Abstract
A randomized, three-way crossover study was carried out to determine the effects of food ingestion on the pharmacokinetics of stavudine (d4T). Fifteen subjects with human immunodeficiency virus (HIV) infection and CD4+ cell counts of ≥200/μl received 70 mg of d4T in a fasting state or 1 h before or 5 min after a standardized high-fat breakfast. A 7- to 15-day washout period was included between treatments. Blood and urine were collected before and for 10 h after dosing, and plasma and urine d4T concentrations were determined with a validated radioimmunoassay. Plasma drug concentration-time data were analyzed with a noncompartmental model. The mean maximum plasma drug concentration (Cmax) and the time to Cmax (Tmax) for administration of d4T after a meal were significantly lower and longer (P = 0.0001 for both measures) than those observed in the fasting state, although the area under the concentration-time curve from time zero to infinity (AUC0–∞) was not significantly different. Neither of these parameters was significantly altered when d4T was taken 1 h before a meal. The bioavailability of d4T taken after a meal was 95% of that observed in the fasting state, and it was 97% when d4T was administered before a meal (P > 0.05 for both comparisons with the fasting state). The results of this study indicate that (i) ingestion of food does not affect the bioavailability of d4T and that patients with HIV infection can take it without regard to meals, and (ii) absorption is essentially complete within 1 h when d4T is administered in the fasted state.
Stavudine (d4T) is a nucleoside analog antiretroviral agent that has been used effectively as monotherapy and in combination with other nucleosides and protease inhibitors for the treatment of patients with human immunodeficiency virus (HIV) infection (4, 12, 17, 18, 23, 30–32, 38). In these mono- or combination therapy trials, d4T (1 mg/kg of body weight/day) has consistently been shown to decrease viral load in both plasma and peripheral blood mononuclear cells and to increase CD4+ cell counts (4, 12, 17, 18, 30–32, 38). In addition, d4T is generally well tolerated. Peripheral neuropathy, the only major toxicity, increases with higher doses and longer duration of therapy (4, 30, 31, 33, 38). Analyses of viral isolates from patients who have undergone long-term therapy with d4T indicate that such treatment is not associated with the selection of mutations that confer high-level resistance as has been observed for other nucleoside analogs, particularly zidovudine (ZDV), in which such mutations have been associated with increased risk of disease progression (6, 25, 26).
The single- and multiple-dose pharmacokinetics of d4T have been extensively studied in nonhuman primates and patients with HIV infection. It has been demonstrated that d4T is rapidly and nearly completely absorbed after oral dosing, with a time (Tmax) to maximum plasma drug concentration (Cmax) of <1 h and a mean absolute bioavailability after oral administration ranging between 80 and 99% (8, 16, 19, 21). The elimination half-life (t1/2) for d4T has been reported to range between 1 and 2 h (8, 16, 21). The pharmacokinetics of d4T are also characterized by lower interpatient variability in absorption and clearance (8, 16).
Antiretroviral therapy for patients with HIV infection is long term and may even be lifelong. It is therefore important that such treatment be as convenient as possible in order to promote high patient compliance and maximum suppression of viral replication. Convenience of treatment for HIV-infected patients would be enhanced if antiretroviral agents could be taken without regard to meals. The present study was therefore undertaken to determine the effects of food ingestion on the pharmacokinetic profile of d4T.
MATERIALS AND METHODS
Subjects.
The subjects in this study were adults (18 to 40 years of age, ≥40 kg in body weight) with asymptomatic HIV infection, as determined by enzyme-linked immunosorbent assay and Western blot assay, and with CD4+ cell counts of ≥200/μl. All subjects were within 15% of normal weight for height and had not experienced >10% weight loss over the previous 8 months. All were free of acute illness and significant chronic organ dysfunction, and none had recent diarrheal illness. Prior therapy with ZDV was allowed if it had been discontinued for at least 1 week prior to enrollment, but previous treatment with any other antiretroviral drug was not permitted. Amylase was required to be within normal limits, and liver function test results (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, γ-glutamyltransferase, alkaline phosphatase, and bilirubin) were required to be ≤2 times the upper limit of normal. Serum creatinine was required to be ≤1.4 mg/dl, and blood urea nitrogen was required to be ≤24 mg/dl. Women were required to be negative by a test for human chorionic gonadotropin and to be using an accepted method of contraception. Approval of the protocol was obtained from the Human Investigation Committee of the Rush-Presbyterian-St. Lukes Medical Center, Chicago, Ill. All subjects gave written informed consent.
Study design.
The study employed an open-label, randomized, three-way crossover design. Subjects were initially assigned to one of three treatments, all of which followed an overnight fast: (i) 70 mg of d4T administered as a single dose under protracted fasting conditions (8 h before and 4 h after dosing), (ii) 70 mg of d4T administered 5 min after consumption of a standard high-fat breakfast (two boiled eggs, one slice of toast with butter and jelly, two strips of bacon, 4 oz of hashed brown potatoes, and 8 oz of whole milk; 32% carbohydrate, 15% protein, 53% fat; [773 kcal]), and (iii) 70 mg of d4T administered 1 h before the same standard high-fat breakfast. In all three study arms, d4T was administered with 200 ml of tap water. A light lunch was provided 4 h after d4T administration in each arm. All treatments were separated by 7 to 15 days. The dose of d4T selected for the present investigation was 70 mg (approximately 1 mg/kg), which was potentially the maximum unit dose for further clinical evaluation. However, future results proved that the optimal unit dose of d4T was 40 mg administered as two equally divided doses (total dose, 80 mg [1 mg/kg/day]) 12 h apart (29).
Blood and urine sampling.
Blood samples (∼3 ml) were collected just prior to and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, and 10 h after d4T administration. Within 60 min of collection, samples were centrifuged at <2,000 × g for 10 min at 0 to 4°C, and plasma was separated and stored at −20°C until assayed for d4T. Urine was collected before d4T administration, in 2-h blocks for the first 6 h after dosing, and in a single block for 6 to 10 h. All urine collections were measured for volume, and a 2-ml aliquot of urine from each collection was stored at −20°C until assayed.
Assay for d4T.
d4T concentrations in plasma and urine were measured with a validated radioimmunoassay (20). Quality-control samples were included in each analytical sequence in order to verify the stability of study samples during shipment and storage as well as the accuracy and precision of the analysis. The between-day error and within-day precision estimates for the error of the plasma assay were no greater than 10.2 and 18.1% relative standard deviation (RSD), respectively, with an average deviation from nominal of no more than 9.1%. The respective values for the urine assay were no greater than 9.5 and 17.0% RSD and no more than 11.0%.
Pharmacokinetic analysis.
Plasma drug concentration-time data were analyzed with a noncompartmental model (9, 34). The terminal slope (k) of the concentration-time curve was determined by log-linear regression of at least the last three data points, which yielded a minimum mean square error (MSE). The t1/2 of the terminal log-linear phase was established as 0.693/k. The area under the concentration-time curve extrapolated to infinity (AUC0–∞) was determined by summing the areas from time zero to the time of the last quantifiable concentration by trapezoidal and log-trapezoidal methods and the extrapolated area. The extrapolated area was determined by dividing the final concentration (interpolated from the log-linear regression analysis) by the slope of the terminal log-linear phase. Values for Cmax, Tmax, and urinary recovery (UR) were obtained directly from the experimental observations. Renal clearance (CLR) was estimated as: CLR = UR0–T/AUC0–T, where T is a specified time point after dosing.
Statistical analysis.
An analysis of variance (ANOVA) model for a three-way crossover design was performed with the results for Cmax, AUC0–∞, t1/2, and CLR. The sequence and subjects-within-sequence effects were estimated by using the type I sums of squares. The treatment and period effects were estimated by using type III sums of squares. The values for Cmax and AUC0–∞ were log transformed a priori before analysis. For all other parameters, Box-Cox (3) analysis was used to determine whether the ANOVA was accomplished with the raw or log-transformed data. If the likelihood ratio test results from comparison of the MSEs from the two analyses were significant, then the analysis based on the log transformation was reported. The relative bioavailability assessments for d4T administered under the different treatment conditions were evaluated by using the two one-sided tests procedure (37). If the analysis was performed with log-transformed data, the antilogs of the limits for the difference between the log means provided the confidence limits of interest. Confidence intervals for the difference between means for untransformed data were scaled to the reference mean. The Wilcoxon’s signed-rank test was used to compare Tmax values for the different treatments (15). The accepted level of significance for all analyses was P = 0.05.
Safety.
Safety evaluation consisted of medical history, symptom assessment, physical examination, vital signs, electrocardiogram, and clinical laboratory tests performed at screening and before discharge from the testing facility after the final dose of d4T. Symptom assessments and physical examinations were also performed after each dose. Blood pressure and heart rate were recorded before and at 1, 2, 4, and 10 h after each d4T administration. Assessment of subjects for evidence of adverse events was carried out throughout the study.
RESULTS
Subject demographics.
Seventeen subjects entered the trial between 25 February and 19 November 1992, and 15 completed all dosing and evaluations. The average age (mean ± standard deviation) for the subjects was 30.1 ± 4.5 years, the average weight was 66.8 ± 4.4 kg, and the average CD4+ cell count was 502 ± 252/μl. Thirteen subjects were men, and 4 were women; 9 were Caucasian, 3 were Afro-American, and 5 were Hispanic. Two subjects withdrew voluntarily for reasons unrelated to the study; both subjects completed all study discharge evaluations, and there were no abnormal findings.
Pharmacokinetic results.
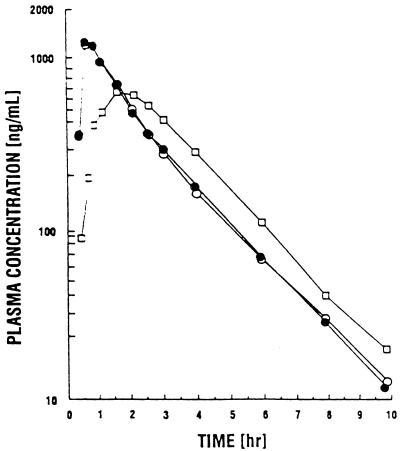
The mean plasma concentration-time curves of d4T are shown in Fig. 1, and the average pharmacokinetic parameters for subjects who completed all three treatment conditions are summarized in Table 1. Under fasting conditions, the Cmax was 1,439 ± 490 ng/ml, the Tmax was 0.65 ± 0.28 h, AUC0–∞ was 2,527 ± 726 ng · h/ml, and t1/2 was 1.56 ± 0.27 h (all values are means ± standard deviations). For administration of d4T after a meal, these values were 756 ± 166 ng/ml, 1.73 ± 0.64 h, 2,359 ± 537 ng · h/ml, and 1.55 ± 0.22 h, and for administration of d4T 1 h before a meal, they were 1,532 ± 673 ng/ml, 0.75 ± 0.37 h, 2,459 ± 721 ng · h/ml, and 1.67 ± 0.39 h, respectively. The Cmax and Tmax values for administration of d4T after a meal were significantly lower and longer, respectively (P = 0.0001 for both measures), than those observed in the fasting state, but AUC was not significantly affected. Neither of these parameters was significantly altered when d4T was taken 1 h before a meal. There were no significant differences among treatments with respect to CLR, percent UR, and t1/2, which ranged from an average of 166 to 211 ml/min, 32.2 to 39.8%, and 1.55 to 1.67 h, respectively, for the three treatment conditions.
FIG. 1.
Mean plasma drug concentration-time curves of d4T in 15 HIV-infected subjects after dosing with 70 mg of d4T in the fasting state (•) or 5 min after (□) and 1 h prior to (○) a high-fat meal.
TABLE 1.
Average pharmacokinetic parameters for d4T administered while fasting and after and before a meala
| Food condition | Cmax (ng/ml) | Tmax (h) | AUC0–∞ (ng · h/ml) | t1/2 (h) | CLR (ml/min) | UR (%) |
|---|---|---|---|---|---|---|
| Fasting | 1,439 ± 490 | 0.65 ± 0.28 | 2,527 ± 726 | 1.56 ± 0.27 | 179 ± 76 | 35.2 ± 11.9 |
| 5 min after a meal | 756 ± 166 | 1.73 ± 0.64 | 2,359 ± 537 | 1.55 ± 0.22 | 211 ± 63 | 39.8 ± 11.9 |
| 1 h before a meal | 1,532 ± 673 | 0.75 ± 0.37 | 2,459 ± 721 | 1.67 ± 0.39 | 166 ± 53 | 32.2 ± 7.1 |
Values are means ± standard deviations.
The relative bioavailability estimates for d4T dosed before or after a meal versus that while fasting for subjects who received all three d4T administrations are as follows: The point estimate (90% confidence interval) of Cmax for d4T taken after a meal was 54% (47%, 62%) of that observed in the fasted state, and that for d4T administered before a meal was 104% (91%, 118%). On the basis of AUC0–∞, the point estimates (90% confidence intervals) for d4T taken after a meal and for d4T administered before a meal were 95% (89%, 103%) and 97% (92%, 103%), respectively.
Safety.
No serious clinical adverse events were reported during the study. Only two events (headache occurring on two occasions in the same subject) were considered to be related to d4T. There were no significant changes in electrocardiograms during the trial, and all vital signs remained within normal limits. Four subjects had abnormal clinical laboratory values that met the criteria for toxicity grading. These included grade 1 elevations in (aspartate aminotransferase, alanine aminotransferase, γ-glutamyltransferase, and alkaline phosphatase, none of which was considered to be clinically significant or clearly related to d4T administration.
DISCUSSION
The present results demonstrate that (i) the bioavailability of orally administered d4T is not altered by administration of d4T just before or after a high-fat breakfast and (ii) confirm that absorption is nearly complete in <1 h when administered in the fasted state. The rate of absorption was significantly slowed and the Cmax was significantly reduced when d4T was administered after a meal, but AUC was unchanged. The lack of a clinically significant effect of food on the bioavailability for d4T is consistent with results from a previous population study of the pharmacokinetics of this nucleoside reported by Grasela et al. (11).
The pharmacokinetic parameters for d4T described in the present trial are also consistent with those from other previous pharmacokinetic studies of this nucleoside analog. Dudley et al. (8) studied the single-dose pharmacokinetics of d4T in patients treated with 0.67 to 4.0 mg/kg. In their study, a 1.33-mg/kg dose of d4T resulted in a Cmax of 1,560 ng/ml, a Tmax of about 0.75 h, an AUC of 2,320 ng · h/ml, and a t1/2 of 1.0 h. All of these results are remarkably similar to those obtained for the fasted subjects in the present trial, who received doses ranging from 0.9 to 1.2 mg/kg. It is important to note that the clinical characteristics of the patients studied by Dudley et al. (8) differed substantially from those of the subjects reported on here. The patients studied by Dudley et al. (8) had AIDS or AIDS-related complex and a median CD4+ cell count of 140/μl. The subjects in the present trial were asymptomatic and had a mean CD4+ cell count of 502/μl. The similarity between the pharmacokinetic parameters for d4T in these two groups of HIV-infected individuals with very different clinical characteristics is consistent with previous conclusions regarding the low interpatient variability in the pharmacokinetic properties of this nucleoside analog (8, 16).
The present results indicate that about 35% of an administered dose of d4T is recovered unchanged in the urine. This result is in agreement with findings from other clinical pharmacokinetic studies and those obtained after administration of d4T to nonhuman primates (8, 19). While results are not yet available for humans, findings from monkeys indicate that none of a radiolabeled dose of d4T is recovered in feces (5). Thus, d4T is not eliminated in significant amounts by hepatobiliary mechanisms. This is consistent with the suggestion that primary d4T metabolites are further metabolized by salvage pathways and/or converted to biological macromolecules (5). The elimination of d4T appears to differ from that of either ZDV or zalcitabine (ddC), but is similar to that of didanosine (ddI). ZDV is extensively metabolized in the liver, and its primary metabolite, a 5′-O-glucuronide of the parent drug, is excreted in the urine (2). Similar to d4T, only about one-third of a dose of ddI is recovered unchanged in the urine (13). In contrast, ddC is largely excreted in the urine as unchanged drug (22).
The effect of food on the bioavailability of nucleoside analogs has been previously investigated. Several investigators have evaluated the influence of food on the bioavailability of ZDV. Ruhnke et al. (35) evaluated the influence of a standard breakfast on the pharmacokinetics of ZDV in patients with advanced HIV infection and reported a complicated interaction between ZDV dose and the effects of food. Coadministration of food with 100 mg of ZDV resulted in a 28% increase in Tmax, a 37% reduction in Cmax, and a 33% decrease in AUC. Delivery of a 200-mg dose of ZDV along with the same meal resulted in a 363% increase in Tmax, a 73% fall in Cmax, and a 14% reduction in AUC compared with administration in a fasting state. These investigators concluded that the absorption of ZDV in patients with HIV infection is quite variable and is markedly affected by a standard breakfast. They recommended that ZDV should be taken in the fasting state. Other investigators have reported similar results (28, 39). However, Sahai et al. (36) reported that a liquid protein-based meal did not have a clinically significant effect on ZDV extent of absorption; although Cmax was significantly reduced by 34%, AUC was not significantly altered. These findings with ZDV underscore the variable influence that different types of meals may have on the bioavailability of a drug. Prescription information for ddC indicates that its absorption is slowed and its extent of absorption is decreased 14% by coadministration of food (29). The bioavailability of ddI is markedly reduced when it is taken after a meal (24).
The in vitro 50% inhibitory concentration (IC50) of d4T against HIV-1 is reported to be quite variable because of significant differences in methodology (14). The IC50 of d4T ranges from 0.009 to 0.04 μM (approximately 2 to 9 ng/ml), with human peripheral blood mononuclear cells used as the test system (14). In the present study, plasma d4T concentrations were above the target concentration for approximately 10 h irrespective of the study treatment, suggesting that the significant reduction of d4T Cmax in the presence of food will not compromise its antiviral activity.
Compliance with treatment has been identified as an important determinant of the long-term success of antiretroviral therapy (1, 10, 27), and research on antimicrobial therapy has already taught us that convenience of dosing regimen enhances compliance and may also improve outcome (7). Thus, the ability to administer d4T without regard to meals might be expected to enhance patient compliance in patients taking this antiretroviral agent.
ACKNOWLEDGMENTS
We are grateful to Robert E. Kates, Analytical Solutions, Inc., Sunnyvale, Calif., for assaying the study samples and the reviewers for their insightful comments.
REFERENCES
- 1.Berger P B. Hope and caution: report from the XI International Conference on AIDS. Can Med Assoc J. 1996;155:717–721. . (Comment.) [PMC free article] [PubMed] [Google Scholar]
- 2.Blum, M. R., S. H. T. Liao, S. S. Good, and P. DeMiranda. 1988. Pharmacokinetics and bioavailability of zidovudine in humans. Am. J. Med. 85(Suppl. 2A):189–194. [PubMed]
- 3.Box G E P, Cox D R. Analysis of transformation. J R Stat Soc B. 1982;265:211–252. [Google Scholar]
- 4.Browne M J, Mayer K H, Chafee S B D, Dudley M N, Posner M R, Steinberg S M, Graham K K, Geletko S M, Zinner S H, Denman S L, Dunkle L M, Kaul S, McLaren C, Skowron G, Kouttab N M, Kennedy T A, Weitberg A B, Curt G A. 2′,3′-Didehydro-3′-deoxythymidine (d4T) in patients with AIDS or AIDS-related complex: a phase I trial. J Infect Dis. 1993;167:21–29. doi: 10.1093/infdis/167.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Cretton E M, Zhou Z, Kidd L B, McClure H M, Kaul S, Hitchcock M J M, Sommadossi J-P. In vitro and in vivo disposition and metabolism of 3′-deoxy-2′,3′-didehydrothymidine. Antimicrob Agents Chemother. 1993;37:1816–1825. doi: 10.1128/aac.37.9.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Aquila R T, Johnson V A, Welles S L, Japour A J, Kuritzkes D R, DeGruttola V, Reichelderfer P S, Coombs R W, Crumpacker C S, Kahn J O, Richman D D for the AIDS Clinical Trials Group Protocol 116B/117 Team and the Virology Committee Resistance Working Group. Zidovudine resistance and HIV-1 disease progression during antiretroviral therapy. Ann Intern Med. 1995;122:401–408. doi: 10.7326/0003-4819-122-6-199503150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Davey P, Parker S. Cost effectiveness of once-daily oral antimicrobial therapy. J Clin Pharmacol. 1992;32:706–710. doi: 10.1002/j.1552-4604.1992.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 8.Dudley M N, Graham K K, Kaul S, Geletko S, Dunkle L, Browne M, Mayer K. Pharmacokinetics of stavudine in patients with AIDS or AIDS-related complex. J Infect Dis. 1992;166:480–485. doi: 10.1093/infdis/166.3.480. [DOI] [PubMed] [Google Scholar]
- 9.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel-Dekker; 1982. pp. 409–417. [Google Scholar]
- 10.Goebel, F. D. 1995. Combination therapy from a clinician’s perspective. J. Acquired Immune Defic. Syndr. 10(Suppl. 1):S62–S68. [PubMed]
- 11.Grasela T H, Haworth S J, Fiedler-Kelley J, Christofalo B. Population pharmacokinetic (PK) analysis of stavudine (d4T) in HIV-infected patients with CD4 counts between 50 and 500 cells/mm3. Int Conf AIDS. 1996;11:287. . (Abstract Tu.B. 2134.) [Google Scholar]
- 12.Griffith B P, Brett-Smith H, Kim G, Mellors J W, Chacko T M, Garner R B, Chen Y-C, Alcabes P, Friedland G. Effect of stavudine on human immunodeficiency virus type I virus load as measured by quantitative mononuclear cell culture, plasma RNA, and immune complex-dissociated antigenemia. J Infect Dis. 1996;173:1252–1255. doi: 10.1093/infdis/173.5.1252. [DOI] [PubMed] [Google Scholar]
- 13.Hartman N R, Yarchoan R, Pluda J M, Thomas R V, Marczyk K S, Broder S, Johns D G. Pharmacokinetics of 2′,3′-dideoxyadenosine and 2′,3′-dideoxyinosine in patients with severe human immunodeficiency virus infection. Clin Pharmacol Ther. 1990;47:647–654. doi: 10.1038/clpt.1990.86. [DOI] [PubMed] [Google Scholar]
- 14.Hitchcock M J M. 2′,3′-Didehydro-2′,3′-dideoxythymidine (D4T), an anti-HIV agent. Antivir Chem Chemother. 1991;2:125–132. [Google Scholar]
- 15.Hollander M, Wolfe D A. Nonparametric statistical methods. New York, N.Y: John Wiley; 1973. pp. 50–53. [Google Scholar]
- 16.Horton C M, Dudley M N, Kaul S, Mayer K H, Squires K, Dunkle L, Anderson R. Population pharmacokinetics of stavudine (d4T) in patients with AIDS or advanced AIDS-related complex. Antimicrob Agents Chemother. 1995;39:2309–2315. doi: 10.1128/aac.39.10.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katlama, C. Efficacy and tolerability of stavudine plus lamivudine in antiretroviral-naive and previously treated patients with HIV infection. In press. [DOI] [PubMed]
- 18.Katlama, C. A randomized trial to assess antiviral activity of two doses of stavudine (d4T) vs placebo in patients with early asymptomatic HIV-1 infection. In press.
- 19.Kaul S, Dandekar K A. Pharmacokinetics of the anti-human immunodeficiency virus nucleoside analog stavudine in cynomolgus monkeys. Antimicrob Agents Chemother. 1993;37:1160–1162. doi: 10.1128/aac.37.5.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul S, Stouffer B, Mummaneni V, Turabi N, Mantha S, Jayatilak P, Barbhaiya R. Specific radioimmunoassays for the measurement of stavudine in human plasma and urine. J Pharm Biomed Anal. 1996;15:165–174. doi: 10.1016/0731-7085(96)01839-0. [DOI] [PubMed] [Google Scholar]
- 21.Kaul S, Mummaneni V, Barbhaiya R H. Dose proportionality of stavudine in HIV seropositive asymptomatic subjects: application to bioequivalence assessment of various capsule formulations. Biopharm Drug Dispos. 1995;16:125–136. doi: 10.1002/bdd.2510160207. [DOI] [PubMed] [Google Scholar]
- 22.Klecker R W, Jr, Collins J M, Yarchoan R C, Thomas R, McAtee N, Broder S, Myers C E. Pharmacokinetics of 2′,3′-dideoxycytidine in patients with AIDS and related disorders. J Clin Pharmacol. 1988;28:837–842. doi: 10.1002/j.1552-4604.1988.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 23.Kline M W, Dunkle L M, Church J A, Goldsmith J C, Harris A T, Federici M E, Schultze M E, Woods L, Loewen D F, Kaul S, Cross A, Rutkiewicz V L, Rosenblatt H M, Hanson I C, Shearer W T. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96:247–252. [PubMed] [Google Scholar]
- 24.Knupp C A, Milbrath R, Barbhaiya R H. Effect of time of food administration on the bioavailability of didanosine from a chewable tablet formulation. J Clin Pharmacol. 1993;33:568–573. doi: 10.1002/j.1552-4604.1993.tb04705.x. [DOI] [PubMed] [Google Scholar]
- 25.Larder B A, Kohli A, Bloor S, Kemp S D, Harrigan P R, Schooley R T, Lange J M A, Pennington K N, St. Clair M H the Protocol 34,225-02 Collaborative Group. Human immunodeficiency virus type 1 drug susceptibility during zidovudine (AZT) monotherapy compared with AZT plus 2′,3′-dideoxyinosine or AZT plus 2′,3′-dideoxycytidine combination therapy. J Virol. 1996;70:5922–5929. doi: 10.1128/jvi.70.9.5922-5929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin P-F, Samanta H, Rose R E, Patick A K, Trimble J, Bechtold C M, Revie D R, Khan N C, Federici M E, Li H, Lee A, Anderson R E, Colonno R J. Genotypic and phenotypic analysis of human immunodeficiency virus type 1 isolates from patients on prolonged stavudine therapy. J Infect Dis. 1994;170:1157–1164. doi: 10.1093/infdis/170.5.1157. [DOI] [PubMed] [Google Scholar]
- 27.LoCaputo S, Maggi P, Buccoliero G, Federico M, Angarano G, Pastore G. Antiretroviral therapy with zidovudine (AZT): evaluation of the compliance in a cohort of HIV+ patients. Int Conf AIDS. 1993;9:489. . (Abstract PO-B26-2124.) [Google Scholar]
- 28.Lotterer E, Ruhnke M, Trautmann M, Beyer R, Bauer F E. Decreased and variable systemic availability of zidovudine in patients with AIDS if administered with a meal. Eur J Clin Pharmacol. 1990;40:305–308. doi: 10.1007/BF00315215. [DOI] [PubMed] [Google Scholar]
- 29.Medical Economics. Physicians’ desk reference. 51st ed. Montvale, N.J: Medical Economics; 1997. pp. 2981–2988. [Google Scholar]
- 30.Murray, H. W., K. E. Squires, W. Weiss, S. Sledz, H. S. Sacks, J. Hassett, A. Cross, R. E. Anderson, and L. M. Dunkle. 1995. Stavudine in patients with AIDS and AIDS-related complex: AIDS Clinical Trials Group 089. J. Infect. Dis. 171(Suppl. 2):S123–S130. [DOI] [PubMed]
- 31.Petersen, E. A., C. H. Ramírez-Ronda, W. D. Hardy, R. Schwartz, H. S. Sacks, S. Follansbee, D. M. Petersen, A. Cross, R. E. Anderson, and L. M. Dunkle. 1995. Dose-related activity of stavudine in patients infected with human immunodeficiency virus. J. Infect. Dis. 171(Suppl. 2):S131–S139. [DOI] [PubMed]
- 32.Pollard R, Peterson D, Hardy D, Pedneault L, Rutkiewicz V, Pottage J, Murphy R, Gathe J, Beall G, Skovronski J, Cross A, Dunkle L. Stavudine (d4T) and didanosine (ddI) combination therapy in HIV-infected subjects: antiviral effect and safety in an on-going pilot randomized double-blinded trial. Int Conf AIDS. 1996;11:225. . (Abstract Th.B. 293.) [Google Scholar]
- 33.Pottage J C, Benson C A, Sha B E, Urbanski P A, Kessler H. Stavudine (d4T) in the therapy of patients with advanced AIDS and less than 50 CD4 cells. Natl Conf Hum Retroviruses Relat Infect. 1995;2:104. [Google Scholar]
- 34.Riegelman S, Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980;8:509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- 35.Ruhnke M, Bauer F E, Seifert M, Trautmann M, Hille H, Koeppe P. Effects of standard breakfast on pharmacokinetics of oral zidovudine in patients with AIDS. Antimicrob Agents Chemother. 1993;37:2153–2158. doi: 10.1128/aac.37.10.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahai J, Gallicano K, Garber G, McGilveray I, Hawley-Foss N, Turgeon N, Cameron D W. The effect of a protein meal on zidovudine pharmacokinetics in HIV-infected patients. Br J Clin Pharmacol. 1992;33:657–660. doi: 10.1111/j.1365-2125.1992.tb04097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuirmann D J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 38.Skowron, G. 1995. Biologic effects and safety of stavudine: overview of phase I and II clinical trials. J. Infect. Dis. 171(Suppl. 2):S113–S117. [DOI] [PubMed]
- 39.Unadkat J D, Collier A C, Crosby S S, Cummings D, Opheim K E, Corey L. Pharmacokinetics of oral zidovudine (azidothymidine) in patients with AIDS when administered with and without a high-fat meal. AIDS. 1990;4:229–232. doi: 10.1097/00002030-199003000-00008. [DOI] [PubMed] [Google Scholar]