Abstract
Background
Dravet syndrome (DS) is a severe developmental and epileptic encephalopathy characterized by drug-resistant, lifelong seizures. The management of seizures in DS has changed in recent years with the approval of new antiseizure medications (ASMs).
Objective
The aim of this study was to estimate the comparative efficacy and tolerability of the ASMs for the treatment of seizures associated with DS using a network meta-analysis (NMA).
Methods
Studies were identified by conducting a systematic search (week 4, January 2023) of the MEDLINE (accessed by PubMed), EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov) databases. Any randomized, controlled, double- or single-blinded, parallel-group study comparing at least one ASM therapy against placebo, another ASM, or a different dose of the same ASM in participants with a diagnosis of DS was identified. The efficacy outcomes were the proportions of participants with ≥ 50% (seizure response) and 100% reduction (seizure freedom) in baseline convulsive seizure frequency during the maintenance period. The tolerability outcomes included the proportions of patients who withdrew from treatment for any reason and who experienced at least one adverse event (AE). Effect sizes were estimated by network meta-analyses within a frequentist framework.
Results
Eight placebo-controlled trials were included, and the active add-on treatments were stiripentol (n = 2), pharmaceutical-grade cannabidiol (n = 3), fenfluramine hydrochloride (n = 2), and soticlestat (n = 1). The studies recruited 680 participants, of whom 409 were randomized to active treatments (stiripentol = 33, pharmaceutical-grade cannabidiol = 228, fenfluramine hydrochloride = 122, and soticlestat = 26) and 271 to placebo. Pharmaceutical-grade cannabidiol was associated with a lower rate of seizure response than fenfluramine hydrochloride (odds ratio [OR] 0.20, 95% confidence interval [CI] 0.07–0.54), and stiripentol was associated with a higher seizure response rate than pharmaceutical-grade cannabidiol (OR 14.07, 95% CI 2.57–76.87). No statistically significant differences emerged across the different ASMs for the seizure freedom outcome. Stiripentol was associated with a lower probability of drug discontinuation for any reason than pharmaceutical-grade cannabidiol (OR 0.45, 95% CI 0.04–5.69), and pharmaceutical-grade cannabidiol was associated with a lower proportion of participants experiencing any AE than fenfluramine hydrochloride (OR 0.22, 95% CI 0.06–0.78). Stiripentol had a higher risk of AE occurrence than pharmaceutical-grade cannabidiol (OR 75.72, 95% CI 3.59–1598.58). The study found high-quality evidence of efficacy and tolerability of the four ASMs in the treatment of convulsive seizures in DS.
Conclusions
There exists first-class evidence that documents the efficacy and tolerability of stiripentol, pharmaceutical-grade cannabidiol, fenfluramine hydrochloride, and soticlestat for the treatment of seizures associated with DS, and allows discussion about the expected outcomes regarding seizure frequency reduction and tolerability profiles.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-023-01936-y.
Key Points
| Placebo-controlled trials assessed stiripentol, pharmaceutical-grade cannabidiol, fenfluramine hydrochloride, and soticlestat as treatments for seizures associated with Dravet syndrome (DS). |
| There is high-quality evidence that documents the efficacy and tolerability profiles of either stiripentol, cannabidiol, fenfluramine, or soticlestat in people with DS. |
| Cannabidiol was associated with a lower rate of seizure response than fenfluramine and stiripentol. |
| Stiripentol was associated with a lower probability of drug discontinuation for any reason than cannabidiol. |
| Cannabidiol was associated with a lower proportion of participants experiencing any adverse event than fenfluramine and stiripentol. |
Introduction
Dravet syndrome (DS), formerly known as severe myoclonic epilepsy of infancy, is a severe developmental and epileptic encephalopathy with onset in the first year of life in previously healthy infants [1]. The syndrome is characterized by drug-resistant, lifelong seizures and is associated with comorbidities such as intellectual disability, behavior disturbances, sleep disorders, and gait problems that negatively impact the quality of life of the patients and their families [1]. Pathogenic variants in SCN1A, the gene that encodes the α-1 subunit of voltage-gated sodium channel Nav1.1, may lead to loss of channel function and are identified in over 80–90% of the individuals with DS [2]. In the central nervous system, NaV1.1 is highly expressed in many GABAergic inhibitory neurons, and the loss of function of the channel leads to hyperexcitability of the neuronal networks [3].
The pharmacological treatment of seizures associated with DS remains challenging. Seizures are highly pharmacoresistant, people usually require polytherapy to achieve a reduction in the seizure burden, and some drugs such as carbamazepine, oxcarbazepine, lamotrigine, phenytoin, and vigabatrin can even exacerbate seizures [4]. The management of seizures in DS has changed in recent years with the approval of new antiseizure medications (ASMs) for this condition, and the therapeutic landscape is still growing with treatment options in clinical development.
This study aims to systematically evaluate and summarize the available evidence about the efficacy and tolerability of the ASMs for managing seizures in DS and to assess their comparative efficacy and tolerability by performing a network meta-analysis (NMA) of randomized controlled trials (RCTs). Compared with a recent NMA that also assessed the efficacy and safety of adjunctive ASMs for DS [5], the present study aims to consider additional outcomes and provide subgroup analyses.
Methods
Search Strategy
The results were reported following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analyses [6]. Studies were identified by a systematic search (week 4, January 2023) of the MEDLINE (accessed by PubMed), EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and the US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov) databases. The search terms included ‘severe myoclonic epilepsy in infancy’, ‘Dravet syndrome’, ‘epilepsy’, and ‘seizure’; the search strategies are reported in Appendix I of the electronic supplementary material (ESM). There were no date or language restrictions. We reviewed the reference lists of retrieved studies to identify additional reports of relevant trials. Additional data were sought from the regulatory websites (i.e., the US Food and Drug Administration [FDA] and the European Medicines Agency [EMA]). The review protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42023404934).
Eligibility Criteria
We included any randomized, controlled, double- or single-blinded, parallel-group studies that compared at least one ASM therapy against placebo, another ASM, or a different dose of the same ASM. We considered participants with a diagnosis of DS regardless of age, sex, and ethnicity.
Outcome Measures
Efficacy outcomes were the proportion of individuals who achieved ≥ 50% reduction (seizure response) and the proportion of individuals who achieved 100% reduction (seizure freedom) from baseline in convulsive seizure frequency during the treatment period. Tolerability and safety outcomes were the proportions of individuals who withdrew from treatment for any reason and any adverse events (AEs), and who experienced at least one AE and at least one serious AE. Changes from baseline to the end of treatment in clinical global impression rated through the caregiver-reported Clinical Global Impression of Change (C-CGIC) were also reviewed.
Study Selection, Data Extraction, Assessment of the Risk of Bias, and Confidence in the Evidence
Two authors independently screened the records identified by the literature search and extracted the following data from the included studies: main study author, year of publication, study design, main inclusion criteria, treatment arms, number and demographics of participants, and number of participants experiencing each outcome. We solved any disagreement through discussion with a third review author. The risk of bias in the included studies was evaluated following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [7]. The confidence in the evidence derived from the NMA was judged through the online application CINeMA (http://cinema.ispm.ch/) [8].
Statistical Analysis
Pairwise meta-analyses were performed and heterogeneity among the trials was evaluated by means the Chi-square test and the I2 statistics for heterogeneity [9, 10]. If no substantial heterogeneity was present (p > 0.10), results were synthesized through a fixed-effects model; if the probability value was ≤ 0.10, a fixed- or random-effects model was adopted for I2 < 40% or ≥ 40%, respectively [11–13]. We presented heterogeneity statistics unless only one study contributed data and heterogeneity was not applicable.
Network meta-analyses within a frequentist framework were then performed assuming equal heterogeneity across all comparisons [14, 15]. We evaluated the assumption of transitivity (distributions of the potential effect modifiers, such as study and patient-level covariates, across pairwise comparisons) by looking at the similarities of studies in each comparison [16]. Local coherence, i.e., the statistical agreement between direct and indirect evidence for a specific comparison (coherence assumption), could not be assessed as there were no closed loops [17]. Whenever data were available, results were also provided by daily doses, with each dose of any ASM representing a separate node in the network. Secondary analyses were performed including only trials with a maintenance phase of 12 weeks or longer. Although there is no consensus about the optimal length of the treatment period, the guideline on clinical investigation of medicinal products in the treatment of epileptic disorders recommends that the maintenance phase should last at least 12 weeks to establish that efficacy is not short-lasting [18]. Effect sizes were estimated as odds ratios (ORs) with their 95% confidence intervals (CIs). Data analysis was performed using STATA®/IC 13.1 (StataCorp LLC, College Station, TX, USA).
Compliance with Ethics Guidelines
This manuscript is based on previously performed studies and does not contain any new studies with human participants or animals.
Results
Search Results and Characteristics of the Included Studies
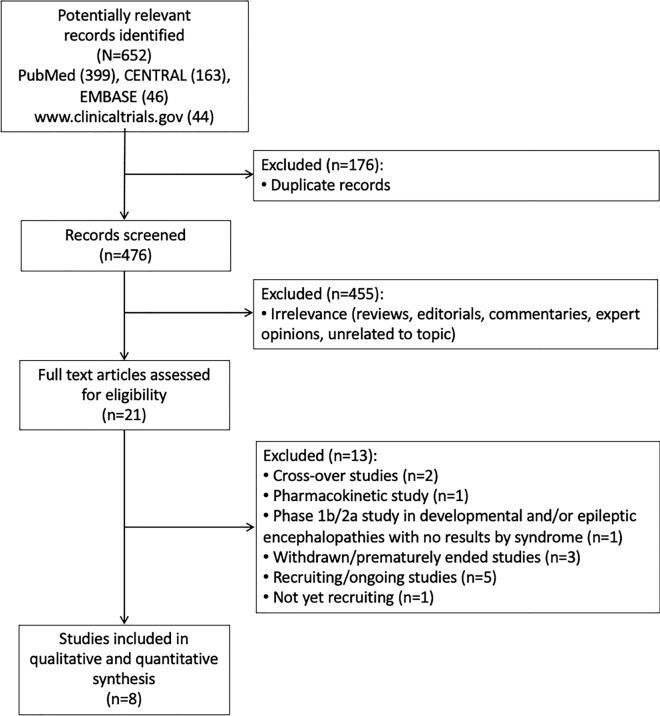
The literature search identified a total of 652 records, of which 8 RCTs were included in the qualitative and quantitative synthesis (Fig. 1) [19–26]. All the included trials were placebo-controlled studies, and the active add-on treatments were stiripentol (n = 2), pharmaceutical-grade cannabidiol (n = 3), fenfluramine hydrochloride (n = 2), and soticlestat (n = 1). All trials evaluated both the efficacy and safety of the active comparators, except one, which evaluated the safety and pharmacokinetics of cannabidiol [21]. The results of STICLO-Italy were published only in a preliminary form [20], but they have been reported in the FDA [27] and EMA [28] clinical review of the drug. Details about the RCTs are summarized in Table 1. In the STICLO studies, stiripentol was administered at a dosage of 50 mg/kg/day [19, 20]. Participants were randomized to receive pharmaceutical-grade cannabidiol at dosages of 5, 10, or 20 mg/kg/day in the GWPCARE1 Part A study [21], 20 mg/kg/day in the GWPCARE1 Part B study [22], and 10 or 20 mg/kg/day in the GWPCARE2 study [23]. Fenfluramine hydrochloride was administered at dosages of 0.2 mg and 0.8 mg/kg/day in the study by Lagae et al. [24], and at a dosage of 0.5 mg/kg/day in the study by Nabbout et al. [25]. In the study by Hahn et al. [26], soticlestat was titrated up to 600 mg/day for participants weighing ≥ 60 kg, with weight-based dosing used for those weighing < 60 kg. Overall, 80.3% of participants receiving soticlestat were included in dose level 3 (weight-adjusted equivalent of adult 300 mg twice daily), 9.9% in dose level 2 (weight-adjusted equivalent of adult 200 mg twice daily), and 7.0% in dose level 1 (weight-adjusted equivalent of adult 100 mg twice daily); no data regarding the study outcomes were provided according to the dose levels [26].
Fig. 1.
Study flow diagram. CENTRAL Cochrane Central Register of Controlled Trials
Table 1.
Characteristics of the included studies
| Study | Study design | Main inclusion criteria | Treatment arms |
|---|---|---|---|
| STICLO–France [19] |
Multicenter (France) Parallel-group, randomized, placebo-controlled clinical trial: 4-week observational baseline period 8-week double-blind treatment period |
Age 3–18 years Clinical diagnosis of Dravet syndrome Maximum weight 60 kg At least four generalized tonic-clonic or clonic seizures per month Current treatment with valproic acid and clobazam |
Placebo STP: 50 mg/kg/day |
| STICLO–Italy [20] |
Multicenter (Italy) Parallel-group, randomized, placebo-controlled clinical trial: 4-week observational baseline period 8-week double-blind treatment period |
Age 3–18 years Clinical diagnosis of Dravet syndrome Maximum weight 60 kg At least four generalized tonic-clonic or clonic seizures per month Current treatment with valproic acid and clobazam |
Placebo STP: 50 mg/kg/day |
| GWPCARE1 Part A [21] |
Phase III Multicenter (UK, USA) Parallel-group, randomized, placebo-controlled trial: 4-week observational baseline period 3-week double-blind treatment period (starting at 2.5 mg/kg/day and increasing by 2.5–5.0 mg/kg every other day to randomized dose) |
Age 4–10 years Documented history of Dravet syndrome not completely controlled by current ASMs Fewer than four convulsive seizures during the 4-week baseline period Current treatment with one or more ASMs at a stable dose for at least 4 weeks prior to screening |
Placebo CBD: 5, 10, and 20 mg/kg/day |
| GWPCARE1 Part B [22] |
Phase III Multicenter (Europe, USA) Parallel-group, randomized, placebo-controlled trial: 4-week observational baseline period 14-week double-blind treatment period (2-week titration, 12-week maintenance) |
Age 2–18 years Documented history of Dravet syndrome not completely controlled by current ASMs At least four convulsive seizures during the 4-week baseline period Current treatment with one or more ASMs at a stable dose for at least 4 weeks prior to screening |
Placebo CBD: 20 mg/kg/day |
| GWPCARE2 [23] |
Phase III Multicenter (Australia, Europe, Israel, USA) Parallel-group, randomized, placebo-controlled trial: 4-week observational baseline period 14-week double-blind treatment period (2-week titration, 12-week maintenance) |
Age 2–18 years Documented history of Dravet syndrome not completely controlled by current ASMs At least four convulsive seizures during the 4-week baseline period Current treatment with one or more ASMs at a stable dose for at least 4 weeks prior to screening |
Placebo CBD: 10 and 20 mg/kg/day |
| Lagae et al., 2019 [24] |
Phase III Multicenter (Australia, Canada, Europe, USA) Parallel-group, randomized, placebo-controlled clinical trial: 6-week observational baseline period 14-week double-blind treatment period (2-week titration, 12-week maintenance) |
Age 2–18 years Clinical diagnosis of Dravet Syndrome Convulsive seizures not completely controlled by current regimen of ASMs or other therapies At least six convulsive seizures during the baseline period, with at least two in the first 3 weeks and at least two in the second 3 weeks All medications or interventions for epilepsy stable for at least 4 weeks before screening and expected to remain stable throughout the trial |
Placebo FFA: 0.2 mg/kg/day FFA: 0.8 mg/kg/day |
| Nabbout et al., 2019 [25] |
Phase III Multicenter (Canada, Europe, UK, USA) Parallel-group, randomized, placebo-controlled clinical trial: 6-week observational baseline period 15-week double-blind treatment period (3-week titration, 12-week maintenance) |
Age 2–18 years Clinical diagnosis of Dravet Syndrome Convulsive seizures not completely controlled by current regimen of ASMs or other therapies, including stiripentol (plus valproate or clobazam, at a minimum) at a therapeutically relevant dose At least six convulsive seizures during the baseline period with at least two in the first 3 weeks and at least two in the second 3 weeks All medications or interventions for epilepsy stable for at least 4 weeks before screening and expected to remain stable throughout the trial |
Placebo FFA: 0.5 mg/kg/day |
| ELEKTRA [26] |
Phase II Multicenter (Australia, Canada, China, Israel, Poland, Portugal, Spain, USA) Parallel-group, randomized, placebo-controlled trial: 4- to 6-week observational screening/baseline period 20-week double-blind treatment period (8-week dose optimization, 12-week maintenance) |
Age 2–17 years Clinical diagnosis of Dravet syndrome Weight ≥ 10 kg at the screening visit History of inadequate response to at least two ASMs Current treatment with one to four ASMs at a stable dose for 4 weeks before screening At least three convulsive seizures per month during the past 3 months and at least three convulsive seizures during a minimum of 4 weeks during the baseline period |
Placebo Soticlestat: ≤ 600 mg/day |
ASMs antiseizure medications, CBD pharmaceutical-grade cannabidiol, FFA fenfluramine hydrochloride, STP stiripentol
The studies recruited 680 participants, of whom 409 were randomized to active treatments (stiripentol = 33, pharmaceutical-grade cannabidiol = 228, fenfluramine hydrochloride = 122, and soticlestat = 26) and 271 to placebo. Characteristics of the participants are summarized in Table 2.
Table 2.
Characteristics of the study participants
| Study | Treatment arm | No. of participants | Age, years | Male sex, % | No. of prior ASMs | No. of concomitant ASMs | Baseline monthly convulsive seizures frequency |
|---|---|---|---|---|---|---|---|
|
STICLO–France [19] |
Placebo | 20 | 9.3 (4.9) | 55.0 | 7.5 (2.9) | Valproate and clobazam ongoing drugs | 18.5 (17.0) |
| STP: 50 mg/kg | 21a | 9.4 (4.0) | 28.6 | 6.6 (2.5) | Valproate and clobazam ongoing drugs | 17.9 (17.3) | |
|
STICLO–Italy [20] |
Placebo | 11 | 8.7 (4.4) | 83.3 | – | Valproate and clobazam ongoing drugs | 27.4 (28.6) |
| STP: 50 mg/kg | 12 | 9.2 (3.6) | 66.7 | – | Valproate and clobazam ongoing drugs | 33.6 (28.2) | |
| GWPCARE1A [21] | Placebo | 7 | 7.0 (0.9) | 71.4 | 5 (1–5)b | 2 (1–3)b | – |
| CBD: 5 mg/kg | 10 | 7.2 (1.9) | 50.0 | 3 (0–11)b | 3 (1–4)b | – | |
| CBD: 10 mg/kg | 8 | 7.4 (2.1) | 37.5 | 3.5 (0–5)b | 3 (2–3)b | – | |
| CBD: 20 mg/kg | 9 | 8.7 (1.8) | 33.3 | 4 (2–8)b | 3 (1–4)b | – | |
| GWPCARE1B [22] | Placebo | 59 | 9.8 (4.8) | 45.8 | 4 (0–14)b | 3 (1–5)b | 14.9 (7.0–36.0)c |
| CBD: 20 mg/kg | 61 | 9.7 (4.7) | 57.4 | 4 (0–26)b | 3 (1–5)b | 12.4 (6.2–28.0)c | |
| GWPCARE2 [23] | Placebo | 65 | 9.6 (range 2.2–18.1) | 47.7 | 4 (0–11)b | 3 (1–5)b | 16.6 (7.0–51.1)c |
| CBD: 10 mg/kg | 66 | 9.2 (range 2.3–17.7) | 40.9 | 4 (0–19)b | 3 (1–5)b | 13.5 (6.0–31.2)c | |
| CBD: 20 mg/kg | 67 | 9.3 (range 2.2–18.9) | 53.7 | 4 (0–11)b | 3 (1–4)b | 9.0 (6.3–21.2)c | |
| Lagae et al., 2019 [24] | Placebo | 40 | 9.2 (5.1) | 52.5 | – | 2.5 (0.9) | 27.3 (3.3–147.3)c |
| FFA: 0.2 mg/kg | 39 | 9.0 (4.5) | 56.4 | – | 2.5 (1.1) | 17.5 (4.7–623.5)c | |
| FFA: 0.8 mg/kg | 40 | 8.8 (4.4) | 52.5 | – | 2.3 (0.9) | 20.7 (4.8–124)c | |
| Nabbout et al., 2019 [25] | Placebo | 44 | 9.4 (5.1) | 61.4 | – | 3.4 (0.6) | 10.7 (3–163)c |
| FFA: 0.5 mg/kg | 43 | 8.8 (4.6) | 53.5 | – | 3.7 (0.8) | 14.0 (3–213)c | |
| ELEKTRA [26] | Placebo | 25 | 8.8 (4.5) | 56.0 | – | – | 13.2 (23.9) |
| Soticlestat: ≤ 600 mg | 26 | 8.7 (3.9) | 65.4 | – | – | 13.8 (11.0) |
Data are expressed as mean (SD) unless otherwise specified
ASMs antiseizure medications, CBD pharmaceutical-grade cannabidiol, FFA fenfluramine hydrochloride, IQR interquartile range, SD standard deviation, STP stiripentol
aThe number of patients originally allocated to stiripentol was 22 but one patient was considered not evaluable as they were not compliant
bMedian (range)
cMedian (IQR)
All trials were judged to have used adequate methods of random sequence generation and allocation concealment. We rated six trials at low risk of performance and detection bias since blinding was ensured by matching placebo, and participants, investigators, and study personnel were masked to the assigned treatment; we considered the STICLO studies at unclear risk of performance and detection bias because the trials were defined as ‘double-blind’ but with no further information to permit judgment [19, 20]. The risk of attrition bias was low as participants lost to follow-up, withdrawals, and corresponding reasons were documented, and there was no suspicion of selective outcome reporting across the trials. A summary of bias assessment risk is shown in ESM Table e-1.
Efficacy Outcomes
Data regarding the proportion of participants who achieved seizure response and seizure freedom were available for all studies. Most studies reported efficacy outcomes over the whole treatment period, apart from the STICLO studies, which assessed the frequency of seizures during the second month of the 8-week double-blind treatment period compared with baseline. The definitions of ‘convulsive seizures’ adopted in any of the included trials are summarized in ESM Table e-2. The network plots of treatment comparisons for the efficacy outcomes are shown in ESM Fig. e-1.
At the pairwise meta-analyses, all ASMs were associated with a higher responder rate than placebo, and stiripentol was associated with a higher rate of seizure freedom than placebo (ESM Table e-3).
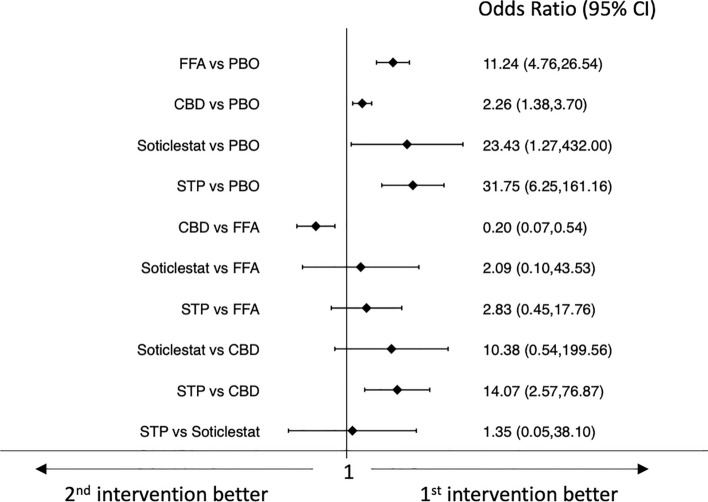
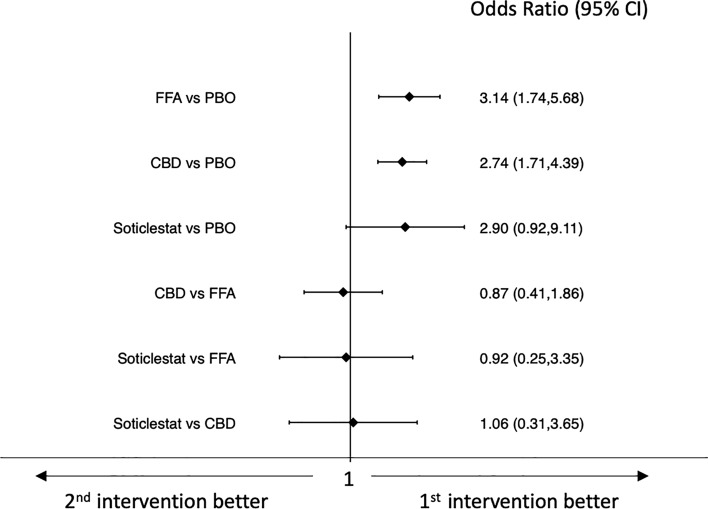
Results of the network meta-analyses of efficacy outcomes are shown in Figs. 2 and 3. All drugs were associated with higher efficacy than placebo for the achievement of seizure response. Pharmaceutical-grade cannabidiol was associated with a lower rate of seizure response than fenfluramine hydrochloride (OR 0.20, 95% CI 0.07–0.54) and stiripentol was associated with a higher seizure response rate than pharmaceutical-grade cannabidiol (OR 14.07, 95% CI 2.57–76.87) (Fig. 2).
Fig. 2.
Interval plot for the efficacy outcome: seizure response. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo, STP stiripentol
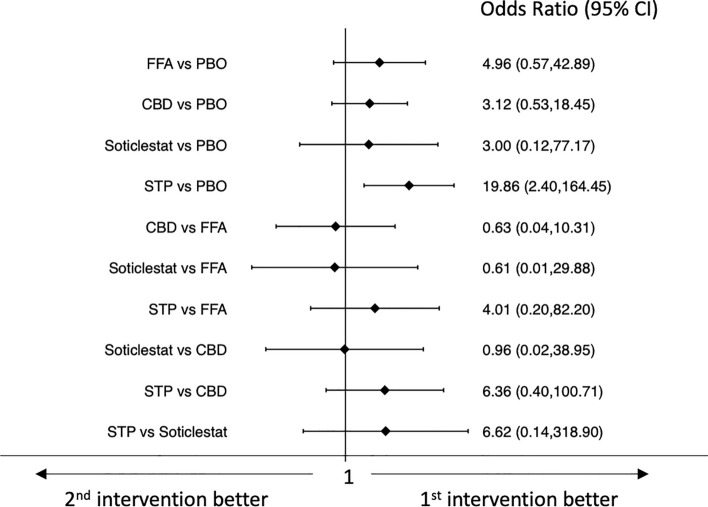
Fig. 3.
Interval plot for the efficacy outcome: seizure freedom. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo, STP stiripentol
Stiripentol was the only drug associated with a higher rate of seizure freedom than placebo (OR 19.86, 95% CI 2.40–164.45); no statistically significant differences emerged in the comparisons between the different ASMs (Fig. 3). Confidence in the evidence for the efficacy outcomes is summarized in ESM Appendix II.
Results by daily doses are shown in ESM Fig. e-2 and ESM Fig. e-3. The rates of seizure response were higher with fenfluramine hydrochloride 0.8 mg/kg/day than 0.2 mg/kg/day (OR 3.32, 95% CI 1.32–8.37), and with stiripentol 50 mg/kg/day than pharmaceutical-grade cannabidiol 10 mg/kg/day (OR 15.64, 95% CI 2.69–90.92) and 20 mg/kg/day (OR 13.47, 95% CI 2.44–74.39). Pharmaceutical-grade cannabidiol at dosages of 10 mg/kg/day (OR 0.08, 95% CI 0.02–0.45) and 20 mg/kg/day (OR 0.10, 95% CI 0.02–0.50) was associated with a lower rate of seizure responder than fenfluramine hydrochloride 0.5 mg/kg/day; the treatment with pharmaceutical-grade cannabidiol at dosages of 10 mg/kg/day (OR 0.14, 95% CI 0.04–0.53) and 20 mg/kg/day (OR 0.16, 95% CI 0.05–0.57) was associated with a lower probability of seizure response than fenfluramine hydrochloride 0.8 mg/kg/day (ESM Fig. e-2). There were no differences between the ASMs in the proportions of participants who reached seizure freedom (ESM Fig. e-3).
Tolerability Outcomes
All RCTs provided data on the proportions of participants who discontinued treatment for any reason and for AEs. Data regarding the proportions of participants who experienced any AE and any serious AE were available for six and seven trials, respectively. ESM Fig. e-1 shows the network plots of treatment comparisons for the tolerability outcomes.
At the pairwise meta-analyses, pharmaceutical-grade cannabidiol was associated with higher rates of treatment discontinuation for any reason and for AEs than placebo. Fenfluramine hydrochloride and stiripentol were associated with a higher rate of AEs in comparison with placebo (ESM Table e-3).
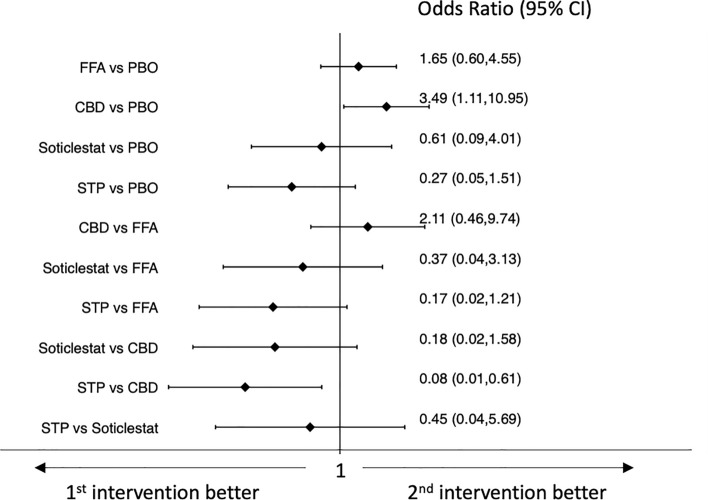
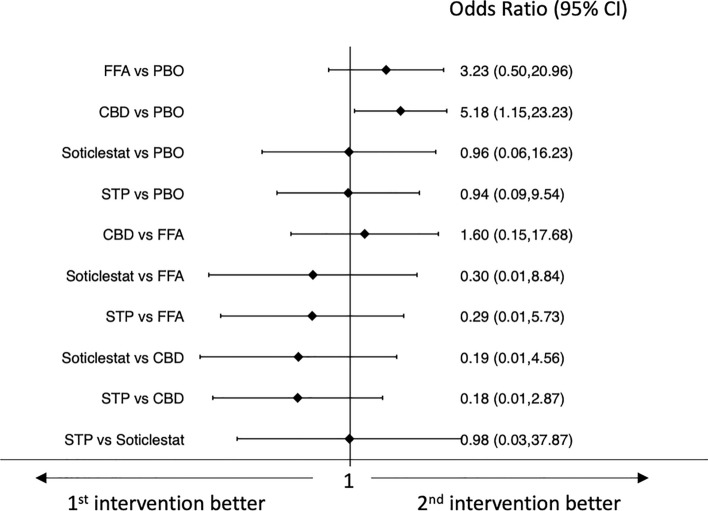
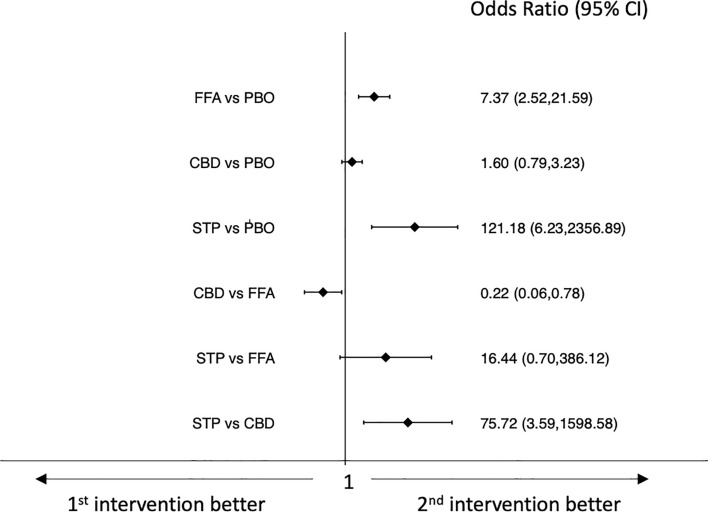
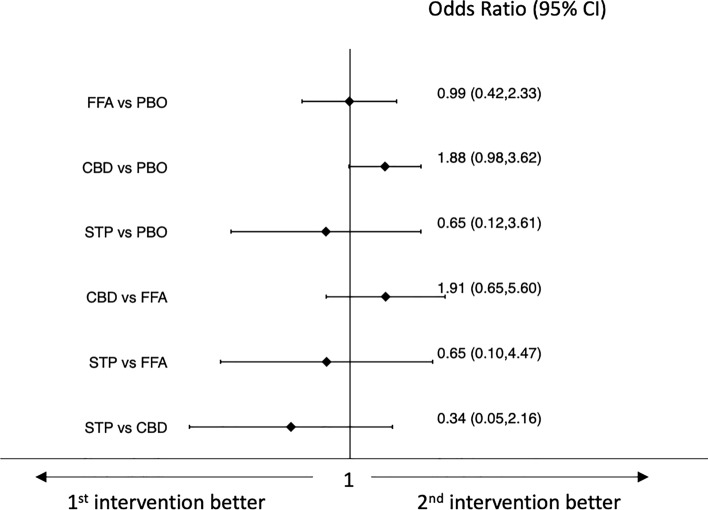
Results of the network meta-analyses of tolerability outcomes are shown in Figs. 4, 5, 6, 7. Pharmaceutical-grade cannabidiol was associated with higher rates of treatment discontinuation for any reason (OR 3.49, 95% CI 1.11–10.95) and for AEs (OR 5.18, 95% CI 1.15–23.23) than placebo, and stiripentol was associated with a lower probability of drug discontinuation for any reason than pharmaceutical-grade cannabidiol (OR 0.08, 95% CI 0.01–0.61) (Figs. 4 and 5). Fenfluramine hydrochloride (OR 7.37, 95% CI 2.52–21.59) and stiripentol (OR 121.18, 95% CI 6.23–2356.89) were associated with a higher risk of experiencing AEs than placebo; pharmaceutical-grade cannabidiol was associated with a lower proportion of participants experiencing any AE than fenfluramine hydrochloride (OR 0.22, 95% CI 0.06–0.78); and stiripentol had a higher risk of AE occurrence than pharmaceutical-grade cannabidiol (OR 75.72, 95% CI 3.59–1598.58) (Fig. 6). There were no statistically significant differences between the ASMs and placebo and across the individual ASMs in the risk of the occurrence of serious AEs (Fig. 7). Confidence in the evidence for the tolerability outcomes is summarized in ESM Appendix II.
Fig. 4.
Interval plot for the tolerability outcome: discontinuation for any reason. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo, STP stiripentol
Fig. 5.
Interval plot for the tolerability outcome: discontinuation for adverse events. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo, STP stiripentol
Fig. 6.
Interval plot for the tolerability outcome: occurrence of adverse events. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo, STP stiripentol
Fig. 7.
Interval plot for the tolerability outcome: occurrence of serious adverse events. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo, STP stiripentol
Results by daily doses are shown in ESM Figs. e-4–7. Pharmaceutical-grade cannabidiol at a dosage of 20 mg/kg/day was associated with a higher risk of treatment discontinuation than fenfluramine hydrochloride 0.2 mg/kg/day (OR 29.93, 95% CI 1.21–741.84), and stiripentol at a dosage of 50 mg/kg/day was associated with a lower rate of treatment withdrawal than fenfluramine hydrochloride 0.5 mg/kg/day (OR 0.10, 95% CI 0.01–0.95) and pharmaceutical-grade cannabidiol 20 mg/kg/day (OR 0.07, 95% CI 0.01–0.53). The risk of occurrence of AEs was lower with pharmaceutical-grade cannabidiol at a dosage of 10 mg/kg/day than with fenfluramine hydrochloride 0.2 mg/kg/day (OR 0.10, 95% CI 0.02–0.63) and 0.8 mg/kg/day (OR 0.10, 95% CI 0.02–0.61). Stiripentol administered at a dosage of 50 mg/kg/day was associated with a higher rate of participants who experienced any AEs than fenfluramine hydrochloride 0.5 mg/kg/day (OR 60.59, 95% CI 1.30–2821.73) and pharmaceutical-grade cannabidiol at dosages of 5 mg/kg/day (OR 66.14, 95% CI 1.94–2252.18), 10 mg/kg/day (OR 118.91, 95% CI 5.31–2663.41), and 20 mg/kg/day (OR 61.35, 95% CI 2.86–1315.81).
Global Functioning
Five trials reported data on the proportion of participants who had any improvement (slightly improved, much improved, or very much improved) from baseline in overall condition at the last visit according to C-CGIC (ESM Fig. e-1). At the pairwise meta-analyses, pharmaceutical-grade cannabidiol and fenfluramine hydrochloride were associated with higher rates of improvement at the C-CGIC than placebo (ESM Table e-3). The NMA did not identify differences between the ASMs (Fig. 8) and the different doses of the ASMs (ESM Fig. e-8) for the improvement observed at the C-CGIC. The confidence in the evidence for the outcome of global functioning is summarized in ESM Appendix II.
Fig. 8.
Interval plot for the global functioning outcome: improvement at caregiver-reported Clinical Global Impression of Change. CBD pharmaceutical-grade cannabidiol, CI confidence interval, FFA fenfluramine hydrochloride, PBO placebo
Study Outcomes in Trials with a Maintenance Period of at Least 12 Weeks
Five RCTs had a maintenance period of 12 weeks or longer and were included in the secondary analyses [22–26]. Either pharmaceutical-grade cannabidiol, fenfluramine hydrochloride, or soticlestat were associated with higher rates of seizure response than placebo, and pharmaceutical-grade cannabidiol was associated with a lower probability of achieving the seizure response than fenfluramine hydrochloride (OR 0.20, 95% CI 0.07–0.54) [ESM Fig. e-9]. No differences were identified between the ASMs regarding the outcome of seizure freedom (ESM Fig. e-10).
Pharmaceutical-grade cannabidiol was associated with a higher risk of treatment discontinuation for any reason (OR 3.98, 95% CI 1.17–13.59) [ESM Fig. e-11] and for AEs (OR 7.51, 95% CI 1.36–41.48) [ESM Fig. e-12] than placebo. Fenfluramine hydrochloride was associated with a higher risk of experiencing AEs than placebo (OR 7.37, 95% CI 2.52–21.59) and pharmaceutical-grade cannabidiol had a lower risk of occurrence of AEs than fenfluramine hydrochloride (OR 0.25, 95% CI 0.07–0.91) [ESM Fig. e-13]. There were no differences between the interventions in the proportions of participants who experienced serious AEs (ESM Fig. e-14). Either pharmaceutical-grade cannabidiol or fenfluramine hydrochloride were associated with higher rates of improvement at the C-CGIC than placebo (ESM Fig. e-15).
Discussion
There is high-quality evidence from randomized, placebo-controlled trials about the efficacy of either stiripentol, pharmaceutical-grade cannabidiol, fenfluramine hydrochloride, or soticlestat as treatments for convulsive seizures associated with DS. Each of these drugs acts by different mechanisms of action. The antiseizure activities of cannabidiol are mainly due to the inhibition of the reuptake of adenosine and modulation of the Ca2+ intracellular levels by inhibiting the G-protein-coupled receptor GPR55, desensitizing the transient receptor channel TRPV1 and modulating the M-current of Kv7.2/7.3 [29, 30]. Fenfluramine has a multimodal mechanism of action enhancing serotoninergic transmission both directly by acting as an agonist of 5-HT1D, 5-HT2A, and 5-HT2C, and indirectly by increasing extracellular serotonin, while it also positively modulates σ-1 receptors [31–34]. Stiripentol potentiates the GABAergic transmission by inhibiting the synaptosomal uptake of GABA and/or GABA transaminase and by acting as a positive allosteric modulator of GABAA receptors at a site that is different from benzodiazepines [27] or inhibiting spike-and-wave discharges by targeting T-type calcium channels [35]. Moreover, stiripentol potentiates the efficacy of other ASMs such as clobazam, sodium valproate, phenobarbital, carbamazepine, and phenytoin through the metabolic inhibition of several isoenzymes, in particular cytochrome P450 (CYP) 2C19 and CYP3A4 [27]. Soticlestat is a selective inhibitor of cholesterol 24-hydroxylase, which plays a role in the homeostasis of cholesterol in the brain [36]. The reduction in the levels of 24S-hydroxycholesterol induced by soticlestat treatment has been shown to decrease glutamatergic signaling via multimodal mechanisms and potentially reduce inflammation, which may affect seizure susceptibility [36]. The NMA suggested that fenfluramine hydrochloride may have greater efficacy than pharmaceutical-grade cannabidiol in the reduction of convulsive seizure frequency, and fenfluramine hydrochloride at dosages of 0.5 and 0.8 mg/kg/day was associated with a higher probability of seizure response than pharmaceutical-grade cannabidiol at either the daily dosages of 10 or 20 mg/kg/day. Interestingly, physicians who participated in the international consensus on the management of DS perceived fenfluramine hydrochloride as the most efficacious maintenance medication for focal or generalized convulsive seizures, and 84% of them believed it should be used as a first or second line [4].
Differences can be identified in the tolerability profiles of the ASMs. The AEs most frequently encountered in clinical trials with the use of pharmaceutical-grade cannabidiol included somnolence, decreased appetite, and diarrhea [21–23, 37]. The rate of somnolence was higher when cannabidiol was coadministered with clobazam, likely due to the pharmacokinetic interaction that leads to an increase in the serum levels of clobazam and its active metabolite. Elevation in the concentrations of transaminases by threefold or more of the upper limit of the normal range occurred in approximately 15% of the participants randomized to pharmaceutical-grade cannabidiol and was the main cause for treatment discontinuation [21–23]. Importantly, in all cases, laboratory abnormalities reversed either spontaneously, when the dose of a concomitant ASM was decreased, or when pharmaceutical-grade cannabidiol was tapered or discontinued. The concomitant administration of valproic acid and high levels of transaminases at baseline can increase the risk of hepatotoxicity, which usually develops during the first 30 days and rarely after 100 days of pharmaceutical-grade cannabidiol use [38].
The non-cardiovascular AEs associated with fenfluramine hydrochloride treatment during the clinical trials were decreased appetite, diarrhea, fatigue, and weight loss [24, 25, 39]. Fenfluramine was originally developed as an anorectic drug, and decreased appetite represented the most common AE. Clinically meaningful weight loss, defined as a loss above 7% of body weight, occurred in around 5–20% of participants randomized to fenfluramine hydrochloride and up to 5% of participants randomized to placebo; of note, weight loss did not result in treatment withdrawal in any case [24, 25, 39]. Regarding the cardiovascular safety of fenfluramine hydrochloride, the echocardiographic monitoring during the trials did not identify pathological functional changes in cardiac valves and no signs of pulmonary arterial hypertension in any participant at any time [24, 25]; however, it is worth noting the short observation period in RCTs.
The main AEs associated with the addition of stiripentol in STICLO trials were somnolence, decreased appetite, and decreased weight [19, 27], and in some cases, these AEs disappeared after the decrease in the dose of comedications as planned by the protocol. During the treatment period, an adjustment in sodium valproate and clobazam dose was allowed: the sodium valproate dose could be decreased by 10 mg/kg/day in the event of a severe decrease in appetite or weight loss, and clobazam dose could be decreased by 25% (and then an additional 25% if the AE persists) in the case of drowsiness or hyperexcitability [19, 27]. Hematological AEs observed in the STICLO studies included declining neutrophil and platelet counts. Furthermore, blood counts should be assessed before starting treatment with stiripentol and checked every 6 months unless otherwise clinically indicated [40]. In the trial of soticlestat included in this NMA, lethargy and constipation were the only AEs that occurred, with a difference of ≥ 5% compared with placebo in the pooled cohort of participants with DS and Lennox–Gastaut syndrome; no details were available for the AEs that developed in the DS population only [26]. In a phase Ib/IIa randomized, double-blind, placebo-controlled trial of soticlestat in adults with developmental and epileptic encephalopathies, the only non-serious AEs that occurred in more than one participant in the soticlestat group were dysarthria, lethargy, upper respiratory tract infection, fatigue, and headache. Of these, fatigue and headache were each reported in one placebo-treated participant. Although the trial included one individual with DS, no details about the tolerability were provided according to the diagnoses of participants [41]. The NMA suggested that pharmaceutical-grade cannabidiol could be better tolerated than stiripentol, and pharmaceutical-grade cannabidiol at a dosage of 10 mg/kg/day was associated with a lower proportion of participants experiencing AEs than fenfluramine hydrochloride.
Considerations are required according to known and potential drug–drug interactions, which can significantly influence the efficacy and tolerability profiles of ASMs. Stiripentol is known to inhibit CYP2C19, CYP3A4, CYP2D6, and likely other enzymes, leading to an increase in the plasma levels of several drugs [40]. The combination of stiripentol with clobazam, which leads to increased levels of clobazam and its active metabolite N-desmethylclobazam, represents a mainstay of DS treatment. Stiripentol also inhibits the metabolism of fenfluramine hydrochloride and leads to an increase in the plasma concentrations of the latter; this interaction has imposed a limitation on the maximum fenfluramine hydrochloride dose to be used when combined with stiripentol to reduce the risk of adverse effects [42]. Of note, the use of fenfluramine hydrochloride at a dosage of 0.5 mg/kg/day relied on the use of concomitant stiripentol (plus valproate or clobazam, at a minimum), which is known to increase fenfluramine plasma levels, as an entry criterion to the trial [25]. Pharmaceutical-grade cannabidiol can both induce and inhibit several enzymes involved in the metabolism of drugs and cause drug–drug interactions. By inhibiting the catalytic activity of CYP2C19, cannabidiol determines a two- to fourfold increase in plasma concentrations of N-desmethylclobazam [21, 43, 44]. An interaction also exists with fenfluramine hydrochloride, whose levels are increased by the concomitant use of cannabidiol; however, dose adjustments are unlikely to be required and there are no restrictions in the concomitant use of these two drugs [42]. Of note, the potential impact of the interaction between fenfluramine and cannabidiol was not explored in the RCTs as people taking cannabidiol were excluded. Fenfluramine is reported to induce CYP3A4 and CYP2B6 and minimally inhibit CYP2D6, suggesting potential interactions with other drugs [42].
This systematic review represents a comprehensive analysis of the currently available evidence from RCTs regarding the ASMs for treating convulsive seizures in DS. It performs a comparative assessment of the efficacy and tolerability of the different drugs and considers additional outcomes and subgroup analyses compared with prior work in the literature [5]. The results by daily doses may provide clinically useful information, and the subgroup analyses of trials with a maintenance phase of at least 12 weeks may minimize the risk to assess effects that are short-lasting [18]. However, some limits need to be considered in the interpretation of the results. Only one single RCT of soticlestat was included and some of the estimates were associated with wide CIs. Furthermore, data regarding AEs associated with soticlestat were only available for the pooled population of participants with DS and Lennox–Gastaut syndrome and could not be considered in the NMA. In this regard, a global trial testing soticlestat as add-on therapy in a larger cohort of people with DS is ongoing and is expected to enrol approximately 142 participants worldwide (ClinicalTrials.gov: NCT04940624). Randomized, controlled trials were available for pharmaceutical-grade CBD oral solution, and results cannot be translated to other cannabis-based or non-purified products. The low rates of seizure-free individuals across the trials may have hampered the likelihood to reach a statistical power adequate to detect differences between the interventions and led to extreme results due to the imprecision and uncertainty. None of the included trials were rated as having a high risk of bias, but pharmaceutical companies sponsored all of them. Some differences in the characteristics of studies and populations as well as the broad interpretation and different definition of ‘convulsive seizures’ across the trials need to be acknowledged. The general shortcomings of the clinical trials, such as their short duration, may also limit the external validity of the findings. A further overall weakness of the RCTs is that they have all been conducted in children and did not include adults with DS. Real-life and cost-effectiveness data will complement the current evidence and better clarify the therapeutic potentialities and positioning of these ASMs.
Conclusion
According to the practical guides, valproic acid and clobazam represent the first-line drugs for treating DS, although no randomized, placebo-controlled studies have specifically investigated these medications in people with this developmental and epileptic encephalopathy [45]. Nowadays, there exists first-class evidence that documents the efficacy and tolerability of stiripentol, pharmaceutical-grade cannabidiol, fenfluramine hydrochloride, and soticlestat for the treatment of seizures associated with DS, and allows physicians to have reliable data and discuss with the caregivers of people with DS about the expected outcomes regarding seizure frequency reduction and tolerability profiles. Three of these drugs have already been approved by regulatory agencies, and the demonstrated efficacy may support their earlier use in the treatment paradigm [4]. Although only head-to-head trials may draw definitive evidence about how one drug compares with the others, similar studies are unlikely to be conducted. In this scenario, NMAs can offer reliable cues to decipher the comparative efficacy and tolerability of ASMs and inform evidence-based healthcare decision making [46–49]. The indirect comparative analyses suggested fenfluramine hydrochloride and stiripentol to be more efficacious therapeutic options than pharmaceutical-grade cannabidiol, and pharmaceutical-grade cannabidiol to be overall better tolerated than stiripentol and fenfluramine hydrochloride. These data support the recently proposed therapeutic algorithm, which considers fenfluramine hydrochloride and stiripentol as first- or second-line and pharmaceutical-grade cannabidiol as third-line maintenance therapies for seizures due to DS [4]. Importantly, treatment needs to be individualized on a case-by-case basis and the selection of the drug for any person with DS should rely on an evidence-based framework, integrating the best available evidence for efficacy and tolerability, risk of drug interactions, presence of comorbidities, and preferences of the caregivers [4, 51].
New options are under development for the treatment of DS. Randomized trials to investigate serotonin modulators such as clemizole (ClinicalTrials.gov: NCT04462770), lorcaserin (ClinicalTrials.gov: NCT04572243), and LP352 (ClinicalTrials.gov: NCT05364021) are recruiting participants. Furthermore, strategies targeting the underlying cause of DS, such as antisense oligonucleotides [50] and gene therapy (ClinicalTrials.gov: NCT05419492) are on the horizon. Although current treatments focused mainly on seizure reduction, non-seizure-related comorbidities should also be addressed. Disease-modifying therapies that can interfere with the neurobiological process of the disease may have favorable impacts on the overall quality of life for both people with DS and caregivers. Functional analysis for evaluating the consequences of pathogenic variants may also be relevant to tailor the appropriate treatment [52]. Finally, the research activity and progress for DS may lead to further advances in the treatment of other genetic epilepsy syndromes and developmental epileptic encephalopathies.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement.
Conflicts of interest
Simona Lattanzi has received speaker’s or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, and UCB Pharma, and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, Eisai, GW Pharmaceuticals and Rapport Therapeutics outside the submitted work. Eugen Trinka received speaker’s honoraria from UCB Pharma, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, Sunovion Pharmaceuticals Inc., LivaNova and Novartis; consultancy funds from UCB Pharma, Biogen, Gerot-Lannach, Bial, Eisai, Takeda, Newbridge, GW Pharmaceuticals, Sunovion Pharmaceuticals Inc., and Novartis outside the submitted work; and directorship funds from Neuroconsult GmbH. Furthermore, his institution received grants from Biogen, Red Bull, Merck, UCB Pharma, European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, and Bundesministerium für Wissenschaft und Forschung. Emilio Russo has received speaker’s fees or funding from, and has participated in advisory boards for, Angelini, Arvelle Therapeutics, Eisai, Kolfarma, Pfizer, GW Pharmaceuticals, UCB Pharma, and Lundbeck outside the submitted work. Sara Matricardi has served on the advisory board for UCB Pharma, and has received consultancy fees from Eisai outside the submitted work. Stefano Meletti received research grant support from the Ministry of Health and the non-profit organization Foundation ‘Fondazione Cassa di Risparmio di Modena—FCRM’; and has received personal compensation as a scientific advisory board member for UCB and Eisai outside the submitted work. Pasquale Striano received fees and research grants from GW Pharmaceuticals, Zogenyx, Biomarin, and Kolfarma outside the submitted work. Cinzia Del Giovane, Payam Tabaee Damavandi, Mauro Silvestrini, and Francesco Brigo have no conflicts of interest to declare that are directly relevant to the contents of this study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
SL designed and conceptualized the study, carried out the data analyses, and drafted the manuscript. CDG carried out the data analyses. All authors critically revised the manuscript for important intellectual content, approved the final manuscript for submission, and agree to be accountable for all aspects of this work.
References
- 1.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl. 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 2.Cetica V, Chiari S, Mei D, Parrini E, Grisotto L, Marini C, et al. Clinical and genetic factors predicting Dravet syndrome in infants with SCN1A mutations. Neurology. 2017;88:1037–1044. doi: 10.1212/WNL.0000000000003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588(Pt 11):1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirrell EC, Hood V, Knupp KG, Meskis MA, Nabbout R, Scheffer IE, Wilmshurst J, Sullivan J. International consensus on diagnosis and management of Dravet syndrome. Epilepsia. 2022;63:1761–1777. doi: 10.1111/epi.17274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Zhang L, Zhou X, Wang J, Zheng X, Hu H, Wu D. Efficacy and safety of adjunctive antiseizure medications for Dravet syndrome: a systematic review and network meta-analysis. Front Pharmacol. 2022;13:980937. doi: 10.3389/fphar.2022.980937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. In: Higgins JPT, Green S, editors. The Cochrane Collaboration; 2011. http://handbook-5-1.cochrane.org/. Accessed Mar 2023.
- 8.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082. doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, Silvestrini M. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78:1791–1804. doi: 10.1007/s40265-018-0992-5. [DOI] [PubMed] [Google Scholar]
- 12.Lattanzi S, Trinka E, Zaccara G, Striano P, Del Giovane C, Silvestrini M, Brigo F. Adjunctive cenobamate for focal-onset seizures in adults: a systematic review and meta-analysis. CNS Drugs. 2020;34:1105–1120. doi: 10.1007/s40263-020-00759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lattanzi S, Grillo E, Brigo F, Silvestrini M. Efficacy and safety of perampanel in Parkinson’s disease. A systematic review with meta-analysis. J Neurol. 2018;265:733–740. doi: 10.1007/s00415-017-8681-y. [DOI] [PubMed] [Google Scholar]
- 14.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 15.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159:130–137. doi: 10.7326/0003-4819-159-2-201307160-00008. [DOI] [PubMed] [Google Scholar]
- 17.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42:332–345. doi: 10.1093/ije/dys222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Medicines Agency. Committee for medicinal products for human use. Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-epileptic-disorders-revision-2_en.pdf. Accessed Mar 2023.
- 19.Chiron C, Marchand MC, Tran A, Rey E, d'Athis P, Vincent J, Dulac O, Pons G. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000;356:1638–1642. doi: 10.1016/S0140-6736(00)03157-3. [DOI] [PubMed] [Google Scholar]
- 20.Guerrini R, Tonnelier S, d'Athis P, Rey E, Vincent J, Pons G, et al. Stiripentol in severe myoclonic epilepsy in infancy (SMEI): a placebo-controlled Italian trial. Epilepsia. 2002;43(Suppl. 8):155. [Google Scholar]
- 21.Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al.; GWPCARE1 Part A Study Group. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–11. [DOI] [PMC free article] [PubMed]
- 22.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al.; Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the Dravet syn-drome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed]
- 23.Miller I, Scheffer IE, Gunning B, Sanchez-Carpintero R, Gil-Nagel A, Perry MS, Saneto RP, Checketts D, Dunayevich E, Knappertz V; GWPCARE2 Study Group. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77:613–21. [DOI] [PMC free article] [PubMed]
- 24.Lagae L, Sullivan J, Knupp K, Laux L, Polster T, Nikanorova M, Devinsky O, Cross JH, Guerrini R, Talwar D, Miller I, Farfel G, Galer BS, Gammaitoni A, Mistry A, Morrison G, Lock M, Agarwal A, Lai WW, Ceulemans B; FAiRE DS Study Group. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:2243–54. [DOI] [PubMed]
- 25.Nabbout R, Mistry A, Zuberi S, Villeneuve N, Gil-Nagel A, Sanchez-Carpintero R, Stephani U, Laux L, Wirrell E, Knupp K, Chiron C, Farfel G, Galer BS, Morrison G, Lock M, Agarwal A, Auvin S; FAiRE, DS Study Group. Fenfluramine for treatment-resistant seizures in patients with Dravet syndrome receiving stiripentol-inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77:300–08. [DOI] [PMC free article] [PubMed]
- 26.Hahn CD, Jiang Y, Villanueva V, Zolnowska M, Arkilo D, Hsiao S, Asgharnejad M, Dlugos D. A phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of soticlestat as adjunctive therapy in pediatric patients with Dravet syndrome or Lennox-Gastaut syndrome (ELEKTRA) Epilepsia. 2022;63:2671–2683. doi: 10.1111/epi.17367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center for Drug Evaluation and Research. Application number:206709Orig1s000, 207223Orig1s000. Clinical Reviews 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/206709Orig1s000,207223Orig1s000MedR.pdf. Accessed Mar 2023.
- 28.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). International non-proprietary name: stiripentol. Scientific Discussion. https://www.ema.europa.eu/en/documents/scientific-discussion/diacomit-epar-scientific-discussion_en.pdf. Accessed Mar 2023.
- 29.Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730. doi: 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Pozo A, Barker-Haliski M. Cannabidiol reveals a disruptive strategy for 21st century epilepsy drug discovery. Exp Neurol. 2023;360:114288. doi: 10.1016/j.expneurol.2022.114288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin P, de Witte PAM, Maurice T, Gammaitoni A, Farfel G, Galer B. Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav. 2020;105:106989. doi: 10.1016/j.yebeh.2020.106989. [DOI] [PubMed] [Google Scholar]
- 32.Sourbron J, Smolders I, de Witte P, Lagae L. Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in scn1a mutant zebrafish. Front Pharmacol. 2017;8:191. doi: 10.3389/fphar.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin P, Reeder T, Sourbron J, de Witte PAM, Gammaitoni AR, Galer BS. An emerging role for sigma-1 receptors in the treatment of developmental and epileptic encephalopathies. Int J Mol Sci. 2021;22:8416. doi: 10.3390/ijms22168416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samanta D. Fenfluramine: a review of pharmacology, clinical efficacy, and safety in epilepsy. Children (Basel). 2022;9:1159. doi: 10.3390/children9081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riban V, Heulard I, Reversat L, Si Hocine H, Verleye M. Stiripentol inhibits spike-and-wave discharges in animal models of absence seizures: a new mechanism of action involving T-type calcium channels. Epilepsia. 2022;63:1200–1210. doi: 10.1111/epi.17201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishi T, Kondo S, Miyamoto M, Watanabe S, Hasegawa S, Kondo S, et al. Soticlestat, a novel cholesterol 24-hydroxylase inhibitor shows a therapeutic potential for neural hyperexcitation in mice. Sci Rep. 2020;10:17081. doi: 10.1038/s41598-020-74036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattanzi S, Brigo F, Trinka E, Zaccara G, Striano P, Del Giovane C, Silvestrini M. Adjunctive cannabidiol in patients with dravet syndrome: a systematic review and meta-analysis of efficacy and safety. CNS Drugs. 2020;34:229–241. doi: 10.1007/s40263-020-00708-6. [DOI] [PubMed] [Google Scholar]
- 38.Lattanzi S, Zaccara G, Russo E, La Neve A, Lodi MAM, Striano P. Practical use of pharmaceutically purified oral cannabidiol in Dravet syndrome and Lennox-Gastaut syndrome. Expert Rev Neurother. 2021;21:99–110. doi: 10.1080/14737175.2021.1834383. [DOI] [PubMed] [Google Scholar]
- 39.Tabaee Damavandi P, Fabin N, Giossi R, Matricardi S, Del Giovane C, Striano P, Meletti S, Brigo F, Trinka E, Lattanzi S. Efficacy and safety of fenfluramine in epilepsy: a systematic review and meta-analysis. Neurol Ther. 2023;12:669–686. doi: 10.1007/s40120-023-00452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Medicines Agency. Diacomit, INN-stiripentol. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/diacomit-epar-product-information_en.pdf. Accessed Mar 2023.
- 41.Halford JJ, Sperling MR, Arkilo D, Asgharnejad M, Zinger C, Xu R, During M, French JA. A phase 1b/2a study of soticlestat as adjunctive therapy in participants with developmental and/or epileptic encephalopathies. Epilepsy Res. 2021;174:106646. doi: 10.1016/j.eplepsyres.2021.106646. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Fintepla, INN-fenfluramine. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/fintepla-epar-product-information_en.pdf. Accessed Mar 2023.
- 43.Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–1251. doi: 10.1111/epi.13060. [DOI] [PubMed] [Google Scholar]
- 44.Lattanzi S, Trinka E, Striano P, Zaccara G, Del Giovane C, Nardone R, Silvestrini M, Brigo F. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia. 2020;61:1090–1098. doi: 10.1111/epi.16546. [DOI] [PubMed] [Google Scholar]
- 45.Strzelczyk A, Schubert-Bast S. A practical guide to the treatment of Dravet syndrome with anti-seizure medication. CNS Drugs. 2022;36:217–237. doi: 10.1007/s40263-022-00898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lattanzi S, Trinka E, Zaccara G, Striano P, Russo E, Del Giovane C, Silvestrini M, Brigo F. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. 2022;82:199–218. doi: 10.1007/s40265-021-01661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S. Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav. 2019;101(Pt B):106466. doi: 10.1016/j.yebeh.2019.106466. [DOI] [PubMed] [Google Scholar]
- 48.Lattanzi S, Trinka E, Del Giovane C, Nardone R, Silvestrini M, Brigo F. Antiepileptic drug monotherapy for epilepsy in the elderly: a systematic review and network meta-analysis. Epilepsia. 2019;60:2245–2254. doi: 10.1111/epi.16366. [DOI] [PubMed] [Google Scholar]
- 49.Lattanzi S, Zaccara G, Giovannelli F, Grillo E, Nardone R, Silvestrini M, Trinka E, Brigo F. Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta-analysis. Acta Neurol Scand. 2019;139:33–41. doi: 10.1111/ane.13025. [DOI] [PubMed] [Google Scholar]
- 50.Strzelczyk A, Schubert-Bast S. Therapeutic advances in Dravet syndrome: a targeted literature review. Expert Rev Neurother. 2020;20:1065–1079. doi: 10.1080/14737175.2020.1801423. [DOI] [PubMed] [Google Scholar]
- 51.Asadi-Pooya AA, Brigo F, Lattanzi S, et al. Adult epilepsy. Lancet. 2023;402:412–424. doi: 10.1016/S0140-6736(23)01048-6. [DOI] [PubMed] [Google Scholar]
- 52.Matricardi S, Cestèle S, Trivisano M, Kassabian B, Leroudier N, Vittorini R, et al. Gain of function SCN1A disease-causing variants: Expanding the phenotypic spectrum and functional studies guiding the choice of effective antiseizure medication. Epilepsia. 2023;64:1331–1347. doi: 10.1111/epi.17509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.