Abstract
Objective
To examine the association of COVID-19 convalescent plasma transfusion with mortality and the differences between subgroups in hospitalized patients with COVID-19.
Patients and Methods
On October 26, 2022, a systematic search was performed for clinical studies of COVID-19 convalescent plasma in the literature from January 1, 2020, to October 26, 2022. Randomized clinical trials and matched cohort studies investigating COVID-19 convalescent plasma transfusion compared with standard of care treatment or placebo among hospitalized patients with confirmed COVID-19 were included. The electronic search yielded 3841 unique records, of which 744 were considered for full-text screening. The selection process was performed independently by a panel of 5 reviewers. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Data were extracted by 5 independent reviewers in duplicate and pooled using an inverse-variance random effects model. The prespecified end point was all-cause mortality during hospitalization.
Results
Thirty-nine randomized clinical trials enrolling 21,529 participants and 70 matched cohort studies enrolling 50,160 participants were included in the systematic review. Separate meta-analyses reported that transfusion of COVID-19 convalescent plasma was associated with a decrease in mortality compared with the control cohort for both randomized clinical trials (odds ratio [OR], 0.87; 95% CI, 0.76-1.00) and matched cohort studies (OR, 0.76; 95% CI, 0.66-0.88). The meta-analysis of subgroups revealed 2 important findings. First, treatment with convalescent plasma containing high antibody levels was associated with a decrease in mortality compared with convalescent plasma containing low antibody levels (OR, 0.85; 95% CI, 0.73 to 0.99). Second, earlier treatment with COVID-19 convalescent plasma was associated with a decrease in mortality compared with the later treatment cohort (OR, 0.63; 95% CI, 0.48 to 0.82).
Conclusion
During COVID-19 convalescent plasma use was associated with a 13% reduced risk of mortality, implying a mortality benefit for hospitalized patients with COVID-19, particularly those treated with convalescent plasma containing high antibody levels treated earlier in the disease course.
The COVID-19 pandemic created a humanitarian crisis that prompted an expeditious search for safe and effective COVID-19 therapies. Before identifying effective antispike monoclonal antibodies and small molecule antivirals, COVID-19 convalescent plasma was proposed as a safe treatment with a promising efficacy profile in early reports.1, 2, 3 More recently, as new SARS-CoV-2 variants emerged and evaded antispike monoclonal antibodies,4,5 interest has been renewed in understanding the clinical efficacy of COVID-19 convalescent plasma,6 particularly among patients who are immunocompromised.7 Although COVID-19 convalescent plasma has been widely available and used to treat over half a million patients with COVID-19,8 uncertainty remains about the utility of COVID-19 convalescent plasma and its association with mortality because of heterogenous findings from individual studies.9 Heterogeneity in clinical studies is likely due to several key factors: biological diversity of COVID-19 convalescent plasma, evolving and nonstandard treatment protocols, and a wide spectrum of clinical use of COVID-19 convalescent plasma, from postexposure prophylaxis to therapy of last resort in patients with multiorgan failure. In this framework, many different approaches have been used to study the mortality of patients with COVID-19 treated with COVID-19 convalescent plasma, including randomized clinical trials (RCTs),10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 real-time pooling of individual patient data from RCTs,6,49 and meta-analyses.41,50, 51, 52, 53 The present work was performed to provide an updated, high-quality systematic review and meta-analysis on the use of COVID-19 convalescent plasma.
This systematic review and meta-analysis aimed to evaluate the association of COVID-19 convalescent plasma with mortality among hospitalized patients with COVID-19 by pooling data from RCTs and matched cohort studies. Moreover, prespecified analyses aimed to determine if the potential association between COVID-19 convalescent plasma and mortality benefit differs across patient subgroups on the basis of anti-SARS-CoV-2 antibody levels within COVID-19 convalescent plasma and the timing of the convalescent plasma transfusion in relation to the disease course.54 By integrating information from many studies and lines of evidence, we hope that this work provides new insights, clarifies ambiguous areas, and removes biases from the scientific corpus.55
Methods
This systematic review and meta-analysis followed the recommendations in the Cochrane Handbook for Systematic Review of Interventions56 and reported findings according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guidelines. The study protocol has been registered in the International Prospective Register of Systematic Reviews (CRD42022316321); all changes to the protocol are reported in the Methods section. In accordance with the Code of Federal Regulations, 45 CFR 46.102, this study was exempt from obtaining institutional review board approval from Mayo Clinic and the requirement to obtain informed patient consent because it is a secondary use of publicly available data sets.
Eligibility Criteria
Eligible patients were hospitalized with COVID-19. The intervention investigated was transfusion with COVID-19 convalescent plasma of any dosage. The control group was treated with standard of care according to local treatment guidelines, with or without a placebo. The primary outcome was all-cause mortality during hospitalization. Randomized clinical trials and matched cohort studies were eligible for all analyses. For subgroup analyses, where the focus is on differences among patients treated with COVID-19 convalescent plasma, case series were also eligible.
Information Sources
On October 26, 2022, PubMed, MEDLINE, Google Scholar, and medRiv were searched for eligible studies published beginning with January 1, 2020—approximating the origins of the COVID-19 pandemic. Keywords used in the search included ([convalescent plasma] or [convalescent serum]) COVID-19 (and medical subject headings).
Selection and Data Collection Processes
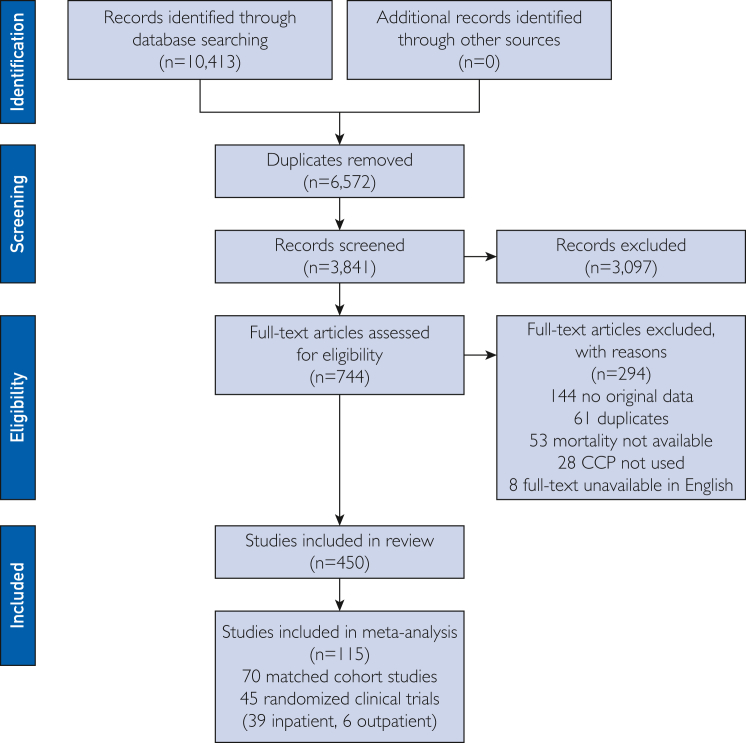
Both the selection process and data collection process were performed in duplicate and independently by 2 reviewers from a cohort of 5 potential reviewers (J.W.S., E.K.G., M.E.M., S.A.K., and O.H.M.), and all data were independently verified by review from a third reviewer. Disagreements were discussed until consensus. Data abstraction was performed using a standardized data abstraction form. Abstracted data included patient demographic characteristics (sample size, age, sex, and need for mechanical ventilation at the time of COVID-19 convalescent plasma transfusion) and COVID-19 convalescent plasma transfusion characteristics (volume transfused, antibody level, and time to transfusion in relation to disease course) as available. Data were abstracted corresponding to the latest available follow-up time for mortality. Further information on the selection process is presented in Figure 1.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Study Risk of Bias Assessment
A risk of bias assessment was conducted using the Cochrane risk of bias 2.0 tool for RCTs57,58 and the Newcastle-Ottawa Scale for matched cohort studies. Two reviewers from a cohort of 3 potential reviewers (J.W.S., E.K.G., and S.A.K.) applied the risk of bias assessment independently and it was verified by a third reviewer. Discrepancies were discussed until a consensus was reached.
Statistical Analyses
For the primary, dichotomous outcome of mortality, we performed a meta-analysis using a random effects model. We extracted raw data on mortality events and the number of patients in each group. For each study, we compared the observed number of deaths among patients transfused with COVID-19 convalescent plasma with the expected number if all patients were at equal risk using standard formulas for 2×2 contingency tables. Trial results were combined and weighted using an inverse-variance model. Analyses were done using Comprehensive Meta-analysis software (CMA 2.0, Biostat). Results are reported with 95% CIs, statistical significance was set at α=.05, and all tests were 2-tailed.
Primary meta-analyses were performed separately for RCTs and matched cohort studies. We performed prespecified subgroup analyses, including a subgroup analysis on the basis of the timing of convalescent plasma transfusion (early vs late treatment) in relation to the COVID-19 disease course and a subgroup analysis on the basis of antibody concentration in the transfused COVID-19 convalescent plasma (high vs low antibody levels).
In addition, an exploratory meta-analysis was performed on hospitalization rates among outpatients with recent SARS-CoV-2 exposure or infection.
Results
Study Selection and Characteristics
The process of study selection is represented in the Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram (Figure 1). Thirty-nine RCTs10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 enrolling 21,529 participants and 70 matched cohort studies2,59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127 enrolling 50,160 participants were included in the primary analyses. Although controlled studies were the focus of this systematic review and meta-analysis, secondary analyses on COVID-19 convalescent plasma antibody levels and timing of COVID-19 convalescent plasma transfusion encompass findings from case series, as delineated below.
Risk Assessment
The results of the risk of bias assessment for RCTs and matched cohort studies are presented in Supplemental Tables 2 and 3, respectively, available online at http://www.mcpiqojournal.org. Because our analyses primarily included controlled trials and focused on a discrete, dichotomous outcome that is unlikely to be influenced by the implicit biases of research personnel (all-cause mortality), many studies were determined to have low risk of bias. Matched cohort studies were associated with higher risk of bias because of the open-label trial design.
Association Between Convalescent Plasma Transfusion and Mortality in Hospitalized Patients with COVID-19
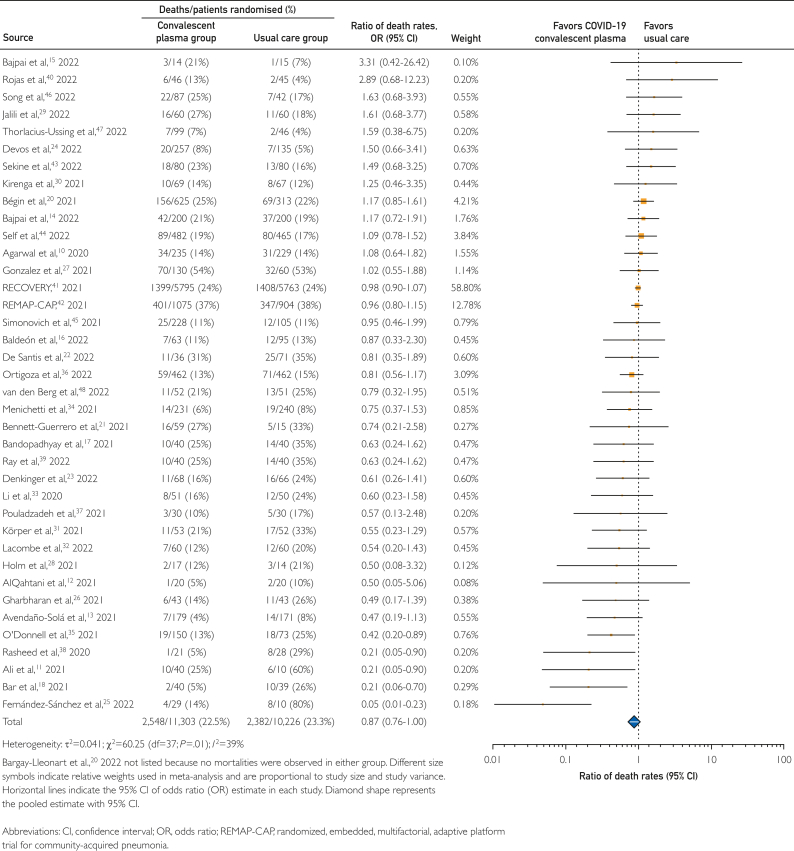
Key findings of the primary meta-analysis of 39 RCTs including 11,303 patients treated with COVID-19 convalescent plasma and 10,226 patients treated with usual care (controls), are displayed in Figure 2. Treatment with COVID-19 convalescent plasma was associated with a 13% reduced risk in mortality rates compared with usual care, with a pooled risk ratio estimate of 0.87 (95% CI, 0.76-1.00).
Figure 2.
Forest plot of mortality among randomized clinical trials.
The clinical benefit associated with COVID-19 convalescent plasma observed in 39 RCTs was also supported by a meta-analysis of 70 matched cohort studies, which included 14,541 patients treated with COVID-19 convalescent plasma and 35,619 patients treated with usual care (controls). In this meta-analysis of matched cohort studies, treatment with COVID-19 convalescent plasma was associated with a 23% reduced risk in mortality rates compared with usual care, with a pooled risk ratio estimate of 0.76 (95% CI, 0.66-0.88). A forest plot associated with these findings is displayed in Supplemental Figure 1, available online at http://www.mcpiqojournal.org. Although there was heterogeneity between individual studies, there was a high level of concordance between these 2 separate meta-analyses.
Exploratory Analyses of Outpatient COVID-19 Convalescent Plasma
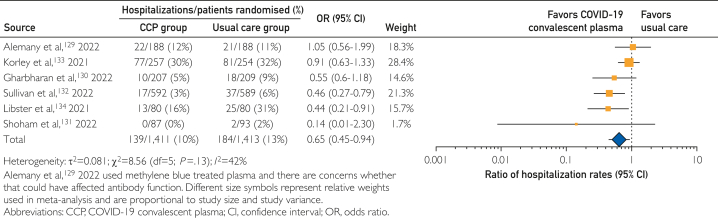
Six RCTs128, 129, 130, 131, 132, 133 enrolling 2824 participants were included in an exploratory analysis of hospitalization or mortality rates among outpatients with recent SARS-CoV-2 exposure or infection. In this meta-analysis, treatment with COVID-19 convalescent plasma among outpatients was associated with a 35% decrease in hospitalization rate, with a pooled risk ratio estimate of 0.65 (95% CI, 0.45-0.94). Results of this exploratory meta-analysis are shown in the forest plot in Figure 3. However, there was no apparent mortality benefit associated with convalescent plasma among outpatients, with a pooled risk ratio estimate of 0.60 (95% CI, 0.16-2.28; Supplemental Figure 2, available online at http://www.mcpiqojournal.org.)
Figure 3.
Forest plot of hospitalization among outpatients with recent SARS-CoV-2 exposure or infection in randomized clinical trials.
Subgroup Analyses
COVID-19 Convalescent Plasma Antibody Levels
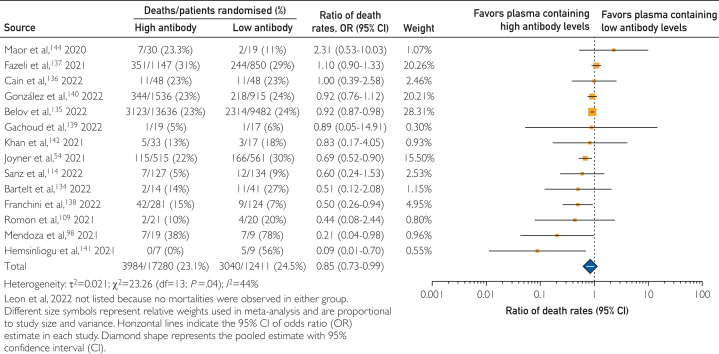
Among patients treated with COVID-19 convalescent plasma, receipt of convalescent plasma with higher levels of antibodies has been suggested to be associated with reduced mortality.20,54 Thus, heterogeneity of antibody levels in the COVID-19 convalescent plasma used to treat patients may affect the mortality rates reported. Hence, we examined within-study mortality rates among patients treated with convalescent plasma containing high or low antibody levels. Because several assays were authorized for use in the manufacture of high antibody-titer convalescent plasma and the cutpoints used to qualify high antibody-titer convalescent plasma have changed over time, we used study-defined cutpoints to define high and low antibody levels. Information on assay systems and cutpoints used to delineate high and low antibody levels are provided in Supplemental Table 4, available online at http://www.mcpiqojournal.org.
In this framework, 3 matched cohort studies98,109,114 enrolling 330 participants and 12 case series54,134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144 enrolling 29,361 participants were included in this subgroup analysis. In this subgroup meta-analysis, treatment with COVID-19 convalescent plasma containing high antibody levels was associated with a 15% decrease in mortality rates compared with convalescent plasma containing low antibody levels, with a pooled risk ratio estimate of 0.85 (95% CI, 0.73-0.99). The results of this subgroup meta-analysis are shown in the forest plot in Figure 4.
Figure 4.
Forest plot of mortality among studies investigating COVID-19 convalescent plasma containing high compared with low antibody levels.
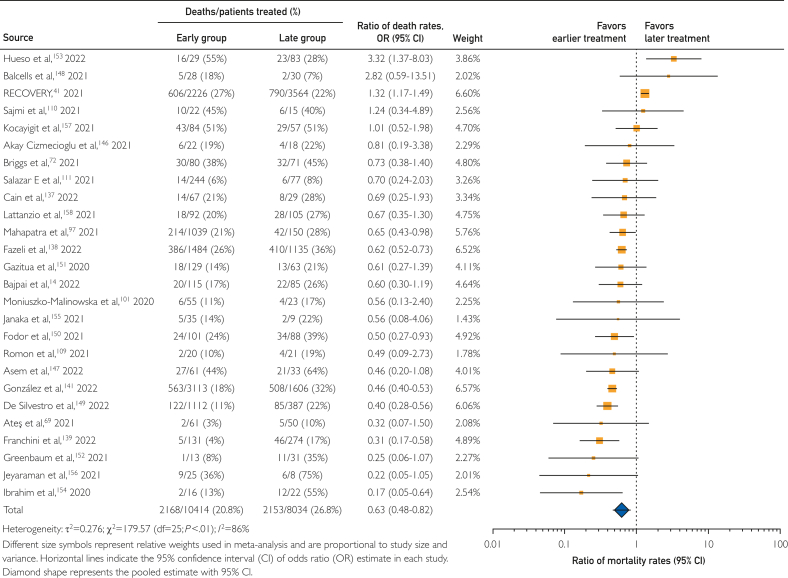
Timing of COVID-19 convalescent plasma transfusion
Among hospitalized patients transfused with COVID-19 convalescent plasma, transfusion earlier in the COVID-19 disease course has been suggested to be associated with reduced mortality,52,54,72 thus, heterogeneity of the time between COVID-19 diagnosis and convalescent plasma transfusion may affect mortality rates. Because there was no standard to define ‘early treatment’ and individual studies used diverse criteria to define ‘early treatment’, we used study-defined cutpoints to delineate early and late treatment with COVID-19 convalescent plasma. Information on cutpoints used to define earlier and later treatment with COVID-19 convalescent plasma are provided in Supplemental Table 5, available online at http://www.mcpiqojournal.org.
In this context, we examined within-study mortality rates among patients treated with convalescent plasma earlier compared with those treated later in the COVID-19 disease course. This subgroup analysis examined 26 studies, including 2 RCTs14,41 enrolling 5990 participants, 7 matched cohort studies69,72,97,101,109, 110, 111 enrolling 1928 participants, and 17 case series136, 137, 138,140,145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157 enrolling 10,530 participants. In this subgroup meta-analysis, treatment with convalescent plasma earlier in the COVID-19 disease course was associated with a 37% decrease in mortality rates compared with treatment later in the disease course, with a pooled risk ratio estimate of 0.63 (95% CI, 0.48-0.82). The results of this subgroup meta-analysis are shown in the forest plot in Figure 5.
Figure 5.
Forest plot of mortality among studies investigating earlier compared with later transfusion of COVID-19 convalescent plasma.
Case Series and Reports
In the United States and many other countries, the regulatory framework during the COVID-19 pandemic enabled broad access to COVID-19 convalescent plasma.158 In this context, a large number of single-arm studies evaluated the risk of death from COVID-19 after transfusion with convalescent plasma, including over 300 case series or case reports. The case series and case reports treated 135,949 participants with COVID-19 convalescent plasma, and mortality was observed in 33,771 participants (∼25% mortality rate). Study level data are provided in Supplementary Table 6, and references associated with these case series and case reports are provided in the supplementary references.
Discussion
This systematic review and meta-analysis of 39 RCTs and 70 matched cohort studies of the use of COVID-19 convalescent plasma for hospitalized patients with COVID-19 provides new insights into the therapeutic use of convalescent plasma and removes biases from the scientific and clinical literature. This meta-analysis found that convalescent plasma was associated with a clinically meaningful mortality benefit among hospitalized patients with COVID-19. However, given the large diversity of the included trials and the heterogeneity of findings, the results must be analyzed and interpreted critically.
Overall, COVID-19 convalescent plasma transfusion was associated with a 13% decrease in mortality rates compared with the (usual care) control group. Subgroup analyses revealed that patients treated with high-titer plasma and patients treated earlier in the course of the disease benefited more from COVID-19 convalescent plasma transfusion than patients treated with lower-titer plasma or patients treated later in the course of the disease. Exploratory analyses also found that transfusion of COVID-19 convalescent plasma among outpatients was associated with a 35% decrease in hospitalization rates compared with the control group—a finding that is consistent with pooled individual patient data.159 Our finding that convalescent plasma transfusion reduced mortality is consistent with the epidemiologic data showing an inverse correlation between plasma use and COVID-19 death in the United States.8
Although data were directionally consistent when pooling RCTs and matched cohort studies, findings of individual trials were heterogeneous. This heterogeneity between trials is likely associated with several factors. First, clinical heterogeneity may be on the basis of patient characteristics at the time of COVID-19 convalescent plasma therapy. It is important to consider COVID-19 disease severity, which is directly related to higher odds of death. Among studies in which the patients had more severe disease, mortality was higher, and there was often no clinical benefit associated with COVID-19 convalescent plasma transfusion.160 This meta-analysis found that treatment with COVID-19 convalescent plasma earlier in the course of the disease was associated with a 37% decrease in mortality rates. Thus, treatment later in the course of the disease may not confer a mortality benefit unless the patients are immunocompromised.161
Second, clinical heterogeneity may result from differences in the COVID-19 convalescent plasma intervention itself, particularly the antibody content and geographic provenance of plasma supplies.162 Several assays are authorized for use in the manufacture of high antibody-titer convalescent plasma, and there are established, assay-specific cutpoints used to define high antibody-titer convalescent plasma. However, there is discordance between assays, and the cutpoints to define high antibody-titer convalescent plasma are not directly comparable across assays.163 In addition, the cutpoints used to qualify high antibody-titer convalescent plasma have changed over time, and generally, the required antibody content has increased. In this context, the discordance between assays and changing clinical guidelines may have contributed to the heterogeneity of individual trial findings. This meta-analysis found that treatment with high-titer convalescent plasma was associated with a 15% decrease in mortality rates, and treatment with low-titer convalescent plasma may not confer a clinically meaningful mortality benefit.
Critical reporting and sufficient analyses are crucial when it comes to investigating heterogeneity of meta-analyses in systematic reviews. Failure to fully reflect heterogeneity of results may lead to misinterpretations, incorrect assumptions, and incorrect and potentially harmful clinical recommendations. In this framework, this meta-analysis may provide new insights into the therapeutic use of convalescent plasma. Although this meta-analysis revealed that convalescent plasma was associated with a clinically meaningful overall mortality benefit among hospitalized patients with COVID-19, convalescent plasma is not a panacea because it is a nonstandardized therapy that requires early use for efficacy. COVID-19 convalescent plasma may be unlikely to confer a mortality benefit among patients treated with low antibody-titer convalescent plasma later in the COVID-19 disease course. The finding that COVID-19 convalescent plasma was associated with reduced mortality is consistent with the historical experience of the 1918 pandemic, where convalescent serum therapy was associated with reduced mortality.164
Limitations
This systematic review and meta-analysis has several limitations. First, the empirical and clinical studies were associated with biological diversity and heterogeneous comparator groups. Key factors were continuously evolving during the pandemic, including the pathogen of interest (SARS-CoV-2), contemporary treatment strategies for the disease of interest (COVID-19), and antibody content of the treatment of interest (COVID-19 convalescent plasma). In this context, we believe the high level of concordance among study outcomes despite the biological heterogeneity between studies offers compelling evidence for the therapeutic value of convalescent plasma among COVID-19 patients overall. Second, we did not have access to patient-level data for the studies included in this article. Thus, our subgroup analyses that separated patients by a single baseline characteristic were simplistic.165 Lack of patient-level data does not allow analyses using more complex statistical models that incorporate multiple characteristics,166 and previous studies have pooled patient-level data from clinical trials of COVID-19 convalescent plasma.6,49 Third, we limited our focus to a single outcome. Finally, we note that the preponderance of data in this analysis came from studies in the first years of the pandemic involving unvaccinated populations that were immunologically naïve to SARS-CoV-2, and today most individuals in the countries contributing the most studies are vaccinated and/or have previous experience with COVID-19, making the populations then and now immunologically different.
Conclusion
This systematic review and meta-analysis found that convalescent plasma was associated with a 13% decrease in mortality rates in hospitalized patients with COVID-19 compared with a control cohort. Subgroup analyses revealed that patients treated with high-titer plasma and patients treated earlier in the course of the disease benefited more from COVID-19 convalescent plasma transfusions. Thus, reasonable concerns about the use of low antibody-titer convalescent plasma later in the course of the disease remain. These findings can offer experts a new starting point in forming their judgment of the therapeutic effectiveness of COVID-19 convalescent plasma and may help transform subjective and nebulous insider views into a more transparent and reliable knowledge base. Finally, our findings support the deployment of convalescent plasma in future epidemic emergencies until better therapies are available, with the caveat that convalescent plasma should be administered early in disease using units with the highest antibody content available.
Potential Competing Interests
Drs Senefeld, Carter, Joyner, Fairweather, Bruno, and Wright reported being investigators in the US Expanded Access Program of COVID-19 convalescent plasma. Drs Paneth, Casadevall, and Joyner reported serving as leadership for the COVID-19 Convalescent Plasma Project outside the submitted work.
Acknowledgment
The authors requested to waive the reference limit for inclusion of all references included and data analyzed for this systematic review and meta-analysis. Dr Senefeld and Ms Gorman contributed equally as first authors to the work of the study and manuscript. Drs Casadevall and Joyner contributed equally as senior authors to the work of the study and manuscript.
Footnotes
Grant Support: This work was supported by the United Health Group, David and Lucile Packard Foundation, Schwab Charitable Fund (Eric E. Schmidt, Wendy Schmidt donors), National Basketball Association, and Mayo Clinic.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye M., Fu D., Ren Y., et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pommeret F., Colomba J., Bigenwald C., et al. Bamlanivimab + etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies. Ann Oncol. 2021;32(11):1445–1447. doi: 10.1016/j.annonc.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jary A., Marot S., Faycal A., et al. Spike gene evolution and immune escape mutations in patients with mild or moderate forms of COVID-19 and treated with monoclonal antibodies therapies. Viruses. 2022;14(2) doi: 10.3390/v14020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troxel A.B., Petkova E., Goldfeld K., et al. Association of convalescent plasma treatment with clinical status in patients hospitalized with COVID-19: a meta-analysis. JAMA Netw Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.47331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senefeld J.W., Franchini M., Mengoli C., et al. COVID-19 convalescent plasma for the treatment of immunocompromised patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1) doi: 10.1001/jamanetworkopen.2022.50647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall A., Dragotakes Q., Johnson P.W., et al. Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality. eLife. 2021:10:e69866. doi: 10.7554/eLife.69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piechotta V., Iannizzi C., Chai K.L., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A., Mukherjee A., Kumar G., et al. Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali S., Uddin S.M., Shalim E., et al. Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: a phase I/II randomized control trial. EClinicalmedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AlQahtani M., Abdulrahman A., Almadani A., et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep. 2021;11(1):9927. doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avendaño-Solá C., Ramos-Martínez A., Muñez-Rubio E., et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021;(20):131. doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajpai M., Maheshwari A., Dogra V., et al. Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial) BMJ, (Open) 2022;12(4) doi: 10.1136/bmjopen-2021-055189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajpai M., Maheshwari A., Kumar S., et al. Comparison of safety and efficacy of convalescent plasma with fresh frozen plasma in severe Covid-19 patients. An Acad Bras Cienc. 2022;94(4) doi: 10.1590/0001-3765202220210202. [DOI] [PubMed] [Google Scholar]
- 16.Baldeón M.E., Maldonado A., Ochoa-Andrade M., et al. Effect of convalescent plasma as complementary treatment in patients with moderate COVID-19 infection. Transfus Med. 2022;32(2):153–161. doi: 10.1111/tme.12851. [DOI] [PubMed] [Google Scholar]
- 17.Bandopadhyay P., D’Rozario R., Lahiri A., et al. Nature and dimensions of systemic hyperinflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis. 2021;224(4):565–574. doi: 10.1093/infdis/jiab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar K.J., Shaw P.A., Choi G.H., et al. A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J Clin Invest. 2021;131(24) doi: 10.1172/JCI155114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bargay-Lleonart J., Sarubbo F., Arrizabalaga M., et al. Reinforcement of the standard therapy with two infusions of convalescent plasma for patients with COVID-19: a randomized clinical trial. J Clin Med. 2022;11(11) doi: 10.3390/jcm11113039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bégin P., Callum J., Jamula E., et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27(11):2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett-Guerrero E., Romeiser J.L., Talbot L.R., et al. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind randomized trial. Crit Care Med. 2021;49(7):1015–1025. doi: 10.1097/CCM.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Santis G.C., Oliveira L.C., Garibaldi P.M.M., et al. High-dose convalescent plasma for treatment of severe COVID-19. Emerg Infect Dis. 2022;28(3):548–555. doi: 10.3201/eid2803.212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denkinger C.M., Janssen M., Schäkel U., et al. Anti-SARS-CoV-2 antibody containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19 via increased neutralizing antibody activity – a randomized clinical trial. Preprint. Posted online October 13, 2022. medRxiv. 2022 doi: 10.1101/2022.10.10.22280850. 2022.2010.2010.22280850. [DOI] [Google Scholar]
- 24.Devos T., Van Thillo Q., Compernolle V., et al. Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma. Eur Respir J. 2022;59(2) doi: 10.1183/13993003.01724-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Sánchez V, Ventura-Enríquez Y, Cabello-Gutiérrez C, et al. Convalescent plasma to treat Covid-19: a randomized double blind 2 centers trial. Preprint. Posted online April 4, 2022. Research square. https://doi.org/10.21203/rs.3.rs-1277990/v1
- 26.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12(1):3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez J.L.B., González Gámez M., Mendoza Enciso E.A., et al. Efficacy and safety of convalescent plasma and intravenous immunoglobulin in critically ill COVID-19 patients. a controlled clinical trial. Preprint. Posted online March 31, 2021. medRxiv. 2021 doi: 10.1101/2021.03.28.21254507. 2021.2003.2028.21254507. [DOI] [Google Scholar]
- 28.Holm K., Lundgren M.N., Kjeldsen-Kragh J., et al. Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden. BMC Res Notes. 2021;14(1):440. doi: 10.1186/s13104-021-05847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jalili E., Khazaei S., Mohammadi A., et al. Effect of convalescent plasma therapy on clinical improvement of COVID-19 patients: a randomized clinical trial. Tanaffos. 2022;21(1):24–30. [PMC free article] [PubMed] [Google Scholar]
- 30.Kirenga B., Byakika-Kibwika P., Muttamba W., et al. Efficacy of convalescent plasma for treatment of COVID-19 in Uganda. BMJ Open Respir Res. 2021;8(1) doi: 10.1136/bmjresp-2021-001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Körper S., Weiss M., Zickler D., et al. Results of the capsid randomized trial for high-dose convalescent plasma in patients with severe COVID-19. J Clin Invest. 2021;(20):131. doi: 10.1172/JCI152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacombe K., Hueso T., Porcher R., et al. Efficacy and safety of convalescent plasma to treat hospitalised COVID-19 patients with or without underlying immunodeficiency. Preprint. Posted online August 10, 2022. medRxiv. 2022 doi: 10.1101/2022.08.09.22278329. [DOI] [Google Scholar]
- 33.Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menichetti F., Popoli P., Puopolo M., et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell M.R., Grinsztejn B., Cummings M.J., et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest. 2021;131(13) doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortigoza M.B., Yoon H., Goldfeld K.S., et al. Efficacy and safety of COVID-19 convalescent plasma in hospitalized patients: a randomized clinical trial. JAMA Intern Med. 2022;182(2):115–126. doi: 10.1001/jamainternmed.2021.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pouladzadeh M., Safdarian M., Eshghi P., et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Intern Emerg Med. 2021;16(8):2181–2191. doi: 10.1007/s11739-021-02734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasheed A.M., Fatak D.F., Hashim H.A., et al. The therapeutic potential of convalescent plasma therapy on treating critically ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28(3):357–366. [PubMed] [Google Scholar]
- 39.Ray Y., Paul S.R., Bandopadhyay P., et al. A phase 2 single center open label randomised control trial for convalescent plasma therapy in patients with severe COVID-19. Nat Commun. 2022;13(1):383. doi: 10.1038/s41467-022-28064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas M., Rodríguez Y., Hernández J.C., et al. Safety and efficacy of convalescent plasma for severe COVID-19: a randomized, single blinded, parallel, controlled clinical study. BMC Infect Dis. 2022;22(1):575. doi: 10.1186/s12879-022-07560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Writing Committee for the REMAP-CAP Investigators. Estcourt L.J., Turgeon A.F., et al. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2021;326(17):1690–1702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekine L., Arns B., Fabro B.R., et al. Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial. Eur Respir J. 2022;59(2) doi: 10.1183/13993003.01471-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Self W.H., Wheeler A.P., Stewart T.G., et al. Neutralizing COVID-19 convalescent plasma in adults hospitalized with COVID-19: a blinded, randomized, placebo-controlled trial. Chest. 2022;162(5):982–994. doi: 10.1016/j.chest.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song A.T.W., Rocha V., Mendrone-Júnior A., et al. Treatment of severe COVID-19 patients with either low- or high-volume of convalescent plasma versus standard of care: a multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI) Lancet Reg Health Am. 2022;10 doi: 10.1016/j.lana.2022.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorlacius-Ussing L., Brooks P.T., Nielsen H., et al. A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-19629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Berg K., Glatt T.N., Vermeulen M., et al. Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial) Sci Rep. 2022;12(1):2552. doi: 10.1038/s41598-022-06221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park H., Tarpey T., Liu M., et al. Development and validation of a treatment benefit index to identify hospitalized patients with COVID-19 who may benefit from convalescent plasma. JAMA Netw Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.47375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Axfors C., Janiaud P., Schmitt A.M., et al. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis. 2021;21(1):1170. doi: 10.1186/s12879-021-06829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siemieniuk R.A., Bartoszko J.J., Díaz Martinez J.P., et al. Antibody and cellular therapies for treatment of Covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klassen S.A., Senefeld J.W., Johnson P.W., et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc. 2021;96(5):1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Millat-Martinez P., Gharbharan A., Alemany A., et al. Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients. Nat Commun. 2022;13(1):2583. doi: 10.1038/s41467-022-29911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joyner M.J., Carter R.E., Senefeld J.W., et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384(11):1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ioannidis J.P.A. Systematic reviews for basic scientists: a different beast. Physiol Rev. 2023;103(1):1–5. doi: 10.1152/physrev.00028.2022. [DOI] [PubMed] [Google Scholar]
- 56.Higgins J.P., Thomas J., Chandler J., et al. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. version 6.2. [Google Scholar]
- 57.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 58.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abolghasemi H., Eshghi P., Cheraghali A.M., et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;59(5) doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abuzakouk M., Saleh K., Algora M., et al. Convalescent plasma efficacy in life-threatening COVID-19 patients admitted to the ICU: a retrospective cohort study. J Clin Med. 2021;10(10) doi: 10.3390/jcm10102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acosta-Ampudia Y., Monsalve D.M., Rojas M., et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J Autoimmun. 2021;118 doi: 10.1016/j.jaut.2021.102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alamgir J., Abid M.R., Garibaldi B., et al. Lack of association between convalescent plasma administration and length of hospital stay: a hospital-day stratified multi-center retrospective cohort study. Preprint. Posted online May 8, 2021. medRxiv. 2021 doi: 10.1101/2021.05.04.21256627. 2021.2005.2004.21256627. [DOI] [Google Scholar]
- 63.Al Harthi S., Osali M.A., Kindi N.A., et al. Characteristics of the first 102 severe COVID-19 cases treated with convalescent plasma or tocilizumab or both in Al Nahdha Hospital, Oman. Health Serv Res Manag Epidemiol. 2021;8 doi: 10.1177/2333392820986639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allahyari A., Seddigh-Shamsi M., Mahmoudi M., et al. Efficacy and safety of convalescent plasma therapy in severe COVID-19 patients with acute respiratory distress syndrome. Int Immunopharmacol. 2021;93 doi: 10.1016/j.intimp.2020.107239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsharidah S., Ayed M., Ameen R.M., et al. COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: a multicenter interventional study. Int J Infect Dis. 2021;103:439–446. doi: 10.1016/j.ijid.2020.11.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.AlShehry N., Zaidi S.Z.A., AlAskar A., et al. Safety and efficacy of convalescent plasma for severe COVID-19: interim report of a multicenter Phase II study from Saudi Arabia. Saudi J Med Med Sci. 2021;9(1):16–23. doi: 10.4103/sjmms.sjmms_731_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altuntas F., Ata N., Yigenoglu T.N., et al. Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci. 2021;60(1) doi: 10.1016/j.transci.2020.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnold Egloff S.A., Junglen A., Restivo J.S., et al. Convalescent plasma associates with reduced mortality and improved clinical trajectory in patients hospitalized with COVID-19. J Clin Invest. 2021;131(20) doi: 10.1172/JCI151788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ateş İ., Erden A., Güven S.C., et al. Should timing be considered before abandoning convalescent plasma in Covid-19? Results from the Turkish experience. Transfus Apher Sci. 2021;60(6) doi: 10.1016/j.transci.2021.103238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biernat M.M., Kolasińska A., Kwiatkowski J., et al. Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID-19. Viruses. 2021;13(3) doi: 10.3390/v13030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bihariesingh R., Bansie R., Froberg J., et al. Mortality reduction in ICU-admitted COVID-19 patients in Suriname after treatment with convalescent plasma acquired via gravity filtration. J Anesth Clin Res. 2021;2(2):2–12. [Google Scholar]
- 72.Briggs N., Gormally M.V., Li F., et al. Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. PLoS ONE. 2021;16(7) doi: 10.1371/journal.pone.0254453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Budhiraja S., Dewan A., Aggarwal R., et al. Effectiveness of convalescent plasma in Indian patients with COVID-19. Blood Cells Mol Dis. 2021;88 doi: 10.1016/j.bcmd.2021.102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cacilhas P., Caberlon E., Angoleri L., Fassina K., Ribeiro R.N., Pinto L.C. Convalescent plasma therapy in COVID-19 patients: a non-randomized case-control study with concurrent control. Braz J Med Biol Res. 2022;55 doi: 10.1590/1414-431X2022e12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chauhan L., Pattee J., Ford J., et al. A multicenter, prospective, observational, cohort-controlled study of clinical outcomes following coronavirus disease 2019 (COVID-19) convalescent plasma therapy in hospitalized patients with COVID-19. Clin Infect Dis. 2022;75(1):e466–e472. doi: 10.1093/cid/ciab834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho K., Keithly S.C., Kurgansky K.E., et al. Early convalescent plasma therapy and mortality among US veterans hospitalized with nonsevere COVID-19: an observational analysis emulating a target trial. J Infect Dis. 2021;224(6):967–975. doi: 10.1093/infdis/jiab330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cristelli M.P., Langhi Junior D.M., Viana L.A., et al. Efficacy of convalescent plasma to treat mild to moderate COVID-19 in kidney transplant patients: a propensity score matching analysis. Transplantation. 2022;106(1):e92–e94. doi: 10.1097/TP.0000000000003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai W., Wu J., Li T., et al. Clinical outcomes for COVID-19 patients with diabetes mellitus treated with convalescent plasma transfusion in Wuhan, China. J Med Virol. 2021;93(4):2321–2331. doi: 10.1002/jmv.26712. [DOI] [PubMed] [Google Scholar]
- 79.Donato M.L., Park S., Baker M., et al. Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma. JCI Insight. 2021;6(6) doi: 10.1172/jci.insight.143196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eren E., Ulu-Kılıç A., Korkmaz S., et al. Retrospective analysis on efficacy of convalescent plasma in acute respiratory distress syndrome due to COVID-19. Sao Paulo Med J. 2022;140(1):12–16. doi: 10.1590/1516-3180.2021.0200.R1.03052021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia-Muñoz R., Farfán-Quiroga G., Ruiz-de-Lobera N., et al. Serology-based therapeutic strategy in SARS-CoV-2-infected patients. Int Immunopharmacol. 2021;101(B) doi: 10.1016/j.intimp.2021.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hatzl S., Posch F., Sareban N., et al. Convalescent plasma therapy and mortality in COVID-19 patients admitted to the ICU: a prospective observational study. Ann Intensive Care. 2021;11(1):73. doi: 10.1186/s13613-021-00867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hegerova L., Gooley T.A., Sweerus K.A., et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136(6):759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoepler W.P., Weidner L., Traugott M.T., et al. Adjunctive treatment with high-titre convalescent plasma in severely and critically ill COVID-19 patients – a safe but futile intervention. A comparative cohort study. Infect Dis (Lond) 2021;53(11):820–829. doi: 10.1080/23744235.2021.1940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang L., Zhang C., Zhou X., et al. Convalescent plasma is of limited clinical benefit in critically ill patients with coronavirus disease-2019: a cohort study. J Transl Med. 2021;19(1):365. doi: 10.1186/s12967-021-03028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang W., Li W., Xiong L., et al. Clinical efficacy of convalescent plasma therapy on treating COVID-19 patients: evidence from matched study and a meta-analysis. Clin Transl Med. 2020;10(8):e259. doi: 10.1002/ctm2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khamis F., Al Arimi Z., Al Naamani H., et al. Convalescent plasma therapy in critically ill COVID-19 patients: an open label trial. Oman Med J. 2021;36(5):e296. doi: 10.5001/omj.2021.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klapholz M., Pentakota S.R., Zertuche J.P., et al. Matched cohort study of convalescent COVID-19 plasma treatment in severely or life threateningly ill COVID-19 patients. Open Forum Infect Dis. 2021;8(2) doi: 10.1093/ofid/ofab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klein M.N., Wang E.W., Zimand P., et al. Kinetics of SARS-CoV-2 antibody responses pre-COVID-19 and post-COVID-19 convalescent plasma transfusion in patients with severe respiratory failure: an observational case-control study. J Clin Pathol. 2022;75(8):564–571. doi: 10.1136/jclinpath-2020-207356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koirala J., Gyanwali P., Gerzoff R.B., et al. Experience of treating COVID-19 with remdesivir and convalescent plasma in a resource-limited setting: a prospective, observational study. Open Forum Infect Dis. 2021;8(8):ofab391. doi: 10.1093/ofid/ofab391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuno T., Takahashi M., Egorova N.N. The association between convalescent plasma treatment and survival of patients with COVID-19. J Gen Intern Med. 2021;36(8):2528–2531. doi: 10.1007/s11606-021-06894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurnianda J., Hardianti M.S., Triyono T., et al. Efficacy and safety of convalescent plasma therapy in patients with moderate-to-severe COVID-19: a non-randomized comparative study with historical control in a referral hospital in Indonesia. J Infect Public Health. 2022;15(1):100–108. doi: 10.1016/j.jiph.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurtz P., Righy C., Gadelha M., et al. Effect of convalescent plasma in critically ill patients with COVID-19: an observational study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.630982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lanza F., Monaco F., Ciceri F., et al. Lack of efficacy of convalescent plasma in COVID-19 patients with concomitant hematological malignancies: an Italian retrospective study. Hematol Oncol. 2022;40(5):857–863. doi: 10.1002/hon.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liao M., Liao X., Yuan J., et al. The concentrated antibody from convalescent plasma balanced the dysfunctional immune responses in patients with critical COVID-19. Clin Transl Med. 2021;11(11) doi: 10.1002/ctm2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu S.T.H., Lin H.M., Baine I., et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 97.Mahapatra S., Rattan R., Mohanty C.B.K. Convalescent plasma Therapy in the management of COVID-19 patients-The newer dimensions. Transfus Clin Biol. 2021;28(3):246–253. doi: 10.1016/j.tracli.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mendoza R.P., Fyke W., Daniel D., et al. Administration of high titer convalescent anti-SARS-CoV-2 plasma: from donor selection to monitoring recipient outcomes. Hum Immunol. 2021;82(4):255–263. doi: 10.1016/j.humimm.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mesina F.Z., Mangahas C.G., Gatchalian E.M., Ariola-Ramos M.S., Torres R.P. Use of convalescent plasma therapy among hospitalized coronavirus disease 2019 (COVID-19) patients: a single-center experience. Preprint. Posted online February 17, 2021. medRxiv. 2021 doi: 10.1101/2021.02.16.21251824. 2021.2002.2016.21251824. [DOI] [Google Scholar]
- 100.Mesina F., Julian J., Relos J., et al. Use of convalescent plasma therapy with best available treatment (BAT) among hospitalized COVID-19 patients: a multi-center study. Preprint. Posted online March 1, 2022. medRxiv. 2022 doi: 10.1101/2022.02.23.22271424. 2022.2002.2023.22271424. [DOI] [Google Scholar]
- 101.Moniuszko-Malinowska A., Czupryna P., Zarębska-Michaluk D., et al. Convalescent plasma transfusion for the treatment of COVID-19-experience from Poland: a multicenter study. J Clin Med. 2020;10(1) doi: 10.3390/jcm10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Novacescu A.N., Duma G., Buzzi B., et al. Therapeutic plasma exchange followed by convalescent plasma transfusion in severe and critically ill COVID-19 patients: a single centre non-randomized controlled trial. Exp Ther Med. 2022;23(1):76. doi: 10.3892/etm.2021.10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Omrani A.S., Zaqout A., Baiou A., et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: a preliminary report. J Med Virol. 2021;93(3):1678–1686. doi: 10.1002/jmv.26537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan C., Chen H., Xie J., et al. The efficiency of convalescent plasma therapy in the management of critically ill patients infected with COVID-19: a matched cohort study. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.822821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pappa V., Bouchla A., Terpos E., et al. A Phase II study on the use of convalescent plasma for the treatment of severe COVID-19- a propensity score-matched control analysis. Microorganisms. 2021;9(4) doi: 10.3390/microorganisms9040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perotti C., Baldanti F., Bruno R., et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. a proof of concept single arm multicenter trial. Haematologica. 2020;105(12):2834–2840. doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rogers R., Shehadeh F., Mylona E.K., et al. Convalescent plasma for patients with severe coronavirus disease 2019 (COVID-19): a matched cohort study. Clin Infect Dis. 2021;73(1):e208–e214. doi: 10.1093/cid/ciaa1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rollas K., Emgin Ö., Çalışkan T., et al. Convalescent plasma for COVID-19 in the intensive care unit. Anaesthesiol Intensive Ther. 2021;53(5):398–402. doi: 10.5114/ait.2021.111551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romon I., Dominguez-Garcia J.J., Arroyo J.L., et al. Convalescent plasma treatment for patients of 80 years and older with COVID-19 pneumonia. BMC Geriatr. 2021;21(1):566. doi: 10.1186/s12877-021-02447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sajmi S., Goutham K., Arumugam V., et al. Efficacy and safety of convalescent plasma therapy in SARS-CoV2 patients on hemodialysis. Hemodial Int. 2021;25(4):515–522. doi: 10.1111/hdi.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salazar E., Christensen P.A., Graviss E.A., et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol. 2021;191(1):90–107. doi: 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salazar M.R., González S.E., Regairaz L., et al. Risk factors for COVID-19 mortality: the effect of convalescent plasma administration. PLoS ONE. 2021;16(4) doi: 10.1371/journal.pone.0250386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sammartino D., Jafri F., Cook B., et al. Predictors for inpatient mortality during the first wave of the SARS-CoV-2 pandemic: a retrospective analysis. PLoS ONE. 2021;16(5) doi: 10.1371/journal.pone.0251262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanz C., Nomdedeu M., Pereira A., et al. Efficacy of early transfusion of convalescent plasma with high-titer SARS-CoV-2 neutralizing antibodies in hospitalized patients with COVID-19. Transfusion. 2022;62(5):974–981. doi: 10.1111/trf.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Semedi B.P., Ramadhania N.N., Tambunan B.A., Bintoro S.U.Y., Soedarsono S., Prakoeswa C.R.S. Prolonged ICU stay in severe and critically ill COVID-19 patients who received convalescent plasma therapy. Crit Care Res Pract. 2022;2022 doi: 10.1155/2022/1594342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shenoy A.G., Hettinger A.Z., Fernandez S.J., Blumenthal J., Baez V. Early mortality benefit with COVID-19 convalescent plasma: a matched control study. Br J Haematol. 2021;192(4):706–713. doi: 10.1111/bjh.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sostin O.V., Rajapakse P., Cruser B., Wakefield D., Cruser D., Petrini J. A matched cohort study of convalescent plasma therapy for COVID-19. J Clin Apher. 2021;36(4):523–532. doi: 10.1002/jca.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sturek J.M., Thomas T.A., Gorham J.D., et al. Convalescent plasma for preventing critical illness in COVID-19: a Phase 2 trial and immune profile. Microbiol Spectr. 2022;10(1) doi: 10.1128/spectrum.02560-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang J., Grubbs G., Lee Y., Golding H., Khurana S. Impact of convalescent plasma therapy on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody profile in coronavirus disease 2019 (COVID-19) patients. Clin Infect Dis. 2022;74(2):327–334. doi: 10.1093/cid/ciab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thompson M.A., Henderson J.P., Shah P.K., et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. 2021;7(8):1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tworek A., Jaroń K., Uszyńska-Kałuża B., et al. Convalescent plasma treatment is associated with lower mortality and better outcomes in high-risk COVID-19 patients – propensity-score matched case-control study. Int J Infect Dis. 2021;105:209–215. doi: 10.1016/j.ijid.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weisser M., Khanna N., Hedstueck A., et al. Characterization of pathogen-inactivated COVID-19 convalescent plasma and responses in transfused patients. Transfusion. 2022;62(10):1997–2011. doi: 10.1111/trf.17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xia X., Li K., Wu L., et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136(6):755–759. doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiao K., Lin Y., Fan Z., et al. Effect of transfusion convalescent recovery plasma in patients with coronavirus disease 2019. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(5):565–570. doi: 10.11817/j.issn.1672-7347.2020.200318. [DOI] [PubMed] [Google Scholar]
- 125.Yoon H.A., Bartash R., Gendlina I., et al. Treatment of severe COVID-19 with convalescent plasma in Bronx, NYC. JCI Insight. 2021;6(4) doi: 10.1172/jci.insight.142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeng Q.L., Yu Z.J., Gou J.J., et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou C.K., Bennett M.M., Villa C.H., et al. Multi-center matched cohort study of convalescent plasma for hospitalized patients with COVID-19. PLoS ONE. 2022;17(8) doi: 10.1371/journal.pone.0273223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alemany A., Millat-Martinez P., Corbacho-Monné M., et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med. 2022;10(3):278–288. doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gharbharan A., Jordans C., Zwaginga L., et al. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial. Clin Microbiol Infect. 2023;29(2):208–214. doi: 10.1016/j.cmi.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shoham S., Bloch E.M., Casadevall A., et al. Transfusing convalescent plasma as post-exposure prophylaxis against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a double-blinded, phase 2 randomized, controlled trial. Clin Infect Dis. 2023;76(3):e477–e486. doi: 10.1093/cid/ciac372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sullivan D.J., Gebo K.A., Shoham S., et al. Early outpatient treatment for Covid-19 with convalescent plasma. N Engl J Med. 2022;386(18):1700–1711. doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Korley F.K., Durkalski-Mauldin V., Yeatts S.D., et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. 2021;385(21):1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Libster R., Pérez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bartelt L.A., Markmann A.J., Nelson B., et al. Outcomes of convalescent plasma with defined high versus lower neutralizing antibody titers against SARS-CoV-2 among hospitalized patients: CoronaVirus Inactivating Plasma (CoVIP) study. mBio. 2022;13(5) doi: 10.1128/mbio.01751-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Belov A., Huang Y., Villa C.H., et al. Early administration of COVID-19 convalescent plasma with high titer antibody content by live viral neutralization assay is associated with modest clinical efficacy. Am J Hematol. 2022;97(6):770–779. doi: 10.1002/ajh.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cain W.V., Sill A.M., Solipuram V., Weiss J.J., Miller C.B., Jelsma P.F. Efficacy of COVID-19 convalescent plasma based on antibody concentration. Adv Hematol. 2022;2022 doi: 10.1155/2022/7992927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fazeli A., Sharifi S., Behdad F., et al. Early high-titer convalescent plasma therapy in patients with moderate and severe COVID-19. Transfus Apher Sci. 2022;61(2) doi: 10.1016/j.transci.2021.103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Franchini M., Glingani C., Donno G., et al. Convalescent plasma for hospitalized COVID-19 patients: A single-center experience. Life (Basel) 2022;12(3) doi: 10.3390/life12030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gachoud D., Pillonel T., Tsilimidos G., et al. Antibody response and intra-host viral evolution after plasma therapy in COVID-19 patients pre-exposed or not to B-cell-depleting agents. Br J Haematol. 2022;199(4):549–559. doi: 10.1111/bjh.18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.González S.E., Regairaz L., Salazar M.R., et al. Timing of convalescent plasma administration and 28-day mortality in COVID-19 pneumonia. J Investig Med. 2022;70(5):1258–1264. doi: 10.1136/jim-2021-002158. [DOI] [PubMed] [Google Scholar]
- 141.Hemsinlioglu C., Pelit N., Yalcin K., et al. The effectiveness of ACB-IP 1.0 universal pathogen free concentrated cocktail convalescent plasma in COVID-19 infection. Preprint. Posted online March 22, 2021. medRxiv. 2021 doi: 10.1101/2021.03.05.21251413. 2021.2003.2005.21251413. [DOI] [Google Scholar]
- 142.Khan T.N.S., Mukry S.N., Masood S., et al. Usefulness of convalescent plasma transfusion for the treatment of severely ill COVID-19 patients in Pakistan. BMC Infect Dis. 2021;21(1):1014. doi: 10.1186/s12879-021-06451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leon J., Merrill A.E., Rogers K., et al. SARS-CoV-2 antibody changes in patients receiving COVID-19 convalescent plasma from normal and vaccinated donors. Transfus Apher Sci. 2022;61(2) doi: 10.1016/j.transci.2021.103326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maor Y., Cohen D., Paran N., et al. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG antibody levels in donated plasma. EClinicalmedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Akay Cizmecioglu H., Goktepe M.H., Demircioglu S., et al. Efficacy of convalescent plasma therapy in severe COVID-19 patients. Transfus Apher Sci. 2021;60(4) doi: 10.1016/j.transci.2021.103158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Asem N., Massoud H.H., Serag I., et al. Clinical efficacy of early administration of convalescent plasma among COVID-19 cases in Egypt. Open Access Maced J Med Sci. 2022;10(B):1698–1705. [Google Scholar]
- 147.Balcells M.E., Rojas L., Le Corre N., et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18(3) doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Silvestro G., Marson P., La Raja M., et al. Outcome of SARS CoV-2 in patients treated with convalescent plasma: one-year of data from the Veneto region (Italy) registry. Eur J Intern Med. 2022;97:42–49. doi: 10.1016/j.ejim.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fodor E., Müller V., Iványi Z., et al. Early transfusion of convalescent plasma improves the clinical outcome in severe SARS-CoV2 infection. Infect Dis Ther. 2022;11(1):293–304. doi: 10.1007/s40121-021-00514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gazitúa R., Briones J.L., Selman C., et al. Convalescent plasma in COVID-19. Mortality-Safety First results of the prospective multicenter FALP 001-2020 trial. Preprint. Posted online December 2, 2020. medRxiv. 2020 doi: 10.1101/2020.11.30.20218560. 2020.2011.2030.20218560. [DOI] [Google Scholar]
- 151.Greenbaum U., Klein K., Martinez F., et al. High levels of common cold coronavirus antibodies in convalescent plasma are associated with improved survival in COVID-19 patients. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.675679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hueso T., Godron A.S., Lanoy E., et al. Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis. Leukemia. 2022;36(4):1025–1034. doi: 10.1038/s41375-022-01511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ibrahim D., Dulipsingh L., Zapatka L., et al. Factors associated with good patient outcomes following convalescent plasma in COVID-19: a prospective Phase II clinical trial. Infect Dis Ther. 2020;9(4):913–926. doi: 10.1007/s40121-020-00341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Janaka S.K., Hartman W., Mou H., et al. Donor anti-spike immunity is related to recipient recovery and can predict the efficacy of convalescent plasma units. Preprint. Posted online March 1, 2021. medRxiv. 2021 doi: 10.1101/2021.02.25.21252463. 2021.2002.2025.21252463. [DOI] [Google Scholar]
- 155.Jeyaraman P., Agrawal N., Bhargava R., et al. Convalescent plasma therapy for severe Covid-19 in patients with hematological malignancies. Transfus Apher Sci. 2021;60(3) doi: 10.1016/j.transci.2021.103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kocayiğit H., Demir G., Karacan A., et al. Effects on mortality of early vs late administration of convalescent plasma in the treatment of Covid-19. Transfus Apher Sci. 2021;60(4) doi: 10.1016/j.transci.2021.103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lattanzio N., Acosta-Diaz C., Villasmil R.J., et al. Effectiveness of COVID-19 convalescent plasma infusion within 48 hours of hospitalization with SARS-CoV-2 infection. Cureus. 2021;13(7) doi: 10.7759/cureus.16746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Senefeld J.W., Johnson P.W., Kunze K.L., et al. Access to and safety of COVID-19 convalescent plasma in the United States Expanded Access Program: a national registry study. PLoS Med. 2021;18(12) doi: 10.1371/journal.pmed.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Levine A.C., Fukuta Y., Huaman M.A., et al. COVID-19 convalescent plasma outpatient therapy to prevent outpatient hospitalization: a meta-analysis of individual participant data from five randomized trials. Preprint. Posted online December 18, 2022. medRxiv. 2022 doi: 10.1101/2022.12.16.22283585. 2022.2012.2016.22283585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma T., Wiggins C.C., Kornatowski B.M., et al. The role of disease severity and demographics in the clinical course of COVID-19 patients treated with convalescent plasma. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.707895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ripoll J.G., Gorman E.K., Juskewitch J.E., et al. Vaccine-boosted convalescent plasma therapy for patients with immunosuppression and COVID-19. Blood Adv. 2022;6(23):5951–5955. doi: 10.1182/bloodadvances.2022008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kunze K.L., Johnson P.W., van Helmond N., et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat Commun. 2021;12(1):4864. doi: 10.1038/s41467-021-25113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Larkey N.E., Ewaisha R., Lasho M.A., et al. Limited correlation between SARS-CoV-2 serologic assays for identification of high-titer COVID-19 convalescent plasma using FDA thresholds. Microbiol Spectr. 2022;10(4) doi: 10.1128/spectrum.01154-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 165.Kent D.M., Steyerberg E., van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ. 2018;363:k4245. doi: 10.1136/bmj.k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kent D.M., Paulus J.K., van Klaveren D., et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement. Ann Intern Med. 2020;172(1):35–45. doi: 10.7326/M18-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.