Abstract
Emerging literature supports removal of chronic indwelling IVC filters when they are contributing to complications for a patient and are no longer indicated. We present an interesting case of an elderly patient who had a history of DVT and underwent spinal surgery, which required cessation of his anticoagulation and placement of an IVC filter pre-operatively. Approximately 15 years later the patient presented to our institution with chronic occlusion of his IVC at the level of his filter which had never been removed, with bilateral lower extremity DVT and symptoms of phlegmasia cerulea dolens. Despite a previous unsuccessful attempt at DVT thrombectomy at an outside institution, interventional radiology was consulted, and he subsequently underwent successful laser sheath assisted removal of his 15-year-old permanent Greenfield filter with bilateral lower extremity DVT thrombectomy and venous stenting with significant improvement in his presenting symptoms. Clinical presentation, diagnostic workup, case findings, and outcomes are described.
Keywords: Greenfield Filter, IVC, Permanent filter, Thrombosis
Introduction
Inferior vena cava (IVC) filters are placed to prevent thrombus embolization to the lungs from deep venous thromboses (DVT) occurring in the lower extremity veins. IVC filters have been in use since 1973 and can be categorized broadly into those that are designed to be retrievable, versus those that are placed permanently. Permanent IVC filters are not designed with any retrieval mechanism built into their inherent design. Despite the classification, however, there are reports of successful removal of permanent IVC filters using advanced retrieval techniques [1], [2], [3], [4]. Indications for IVC filter retrieval include but are not limited to successful initiation of pharmacologic anticoagulation, clinically judged low risk of pulmonary embolism (PE), filter thrombosis, filter fracture, filter migration, and tilt, pain presumed to be due to the IVC filter, and perforation of adjacent structures [3,5,6]. Retrieval should ideally occur within 29-54 days after implantation [5,7,8]. We report a case of a 76-year-old male who underwent successful removal of an indwelling permanent infrarenal Greenfield IVC filter with ileocaval stent reconstruction following filter thrombosis and complete occlusion of the IVC and iliofemoral venous system.
Case report
Our case describes a 76-year-old male with history of recurrent DVT's on chronic anticoagulation therapy who had a permanent Greenfield IVC filter (Boston Scientific, Marlborough, MA) placed approximately 15 years prior preoperatively for spinal surgery. His filter was never removed after resuming his anticoagulation following his successful surgery.
The patient presented approximately 15 years later to an outside facility after his chronic anticoagulation was discontinued due to gastrointestinal hemorrhage with significant bilateral lower extremity swelling. Computed tomography venography (CTV) and ultrasound (US) were performed and revealed extensive bilateral iliofemoral DVT with extension to the level of the IVC filter (Fig. 1). Vascular surgical consultation was obtained, but due to his history of GI bleeding, thrombolysis was not recommended, and mechanical thrombectomy was not offered. On exam, the patient displayed symptoms of phlegmasia cerulea dolens, including cyanosis of his feet, cutaneous coolness, and necrosis of several of the digits of his lower extremities, and he was restarted on anticoagulation as tolerated given his GI bleeding history.
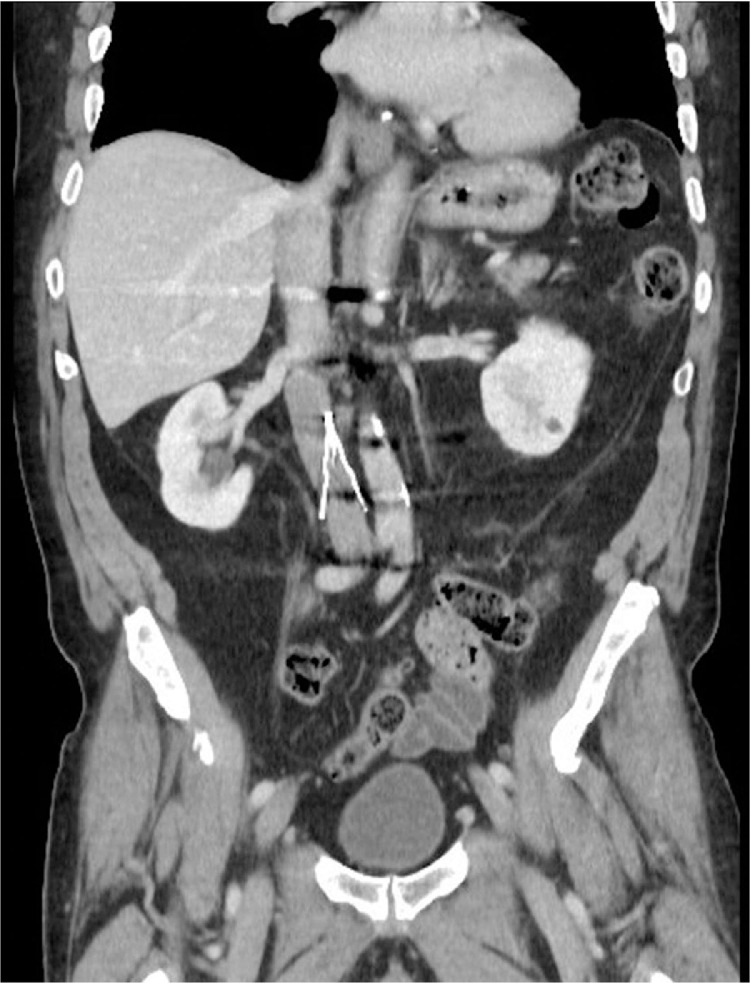
Fig. 1.
Preprocedure coronal CT of the abdomen demonstrates Greenfield filter and occluded IVC below level of filter.
Two months later the patient re-presented and was admitted to a nearby facility for weakness and was found to have a urinary tract infection. Given his history of chronic lower extremity swelling, radiology was consulted for evaluation of his chronic ileocaval DVT's, and after work up the decision was made to perform a mechanical thrombectomy. Thrombectomy was performed of his bilateral lower extremities, iliac veins, and distal IVC to the level just below his IVC filter with Inari ClotTriever and FlowTriever devices (Inari Medical, Irvine, CA) from bilateral popliteal approaches, however, his IVC filter was found to be nearly completely thrombosed despite attempts at re-cannalization (Figs. 2A and B). The facility at which he presented was unable to perform complex IVC filter removal, so an outpatient referral was made to our facility for consideration of complex IVC filter retrieval. He was started on low-dose rivaroxaban as anticoagulation and discharged to home following treatment of his presenting urinary tract infection with improvement in his lower extremity swelling. The patient was then evaluated as an outpatient in our interventional radiology (IR) clinic after discharge from the outside facility and was scheduled for a complex IVC filter retrieval with anesthesia support. He described a significant reduction in his symptomatic lower extremity swelling following his initial thrombectomy, despite residual thrombus in his filter noted at the time of his thrombectomy.
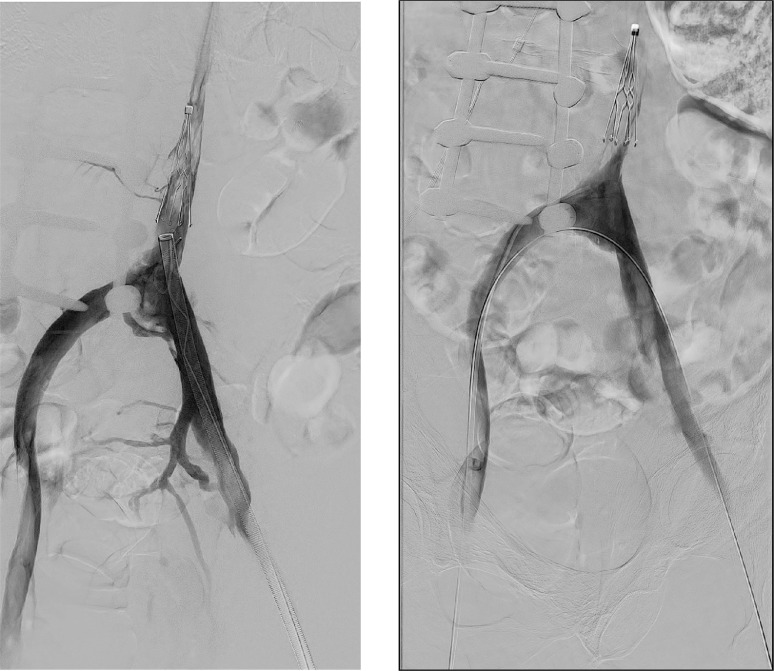
Fig. 2.
(A and B) Digital subtraction venography performed during mechanical thrombectomy shows persistent thrombus in the patient's IVC filter despite attempts at removal using an Inari Flowtreiver device (A), and final pelvic venography following mechanical thrombectomy using the Clotreiver device from a unilateral approach over wire placed across the iliac vein confluence (B).
Prior to his scheduled IVC Filter retrieval, however, the patient was admitted to our institution due to significant recurrent bilateral lower extremity swelling and was again found to have complete ileocaval thrombosis below the level of his filter despite his outpatient anticoagulation therapy. Following the IR team evaluation, the initial treatment consideration was pharmacologic thrombolysis of both lower extremity DVT's and IVC filter with infusion catheters using intravenous tPA, followed by IVC filter removal using advanced filter removal techniques.
The patient was brought to the IR suite and placed prone on the fluoroscopic table. Moderate sedation was used during the case for patient comfort. Ultrasound was used to access the bilateral popliteal veins and 6F venous sheaths (Terumo, Somerset, NJ) were placed bilaterally. Initially, a 5F Kumpe catheter (AngioDynamics, Latham, NY) and 0.035 Glidewire (Terumo, Somerset, NJ) were used to cross through the thrombosed right lower extremity deep veins from the popliteal access into the IVC above the level of the Greenfield filter. Next, working through the left popliteal venous sheath, a second 5F Kumpe catheter was used to cannulate the left lower extremity deep veins. Despite multiple attempts, the catheter could not be advanced across the left common femoral vein into the pelvis or IVC due to an encountered occlusion, with some contrast extravasation noted at the site upon further venography. Due to the concern for bleeding at this site during planned tPA administration, the procedure was terminated, and the wires, catheters, and bilateral sheaths were removed. Pressure was held to achieve hemostasis. Given the unexpected finding of stricture and contrast extravasation during this initial procedure, the patient returned to his hospital room for further anticoagulation therapy with significant lower extremity discomfort due to his persistent swelling. After discussing the case further with the patient and his family, the decision was made to re-attempt his case the following day using advanced filter removal techniques, general anesthesia, and an additional attempt at performing mechanical thrombectomy.
The following morning, the patient returned to interventional radiology and was placed supine on the fluoroscopic table following induction of general anesthesia. Ultrasound was used to access the right internal jugular vein and under fluoroscopic guidance an 18F sheath (Cook Medical, Bloomington, IN) was advanced into the suprarenal IVC superior to the Greenfield IVC filter (Fig. 3). Working through the 18F sheath, a 16F CavaClear laser sheath (Philips, Cambridge, MA) and a 5F loop snare (Merit, Salt Lake City, UT) were advanced. The loop snare was used to grasp the cone of the filter (Fig. 4A) and the laser sheath was advanced subsequently over the cone of the filter (Fig. 4B). Gentle traction was then applied to the snare and the laser sheath was activated. After serial activation of the laser sheath, the sheath was eventually able to collapse the filter away from the caval walls, and ultimately the filter was retrieved into and removed through the 18F sheath in its entirety. Venography was then performed of the IVC which was remarkable for complete thrombosis of the cava below the level of the filter with no evidence of complications to suggest IVC rupture or hemorrhage (Fig. 5).
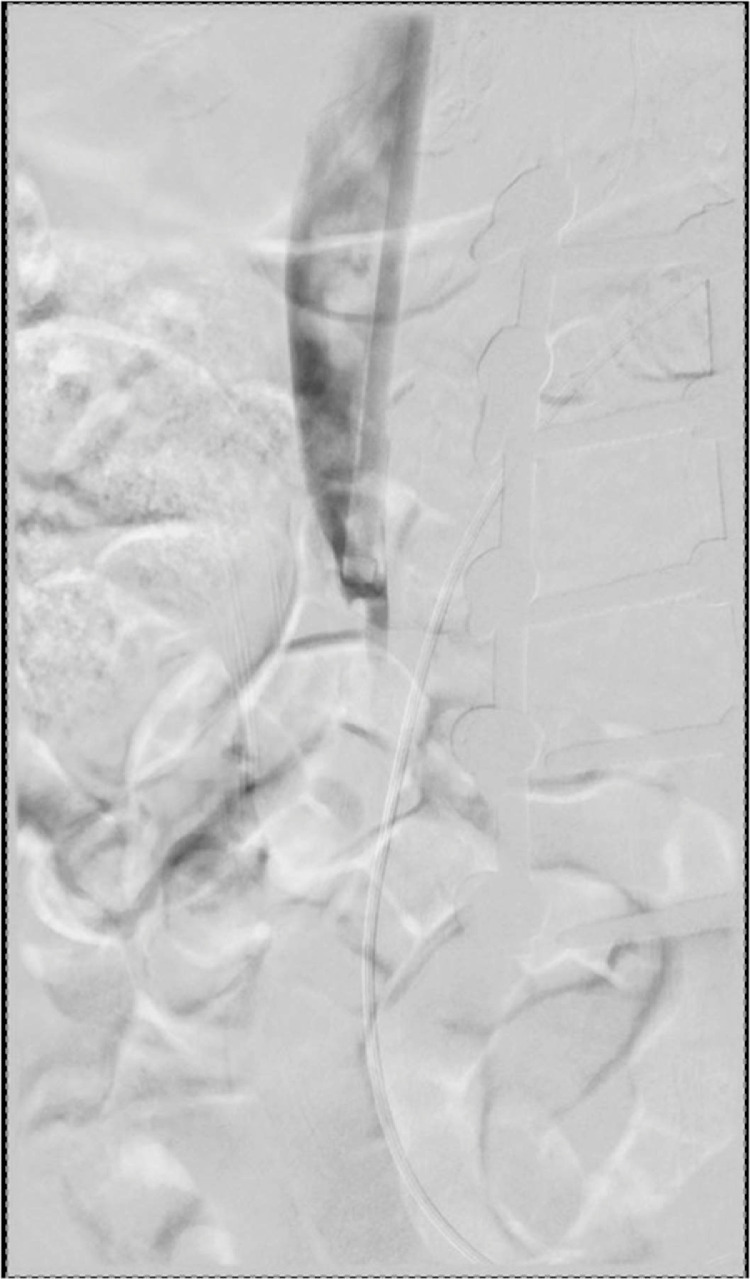
Fig. 3.
Prefilter removal DSA demonstrates occlusion of the infrarenal IVC below the level of the filter.
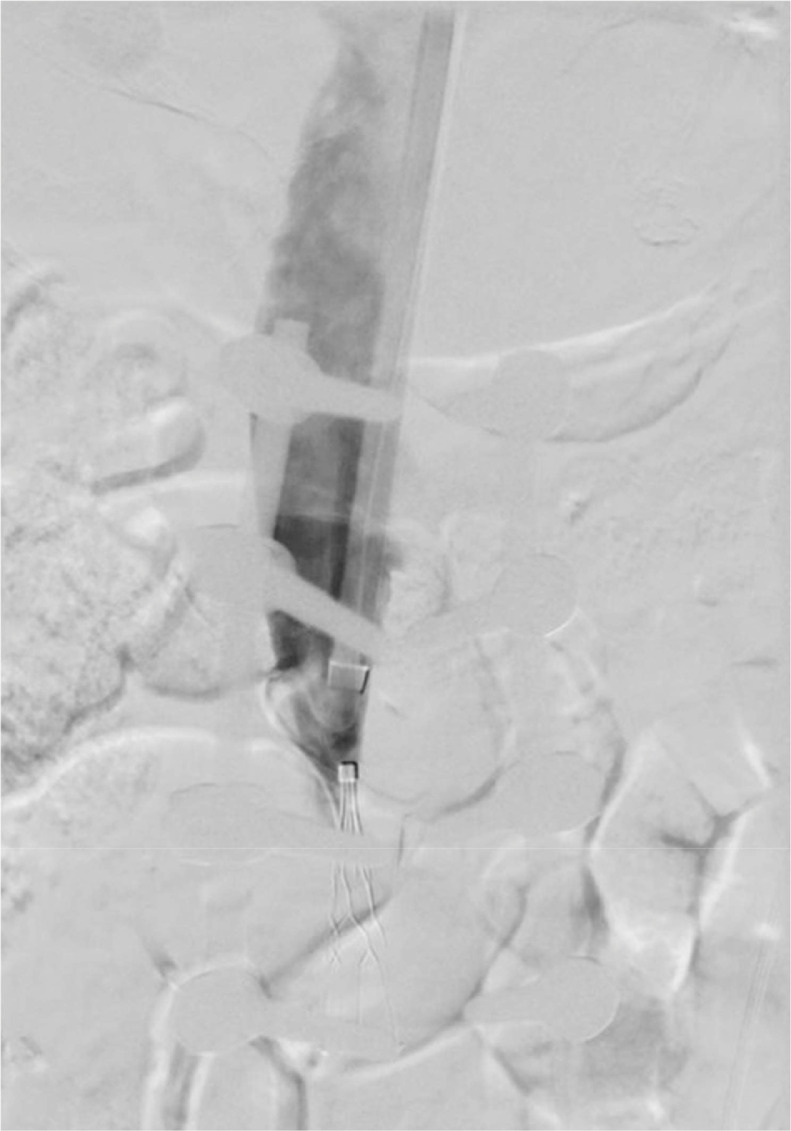
Fig. 4.
(A and B) Capturing of the Greenfield IVC filter with an end snare with subsequent capturing of the filter using a laser sheath and 18F sheath.
Fig. 5.
Postfilter removal DSA demonstrates occlusion of the infra renal IVC additionally no evidence of extra luminal contrast to suggest complications of complex removal of Greenfield IVC filter.
Attention was then turned toward DVT thrombectomy. The bilateral popliteal veins were accessed using ultrasound guidance and 5F micropuncture sets. A 16F Inari sheath (Inari Medical, Irvine, CA) was placed in the right popliteal vein and a 13F Inari sheath (Inari Medical, Irvine, CA) in the left popliteal vein. A 5F Kumpe catheter was then successfully advanced through the right lower extremity deep venous system into the IVC above the level of the chronic occlusion. An exchange length of 0.035 Amplatz wire was advanced from the right popliteal sheath and “flossed” out through the RIJV sheath creating a through/through wire access. Multiple attempts were made to cross the occluded left lower extremity deep venous system with the Kumpe catheter but were unsuccessful due to the apparent occlusion in the common femoral vein noted the day prior. Venography of the left lower extremity deep venous system was remarkable for a large greater saphenous vein seen draining freely into the left external iliac vein above the level of the chronic occlusion.
Mechanical thrombectomy of the IVC, right iliac system, and right lower extremity deep veins was then performed with an Inari ClotTriever device following balloon angioplasty during which a large amount of chronic thrombus was removed. Venography was then performed which revealed a patent right lower extremity deep venous system, right iliac venous system, and patent, though stenosed IVC.
Given the failed attempts at passing a wire across the distal left common femoral occlusion, the Kumpe catheter was advanced from the right jugular venous access site inferiorly into the IVC. Using the Kumpe catheter and Glidewire, the left common and external iliac veins were successfully crossed, and the catheter was advanced readily into the patent greater saphenous vein with ease. Follow-up venography revealed “inline” flow from the lower extremity via the greater saphenous vein into the iliac venous system and IVC.
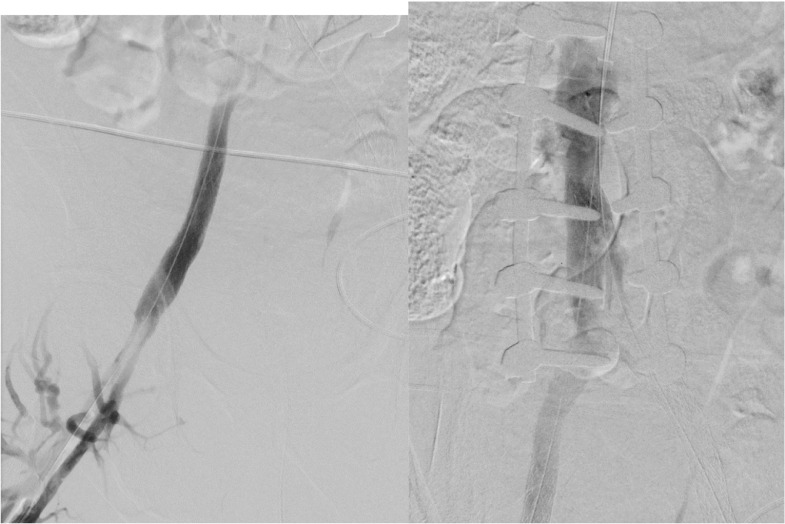
Multiple Abre self-expanding venous stents (Medtronic, Minneapolis, MN) were then placed from the infrarenal IVC distally into the bilateral external iliac veins in a paired “double barrel” fashion (Fig. 6). The left external iliac limb was extended into the left greater saphenous vein as this appeared to be the dominant left leg venous outflow vessel. The newly placed venous stents were then angioplastied to ensure good wall apposition. Post-stenting venography was then completed and revealed robust inline flow from each lower extremity (the left via the greater saphenous vein) (Figs. 7A, B and 8A, B). Given the results, the procedure was halted, and the patient was then extubated and admitted to the ICU for overnight monitoring. The next day, it was noted that his bilateral leg swelling had significantly improved, and he was discharged several days later to a skilled nursing facility for rehabilitation due to his chronic debilitated state on full anticoagulation and an antiplatelet regimen for his recently placed stents.
Fig. 6.
Following filter removal, the IVC and bilateral iliac venous systems were recanulated and stented followed by percutaneous transluminal angioplasty of the newly placed venous stents.
Fig. 7.
(A and B) Postintervention DSA from the right popliteal sheath demonstrated inline flow from the right femoral vein antegrade into the IVC.
Fig. 8.
(A and B) Postintervention DSA of the left greater saphenous vein demonstrates brisk inline flow from the left greater saphenous vein antegrade into the IVC.
The patient returned to outpatient IR clinic 1 month following his procedure and was noted to be ambulating for the first time in over 3 months with the assistance of a walker. A CTV at that time revealed patent bilateral ileocaval stents, with inline flow from both of his lower extremities, the left via his greater saphenous vein. On exam, his bilateral lower extremity swelling was markedly improved.
Follow-up
After the patient's 1 month follow-up, he continued aggressive physical therapy and returned home for his recovery. Interventional radiology followed the patient at the 3-month and 6-month intervals post-procedure. At both visits, the patient's strength continued to improve in addition to improvement in the physical appearance of his bilateral lower extremities. The patient remained on rivaroxaban and clopidogrel for 6 months postprocedure. At the 6-month mark, clopidogrel was discontinued and he continues rivaroxaban for anticoagulation. The patient now follows-up with the interventional radiology clinic on an as-needed basis.
Discussion
Primary management of venous thromboembolism (VTE) is anticoagulant therapy, assuming the patient can safely tolerate therapy [7]. Placement of an IVC filter is an alternative method to prevent PE in patients with absolute contraindications to anticoagulation, which include but are not limited to history of hemorrhagic stroke, major trauma, active hemorrhage, history of head injury within the prior 3 months, bleeding diathesis, and recent brain or spinal surgery [8,9]. In the case discussed, placement of an IVC filter was indicated due to patients' history of DVTs and high perioperative risk for development of DVT.
Since the introduction of IVC filters in 1967, many professional societies have published various guidelines for indications for placement of IVC filters. IVC filter placement is not routine, but current indications per the American College of Radiology (ACR) for placement in the setting of documented VTE include absolute contraindication to anticoagulant therapy, failure of anticoagulation therapy, recurrent PE despite adequate therapy, perioperatively when discontinuation of anticoagulation is necessary, free-floating iliofemoral or IVC thrombus, and severe cardiopulmonary disease [10].
Although generally regarded as a safe procedure, IVC filter placement is associated with some risk of intraoperative and postoperative complications. Intraoperative complications typically occur during vascular access, IVC filter deployment, or due to operator error. The most common vascular access complications are bleeding and thrombosis at the access site which occurs in 2%-35% of patients, with access site thrombosis more commonly occurring in patients with an underlying hypercoagulability [11]. Trauma may occur to adjacent arteries during access for placement of an IVC filter, which may result in arteriovenous fistula—a rare complication [12]. Complications associated with IVC filter deployment include filter tilt during placement, filter migration, and incomplete opening of the filter during placement [6]. Factors that contribute to filter migration include under-sizing of the filter for the size of the IVC and placement of central lines during or after filter placement. Complications that arise due to operator error include filter placement in nontarget veins, incomplete opening of the filter, and inappropriate orientation of the filter [6].
Postoperative complications are frequently discovered incidentally and usually identified greater than 30 days after placement [13]. IVC filter thrombosis, as discussed in this case, can occur in 2%-30% of patients, [6,11,14] and this variation in part may be due to differences in patient screening. In fact, symptomatic IVC thrombosis occurs in up to 13% of patients after 8 years of follow-up per the PRECIP (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) study [15]. Severity of filter thrombosis can vary from clinically insignificant clot isolated to the filter cone to extensive thrombosis of the filter leading to lower extremity and complete caval occlusion.[6] Hypercoagulability, intrinsic thrombogenicity of the filter, and migration of a large extremity thrombus all may lead to occlusion of the filter. Additionally, IVC filters intrinsically increase the risk of DVTs, which is dependent upon the design of the filter and duration of filter placement [13]. Fractures are also commonly observed in filters that have been in place for more than 1 year, [16] and fragments potentially may migrate centrally into the renal veins, cardiac, or pulmonary vasculature. Additionally, filter struts can perforate into the peri-caval space and adjacent structures such as the aorta, duodenum, and lumbar spine which may lead to pain or other complications related to strut location [6]. In general, most identified complications related to IVC filters are associated with those that have been in place for a duration longer than the manufacturer's recommended dwell time [17].
In 2010, the FDA issued a safety communication urging the removal of retrievable filters in patients who were no longer at risk for PE [18]. Additional indications for filter retrieval include those related to filter dwell time, the need for lifelong anticoagulation in a patient with an indwelling filter, or when the original indication for filter placement is no longer clinically relevant, [3,6,19]. Traditional methods for retrieval include the use of loop snares to capture the cone of the filter with subsequent re-sheathing and removal of the filter. Advanced techniques are used when traditional techniques have failed and they include removal using endobronchial forceps, balloon displacement techniques, realignment of the filter with an angle-guided catheter, the “sling” technique using a curved wire, and stiff wire displacement [20,3] Recently, laser sheath assisted photoablation has also been described for removal of filters. Success rates of laser sheath-assisted retrieval of embedded IVC filters range from 95% to 100% with a 4% risk of major device-related complications, which suggests the effectiveness and safety of this retrieval method [21,22]. Additionally, preprocedural catheter-directed administration of tissue plasminogen activator (tPA) and recanalization of the IVC may also facilitate successful retrievals [3]. Although permanent IVC filters are not often removed, temporary filters should ideally be retrieved within 29-54 days after placement or per the device manufacturer's indications for use [13,23]. The most cited reason for failure to successfully retrieve IVC filters is due to excessive filter tilt, which is defined as an angle greater than 15 degrees relative to the vertical axis of the IVC [24]. Additionally, the presence of a thrombus within the filter and adherence of the filter to the caval wall may also prevent successful retrieval [3,13,25].
Complications associated with IVC filter retrieval include filter fracture, filter migration, and IVC injury including caval intussusception, dissection, or hemorrhage [13]. Tamrazi et al. [3] reported postretrieval complications of permanent IVC filters in 2 of 12 total patients, including caval stenosis and a large groin hematoma. Morrow et al. [17] demonstrated no significant postretrieval complications including IVC rupture or death in 294 patients at their institution whose filters were removed 30 days following placement. The lack of common complications from retrieval of temporary filters supports the recommendations to remove these devices, including in patients who have had filters with longer dwell times [17,26]. Further research to investigate techniques and complications associated with the retrieval of permanent IVC filters may guide clinical decision-making and help improve patient outcomes when considering the removal of permanent filters.
Following the release of FDA safety recommendations for filter retrieval in 2010, the utilization of IVC filters has decreased; nevertheless, retrieval rates continue to remain low at 22% [27]. To improve retrieval rates, healthcare systems may consider implementation of a combination of patient and provider education along with systems such as filter recipient tracking, automated scheduling, multidisciplinary pulmonary embolism response teams, and the use of convertible or bioconvertible filters [17,28,29]. As an alternative to filter placement, the use of a temporary central venous catheter with a deployable IVC filter has also been proven to lower the risk of fatal PE in critically ill patients with a contraindication to anticoagulants [30].
The likelihood of unsuccessful filter retrieval rises with filter dwell time exceeding 375 days, filter tilt, fibrosis, perforation of the caval wall, hypercoagulable states, significant clot burden leading to IVC occlusion, and placement of a permanent filter [13,17]. Morrow et al. observed an improvement in filter retrieval success rates in individual physicians over time, suggesting a learning curve linked to the retrieval procedure. In cases where IVC filters cannot be retrieved due to chronic caval occlusion and fibrosis, stent placement through the IVC filter to exclude filter material from the main flow channel may be considered to alleviate the patients’ symptoms [3].
Conclusion
Despite classic literature suggesting that permanent Greenfield IVC filters cannot be removed, we present a case in which advanced techniques were successfully used to remove an indwelling permanent infrarenal Greenfield IVC filter and reconstruct the IVC and iliac veins. As presented, multiple attempts are often necessary to remove indwelling IVC filters, and IVC and iliac vein reconstruction are often lifesaving in patients who present with symptoms of phlegmasia cerulea dolens.
Disclaimer
None
Patient consent
Written, informed consent was obtained from the patient for publication prior to submission of this manuscript.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ghatan C, Ryu R. Permanent versus retrievable inferior vena cava filters: rethinking the “one-filter-for-all” approach to mechanical thromboembolic prophylaxis. Semin Intervent Radiol. 2016;33(02):075–078. doi: 10.1055/s-0036-1582123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBride JJ, Duncan AA, Shields R, Gfeller B, Stanson AW. Percutaneous blunt dissection technique for retrieval of over-the-wire Greenfield filters. J Vasc Interv Radiol. 2010;21(1):144–147. doi: 10.1016/j.jvir.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Tamrazi A, Wadhwa V, Holly B, et al. Percutaneous retrieval of permanent inferior Vena cava filters. Cardiovasc Intervent Radiol. 2016;39(4):538–546. doi: 10.1007/s00270-015-1214-0. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Bold M, Rajebi MR. Endovascular retrieval of Greenfield IVC filters 13 and 19 years post placement without major complication. J Radiol Case Rep. 2017;11(6) doi: 10.3941/jrcr.v11i6.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grewal S, Lewandowski RJ, Ryu RKW, Desai KR. Inferior Vena Cava filter retrieval: patient selection, procedural planning, and postprocedural complications. Am J Roentgenol. 2020;215(4):790–794. doi: 10.2214/AJR.19.22387. [DOI] [PubMed] [Google Scholar]
- 6.Grewal S, Chamarthy MR, Kalva SP. Complications of inferior vena cava filters. Cardiovasc Diagn Ther. 2016;6(6):632–641. doi: 10.21037/cdt.2016.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartholomew JR. Update on the management of venous thromboembolism. Clev Clin J Med. 2017;84(12 Suppl 3):39–46. doi: 10.3949/ccjm.84.s3.04. [DOI] [PubMed] [Google Scholar]
- 8.Becattini C, Agnelli G. Acute treatment of venous thromboembolism. Blood. 2020;135(5):305–316. doi: 10.1182/blood.2019001881. [DOI] [PubMed] [Google Scholar]
- 9.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 10.DeYoung E, Minocha J. Inferior Vena Cava filters: guidelines, best practice, and expanding indications. Semin Intervent Radiol. 2016;33(02):065–070. doi: 10.1055/s-0036-1581088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MJ, Blair KS, Curry TK, Singh N. Vena cava filters: current concepts and controversies for the surgeon. Curr Probl Surg. 2010;47(7):524–618. doi: 10.1067/j.cpsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Hann CL, Streiff MB. The role of vena caval filters in the management of venous thromboembolism. Blood Rev. 2005;19(4):179–202. doi: 10.1016/j.blre.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Angel LF, Tapson V, Galgon RE, Restrepo MI, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol. 2011;22(11):1522–1530. doi: 10.1016/j.jvir.2011.08.024. e3. [DOI] [PubMed] [Google Scholar]
- 14.Milovanovic L, Kennedy S, Midia M. Procedural and indwelling complications with inferior Vena Cava filters: frequency, etiology, and management. Semin Intervent Radiol. 2015;32(01):034–041. doi: 10.1055/s-0034-1396962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PRECIP Study Group Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism. Circulation. 2005;112(3):416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 16.Kalva SP, Wicky S, Waltman AC, Athanasoulis CA. TrapEase Vena Cava filter: experience in 751 patients. J Endovascr Ther. 2006;13(3):365–372. doi: 10.1583/05-1741.1. [DOI] [PubMed] [Google Scholar]
- 17.Morrow KL, Bena J, Lyden SP, Parodi E, Smolock CJ. Factors predicting failure of retrieval of inferior vena cava filters. J Vasc Surg Venous Lymphat Disord. 2020;8(1):44–52. doi: 10.1016/j.jvsv.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. Inferior vena cava (IVC) filters: initial communication: risk of adverse events with long-term use. 2010. https://wayback.archive-it.org/7993/20161022180008/http:/www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676.htm Accessed October 2, 2023.
- 19.McLoney ED, Krishnasamy VP, Castle JC, Yang X, Guy G. Complications of Celect, Günther Tulip, and Greenfield inferior Vena Cava filters on CT follow-up: A single-institution experience. J Vasc Interv Radiol. 2013;24(11):1723–1729. doi: 10.1016/j.jvir.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hakim R, ST Kee, Olinger K, Lee EW, Moriarty JM, McWilliams JP. Inferior Vena Cava filter retrieval: effectiveness and complications of routine and advanced techniques. J Vasc Interv Radiol. 2014;25(6):933–939. doi: 10.1016/j.jvir.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Desai KR, Kaufman J, Truong P, Lindquist J, Ahmed O, Flanagan S, et al. Safety and success rates of excimer laser sheath–Assisted retrieval of embedded inferior vena cava filters. JAMA Netw Open. 2022;5(12) doi: 10.1001/jamanetworkopen.2022.48159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhussaini A, Alahmad MA, Alomaim MM, Alzahrani MY, Alghamdi AS, Arabi M. Effectiveness and safety of laser-assisted removal of inferior Vena Cava (IVC) filters in a single tertiary care center. Cureus. 2022;14(12):e32809. doi: 10.7759/cureus.32809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales JP, Li X, Irony TZ, Ibrahim NG, Moynahan M, Cavanaugh KJ. Decision analysis of retrievable inferior vena cava filters in patients without pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2013;1(4):376–384. doi: 10.1016/j.jvsv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Kinney TB. Update on inferior Vena Cava filters. J Vasc Interv Radiol. 2003;14(4):425–440. doi: 10.1097/01.RVI.0000064860.87207.77. [DOI] [PubMed] [Google Scholar]
- 25.Andreoli JM, Thornburg BG, Hickey RM. Inferior Vena cava filter-related thrombus/deep vein thrombosis: data and management. Semin Intervent Radiol. 2016;33(2):101–104. doi: 10.1055/s-0036-1581087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Haddadin I, McLennan G, et al. Inferior vena cava filter – comprehensive overview of current indications, techniques, complications and retrieval rates. VASA. 2020;49(6):449–462. doi: 10.1024/0301-1526/a000887. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed O, Wadhwa V, Patel K, Patel MV., Turba UC, Arslan B. Rising retrieval rates of inferior Vena Cava filters in the United States: insights from the 2012 to 2016 summary Medicare Claims data. J Am Coll Radiol. 2018;15(11):1553–1557. doi: 10.1016/j.jacr.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Goei A, Josephs S, Kinney T, Ray C, Sacks D. Improving the tracking and removal of retrievable inferior Vena Cava filters. Semin Intervent Radiol. 2011;28(01):118–127. doi: 10.1055/s-0031-1273946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutphin PD, Reis SP, McKune A, Ravanzo M, Kalva SP, Pillai AK. Improving inferior Vena cava filter retrieval rates with the define, measure, analyze, improve, control methodology. J Vasc Interv Radiol. 2015;26(4):491–498. doi: 10.1016/j.jvir.2014.11.030. e1. [DOI] [PubMed] [Google Scholar]
- 30.Tapson VF, Hazelton JP, Myers J, Robertson C, Gilani R, Dunn J, et al. Evaluation of a device combining an inferior Vena Cava filter and a Central venous catheter for preventing pulmonary embolism among critically ill trauma patients. J Vasc Interv Radiol. 2017;28(9):1248–1254. doi: 10.1016/j.jvir.2017.05.001. [DOI] [PubMed] [Google Scholar]