Abstract
Introduction
Systemic sclerosis is a chronic and progressive connective tissue disease with various manifestation. Inflammatory status is developed in early stages and is followed by major organs’ dysfunction. Disease severity is evaluated mostly through Medsger scale. There is not any single laboratory test to evaluate disease severity, although some hematologic can reflect disease severity. In this study, we evaluated the association between hematologic indices (specially Neutrophil/Lymphocyte ratio) and Medsger score of disease severity.
Materials and methods
One hundred and twenty-three patients along with the same number of healthy controls were enrolled in this study. Demographic information and past medical records were gathered in first appointment. Hematologic indices were calculated based on the laboratory findings and the association between these indices and Medsger score of disease severity was evaluated.
Results
One hundred and twenty-three patients with mean disease duration of 9.54 and mean Medsger score of 7.42 were investigated in this study. Neutrophil count, erythrocyte sedimentation rate, red cell distribution width and NLR were significantly higher and mean platelets volume was significantly lower in SSc patients in comparison to controls. NLR was significantly correlated with pulmonary and cardiac involvements and Monocyte/Lymphocyte ratio was significantly correlated with the involvement of joint and tendons. We showed that NLR is a predictive factor for the severity of systemic sclerosis. We also found a cut off Value of 1.9 for NLR as a predictor for disease severity in our patients.
Conclusion
Our study shows that SSc and its severity is associated with some hematologic indices like NLR, MLR, platelets and hemoglobin. These indices can also specifically predict the involvement of some organs.
1. Introduction
Systemic sclerosis is a chronic and progressive connective tissue disease with various manifestation. It is characterized by skin thickening and internal organs' involvement such as lung, heart, kidneys and gastrointestinal system. Inflammatory status is developed in early stages of systemic sclerosis. This condition is followed by major dysfunction in visceral organs as a result of fibrosis. Skin thickening distinguishes SSc from other connective tissue disorders [[1], [2], [3], [4]]. Many studies recommend that both genetic and environmental factors are responsible for this disease. These factors are believed to affect DNA and micro-RNA expression through epigenetic regulation mechanism [[5], [6], [7]]. SSc is diagnosed clinically based on patient's present history and physical examinations. Skin thickening accompanied by manifestations of internal organs' involvements make the diagnosis almost definite. Laboratory findings consist of mild anemia (normocytic or microcytic) and elevated inflammatory markers (such as Erythrocyte sedimentation rate and C-reactive protein levels in serum). These markers can play a role in choosing treatment strategies and determining disease prognosis [[8], [9], [10]]. Disease severity is evaluated mostly through Medsger scale, which is assessed based on the severity of involvement in nine organs (Medsger et al.) [11]. there is not any single laboratory test to evaluate disease severity, although some hematologic indices (including neutrophil/lymphocyte ratio (NLR) and monocyte/lymphocyte ratio (MLR)) can reflect disease severity [12,13]. NLR is routinely used as a prognostic factor in cardiovascular disease, infections, inflammatory disease and cancers [14]. It may also be used to predict mortality and morbidity in SSc patients according to its inflammatory nature. In this study, we evaluated the association between hematologic indices and Medsger score of disease severity.
2. Materials and methods
One hundred and twenty-three patients were enrolled in this study via consecutive sampling from February 2020 until February 2021. All patients were diagnosed with systemic sclerosis (confirmed by a rheumatologist based on ACR/EULAR 2013 criteria), they were above the age of 18 and registered to rheumatology clinic in Razi hospital, Rasht. Patients who underwent high-dose corticosteroid treatments and the ones with uncontrolled diabetes mellitus, neoplasms, recent infections and other hematologic diseases were excluded. Also 123 healthy people, who were referred to our clinic with laboratory test results, were chosen as control group in this study through consecutive sampling. Their past medical records were checked to ensure absence of registered rheumatologic diseases and they also underwent clinical and paraclinical investigation by a rheumatologist to exclude patients who are likely to be involved with rheumatologic disorders.
Demographic information and past medical records were gathered in first appointment. Patients' organ involvements were noted in Razi clinic's data registration and Medsger score of disease severity was calculated based on the severity of the involvement of nine major organs. Each organ involvement is evaluated with a severity score of zero (normal), one (mild), two (moderate), three (severe) and four (end stage) and the total score indicates Medsger score of disease severity. A blood sample was sent for each patient to determine following laboratory findings; complete blood count with cell differentiation, ESR and serum levels of CRP. NLR and MLR were calculated for each patient based on the results of the mentioned tests.
In this survey, we used mean, standard deviation, median, minimum and maximum to define quantitative data and frequency (percentile) for qualitative data. Independent T-test (or Mann Whitney U test) was used to compare the results in case and control groups. Correlation between hematologic indices and Medsger score (and its 9 components separately) was assessed via Pearson correlation coefficient (or spearman correlation coefficient). Multiple logistic regression and multiple linear regression models were used to determine predicting factors of the existence of SSc and its severity, respectively. All results were analyzed with 95% Confidence Interval (α = 0.05).
3. Ethical Considerations
This study was approved by Ethics Committee of Guilan University of Medical Sciences (Code: IR. GUMS.REC.1400.160, Date: 14-07-2021).
4. Results
One hundred and twenty-three patients with definite diagnosis of SSc were enrolled in this study, along with 123 healthy controls. Majority of patients (61.79%) were in the age group of 40–60 years. 89% of patients were female and 6% of patients had a history of smoking or alcohol consumption. 16% of patients had diabetes mellitus, 34% had cardiovascular disease, 27% had HTN, 59% had dyslipidemia and 14% had thyroid dysfunction. Mean disease duration (from the onset of first non-Raynaud symptom) was 9.54 (Standard Deviation = 7.26) years and mean Medsger score for patients was 7.42 (SD = 2.77). Age group distribution was significantly different in two groups. furthermore, frequency of female sex, cardiovascular disease, HTN, dyslipidemia and hypothyroidism were significantly higher in SSc patients in comparison to control group. On the contrary, smoking and alcohol consumption was significantly more frequent in control group. Patients’ demographic and clinical information is available in Table 1 and Table 2.
Table 1.
Demographic and clinical characteristics of individuals divided by groups.
| Cases (n = 123) | Controls (n = 123) | Total (n = 246) | P Value | ||
|---|---|---|---|---|---|
| Age | <40 years | 17 (13.82%) | 37 (30.08%) | 54 (21.9%) | 0.01 |
| 40–60 | 76 (61.79%) | 58 (47.15%) | 134 (54.47%) | ||
| >60 years | 30 (24.39%) | 28 (22.76%) | 58 (23.58%) | ||
| Sex | Male | 14 (11%) | 57 (46%) | 71 (29%) | <0.01 |
| Female | 109 (89%) | 66 (54%) | 175 (71%) | ||
| Smoking | 5 (4%) | 30 (24%) | 35 (14%) | <0.01 | |
| Alcohol consumption | 2 (2%) | 16 (13%) | 18 (7%) | <0.01 | |
| Diabetes mellitus | 19 (15%) | 20 (16%) | 39 (16%) | 0.86 | |
| Cardiovascular disease | 42 (34%) | 21 (17%) | 63 (26%) | <0.01 | |
| HTN | 33 (27%) | 20 (16%) | 53 (22%) | 0.04 | |
| Dyslipidemia | 73 (59%) | 20 (16%) | 93 (38%) | <0.01 | |
| Hypothyroidism | 17 (14%) | 7 (6%) | 24 (10%) | 0.03 | |
| Hyperthyroidism | 0 | 0 | 0 | – | |
Table 2.
Distribution of disease duration and Medsger score.
| Mean ± SD | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Medsger score | 7.4 ± 2.8 | 7 (IQR: 5–9) | 1 | 17 |
| Disease duration (years) | 9.5 ± 7.2 | 8 | 1 | 33 |
Table 3 demonstrates the comparison of laboratory findings between two groups. Neutrophil count, ESR, RDW and NLR were significantly higher in SSc patients in comparison to controls. In contrast, serum levels of hemoglobin and MPV were significantly lower in SSc patients.
Table 3.
Comparison of laboratory results between two groups.
| Cases (n = 123) | Controls (n = 123) | P Value | |
|---|---|---|---|
| ESR | 28.2 ± 21.6 | 7.8 ± 3.2 | < 0.01 |
| CRP | 6.3 ± 6 | 4.5 ± 2.1 | 0.87 |
| WBC ( × 1000) | 8.4 ± 1.6 | 7.5 ± 1.7 | 0.23 |
| RBC ( × 106) | 4.6 ± 0.6 | 4.6 ± 0.6 | 0.89 |
| Platelets ( × 1000) | 272 ± 95 | 266 ± 72 | 0.87 |
| Hemoglobin | 12.3 ± 1.5 | 13.6 ± 1.7 | < 0.01 |
| Neutrophil (%) | 62.4 ± 10.4 | 59.7 ± 8.5 | 0.03 |
| Lymphocyte (%) | 29.8 ± 9.9 | 31.9 ± 7.4 | 0.07 |
| Monocyte (%) | 5.1 ± 3.2 | 5.3 ± 2.8 | 0.41 |
| MPV | 8.5 ± 1.2 | 9.2 ± 5.3 | 0.03 |
| RDW | 14.8 ± 6.2 | 13.2 ± 1.4 | < 0.01 |
| NLR | 2.5 ± 1.4 | 2.1 ± 0.8 | 0.02 |
| MLR | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.82 |
Table 4 shows the correlation between the components of Medsger score and NLR and MLR. While NLR was significantly correlated with pulmonary and cardiac involvements, MLR was significantly correlated with the involvement of joint and tendons. There was not any significant correlation between other organs’ involvements and mentioned hematologic indices.
Table 4.
Correlation between the components of Medsger score and NLR and MLR.
| NLR | MLR | ||
|---|---|---|---|
| General | R | −0.078 | 0.034 |
| P Value | 0.39 | 0.71 | |
| Peripheral vessels | R | −0.039 | 0.016 |
| P Value | 0.67 | 0.86 | |
| Skin | r | 0.023 | 0.103 |
| P Value | 0.80 | 0.26 | |
| Joints and tendon | r | 0.094 | 0.185 |
| P Value | 0.30 | 0.04 | |
| Musculoskeletal | r | −0.004 | −0.112 |
| P Value | 0.97 | 0.22 | |
| Gastrointestinal system | r | −0.094 | −0.014 |
| P Value | 0.30 | 0.89 | |
| Lung | r | 0.304 | 0.159 |
| P Value | < 0.01 | 0.08 | |
| Heart | r | 0.335 | 0.073 |
| P Value | < 0.01 | 0.42 | |
| Kidneys | r | 0.139 | 0.107 |
| P Value | 0.12 | 0.24 | |
As shown in Table 5, after controlling the effect of intervening variables, NLR is a predicting factor of disease severity (P = 0.041, β = 0.347). Hemoglobin (P = 0.012, β = −0.437), ESR (P = 0.010, β = 0.030) and male to female ratio (P = 0.043, β = −1.71) were also a predicting factor for disease severity.
Table 5.
Predicting factors of disease severity based on Medsger score.
| Unstandardized coefficient |
Standardized coefficient |
P Value | 95% CI for β |
|||
|---|---|---|---|---|---|---|
| β | SE | Β | Lower | Upper | ||
| Sex (M/F ratio) | −1.708 | 0.834 | −0.197 | 0.04 | −3.360 | −0.057 |
| WBC | −4.056E-5 | 0.000 | −0.170 | 0.06 | 0.000 | 0.000 |
| Hemoglobin | −0.437 | 0.172 | −0.236 | 0.01 | −0.777 | −0.097 |
| ESR | 0.030 | 0.011 | 0.231 | 0.01 | 0.007 | 0.052 |
| NLR | 0.347 | 0.168 | 0.179 | 0.04 | 0.015 | 0.679 |
| Constant | 14.640 | 3.213 | – | 0.00 | 8.276 | 21.004 |
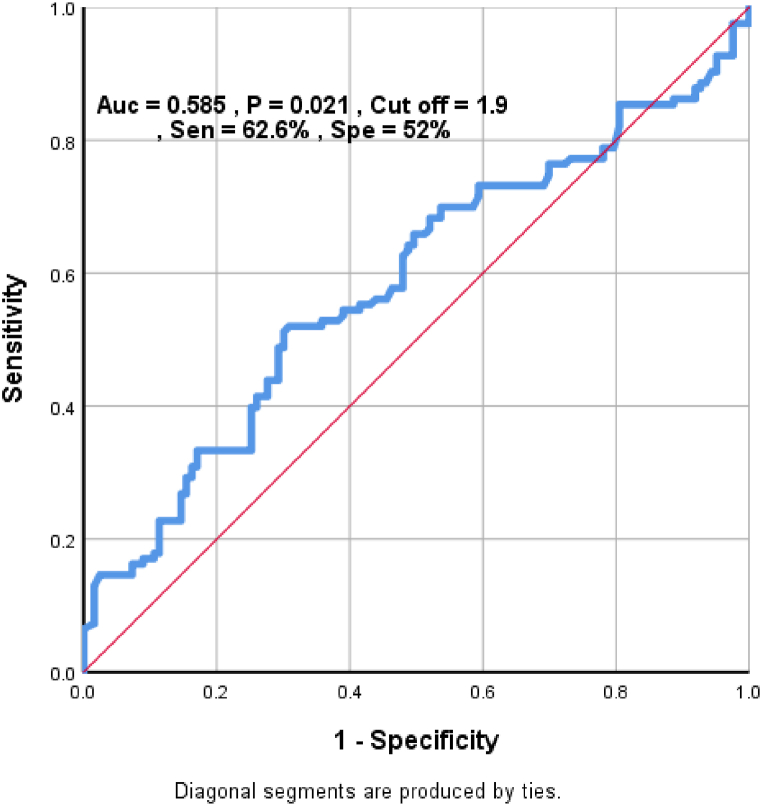
In this study, we found a cut off Value of 1.9 for NLR as a predictor for disease severity in our patients with 62.6% sensitivity and 52% specificity. The result is shown as a ROC curve in Fig. 1.
Fig. 1.
ROC curve to determine a cut off value for NLR to predict disease severity.
5. Discussion
One hundred and twenty-three patients with mean Medsger score of 7.42 were enrolled in this study along with 123 healthy controls. Of 123 patients, 61.8% were between the age of 40 and 60, 88.6% were female and 5.7% had a history of smoking or alcohol consumption. Rates of diabetes mellites, alcohol consumption and smoking were higher in controls, while other underlying diseases were more prevalent in SSc patients.
In this study, NLR was significantly higher in SSc patients in comparison to control group. This result is compatible with the ones reached in many previous studies [[15], [16], [17], [18], [19], [20]]. ME Yayla et al. stated that inflammatory process can lead to a rise in neutrophil count and a decrement in lymphocyte count, which will result in increased NLR [17]. Hussein et al. also stated that neutrophils may increase in SSc patients as a result of inhibition of inactivators of chemotactic factors [21]. Some recent studies proposed NLR as a novel marker for infection and inflammation [22,23]. This can be justified by considering the great role of neutrophils in inflammatory process through inducing inflammatory cytokines' production [24]. Neutrophils have multiple roles in the pathogenesis of SSc including induction of endothelial cells’ apoptosis [25] and increasing fibrosis via Reactive oxygen species production [26,27]. Formation of NETs, which contain large amount of intra-cellular and extra-cellular items, by activated neutrophils can lead to activation of immune system against potential autoantigens and development of endothelial injury. Both mentioned processes play a vital role in pathogenesis of SSc [[28], [29], [30]]. Increased production of inflammatory cytokines (such as GM-CSF, IFN-c, TNF-α and IL-6), which evidently stir up the activation of neutrophils, confirm the role of neutrophils in pathogenesis of SSc [31].
In our study, NLR was a significant predictor for disease severity (based on Medsger score) after controlling confounding variables (including past medical records, smoking and etc.). Previous studies have shown the correlation between NLR and disease severity or activity based on different scales (e.g., Medsger, Rodnan, Valentini, EUSTAR) [17,32]. severity of other autoimmune diseases was also confirmed by some studies to be related with NLR [33,34]. Our study also presented that NLR had a significant correlation with cardiac and pulmonary involvement in SSc patients. Many studies have confirmed the correlation between NLR and various organs’ involvements like muscle weakness [16], peripheral vascular ischemia or digital ulcers [16,17], cardiac involvement [35] and pulmonary involvement [[18], [19], [20],32,35]. G Cuomo et al. presented a positive significant correlation between NLR and sPAP [32]. A Kim et al. study showed no significant correlation between NLR and Pulmonary hypertension, Rodnan score and presence of digital ulcers [18].
Previous studied have shown increased MLR in patients with cardiovascular and inflammatory diseases like SLE, RA and AS [36,37]. ME Yayla et al. study showed a significant correlation between MLR and Medsger, Rodnan and EUSTAR scores [17]. Yang Z et al. showed significantly higher MLR in SSc patients in comparison to healthy controls, but it didn't confirm the correlation between MLR and disease severity [36]. In our study, there was no significant difference in MLR between two groups. There wasn't any correlation between MLR and Medsger score in our patients either. However, it was correlated to the involvement of joints and tendon.
In our study, platelets count was not significantly different between SSc patients and healthy controls. Many studies have shown similar results [15,17,20] while others demonstrated a significant difference between two groups [16,38]. Platelets play a crucial role in the pathogenesis of SSc with overproduction and release of ROS, cytokines, growth factors, High mobility group box 1 and serotonin [[39], [40], [41]]. These inflammatory mediators lead to increased production of collagen, activation of myofibroblasts, endothelial cell damage and impaired vascular repair. Mentioned events result in peripheral vascular damage and its ischemic complications such as digital ulcers [[40], [41], [42], [43], [44]]. Furthermore, an association is known to be between platelets activation and Raynaud phenomenon, which is mostly the first manifestation of SSc [45,46].
It was also shown in our study that MPV was significantly lower in SSc patients in comparison to the healthy controls. The result on changes of MPV in SSc in patients is controversy in previous records. While some records support our results [38], others show higher MPV in SSc patients in comparison to the control group [16,47]. There also some studies that show no significant difference in MPV between SSc patients and healthy controls [17]. Our results showed no correlation between MPV and disease severity. Previous studies mostly showed a negative correlation between MPV and disease severity [38,47]. Studies on other rheumatologic disorders (like RA and AS) show a negative correlation between MPV and disease activity, too [48]. Decreased MPV may be present due to impaired thrombopoiesis in systemic inflammation in rheumatologic diseases [49].
We found higher RDW in SSc patients compared to healthy controls. There are many studies showing the same result accompanied by a significant correlation between RDW and disease severity [16,17,50]. ME Yayla et al. and N Farkas et al. suggested that RDW can be used as a severity and prognostic factor in SSc patients [17,50]. RDW reflects numerous pathological processes in SSc, like oxidative stress, thrombosis, inflammatory state and endothelial dysfunction [47,[51], [52], [53], [54], [55], [56]]. Increased levels of cytokines can also affect the function of erythropoietin and lead to production of immature RBCs. This process ends in elevated RDW [57]. It's proposed that higher RDW, which is caused by inflammatory processes, can be associated with activating coagulative state [58].
Other factors like hemoglobin, ESR and female gender were shown to be a predictor for disease severity, too. Previous studies also show the effect of decreased hemoglobin on disease severity and its mortality [16,17,59].
We reached a cut-off value of 1.9 for NLR to predict higher Medsger scores. Jung et al. proposed higher risk of SSc-ILD in NLR above 2.59 [20]. N Atilla et al. suggested cut-off value of 3.21 for NLR to predict pulmonary involvement [19]. The mentioned cut-off values are both higher than the one proposed in our study. On the other hand, they are predicting only one component of Medsger score.
6. Limitations
A longer follow-up and a larger sample size can always improve the accuracy of results.
Author contribution statement
Fatemeh Nejatifar; Habib Zayeni: Conceived and designed the experiments; Wrote the paper. Neda Mirbolouk; Irandokht Shenavar Masooleh: Performed the experiments. Ehsan Kazemnejad: Analyzed and interpreted the data. Banafsheh Ghavidel-Parsa: Contributed reagents, materials, analysis tools or data. Amir Mohammad Ghanbari: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Authors also declare that there was no funding and investments in this survey.
Acknowledgements
We are grateful to all the staff in the Rheumatology clinic of Razi Hospital for their great cooperation in this survey.
References
- 1.Gilbane A., Denton C., Holmes A. Scleroderma pathogenesis: a pivotal role for fibroblasts as effector cells. Arthritis Res. Ther. 2013;15:215. doi: 10.1186/ar4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayes M.D. Scleroderma epidemiology. Rheum. Dis. Clin. N. Am. 2003;29(2):239–254. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 3.Simeón-Aznar C.P., Fonollosa-Plá V., Tolosa-Vilella C., Selva-O'Callaghan A., Soláns-Laqué R., Vilardell Tarrés M. Effect of mycophenolate sodium in scleroderma-related interstitial lung disease. Clin. Rheumatol. 2011;30:1393–1398. doi: 10.1007/s10067-011-1823-1. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan K., Keyes-Elstein L., McSweeney P., Pinckney A., Welch B., Mayes M., et al. Myeloablative autologous transplantation of CD34 + selected hematopoietic stem cells (HSCT) vs monthly intravenous cyclophosphamide (CY) for severe scleroderma with internal organ involvement: outcomes of a randomized north American clinical trial. Biol. Blood Marrow Transplant. 2017;23:S23–S24. [Google Scholar]
- 5.Barsotti S., Bruni C., Orlandi M., Della Rossa A., Marasco E., Codullo V., et al. One year in review 2017: systemic sclerosis. Clin. Exp. Rheumatol. 2017;35(4):3–20. Suppl 106. [PubMed] [Google Scholar]
- 6.Iniesta Arandia N., Simeón-Aznar C.P., Guillén del Castillo A., Colunga Argüelles D., Rubio-Rivas M., Trapiella Martínez L., et al. Influence of antibody profile in clinical features and prognosis in a cohort of Spanish patients with systemic sclerosis. Clin. Exp. Rheumatol. 2017;35(Suppl 106 4):98–105. [PubMed] [Google Scholar]
- 7.Li H., Yang R., Fan X.Y., Gu T., Zhao Z., Chang D., et al. MicroRNA array analysis of microRNAs related to systemic scleroderma. Rheumatol. Int. 2010;32:307–313. doi: 10.1007/s00296-010-1615-y. [DOI] [PubMed] [Google Scholar]
- 8.Bryan C., Knight C.J., Black C.M., Silman A.J. Prediction of five-year survival following presentation with scleroderma: development of a simple model using three disease factors at first visit. Arthritis Rheum. 1999;42 12:2660–2665. doi: 10.1002/1529-0131(199912)42:12<2660::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Czirják L., Kumánovics G., Varjú C., Nagy Z.Z., Pákozdi A., Szekanecz Z. Survival and causes of death in 366 Hungarian patients with systemic sclerosis. Ann. Rheum. Dis. 2008;67:59–63. doi: 10.1136/ard.2006.066340. [DOI] [PubMed] [Google Scholar]
- 10.Joven B.E., Almodovar R., Carmona L., Carreira P.E. Survival, causes of death, and risk factors associated with mortality in Spanish systemic sclerosis patients: results from a single university hospital. Semin. Arthritis Rheum. 2010;39(4):285–293. doi: 10.1016/j.semarthrit.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Medsger T., Bombardieri S., Czirjak L., Scorza R., Rossa A., Bencivelli W. Assessment of disease severity and prognosis. Clin. Exp. Rheumatol. 2003;21(3):S42–S46. SUPP/29) [PubMed] [Google Scholar]
- 12.Mercan R., Bitik B., Tufan A., Bozbulut U.B., Atas N., Ozturk M.A., et al. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J. Clin. Lab. Anal. 2016;30(5):597–601. doi: 10.1002/jcla.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., Chen Y., Yang X., Chen L., Yang Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharm. 2016;36:94–99. doi: 10.1016/j.intimp.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bernardino V., Rodrigues A., Fernandes M., Lladó A., Panarra A. 2020. Neutrophil To Lymphocyte Ratio And Systemic Sclerosis Clinical Impact. [Google Scholar]
- 15.Tezcan D., Turan Ç., Yılmaz S., Sivrikaya A., Gülcemal S., Limon M., et al. What do simple hematological parameters tell us in patients with systemic sclerosis. Acta Dermatovenerol. Alpina Pannonica Adriatica. 2020;29(3):101–107. [PubMed] [Google Scholar]
- 16.Sakr B.R., Rabea R.E., ElHamid S.M. Value of hematological parameters as biomarkers of disease manifestations and severity in systemic sclerosis. The Egyptian Rheumatologist. 2021;43(2):159–165. [Google Scholar]
- 17.Yayla M.E., İlgen U., Okatan İ.E., UsluYurteri E., Torgutalp M., Keleşoğlu Dinçer A.B., et al. Association of simple hematological parameters with disease manifestations, activity, and severity in patients with systemic sclerosis. Clin. Rheumatol. 2020;39(1):77–83. doi: 10.1007/s10067-019-04685-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim A., Kim Y., Kim G.-T., Ahn E., So M.W., Sohn D.H., et al. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as potential makers for digital ulcers and interstitial lung disease in patients with systemic sclerosis: cross-sectional analysis of data from a prospective cohort study. Rheumatol. Int. 2020;40(7):1071–1079. doi: 10.1007/s00296-020-04604-6. [DOI] [PubMed] [Google Scholar]
- 19.Atilla N., Ceti̇n G.Y., Balkarli A. Association of neutrophil/lymphocyte ratio with the degree ofinterstitial lung disease in systemic sclerosis. Turk. J. Med. Sci. 2016;46(6):1871–1874. doi: 10.3906/sag-1601-87. [DOI] [PubMed] [Google Scholar]
- 20.Jung J.-H., Lee Y.-M., Lee E.-G., Yoo W.-H., Lee W.-S. Neutrophil-to-lymphocyte ratio in diagnosis of systemic sclerosis for prediction of interstitial lung disease. Journal of Rheumatic Diseases. 2017;24(3):138–142. [Google Scholar]
- 21.Hussein M., Hassan H., Hofny E., Elkholy M., Fatehy N., Abd Elmoniem A., et al. Alterations of mononuclear inflammatory cells, CD4/CD8+ T cells, interleukin 1β, and tumour necrosis factor α in the bronchoalveolar lavage fluid, peripheral blood, and skin of patients with systemic sclerosis. J. Clin. Pathol. 2005;58(2):178–184. doi: 10.1136/jcp.2004.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polat N., Yildiz A., Yuksel M., Bilik M.Z., Aydin M., Acet H., et al. Association of neutrophil–lymphocyte ratio with the presence and severity of rheumatic mitral valve stenosis. Clinical and Applied Thrombosis/Hemostasis. 2014;20(8):793–798. doi: 10.1177/1076029613514131. [DOI] [PubMed] [Google Scholar]
- 23.de Jager C.P., van Wijk P.T., Mathoera R.B., de Jongh-Leuvenink J., van der Poll T., Wever P.C. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit. Care. 2010;14(5):1–8. doi: 10.1186/cc9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moodley D., Mody G.M., Chuturgoon A.A. Initiation but no execution-modulation of peripheral blood lymphocyte apoptosis in rheumatoid arthritis-a potential role for heat shock protein 70. J. Inflamm. 2011;8(1):1–11. doi: 10.1186/1476-9255-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes T.C., Spiller D.G., Anderson M.E., Edwards S.W., Moots R.J. Endothelial activation and apoptosis mediated by neutrophil-dependent interleukin 6 trans-signalling: a novel target for systemic sclerosis? Ann. Rheum. Dis. 2011;70(2):366–372. doi: 10.1136/ard.2010.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagnato G., Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur. Respir. Rev. 2015;24(135):102–114. doi: 10.1183/09059180.00003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doridot L., Jeljeli M., Chêne C., Batteux F. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019;25 doi: 10.1016/j.redox.2019.101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmona-Rivera C., Zhao W., Yalavarthi S., Kaplan M.J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 2015;74(7):1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radic M., Marion T.N., editors. Seminars in Immunopathology. Springer; 2013. Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y.-F., Pan Y., Pan G.-G., Wei Y.-S., Luo B. Platelet to lymphocyte ratio in polymyositis as a marker of disease activity. Clin. Lab. 2016;62(5):915–919. doi: 10.7754/clin.lab.2015.150941. [DOI] [PubMed] [Google Scholar]
- 32.Cuomo G., Masini F., Gjeloshi K., Guarino F., Danzo F., Adinolfi L.E., et al. BMJ Publishing Group Ltd; 2019. Ab0648 Correlations Between Neutrophil/Lymphocyte Ratio And Clinical Characteristics Of Patients With Systemic Sclerosis. [Google Scholar]
- 33.Greisenegger S., Endler G., Hsieh K., Tentschert S., Mannhalter C., Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35(7):1688–1691. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 34.Qin B., Ma N., Tang Q., Wei T., Yang M., Fu H., et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod. Rheumatol. 2016;26(3):372–376. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 35.Esheba N., Shahba A. Assessment of neutrophil lymphocyte ratio in systemic sclerosis patients in Tanta university hospital: a promising marker in predicting disease severity. Egyptian Journal of Rheumatology and Clinical Immunology. 2016;4(1):33–41. [Google Scholar]
- 36.Yang Z., Zhang Z., Lin F., Ren Y., Liu D., Zhong R., et al. Comparisons of neutrophil‐, monocyte‐, eosinophil‐, and basophil‐lymphocyte ratios among various systemic autoimmune rheumatic diseases. Apmis. 2017;125(10):863–871. doi: 10.1111/apm.12722. [DOI] [PubMed] [Google Scholar]
- 37.Xiang F., Chen R., Cao X., Shen B., Liu Z., Tan X., et al. Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all‐cause mortality in hemodialysis patients: a prospective cohort study. Hemodial. Int. 2018;22(1):82–92. doi: 10.1111/hdi.12549. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim S.E., Morad C.S., Farouk N., Louis A. Platelet indices as markers of inflammation in systemic sclerosis patients: relation to vascular endothelial growth factor and flow mediated dilatation. The Egyptian Rheumatologist. 2018;40(4):239–242. [Google Scholar]
- 39.Yuri Gasparyan A., Ayvazyan L., Pretorius E., D Kitas G. Platelets in rheumatic diseases: friend or foe? Curr. Pharmaceut. Des. 2014;20(4):552–566. doi: 10.2174/138161282004140213143843. [DOI] [PubMed] [Google Scholar]
- 40.Ntelis K., Solomou E.E., Sakkas L., Liossis S.-N., Daoussis D., editors. Seminars in Arthritis and Rheumatism. Elsevier; 2017. The role of platelets in autoimmunity, vasculopathy, and fibrosis: implications for systemic sclerosis. [DOI] [PubMed] [Google Scholar]
- 41.Scherlinger M., Guillotin V., Truchetet M.-E., Contin-Bordes C., Sisirak V., Duffau P., et al. Systemic lupus erythematosus and systemic sclerosis: all roads lead to platelets. Autoimmun. Rev. 2018;17(6):625–635. doi: 10.1016/j.autrev.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 42.León-Ponte M., Ahern G.P., O'Connell P.J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109(8):3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z., Sun Y., Wang Q., Han Z., Huang Y., Liu X., et al. Red blood cell distribution width is a potential prognostic index for liver disease. Clin. Chem. Lab. Med. 2013;51(7):1403–1408. doi: 10.1515/cclm-2012-0704. [DOI] [PubMed] [Google Scholar]
- 44.Sacchetti C., Bai Y., Stanford S.M., Di Benedetto P., Cipriani P., Santelli E., et al. PTP4A1 promotes TGFβ signaling and fibrosis in systemic sclerosis. Nat. Commun. 2017;8(1):1–14. doi: 10.1038/s41467-017-01168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silveri R.D.A., Poggi Andreina, Muti Stefania, Giuseppe Bonapace, Franco Argentati, Cervini Claudio, Ferdinando Relative roles of endothelial cell damage and platelet activation in primary Raynaud's phenomenon (RP) and RP secondary to systemic sclerosis. Scand. J. Rheumatol. 2001;30(5):290–296. doi: 10.1080/030097401753180372. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca C., Abraham D., Ponticos M. Neuronal regulators and vascular dysfunction in Raynaud's phenomenon and systemic sclerosis. Curr. Vasc. Pharmacol. 2009;7(1):34–39. doi: 10.2174/157016109787354105. [DOI] [PubMed] [Google Scholar]
- 47.Soydinc S., Turkbeyler I.H., Pehlivan Y., Soylu G., Goktepe M.F., Bilici M., et al. Mean platelet volume seems to be a valuable marker in patients with systemic sclerosis. Inflammation. 2014;37(1):100–106. doi: 10.1007/s10753-013-9716-x. [DOI] [PubMed] [Google Scholar]
- 48.Sahin A., Yetisgin A., Sahin M., Durmaz Y., Cengiz A. Can mean platelet volume be a surrogate marker of inflammation in rheumatic diseases? W. Indian Med. J. 2016;65(1) doi: 10.7727/wimj.2014.202. [DOI] [PubMed] [Google Scholar]
- 49.Stürzebecher C.-S., Losert W. Springer; 1987. Effects of Iloprost on Platelet Activation in Vitro. Prostacyclin and its Stable Analogue Iloprost; pp. 39–45. [Google Scholar]
- 50.Farkas N., Szabó A., Lóránd V., Sarlós D.P., Minier T., Prohászka Z., et al. Clinical usefulness of measuring red blood cell distribution width in patients with systemic sclerosis. Rheumatology. 2014;53(8):1439–1445. doi: 10.1093/rheumatology/keu022. [DOI] [PubMed] [Google Scholar]
- 51.Fatini C., Mannini L., Sticchi E., Rogai V., Guiducci S., Conforti M.L., et al. Hemorheologic profile in systemic sclerosis: role of NOS3− 786T> C and 894G> T polymorphisms in modulating both the hemorheologic parameters and the susceptibility to the disease. Arthritis Rheum.: Official Journal of the American College of Rheumatology. 2006;54(7):2263–2270. doi: 10.1002/art.21933. [DOI] [PubMed] [Google Scholar]
- 52.Jinjuvadia R., Liangpunsakul S. Association between metabolic syndrome and its individual components with viral hepatitis B. Am. J. Med. Sci. 2014;347(1):23–27. doi: 10.1097/MAJ.0b013e31828b25a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rustin M., Kovacs I., Sowemimo‐Coker S., Maddison P., Kirby J. Differences in red cell behaviour between patients with Raynaud's phenomenon and systemic sclerosis and patients with Raynaud's disease. Br. J. Dermatol. 1985;113(3):265–272. doi: 10.1111/j.1365-2133.1985.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 54.Subhashree A., Shanthi B., Parameaswari P. The red cell distribution width as a sensitive biomarker for assessing the pulmonary function in automobile welders-a cross sectional study. J. Clin. Diagn. Res.: J. Clin. Diagn. Res. 2013;7(1):89. doi: 10.7860/JCDR/2012/5051.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippi G., Targher G., Montagnana M., Salvagno G.L., Zoppini G., Guidi G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab Med. 2009;133(4):628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 56.Förhécz Z., Gombos T., Borgulya G., Pozsonyi Z., Prohászka Z., Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am. Heart J. 2009;158(4):659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Barnes T.C., Anderson M.E., Edwards S.W., Moots R.J. Neutrophil-derived reactive oxygen species in SSc. Rheumatology. 2012;51(7):1166–1169. doi: 10.1093/rheumatology/ker520. [DOI] [PubMed] [Google Scholar]
- 58.Rezende S.M., Lijfering W.M., Rosendaal F.R., Cannegieter S.C. Hematologic variables and venous thrombosis: red cell distribution width and blood monocyte count are associated with an increased risk. Haematologica. 2014;99(1):194. doi: 10.3324/haematol.2013.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sehra S.T., Kelly A., Baker J.F., Derk C.T. Predictors of inpatient mortality in patients with systemic sclerosis: a case control study. Clin. Rheumatol. 2016;35(6):1631–1635. doi: 10.1007/s10067-016-3245-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.