Abstract
Biological invasions, particularly of fish species, significantly threaten aquatic ecosystems. Among these invaders, the introduction of the European perch (Perca fluviatilis) can have particularly detrimental effects on native communities, affecting both ecosystem functioning and human well-being. In this study, carbon and nitrogen stable isotope analysis was employed, using perch originating from five different ecosystems, to model the effects of their hypothetical introduction into İznik Lake, an economically and ecologically important, biodiversity-rich lake in northern Turkey, to ultimately assess their potential predation impact and competition with native predators. The results revealed that if perch were introduced to the community, they would – considering gape size limitations – primarily prey upon Vimba vimba and Rutilus rutilus, indicating a significant feeding pressure on these species. Furthermore, the study identified a potential overlap and competition for resources between commonly mesopredator perch and the European catfish Silurus glanis, the current top predator in the ecosystem. Both species would occupy top predatory positions, emphasizing the potential disruption of predator–prey dynamics. Our findings underscore the potential ecological repercussions of perch invasions. The selective predation on V. vimba and R. rutilus, with the latter being consumed to a lesser extent by perch, could lead to cascading effects throughout the food web, altering the community structure, and ecosystem dynamics. Additionally, the competition between perch and S. glanis raises concerns about effects on the stability and functioning of the fish community. These results highlight the need for proactive management strategies to mitigate the risk of perch introductions. Strict regulations on the movement and introduction of invasive species, along with comprehensive monitoring, are crucial for preserving native communities and maintaining the ecological integrity of freshwater ecosystems. Our study demonstrates the potential predation impact of perch on vulnerable fish species and the competition with the established apex predator, emphasizing the importance of considering the ecological consequences of perch invasions and informing management decisions to ensure the conservation and sustainability of aquatic ecosystems.
Subject terms: Animal migration, Behavioural ecology, Community ecology, Ecological modelling, Ecosystem ecology, Invasive species
Introduction
Biological invasions, specifically those occurring in freshwater ecosystems1, have emerged as a significant environmental concern in recent years2. Invasive species, i.e. organisms introduced into an ecosystem outside their natural range, often because of human activities where they establish self-sustaining populations and spread, have the potential to disrupt native ecosystems, leading to detrimental ecological and economic consequences3–5. These species often possess competitive advantages, allowing them to outcompete native species for resources6. Freshwater invasions are distinctive within the broader context of biological invasions due to the insular nature of lake ecosystems and high connectivity of rivers and streams. This susceptibility to invasive species' impacts presents a challenge for invasion risk and impact assessments7.
Due to its natural biogeographical frontiers8, freshwater ecosystems in Turkey hold significant ecological and economic importance, harboring diverse species and supporting numerous human activities9. However, these ecosystems face escalating threats from biological invasions, which pose a serious risk to their integrity and functionality10. This is mainly because of the large transboundary river systems running through the country that increase the risk of non-native fish introductions from both Asia and Europe, a hotspot of freshwater fish diversity and endemism8. To date, 384 freshwater fish species have been recorded from Turkey, of which 208 (54%) are endemic and 15 (4%) non-native11. Often overlooked, nationally translocated species—those that are moved from one region within a country where they are native to another region where they are not—can pose a significant risk to native species and ecosystems12–15. Translocated fish are of potential concern, as many demonstrate a high invasiveness in Europe (e.g. Gobiidae)12. Despite being particularly common in some countries such as Turkey10, translocated species are probably the least regulated among fish movements, whether they are for fisheries, aquaculture or ornamental (i.e. aquaria or garden ponds) purposes, with secondary spread being common in most cases16,17. Nonetheless, frequent translocations occur within Anatolia, involving Alburnus alburnus, Garra rufa, Rutilus rutilus, Esox lucius and, recently, the European perch Perca fluviatilis9.
Fish invasions can have wide-ranging and often severe impacts on native communities and ecosystem functioning18–20. Predation and competition are among the most common effects associated with fish invasions21,22. Predatory fish can alter prey populations, leading to cascading effects throughout the food web23. Similarly, competition between invasive and native species for limited resources can disrupt ecological balances, potentially driving native species to local extinctions7. The European perch, which is widely distributed throughout Eurasia, has been introduced to a few small reservoirs in Portugal mainland and river systems in Portuguese Azores islands, Catalonian river basins including the Ebro delta in Spain, central and southern Italy, Lake Skadar (Montenegro, Albania), Amur (Siberia), Australia and – among other regions – South Africa, where it has been recognized as an invasive species, posing significant threats to native biodiversity24,25. Its aggressive feeding behavior often results in the extirpation of native fish species26, as for instance anglers’ observations indicate that the aggressiveness of the species is more pronounced in artificial ecosystems (i.e. reservoirs) where it has been translocated compared to its native populations in natural lakes. Concomitantly, when introduced, perch may become a preferred prey species for the native European catfish Silurus glanis27,28, affecting currently established trophic networks and predator prey dynamics29.
To better understand the ecological dynamics of invasive fish species and their impacts on native communities, stable isotope analysis has become a valuable tool30–32, as nitrogen and carbon stable isotopes provide unique signatures that can be used to trace energy flow within food webs and elucidate trophic interactions, allowing researchers to investigate the feeding habits, trophic positions, and dietary preferences of invasive species, as well as their potential impacts on native communities30. Understanding trophic positions, which denote an organism's place in the food web, ranging from primary producers to consumers and higher-level predators, is crucial in this context33. This information is provided by the nitrogen stable isotope signature of a certain consumer, which is enriched by a predictable factor (the trophic discrimination factor) compared to its prey34, providing essential insights into the flow of energy and nutrients within an ecosystem. Together with carbon stable isotope values, which inform about the carbon pathway of the nutrient and biomass flow33 and allow to define an isotopic niche35, this comprehensive understanding sheds light on the potential impacts of species reintroduction and invasion events on ecological dynamics20,30. In recent years, stable isotope analyses have been applied to predict the ecological consequences of species reintroduction and invasion events20. Notably, Balzani and Haubrock35 developed a conceptual framework to use stable isotopes data to approximate potential impacts of biological invasions on trophic webs. This approach has concomitantly proven valuable in assessing the potential ecological repercussions of reintroducing a species that had previously gone extinct and its subsequent effects on the lake community20.
Understanding the potential impacts of fish invasions on often highly anthropogenically affected lake ecosystems is more important than ever, as they are home to often unique species communities and in many cases, endemic species, while forming the basis of effective management and conservation efforts36,37. Hence, in the present study, we adopted the approach proposed by Balzani and Haubrock35 to evaluate the potential impacts of perch introductions from two types of source populations (reservoirs and natural lakes) on native communities, using as a model site the İznik Lake in Turkey. Specifically, we investigated the predation on lower trophic levels and the competition with other species occupying similar trophic positions. Considering the ethological differences between the source populations of perch, we hypothesized that the theoretical impacts would vary, resulting in differences in the predation impact and competition with other predators. The findings from our study will have important implications for ecosystem managers, stakeholders, and policymakers, highlighting the significance of proactive measures in mitigating biological invasions before they occur. By understanding the potential impacts of invasive species introductions, we can develop targeted strategies for invasive species management and prioritize the preservation of native communities and the ecological integrity of freshwater ecosystems.
Results
In İznik Lake, Silurus glanis was considered the only apex predator, with only Gambusia holbrooki distinguishing from the otherwise overlapping fish species (Fig. 1). δ15N values were on average higher in lakes (15.5‰) than in reservoirs (11.8‰), while the variance in both δ13C and δ15N were wider in reservoirs (Table 1). Projecting the stable isotope signatures of the two groups of Perca fluviatilis indicated a strong potential overlap with the S. glanis population (Fig. 1). Although not yet occurring in syntopy, the trophic position of the fish species in İznik Lake, indicated that potentially introduced P. fluviatilis from both lakes (TP = 5.39) and reservoirs (TP = 4.28), as well as S. glanis (TP = 4.46) would be occupying top predator positions. The TP of other fish species ranged from 3.95 (G. holbrooki) and 3.60 (Vimba vimba) to as low as 2.82 (Carassius gibelio).
Figure 1.
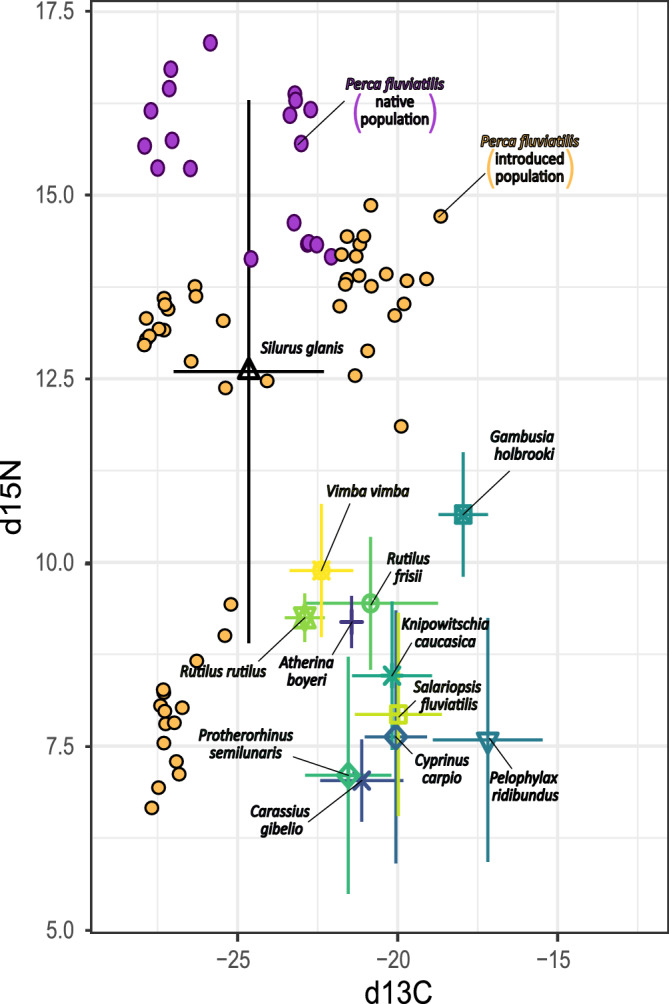
Carbon (δ13C) and nitrogen (δ15N) stable isotopes biplot of the İznik Lake fish community, with potential prey species for native and possibly introduced Perca fluvialitis.
Table 1.
Average total length (TL), weight (W), δ15N and δ13C of native and translocated Perca fluviatilis from lakes and reservoirs.
| System | Status | Site | δ15N [‰] | δ13C [‰] | TL [mm] | W [g] |
|---|---|---|---|---|---|---|
| Lake | Native | Gala | 15.8 | − 27.5 | 153.0 | 59.9 |
| Lake | Native | Sigirci | 15.2 | − 23.6 | 172.6 | 111.8 |
| Mean | 15.5 | − 25.4 | 163.3 | 87.2 | ||
| Reservoir | Translocated | Bayat | 7.9 | − 28.5 | 158.0 | 44.8 |
| Reservoir | Translocated | Ozburun | 13.2 | − 28.4 | 169.3 | 61.4 |
| Reservoir | Translocated | Seyitler | 13.8 | − 21.9 | 229.3 | 174.2 |
| Mean | 11.8 | − 25.8 | 189.9 | 101.5 | ||
| Mean (overall) | 12.9 | − 25.7 | 182.6 | 97.6 | ||
Potential dietary impact
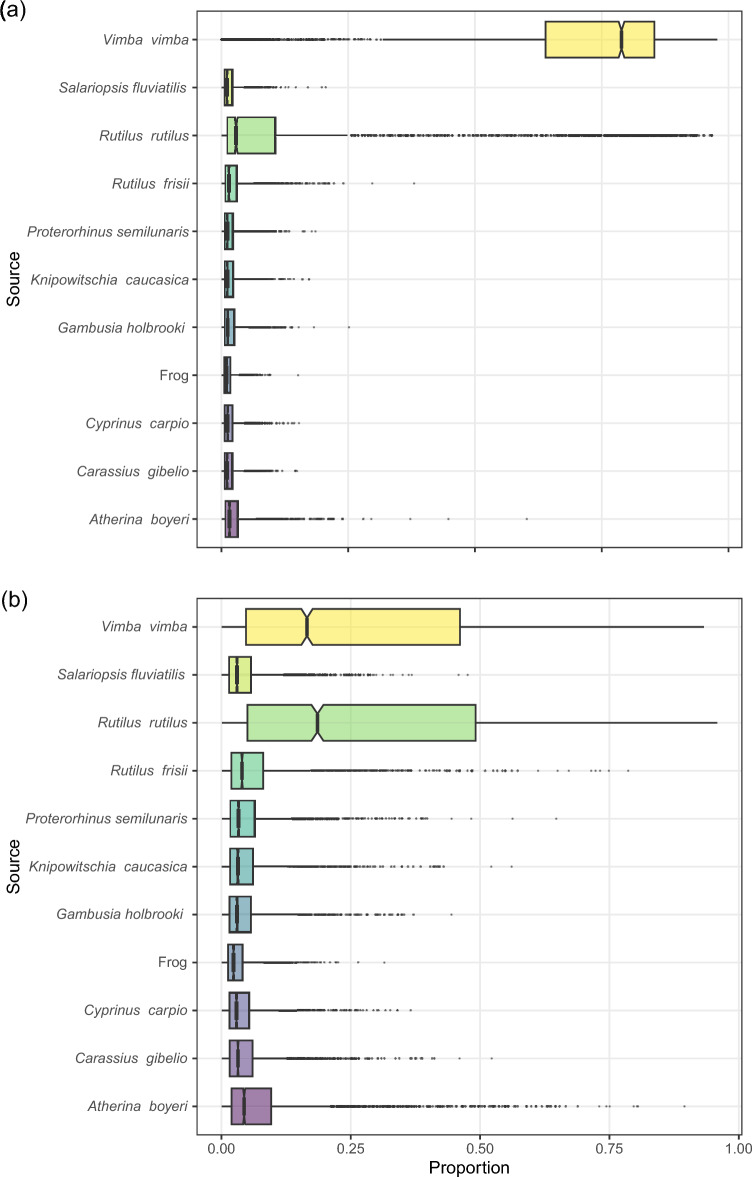
Stable isotope mixing models suggested that if introduced to İznik, both P. fluviatilis from reservoirs where it was previously introduced to (Fig. 2a) and lakes where it was native to (Fig. 2b) would result in the primary predation of Vimba vimba and Rutilus rutilus, albite the latter appearing to a lesser degree in P. fluviatilis from reservoirs. All other species were indicated as unlikely to be consumed by P. fluviatilis in both cases. However, running stable isotopes mixing models separately for each source population of perch indicated variations, with predated species, such as Proterorhinus semilunaris, C. gibelio, and Atherina boyeri, may be targeted by perch predation, albeit to a lesser degree (Fig. S1). Our predictive models also suggest that C. gibelio, the most common invasive fish species in Turkish freshwaters, would be preyed upon by translocated perch in Bayat reservoir (Fig. S2).
Figure 2.
Mixing models results for (a) introduced perch vs (b) native perch.
Niche overlap
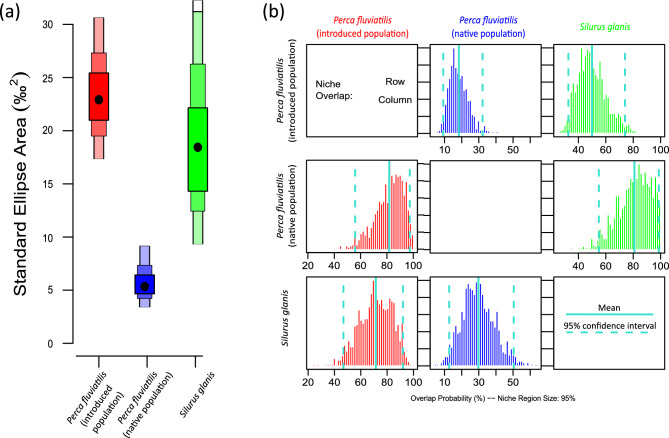
With regard to the possibility of competition between introduced perch and other predators (i.e. S. glanis) in İznik Lake, we identified considerable overlap potential if P. fluviatilis was to be introduced. We found that, following the theoretic introduction of P. fluviatilis, S. glanis would overlap in terms of the 95% Bayesian standard ellipse area (SEAb) with both introduced P. fluviatilis from reservoirs (27.2% overlap) and native P. fluviatilis from lakes (29.4% overlap). Silurus glanis furthermore expressed the widest SEAb, followed by introduced P. fluviatilis from reservoirs and native P. fluvialits from lakes, with the latter expressing a comparably small and condensed niche (Fig. 3a). Considering SEAb, the highest potential of directional overlap was between native and introduced P. fluviatilis (82.5%), followed by the likelihood of introduced P. fluviatilis overlapping with native S. glanis (81.0%). Considering the 40% corrected standard ellipse areas (SEAc), the overlap potential was much lower. Perca fluviatilis from lakes and reservoirs both had a considerable potential to overlap with S. glanis (18.1 to 21.9%). The probability of S. glanis overlapping ranged from 29.9% (P. fluviatilis from reservoirs) to 8.6% (P. fluviatilis from lakes; Fig. 3b; Table 2).
Figure 3.
A posteriori distributions for the Bayesian standard ellipse areas (SEAb) (a) and the niche overlap based on the 95% confidence interval (SEAb) (b) for native Perca fluviatilis from lakes, introduced P. fluviatilis from reservoirs, and Silurus glanis native to İznik Lake.
Table 2.
Directional pairwise probability of niche overlap (i.e. the probability of an individual of species A to fall within the niche of species B) for the Bayesian standard ellipse areas (SEAb, 95% confidence interval) and corrected standard ellipse areas (SEAc, 40% confidence interval) between the established native Silurus glanis and the two potential populations of Perca fluviatilis.
| Species B | ||||
|---|---|---|---|---|
| Perca fluviatilis (introduced) | Perca fluviatilis (native) | Silurus glanis | ||
| SEAb | ||||
| Species A | Perca fluviatilis (introduced) | NA | 18.46 | 49.62 |
| Perca fluviatilis (native) | 82.50 | NA | 81.00 | |
| Silurus glanis | 70.98 | 29.71 | NA | |
| SEAc | ||||
| Species A | Perca fluviatilis (introduced) | NA | 3.30 | 18.06 |
| Perca fluviatilis (native) | 8.37 | NA | 21.94 | |
| Silurus glanis | 29.87 | 8.49 | NA | |
Discussion
The results of our study provide valuable insights into the potential predation impact and competition of the introduced European perch Perca fluviatilis on the fish community of İznik Lake. Our findings support our hypothesis that the impacts of perch introductions would vary based on the source population and manifest as differences in predation impact and competition with other apex predators38. These differences in dietary preferences and trophic positions confirm that the source population of perch influences their ecological impacts. Furthermore, our results also confirm that the introduction of P. fluviatilis could lead to competition with other apex predators, such as Silurus glanis, as the overlap analysis and trophic position calculations revealed the potential for competition between these species, highlighting the potential disruption of predator–prey dynamics and the need for considering the consequences of introductions in freshwater ecosystems.
Predation impact based on the mixing models
Stable isotope mixing models revealed important information about potential dietary impacts of introduced P. fluviatilis on the fish community of İznik Lake. The models indicated that if perch were introduced to the lake, they would primarily prey upon Vimba vimba and Rutilus rutilus, with R. rutilus being consumed to a lesser extent by perch from reservoirs, thereby confirming the results of previous work39,40. These findings can be attributed to several ecological factors, both species being likely preferred prey for perch due to their ecological traits and availability41. Both species are known to occupy lower trophic levels within the fish community42, feeding on detritus, benthic invertebrates, and zooplankton43,44. These prey items provide an energy-rich food source for perch. Vimba vimba and R. rutilus are commonly found in the shallow areas of İznik Lake, where perch are known to be efficient predators due to their hunting strategies and adaptability to different habitats45. It should however be acknowledged that P. fluviatilis prefers slightly deeper zones compared to R. rutilus, meaning that their realized spatial overlap will depend on oxygen availability46. On the other hand, other fish species in the community were deemed unlikely to be consumed by perch. This could be attributed to a combination of factors, including differences in habitat preferences, feeding behavior, and size47,48. Some species, such as Gambusia holbrooki, Capoeta tinca, and Salaria fluviatilis, occupy different ecological niches or exhibit specific adaptations that reduce their vulnerability to predation by perch49–52. It is important to note that gobiid species like Proterorhinus semilunaris, are highly unlikely to occur in reservoirs located in remote areas established for irrigation purposes due to their incompatible habitat requirements53. On the other hand, Atherina boyeri, another translocated species, appears to be an important component of perch diet in both reservoirs and natural lakes, as evidenced by reports of perch preying on A. boyeri in Seyitler reservoir54. Additionally, larger species like Cyprinus carpio or Silurus glanis (this latter also being protected by its pectoral spines55) may exceed the preferred prey size range of perch, making them less suitable prey26. While we acknowledge that we only considered adult individuals as prey, unable to assess the potential impacts of perch on the young-of-the-year populations56, our findings highlight the potential impact of perch introductions on the prey populations in İznik Lake, with V. vimba and R. rutilus being particularly susceptible to predation. The selective predation on these species could have cascading effects throughout the food web, altering the community structure and ecosystem dynamics57.
Competition with Silurus glanis for the position of apex predator
Our study identified the European catfish S. glanis as the sole apex predator in İznik Lake, with only G. holbrooki showing differentiation from the otherwise all-overlapping fish species. Silurus glanis occupied the top trophic position in the ecosystem, therefore exerting top-down control over the fish community dynamics. However, projecting the isotopic niche of the two groups of P. fluviatilis indicated a theoretical overlap with the S. glanis population, suggesting a potential shift in the apex predator status and competition for feeding resources within the ecosystem58,59.
Such competition for limited food resources among top predators can have profound ecological implications, potentially leading to changes in the community structure and dynamics60,61. The overlap potential and niche width analysis further supported the likelihood of competition between P. fluviatilis and S. glanis. The considerable overlapping potential between the two species in terms of standard ellipse areas (SEAb) suggests that they may have similar dietary preferences and utilize similar feeding habitats within the lake. This implies that the introduction of P. fluviatilis could potentially disrupt the existing trophic interactions and resource partitioning, leading to increased competition for prey resources and potential changes in the distribution and abundance of both species59. When examining congruence, it is however important to take into account not only the contrasting sizes of P. fluviatilis and S. glanis, but also the varying sizes of their potential prey. Silurus glanis, in addition to actively hunting throughout the water column, also consumes decaying matter such as dead fish found on the lake bottom58. On the other hand, due to limitations in their mouth size, P. fluviatilis is likely to primarily target smaller prey fish, including young-of-the-year and juveniles, which subsequently prevents them from growing and becoming prey for S. glanis.
These findings highlight the importance of considering the potential ecological consequences of P. fluviatilis introductions, especially in relation to the existing apex predator, S. glanis, in the ecosystem. The potential competition between these two top predators raises concerns about the stability and functioning of the fish community in İznik Lake. Understanding the ecological implications of such interactions is crucial for making informed decisions regarding the introduction of non-native species and managing the conservation and sustainability of aquatic ecosystems.
Caveats
One of the challenges in our study was standardization of the isotope data from the target community and the different perch populations, as the baseline organisms differed. Considering that even standardization with baseline organisms can introduce a certain bias based on differences in baseline organisms, which may locally differ even within ecosystems (see Ref.35 for a detailed discussion), we employed a novel approach by superimposing raw stable isotopes data from multiple perch populations into the recipient community stable isotopes biplot, assuming that the focal species niche would position itself somewhere in between the range of the superimposed data35,61. While this approach may introduce some uncertainties, it provides a rough estimation of the predation impact and competition dynamics, and—due to data availability limitations—such a situation is often the best compromise35. Concomitantly, we found similar results between P. fluviatilis originating from lakes and reservoirs, underlining the reliability of our results.
In light of the intricate web of interactions within the fish community, we acknowledge the critical role played by size guilds and body characteristics in shaping the potential impacts of P. fluviatilis introductions. Species-specific attributes, such as body depth and size can prevent perch predation, as exemplified by the deep-bodied morph observed in C. gibelio54. This adaptation provides a degree of protection even in the presence of larger perch. Conversely, smaller-sized species and younger individuals, as indicated in Table 3, may face increased vulnerability to predation. While our study primarily focuses on average food niche dynamics, we recognize the need for a more nuanced consideration of these factors in future studies by investigating empirical evidence regarding the post-introduction trends in these vulnerable species, seeking to provide a more comprehensive understanding of the ecological consequences of perch introduction.
Table 3.
Status (Inv= invasive, Trans= translocated, Nat= native), number of samples (n), average and standard deviation (SD) of δ15N and δ13C, mean total length (TL), and weight (W) of fish species and other groups collected from İznik Lake.
| Species | Status | n | Average δ15N | SD δ15N | Average δ13C | SD δ13C | Mean TL [mm] | Mean W [g] |
|---|---|---|---|---|---|---|---|---|
| Atherina boyeri | Trans | 10 | 9.19 | 0.35 | – 21.44 | 0.29 | 93.10 | 5.15 |
| Capoeta tinca | Nat | 1 | 15.72 | NA | – 19.88 | NA | 256.00 | 162.00 |
| Carassius gibelio | Inv | 10 | 7.03 | 0.56 | – 21.12 | 1.30 | 137.80 | 44.35 |
| Cyprinus carpio | Nat | 4 | 7.62 | 1.72 | – 20.06 | 0.97 | 104.00 | 17.63 |
| Gambusia holbrooki | Inv | 10 | 10.65 | 0.85 | – 17.95 | 0.77 | 40.60 | 0.90 |
| Knipowitschia caucasica | Nat | 7 | 8.46 | 1.01 | – 20.18 | 1.25 | 27.86 | 0.19 |
| Proterorhinus semilunaris | Nat | 12 | 7.10 | 1.61 | – 21.54 | 1.35 | 42.55 | 1.03 |
| Rutilus frisii | Nat | 10 | 9.44 | 0.91 | – 20.85 | 2.11 | 218.40 | 117.45 |
| Rutilus rutilus | Nat | 10 | 9.25 | 0.33 | – 22.90 | 0.62 | 143.20 | 27.80 |
| Salariopsis fluviatilis | Nat | 20 | 7.93 | 1.38 | – 19.98 | 1.36 | 30.33 | 0.27 |
| Silurus glanis | Nat | 10 | 12.60 | 3.70 | – 24.65 | 2.35 | ||
| Vimba vimba | Nat | 10 | 9.89 | 0.91 | – 22.38 | 0.99 | 143.10 | 26.60 |
| Pelophylax ridibundus | Nat | 3 | 7.58 | 1.66 | – 17.19 | 1.72 | ||
| Macrozoobenthos | 1 | 5.25 | NA | – 20.25 | NA | |||
| Phytoplankton | 2 | 3.79 | 0.23 | – 22.00 | 0.07 | |||
| Macrophytes | 2 | 1.23 | 3.48 | – 10.10 | 0.56 | |||
| Algae | 1 | 2.09 | NA | – 20.58 | NA | |||
| Detritus | 3 | 5.63 | 4.65 | – 16.60 | 4.73 | |||
| Zooplankton | 2 | 5.63 | 0.43 | – 24.27 | 1.07 |
Management implications: steps to be taken for invasive perch
By utilizing stable isotope analyses and considering the ethological differences in behavior between perch source populations, we were able to gain valuable insights into the potential ecological consequences or repercussions of potential perch introductions. Our approach, although not perfect, contributes to our understanding of the potential impacts of invasive species introductions and serves as a starting point for further research and management decisions.
Perca fluviatilis has been recognized as an invasive species with the potential to disrupt native biodiversity24,62. Considering the aggressive feeding behavior and competitive advantage of perch63, proactive measures should be taken to mitigate the risk of perch introductions before they occur. The recent surge in popularity of perch as a target species for angling has led to widespread introductions, particularly in reservoirs54. The active involvement and support of local and national angling clubs in this initiative suggest that the spread of perch may occur rapidly and effectively in the coming years. Being a large natural lake, it is possible that the results obtained from P. fluviatilis originating from lakes may be behaviourally—and possibly trophically—more similar than individuals from reservoirs54. It is however possible that observed behavioral differences (i.e. higher aggressiveness in reservoirs; pers. communication Necati Ayvaz) could be due to the presence of schooling prey64, which could lead to different impacts based on the origin of the source population – in the case P. fluviatilis was to be introduced into İznik Lake. Additionally, recent research indicates that perch may become a preferred prey species for European catfish, potentially leading to a mesopredator status and introducing a new layer of complexity to the ecological dynamics, with S. glanis functioning as natural control agent27,28. This relationship underscores the need for a comprehensive assessment of species interactions in the context of perch introductions. Hence, our study highlights the need for targeted strategies for invasive species management, focusing on the preservation of native communities and the ecological integrity of freshwater ecosystems. Implementing strict regulations on the movement and introduction of invasive species, along with comprehensive monitoring.
The findings from our study will have important implications for ecosystem managers, stakeholders, and policymakers, highlighting the significance of proactive measures in mitigating biological invasions before they occur. By understanding the potential impacts of invasive species introductions, we can develop targeted strategies for invasive species management and prioritize the conservation of native communities and the ecological integrity of freshwater ecosystems.
Methods
Study sites
İznik Lake is situated between the towns of Orhangazi and İznik, with coordinates ranging from 40° 23′ to 40° 30′ N and 29° 30′ to 29° 42′ E. It is located at 80 m a.s.l. and, with an area of 313 km2, is the largest water body in the Marmara Region and the fifth largest lake in Turkey (Fig. 4). This deep tectonic lake is fed by small streams, creeks, and groundwater. However, due to high agricultural activity in the surrounding areas and the discharge of wastewater from nearby residential areas, the lake has experienced an increase in nutrient concentrations65. Consequently, its trophic status has shifted from oligotrophic to mesotrophic in recent decades38. İznik Lake is home to more than 20 fish species, predominantly cyprinids45 (Table 3).
Figure 4.
Sampling sites in western Anatolia, Turkey. The map was created with Arcgis 10.8.
Sample collection and laboratory processing
Sampling was conducted during autumn (October–November) of 2021. Fish were captured using multi-mesh gillnets consisting of 12 panels with varying mesh sizes ranging from 5 to 55 mm. Once captured, fish were transported to the laboratory in an ice water slurry. Samples of potential prey resources, including submerged macrophytes, algae, phytoplankton, zooplankton, macroinvertebrates (i.e. bivalves, gastropods), and detritus were collected from each sampling site, whenever available. Macroinvertebrates and macrophytes were collected with a grab and a scoop from the lake bottom and surface, while phytoplankton and zooplankton were collected with a plankton net (50 µm).
In total, twelve fish species, three individuals of the marsh frog Pelophylax ridibundus and other organisms (Table 3) were collected. For fish species, we used a caliper with an accuracy of 0.01 mm to measure the total length (TL). The weight (W) was measured using an electronic balance with an accuracy of 0.01 g. For all individuals of each fish species and frog, a sample of muscle tissue was extracted for stable isotopes analysis. The muscle tissue was thoroughly cleaned to remove any fat, skin, scales, and bones. Macrophyte samples were rinsed with tap water and, after removing insects and other organic materials. All samples were stored at − 20 °C for stable isotope analysis. Other organisms were processed as a whole, except for mollusks for which the shell was removed. The samples were placed in separate glass trays and dried for 48 h in an oven set at 60 °C. After drying, the samples were finely ground into a powder using an agate mortar and pestle. The combusted samples were subsequently analyzed using a Thermo Finnigan Delta Plus Advantagean isotope ratio mass spectrometer at the Biological and Chemical Research Centre in Warsaw, Poland. The isotope compositions were expressed as ‰ using the δ notation, calculated based on δ13C or δ15N = ((Rsample/Rstandard) − 1) × 1000, where R represents the 13C:12C or 15N:14N ratios, and the standards were Vienna Pee Dee Belemnite for carbon and atmospheric N2 for nitrogen.
Ethical approval for research involving human participants and/or animal
This article does not contain any studies with human participants performed or experiments conducted on animals by any of the authors. The legal permission for collecting fish was provided by the Republic of Türkiye Ministry of Agriculture and Forestry (E-67852565-140.03.03-1800883). All care and use of experimental animals were complied with animal welfare international/national laws, guidelines and policies. Some fish were obtained from the fisherman (Nazmi Tuna) with his consent.
Obtaining perch isotope data
To investigate the potential effects of introducing P. fluviatilis on the fish community of İznik Lake, we collected perch δ13C and δ15N data from five water bodies in western Anatolia. Three of these water bodies are reservoirs (Seyitler, Bayat, and Ozburun reservoirs) established for mainly irrigation purposes, ranging in size from 0.05 to 4.5 km2, with average maximum depths of approximately 25 m. The remaining two study sites are natural lakes (Gala and Sigirci lakes) of similar sizes, around 6 km2, and primarily shallow, with depths ranging between 2 and 6 m. The reservoirs have a limited fish fauna, with perch as the only predator species, along with a few other native and non-native species. On the other hand, the two native lakes harbor a higher number of species, including several native predator species such as Silurus glanis and Sander lucioperca, in addition to perch.
Statistical analyses
To calculate the trophic position (TP) for each species, we used a two-baseline model (baseline 1: detritus; baseline 2: phyto- and zooplankton66) implemented in the R package tRophicPosition, which uses Markov Chain Monte Carlo simulations67. Because species-specific trophic discrimination factors (TDFs) were not available for all species, we generated values based on the available data using the R function simulateTDF67. We analyzed the stable isotope data of P. fluviatilis and observed differences between the signatures found in lakes versus reservoirs. This led us to consider the potential effects of both lake and reservoir environments on the fish fauna of İznik Lake, individually assessing their impacts on the fish community.
The application of dual plot graphs for δ15N versus δ13C of consumer tissues and food sources enabled the determination of probable prey sources and combinations of prey contributing to the diet of predators68. To estimate how sampled species would contribute to the diet of possibly introduced P. fluviatilis from reservoirs and lakes, stable isotope mixing models were applied using the R package simmr69. These models were conducted for the two groups of P. fluviatilis (native specimens from lakes and introduced individuals from reservoirs) possibly introduced to İznik Lake. As potential prey items, we considered all fish species except Silurus glanis, which was, based on its apex position and size, considered as unsuitable70,71. Results of the mixing models are presented as a posteriori distribution for the proportion of each prey item in the diet.
The standard ellipse areas corresponding to the Bayesian 95% standard ellipse area (SEAb) and the area corresponding to 40% of central data points (SEAc) were calculated using the R package SIBER72 for both groups of P. fluviatilis and S. glanis72. Using the same package, we computed the degree of isotopic niche overlap (ranging from 0: no overlap, to 1: complete overlap) as a proxy for trophic competition among populations72. Finally, the directional pairwise probability of the two groups of P. fluviatilis and S. glanis to overlap their niche was estimated using the R package nicheROVER, which applies a Monte Carlo estimation (chain length: 10,000 steps) on the potential overlap, thus computing the directional pairwise probability of the niche of one species (not either species) overlapping onto the niche of another to further assess the direction of the potential trophic competition32,67.
Supplementary Information
Author contributions
A.S.T. and P.J.H. conceptually designed the study. P.J.H. and I.K. performed the analyses. P.J.H. wrote the first version of the manuscript. P.B., A.S.T., and I.K. provided valuable input to an earlier version of the manuscript. S.A. helped with the visualization of the results. S.A., O.M., Ö.E., E.K., S.B., E.Ç., and P.O.V. contributed to the writing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data used in this study can be obtained from Ali Serhan Tarkan upon reasonable request by contacting the lead author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ali Serhan Tarkan and Phillip J. Haubrock.
Contributor Information
Ali Serhan Tarkan, Email: serhantarkan@gmail.com.
Phillip J. Haubrock, Email: phillip.haubrock@senckenberg.de
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44865-2.
References
- 1.Poulin R, Paterson RA, Townsend CR, Tompkins DM, Kelly DW. Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshw. Biol. 2011;56(4):676–688. doi: 10.1111/j.1365-2427.2010.02425.x. [DOI] [Google Scholar]
- 2.Cassini MH. A review of the critics of invasion biology. Biol. Rev. 2020;95(5):1467–1478. doi: 10.1111/brv.12624. [DOI] [PubMed] [Google Scholar]
- 3.Gozlan RE, Britton JR, Cowx I, Copp GH. Current knowledge on non-native freshwater fish introductions. J. Fish Biol. 2010;76(4):751–786. doi: 10.1111/j.1095-8649.2010.02566.x. [DOI] [Google Scholar]
- 4.Haubrock PJ, et al. Invasion impacts and dynamics of a European-wide introduced species. Glob. Change Biol. 2022;28(15):4620–4632. doi: 10.1111/gcb.16207. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn TM, et al. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011;26(7):333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Warren RJ, et al. Release from intraspecific competition promotes dominance of a non-native invader. Biol. Invasions. 2019;21:895–909. doi: 10.1007/s10530-018-1868-z. [DOI] [Google Scholar]
- 7.Marr SM, et al. A global assessment of freshwater fish introductions in mediterranean-climate regions. Hydrobiologia. 2013;719:317–329. doi: 10.1007/s10750-013-1486-9. [DOI] [Google Scholar]
- 8.Fricke R, Bilecenoğlu M, Sarı HM. Annotated checklist of fish and lamprey species (Gnathostomata and Petromyzontomorphi) of Turkey, including a Red List of threatened and declining species. Stuttg. Beitr. Naturkd. 2007;706:1–172. [Google Scholar]
- 9.Tarkan AS, Marr SM, Ekmekçi FG. Non-native and translocated freshwater fish. FISHMED Fish. Mediterr. Environ. 2015;3:1–28. doi: 10.29094/FiSHMED.2015.003. [DOI] [Google Scholar]
- 10.Tarkan AS, et al. Identification of potentially invasive freshwater fishes, including translocated species, in Turkey using the aquatic species invasiveness screening kit (AS-ISK) Int. Rev. Hydrobiol. 2017;102:47–56. doi: 10.1002/iroh.201601877. [DOI] [Google Scholar]
- 11.Çiçek E, Sungur S, Fricke R. Freshwater lampreys and fishes of Turkey; a revised and updated annotated checklist 2020. Zootaxa. 2020;4809(2):241–270. doi: 10.11646/zootaxa.4809.2.2. [DOI] [PubMed] [Google Scholar]
- 12.Copp GH, et al. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005;21:242–262. doi: 10.1111/j.1439-0426.2005.00690.x. [DOI] [Google Scholar]
- 13.Hodder KH, Bullock JM. Translocation of native species in the UK: Implications for biodiversity. J. Appl. Ecol. 1997;34:547–565. doi: 10.2307/2404906. [DOI] [Google Scholar]
- 14.Glamuzina B, et al. Comparison of taxon-specific and taxon-generic risk screening tools for identifying potentially invasive non-native fishes in the River Neretva catchment (Bosnia & Herzegovina and Croatia) River Res. Appl. 2017 doi: 10.1002/rra.3124. [DOI] [Google Scholar]
- 15.Piria M, et al. Risk screening of non-native freshwater fishes in Croatia and Slovenia using FISK (Fish Invasiveness Screening Kit) Fish. Manag. Ecol. 2016;23:21–31. doi: 10.1111/fme.12147. [DOI] [Google Scholar]
- 16.Vander Zanden MJ, Olden JD. A management framework for preventing the secondary spread of aquatic invasive species. Can. J. Fish. Aquat. Sci. 2008;65:1512–1522. doi: 10.1139/F08-099. [DOI] [Google Scholar]
- 17.Tricarico E. A review on pathways and drivers of use regarding non-native freshwater fish introductions in the Mediterranean region. Fish. Manag. Ecol. 2012;19:133–141. doi: 10.1111/j.1365-2400.2011.00834.x. [DOI] [Google Scholar]
- 18.Strayer DL. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010;55:152–174. doi: 10.1111/j.1365-2427.2009.02380.x. [DOI] [Google Scholar]
- 19.Simberloff D, et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013;28:58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Haubrock PJ, et al. Predicting the effects of reintroducing a native predator (European eel, Anguilla anguilla) into a freshwater community dominated by alien species using a multidisciplinary approach. Manag. Biol. Invasions. 2019;10(1):171–191. doi: 10.3391/mbi.2019.10.1.11. [DOI] [Google Scholar]
- 21.Sih A, et al. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos. 2010;119(4):610–621. doi: 10.1111/j.1600-0706.2009.18039.x. [DOI] [Google Scholar]
- 22.Balzani P, Gozlan RE, Haubrock PJ. Overlapping niches between two co-occurring invasive fish: The topmouth gudgeon Pseudorasbora parva and the common bleak Alburnus alburnus. J. Fish Biol. 2020;97(5):1385–1392. doi: 10.1111/jfb.14499. [DOI] [PubMed] [Google Scholar]
- 23.Elvira B, Almodóvar A. Freshwater fish introductions in Spain: Facts and figures at the beginning of the 21st century. J. Fish Biol. 2001;59:323–331. doi: 10.1111/j.1095-8649.2001.tb01393.x. [DOI] [Google Scholar]
- 24.Bakiu R, et al. Invasiveness assessment of European perch (Perca fluviatilis), pike-perch (Sander lucioperca) and northern pike (Esox lucius) in Albanian freshwater ecosystems by using the aquatic species invasiveness screening kit (AS-ISK) Stud. Mar. 2022;35(2):12–18. doi: 10.5281/zenodo.7414001. [DOI] [Google Scholar]
- 25.Freyhof, J. & Kottelat, M. Perca fluviatilis. The IUCN Red List of Threatened Species 2008: e.T16580A6135168. 10.2305/IUCN.UK.2008.RLTS.T16580A6135168.en. (Accessed 10 June 2023) (2008).
- 26.Adámek Z, Musil J, Sukop I. Diet composition and selectivity in O+ perch (Perca fluviatilis L.) and its competition with adult fish and carp (Cyprinus carpio L.) stock in pond culture. Agric. Conspec. Sci. 2004;69(1):21–27. [Google Scholar]
- 27.Vejřík L, et al. European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci. Rep. 2017;7:15970. doi: 10.1038/s41598-017-16169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Šmejkal M, Ricard D, Sajdlová Z, et al. Can species-specific prey responses to chemical cues explain prey susceptibility to predation? Ecol. Evol. 2018;8:4544–4551. doi: 10.1002/ece3.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hempel M, Neukamm R, Thiel R. Effects of introduced round goby (Neogobius melanostomus) on diet composition and growth of zander (Sander lucioperca), a main predator in European brackish waters. Aquat. Invasions. 2016;11:167–178. doi: 10.3391/ai.2016.11.2.06. [DOI] [Google Scholar]
- 30.Cucherousset J, et al. Using stable isotope analyses to determine the ecological effects of non-native fishes. Fish. Manag. Ecol. 2012;19:111–119. doi: 10.1111/j.1365-2400.2011.00824.x. [DOI] [Google Scholar]
- 31.Trueman CN, MacKenzie KM, Palmer MR. Identifying migrations in marine fishes through stable-isotope analysis. J. Fish Biol. 2012;81:826–847. doi: 10.1111/j.1095-8649.2012.03361.x. [DOI] [PubMed] [Google Scholar]
- 32.Haubrock PJ, et al. Spatio-temporal niche plasticity of a freshwater invader as a harbinger of impact variability. Sci. Total Environ. 2021;777:145947. doi: 10.1016/j.scitotenv.2021.145947. [DOI] [PubMed] [Google Scholar]
- 33.Boecklen WJ, Yarnes CT, Cook BA, James AC. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011;42:411–440. doi: 10.1146/annurev-ecolsys-102209-144726. [DOI] [Google Scholar]
- 34.Post DM. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology. 2002;83:703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2. [DOI] [Google Scholar]
- 35.Balzani P, Haubrock PJ. Expanding the invasion toolbox: Including stable isotope analysis in risk assessment. NeoBiota. 2022;76:191–210. doi: 10.3897/neobiota.76.77944. [DOI] [Google Scholar]
- 36.Top Karakuş N, Tarkan AS. Does non-native pumpkinseed Lepomis gibbosus affect endemic algae-scraping Capoeta aydinensis in case of introduction to a small stream? An ex-situ growth experiment. Ecol. Freshw. Fish. 2022;31:81–86. doi: 10.1111/eff.12614. [DOI] [Google Scholar]
- 37.Emiroğlu Ö, Aksu S, Başkurt S, Britton JR, Tarkan AS. Predicting how climate change and globally invasive piscivorous fishes will interact to threaten populations of endemic fishes in a freshwater biodiversity hotspot. Biol. Invasions. 2023;25:1907–1920. doi: 10.1007/s10530-023-03016-4. [DOI] [Google Scholar]
- 38.Jacobson P, Bergström U, Eklöf J. Size-dependent diet composition and feeding of Eurasian perch (Perca fluviatilis) and northern pike (Esox lucius) in the Baltic Sea. Boreal Environ. Res. 2019;24:137–153. [Google Scholar]
- 39.Persson L, Eklov P. Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology. 1995;76:70–81. doi: 10.2307/1940632. [DOI] [Google Scholar]
- 40.Persson L, De Roos AM, Byström P. State-dependent invasion windows for prey in size-structured predator-prey systems: Whole lake experiments. J. Anim. Ecol. 2007;76:94–104. doi: 10.1111/j.1365-2656.2006.01190.x. [DOI] [PubMed] [Google Scholar]
- 41.Dörner H, Wagner A. Size-dependent predator-prey relationships between perch and their fish prey. J. Fish Biol. 2003;62:1021–1032. doi: 10.1046/j.1095-8649.2003.00092.x. [DOI] [Google Scholar]
- 42.Hayden B, et al. Trophic flexibility by roach Rutilus rutilus in novel habitats facilitates rapid growth and invasion success. J. Fish Biol. 2014;84(4):1099–1116. doi: 10.1111/jfb.12351. [DOI] [PubMed] [Google Scholar]
- 43.Đikanović, V., Čanak Atlagić, J., Zorić, K., Ilić, M. & Skorić, S. The diet of 22 fish species in the Belgrade sector of the Danube River. In Abstracts of the 3rd CESAMIR, Central Europen Symposium for Aquatic Macroinvertebrates Research, 99–99. Department of Invertebrate Zoology & Hydrobiology University of Łódź (2018).
- 44.Ostaszewska T, Dabrowski K, Hliwa P, Gomółka P, Kwasek K. Nutritional regulation of intestine morphology in larval cyprinid fish, silver bream (Vimba vimba) Aquac. Res. 2008;39(12):1268–1278. doi: 10.1111/j.1365-2109.2008.01989.x. [DOI] [Google Scholar]
- 45.Özuluğ M, Altun Ö, Meriç N. On the fish fauna of İznik Lake (Turkey) Turk. J. Zool. 2005;29:371–375. [Google Scholar]
- 46.Prchalová M, et al. The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecol. Freshw. Fish. 2009;18:247–260. doi: 10.1111/j.1600-0633.2008.00342.x. [DOI] [Google Scholar]
- 47.Truemper HA, Lauer TE. Gape limitation and piscine prey size-selection by yellow perch in the extreme southern area of Lake Michigan, with emphasis on two exotic prey items. J. Fish Biol. 2005;66:135–149. doi: 10.1111/j.0022-1112.2005.00588.x. [DOI] [Google Scholar]
- 48.Mihalitsis M, Bellwood DR. A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS One. 2017;12(9):e0184679. doi: 10.1371/journal.pone.0184679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanco-Garrido F, Clavero M, Prenda J. Jarabugo (Anaecypris hispanica) and freshwater blenny (Salaria fluviatilis): Habitat preferences and relationship with exotic fish species in the middle Guadiana basin. Limnetica. 2009;28(1):139–148. doi: 10.23818/limn.28.10. [DOI] [Google Scholar]
- 50.Murphy CA, Grenouillet G, García-Berthou E. Natural abiotic factors more than anthropogenic perturbation shape the invasion of Eastern Mosquito fish (Gambusia holbrooki) Freshw. Sci. 2015;34(3):965–974. doi: 10.1086/681948. [DOI] [Google Scholar]
- 51.Kaya, C. Taxonomic revision of the species belong to genus Capoeta distributed in Turkey. PhD Thesis. Recep Tayyip Erdogan University, Institute of Science and Technology, 126 (2019).
- 52.Kurtul I, Tarkan AS, Sarı HM, Britton JR. Climatic and geographic variation as a driver of phenotypic divergence in reproductive characters and body sizes of invasive Gambusia holbrooki. Aquat. Sci. 2022;84:29. doi: 10.1007/s00027-022-00862-7. [DOI] [Google Scholar]
- 53.Top N, Karakuş U, Tepeköy EG, Britton JR, Tarkan AS. Plasticity in habitat preferences of two native Ponto-Caspian gobies, Proterorhinus semilunaris and Neogobius fluviatilis: Implications for invasive populations. Knowl. Manag. Aquat. Ecosyst. 2019;420:40. doi: 10.1051/kmae/2019031. [DOI] [Google Scholar]
- 54.Tarkan AS, et al. Phenotypic responses to piscivory in invasive gibel carp populations. Aquat. Sci. 2023;85:75. doi: 10.1007/s00027-023-00974-8. [DOI] [Google Scholar]
- 55.Kalish-Achrai N, Monsonego-Ornan E, Shahar R. Structure, composition, mechanics and growth of spines of the dorsal fin of blue tilapia Oreochromis aureus and common carp Cyprinus carpio. J. Fish Biol. 2017;90(5):2073–2096. doi: 10.1111/jfb.13287. [DOI] [PubMed] [Google Scholar]
- 56.Westrelin S, Balzani P, Haubrock PJ, Santoul F. Interannual variability in the trophic niche of young-of-year fish belonging to four piscivorous species coexisting in a natural lake. Freshw. Biol. 2023;68(3):487–501. doi: 10.1111/fwb.14041. [DOI] [Google Scholar]
- 57.Diehl S. Fish predation and benthic community structure: The role of omnivory and habitat complexity. Ecology. 1992;73(5):1646–1661. doi: 10.2307/1940017. [DOI] [Google Scholar]
- 58.Copp GH, et al. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009;10:252–282. doi: 10.1111/j.1467-2979.2008.00321.x. [DOI] [Google Scholar]
- 59.Sicuro B, Tarantola M, Valle E. Italian aquaculture and the diffusion of alien species: Costs and benefits. Aquac. Res. 2016;47(12):3718–3728. doi: 10.1111/are.12997. [DOI] [Google Scholar]
- 60.Guillerault N, et al. Does the non-native European catfish Silurus glanis threaten French river fish populations? Freshw. Biol. 2015;60(5):922–928. doi: 10.1111/fwb.12545. [DOI] [Google Scholar]
- 61.Haubrock PJ, Azzini M, Balzani P, Inghilesi AF, Tricarico E. When alien catfish meet—Resource overlap between the North American Ictalurus punctatus and immature European Silurus glanis in the Arno River (Italy) Ecol. Freshw. Fish. 2020;29(1):4–17. doi: 10.1111/eff.12481. [DOI] [Google Scholar]
- 62.UK, C. Perca fluviatilis (Linnaeus, 1758), perch.[invasive species]. Perca fluviatilis (Linnaeus, 1758), perch. [invasive species], (AQB ISC record) (2014).
- 63.Beeck, P. The early piscivory of European perch (Perca fluviatilis)—A neglected phenomenon with notable consequences for the population structure and fish community in lake ecosystems. PhD Thesis, University of Cologne, 115 (2003).
- 64.Neill S, Cullen JM. Experiments on whether schooling by their prey affects the hunting behaviour of cephalopods and fish predators. J. Zool. 1974;172(4):549–569. doi: 10.1111/j.1469-7998.1974.tb04385.x. [DOI] [Google Scholar]
- 65.Akçaalan R, Mazur-Marzec H, Zalewska A, Albay M. Phenotypic and toxicological characterization of toxic Nodularia spumigena from a freshwater lake in Turkey. Harmful Algae. 2009;8:273–278. doi: 10.1016/j.hal.2008.06.007. [DOI] [Google Scholar]
- 66.Haubrock PJ, Balzani P, Britton JR, Haase P. Using stable isotopes to analyse extinction risks and reintroduction opportunities of native species in invaded ecosystems. Sci. Rep. 2020;10(1):21576. doi: 10.1038/s41598-020-78328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swanson HK, et al. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology. 2015;96(2):318–324. doi: 10.1890/14-0235.1. [DOI] [PubMed] [Google Scholar]
- 68.Phillips DL, Gregg JW. Source partitioning using stable isotopes: Coping with too many sources. Oecologia. 2003;136:261–269. doi: 10.1007/s00442-003-1218-3. [DOI] [PubMed] [Google Scholar]
- 69.Parnell A. & Inger R. Simmr: A stable isotope mixing model. R package version 0.3. https://cran.r-project.org/web/packages/simmr/index.html (2016).
- 70.Boulêtreau S, Santoul F. The end of the mythical giant catfish. Ecosphere. 2016;7(11):1–5. doi: 10.1002/ecs2.1606. [DOI] [Google Scholar]
- 71.Bergström K, et al. Exceptional longevity in northern peripheral populations of Wels catfish (Silurus glanis) Sci. Rep. 2022;12(1):8070. doi: 10.1038/s41598-022-12165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER-stable isotope Bayesian ellipses in R. J. Anim. Ecol. 2011;80:595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study can be obtained from Ali Serhan Tarkan upon reasonable request by contacting the lead author.