100 | ||
| DTC reccurence risk low & side effect risk high or medium (TSH target 0.5–1.0) | 0.1–0.5 | Decrease by 12.5 | |
| 0.5–1.0 | Unchange | ||
| 1.0–5.0 | Increase by 25.0 | ||
| 5.0–10 | Increase by 50.0 | ||
| 10–30 | Increase by 75.0 | ||
| 30–100 | Increase by 100 | ||
| 0.1–0.5 | Unchange | ||
| DTC reccurence risk low & side effect risk low (TSH target 0.1–0.5) | (2) If it exceeds the upper bound of normal values, follow the recommendation of the previous line; If the current line is the first line, maintain the original dose | 0.5–1.0 | Increase by 12.5 |
| 1.0–5.0 | Increase by 25.0 | ||
| 5.0–10 | Increase by 50.0 | ||
| 10–30 | Increase by 75.0 | ||
| 30–100 | increase by 100 |
In addition to the new dose of the medicine and the suggested date for the next follow-up, ThyroidKeeper also provides some tips for keeping healthy. For example, for a patient with thyroid papillary cancer, the tips may include but not limited to:
Taking medicine with an empty stomach before breakfast is most conducive to maintaining a stable TSH level.
If there is any missed dose, double doses should be taken until all missed doses are taken.
Some special medicines or foods should be taken after a sufficient interval: 1 h for vitamins and supplements, 2 h for iron and calcium foods or medicines, 4 h for milk and legumes; 12 h for cholestyramine or fat-reducing resin.
After each adjustment of the L-T4 dose, TSH can gradually reach a steady state in about 4 weeks, and it may take longer for the elderly.
Symptom and diagnosis forecast
As mentioned above, ThyroidKeeper has other core values for both patients and doctors: for patients, it has the ability to predict the complications, and for general practitioners or interns, it helps them improve their professional capability. To this end, we need to model patients and their associated clinical events, and use models (based on historical medical records of a large number of patients) to predict their missing attributes (such as possible future symptoms or diagnostic conclusions). Similarly, based on these models, correlation analysis of similar cases can be done to assist diagnosis. This section presents our heterogeneous network pair embedding model for medical diagnosis.
Motivations
Predictive modeling using traditional machine learning methods (such as Naive Bayes, decision trees, SVM, etc.) requires careful design (extraction [36], selection [37], and transformation [38]) of the features of training samples. However, this approach of relying on feature engineering has encountered great difficulties in the field of medical diagnosis and prediction [39–41]. First, the medical records of patients are multiple-sourced and heterogeneous, including various representations of demographic features, laboratory test indicators, imaging features, clinical symptom descriptions, and so on, which can hardly be stacked together to obtain good models. Well-trained experts may have better feature engineering solutions, but this undoubtedly increases the cost of model construction. Therefore, in a big data environment, unsupervised or weakly supervised solutions are the ideal choice. Then, there are a lot of missing values in the patient’s medical records. Even if patients have the same disease, there is no guarantee that they have all undergone identical examinations. In addition, the correlation between the patient and the patient and between the patient and the diagnosis plays a crucial role in the performance of models, but traditional methods are based on an assumption that they are independent of each other.
In recent years, representation learning techniques based on graph neural networks have performed well in complex data modeling [42], which has been successfully applied to medical domains. For example, RETAIN [43] and Dipole [44] and focus on the use of admission history and diagnosis history in electronic health record (EHR) data to predict future diseases by the way of graph networks. However, these models are too rough for the processing of EHRs. Another famous work, MedVec [45], considers the co-occurrence of clinical events in diferent patient records to extract latent embeddings of these entities. Although it has begun to utilize the detailed information in EHRs, its representations learned are general and not tailored to the goal of diagnosis prediction.
As we know, a diagnostic process usually involves careful consideration of clinical observations (such as symptoms and diagnostic tests). A clinical observation is generally non-speciic to a single disease. Its relation or co-occurrence with other observations can be indicative of a disease [46]. Moreover, the presence of multiple diseases can cause complexity in observations. Therefore, to address the problem of capturing the rich structure of EHR data and semantics of various relations it contains, ThyroidKeeper employs a thyroid disease diagnosis model based on heterogeneous graph neural networks. Heterogeneous graph neural networks, as known as heterogeneous information networks (HINs) [47], with various types of nodes and relations, have strong ability in distinguishing and learning the diferent semantics of relations among entities. HINs have been applied to medical domains to construct heterogeneous embedding models [48]. In ThyroidKeeper, we focus on patient-diagnosis pairwise relationship, proposing a novel Heterogeneous network Pair Embedding model for Medical application (HPEMed), which captures closer relation between patients and their diagnosis, compared with the patient-centric model like [49]. ThyroidKeeper uses the HPEMed model to predict the occurrence of symptoms and verify the consistency of the doctor’s diagnosis through the similarity between cases. Here, we provide the outlines of our HPEMed model.
Definitions
We first provide the definitions and clinical terminology used in our work. Then, we describe how EHRs can be modeled as a heterogeneous network. Finally, we formalize the disease diagnosis task.
Clinical events and patient features
Clinical events summarize a series of basic information, admission data, treatment results of a patient. In terms of the composition of nodes in a heterogeneous network, a clinical event e can be formulized as , where t, n, and v represent its node type, name (terminology), and value, respectively. For example, the TSH level of 1.0 can be viewed as , , and .
From the perspective of the division of medical events, clinical events of a patient (E(p)) shown in Fig 4 consists of a set of observation events, a set of treatment events and a diagnostic event. That is, given a clinical event e, we have , where . Observation events are the source of information for patients and can be treated as the features of patient nodes.
-
(2)
Concepts related to heterogeneous information networks
We provide some concepts related to HINs, which formalizes EHRs from a HIN perspective.
Fig. 4.

Clinical Events for a patient from a HIN viewpoint
Definition 1
Heterogeneous information network. A heterogeneous network can be defined as a graph , where nodes V and edges E between them have different types. Nodes are mapped to their type by the defined node mapping function , where is the union of different types of nodes. Meanwhile, each edge is also mapped to edge type mapping function where is the union of various types of edges. Due to E is a heterogeneous network, we define and .
Different patients and clinical events form the nodes of the HPEMed model. The type of a node is deined by the type of the clinical event mapped to it. Links are designed based on the EHR relations, which are mainly between a patient and a clinical event. Fig 4 also shows the abstract schema of the network illustrating node types and basic links. The network can enrich the semantics of relations by defining new compositional relations via Meta-paths [50].
Definition 2
Meta-path. A meta-path in HIN deines a higher order relation between two node types. Set and constitute a meta-path schema , which shows a composite relation R between objects and . That is, , where denotes the composition operator on relations in a given meta-path.
Definition 3
Context path. Given two nodes , we have a set of context paths for all random walks from to , where is a set of collected random walks and P represents a specific meta-path. A context path from to is denoted by .
Definition 4
Diagnostic task. Input: Node sets that consist of patient-diagnosis pairs (each of which is denoted by ) and context path sets (each of which is denoted by ) affliated to the patient-diagnosis pairs and distilled from the meta-path scheme S(P) derived from random walks. Output: Probability of the pairwise relationship between a specific pair of nodes in V.
Construction of clinical events for HINs
Since we have an outline to map clinical events to HIN constructs, this section will go to the details of generating clinical events.
Data preprocessing
For diagnosis nodes, we map diagnosis clinical events based on the WHO International Classification of Diseases (ICD-11) coding system. We also utilize Autophrase [51] to extract the common symptoms in the original clinical records and provide clinical phrases for Autophrase, which come from Medical Subject Headings (MeSH) and the (ICD-11) database.
-
(2)
Feature representation of patient nodes
To acquire latent embeddings of patients from context of words in medical records, we apply metapath2vec [48] to accomplish the meta-path guided random walk in conjunction with the heterogeneous skip-gram model [52]. We define a graph and an embedding function that projects each node to a d-dimensional vector. The purpose of f(v) is to maximize the probability of visiting the neighborhood N(v) of a node v:
| 1 |
where is defined as a softmax function normalized regard to node representation. More precisely, the probability of visiting a neighbor s of a node v under path r with route is represented as:
| 2 |
| 3 |
We apply negative sampling [53] to attain the objective function in Eq. (3). One training step stochastically samples m negative nodes, path r, two nodes v and s connected under r. Finally, we use Stochastic Gradient Descent to update the embeddings as new representations of the patients.
Having the representation of patients, we could perform some tasks such as the completion of missing values and calculation of the similarity of patients. Clinically, the symptoms of many diseases are very similar, and only one or two indicators are different to represent different diagnostic conclusions. Then, in such cases, using the above simple model will cause misjudgment. Because the difference between one or two indicators is not enough to produce a significant difference in similarity. In addition, our ThyroidKeeper is not designed for diagnosis. Thus, in our original data, the patient’s condition is bound to the existing diagnosis (note that most of our users are patients after surgery). Therefore, we further model patients and their diagnoses jointly to improve the performance of the model. That is, we propose a patient-diagnosis pairwise embedding network model, namely HPEMed.
The proposed HPEMed model
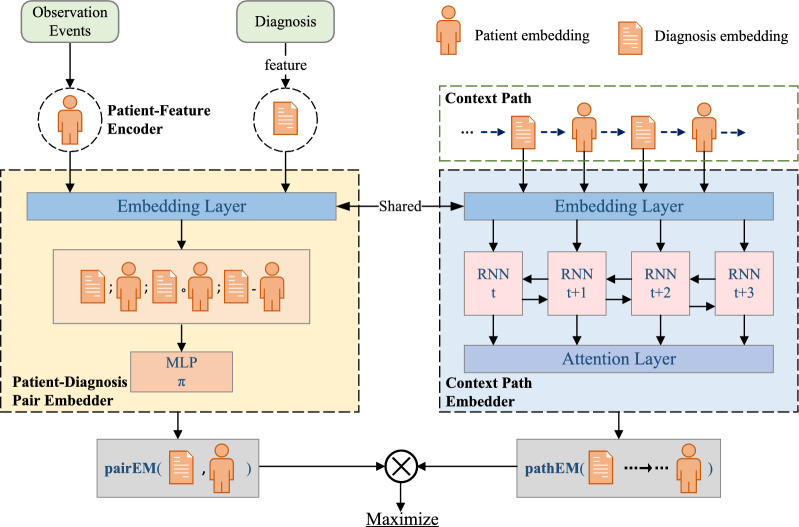
We present our proposed HPEMed model in top-down manner, focusing on the principle of the model.
Framework
Figure 5 shows the framework of our proposed HPEMed model. First, we utilize the method in Sect. 4.3 to obtain the representation of patient features. Second, by performing random walk in the network, we can obtain a set of context-paths. Because a patient can have multiple different diagnoses and a diagnosis can also be given to different patiets, we can extract multiple patient-diagnosis pairs from the context paths. Both patient-diagnosis pair and context path also will be embedded into vectors, respectively. They share the same embedding layer. Finally, the training of the network will maximize the probability of the patient-diagnosis pairs within the corresponding context paths.
-
(2)
Patient-diagnosis pair embedding
Fig. 5.
The framework of the proposed HPEMed model
We define a pair of patient embedding and diagnosis embedding as a . The pair embedder pairEM: is defined as a n-layer multi-layer perceptron (MLP) that generates a d-dimensional embedding for a patient-diagnosis pair(v, u). Thus, we have
| 4 |
| 5 |
| 6 |
We define . is a MLP with two layers. Each layer has 100 hidden units. Operator is element-wise vector multiplication. In addition, we use the Dropout [54] method to prevent overfitting in the hidden layers. Finally, we will obtain the pair embedding from the output layer of MLP.
-
(3)
Context path embedding
Referring to Definition 3, we define a set of context paths of patient-diagnosis pair(v, u) as . We use a bi-directional Gated Recurrent Unit (GRU) model to embed a context path composed of a sequence of nodes.
A context path can be represented as a sequence of nodes, i.e. . We transform this node sequence into a K-dimensional embedding vector , where we have and . For a metapath , if , then we have and . At unit t, GRU calculates the hidden state in view of the last hidden state and the current context path input . Thus, it can be understood as . The computation of the GRU is as follows:
| 7 |
| 8 |
where is the ratio of update gate and is the ratio of reset gate. The operator | | is the concatenation. , and are parameters for model update. HPEMed conducts bidirectional GRU computation, denoted by:
| 9 |
| 10 |
where the top arrows stand for the direction of calculation. and stand for the parameters on the projection layer. The operator stands for vector concatenation.
The purpose is to embed nodes along the context path into the matrix representation h. To better measure the importance of different nodes in the and represent the whole context path, we use attension pooling [55] to define the context path embedder as follows:
| 11 |
| 12 |
The adoption of the attention mechanism allows the focus to be concentrated on the context path whose contribution is much higher, referring to Eq. (12), where and
-
(4)
Joint optimization
. Our objective is to maximize the probability of the pair(v, u) within the context path c. We transform the objective into calculating the minimum of the negative log probability
| 13 |
More precisely, we generate different context paths from random walks as many as possible and the probability of is defined as:
| 14 |
In the same way, as directly computing the above probability is time-comsuming in large networks, we select k () randomly sampled context paths and employ negative sampling to achieve further optimization as follows:
| 15 |
To sum up, ThyriodKeeper can use the HPEMed model to perform different tasks, such as predicting some symptoms for patients (treating them as missed values), finding similar patients in the system, and validating the consistence of doctors’ diagnoses.
Implementation of human—machine interaction
At present, we only implemented the Android OS version of ThyroidKeeper, because in the hospital where the author works, most patients use cell phones with Android OS. In this section, we briefly introduce the techniques used in system implementation.
ThyroidKeeper uses the Model-View-ViewModel (MVVM) software architecture, which separates the data processing and business logic. It uses ViewModel as a connector between Activity/Fragment and other components, which is responsible for transforming and aggregating the data returned in the Model, making these data easy to display, and immediately notifying Activity/Fragment of these data changes. ThyroidKeeper uses life cycle awareness technology, which utilizes LiveData (an observable data holder class in Android) to hold data, and destroys data when Activity/Fragment is inactive to prevent memory leaks.
In terms of UI design, ThyroidKeeper uses the Android Jetpack component package to achieve texture design. It implements the bottom navigation buttons through the Navigation component, optimizes parts of input boxes through the TextInputLayout component, and finally, achieves multi-entry data display through the current popular Recyclerview component and uses a data adapter to achieve two-way binding of data and controls. Some examples of the UI design are shown in Fig. 6.
Fig. 6.
The user interface of the proposed ThyroidKeeper app
In terms of messaging, ThyroidKeeper adopts a precise instant messaging and message push platform based on UMeng Plus Global Data,7 which has rich communication forms, stable messaging channels, and supports high concurrent loads. As for network requests, ThyroidKeeper adopts the Retrofit8 framework, the currently most popular network request library, which is based on the OkHttp9 network request encapsulation. The Retrofit framework makes the code concise and easy to use in conjunction with other frameworks to improve request performance. The backend of ThyroidKeeper uses the J2EE Spring Boot10 framework, which shields complex dependencies, configuration and deployment, allowing us to focus more on business logic.
In terms of implementing OCR and image processing, ThyroidKeeper employs on Baidu AI open platform,11 which can provide cloud services with high reliability, elastic scalability, and high concurrency. It realizes the accurate recognition of images and text, carrying out special optimization for the blurry, slanted, flipped, and other situations of images. In order to realize the diversified processing of images, the application uses the Glide image loading and caching library to display and crop images. At the same time, relying on custom components, it realizes zooming and dragging images by gestures.
Finally, in order to improve development efficiency, ThyroidKeeper also uses ButterKnife12 to implement dependency injection and binding of controls and methods, and uses third-party libraries such as Image Picker13 to implement image, address and time selector functions.
Preliminary evaluation and discussion
Our ThyroidKeeper system is designed and developed to effectively solve the problems of healthcare management of postoperative patients. At present, the development of the first version of the ThyroidKeeper system has just been completed. The system is being tested in the hospital where one of the authors of this paper works. We use subjective and objective evaluation methods to preliminarily evaluate the system. In this section, we present the results of our preliminary evaluation and make some discussion on our findings.
Evaluation of patient acceptance
Setup
Because ThyroidKeeper involves medication dosage recommendations and other functions related to disease treatment, clinicians or nurses should guide the patients to use it, who will introduce the software to patients and complete the binding of patients and doctors in the system. We carried out the software promotion trial in the thyroid surgery of the tertiary hospital where some authors of this article works. In Fig. 7, a clinical nurse is introducing the use of the software to patients undergoing thyroid surgery. We spent 3 months persuading a total of 296 patients after thyroid surgery to install this app. These patients were all newly operated patients, and they were required frequent follow-up visits (at least once a month) for the following 3 months. Such patients could help us quickly assess the patient acceptance of the software.
-
(2)
Results and discussion
In the following 3 months for each recruited patient, we observed the use of this software in these patients. We found that 221 patients (74.7%) used this software in the following 3 months (including the use of automated medication dosage adjustment function and patient-doctor communication function). Those patients who used the automated medication dosage adjustment feature would choose to communicate with their doctors online at least once. This indicated that patients have a process of doubting and accepting to new things. Among these 221 patients, 49 (22.2%) of them only had an online consultation and did not use the automated medication dosage adjustment function. Possible reasons include doubts about the accuracy and professionalism of the automated medication dosage adjustment or inconvenience of software input. Among the 172 patients who used the dosage adjustment function, 67 of them used the same function twice or more, indicating they are compliant to the function. In summary, we believe that the patient acceptance rate of the software is 74.7% (221/296), and the patient compliance rate is 39.0% (67/172).14 In addition, we asked those 221 patients a question about whether the tips we provided on the app can remind them to take medicine more correctly. About 92.8% of patients said that they would read the tips on the app before they took medicine. These tips really reminded them to correctly take medicine.
Fig. 7.

A nurse is educating a patient to use ThyroidKeeper app
Limitations: In this preliminary study, we did not design a formal questionnaire to collect patients’ subjective evaluations of the software. In addition, since the evaluation was not a clinical trail, for those patients who have used automated medication dosage adjustment, we have not confirmed whether they were taking the medicine according to the dosage and medication method recommended by the software, because their dosage was still eventually determined by doctors. We currently only objectively reflect some facts based on the usage of the software. We will conduct in-depth research on these issues in the future.
Evaluation of automated adjustment of medication dosage
Setup
TSH-suppressive therapy is applied as part of the standardized treatment and long-term management for differentiated thyroid cancer. Although most thyroid surgeons or physicians adjust the THS suppression medication dosage according to relevant guidelines and the clinical characteristics of patients, individual differences of patients and experience of doctors will still lead to some differences in the dosage of medication given by different doctors for the same patient. ThyroidKeeper’s scheme is based on the summary of most clinical practices in the author’s department. It is worth mentioning that for experienced doctors, the differences between them usually do not have a significant impact on the recovery and health of patients. Because for individuals, how much dosage they need to take to suppress TSH to a certain extent cannot be precisely calculated by some rules (the experience of doctors still plays a critical role in the assessment). This is why patients need to adhere to follow-up.
To evaluate the accuracy of the automated medication dosage adjustment, we adopted a combination of subjective and objective methods. First, we selected 1000 patients with the six types of thyroid diseases mentioned above, and the proportion of patients with each type of disease is roughly the same. All patients had a follow-up record of at least 1 year. We started with their initial doses, extracted the subsequent adjusted doses from their follow-up records, and used these adjusted doses as the Ground Truths. Then, we used ThyroidKeeper to calculate the doses for these 1000 patients and compared the values obtained by the software with the ground truths. For the each recommended medication dose, if it was equal to the ground truth, then it was considered Correct. If they were not equal, then we needed to consult a clinician (the clinician being consulted was not involved in the development of ThyroidKeeper and did not know its existence). If the clinician approves the recommended medication dose, then it was considered Acceptable, otherwise, it was recorded as Unacceptable.
The automated medication dosage adjustment is based on the judgment of the DTC Recurrence Risk and the Side Effect Risk of TSH Suppression. However, the judgment of these two risks partially depends on the clinical experiences of doctors. However, the judgment of these two risks partially depends on the clinical experiences of doctors. Our system summarizes complex conditions so that patients can make their own decisions. We still used the judgment results of the above 221 patients who participated in the evaluation. We sent the medical records of these 221 patients to three clinicians and asked them to make judgments on the levels of these two risks. We used majority voting to make a consensus among clinicians. Finally, we compared the patients’ judgment and the clinicians’ judgment.
-
(2)
Results and discussion
We performed a total of 3153 dose adjustment calculations. Among them, 2245 calculations (71.2%) were marked as Correct, and 612 calculations (19.4%) were marked as Acceptable. Therefore, it can be considered that the acceptable rate of the medication dosage adjustment output provided by ThyroidKeeper is 90.6%. According to the author’s experience, this acceptance rate has reached the acceptance rate of recommendations provided by general clinicians (not domain experts). This shows that it is quite reasonable for patients to rely on the recommended dosage provided by the software to take medication.
We analyzed 221 patients’ judgments of two risk levels and compared them with clinicians’ judgments. For the Side Effect Risk of TSH Suppression, the patient judgment accuracy for the low level is 83.6%, for the medium level is 94.4%, and for the high level is 97.4%. For the DTC Recurrence Risk, the patient judgment accuracy for the low level is 73.5%, for the medium level is 91.6%, and for the high level is 95.0%. We found that patients’ judgments of the two risk levels followed the same pattern. First, the accuracy of judging medium and high risks is very high (more than 90%). In particular, the judgment of high risk is higher than 95%. They did not judge medium and high risks as low risk. Second, since low risk needs to meet all conditions, the judgment of low risk is less accurate. There are some low-risk instances that are classified as medium or high risk. Therefore, our design will induce a more conservative judgment, which is more reasonable for self-monitoring of the condition. Finally, compared with the Side Effect Risk of TSH Suppression, it is more difficult for the patient to judge the DTC Recurrence Risk because it relies on more professional knowledge.
Limitations: At present, we have only conducted a preliminary assessment of the correctness and acceptability of the automated dosage adjustment of ThyroidKeeper based on historical data and the experience of clinicians. The effectiveness and safety of the software require further formal clinical trials to obtain reliable and multi-dimensional conclusions. In addition, the evaluation of Acceptable dose recommendations needs to be investigated continuously. Will these Acceptable dose recommendations cause cumulative errors? Can these cumulative errors be automatically corrected in subsequent calculations? Will these accumulated errors have a negative impact on patients? These issues need to be evaluated by more rigorous clinical trials.
Evaluation of diagnosis prediction
We use objective methods to evaluate the performance of our HPEMed model.
Setup
Currently, the 1000 patients that we processed include six different types of thyroid diseases. Because every patient in the dataset has a confirmed diagnostic conclusion we use the diagnostic types of the disease as the prediction objective. We extract 30% instances from every types of patients to form the test set, which has 300 instances in total. We also generate 200 normal cases (i.e., who has no thyroid diseases) and add a half of them into the training set and the remainder into the test set. Finally, the training set and test set have 800 and 400 instances with seven disease types, respectively.
In the HPEMed network, node types can be designed to have different granularities. For example, if demographic features of a patient are treated as node type (the node will have multiple fields), there will be a large number of nodes with this type in the network because every patient may have something different from the others. The nodes also have redundancy information such as gender and age group. Therefore, some information can be treated as an independent node type. For example, gender will be a node type, which results in only two nodes with type gender in the network, standing for male and female. Similarly, when the age group is a node type, there are several nodes with the type age_group in the network. Splitting too many node types will increase the complexity of the network. Therefore, we must balance information redundancy and node types. In our experiment, the node types include basic_info, comorbidity, gender, age_group, symptom, laboratory_test, tnm_info, ultrasound_test, and surgery_info.
We compare our HPEMed model with three state-of-the-art models that are popular in medical diagnosis prediction:
Med2Vec [45]: A multi-layer embedding neural network that uses a method inspired by word2vec to learn the embedding of medical events and visits.
Skip-gram [53]: In this model, all clinical events of patients are treated as words and connected into sentences. Skip-gram is able to capture the subtle relationships between words.
HeteroMed [49]: This method uses the unsupervised representation learning to learn node embeddings as the former methods, and then applies supervised diagnosis model to rank the diagnosis code.
-
(2)
Results and discussion
Since our system focuses on the health management of postoperative patients, rather than pre-operative pre-diagnosis as the goal. That is, we are concerned about the accuracy of various diagnostic predictions. In other words, we hope that the system can make accurate judgments about the patient’s condition. Therefore, we use accuracy as an evaluation metric. At the same time, in addition to the overall accuracy, we pay more attention to the prediction accuracy of those malignant tumors, because the cost of misdiagnosis of malignant tumors is much higher than that of benign nodules and common thyroid diseases. In our system, the types of malignant diseases include thyroid papillary cancer, thyroid vesicular cancer, medullary thyroid cancer, and undifferentiated cancer. The other two (benign thyroid tumor and non-operation-related hypothyroidism) are non-malignant diseases. Table 3 shows the overall accuracy and the accuracy of the malignant and non-malignant diseases of the models in comparison.
Table 3.
Comparison results of different models in terms of accuracy on different sub-test sets
| Accuracy | Med2Vec | Skip-gram | HeteroMed | HPEMed (ours) |
|---|---|---|---|---|
| Overall | 0.79 | 0.77 | 0.80 | 0.86 |
| Malignant | 0.82 | 0.84 | 0.81 | 0.92 |
| Non-malignant | 0.76 | 0.72 | 0.78 | 0.81 |
For the overall accuracy, our proposed HPEMed model is 6 percentage points higher than the best existing model (HeteroMed). The main difference between our model and the HeteroMed model is that we embed the diagnosis and the patient in pairs. It can be seen that this paired embedding tightly binds the diagnosis and the patient, which significantly improves the overall accuracy of the model. Furthermore, it also indicates that heterogeneous networks (HeteroMed and HPEMed) are more advantageous than homogeneous models (Med2Vec and Skip-gram) in diagnosis modeling.
For the four models compared, their accuracies on malignant diseases are obviously higher than their accuracies on non-malignant diseases. On one hand, this shows that compared with those of non-malignant diseases, malignant diseases usually have more obvious indications. On the other hand, it shows that the graph neural network model does have advantages in the effectiveness of using sample information for modeling. Clinically, a change in an indicator that deviates from the normal range does not necessarily mean that the patient has a serious disease (usually malignant). However, if the indicator deviates too much from the normal range, or if multiple indicators deviate significantly from their normal ranges at the same time, it often indicates a bad outcome. The sensitivity of our proposed model for modeling malignant diseases is greatly improved than existing methods. In addition, we must point out that in our evaluation, in order to test the system in an all-round way, we set the number of each type of patients to be approximately equal. However, in the real world, the prevalence of diseases in different types is usually significantly different. For example, the population of thyroid papillary cancer is much higher than the that of undifferentiated thyroid cancer. This is the problem of imbalanced learning [56]. As the real patients accumulate in the system, we will continue to study this issue in the context of heterogeneous information networks in the future.
Conclusions
Patients with thyroid disease (especially tumors) usually require lifelong medication and regular follow-up. In order to reduce the frequency of patients going to tertiary hospitals as well as reduce the workload of doctors in tertiary hospitals to follow-up patients. We designed and developed a thyroid disease healthcare management system ThyroidKeeper. Thyroid has three main functions: automatic medication dosage adjustment, complication prediction with heath intervention suggestions, and online communication between doctors and patients. We have started to roll out and used this system in some hospital. Judging from the objective feedback collected so far, the ThyroidKeeper is able to benefit both patients and doctors. In the future, we will continue to improve the function of the system, and conduct formal clinical trials for the system to comprehensively evaluate its role in promoting the health of patients with thyroid disease.
Acknowlegements
This work was sponsored by the National Natural Science Foundation of China (Grant Nos. 62076130 and 91846104), the Start-up Research Fund of Southeast University (Grant No. RF1028623059), the Fundamental Research Funds for the Central Universities (Grant No. 2242023K30034), and the Hospital Research Fund of the Second Affiliated Hospital of Nanjing University of Chinese Medicine (Grant Nos. SEZ202121 and SEZJY2023018).
Declarations
Conflict of interest
No conflict of interest exists for all participating authors.
Footnotes
Although the terms mHealth and telemedicine overlap in content, they emphasize different points: mHealth emphasizes that the medium for obtaining and managing health resources is mobile devices, while telemedicine emphasizes the participation of professional medical staff in the process (mHealth does not necessarily require the participation of medical staff). In this paper, we use both terms depending on the context.
http://ai.baidu.com/tech/ocr (In Chinese)
https://github.com/wildfirechat/ (In Chinese)
For those undifferentiated cancers that are in advanced stages, surgical treatment may have been ineffective, and other therapies such as radiation therapy might be chosen.
The T refers to the size and extent of the main tumor. The main tumor is usually called the primary tumor. The N refers to the the number of nearby lymph nodes that have cancer. The M refers to whether the cancer has metastasized. This means that the cancer has spread from the primary tumor to other parts of the body.
https://www.umeng.com/ (In Chinese)
https://ai.baidu.com/easydl/ocr/ (In Chinese)
We did not conducted rigorous clinical trials. Therefore, for those patients who used the software to adjust the medication dosage, their doctors eventually contacted them to determine the medication dosage.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zhang and Jianhua Li contributed equally to this work.
References
- 1.Vrachimis A, Iakovou I, Giannoula E, et al. Endocrinology in the time of COVID-19: management of thyroid nodules and cancer. Eur J Endocrinol. 2020;183:G41–G48. doi: 10.1530/EJE-20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scappaticcio L, Pitoia F, Esposito K, et al. Impact of COVID-19 on the thyroid gland: an update. Rev Endocrin Metabol Disorders. 2020;1–13. [DOI] [PMC free article] [PubMed]
- 3.Zhang D, Fu Y, Zhou L, et al. Thyroid surgery during coronavirus-19 pandemic phases I, II and III: lessons learned in China, South Korea, Iran and Italy. J Endocrinol Invest. 2021;44:1065–1073. doi: 10.1007/s40618-020-01407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisco G, Tullio AD, Jirillo E, et al. Thyroid and COVID-19: a review on pathophysiological, clinical and organizational aspects. J Endocrinol Invest. 2021;1–14. [DOI] [PMC free article] [PubMed]
- 5.Li X, Krumholz HM, Yip W, et al. Quality of primary health care in china: challenges and recommendations. Lancet. 2020;395:1802–1812. doi: 10.1016/S0140-6736(20)30122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Shi L, Liang H, et al. Urban-rural disparities in health care utilization among Chinese adults from 1993 to 2011. BMC Health Serv Res. 2018;18:1–9. doi: 10.1186/s12913-018-2905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klain M, Nappi C, Maurea S, et al. Management of differentiated thyroid cancer through nuclear medicine facilities during COVID-19 emergency: the telemedicine challenge. Eur J Nucl Med Mol Imaging. 2021;48:831–836. doi: 10.1007/s00259-020-05041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borzouei S, Mahjub H, Sajadi NA, et al. Diagnosing thyroid disorders: comparison of logistic regression and neural network models. J Family Med Primary Care. 2020;9:1470–1476. doi: 10.4103/jfmpc.jfmpc_910_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaubey G, Bisen D, Arjaria S, et al. Thyroid disease prediction using machine learning approaches. Natl Acad Sci Lett. 2021;43:233–238. doi: 10.1007/s40009-020-00979-z. [DOI] [Google Scholar]
- 10.Liu D, Chen HL, Yang B, et al. Design of an enhanced fuzzy k-nearest neighbor classifier based computer aided diagnostic system for thyroid disease. J Med Syst. 2011;36:3243–3254. doi: 10.1007/s10916-011-9815-x. [DOI] [PubMed] [Google Scholar]
- 11.Temurtas F. A comparative study on thyroid disease diagnosis using neural networks. Expert Syst Appl. 2009;36:944–949. doi: 10.1016/j.eswa.2007.10.010. [DOI] [Google Scholar]
- 12.Azar AT, Hassanien AE, hoon Kim T. Expert system based on neural-fuzzy rules for thyroid diseases diagnosis. In: Computer applications for bio-technology, multimedia, and ubiquitous city, 2012;94–105.
- 13.Chen HL, Yang B, Wang G, et al. A three-stage expert system based on support vector machines for thyroid disease diagnosis. J Med Syst. 2011;36:1953–1963. doi: 10.1007/s10916-011-9655-8. [DOI] [PubMed] [Google Scholar]
- 14.Bibi Amina B, Parkavi A . Prediction of thyroid disease using data mining techniques. In: The 5th international conference on advanced computing and communication systems, 2019;342–5.
- 15.Sim JZ, Zang Y, Nguyen PV, et al. Thyroid-spot for mobile devices: personalised thyroid treatment management app. Sci Phone Apps Mob Devices. 2017;3:1–5. [Google Scholar]
- 16.Tarakčija A, Terzić V, Vardo A, et al . Development of a diagnostic support software in the clinicobiochemical evaluation of thyroid disease diagnosis. In: Proceedings of the international conference on medical and biological engineering, 2019;475–80.
- 17.Zhang B, Tian J, Pei S, et al. Machine learning-assisted system for thyroid nodule diagnosis. Thyroid. 2019;29:858–867. doi: 10.1089/thy.2018.0380. [DOI] [PubMed] [Google Scholar]
- 18.Anand V, Koundal D . Computer-assisted diagnosis of thyroid cancer using medical images: a survey. In: Proceedings of the 2nd international conference on recent innovations in computing, 2020;543–59.
- 19.McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database System Rev. 2017;5. [DOI] [PMC free article] [PubMed]
- 20.Anastasiadou D, Folkvord F, Serrano-Troncoso E, et al. Mobile health adoption in mental health: user experience of a mobile health app for patients with an eating disorder. JMIR Mhealth Uhealth. 2019;7:e12920. doi: 10.2196/12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martos-Cabrera MB, Velando-Soriano A, Pradas-Hernández L, et al. Smartphones and apps to control glycosylated hemoglobin (hba1c) level in diabetes: a systematic review and meta-analysis. J Clin Med. 2020;9:693. doi: 10.3390/jcm9030693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannoula E, Iakovou I, Katsikavelas I, et al. A mobile app for thyroid cancer patients aiming to enhance their quality of life: protocol for a quasi experimental interventional pilot study. JMIR Res Protocols. 2020;9:e13409. doi: 10.2196/13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabor VH, Tabor MH, Keestra S, et al. Improving the quality of life of patients with an underactive thyroid through mhealth: a patient-centered approach. Women’s Health Reports. 2021;2:182–194. doi: 10.1089/whr.2021.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng L, Zhao Y, Xu X. Wechat app in the follow up of thyroid cancer patients after thyroidectomy during the COVID-19 pandemic. Br J Surg. 2020;107:e533. doi: 10.1002/bjs.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han SX, Eisenberg MC, Larsen P, et al .: Thyrosim app for education and research predicts potential health risks of over-the-counter thyroid supplements. Thyroid. 2016; 26:489–98. [DOI] [PubMed]
- 26.Lee J, Yoon W, Kim S, et al. Biobert: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234–1240. doi: 10.1093/bioinformatics/btz682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin J, Chang MW, Lee K, et al. Bert: pre-training of deep bidirectional transformers for language understanding. In: Conference of the North American chapter of the association for computational linguistics (NAACL), 2019.
- 28.Wei Q, Ji Z, Si Y, et al. Relation extraction from clinical narratives using pre-trained language models. AMIA Annu Symp Proc. 2019;1236–45. [PMC free article] [PubMed]
- 29.Karami A, Gangopadhyay A, Zhou B, et al. Fuzzy approach topic discovery in health and medical corpora. Int J Fuzzy Syst. 2018;20:1334–1345. doi: 10.1007/s40815-017-0327-9. [DOI] [Google Scholar]
- 30.Higashinaka R, Funakoshi K, Kobayashi Y, et al. The dialogue breakdown detection challenge: task description, datasets, and evaluation metrics. In: Proceedings of the 10th international conference on language resources and evaluation, 2016;3146–50.
- 31.Kepner J, Arcand W, David Bestor BB, et al. Achieving 100,000,000 database inserts per second using accumulo and d4m. In: 2014 IEEE high performance extreme computing conference (HPEC), 2014;1–6.
- 32.Gan T, Huang B, Chen Q, et al . Risk of recurrence in differentiated thyroid cancer: a population-based comparison of the 7th and 8th editions of the American joint committee on cancer staging systems. Ann Surg Oncol. 2019;1–8 [DOI] [PMC free article] [PubMed]
- 33.Kim TY, Kim WG, Kim WB, et al. Current status and future perspectives in differentiated thyroid cancer. Endocrinol Metab. 2014;29:217–225. doi: 10.3803/EnM.2014.29.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luster M, Weber T, Verburg FA. Differentiated thyroid cancer-personalized therapies to prevent overtreatment. Nat Rev Endocrinol. 2014;10:563–574. doi: 10.1038/nrendo.2014.100. [DOI] [PubMed] [Google Scholar]
- 35.Leite V. The importance of the 2015 American thyroid association guidelines for adults with thyroid nodules and differentiated thyroid cancer in minimising over diagnosis and overtreatment of thyroid carcinoma. Eur Endocrinol. 2018;14:13–14. doi: 10.17925/EE.2018.14.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elnemr HA, Zayed NM, Fakhreldein MA. Feature extraction techniques: fundamental concepts and survey. In: Handbook of research on emerging perspectives in intelligent pattern recognition, analysis, and image processing. 2016;264–94.
- 37.Cai J, Luo J, Wang S, et al. Feature selection in machine learning: a new perspective. Neurocomputing. 2018;300:70–79. doi: 10.1016/j.neucom.2017.11.077. [DOI] [Google Scholar]
- 38.Kusiak A. Feature transformation methods in data mining. IEEE Trans Electron Packag Manuf. 2001;24:214–221. doi: 10.1109/6104.956807. [DOI] [Google Scholar]
- 39.Sun J, Wang F, Hu J, et al. Supervised patient similarity measure of heterogeneous patient records. SIGKDD Exploration. 2012;14:16–24. doi: 10.1145/2408736.2408740. [DOI] [Google Scholar]
- 40.Ghassemi M, Naumann T, Doshi-Velez F, et al. Unfolding physiological state: mortality modelling in intensive care units.2014;75–84 [DOI] [PMC free article] [PubMed]
- 41.Wang Y, Ng K, Byrd RJ, et al. Early detection of heart failure with varying prediction windows by structured and unstructured data in electronic health records. 2015;2530–3 [DOI] [PMC free article] [PubMed]
- 42.Wu Z, Pan S, Chen F, et al. A comprehensive survey on graph neural networks. IEEE Trans Neural Netw Learn Syst. 2019;32:4–24. doi: 10.1109/TNNLS.2020.2978386. [DOI] [PubMed] [Google Scholar]
- 43.Choi E, Bahadori MT, Sun J, et al. Retain: an interpretable predictive model for healthcare using reverse time attention mechanism. In: NIPS 2016.
- 44.Ma F, Chitta R, Zhou J, et al. Dipole: diagnosis prediction in healthcare via attention-based bidirectional recurrent neural networks.2017;1903–11.
- 45.Choi E, Bahadori MT, Searles E, et al . Multi-layer representation learning for medical concepts.2016;1495–504.
- 46.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493–1499. doi: 10.1001/archinte.165.13.1493. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Sun Y, Yan X, et al . Mining knowledge from databases: an information network analysis approach. In: Proceedings of the 2010 ACM SIGMOD international conference on management of data, 2010;1251–2.
- 48.Dong Y, Chawla N, Swami A . metapath2vec: scalable representation learning for heterogeneous networks. In: Proceedings of the 23rd ACM SIGKDD international conference on knowledge discovery and data mining, 2017;135–44.
- 49.Hosseini A, Chen T, Wu W, et al. Heteromed: Heterogeneous information network for medical diagnosis. In: Proceedings of the 27th ACM international conference on information and knowledge management, 2018; 763–72.
- 50.Chang S, Han W, Tang J, et al . Heterogeneous network embedding via deep architectures. In: Proceedings of the 21th ACM SIGKDD international conference on knowledge discovery and data mining, 2015;119–28.
- 51.Shang J, Liu J, Jiang M, et al. Automated phrase mining from massive text corpora. IEEE Trans Knowl Data Eng. 2018;30:1825–1837. doi: 10.1109/TKDE.2018.2812203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grover A, Leskovec J . node2vec: Scalable feature learning for networks. Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining, 2016;855–64. [DOI] [PMC free article] [PubMed]
- 53.Mikolov T, Sutskever I, Chen K, et al.: Distributed representations of words and phrases and their compositionality. In: NIPS, 2013;3111–9.
- 54.Srivastava N, Hinton GE, Krizhevsky A, et al. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res. 2014;15:1929–1958. [Google Scholar]
- 55.Zhou P, Shi W, Tian J, et al . Attention-based bidirectional long short-term memory networks for relation classification. In: Proceedings of the 54th annual meeting of the association for computational linguistics, 2016;207–12.
- 56.Krawczyk B. Learning from imbalanced data: open challenges and future directions. Progress Artif Intell. 2016;5:221–232. doi: 10.1007/s13748-016-0094-0. [DOI] [Google Scholar]