Abstract
The antichlamydial effects of several fatty acids and monoglycerides were studied by incubating Chlamydia trachomatis bacteria with equal volumes of lipid solutions for 10 min and measuring the reduction in infectivity titer compared with that in a control solution without lipid. Caprylic acid (8:0), monocaprylin (8:0), monolaurin (12:0), myristic acid (14:0), palmitoleic acid (16:1), monopalmitolein (16:1), oleic acid (18:1), and monoolein (18:1) at concentrations of 20 mM (final concentration, 10 mM) had negligible effects on C. trachomatis. In contrast, lauric acid (12:0), capric acid (10:0), and monocaprin (10:0) caused a greater than 10,000-fold (>4-log10) reduction in the infectivity titer. When the fatty acids and monoglycerides were further compared at lower concentrations and with shorter exposure times, lauric acid was more active than capric acid and monocaprin was the most active, causing a greater than 100,000-fold (>5-log10) inactivation of C. trachomatis at a concentration of 5 mM for 5 min. The high levels of activity of capric and lauric acids and particularly that of monocaprin are notable and suggest that these lipids have specific antichlamydial effects. The mode of action of monocaprin was further studied by removal of the lipid by centrifugation before inoculation of Chlamydia onto host cells and by electron microscopy. The results indicate that the bacteria are killed by the lipid, possibly by disrupting the membrane(s) of the elementary bodies. A 50% effective concentration of 30 μg/ml was found by incubation of Chlamydia with monocaprin for 2 h. The rapid inactivation of large numbers of C. trachomatis organisms by monocaprin suggests that it may be useful as a microbicidal agent for the prevention of the sexual transmission of C. trachomatis.
Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen. Annually, there are an estimated 50 million new cases of C. trachomatis infection worldwide (4), with more than 4 million occurring in the United States and 3 million occurring in Europe (2). Although they are treatable with antibiotics, many Chlamydia infections go undetected, particularly in women, and can cause severe permanent damage to the female genital tract, which may lead to infertility (18). A vaccine against C. trachomatis has not been developed, and other means of prevention except for the use of condoms are not available. In recent years there has been considerable interest in the use of microbicidal compounds for the prevention of sexually transmitted diseases (STDs) (3). Vaginal spermicides, which have been used as contraceptives for a number of years, have been shown to kill sexually transmissible bacteria and viruses in vitro and in vivo (13). The microbicidal activity of the nonionic surfactant nonoxynol-9 has been studied most extensively. Two studies found that nonoxynol-9 inactivates C. trachomatis in vitro (1, 9), whereas one study found that it did not (8). There is evidence from clinical trials that it may have some effect against chlamydial infections in vivo (12). However, because of the toxicity of nonoxynol-9 to mucosal membranes, particularly when it is applied frequently, there is a need for other less toxic microbicidal compounds which could be used to provide protection against STDs (3).
The microbicidal effects of a variety of lipids have been extensively studied in recent years. A number of free fatty acids and their 1-monoglycerides have been found to kill enveloped viruses and various bacteria, both gram-negative and gram-positive bacteria (5, 7, 14, 15, 17). These lipids are commonly found in natural products, for example, in milk, and can therefore be assumed to be nontoxic to mucosas, at least at low concentrations. It has therefore been suggested that they might be useful as intravaginal microbicides for protection against STDs (6). In order to prevent infections caused by sexually transmitted viruses, it is important that the microbicidal lipid is fast acting and kills the virus before it has time to infect cells of the genital mucosa. The same is true for bacteria such as Chlamydia, which, like viruses, replicate intracellularly. In the present study several fatty acids and their 1-monoglycerides which have previously been found to inactivate enveloped viruses (15) were tested for their microbicidal activities against C. trachomatis. A short inactivation time of 10 min or less was selected as a criterion for a fast and effective killing of the bacteria.
MATERIALS AND METHODS
Cell culture.
Monolayer cultures of McCoy cells, a heteroploid mouse fibroblast cell line, were used for cultivation of C. trachomatis and in antichlamydial assays. They were grown in RPMI 1640 medium (GIBCO) containing 5% (vol/vol) heat-inactivated fetal calf serum, 45 mM sodium bicarbonate, 2 mM l-glutamine, and 0.05 mg of gentamicin per ml. This was called the base medium (BM).
The cell cultures were maintained by weekly trypsinization and passage in 260-ml tissue culture flasks (Nunclon). The cultures were passaged in the following manner. The medium was removed and the monolayer was rinsed twice with 10 ml of Hanks’ balanced salt solution (GIBCO). The monolayer was covered with 1 ml of trypsin-EDTA solution (GIBCO) and was carefully shaken until the cells came into suspension. The trypsin activity was then stopped by adding 10 ml of BM, and the cells were evenly suspended by pipetting. They were counted, and 107 cells in 20 ml of BM were seeded into each flask. Cell cultures were kept at 37°C in a humidified incubator with 5% CO2 in air.
Chlamydial stock cultures.
C. trachomatis serotype K, originally isolated from a human cervix, was obtained from the American Type Culture Collection (strain VR 887). The concentration of bacteria used in the experiments varied from 106.5 to 107.8 inclusion-forming units (IFU) per ml. Two strains of C. trachomatis were recent clinical isolates. One was isolated from a cervical swab, and the other was isolated from a urethral swab from a male patient. They were subcultured once in McCoy cells and were used at concentrations of 105.1 and 105.4 IFU per ml, respectively. All bacterial stocks were kept frozen at −70°C in a mixture of equal volumes of cycloheximide medium (CM) and transport medium. The latter is composed of 0.2 M sucrose buffer supplemented with 10% inactivated fetal calf serum.
Lipids.
Fatty acids and 1-monoglycerides were purchased from Sigma Chemical Co., St. Louis, Mo. (purest grade). Stock solutions of 1 M were made in ethanol.
Assay of antichlamydial activity.
Twenty-four-well (16 mm in diameter) tissue culture plates (Corning, Corning, N.Y.) were seeded with 6 × 105 McCoy cells per well in 2 ml of BM. Twenty-four hours later, when the cells had formed a confluent monolayer, the medium was changed to 1 ml of maintenance medium (MM), which is BM supplemented with 0.5% (wt/vol) glucose. Stock solutions of fatty acids or monoglycerides were diluted to the desired concentration by vortexing at the highest speed for 1 min at 37°C. The solutions showed a little turbidity which varied between lipids but which was less for lipids with shorter fatty acid chains. The solutions were immediately tested against C. trachomatis by thoroughly mixing together 100 μl of a lipid solution and 100 μl of the bacterial stock in a plastic tube. The mixtures were incubated for the desired time at 37°C. Samples were removed and diluted in MM in 10-fold dilutions, and 0.2 ml of each dilution was inoculated into culture plates with McCoy cells (four wells for each dilution). Bacteria mixed with MM alone and with 2% ethanol in MM were used as controls. The plates were centrifuged at 1,100 × g for 75 min at 35°C and were then incubated for 2 h at 37°C in an atmosphere of 5% CO2. Finally, the medium was replaced with 1 ml of MM containing 2 μg of cycloheximide per ml (CM) to inhibit protein synthesis of the host cells, and the incubation was continued for an additional 72 h. The CM was then removed from each well and the monolayers were fixed with 96% ethanol and stained with iodine-glycerol stain for 10 min. Brown inclusions filled with Chlamydia could be distinguished in the cytoplasms of infected cells when the cells were viewed under a microscope.
In a few experiments inclusions were stained with fluorescein-labeled monoclonal antibodies specific for C. trachomatis. A C. trachomatis culture confirmation test (Syva Microtrak; Syva Company, San Jose, Calif.) was performed by the procedure described by the manufacturer.
Inclusions were counted in all four wells of the dilution which contained 10 to 50 inclusions per well. The average number of inclusions in that dilution was evaluated, and the number of IFU per milliliter of undiluted sample was calculated. The number (log10) of IFU in the lipid-treated sample was subtracted from the number (log10) of IFU in the control sample, and the difference was used as a measure of antichlamydial activity, i.e., the ratio of bacteria inactivated by incubation with a lipid solution for a given length of time at 37°C.
Because of toxicity to cell cultures, lipid-bacterium mixtures could not be tested undiluted or at a dilution of 10−1, depending on the lipid or the lipid concentration. In such lipid-bacterium mixtures in which no inclusions were detectable in the 10−1 dilution, it was assumed that the undiluted mixture contained ≤45 IFU per ml, i.e., ≤1.7 log10 IFU. Similarly, when no inclusions were detectable in the 10−2 dilution, the undiluted mixture might contain ≤450 IFU per ml, or ≤2.7 log10 IFU. The antichlamydial activity was therefore calculated by subtracting 1.7 or 2.7, respectively, from the log10 number of IFU for the control sample, and the numbers of IFU expressed the activity as being equal to or greater than this difference.
Washing of monocaprin-treated Chlamydia.
One milliliter of bacterial stock was diluted fivefold with antibiotic-free BM (a-BM) and was pelleted by centrifugation at 2,000 × g in a Sorvall RT 6000D centrifuge at 35°C for 30 min, and the bacterial pellet was resuspended in 1 ml of a-BM. The bacteria were mixed with an equal volume (200 μl) of 10 mM monocaprin in a-BM, and the mixture was incubated at 37°C for both 5 and 10 min. Bacteria incubated in the same way with a-BM without lipid served as a control. After incubation each mixture was diluted 10-fold and the dilution was divided into two samples. One sample was kept at 35°C for the duration of the experiment, whereas the bacteria in the other sample were washed twice by centrifugation at 2,000 × g at 35°C for 30 min and the pellet was resuspended in 2 ml of a-BM. The infectivity titers of all the samples were then determined by inoculation of 10-fold dilutions onto McCoy cells in 24-well culture plates as described above.
Electron microscopy.
A suspension of C. trachomatis was mixed with an equal volume of 10 mM monocaprin in MM, and the mixture was incubated at 37°C. Samples were removed after 1, 5, and 10 min and were immediately diluted 1:10 in MM. Chlamydiae incubated for 10 min with an equal volume of MM without monocaprin were diluted 1:10 and were used as a control. Three hundred microliters of each sample was pipetted into two wells of a 96-well microtiter plate, and the plates were centrifuged at 1,100 × g for 60 min at 35°C so that the samples adhered to Formvar-coated 300-mesh copper grids which had been placed on the bottoms of the wells. After centrifugation the grids were removed, and the bacteria were negatively stained with 1% phosphotungstic acid (pH 7.0) and examined in Philips 300 transmission electron microscope at 80 kV.
Assay of the MIC of monocaprin.
Ninety-six-well microtiter plates (Nunclon) were seeded with 105 McCoy cells per well in 100 μl of BM. Twenty-four hours later the cells had formed a confluent monolayer and were ready for inoculation with the Chlamydia. Monocaprin was diluted at 37°C by vortexing at a high speed so that after the addition of the chlamydial culture at a final concentration of 104 to 105 IFU per ml monocaprin concentrations of 2.5 to 2,500 μg/ml were achieved. The dilutions were tested against the bacteria by thoroughly mixing together 200 μl of a monocaprin dilution and 200 μl of the bacterial suspension. After 2 h of incubation at 37°C, the monocaprin concentrations were reduced by a 100-fold dilution in MM. Then, 200 μl of each diluted mixture was inoculated into six wells of a microtiter plate with McCoy cells. Bacteria mixed with MM served as a control. The plates were centrifuged at 1,100 × g for 75 min at 35°C and were incubated for 2 h at 37°C in an atmosphere of 5% CO2. The medium was then replaced with 200 μl of CM. The cultures were incubated for 72 h before staining with iodine, and the numbers of IFU were counted as described above. The same concentrations (2.5 to 2,500 μg/ml) of monocaprin were tested for cytotoxicity with monolayers of McCoy cells. Cell viability was determined after 2 h at 37°C by trypan blue exclusion.
RESULTS
Activities of fatty acids.
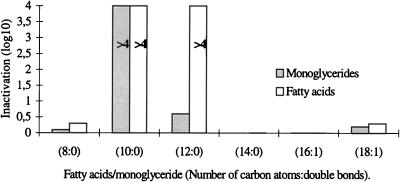
A comparison of the antichlamydial activities of six fatty acids is presented in Fig. 1. The open bars represent the reduction in infectivity titers (log10 IFU) of C. trachomatis after incubation with an equal volume of 20 mM fatty acid for 10 min at 37°C. Caprylic acid (8:0), myristic acid (14:0), and the unsaturated fatty acids palmitoleic (16:1) and oleic acids (18:1) did not cause a significant inactivation of the bacteria, with the reduction in titer varying from zero to 0.3 log10. In contrast, capric acid (10:0) and lauric acid (12:0) reduced the titer by greater than 10,000-fold (≥4 log10).
FIG. 1.
Inactivation of C. trachomatis by incubation with equal volumes of 20 mM fatty acids and monoglycerides for 10 min at 37°C. The bars represent the levels of reduction of the log10 numbers of IFU. The levels of inactivation by capric acid and monocaprin (10:0) and by lauric acid (12:0) are greater than those indicated by the bars (>4 log10). Two percent ethanol had no effect on the infectivity titer.
To compare the activities of capric and lauric acids, they were tested at lower concentrations and with shorter incubation times. The data in Table 1 indicate that capric acid lost most of its activity when it was diluted to a concentration of 10 mM, whereas lauric acid was still very active at that concentration, causing a 500,000-fold reduction in titer (5.7 log10). Lauric acid was therefore more active than capric acid against C. trachomatis. This was confirmed by testing lauric acid at 5 mM, which caused a 2,000-fold (3.3 log10) reduction in infectivity titer. Both acids were active at 20 mM for 5 min, but incubation for 1 min had no effect.
TABLE 1.
Antichlamydial activities of fatty acids
| Fatty acid | Concn (mM)a | Reduction of IFU (log10) at the following times (min):
|
||
|---|---|---|---|---|
| 10 | 5 | 1 | ||
| Capric acid (10:0)b | 20 | ≥5.9c | ≥5.9c | 0.9 |
| 10 | 1.3 | NDd | ND | |
| Lauric acid (12:0) | 20 | ≥4.2e | ≥4.2e | 0.2 |
| 10 | 5.7 | 2.9 | ND | |
| 5 | 3.3 | 0.7 | ND | |
Concentration of fatty acids mixed with C. trachomatis and incubated at 37°C for 1, 5, and 10 min. In the mixtures the concentration was reduced by half due to 1:1 dilution with the bacteria.
Number of carbon atoms:number of double bonds.
No inclusions were detectable in the 10−1 dilution, which was the lowest dilution tested because of toxicity to cell cultures.
ND, not done.
No inclusions were detectable in the 10−2 dilution, which was the lowest dilution tested due to cytotoxicity.
Activities of monoglycerides.
The antichlamydial activities of five 1-monoglycerides were tested, with monomyristin (14:0) being omitted. The results are presented in Fig. 1. Monocaprylin (8:0), monopalmitolein (16:1), and monoolein (18:1) had no significant effect, and monolaurin (12:0) caused only a threefold reduction in titer (0.5 log10). Monocaprin, on the other hand, caused 40,000-fold or greater reductions in the infectivity titer of C. trachomatis (≥4.6 log10). The high level of antichlamydial activity of monocaprin was confirmed by lowering the concentration to 10 and 5 mM and the incubation time to 5 min. As indicated in Table 2, monocaprin was still active at 5 mM with an incubation time of 5 min, causing a greater than 100,000-fold reduction in the infectivity titer (≥5.1 log10). The high level of antichlamydial activity of monocaprin was further confirmed by staining with fluorescein-labeled monoclonal antibodies specific for C. trachomatis. Monocaprin-treated Chlamydia samples showed no inclusions at the lowest dilution tested (10−1), whereas control samples without monocaprin showed fluorescent Chlamydia inclusions at a dilution of 10−6 and had a final infectivity titer of 107.3 IFU per ml. The reduction in titer was therefore 400,000-fold or greater (≥5.6 log10). In order to show that the antichlamydial activity of monocaprin was not limited to the one bacterial strain obtained from the American Type Culture Collection, two C. trachomatis strains recently isolated from patients were also tested. Both strains were inactivated by monocaprin at a concentration of 10 mM and incubation for 5 min, with the reductions in titer being ≥3.4 and ≥3.7 log10, respectively.
TABLE 2.
Antichlamydial activities of monoglycerides
| Monoglycerides | Concn (mM)a | Reduction of IFU (log10) at the following times (min):
|
||
|---|---|---|---|---|
| 10 | 5 | 1 | ||
| Monocaprin (10:0) | 20 | ≥4.6b | ≥4.1b | 1.0 |
| 10 | ≥5.6c | ≥5.1c | 0.6 | |
| 5 | ≥5.6c | ≥5.1c | 0.6 | |
| Monolaurin (12:0) | 20 | 0.5 | NDd | ND |
| 10 | 0.7 | ND | ND | |
| 5 | 0.6 | ND | ND | |
Concentration of monoglycerides mixed with C. trachomatis and incubated at 37°C for 1, 5, and 10 min. The concentration in the mixtures was reduced by half.
No inclusions were detected in the 10−2 dilution, which was the lowest dilution tested.
No inclusions were detected in the 10−1 dilution.
ND, not done.
In another experiment the lipid was removed by centrifugation and the bacteria were washed in culture medium before inoculation of 10-fold dilutions onto cell monolayers. The results are presented in Table 3. By comparing the titers (log10 numbers of IFU) of the untreated control samples that were not washed with those in samples that were washed, it can be seen that most of the bacteria were recovered after washing, that is, recoveries of 100 and 50% in the samples obtained at 5 and 10 min, respectively. As expected from previous experiments (Table 2), no inclusion-forming bacteria were detected in the 1:10 dilution of unwashed samples after treatment with monocaprin for 5 and 10 min. Thus, the loss of infectivity was about 100,000-fold or greater, i.e., ≥4.9 log10 IFU after 5 min and ≥5.0 log10 IFU after 10 min. The same result was obtained after washing of the bacteria treated with monocaprin for 10 min. In this sample no inclusion-forming bacteria were observed in the 1:10 dilution. The titer was therefore ≤1.7 log10 IFU per ml, whereas the titer in the washed untreated control was 6.4 log10 IFU per ml. On the other hand, in the sample treated with monocaprin for 5 min, inclusion-forming bacteria became detectable in the 1:10 dilution after washing. The sample incubated for 5 min still showed an 8,000-fold (3.9 log10) reduction in infectivity titer compared to that for the untreated control.
TABLE 3.
Viability of Chlamydia treated with 10 mM monocaprin at 37°C
| Time (min) | Viability (log10 IFU/ml)
|
|||
|---|---|---|---|---|
| Not washed
|
Washeda
|
|||
| Control | Treated | Control | Treated | |
| 5 | 6.6 | ≤1.7b | 6.6 | 2.7 |
| 10 | 6.7 | ≤1.7 | 6.4 | ≤1.7 |
After treatment the bacteria were washed twice with culture medium (a-BM).
The less than or equal to symbol indicates that no inclusions were detected in the 10−1 dilution, which was the lowest dilution tested.
Electron microscopy of monocaprin-treated Chlamydia.
Figure 2 shows electron micrographs of elementary bodies (EBs) of C. trachomatis treated with 10 mM monocaprin for 1, 5, and 10 min. Untreated bacteria are shown for comparison (Fig. 2A). Bacteria treated for 1 and 5 min (Fig. 2B and C, respectively) are not visibly different from the untreated bacteria. On the other hand, after treatment for 10 min (Fig. 2D) the EBs appear deformed and shrunken, and there is some indication that some of them are disintegrating (Fig. 2D, inset). At a lower magnification it could be seen that in both treated and untreated samples the bacteria are well dispersed on the grid and there is no aggregation of EBs (data not shown).
FIG. 2.
Electron micrographs of negatively stained EBs of C. trachomatis. The EBs were untreated (A) and treated with 10 mM monocaprin for 1 min (B), 5 min (C), and 10 min (D). After treatment for 10 min, the EBs appear deformed and shrunken or partially disrupted (D, inset). Bars, 1 μm.
MICs.
The effects of a series of monocaprin concentrations on Chlamydia upon contact for 2 h at 37°C are presented in Table 4. A concentration of 50 μg per ml (0.2 mM) caused a 96% inactivation of the bacteria, whereas after treatment with 25 μg per ml, 38% of the bacteria were unable to form inclusions. There was less inactivation at lower concentrations. The 50% effective concentration was about 30 μg per ml. Monocaprin at a concentration of 100 μg per ml caused complete lysis of the cell layers in 2 h, whereas at 50 μg per ml and lower concentrations, no lysis was observed by the trypan blue exclusion test. The bacteria were therefore about 2.5 times more sensitive to the lipid than the host cells. It should be noted that mixtures of Chlamydia and monocaprin were diluted 100-fold before inoculation onto McCoy cells. In the tests, the cell monolayers were therefore exposed to monocaprin at concentrations of 25 μg per ml or less.
TABLE 4.
Viability of Chlamydia after treatment with monocaprin at concentrations of 2.5 to 2,500 μg/ml for 2 h
| Monocaprin concn (μg/ml [mM]) | No. of inclusionsa | % Inhibitionb |
|---|---|---|
| 2.5 (0.01) | 151 | 4 |
| 5 (0.02) | 134 | 15 |
| 10 (0.04) | 123 | 22 |
| 25 (0.1) | 98 | 38 |
| 50 (0.2) | 6 | 96 |
| 100 (0.4) | 0 | 100 |
| 200 (0.8) | 0 | 100 |
| 500 (2) | 0 | 100 |
| 1,000 (4) | 0 | 100 |
| 2,500 (10) | 0 | 100 |
Mean number of inclusions in six wells.
Compared with the level of inhibition of the untreated control preparation, in which the mean inclusion count in four wells was 157.
DISCUSSION
Previous studies have shown that medium-chain saturated fatty acids and long-chain unsaturated fatty acids and their 1-monoglycerides are potent inhibitors of enveloped viruses and bacteria, both gram-negative and gram-positive bacteria (5, 14). In this study we have shown that C. trachomatis, a sexually transmitted, gram-negative bacterium, is effectively inactivated by exposure for 10 min to 10 mM (final concentration) lauric acid, a 12-carbon saturated fatty acid (12:0), and to capric acid (10:0) and its 1-monoglyceride. The monoglyceride of lauric acid had much less of an effect, and a number of other fatty acids and their monoglycerides, i.e., caprylic acid (8:0), myristic acid (14:0), and the unsaturated fatty acids palmitoleic acid (16:1) and oleic acid (18:1), had no effect or only a negligible effect. The narrow range of activities of the fatty acids and monoglycerides against Chlamydia is notable and suggests that these lipids have specific antichlamydial effects. A somewhat wider range and higher levels of activity of fatty acids and monoglycerides have been found against herpes simplex virus type 1 (HSV-1), against which, in addition to monocaprin and lauric acid, palmitoleic acid and, to some extent, oleic acid cause a rapid inactivation of the virus (10). Capric acid, on the other hand, had no activity against HSV-1 under the same conditions.
The question of how monocaprin inactivates the infectivity of Chlamydia was addressed by studying whether or not removal of the lipid before inoculation into cell cultures restored the infectivity. The results (Table 3) indicate that the loss of infectivity was not caused by an effect of the lipid on host cells and that the viability of the bacteria was irreversibly lost by treatment with monocaprin. After treatment for 5 min a small viable fraction became detectable by washing, showing that the bacteria were not fully inactivated at this time. This is in agreement with the electron microscopy study (Fig. 2), which showed no visible changes in the EBs after treatment with 10 mM monocaprin for 5 min, whereas after 10 min the EBs appeared deformed and partly disintegrated. We therefore hypothesize that the lipid kills the bacteria by affecting the outer membrane, leading to disruption of the membrane(s) in 5 to 10 min. This is supported by a previous electron microscopy study of the effect of linoleic acid on vesicular stomatitis virus and on Vero cells, in which the viral envelope and the cellular membrane were disrupted by the fatty acid (15).
In a recent study (11) ethers of 6- and 8-carbon fatty acids were tested against C. trachomatis. 2-O-Octylglycerol, which was the most active of the four ethers tested, caused a complete inactivation of the bacteria in 2 h at a concentration of 7.5 mM but was apparently not fully active at lower concentrations or with shorter exposure times. In our study we chose to use short exposure times of 10, 5, or 1 min in order to detect the rapid inactivation of Chlamydia. Monocaprin at a final concentration of 2.5 mM reduced the infectivity titer by >5 log10 IFU in 5 min at 37°C and was the most active of all the lipids tested in this study (Table 2). An exposure time of 1 min had only a minor effect. This is in contrast to the case for HSV-1, which is inactivated 100,000-fold or more (≥5 log10) by exposure to monocaprin for 1 min (10). The rapid in vitro killing of large numbers of sexually transmitted bacteria and viruses by microbicides is an essential prerequisite for their possible use in the prevention of STDs. Even if the number of infectious bacteria or viruses transmitted to genital mucosas is several orders of magnitude lower than the number used in in vitro tests, the conditions for the killing of the microbe in vivo are likely to be less favorable due to less effective mixing with the microbicide and an uneven distribution of the microbe on the mucosa. A large margin of microbicidal activity is therefore necessary. Due to the high in vitro efficacy of monocaprin against C. trachomatis and sexually transmitted enveloped viruses, this lipid may be useful as a microbicidal agent for the prevention of the transmission of STDs. Pharmaceutical formulations which contain monocaprin as the active ingredient have been developed and are potent inactivators of C. trachomatis, HSV-2, and human immunodeficiency virus in vitro (16). In vivo testing of these formulations is needed to establish whether or not they may be used for the prevention of STDs.
Several studies have shown that spermicides such as nonoxynol-9 inactivate C. trachomatis and other sexually transmitted bacteria and viruses (13), and it has been suggested that they may be used for protection against STDs. Benes and McCormack (1) studied the effects of various concentrations of nonoxynol-9 on large numbers of C. trachomatis upon contact for 120 min and found a 50% inhibition of inclusion formation at a concentration of between 500 and 1,000 μg per ml. In a comparable study of the activity of monocaprin against Chlamydia, a greater inhibitory effect was found (Table 4), with a 50% effective concentration of about 30 μg per ml. Although toxic in cell cultures, monocaprin at a concentration of 5 mg per ml (20 mM) has been shown not to cause irritation of the vaginal mucosa of mice and rabbits (16). The low level of toxicity in vivo and the high level of antichlamydial activity in vitro suggest that monocaprin may be more useful than nonoxynol-9 as protection against chlamydial infections.
ACKNOWLEDGMENT
This work was supported by a grant from the Research Fund of the University of Iceland.
REFERENCES
- 1.Benes S, McCormack W M. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob Agents Chemother. 1988;27:724–726. doi: 10.1128/aac.27.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Chlamydia trachomatis infection: policy guidelines for prevention and control. Morbid Mortal Weekly Rep. 1993;42:1–39. [Google Scholar]
- 3.Elias C J, Heise L L. Challenges for the development of female-controlled vaginal microbicides. AIDS. 1994;8:1–9. doi: 10.1097/00002030-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Global Programme on AIDS, World Health Organization. An overview of selected curable sexually transmitted diseases. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]
- 5.Isaacs C E, Litov R E, Thormar H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. Nutr Biochem. 1995;6:362–366. doi: 10.1016/0955-2863(95)80003-u. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs C E, Kim K S, Thormar H. Inactivation of enveloped viruses in human bodily fluids by purified lipids. Ann N Y Acad Sci. 1994;724:457–464. doi: 10.1111/j.1749-6632.1994.tb38947.x. [DOI] [PubMed] [Google Scholar]
- 7.Kabara J J. Fatty acids and derivatives as antimicrobial agents. In: Kabara J J, editor. The pharmacological effect of lipids. St. Louis, Mo: The American Oil Chemists Society; 1978. pp. 1–14. [Google Scholar]
- 8.Kappus E W, Quinn T C. The spermicide nonoxynol-9 does not inhibit Chlamydia trachomatis in vitro. Sex Transm Dis. 1986;3:134–137. doi: 10.1097/00007435-198607000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kelly J P, Reynolds R B, Stagno S, Louv W C, Alexander W J. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob Agents Chemother. 1985;27:760–762. doi: 10.1128/aac.27.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristmundsdóttir, T., and H. Thormar. Unpublished data.
- 11.Lampe M F, Ballweber L M, Isaacs C E, Stamm W E. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. In vitro sensitivity of Chlamydia trachomatis to novel antimicrobial lipids, abstr. D-19; p. 211. [Google Scholar]
- 12.Louv W C, Austin H, Alexander W J, Stagno S, Cheeks J. A clinical trial of nonoxynol-9 for preventing gonococcal and chlamydial infections. J Infect Dis. 1988;158:518–523. doi: 10.1093/infdis/158.3.518. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg M J, Holmes K K, et al. Virucides in prevention of HIV infection. Sex Transm Dis. 1993;20:41–44. doi: 10.1097/00007435-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Shibasaki I, Kato N. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria. In: Kabara J J, editor. The pharmacological effects of lipids. St. Louis, Mo: The American Oil Chemists Society; 1978. pp. 15–24. [Google Scholar]
- 15.Thormar H, Isaacs C E, Brown H R, Barshatzky M R, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thormar, H., et al. Unpublished data.
- 17.Welsh J K, Arsenakis M, Coelen R J, May J T. Effect of antiviral lipids, heat, and freezing on the activity of viruses in human milk. J Infect Dis. 1979;140:322–328. doi: 10.1093/infdis/140.3.322. [DOI] [PubMed] [Google Scholar]
- 18.Westrom L, Wolner-Hanssen P. Pathogenesis of pelvic inflammatory disease. Genitourin Med. 1993;69:9–17. doi: 10.1136/sti.69.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]