Abstract
The second annual 5-day workshop on Principles and Techniques for Improving Preclinical Translation of Alzheimer’s Disease Research was held 7-11 October 2019 at The Jackson Laboratory in Bar Harbor, Maine, USA, and included didactic lectures and hands on training. Participants represented a broad range of research across the Alzheimer’s disease (AD) field, and varied in career stages from trainees and early stage investigators to established faculty, with attendance from the US, Europe, and Asia. In line with the NIH initiative on rigor and reproducibility, the workshop aimed to address training gaps in preclinical drug screening by providing participants with the skills and knowledge required to perform pharmacokinetic, pharmacodynamics, and preclinical efficacy experiments. This innovative and comprehensive workshop provided training in fundamental skill sets for executing in vivo preclinical translational studies with the goal of improving preclinical to clinical translational studies for AD.
Keywords: preclinical translation, Alzheimer’s disease, mouse models, training, best practices, rigor and reproducibility
Introduction
Preclinical testing in animal models is critical for informng clinical trial design and understanding potential for efficacy and safety of therapeutic interventions. Unfortunately, while hundreds of interventions have been demonstrated to have efficacy in animal models of Alzheimer’s disease (AD) and these studies ultimately led to clinical trials, poor preclinical to clinical translation has resulted in an exorbitant failure rate for AD therapeutics1-3. While a wide variety of potential causes of these failures have been proposed, deficiencies in the experimental design, execution, and rigor of preclinical translational experiments are not being sufficiently addressed by common training practices across research laboratories1-4.
Preclinical translation is not typically a course of study available to researchers at their academic institutions, thus it is not surprising that methods for design and study execution are often based on individual knowledge and experiences rather than a vetted curriculum with specific training criteria5,6. While the pharmaceutical industry employs rigorous best practices that align with clinical study design, this knowledge is not typically available as an instructional course, nor are the hands-on training components that allow for development of fundamental skillsets needed for proficient execution of in vivo studies, specific to enable translation. In order to address gaps in training deficiencies in preclinical translational studies for AD, we developed a workshop with the following specific aims: 1) train participants in the rigorous design, experimental execution, analysis, and reporting of data in line with the ARRIVE guidelines for preclinical testing of AD therapies; 2) provide participants with the practical laboratory tools and skills to perform rigorous, reproducible preclinical tests on mouse models of AD; 3) promote honest open discussion to address challenges in the research field to achieving rigorous translational studies that apply best practices. This workshop focused specifically on preclinical testing paradigms for AD translational research that encompassed comprehensive hands-on sessions, didactic lectures, and discussions, in the practices of performing rigorous, reproducible preclinical experiments to enable improved preclinical to clinical translation for AD. As a key component of this workshop which was presented at The Jackson Laboratory in Bar Harbor, Maine (JAX), participants were exposed to discussion forums on historical practices that have led to gaps in preclinical translation to date, new resources and infrastructure now available to better enable improved translation, and SOPs and best practices used for in vivo screening of potential therapeutic agents for Alzheimer’s research. The ultimate goal of this workshop was to enable trainees of this generation of scientists and future generations to accelerate the pace of bringing treatments to patients.
Gaps in historical preclinical studies for AD
Poor translation of preclinical efficacy data in animal models to effective therapies for patients remains a significant roadblock for advancing interventions through clinical trials and FDA approval1-4. Dr. Suzana Petanceska (Director of the Office for Strategic Development and Partnerships, Division of Neuroscience, National Institute on Aging) emphasized a number of key factors including: 1) the failure of the animal models to recapitulate the spectrum of human AD; 2) poor rigor in study design and data analysis; 3) insufficient attention towards best practices; 4) failure to match outcome measures with those of clinical studies; 5) poor reproducibility of published data; and 6) publication bias in favor of reporting positive findings7. Dr. Petanceska described efforts to address these gaps by the development of a publicly available data repository curated by the National Institute on Aging and National Institutes of Health (NIH): Alzheimer’s Preclinical Efficacy Database (AlzPED; https://alzped.nia.nih.gov/)7. AlzPED is a searchable web portal that houses information on preclinical studies including animal models descriptors, key elements of the study design, information related to the therapeutic target and therapeutic agent, principal findings and information related to funding source(s) and financial conflict of interest7. A long-term goal for AlzPED is to provide a platform for reporting unpublished studies in order to mitigate the publication bias that favors the reporting of positive findings. Dr. Stacey Rizzo (University of Pittsburgh) provided an overview of the drug discovery process and provided insight into how traditional cognitive behavioral tests in rodents have failed to translate to cognitive improvement in the clinic8. These include the most frequently reported assays for assessing the potential of AD therapeutics including fear conditioning, novel object recognition, and water maze assays8. Dr. Rizzo emphasized the lack of rigorous experimental design inclusive of lack of vehicle treated controls, unknown drug exposure relationships, absence of dose response, and misinterpretation of data due to confounding variables including hyperactivity and visual impairments related to background strain1,2,8. Dr. Michael Sasner (JAX) discussed the challenges and limitations of historical mouse models that have been frequently used to evaluate therapeutics as a factor in poor translation3. These include the concern that existing animal models have focused on early onset AD (EOAD) genetics while the primary patient population are those with the late onset sporadic form of AD (LOAD); that mice do not spontaneously present with Aβ or tau pathology unless genetically modified and that no single AD model exhibits both Aβ and tau pathology robustly, nor do most models recapitulate the extensive neurodegeneration observed in AD patients; that most existing models demonstrate significant ectopic overexpression of a transgene which introduces non-physiologic effects unrelated to AD; issues with models being generated on hybrid genetic backgrounds and not maintained on congenic and genetically stable backgrounds; issues of legal restrictions, preventing use of many models to for-profit companies for preclinical translational studies; and perhaps most importantly, insufficient attention paid to selection of the appropriate model for a specific combination of drug and physiological readout3,9-12.
New resources for supporting preclinical translational studies of AD
As highlighted by Dr. Petanceska, significant investment in infrastructure and resources for AD have been made by the NIA over the last several years in order to move the field forward in line with the National Alzheimer’s Project Act to identify a treatment by 2025. These include many new funding opportunities such as that which led to the establishment of the Model Organism Development for the Evaluation of Late Onset Alzheimer’s Disease (MODEL-AD) Precision Medicine Consortium1,3. Dr. Sasner provide an overview of MODEL-AD as a resource for generating and comprehensively characterizing up to 50 new mouse models focused on genetic variants of LOAD that are available without restrictions to the research community. Details of variant selection and the characterization pipelines with a focus on best practices were highlighted by Drs. Greg Carter and Gareth Howell (JAX). These models are ultimately being created to be used to evaluate potential therapeutics. Dr. Kelly Dakin (AlzForum) provided a tutorial on how to navigate the AlzForum website, which is a public resource that curates information on historical and new mouse models from MODEL-AD that researchers can access for the most updated information in the field13. In addition, Dr. Kristen Onos (JAX) highlighted work on genetically diverse mouse strains and their utility as improved models for AD over traditional inbred strains14. Finally, Dr. Paul Territo (Indiana University) and Dr. Rizzo described the Preclinical Testing Core of MODEL-AD and the best practices established for preclinical screening of compounds that prioritizes translational PK and PD measures including PET/MR, ‘omics, neuropathology and biomarkers over traditional cognitive assessments in rodents as part of the preclinical screening pipeline. In addition, methods for training of staff including proficiency metrics to ensure rigor and reliability were discussed1,3.
Hands-on practicums for the development of fundamental skill sets for in vivo studies
Following an Animal Welfare orientation requirement, participants were approved by the internal animal care and use committee (IACUC) at JAX to conduct approved procedures on mice. Training over the course of multiple hands-on sessions included serial blood collections using established protocols for in vivo PK sampling studies, practicums on methods for blinding, randomization, and counterbalancing of samples in real-time, and methods for CSF sampling and terminal tissue collections. The trainer to trainee ratio was 1:2. At the conclusion of the hands-on training, Dr. Sara Quinney (Indiana University) described how to interpret bioanalytical data from in vivo PK sampling and the importance of PK/PD modeling. Finally, Dr. Vivek Philip (JAX) provided a statistical practicum including methods and resources for conducting power analyses and development of an appropriate experimental design for rigorous preclinical translational studies. This session also provided the opportunity for participants to get assistance with experimental designs for upcoming studies in their respective laboratories.
Discussion
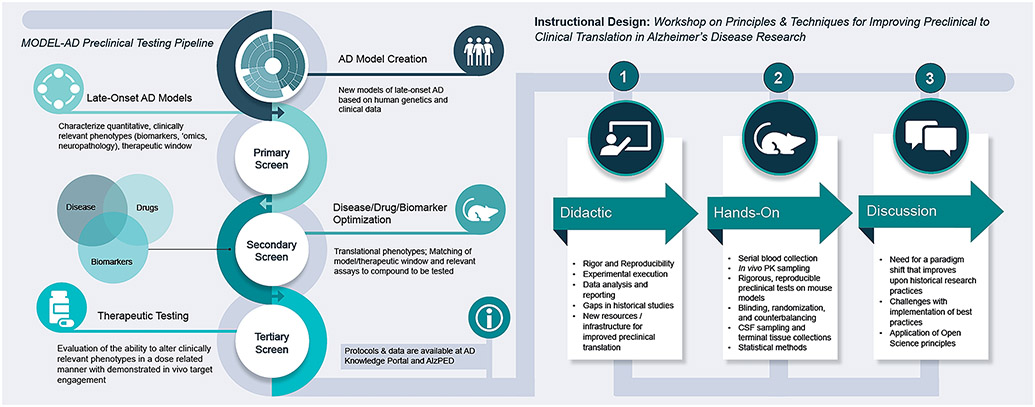
This workshop was developed with the overall goal to provide much needed training in in vivo screening strategies for researchers engaged in translational studies with a focus on development of fundamental hands-on skills in executing rigorous preclinical studies. This format also provided an opportunity for participants to discuss challenges with implementing these types of rigorous best practices in their laboratories. A common concern voiced amongst trainees was the lack of resources and/or funding to be able to conduct rigorous well powered studies, as well as concerns of pressure from their superiors, either perceived or real, towards biasing their experimental design or data manipulation towards a positive result. However, trainees were also optimistic that as they transition to their own independent laboratories this workshop provided them with the essential hands-on skills and knowledge to enable improved translational studies and train the next generation of researchers. In closing, this workshop provided unique instructional strategies with training aimed to improve rigor in preclinical drug-screening experiments and ultimately accelerate the pace of bringing treatments to patients (Figure 1). This hands-on workshop is planned as an annual event at The Jackson Laboratory in Bar Harbor, Maine. The course is open to trainees and experienced researchers and clinicians at all levels, across academia and industry, especially to those new to the field, and are encouraged to register and attend.
Figure 1.
The annual workshop on Principles and Techniques for Improving Preclinical Translation of Alzheimer’s Disease Research provides participants with the skills and knowledge required to perform pharmacokinetic, pharmacodynamics, and preclinical efficacy experiments by leveraging the rigorous best practices and approaches of the MODEL-AD Preclinical Testing Core. This innovative and comprehensive workshop provides training in fundamental skill sets for executing in vivo preclinical translational studies with the goal of improving preclinical to clinical translational studies for AD.
Acknowledgements:
The authors are grateful for the support of Ms. Erin McDevitt, Ms. Alison Kiefer, and The Jackson Laboratory Courses and Conferences team, with special gratitude to Mr. Lothar Holzke for assistance with the graphical abstract.
Funding:
The course was supported by funding from the National Institute on Aging (NIA R13 AG060708, NIA U54 AG054345).
Footnotes
Ethics approval: All animal studies were approved by the institutional animal care and use committee (IACUC) at The Jackson Laboratory prior to initiation. All participants were required to attend an animal welfare orientation and training session prior to initiating studies with animals.
Availability of data and materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the meeting. The MODEL-AD SOPs provided during the hands-on workshop are available through the AD Knowledge Portal: adknowledgeportal.synapse.org
Competing Interests: MS is a full-time employee of The Jackson Laboratory. PRT is a full-time employee of Indiana University School of Medicine. SJSR is a full-time employee of The University of Pittsburgh School of Medicine.
REFERENCES
- 1.Sukoff Rizzo SJ, Masters A, Onos KD, Quinney S, Sasner M, Oblak A, Lamb BT, Territo PR; MODEL-AD consortium. Improving preclinical to clinical translation in Alzheimer's disease research. Alzheimers Dement (N Y). 2020. Jun 14;6(1):e12038. doi: 10.1002/trc2.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shineman DW, Basi GS, Bizon JL, Colton CA, Greenberg BD, Hollister BA, Lincecum J, Leblanc GG, Lee LB, Luo F, Morgan D, Morse I, Refolo LM, Riddell DR, Scearce-Levie K, Sweeney P, Yrjänheikki J, Fillit HM. Accelerating drug discovery for Alzheimer's disease: best practices for preclinical animal studies. Alzheimers Res Ther. 2011. Sep 28;3(5):28. doi: 10.1186/alzrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oblak AL, Forner S, Territo PR, Sasner M, Carter GW, Howell GR, Sukoff-Rizzo SJ, Logsdon BA, Mangravite LM, Mortazavi A, Baglietto-Vargas D, Green KN, MacGregor GR, Wood MA, Tenner AJ, LaFerla FM, Lamb BT; and The MODEL-AD; Consortium. Model organism development and evaluation for late-onset Alzheimer's disease: MODEL-AD. Alzheimers Dement (N Y). 2020. Nov 23;6(1):e12110. doi: 10.1002/trc2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020. Jul 14;18(7):e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyhan AA Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. transl med commun 4, 18 (2019). 10.1186/s41231-019-0050-7 [DOI] [Google Scholar]
- 6.Fernandez-Moure JS. Lost in Translation: The Gap in Scientific Advancements and Clinical Application. Front Bioeng Biotechnol. 2016. Jun 3;4:43. doi: 10.3389/fbioe.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlzPED—Alzheimer’s Preclinical Efficacy Database. https://alzped.nia.nih.gov/ (accessed 04 March 2023).
- 8.Silverman JL, Nithianantharajah J, Der-Avakian A, Young JW, Sukoff Rizzo SJ. Lost in translation: At the crossroads of face validity and translational utility of behavioral assays in animal models for the development of therapeutics. Neurosci Biobehav Rev. 2020. Sep;116:452–453. doi: 10.1016/j.neubiorev.2020.07.008. Epub 2020 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onos KD, Sukoff Rizzo SJ, Howell GR, Sasner M. Toward more predictive genetic mouse models of Alzheimer's disease. Brain Res Bull. 2016. Apr;122:1–11. doi: 10.1016/j.brainresbull.2015.12.003. Epub 2015 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowsky JL, Zheng H. Practical considerations for choosing a mouse model of Alzheimer's disease. Mol Neurodegener. 2017. Dec 22;12(1):89. doi: 10.1186/s13024-017-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukoff Rizzo SJ, McTighe S, McKinzie DL. Genetic Background and Sex: Impact on Generalizability of Research Findings in Pharmacology Studies. Handb Exp Pharmacol. 2020;257:147–162. doi: 10.1007/164_2019_282. [DOI] [PubMed] [Google Scholar]
- 12.Vitek MP, Araujo JA, Fossel M, Greenberg BD, Howell GR, Rizzo SJS, Seyfried NT, Tenner AJ, Territo PR, Windisch M, Bain LJ, Ross A, Carrillo MC, Lamb BT, Edelmayer RM. Translational animal models for Alzheimer's disease: An Alzheimer's Association Business Consortium Think Tank. Alzheimers Dement (N Y). 2021. Jan 11;6(1):e12114. doi: 10.1002/trc2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ALZFORUM - Research Models Database. https://www.alzforum.org/research-models (accessed 04 March 2023).
- 14.Onos KD, Uyar A, Keezer KJ, Jackson HM, Preuss C, Acklin CJ, O'Rourke R, Buchanan R, Cossette TL, Sukoff Rizzo SJ, Soto I, Carter GW, Howell GR. Enhancing face validity of mouse models of Alzheimer's disease with natural genetic variation. PLoS Genet. 2019. May 31;15(5):e1008155. doi: 10.1371/journal.pgen.1008155. [DOI] [PMC free article] [PubMed] [Google Scholar]