Abstract
Objective
This study aimed to estimate the association between number of maternal comorbidities and duration of expectant management and perinatal outcomes in patients with preeclampsia with severe features.
Study Design
Retrospective cohort of patients with preeclampsia with severe features delivering live, nonanomalous singletons at 23 to 342/7 weeks’ gestation at a single center from 2016 to 2018. Patients delivered for an indication other than severe preeclampsia were excluded. Patients were categorized based on the number (0, 1, or ≥2) of comorbidities present: chronic hypertension, pregestational diabetes, chronic kidney disease, and systemic lupus erythematosus. The primary outcome was proportion of potential expectant management time achieved, that is, days of expectant management achieved divided by total potential expectant management time (days from severe preeclampsia diagnosis to 340/7 weeks). Secondary outcomes included delivery gestational age, days of expectant management, and perinatal outcomes. Outcomes were compared in bivariable and multivariable analyses.
Results
Of 337 patients included, 167 (50%) had 0, 151 (45%) had 1, and 19 (5%) had ≥2 comorbidities. Groups differed with respect to age, body mass index, race/ethnicity, insurance, and parity. The median proportion of potential expectant management achieved in this cohort was 1.8% (interquartile range: 0–15.4), and did not differ by number of comorbidities (adjusted β: 5.3 [95% confidence interval [CI]: −2.1 to 12.9] for 1 comorbidity vs. 0 and adjusted β: −2.9 [95% CI: −18.0 to 12.2] for ≥2 comorbidities vs. 0). There was no difference in delivery gestational age or duration of expectant management in days. Patients with ≥2 (vs. 0) comorbidities had higher odds of composite maternal morbidity (adjusted odds ratio: 3.0 [95% CI: 1.1–8.2]). There was no association between number of comorbidities and composite neonatal morbidity.
Conclusion
Among patients with preeclampsia with severe features, the number of comorbidities was not associated with duration of expectant management; however, patients with ≥2 comorbidities had higher odds of adverse maternal outcomes.
Keywords: preeclampsia, hypertension, diabetes, chronic kidney disease, systemic lupus erythematosus, cesarean delivery, placental abruption, pulmonary edema, maternal death
Expectant management of preeclampsia with severe features (SPE) before 34 weeks’ gestation has been shown to improve neonatal outcomes, while maternal risks appear not to worsen.1 Multiple studies demonstrating these benefits, in systematic reviews2 and randomized controlled trials,3–5 led to the 2013 Hypertension in Pregnancy guideline in which the American College of Obstetricians and Gynecologists (ACOG) endorsed expectant management of SPE in appropriate candidates.6 Reinforced in 2019, ACOG detailed recommendations for expectant management of SPE and defined contraindications including pulmonary edema, renal insufficiency, abruption, persistent symptoms, HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, eclampsia, abnormal antenatal testing, critically abnormal umbilical artery Dopplers, and previability.7 A recent Cochrane Review comparing the benefits of expectant management versus immediate delivery of SPE demonstrated improvement in neonatal outcomes, including estimated gestational age (EGA) at delivery, neonatal intensive care unit (NICU) admission, respiratory distress syndrome, and intraventricular hemorrhage; however, there was insufficient evidence to draw reliable conclusions on the risks of perinatal death and maternal morbidity.8
While ACOG recommendations support expectant management of SPE, they do not comment on the suitability of expectant management in medically complicated patients. Medical conditions including chronic hypertension,9–14 pregestational diabetes,15,16 chronic renal disease,17,18 and systemic lupus erythematosus19,20 have been associated with increased risks of preeclampsia and SPE, as well as worsened maternal and neonatal outcomes, especially preterm birth and neonatal death in patients with preeclampsia. In fact, previous trials demonstrating the time gained, improvement in neonatal outcomes, and maternal safety of expectant management of preeclampsia specifically excluded patients with medical comorbidities.3–5 These broad ACOG guidelines for the management of SPE have led to the extrapolation of expectant management in high-risk patients; however, the potential impact of expectant management in this population has not yet been well assessed.
Therefore, we aimed to evaluate the association between number of maternal comorbidities, including chronic hypertension, pregestational diabetes, chronic kidney disease, and systemic lupus erythematosus, and duration of expectant management. We also sought to estimate the impact of the number of medical comorbidities on maternal and neonatal outcomes in those undergoing expectant management. We hypothesized that patients with an increasing number of medical comorbidities would be expectantly managed for a shorter period of time and experience higher rates of adverse maternal and fetal outcomes.
Materials and Methods
We conducted a retrospective cohort study of all patients with SPE who delivered a singleton gestation between 230/7 and 342/7 weeks’ gestational age at our single tertiary care center from January 1, 2016, to December 31, 2018. Institutional review board (IRB) approval was obtained prior to initiation of the study (identifier: 160122004).
Women included for analysis were diagnosed with SPE defined by ACOG criteria6,7 prior to 340/7 weeks. During the study period, it was standard practice to expectantly manage patients with SPE until 340/7 weeks, unless they developed contraindications to further expectant management including uncontrolled blood pressures, persistent severe symptoms, a severe laboratory abnormality, pulmonary edema, eclampsia, HELLP syndrome, fetal growth restriction (FGR) with reversed end diastolic flood on umbilical artery Dopplers, or intrauterine fetal demise. All patients with expectant management of SPE at our institution were hospitalized and cared for both on the antepartum service and on labor and delivery by a single group of Maternal Fetal Medicine providers in an academic, university-based setting. Expectant management included initiating and/or up-titrating antihypertensive medications per the discretion of the clinical team. Routine laboratory assessment was obtained twice weekly until delivery. Daily nonstress tests were performed on patients with SPE, unless there were other indications for more frequent testing such as FGR. Additionally, ultrasounds were performed weekly for amniotic fluid assessment and every 3 to 4 weeks for growth assessment.
Patients were included if they delivered for an indication related to SPE, for example, uncontrolled hypertension, persistent headache, severe laboratory abnormalities (i.e., aspartate aminotransferase [AST] >80 units/L, platelet count <100,000/L, acute kidney injury), HELLP, nonreassuring antenatal testing, achievement of goal gestational age of 34 weeks, eclampsia, pulmonary edema, or abruption. Deliveries of patients with SPE up to 342/7 weeks were included to allow for the possibility of prolonged inductions. However, patients delivered for an indication unrelated to SPE, for example, spontaneous preterm labor or preterm rupture of membranes, were excluded. Patients were also excluded if they had stillbirth identified at the time of SPE diagnosis, a multiple gestation, or known fetal anomalies.
The primary exposure was the number of four different medical comorbidities: chronic hypertension, pregestational diabetes mellitus, chronic kidney disease, and systemic lupus erythematosus. These comorbidities were chosen as they all increase a patient’s risk of adverse pregnancy outcomes.9,13,15,16,18,20 Patients were categorized as having 0, 1, and ≥2 comorbidities. Chronic hypertension in pregnancy was defined by ACOG as a systolic blood pressure of 140 mmHg or more, a diastolic blood pressure of 90 mm Hg or more, or both in patients with no prior history of hypertension at less than 20 weeks’ gestation; generally, this required at least two blood pressure measurements at least 4 hours apart, although on occasion, especially when faced with severe hypertension, the diagnosis was confirmed within a shorter interval to facilitate timely therapy.21 Patients with a previous diagnosis of chronic hypertension outside of pregnancy per patient report or review of electronic medical records, or requiring medications for blood pressure control, were considered to have chronic hypertension even if blood pressure measurements in pregnancy were lower than the aforementioned threshold, likely owing to the physiology of blood pressure decreases as early as the first trimester. More recent guidelines published in 2017 by the American College of Cardiology and the American Heart Association have lowered the threshold for the diagnosis of stage I chronic hypertension in the nonpregnant population.22 However, these guidelines have not yet been widely accepted by ACOG, owing to a lack of data on the risks and benefits of the application of these guidelines for diagnosis and treatment of chronic hypertension in pregnancy,23 and therefore were not applied in this analysis. Both patients with type I and type II pregestational diabetes, as defined by the American Diabetes Association, were included.24 Patients with chronic renal disease diagnosed clinically by a nephrologist or with at least 500 mg protein on a baseline 24-hour urine collection obtained prior to pregnancy or <20 weeks during pregnancy in the setting of pregestational diabetes were included,17 regardless of etiology, for example, glomerular, vascular, tubulointerstitial, cystic, secondary to a systemic etiology, etc. Patients were clinically diagnosed with systemic lupus erythematosus by a rheumatologist.
The primary outcome of this analysis was the proportion of potential expectant management time achieved (%EM), that is, days of expectant management achieved (time in days from SPE diagnosis to delivery) divided by maximum potential expectant management time (days from SPE diagnosis to 340/7 weeks). This was chosen as the primary outcome to avoid bias towards patients with earlier SPE diagnoses who may have a greater likelihood of achieving a longer duration of expectant management. Secondary outcomes included delivery EGA, total days of expectant management, cesarean delivery, length of maternal hospitalization, maternal death, neonatal birth weight, and length of neonatal hospitalization. Additional secondary outcomes included maternal and neonatal composite morbidity, and their individual composite components. The maternal morbidity composite included: maternal intensive care unit (ICU) admission, HELLP syndrome, severe anemia (hematocrit <21 units/L), evidence of hepatotoxicity (AST >80 units/L), thrombocytopenia (platelet count <100,000/μL), acute kidney injury (serum creatinine >1.1 mg/dL in patients with a baseline creatinine ≤1.1 or unknown baseline creatinine, or twice baseline creatinine if baseline creatinine >1.1), clinically diagnosed placental abruption, pulmonary edema, and maternal stroke. Severe anemia represents here a proxy variable for packed red blood cell transfusion at our institution, as transition was not a collected variable available in this analysis. Neonatal morbidity composite, as pragmatically determined by the treating neonatology team or standardized laboratory values, included: mechanical ventilation, need for cardiopulmonary resuscitation, grade III or IV intraventricular hemorrhage, necrotizing enterocolitis, respiratory distress syndrome, arterial cord gas pH <7.1, 5-minute Apgar’s score <3, hypoxic–ischemic encephalopathy, and neonatal death.
The study cohort used for this analysis was created using previously described methods.25 Patients likely to have SPE were identified by a validated algorithm which implemented a search for preeclampsia-related diagnosis descriptors and documented magnesium sulfate usage within our institution’s electronic medical record. Among patients identified, individual chart review by two trained investigators (G.D.C. and A.R.S.) confirmed the SPE diagnosis and eligibility based on the aforementioned inclusion and exclusion criteria. Data regarding maternal demographics and delivery information were extracted electronically from the medical record. Data including maternal comorbidities, timing and indication of decision to deliver, and neonatal complications were abstracted by the two reviewers using standardized abstraction forms. Indications for delivery were not mutually exclusive.
Maternal demographics and primary and secondary outcomes were compared across comorbidity groups using one-way analysis of variance (ANOVA), Kruskal–Wallis, chi-square, and Fisher’s exact tests as appropriate. Multivariable linear regression was used to estimate the association between number of comorbidities and %EM adjusting for maternal age, body mass index (BMI), and race/ethnicity. Adjusted beta (β)-coefficients and 95% confidence intervals (CIs) were estimated for 1 and ≥2 comorbidities with no comorbidities as the referent group. A similar approach was used to estimate the association between number of comorbidities and other continuous outcomes. Multivariable logistic regression was used to estimate odds ratios and 95% CIs for dichotomous outcomes. Covariates that were identified as being statistically significantly different between exposure groups in bivariable analyses with p <0.05 and were not collinear with other variables were included as potential confounders in these multivariable models. A sensitivity analysis was performed including only women who had expectant management for at least 1 day after SPE diagnosis to assess the robustness of the results. All analyses were performed using Stata Version 17.0 (Stata-Corp, College Station, TX). Outcomes were evaluated with at a 0.05 level of significance.
Results
Of 417 patients with SPE who delivered at 230/7 to 342/7 weeks’ gestation during the study time period, 337 met the final inclusion criteria (Fig. 1).
Fig. 1.
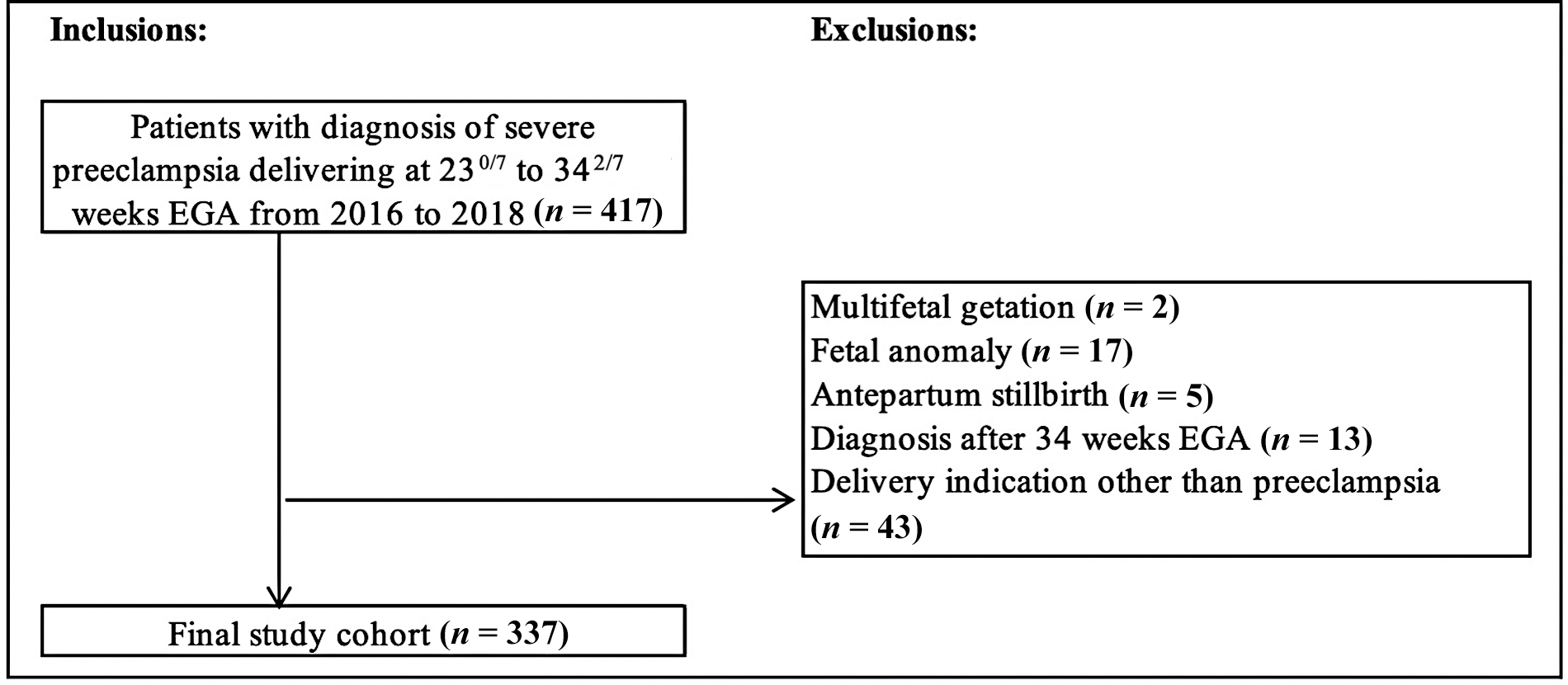
Flow diagram depicting patients identified for inclusion in final analysis. EGA, estimated gestational age.
Of patients included for analysis, 167 (50%) had 0, 151 (45%) had 1, and 19 (5%) had ≥2 comorbidities (Table 1). Chronic hypertension was the most common comorbidity and occurred in 155 (46%) patients (with 67 [43.2%] on antihypertensive medications prior to pregnancy and 111 [71.6%] on medications during pregnancy), followed by 16 (4.7%) patients with chronic kidney disease, 11 (3.3%) patients with pregestational diabetes mellitus, and 9 (2.7%) patients with systemic lupus erythematosus. Groups differed with respect to age, BMI (kg/m2) at delivery, race/ethnicity, insurance, and nulliparity. Patients with ≥2 comorbidities had the highest admission systolic blood pressure, highest admission serum creatinine and platelet count, and highest 24-hour urine protein assessment (collected within 3 days of diagnosis of SPE). While it was not statistically significant, those with ≥2 comorbidities had the earliest median EGA at SPE diagnosis at 29.4 weeks, while those with 0 and 1 comorbidity had a median EGA at diagnosis of 31.1 and 30.9 weeks, respectively (Table 1).
Table 1.
Baseline maternal demographics among women with severe preeclampsia by number of medical comorbidities (chronic hypertension, pregestational diabetes, systemic lupus erythematosus, and chronic kidney disease)
| No comorbidities (n = 167) | 1 comorbidity (n = 151) | ≥2 comorbidities (n = 19) | p-Value | |
|---|---|---|---|---|
| Maternal demographics | ||||
| Maternal age (y) | 26.5 ± 6.0 | 31.2 ± 5.6 | 30.5 ± 5.1 | <0.001 |
| BMI at delivery (kg/m2) | 35.4 ± 8.9 | 38.8 ± 9.6 | 37.8 ± 8.5 | <0.01 |
| Race/ethnicity | 0.03 | |||
| Black, non-Hispanic | 94 (56.3) | 102 (68.0) | 9 (47.4) | |
| White, non-Hispanic | 62 (37.4) | 41 (27.3) | 6 (31.6) | |
| Hispanic | 8 (4.8) | 6 (4.0) | 4 (21.1) | |
| Other | 2 (1.2) | 1 (0.7) | 0 | |
| Private insurance | 60 (36.1) | 43 (28.5) | 2 (10.5) | 0.04 |
| Nulliparous | 103 (61.7) | 44 (29.1) | 4 (21.1) | <0.001 |
| Chronic hypertension | 0 | 139 (92.1) | 16 (84.2) | <0.001 |
| Diabetes | <0.001 | |||
| None | 160 (95.8) | 114 (75.5) | 8 (42.1) | |
| Pregestational (B-RF) | 0 | 6 (4.0) | 5 (26.3) | |
| Gestational (A1–2) | 7 (4.2) | 31 (20.5) | 6 (31.6) | |
| Systemic lupus erythematosus | 0 | 4 (2.7) | 5 (26.3) | <0.001 |
| Chronic kidney disease | 0 | 2 (1.3) | 14 (73.7) | <0.001 |
| EGA at diagnosis of SPE (wk) | 31.1 (28.4–32.6) | 30.9 (28.9–32.7) | 29.4 (27.3–32.3) | 0.56 |
| Admission parameters | ||||
| Systolic blood pressure (mmHg) | 161 ± 21 | 172 ± 22 | 174 ± 27 | <0.001 |
| Diastolic blood pressure (mmHg) | 97 ± 12 | 100 ± 14 | 98 ± 15 | 0.16 |
| Serum creatinine | 0.7 ± 0.2 | 0.6 ± 0.2 | 1.7 ± 1.4 | <0.001 |
| Aspartate aminotransferase | 39 ± 83 | 27 ± 36 | 18 ± 10 | 0.21 |
| Platelets | 207 ± 75 | 250 ± 78 | 282 ±107 | <0.001 |
| Proteinuria (mg/24 h) | 1332 (407–5,522) | 450 (260–1,618) | 5,211 (450–13,432) | <0.001 |
| Use of blood pressure medication for chronic hypertension medication prior to pregnancy | 0 | 59 (39) | 8 (50) | <0.001 |
| Use of blood pressure medication for chronic hypertension during pregnancy | 0 | 101 (66) | 10 (63) | <0.001 |
| Blood pressure medication initiated or increase during pregnancy | 59 (35) | 72 (37) | 5 (31) | 0.08 |
Abbreviations: BMI, body mass index; EGA, estimated gestational age; SPE, preeclampsia with severe features.
Note: Data presented as n (%), median (Q1-Q3), and mean ± SD. Analysis of variance, Kruskal-Wallis, chi-square, and Fisher’s exact tests used as appropriate.
The most common indication for delivery in the cohort was uncontrolled hypertension (70.6%), followed by persistent headache (22.3%), severe laboratory abnormalities (19.3%), nonreassuring antenatal testing (8.3%), and achievement of goal gestational age of 34 weeks (8.0%). Delivery due to new-onset eclampsia (0.6%) was rare, as was pulmonary edema (3.9%), HELLP (2.4%), and abruption (1.2%). In addition, 0% of patients with ≥2 comorbidities were delivered due to achievement of goal gestational age, whereas 4.8 and 12.4% of patients with 0 and 1 comorbidities, respectively, were delivered for this indication. Among patients who were expectantly managed for less than 1 day prior to delivery (n = 161), uncontrolled hypertension remained the most common indication for delivery (67.7%). Laboratory abnormalities (24.8%), headache (25.5%), pulmonary edema (5.6%), HELLP (4.4%), and eclampsia (1.2%) appeared to occur more commonly, although the sample size was limited.
Among the entire cohort, %EM was small with a median of 1.8% (interquartile range [IQR]: 0–15.4%). The median duration of expectant management was 1 day (IQR: 0–3 days). The primary outcome of %EM did not differ significantly between comorbidity groups (Table 2). Duration of expectant management (days) did not differ between groups nor did delivery EGA (Table 2).
Table 2.
Perinatal outcomes by number of medical comorbidities (chronic hypertension, pregestational diabetes, systemic lupus erythematosus, and chronic kidney disease)
| No comorbidities (n = 167) | 1 comorbidity (n = 151) | ≥2 comorbidities (n = 19) | p-Value | |
|---|---|---|---|---|
| Maternal outcomes | ||||
| %EM achieved (%EM)a | 1.4 (0–11.8) | 2.6 (0–25.0) | 0 (0–10.5) | 0.59 |
| EGA at delivery (wk) | 31.7 (28.6–33.1) | 32.1 (29.6–33.3) | 30.4 (27.7–33.1) | 0.19 |
| Duration of EM (d)b | 1 (0–3) | 1 (0–3) | 0 (0–2) | 0.50 |
| Cesarean delivery | 91 (54.5) | 106 (70.7) | 17 (89.5) | 0.001 |
| Length of postpartum hospitalization (d) | 3 (2–4) | 4 (3–4) | 5 (4–7) | <0.001 |
| Maternal death | 2 (1.2) | 0 | 0 | 0.55 |
| Maternal composite morbidity | 55 (32.9) | 33 (21.9) | 14 (73.7) | <0.001 |
| Maternal ICU admission | 3 (1.8) | 3 (2.0) | 2 (10.5) | 0.10 |
| HELLP syndrome | 6 (3.6) | 2 (1.3) | 0 | 0.47 |
| Severe anemia (Hct <21 units/L) | 2 (1.2) | 5 (3.3) | 3 (15.8) | <0.01 |
| Hepatotoxicity (AST >80 units/L) | 26 (15.6) | 10 (6.6) | 0 | 0.01 |
| Thrombocytopenia (Plt <100/μL) | 19 (11.4) | 8 (5.3) | 1 (5.3) | 0.13 |
| Acute kidney injuryc | 13 (7.8) | 9 (6.0) | 6 (31.6) | 0.001 |
| Placental abruption | 3 (1.8) | 1 (0.7) | 1 (5.3) | 0.22 |
| Pulmonary edema | 7 (4.2) | 4 (2.7) | 4 (21.1) | 0.01 |
| Maternal stroke | 1 (0.6) | 0 | 0 | >0.99 |
| Neonatal outcomes | ||||
| Birth weight (g) | 1,406 ± 536 | 1,576 ± 541 | 1,321 ± 464 | <0.01 |
| Length of hospitalization (d) | 33 (20–58) | 32 (16–56) | 34 (20–82) | 0.47 |
| Neonatal ICU admission | 137 (82.0) | 116 (76.8) | 16 (84.2) | 0.45 |
| Neonate composite morbidity | 107 (64.1) | 89 (59.3) | 10 (52.6) | 0.50 |
| Mechanical ventilation | 49 (30.4) | 30 (20.1) | 6 (31.6) | 0.10 |
| Cardiopulmonary resuscitation | 4 (2.5) | 1 (0.7) | 0 | 0.54 |
| Necrotizing enterocolitis | 8 (5.0) | 2 (1.3) | 0 | 0.23 |
| Intraventricular hemorrhage | 6 (3.7) | 3 (2.0) | 0 | 0.71 |
| Respiratory distress syndrome | 91 (56.5) | 79 (53.0) | 9 (47.4) | 0.68 |
| Arterial cord pH <7.1 | 14 (9.8) | 16 (12.3) | 2 (11.8) | 0.80 |
| 5-minute Apgar <3 | 13 (7.8) | 4 (2.7) | 0 | 0.09 |
| Hypoxic-ischemic encephalopathy | 2 (1.2) | 0 | 1 (5.3) | 0.09 |
| Neonatal death | 4 (2.5) | 2 (1.3) | 1 (5.3) | 0.33 |
Abbreviations: EGA, estimated gestational age; EM, expectant management; HELLP, hemolysis, elevated liver enzymes, and low platelets; ICU, intensive care unit; Plt, platelet.
Note: Data presented as n (%), median (Q1-Q3), and mean ± SD. Comorbidity categories compared using analysis of variance, Kruskal-Wallis, chi-square, and Fisher’s exact tests as appropriate.
%EM achieved = duration of EM (days)/34 weeks EGA-EGA at severe features (SPE) diagnosis (days).
Duration of expectant management (EM) = time (in days) from EGA at SPE diagnosis to EGA at delivery.
Acute kidney injury = serum creatinine >1.1 mg/dL in patients with a baseline creatinine ≤1.1 or unknown baseline creatinine, or twice baseline creatinine if baseline creatinine >1.1.
Percentage of EM did not differ significantly by number of comorbidities (Table 3; adjusted β: 5.3 [95% CI: −2.1 to 12.9] for 1 comorbidity vs. 0 and adjusted β: −2.9 [95% CI: −18.0 to 12.2] for ≥2 comorbidities vs. 0) in multivariable analysis adjusting for maternal age, BMI, and race/ethnicity. While creatinine on admission differed between groups, we did not include this in the multivariate model as it may be along the causal pathway for shortened pregnancy duration and other maternal morbidities. Further, the duration of EM (in days) did not differ by number of comorbidities (Table 3; adjusted β: 4.0 [95% CI: −1.5 to 9.5] for 1 comorbidity vs. 0 and adjusted β: 1.1 [95% CI: −10.0 to 12.2] for ≥2 comorbidities vs. 0). There was no difference in delivery gestational age for those with and without comorbidities (Table 3).
Table 3.
Linear and logistic regression of select pregnancy outcomes by number of medical comorbidities (chronic hypertension, pregestational diabetes, systemic lupus erythematosus, and chronic kidney disease)
| No comorbidities (n = 167) | 1 comorbidity (n = 151) | ≥2 comorbidities (n = 19) | |
|---|---|---|---|
| Pregnancy outcomes | |||
| % of EM achieveda | Referent | 5.3 (−2.1 to 12.9) | −2.9 (−18.0 to 12.2) |
| EGA at delivery (wk)a | Referent | 0.7 (−0.003 to 1.4) | −0.5 (−1.9 to 0.9) |
| Duration of EM (d)a | Referent | 4.0 (−1.5 to 9.5) | 1.1 (−10.0 to 12.2) |
| Maternal compositeb | Referent | 0.5 (0.3–0.8) | 3.0 (1.1–8.2) |
| Cesarean deliveryb | Referent | 1.8 (1.1–3.0) | 7.1 (1.5–32.4) |
| Length of maternal postpartum hospitalization (d)a | Referent | 0.2 (−0.2 to 0.6) | 2.2 (1.3–3.0) |
| Neonatal compositeb | Referent | 0.7 (0.4–1.2) | 0.7 (0.2–1.8) |
| Neonatal birth weighta | Referent | 169 (39–300) | −124 (−384 to 135) |
Abbreviations: EGA, estimated gestational age; EM, expectant management.
Note: Referent = no comorbidities.
Beta coefficients with 95% confidence interval (CI), adjusted for maternal age, BMI, and race.
Adjusted odds ratios with 95% CI, adjusted for maternal age, BMI, and race.
A sensitivity analysis including 176 (52%) patients who were expectantly managed for at least 1 day revealed similar results to the primary analysis. The median number of days from diagnosis to delivery was 3 (IQR: 1–7.5) with a median % EM of 14.1% (IQR: 5.9–60.0). Those with 0, 1, and ≥2 comorbidities had a median duration of expectant management of 3, 3, and 1 days, respectively. In multivariable analyses adjusting for maternal age, BMI, and race/ethnicity, there were no significant differences in duration of expectant management for 1 comorbidity or ≥2 comorbidities versus 0 (Supplementary Table S1, available in the online version). Patients with 1 comorbidity had 13.9% longer %EM achieved (95% CI: 1.8–26.1), compared to those with 0 comorbidities. There was no difference in %EM for ≥2 comorbidities compared to none (adjusted β: 1.3 [95% CI: −24.2 to 26.8]). There were also no differences in delivery gestational age; however, the finding of increased maternal morbidity composite in those with ≥2 comorbidities was consistent with the primary analysis (Supplementary Table S1, available in the online version).
With regards to secondary maternal outcomes, composite maternal morbidity differed significantly between groups, with the highest number of adverse outcomes occurring in patients with ≥2 comorbidities (73.7%) compared to those with 0 (32.9%) or 1 (21.9%) comorbidity (p ≤ 0.001, Table 2). Multiple adverse maternal outcomes differed between groups including hematocrit <21, AST >80, acute kidney injury, and pulmonary edema. In multivariable analysis, patients with ≥2 comorbidities had three-fold higher odds of composite morbidity compared to those with 0 comorbidities (95% CI: 1.1–8.2). There was a decreased risk of composite morbidity for those with 1 comorbidity versus 0 (adjusted odds ratio: 0.5 [95% CI: 0.3–0.8]). Patients with 1 and ≥2 comorbidities had a nearly two-fold and seven-fold higher odds for cesarean delivery compared to those with 0 comorbidities, respectively, while only those with ≥2 comorbidities had a longer postpartum hospitalization (Table 3).
With regards to neonatal outcomes, the composite neonatal comorbidity was common, occurring in 61% of patients overall. However, there was no difference in neonatal composite morbidity between the comorbidity groups in bivariable or multivariable analyses (Tables 2 and 3). Neonatal birth weight differed between groups in bivariable analysis, but in multivariable analysis the association was only significant for 1 comorbidity versus 0 (169 g, 95% CI: 39–300). There was no difference in NICU admission or overall length of hospitalization (Table 2).
Discussion
In this retrospective cohort study of patients diagnosed with SPE at less than 340/7 weeks EGA, the number of medical comorbidities, including chronic hypertension, pregestational diabetes, chronic kidney disease, and systemic lupus erythematosus, was not associated with the proportion of time gained with expectant management. Importantly, the duration of expectant management in days was short in all groups. There was no significant difference in EGA at diagnosis of SPE or delivery. However, patients with ≥2 comorbidities frequently had composite maternal morbidity (73.7%) and high rates of severe anemia (15.8%), acute kidney injury (31.6%), and pulmonary edema (21.2%). Neonatal morbidity was similar among groups. These findings of increased maternal composite morbidity did not differ significantly when only women who achieved at least 1 day of expectant management were included.
Previous studies have reported varying amounts of time gained in expectant management of SPE. Published in 1994 by Sibai et al, a seminal randomized controlled trial of patients with SPE at 28 to 32 weeks EGA found an average of 15.4 days of time gained when comparing expectant management to immediate delivery.4 They also found a significantly higher gestational age at delivery, higher birth weight, shorter NICU stay, and lower incidence of neonatal complications among those with expectant management.4 These results have been supported by other trials comparing expectant management to immediate delivery in SPE, demonstrating pregnancy prolongation of 7.1 days3 and 10.3 days.5 In our analysis, patients with 0 and 1 comorbidity had a median duration of expectant management of 1 day while those with ≥2 comorbidities had a median duration of expectant management of 0 days. When excluding those who delivered within 1 day of SPE diagnosis, the median duration of expectant management remained short at 3, 3, and 1 days for those with 0, 1, and ≥2 comorbidities, respectively. While other authors have shown a similarly short duration of expectant management,26 the duration of expectant management in our cohort is vastly shorter than what is reported by other authors who selectively excluded women with certain medical comorbidities such as those included in this study.3–5 This suggests that our patient population, even in those with no comorbidities, may be inherently different than some prior trials.
There have been limited prior studies evaluating the impact of medical comorbidities on expectant management of SPE. A study by Venkatesh et al examined the impact of specific medical comorbidities (defined as chronic hypertension, diabetes, twin gestation, and FGR) on maternal and neonatal outcomes among patients with SPE less than 34 weeks.27 While chronic hypertension was associated with an increased risk of maternal ICU admission and perinatal death, there was generally no other increased risk of other maternal or neonatal adverse outcomes among those with a comorbidity (with the exception of FGR increasing neonatal risks). Our analysis differs from this study not only in the comorbidities chosen but also in the outcomes reported. We demonstrated an increased risk of composite maternal morbidity, as well as select maternal adverse outcomes such as pulmonary edema, among those ≥2 comorbidities. Overall composite neonatal morbidity did not differ by number of comorbidities, suggesting that expectant management of SPE should be undertaken cautiously among those with multiple comorbidities for maternal benefit.
Previous studies have demonstrated that pregnancies complicated by medical comorbidities have increased risks of SPE as well as adverse pregnancy outcomes according to their disease severity. Our study examines pregnancy outcomes in these complicated patients specifically through the lens of SPE. Chronic hypertension is associated with increased risks of SPE, stroke, renal failure, pulmonary edema, abruption, and maternal mortality.9,10,13 Moreover, patients with hypertension with poor blood pressure control or requiring medications have higher risks of preeclampsia compared to those not requiring or well controlled on medications.12,28,29 In a similar vein, Sibai et al demonstrated a higher risk of preeclampsia among those with increasing severity of pregestational diabetes—11% of patients with White’s class B diabetes compared to 21% in class D diabetes and 36% in RF diabetes.16 With regard to renal disease, pregnancy outcomes incrementally worsen as patients increase in their stage of kidney disease,17 and a 2015 meta-analysis of over 500,000 patients with chronic kidney disease demonstrated a more than 10-fold greater odds of preeclampsia, 5-fold odds of preterm delivery, and nearly 3-fold odds of cesarean delivery.18 Lastly, patients with systemic lupus erythematosus have a five-fold increased risk of preeclampsia,19 and the presence of lupus anticoagulant, antihypertensive medication use, thrombocytopenia, maternal flares, and high disease activity were predictive of adverse pregnancy outcomes.30 Our analysis did not demonstrate an earlier delivery gestational age among patients with ≥2 comorbidities; however, we demonstrated worsened maternal morbidity among those with multiple comorbidities, including cesarean delivery, severe anemia, acute kidney injury, and pulmonary edema, and longer postpartum hospitalization.
Since 2013, ACOG has recommended expectant management in particular cases of SPE <34 weeks’ EGA to improve neonatal outcomes.6 A previous retrospective study of 543 women with SPE at our institution comparing perinatal outcomes before and after adopting guidelines for expectant management demonstrated no worsening of maternal outcomes after guideline adoption.25 There remain no clear factors or criteria to predict duration expectant management of SPE, and our study did not demonstrate any predictive ability of duration of expectant management based on medical complexity. However, we did demonstrate that patients with ≥2 comorbidities had an increase in composite maternal morbidity with no difference in pregnancy prolongation or neonatal composite morbidity. In practice, this suggests that decisions regarding continued expectant management versus delivery should take into account these maternal risks, specifically of cesarean, anemia, acute kidney injury, pulmonary edema, and prolonged maternal hospital stay among those with ≥2 comorbidities. It may be that patients with medical comorbidities could benefit, from a maternal standpoint, from a lower threshold for delivery.
Patients with multiple coexisting medical comorbidities have increased risks of developing preeclampsia; for example, patients with pregestational diabetes complicated by hypertension have higher rates of preeclampsia than those without hypertension,15 and patients with systemic lupus erythematosus with lupus nephritis have an increased risk of preeclampsia compared to those without nephritis.20 The impact of multiple comorbidities is further developed in our analysis by evaluating the maternal and neonatal risks of these comorbidities among those with SPE. However, the additive impact of these comorbidities can be challenging to assess as each comorbidity may be causative or contributory for the other, for example, modified White’s class D diabetes (pregestational diabetes and hypertension), diabetic or hypertensive nephropathy, etc. Future larger studies should analyze the impact of these comorbidities on duration of expectant management of SPE and pregnancy outcomes both individually and in specific combinations. Additionally, several comorbidities other than the four we chose to analyze have been identified as risk factors for preeclampsia and adverse perinatal outcomes, such as maternal obesity31,32 or assisted reproductive technology.33,34 Future analyses should also evaluate these additional morbidities and their interplay with other predictors of adverse outcomes.
The strengths of our study include a cohort of 337 patients with SPE who underwent individual detailed chart review by two trained data reviewers, minimizing the risk of misclassification. While the decision to deliver could be impacted by provider preference, all patients were managed by the same group of Maternal Fetal Medicine providers at a single institution and with a standard monitoring plan including twice weekly labs, daily nonstress tests, weekly amniotic fluid assessment, and serial fluid and growths. Additionally, with %EM as the primary outcome instead of total duration of expectant management, bias towards women with earlier SPE diagnoses who may have a greater potential to achieve a longer duration was avoided. Finally, performance of a sensitivity analysis including only women who underwent at least 1 day of expectant management strengthens the validity of our results.
Limitations of our study include the multiple comparison groups and thus the small number of patients with ≥2 comorbidities. As our population of interest included only patients with severe features, we did not assess the impact of comorbidities on those without severe features, who may have been able to undergo expectant management for a longer period of time than those with SPE. We lacked data as to pregnancies complicated by FGR, which is common among patients with SPE and can impact timing of delivery. The pragmatic and retrospective nature of this analysis limited the characterization of these comorbidities by severity of disease, disease control, or timing of diagnosis (prior to pregnancy vs. during pregnancy), which may be important considerations in a patient’s risk for adverse outcomes. Additionally, the characterization and diagnosis of these comorbidities may be altered if the patient had barriers to accessing health care or limited prenatal care. The definition of systemic lupus erythematosus and chronic renal disease made by clinical diagnosis rather than by uniform diagnostic criteria may have created variation in the primary exposure. Additionally, we were unable to assess if particular groupings of ≤2 specific comorbidities had worse outcomes than other groupings due to limited sample size. While we had information on indication for delivery, we did not have information available on specific indications for cesarean if performed, which may have played a role in certain maternal morbidities. Further, these findings are drawn from a single tertiary care center with high rates of preeclampsia and a high proportion of patients with public insurance, obesity, and non-Hispanic Black race/ethnicity, and thus may not be generalizable to all populations.
Lastly, several of the adverse maternal outcomes were higher among those with 0 comorbidities compared to 1 comorbidity; these included composite maternal morbidity, as well as AST >80, acute kidney injury, and pulmonary edema. This may be the result of misclassification, in that those with 0 comorbidities may have actually had SPE while those with 1 comorbidity, for example, hypertension, may have been falsely labelled as having severe features when their actual diagnosis was worsening hypertension or superimposed preeclampsia without severe features. While the possibility of misclassification exists within retrospective studies, future analysis in a larger cohort should evaluate the differences in outcomes between 0 and 1 comorbidity, as this is at odds with other analyses demonstrating worsened outcomes in those with medical comorbidities.
Conclusion
Among patients with SPE, the increasing number of preexisting medical comorbidities was not associated with the proportion of potential time achieved by expectant management, nor was there a worsening of neonatal outcomes. Importantly, the time achieved across all groups was quite low. Despite this, patients with ≥2 comorbidities had higher odds of adverse maternal outcomes, including composite morbidity, cesarean delivery, anemia, acute kidney injury, pulmonary edema, and prolonged hospital stay. While further studies must clarify the risks of expectant management of preeclampsia to medically complicated patients and their fetuses, expectant management of SPE in patients with multiple comorbidities should be done carefully and take into account these maternal risks.
Supplementary Material
Key Points.
Greater number of medical comorbidities were not associated with expectant management duration.
Two or more medical comorbidities were associated with higher odds of adverse maternal outcomes.
Expectant management should be undertaken cautiously in medically complicated patients.
Funding
Effort for A.N.B. was supported by a grant from the The Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number: K23HD103875).
Footnotes
Conflict of Interest
None declared.
References
- 1.Sibai BMPublications Committee, Society for Maternal-Fetal Medicine. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol 2011;205(03):191–198 [DOI] [PubMed] [Google Scholar]
- 2.Magee LA, Yong PJ, Espinosa V, Côté AM, Chen I, von Dadelszen P. Expectant management of severe preeclampsia remote from term: a structured systematic review. Hypertens Pregnancy 2009;28(03):312–347 [DOI] [PubMed] [Google Scholar]
- 3.Odendaal HJ, Pattinson RC, Bam R, Grove D, Kotze TJ. Aggressive or expectant management for patients with severe preeclampsia between 28–34 weeks’ gestation: a randomized controlled trial. Obstet Gynecol 1990;76(06):1070–1075 [PubMed] [Google Scholar]
- 4.Sibai BM, Mercer BM, Schiff E, Friedman SA. Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks’ gestation: a randomized controlled trial. Am J Obstet Gynecol 1994;171(03):818–822 [DOI] [PubMed] [Google Scholar]
- 5.Vigil-De Gracia P, Reyes Tejada O, Calle Miñaca A, et al. Expectant management of severe preeclampsia remote from term: the MEXPRE Latin Study, a randomized, multicenter clinical trial. Am J Obstet Gynecol 2013;209(05):425.e1–425.e8 [DOI] [PubMed] [Google Scholar]
- 6.American College of Ostetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122(05):1122–1131 [DOI] [PubMed] [Google Scholar]
- 7.Gestational Hypertension and Preeclampsia. Gestational hypertension and preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol 2020;135(06):1492–1495 [DOI] [PubMed] [Google Scholar]
- 8.Churchill D, Duley L, Thornton JG, Moussa M, Ali HS, Walker KF. Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks’ gestation. Cochrane Database Syst Rev 2018;10(10):CD003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: a population-based study. J Reprod Med 2007;52(11):1046–1051 [PubMed] [Google Scholar]
- 11.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013;209(06):544.e1–544.e12 [DOI] [PubMed] [Google Scholar]
- 12.Nzelu D, Dumitrascu-Biris D, Kay P, Nicolaides KH, Kametas NA. Severe hypertension, preeclampsia and small for gestational age in women with chronic hypertension diagnosed before and during pregnancy. Pregnancy Hypertens 2018;14:200–204 [DOI] [PubMed] [Google Scholar]
- 13.Panaitescu AM, Syngelaki A, Prodan N, Akolekar R, Nicolaides KH. Chronic hypertension and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol 2017;50(02):228–235 [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003;22(02):203–212 [DOI] [PubMed] [Google Scholar]
- 15.Oppermann MLDR, Alessi J, Hirakata VN, Wiegand DM Reichelt AJ. Preeclampsia in women with pregestational diabetes - a cohort study. Hypertens Pregnancy 2020;39(01):48–55 [DOI] [PubMed] [Google Scholar]
- 16.Sibai BM, Caritis S, Hauth J, et al. ; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Risks of preeclampsia and adverse neonatal outcomes among women with pregestational diabetes mellitus. Am J Obstet Gynecol 2000;182(02):364–369 [DOI] [PubMed] [Google Scholar]
- 17.Hui D, Hladunewich MA. Chronic kidney disease and pregnancy. Obstet Gynecol 2019;133(06):1182–1194 [DOI] [PubMed] [Google Scholar]
- 18.Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015;10(11):1964–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, Yuan F, Dai Z, Wang Z, Zhu Y, Wang B. Preeclampsia in systemic lupus erythematosus pregnancy: a systematic review and meta-analysis. Clin Rheumatol 2020;39(02):319–325 [DOI] [PubMed] [Google Scholar]
- 20.Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 2010;5(11):2060–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy. Obstet Gynecol 2019;133(01):e26–e50 [DOI] [PubMed] [Google Scholar]
- 22.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71(19):e127–e248 [DOI] [PubMed] [Google Scholar]
- 23.Battarbee AN, Sinkey RG, Harper LM, Oparil S, Tita ATN. Chronic hypertension in pregnancy. Am J Obstet Gynecol 2020;222(06):532–541 [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl 1):S14–S31 [DOI] [PubMed] [Google Scholar]
- 25.Sanjanwala AR, Jauk VC, Cozzi GD, et al. Outcomes before and after adopting guidelines for expectant management of severe preeclampsia. Am J Perinatol 2022;39(02):172–179 [DOI] [PubMed] [Google Scholar]
- 26.Duvekot JJ, Duijnhoven RG, van Horen E, et al. ; TOTEM study collaboration group. Temporizing management vs immediate delivery in early-onset severe preeclampsia between 28 and 34 weeks of gestation (TOTEM study): an open-label randomized controlled trial. Acta Obstet Gynecol Scand 2021;100(01):109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesh KK, Strauss RA, Westreich DJ, Thorp JM, Stamilio DM, Grantz KL. Adverse maternal and neonatal outcomes among women with preeclampsia with severe features <34 weeks gestation with versus without comorbidity. Pregnancy Hypertens 2020;20:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecarpentier E, Tsatsaris V, Goffinet F, Cabrol D, Sibai B, Haddad B. Risk factors of superimposed preeclampsia in women with essential chronic hypertension treated before pregnancy. PLoS One 2013;8(05):e62140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nzelu D, Dumitrascu-Biris D, Nicolaides KH, Kametas NA. Chronic hypertension: first-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age. Am J Obstet Gynecol 2018;218(03):337.e1–337.e7 [DOI] [PubMed] [Google Scholar]
- 30.Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015;163(03):153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannakou K, Evangelou E, Papatheodorou SI. Genetic and non-genetic risk factors for pre-eclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol 2018;51(06):720–730 [DOI] [PubMed] [Google Scholar]
- 32.Shen M, Smith GN, Rodger M, White RR, Walker MC Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One 2017;12(04):e0175914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 2004;103(03):551–563 [DOI] [PubMed] [Google Scholar]
- 34.Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril 2016;105(01):73–85.e1, 6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


