Abstract
Background
Cancer is still among the leading causes of death all over the world. Improving chemotherapy and minimizing associated toxicities are major unmet medical needs. Recently, we provided a preliminary preclinical evaluation of a human ferritin (HFt)-based drug carrier (The-0504) that selectively delivers the wide-spectrum topoisomerase I inhibitor Genz-644282 to CD71-expressing tumors. The-0504 has so far been evaluated on four different human tumor xenotransplant models (breast, colorectal, pancreatic and liver cancers).
Methods
Herein, we extend our studies, by: (a) testing DNA damage in vitro, (b) treating eight additional tumor xenograft models in vivo with The-0504; (c) performing pharmacokinetic (PK) studies in rats; and (d) evaluating The-0504 anti-tumor xenotransplant efficacy by optimizing its administration schedule based on PK considerations.
Results
Immunofluorescence demonstrated that The-0504 induces foci expressing the DNA double-strand break marker γH2AX. Expression increases up to 4-fold and is more persistent as compared to free Genz-644282. In vivo studies confirmed a remarkable anti-tumor activity of The-0504, resulting in tumor eradication in most murine xenograft models, regardless of embryological origin (e.g. epithelial, mesenchymal or neuroendocrine), and molecular subtypes. PK studies demonstrated a long persistence of The-0504 in rat serum (half-life of about 40 h as compared to 15 h of the free drug), with a 400-fold increase in peak concentrations as compared to the free drug. On this basis, we reduced The-0504 administration frequency from twice to once per week, with no appreciable loss in therapeutic efficacy in mice.
Conclusion
The results presented here confirm that The-0504 is highly active against several human tumor xenotransplants, even when administered less frequently than previously reported. The-0504 may be a good candidate for further clinical development in a tumor histotype-agnostic setting.
Keywords: Targeted therapy, Solid cancer, Human ferritin, Transferrin receptor (CD71), Genz-644282
1. Introduction
The International Agency for Research on Cancer (IARC) expects that solid tumors will be responsible for 21.7 and 13 million incidences and deaths, respectively, in 2030 worldwide. Whereas surgery may be curative for localized cancer, locally advanced and, more so, metastatic cancers require systemic treatment and/or radiotherapy. Unfortunately, pharmacological resistance (primary and acquired) to both targeted and chemotherapeutic agents limits treatment efficacy, and is the most frequent cause of relapse/progression and cancer-associated deaths [1].
Although we live in an era of precision/personalized oncology, chemotherapy still plays a prominent role. It is widely used in many clinical settings both in indication and as per oncologist's choice, particularly but not exclusively in advanced cancer. Chemotherapy is needed to halt rapidly progressing tumors and to complement/consolidate the results of targeted agents. Unfortunately, toxicity is indeed a major drawback of all anticancer regimens, including chemotherapy, e.g. most patients will experience prominent effects on the gastrointestinal tract, heart, bone marrow, lungs, kidneys etc. Organ failure is a frequent cause of cancer-related deaths [2].
To alleviate toxicity but still take advantage of the wide spectrum of action of cytotoxic agents, other non-genome-informed therapies have been introduced. Two examples are antibody-drug conjugates (ADCs) and therapeutic nanoparticles, typically liposomes and protein-based (e.g. albumin) formulations such as nab-paclitaxel. In these ‘compound’ agents, biologicals and cytotoxic agents work concertedly. The latter are specifically delivered to cancer cells as payloads and are often developed ad hoc not to overlap with the mechanism of action and toxicity of generic chemotherapy.
In summary, all the above treatments have advantages and limitations. They must be considered complementary, and their combined use is foreseen for many years to come. However, despite a rich arsenal of anticancer agents, several medical needs remain unmet.
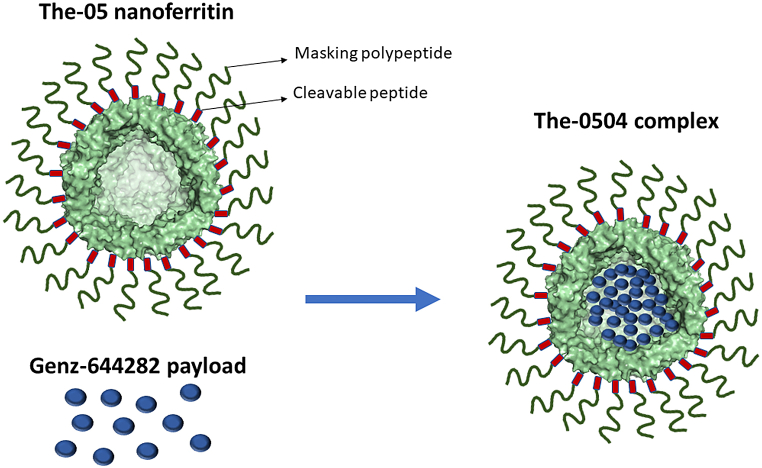
In recent years, we have demonstrated that the ability of the human H-type ferritin (HFt) to bind to and being internalized through the transferrin receptor 1 (CD71 or TfR1) could be exploited to load different chemotherapeutic drugs into the ferritin cavity and deliver them to the cancer cells that often overexpress CD71 [[3], [4], [5], [6], [7], [8], [9], [10], [11]] [[3], [4], [5], [6], [7], [8], [9], [10], [11]] [[3], [4], [5], [6], [7], [8], [9], [10], [11]]. Herein, we propose a first-in-class nanoparticle named The-0504 that incorporates in a single formulation many advantages of each of the above classes of cancer therapeutics, and at the same time has been carefully designed to minimize hurdles. The-0504 is based on the recombinant homopolymeric (n = 24) HFt protein that by spontaneous self-assembly entraps large molar amounts of a selected, cytotoxic drug named Genz-644282 (Scheme 1). Genz-644282, a topoisomerase I inhibitor, had previously showed an appreciable killing efficacy (i.e., IC90 in the submicromolar range) on different cancer cell lines, including camptothecin resistant cell lines [12,13]. Genz-644282 has already done a clinical trial dose-escalation in humans (NCT00942799). Therefore, like chemotherapy, the cytotoxic agent of The-0504 has a wide spectrum of activity against many human tumors. As opposed to standard chemotherapy, The-0504 can spare most of the normal cells because it is a conditionally activated masked drug, unmasked by tumor-associated proteases, thereby promoting the engagement of the target mainly in the tumor milieu. Specifically, The-0504 incorporates a safe-lock mechanism (Scheme 1) that conditionally releases the cytotoxic only following proteolytic cleavage by tumor-restricted metalloproteases (MMP-2/9), favoring local concentration of the therapeutic effect [5,11].
Scheme 1.
Schematic representation of The-0504. The-05 (the ferritin nanocage) contains N-terminal PASE repeats (from the single-letter amino acid codes) and proteolytic cleavage sites (both indicated). The payload is entrapped within the nanoferritin hollow cavity that in nature carries iron. The picture has been generated with PyMol and GNU Image Manipulation Program.
A preliminary evaluation of The-0504 in terms of safety and efficacy outlined the strong anti-tumor activity (i.e. delay in tumor growth or durable complete remissions) as well as manageable toxicities of the nanoferritin compound [5,11]. Herein, we extend our in vivo studies by demonstrating therapeutic efficacy on eight additional xenograft mouse models, which candidates The-0504 for tumor-agnostic applications. In addition, we show that the unique DNA-damaging properties and favorable pharmacokinetics of The-0504 engender comparable therapeutic effects on lower dosages. Therefore, The-0504 may complement naked cytotoxic drugs, ADCs and other nanoparticle-based [[14], [15], [16], [17]] aiming at milder, on-target, low-side-effects chemotherapeutic applications.
2. Results and discussion
2.1. The-0504 production
The production and characterization of the The-0504 protein-drug complex has been thoroughly described [5]. Briefly, The-0504 is based on a modified, recombinant human ferritin molecule, named The-05, that instead of iron contains about 70–80 Genz-644282 molecules entrapped in its hollow cavity (Scheme 1). As reported in Refs. [5,11], The-0504 was found to be highly pure and monodispersed in solution with a zeta-potential value of −4.5 ± 0.9 mV. The mean diameter was about 19.0 nm as assessed by dynamic light scattering (17.0 ± 0.7 nm) and transmission electron microscopy (19.7 ± 1.5 nm). Stability data using size-exclusion liquid chromatography assays did not disclose compound aggregation or drug loss following The-0504 formulation as lyophilized powder, storage at 2–8 °C for 12 months, and reconstitution in water (Table 1) [5].
Table 1.
Free-drug content in lyophilized The-0504 after storage at 2–8 °C for 12 months.
| Time (months) | Free Genz-644282 (%) | Purity of The-0504 by size-exclusion chromatography (%) |
|---|---|---|
| 0 | 4.7 | 98.3 |
| 1 | 4.4 | 98.5 |
| 3 | 4.1 | 98.4 |
| 6 | 5.3 | 97.7 |
| 9 | 4.4 | 98.5 |
| 12 | 4.3 | 98.6 |
2.2. Generation and persistence of γH2AX foci in MiaPaca2 cells in response to Genz-644282 and The-0504
We have previously demonstrated that adding the “masking” PASE polypeptide at the N-terminus of ferritin reduces its binding to the human CD71 receptor by sharply increasing the dissociation constant (Kd) from 10 nM to 120 nM for native HFt and The-05 based compounds, respectively [3]. Of special applicative interest, binding is restored to the original Kd value of 10 nM after in vitro incubation of the purified proteins with site-specific metalloproteases (MMP 2/9) cleaving the proteolytic site immediately downstream of PASE. Thus, reduction in CD71 binding was made transient, reversible, and conditional. In addition, we demonstrated that PASE-masked HFt compounds massively accumulate in the nucleus 3 h after treatment of cell lines at 37 °C [3]. In analogy with previous descriptions [18,19], it then appears that HFt-drug complexes bind CD71 at the cell surface, and are internalized in the endosomal compartment, where a mildly acidic pH (pH 5.5) favours some drug release/translocation in the cytoplasm, diffusion to the nucleus and initial DNA damage [3,20]. This mechanism can further trigger nuclear translocation of HFt-based molecules, due to the well documented DNA-protective activity of HFt itself. Therefore, HFt-based molecules are likely to act as “Trojan horses” delivering high amounts of the drug right to the site of action of the drug itself (cell nucleus).
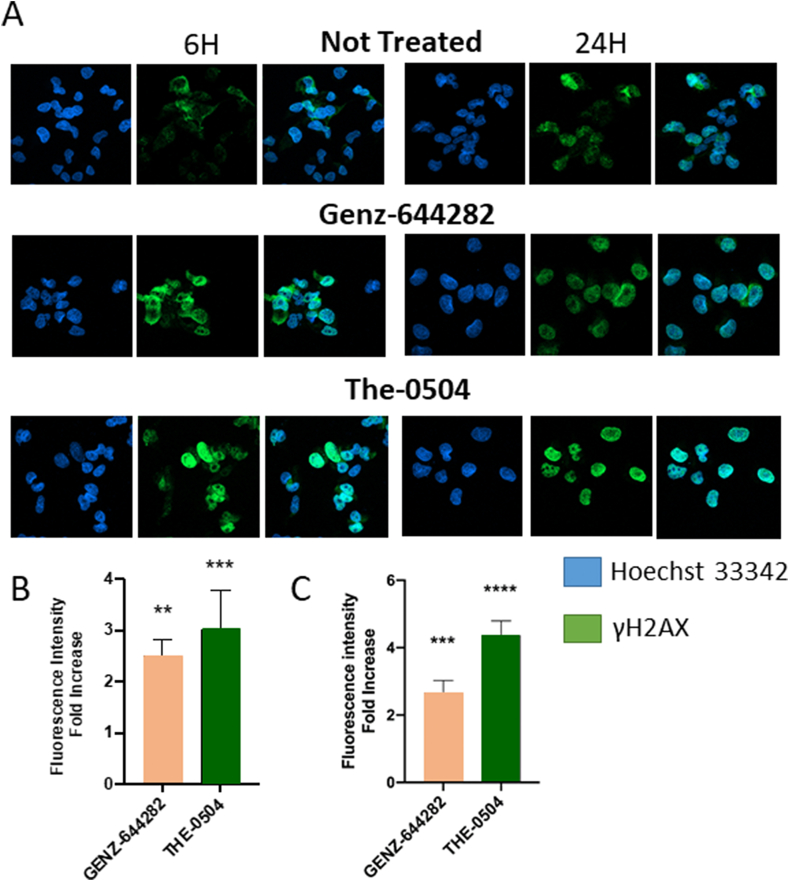
To expand on the mechanism of action of The-0504, we further investigated DNA damage. It was hypothesized that the Topo 1 inhibitor Genz-644282, delivered as The-0504 complex into the nucleus, might induce DNA double-strand breaks (DSBs). DSBs are lethal DNA injuries that can be caused by cytotoxic chemical agents as well as environmental and physical damage. They were assessed by staining cells with an antibody to histone H2AX (S139 phosphorylation), that is a specific DSBs marker. Human pancreatic cancer MiaPaca2 cells were treated with 1 μM of Genz-644282 or The-0504. Immunofluorescence staining revealed clear γH2AX foci after 6 h of treatment with both compounds (Fig. 1A), but H2AX phosphorylation was more evident with The-0504 than free Genz-644282 (Fig. 1B). This reflects relative cytotoxicity of The-0504 and Genz-644282 in this specific cell line [5]. Drugs were washed out after 6 h, but this did not affect DSBs at 24 h, e.g. DNA damage response persists after drug removal. Free Genz-644282 results are in full agreement with previous observation with the naked drug [21]. Notably, DSBs are more persistent upon treatment with HFt-encapsulated Genz-644282 than with the free drug (Fig. 1C). These results altogether are fully consistent with the elective ability of H-ferritins to route and deliver drugs to the nuclear compartment of the cell [3,18,19].
Fig. 1.
Phosphorylation of H2AX in MiaPaca2 cells treated with either free Genz-644282 or the fully encapsulated The-0504 formulation. Treatment was for 6 h (1 μM in Genz-644282 for both the free and encapsulated drug). γH2AX staining: 6h (end of treatment) and 24 h. (A) Immunofluorescence microscopy images of γH2AX foci of MiaPaca2 cells mock-treated vs Genz-644282 vs The-0504 at the indicated times. γH2AX staining: green, nuclei counterstained in blue. (B) and (C) Same results expressed as fold-increase relative to control at 6 and 24 h, respectively. Fluorescence outside the nucleus was considered background and subtracted.
2.3. The-0504 efficacy in vivo: pancreas, breast, lung and sarcoma xenotransplants
In previous studies, The-0504 was evaluated on different human tumor xenotransplant models (triple-negative breast cancer, liver hepatocarcinoma, colorectal and pancreatic cancers) [5,11]. In this paper, we expand our testing on 8 additional in vivo tumor models.
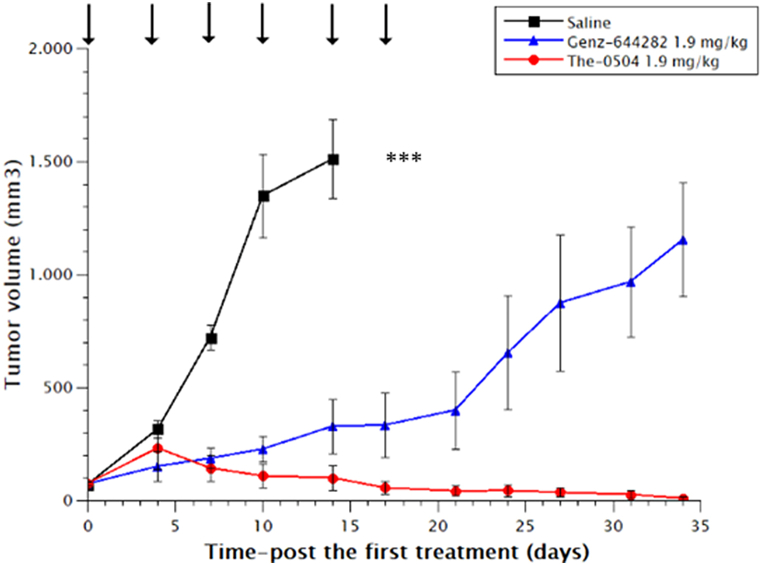
The first model was a subcutaneous tumor xenograft of HT-1080 human fibrosarcoma. The-0504 was administered intravenously at the dose of 1.9 mg/kg (adjusted on the dosage of the active principle Genz-644282), twice a week for three consecutive weeks, and was compared with equimolar administrations of free Genz-644282. These are the same doses and regimens previously used to treat other tumor types. Like in these previous studies, the anticancer activity of The-0504 was very high, leading to complete regression of all HT-1080 tumors, with 100 % values of both Tumor Growth Inhibition (TGI) and Objective Response Rate (ORR) (Fig. 2A and Table 2). Free Genz-644282 also showed antitumor activity, but much lower, failing to achieve complete regression (TGI value of 20.0 % and 0 % ORR; Fig. 2A and Table 2).
Fig. 2.
Anti-tumor activity of The-0504 vs free Genz-644282 on HT-1080 (fibrosarcoma) mouse xenografts. Statistical differences (Student's t-test) are as follows. The-0504 1.9 mg/kg vs control: ***p < 0.0001. Genz-644282 vs control *p < 0.05. Genz-644282 vs The-0504 *p < 0.05. Arrows indicate individual administrations.
Table 2.
Summary of The-0504 and Genz-644282 efficacies in tumor-bearing mice models of several human cell lines using different administration schedules.
| TUMOR MODEL DOSE (mg/kg) |
TGI (%) ORR (%) |
RESPONSE (%) |
||
|---|---|---|---|---|
| Genz-644282 | The-0504 | Genz-644282 | The-0504 | |
|
Pancreatic (PaCa44) 1.9 mg/kg [11] |
64.2 16.7 |
100 100 |
33.3 SD 66.7 PD |
100 CR |
|
Pancreatic (HPAF II) 1.9 mg/kg [5] |
33.3 0 |
93.3 83.3 |
33.3 SD 66.7 PD |
16.7 CR 50.0 PR 16.7 SD |
| Pancreatic (PDX)a [11] | 0 0 |
93.6 83.3 |
100 PD | 50 CR 33.3 PR 16.7 SD |
|
Colorectal (HT29) 1.9 mg/kg [11] |
na 16.6 |
na 100 |
33.3 SD 66.7 PD |
66.7 CR 33.3 PR |
|
Breast (MDA-MB 231) 1.9 mg/kg [11] |
70.1 0 |
100 100 |
16.7 SD 83.3 PD |
100 CR |
|
Liver (HepG2) 1.9 mg/kg [11] |
55.5 25.0 |
100 100 |
25.0 PR 75.0 PD |
100 CR |
|
Fibrosarcoma (HT-1080) 1.9 mg/kg |
20.0 0 |
100 100 |
100 PD | 100 CR |
|
Breast (MDA-MB 157) 2.0 mg/kg |
na | 92.2 66.7 |
na | 33.3 CR 33.3 PR 33.3 SD |
|
Breast (MDA-MB 468) 2.0 mg/kg |
na | 92.2 66.7 |
na | 33.3 CR 33.3 PR 33.3 SD |
|
Lung (H1339) 2.0 mg/kg |
na | 96.5 66.7 |
na | 16.7 CR 50.0 PR 16.7 SD 16.7 PD |
|
Lung (A549) 2.0 mg/kg |
na | 89.0 33.3 |
na | 33.3 PR 50.0 SD 16.7 PD |
|
Pancreatic (MiaPaca2) 2.0 mg/kg |
na | 100 100 |
na | 66.7 CR 33.3 PR |
|
Pancreatic (PaCa44)b 3.0 mg/kg |
na | 100 100 |
na | 100 CR |
|
Pancreatic (PaCa44)b 6.0 mg/kg |
na | 100 100 |
na | 100 CR |
|
Pancreatic (MiaPaca2)b 1.5 mg/kg |
na | 92.2 66.6 |
na | 16.6 CR 50.0 PR 33.3 SD |
Regimen = BIWx3.
Nab-paclitaxel at 10 mg/kg instead of Genz-644282 was used in Patient-Derived Xenograft (PDX) model.
Regimen = Q7dx3.
Combined with the summary in Table 2 and previously published observations of ours [11], these results demonstrate remarkably similar The-0504 efficacy on mesenchymal, treatment-refractory fibrosarcoma and aggressive epithelial xenografts, despite these are usually treated by different drugs in the clinical setting. It is also remarkable that in both cases antitumor responses are complete only with The-0504 but not free Genz-644282, clearly showing the considerable added value of delivering wide-spectrum chemotherapy through a CD71-targeted approach.
Inferiority of free Genz-644282 prompted us to apply the 3R principles of animal ethics. Therefore, no free Genz-644282 treatment groups were planned for the following seven experiments on breast, lung and other pancreatic cancers, that were carried out with The-0504 only.
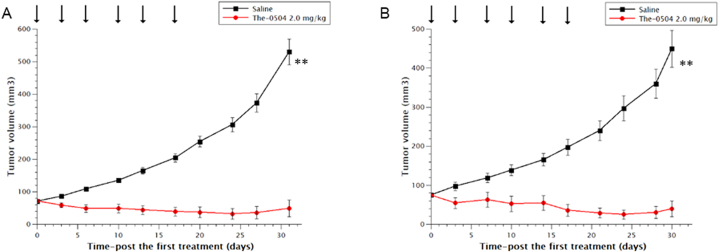
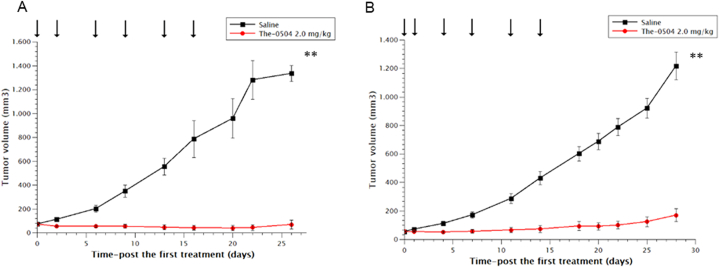
Triple-negative breast cancer is usually more aggressive, harder to treat, and more likely to recur than other breast cancers (hormone receptor-positive or HER2-positive). Two tumor xenografts models were assessed, with similar results. The-0504 displayed a clear antitumor activity on both MDA-MB 468 and MDA-MB 157, complete response (CR), partial response (PR) and stable disease (SD) being equally distributed among the six treated animals (Fig. 3). Overall, the Tumor Growth Inhibition and Overall Response Rate values for The-0504 in both models were 92.2% and 66.7%, respectively.
Fig. 3.
Anti-tumor activity of The-0504 on human breast cancer xenografts. (A) MDA-MB 468; (B) MDA-MB 157. Control vs The-0504 2.0 mg/kg **p < 0.005 (Student's t-test). Arrows: administrations.
The-0504 anticancer activity was also explored in two different histotypes of lung cancer: a small-cell lung cancer cel line (H1339) and a non-small cell lung cancer (A549), the latter of neuroendocrine origin [22]. Anticancer activity was observed, although at lower levels than in previous cancer models evaluated (Fig. 4). This is particularly true for the A549 model, where the Tumor Growth Inhibition and Overall Response Rate values for The-0504 were 89.0 % and 33.3 %. In contrast, the Tumor Growth Inhibition and Overall Response Rate values for The-0504 in the H1339 model were 96.5 % and 66.7 %, respectively. The slightly lower response in the A549 model cannot be ascribed to low expression of the CD71 receptor, as a substantial high expression in this model was previously reported [23]. Primary resistance is more likely and needs further evaluation.
Fig. 4.
Anti-tumor activity of The-0504 on human lung cancer xenografts. (A) H1339; (B) A549. Control vs The-0504 2.0 mg/kg **p < 0.005 (Student's t-test). Arrows: administrations.
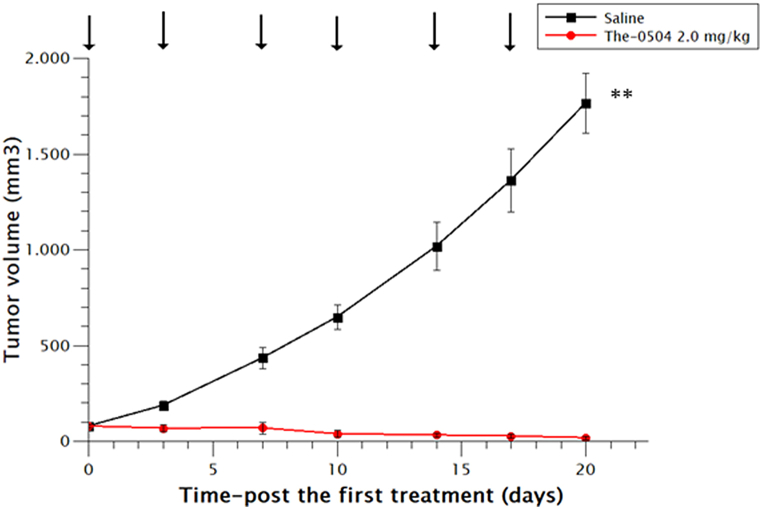
Finally, we tested The-0504 also on the pancreatic MiaPaca2 tumor cells, using the same regimen of 2.0 mg/kg bi-weekly used in the earlier panel of experiments. Like in previous studies on different pancreatic cancer models, e.g. PaCa44 and HPAF II ([5,11], the anticancer activity of The-0504 was very high, leading to a 100 % values of both Tumor Growth Inhibition (TGI) and Objective Response Rate (ORR), confirming that pancreatic cancer is a highly responsive model to The-0504 compound (Fig. 5 and Table 2).
Fig. 5.
Anti-tumor activity of The-0504 on MiaPaca2 pancreas cancer xenografts. Control vs The-0504 2.0 mg/kg **p < 0.005 (Student's t-test). Arrows: administrations.
2.4. Pharmacokinetics in rats
In all the in vivo studies, The-0504 was administered two times a week for three weeks. The rationale for this frequency was essentially dictated by the fast growth kinetics of tumors in murine models. In light of a future use in humans, dosages and schedules will likely require optimization. As a first step to obtain crucial information, we resorted to pharmacokinetic experiments in Wistar Hannover rats and a non-rodent model (cynomolgus monkey). Herein, we report for the first time on single intravenous The-0504 administration in the rat model. As describe above, The-0504 is a complex based on The-05, a modified ferritin which acts as carrier and includes, as payload, the cytotoxic compound Genz-644282. Two bioanalytical techniques were employed to follow the kinetic of the carrier, i.e., an ELISA protocol, using the carrier protein The-05 as standard and a polyclonal anti-The-05 antibody for detection, and an LC/MS method, using the Genz-644282 as reference item.
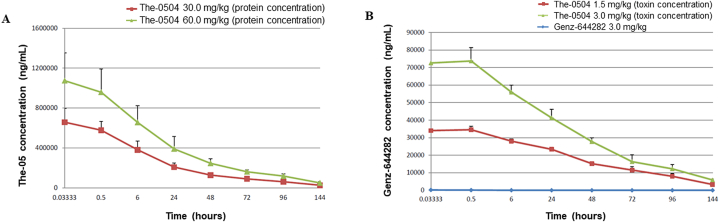
Healthy rats injected with free Genz-644282 at 3.0 mg/kg showed hunched posture, slight to moderate piloerection and moderately decreased activity during the last days of the study. In sharp contrast, no clinical signs were recorded in animals treated with The-0504 at two different doses, 1.5 mg/kg and 3.0 mg/kg (Genz-644282 concentration), corresponding to about 30 mg/kg and 60 mg/kg as The-05 protein concentration, respectively. The dose of 3.0 mg/kg is the maximal concentration of The-0504 that we have previously shown to be safe in rat using multiple administrations [11]. Body weights were within the expected range for this strain and age of animals (not shown). Test item was not detected in rat plasma before the intravenous administration. Pharmacokinetics are illustrated by Fig. 6, that demonstrates at glance the much higher peak levels and persistence of The-0504 compared to free Genz-644282 (Fig. 6B). The mean (N = 6) pharmacokinetic parameters determined from the mean concentration for the analytes are summarised in Table 3. The concentrations of the two analytes, i.e., the payload (Genz-644282) and the carrier protein (The-05), were evaluated in all animals treated with the test item The-0504 and were detected up to the last sampling time point (144 h from administration). Since the values detected were still quite high at the end of the study, half-lives (T1/2) could only be estimated. Mean Cmax values increased with increasing dose, in a dose proportional manner from 657,833 to 1,073,430 ng/mL (Fig. 6A), and from 34,503 to 73,713 ng/mL (Fig. 6B), respectively for the analyte The-05 and Genz-644282 (Table 3). The exposure values showed a good dose correlation since the AUC(0-inf) ranged from 20,316,400 to 37,290,300 ng/mL*h, and from 2,124,120 to 3,812,180 ng/mL*h, respectively for the analyte The-05 and Genz-644282 (Table 3). Clearance values were very low, indicating that both the carrier protein The-05 and encapsulated Genz-644282 remained into the plasma compartment. As expected, free Genz-644282 was characterized by a faster clearance and a Cmax 400-fold lower in comparison to the Genz-644282 complexed with the carrier protein The-05 (Table 3 and Fig. 6B).
Fig. 6.
Mean plasma level of The-0504 following intravenous administration. (A) The-05 protein concentration. (B) Genz-644282 concentration.
Table 3.
Mean pharmacokinetic parameters after The-0504 injection in rats.
| Dose/Analyte | Cmax (ng/mL) | Tmax (h) | AUC(0-inf) (ng/mL*h) |
T1/2 (h) |
|---|---|---|---|---|
| The-0504, 30 mg/kg in The-05 protein | 657,833 | 0.03333 | 20,316,400 | 41.17 |
| The-0504, 60 mg/kg in The-05 protein | 1,073,430 | 0.03333 | 37,290,300 | 41.26 |
| Free Genz-644282, 3.0 mg/kg | 184 | 0.3333 | 983 | 15.02 |
| The-0504, 3.0 mg/kg in Genz-644282 | 34,503 | 0.5 | 2,124,120 | 39.50 |
| The-0504, 6.0 mg/kg in Genz-644282 | 73,713 | 0.5 | 3,812,180 | 48.48 |
Measured analyte is underlined.
Based on the above results and in agreement with the previously reported toxicological studies, it can be concluded that the test item The-0504 was well tolerated by Wistar Hannover rats, when administered intravenously at a concentration of 1.5 and 3.0 mg/kg as a single dose. At the selected dose levels, the test item remained in the plasma compartment and the exposure was from 2000 to 6000-fold higher than free Genz-644282. Furthermore, the half-life and kinetic profiles of the carrier (measured by ELISA method) and of the payload (measured by LC-MS/MS method) are essentially superimposable, suggesting that the circulating protein-drug complex is stable up to 1 week after intravenous administration.
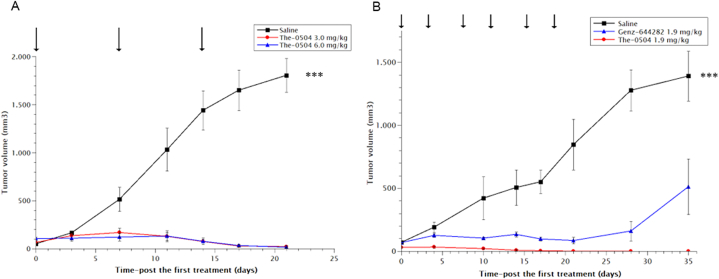
Due to the very long persistence of The-0504 in rat serum, a new set of in vivo experiments were designed for pancreatic PaCa44 tumor xenografts, involving administration once (and not twice) per week for three consecutive weeks. In this case, tumor-bearing mice were treated with The-0504 at 3.0 mg/kg or at 6.0 mg/kg in Genz-644282. The maximal dose of 6.0 mg/kg was applied in agreement with our previous toxicity data reporting the tolerability of The-0504 in mice at this concentration [11]. In Ref. [11] the non-GLP toxicological evaluation in rats is also reported.
The delayed The-0504 schedule still eradicated all tumor masses, although more slowly than in the experiment showed for reference in panel B of the Fig. 7, where a more frequent scheme of administration was applied in the same cancer model (PaCa44). Specifically, tumor growth immediately stalled after the first administration of The-0504 but significant regression occurred only between the second and the third injection. The relative TGI and ORR values at 3.0 and 6.0 mg/kg dose were both 100 % for the two injected doses (Table 2). Therefore, these results are quite identical of the experiment showed in panel B of the Fig. 7 where the more frequent scheme of administration was used. Here, The-0504 induced complete regression, with 100 % values of both TGI and ORR at 1.9 mg/kg dose, whereas free Genz-644282 resulted in much lower TGI and ORR values, 64.2 % and 16.6 %, respectively (Table 2). Mice did not show body weight loss, signs of pain or distress, or abnormal behavior (not shown).
Fig. 7.
Anti-tumor activity of The-0504 in PaCa44 mouse xenografts treated by two different schedules. (A) Once per week; (B) twice per week. Three consecutive weeks in both cases. All p-values calculated by the Student's t-test. Control vs The-0504 1.9 mg/kg and 6.0 mg/kg: ***p < 0.0001: Control vs Genz-644282 and Genz-644282 vs The-0504 1.9 mg/kg: *p < 0.05. Control vs 3.0 mg/kg: **p < 0.005. Arrows: administrations. Panel B was adapted from Ref. [11].
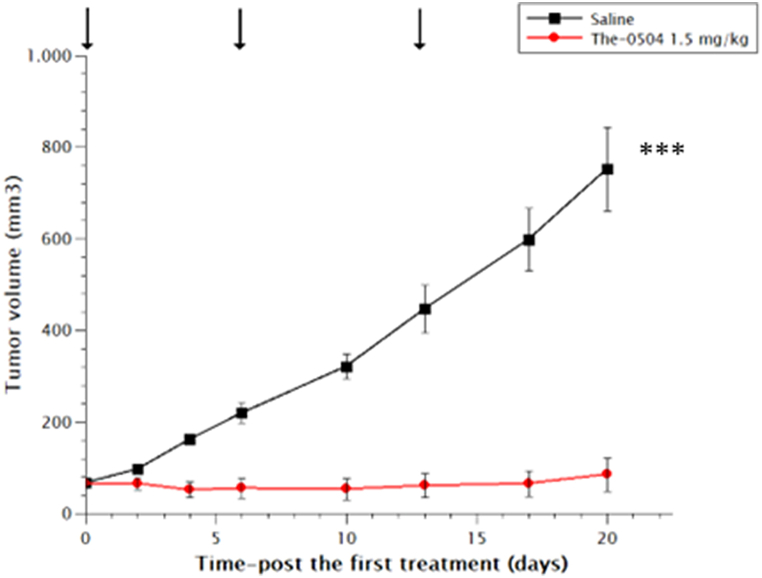
To confirm this novel therapeutic scheme, we tested The-0504 also on MiaPaca2 tumor cells, using a lower dose of 1.5 mg/kg weekly in comparison with the previously used doses of 3.0 and 6.0 mg/kg weekly, or 2.0 mg/kg bi-weekly. As shown in Fig. 8, an excellent anticancer activity was observed, with appreciable TGI and ORR values of 92.2 and 66.6 %, respectively.
Fig. 8.
Anti-tumor activity of The-0504 in MiaPaca2 mouse xenografts. Control vs The-0504 1.5 mg/kg: ***p < 0.0001 (Student's t-test). Arrows: administrations.
A synopsis of all the data from the in vivo efficacy studies performed on The-0504 is provided in Table 2. So far, the minimum efficacious dose (MED, total weekly dose) observed in the in vivo studies (Fig. 8, Table 2) was 1.5 mg/kg. This murine dose translates to a human dose of 0.125 mg/kg. Recently, it was reported that the body surface area (BSA)-corrected mouse efficacious doses can be used to predict human clinical dose [24]. Using this approach, a dose of 4.5 mg/m2 can be considered as predicted MED for humans. As stated before, the free drug Genz-644282 has already undergone a clinical dose-escalation study in humans [https://clinicaltrials.gov/ct2/show/NCT00942799] and the maximum tolerated dose reported was 9 mg/m2 (weekly, 21-day schedule). Therefore, it is rational to expect a significant therapeutic response for The-0504 at the same clinical dose already described for Genz-644282.
3. Conclusions
The present data complement and expand previous information on The-0504. Herein, we show that encapsulation within the The-0504 nanocarrier makes Genz-644282 more cytotoxic due to a combination of quantitative and qualitative effects. Quantitatively, DNA damage becomes 1.5-fold more efficient than that of free Genz-644282. Qualitatively, the improvement is due to the nanoparticle nature of the formulation. This may result in better nuclear dispatch through a positive feedback loop on nuclear import, making DNA damage persistent. Qualitative improvement is most interesting because it alleviates a major shortcoming of small naked drugs, e.g. limited selective uptake and concentration at the target site. Likewise, encapsulation extends the half-life of the drug in the circulation and, most importantly, it protects the payload while it recirculates, ensuring continued payload dispatch. Remarkably, the pharmacokinetics of the protein and Genz-644282 completely overlap, demonstrating tight association in vivo. As a result, encapsulated Genz-644282 achieves blood concentrations 400 times higher than the free drug. Unsurprisingly, leveraging the favorable DNA-damaging, nuclear uptake, and pharmacokinetic properties we were able to de-escalate The-0504 to weekly intervals at the cost of negligible changes in the kinetics of tumor volume reduction, and with no appreciable loss in the overall antitumor effects. These results may help to address dose-limiting toxicity, that is a prominent cause of failure in early-stage clinical trials, and hampers indications and applications of approved cytotoxic drugs. The-0504 has now been tested on several tumor xenografts from very diverse histotypes. It induces up to 100 % objective responses in essentially all tumor xenografts tested so far, regardless of embryological origin, that may be as diverse as epithelial, mesenchymal, and neuroendocrine. This is surprising (most cytotoxic drugs are approved in specific indications), and needs careful verification in true clinical settings. Finally, The-0504 is active on aggressive and hard-to-treat molecular tumor subtypes, as exemplified by triple-negative breast cancer xenografts. These are among the tumors expected to benefit most from rapid outgrowth control, presently achievable by cytoxic agents. It will be of interest to determine complementarity and overlaps of The-0504 with these cytotoxic drugs.
It is clearly impossible to anticipate whether the present results will be reproducible, even to a partial extent, in the clinical setting. However, they hint at a defined prioritization strategy for first-in-human trials with The-0504 as a targeted, potentially low-toxicity, tumor-agnostic chemotherapy in malignancies lacking known alternative therapeutic targets.
4. Materials and methods
4.1. The-0504 production
The production and full characterization of The-0504 is reported [5]. The-0504 was provided as lyophilized powder by Thena Biotech (Latina, Italy). Genz-644282 TFA salt (MedKoo Biosciences, Morrisville, NC, US) was resuspended in DMSO to make a stock solution and then properly diluted in buffer lactate 50 mM pH 6.5.
4.2. Immunofluorescence microscopy detection of γH2AX
The experiment was carried out essentially as described [21]. MIAPaCa-2 cells were seeded at a concentration of 3.5 × 105/well into 6-well dishes in Dulbecco's Modified Eagle's Media (DMEM), and treated with 1 μM Genz-644282 or The-0504 for 6h and 24h. Then, cells were fixed with 4 % formaldehyde solution and permeabilized with 0.01 % Triton X-100 in PBS containing 1 % BSA for 10 min. Cells were incubated with primary rabbit anti-γH2AX antibody (Phospho-gamma-H2AX Ser139 Antibody A300-081A, Thermo Fisher Scientific, Waltham, MA USA) followed by incubation with Alexa fluor 488 (rabbit) conjugated secondary antibodies (Thermo Fisher Scientific). Then, nuclei were counterstained with Hoechst 33,342 (Thermo Fisher Scientific) and were mounted with Vectashield (DBA, Milan, Italy). Cells were visualized under fluorescence confocal microscopy (Zeiss, Wetzlar, Germany). Immunofluorescence intensity was quantified by the ImageJ software plugin. Error bars indicate means ± SEM. **p < 0.01 and ***p < 0.001 as determined by Student's t-test.
4.3. Pharmacokinetics of The-0504 in rat
The exposure to the test item The-0504, after a single intravenous administration in rat, was investigated in a study carried-out at the European Research Biology Center (ERBC, Pomezia, Italy) on 18 Wistar Hannover rats (6 females per group), 7–8 weeks old, body weight approximately 176–225 g. Blood pharmacokinetic time-points were taken from all animals according to the following schedule: pre-dose (0), 2 and 30 min, 6, 24, 48, 72, 96 and 144 h from the end of dosing. Two bioanalytical methods were applied for orthogonal kinetic assessments of the HFt-based carrier (The-05) and the drug, respectively: (a) an ELISA method using a polyclonal antibody anti-The-05 (developed by us), and the purified carrier protein The-05 as standard; and (b) an LC/MS method, using Genz-644282 as reference item. The lower limits of quantitation were 50 ng/mL and 250 ng/mL for the ELISA and LC/MS methods, respectively. The-0504 was intravenously administered at two different doses of 1.5 mg/kg and 3.0 mg/kg, as Genz-644282 concentration, corresponding to about 30 mg/kg and 60 mg/kg as The-05 protein concentration, respectively. The cytotoxic product Genz-644282 was used as reference item and administered at dose of 3.0 mg/kg.
The following pharmacokinetic parameters were obtained or calculated for the injected compounds, when appropriate, from the mean plasma values: Cmax: maximum (peak) observed plasma concentration; Tmax: time to reach peak or maximum concentration; AUC(0-inf): area under the plasma concentration-time curve from time zero to infinity; T1/2: elimination half-life associated with terminal slope (λZ) of a semi logarithmic concentration-time curve; Cl: Clearance.
4.4. Cultured cells
The human cancer cell lines MiaPaca2, HT-1080, MDA-MB 157, MDA-MB 468, H1339, A549 were obtained from American Type Culture Collection (ATCC). PaCa44 cells were provided by Prof. Scarpa (Department of Diagnostics and Public Health University of Verona, Italy). All cell lines were grown as described [11], or as per supplier's instructions.
4.5. Pre-clinical activity of the-on tumor xenotransplants mouse models
The-0504 was administered at MTTlab srl, Trieste, Italy to athymic nude mice carrying tumor xenotransplants of the following human tumor cell lines: PaCa44 and MiaPaca2 (pancreas), HT-1080 (fibrosarcoma), MDA-MB 157 and MDA-MB 468 (breast cancer, triple-negative subtype), H1339 and A549 (lung). Mice were marked with Uno Pico-ID Transponders (1.25 mm × 7 mm) (UNO BV, AA Zevenaar, Netherland) by s. c. Injection, using specific implanter one week prior to the study for accurate individual monitoring.
After the acclimatization period, 5 × 106 tumor cells were inoculated subcutaneously in the right flank of each athymic nude mouse. Mice were observed at least once a day. After a week from cell inoculation, tumor growth was measured by a caliper. Treatments were started when tumor sizes averaged at about 60–100 mm3. Before treatment, all mice were weighted and the size of all tumors was measured. Animals were semi-randomly assigned to the study groups based on the initial tumor size. Animals were divided 4 per cage. The treatment dose normalized to Genz-644282 concentration was 2.0 mg/kg (twice a week) or 1.5, 3.0 or 6.0 mg/kg (once a week). Mice (n = 5 or 6) were treated intravenously once or twice a week for three weeks. All mice were culled by cervical dislocation at the end of the study or when they reached the endpoint tumor volume (i.e., 1500–1700 mm3). A gross autopsy was also performed.
A protocol approved by the Institutional Animal Care and Use Committee of the University of Udine or University of Chieti and authorized by the Italian Ministry of Health (Protocols no. 414/2021-PR and 457/2018-PR) was used for the animal studies. This was in accordance with the principles laid down in the European Community Council Directives (86/609/EEC).
4.6. Response determination
Response codes of the efficacy studies were as reported [11]., e.g. progressive disease (PD); stable disease (SD); partial response (PR); and complete response (CR). The study period was set at 2 weeks from the last treatment. For treated groups only, the percentage of tumor growth inhibition (% TGI) was defined as 100x(MTVcontrol-MTVtreated)/MTVcontrol, where MTV is the median tumor volume. The objective response rate (ORR) represents the percentage of treated animals that show a significative response (PR or CR) to therapy.
4.7. Statistical analysis
A linear mixed-effects model was used to test the tumor volume change rate over time among different groups.
5. Patents
P.C. and E.F. are inventors on patent application EP3186192B1 held by Thena Biotech that covers fusion proteins based on human ferritins and methods of use thereof.
Funding
This research was funded by Associazione Italiana per la Ricerca sul Cancro-AIRC (I.G. Grant 16776 to P·C., and I.G. Grant 25696 to G.S.)
Institutional Review Board statement
The studies were conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of Udine or University of Chieti and authorized by the Italian Ministry of Health (Protocols no. 414/2021-PR and 457/2018-PR), and in accordance with the principles laid down in the European Community Council Directives (86/609/EEC).
Informed consent statement
Not applicable.
Data availability statement
Data associated with this study have been not deposited into a publicly available repository. All data are included in the article.
CRediT authorship contribution statement
Giulio Fracasso: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Elisabetta Falvo: Writing – original draft, Methodology, Formal analysis, Conceptualization. Giada Tisci: Investigation, Formal analysis. Gianluca Sala: Writing – original draft, Resources, Methodology, Investigation, Formal analysis. Gianni Colotti: Investigation, Formal analysis. Sara Cingarlini: Writing – original draft, Investigation. Claudia Tito: Formal analysis. Sandra Bibbo: Investigation. Cristina Frusteri: Investigation, Formal analysis. Elisa Tremante: Formal analysis. Elena Giordani: Investigation. Patrizio Giacomini: Writing – original draft, Investigation, Conceptualization. Pierpaolo Ceci: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Pierpaolo Ceci and Elisabetta Falvo are inventors on patent application EP3186192B1 held by Thena Biotech that covers fusion proteins based on human ferritins and methods of use thereof. Pierpaolo Ceci is a member (free of charge) of the Scientific Advisory Committee of Thena Biotech. All the authors declare no conflict of interest.
Acknowledgements
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments). Skillful technical support by Rocco Fraioli is gratefully acknowledged.
Contributor Information
Giulio Fracasso, Email: giulio.fracasso@unipd.it.
Elisabetta Falvo, Email: elisabetta.falvo@cnr.it.
Giada Tisci, Email: giada.tisci@uniroma1.it.
Gianluca Sala, Email: g.sala@unich.it.
Gianni Colotti, Email: gianni.colotti@cnr.it.
Sara Cingarlini, Email: sara.cingarlini@aovr.veneto.it.
Claudia Tito, Email: claudia.tito@uniroma1.it.
Sandra Bibbo, Email: sandra.bibbo@unich.it.
Cristina Frusteri, Email: cristina.frusteri@univr.it.
Elisa Tremante, Email: elisa.tremante@ifo.it.
Elena Giordani, Email: elena.giordani@ifo.it.
Patrizio Giacomini, Email: patrizio.giacomini@ifo.it.
Pierpaolo Ceci, Email: pierpaolo.ceci@cnr.it.
References
- 1.Yuan R., Hou Y., Sun W., Yu J., Liu X., Niu Y., Lu J.J., Chen X. Natural products to prevent drug resistance in cancer chemotherapy: a review. Ann. N. Y. Acad. Sci. 2017;1401 doi: 10.1111/nyas.13387. [DOI] [PubMed] [Google Scholar]
- 2.Sporn M.B., Suh N. Opinion: chemoprevention: an essential approach to controlling cancer. Nat. Rev. Cancer. 2002;2 doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- 3.Falvo E., Malagrinò F., Arcovito A., Fazi F., Colotti G., Tremante E., di Micco P., Braca A., Opri R., Giuffrè A., Fracasso G., Ceci P. The presence of glutamate residues on the PAS sequence of the stimuli-sensitive nano-ferritin improves in vivo biodistribution and mitoxantrone encapsulation homogeneity. J. Contr. Release. 2018;275:177–185. doi: 10.1016/J.JCONREL.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Falvo E., Tremante E., Arcovito A., Papi M., Elad N., Boffi A., Morea V., Conti G., Toffoli G., Fracasso G., Giacomini P., Ceci P. Serine, and Alanine Elements; Biomacromolecules: 2016. Improved Doxorubicin Encapsulation and Pharmacokinetics of Ferritin-Fusion Protein Nanocarriers Bearing Proline. [DOI] [PubMed] [Google Scholar]
- 5.Falvo E., Arcovito A., Conti G., Cipolla G., Pitea M., Morea V., Damiani V., Sala G., Fracasso G., Ceci P. Engineered human nanoferritin bearing the drug Genz-644282 for cancer therapy. Pharmaceutics. 2020;12:992. doi: 10.3390/pharmaceutics12100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Zhang S., Xu C., Zhao G. Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental conditions. Chem. Commun. 2016;52:7402–7405. doi: 10.1039/C6CC03108K. [DOI] [PubMed] [Google Scholar]
- 7.Chang X., Lv C., Zhao G. A dual function of ferritin (animal and plant) Its Holo Form for Iron Supplementation and Apo Form for Delivery Systems. 2023;14:113–133. doi: 10.1146/ANNUREV-FOOD-060721-024902. [DOI] [PubMed] [Google Scholar]
- 8.Inoue I., Zheng B., Watanabe K., Ishikawa Y., Shiba K., Yasueda H., Uraoka Y., Yamashita I. A novel bifunctional protein supramolecule for construction of carbon nanotube-titanium hybrid material. Chem. Commun. 2011;47:12649–12651. doi: 10.1039/c1cc15221a. [DOI] [PubMed] [Google Scholar]
- 9.Inoue I., Chiba M., Ito K., Okamatsu Y., Suga Y., Kitahara Y., Nakahara Y., Endo Y., Takahashi K., Tagami U., Okamoto N. One-step construction of ferritin encapsulation drugs for cancer chemotherapy. Nanoscale. 2021;13:1875–1883. doi: 10.1039/D0NR04019C. [DOI] [PubMed] [Google Scholar]
- 10.Conti G., Pitea M., Ossanna R., Opri R., Tisci G., Falvo E., Innamorati G., Ghanem E., Sbarbati A., Ceci P., Fracasso G. Mitoxantrone-loaded nanoferritin slows tumor growth and improves the overall survival rate in a subcutaneous pancreatic cancer mouse model. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falvo E., Damiani V., Conti G., Boschi F., Messana K., Giacomini P., Milella M., De Laurenzi V., Morea V., Sala G., Fracasso G., Ceci P. High activity and low toxicity of a novel CD71-targeting nanotherapeutic named The-0504 on preclinical models of several human aggressive tumors. J. Exp. Clin. Cancer Res. 2021;40 doi: 10.1186/s13046-021-01851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzberg L.S., Roth S., Krumbholz R., Crawford J., Bormann C., Dunham S., Yao M., Rouleau C., Bagley R.G., Yu X.-J., Wang F., Schmid S.M., LaVoie E.J., Teicher B.A. Genz-644282, a novel non-camptothecin topoisomerase I inhibitor for cancer treatment. Clin. Cancer Res. 2011;17:2777–2787. doi: 10.1158/1078-0432.CCR-10-0542. [DOI] [PubMed] [Google Scholar]
- 13.Houghton P.J., Lock R., Carol H., Morton C.L., Gorlick R., Anders Kolb E., Keir S.T., Reynolds C.P., Kang M.H., Maris J.M., Billups C.A., Zhang M.X., Madden S.L., Teicher B.A., Smith M.A. Pediatr Blood Cancer; 2012. Testing of the Topoisomerase 1 Inhibitor Genz-644282 by the Pediatric Preclinical Testing Program. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manohar A., Vijayakanth V., Chintagumpala K., Manivasagan P., Jang E.S., Kim K.H. Zn- doped MnFe2O4 nanoparticles for magnetic hyperthermia and their cytotoxicity study in normal and cancer cell lines. Colloids Surf. A Physicochem. Eng. Asp. 2023;675 doi: 10.1016/j.colsurfa.2023.132037. [DOI] [Google Scholar]
- 15.Manohar A., Vijayakanth V., Vattikuti S.V.P., Manivasagan P., Jang E.S., Chintagumpala K., Kim K.H. Ca-doped MgFe2O4Nanoparticles for magnetic hyperthermia and their cytotoxicity in normal and cancer cell lines. ACS Appl. Nano Mater. 2022;5 doi: 10.1021/acsanm.2c01062. [DOI] [Google Scholar]
- 16.Manohar A., Vattikuti S.V.P., Manivasagan P., Jang E.S., Abdelghani H.T.M., Kim K.H. Synthesis and characterization of CeO2/MgFe2O4 nanocomposites for electrochemical study and their cytotoxicity in normal human dermal fibroblast (HDF) and human breast cancer (MDA-MB-231) cell lines. J. Alloys Compd. 2023;968 doi: 10.1016/J.JALLCOM.2023.171932. [DOI] [Google Scholar]
- 17.Manivasagan P., Ashokkumar S., Manohar A., Joe A., Han H.W., Seo S.H., Thambi T., Duong H.S., Kaushik N.K., Kim K.H., Choi E.H., Jang E.S. Biocompatible calcium ion-doped magnesium ferrite nanoparticles as a new family of photothermal therapeutic materials for cancer treatment. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15051555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Li L., Di Penta A., Carmona U., Yang F., Schöps R., Brandsch M., Zugaza J.L., Knez M., Ferritin H-Chain. A natural nuclei targeting and bioactive delivery nanovector. Adv. Healthcare Mater. 2015;4:1305–1310. doi: 10.1002/adhm.201500226. [DOI] [PubMed] [Google Scholar]
- 19.Bellini M., Mazzucchelli S., Galbiati E., Sommaruga S., Fiandra L., Truffi M., Rizzuto M.A., Colombo M., Tortora P., Corsi F., Prosperi D. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J. Contr. Release. 2014;196:184–196. doi: 10.1016/j.jconrel.2014.10.002. S0168-3659(14)00676-2[pii]10.1016/j.jconrel.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L.B., Li L., Di Penta A., Carmona U., Yang F., Schops R., Brandsch M., Zugaza J.L., Knez M., Ferritin H-Chain. A natural nuclei targeting and bioactive delivery nanovector. Adv. Healthcare Mater. 2015;4:1305–1310. doi: 10.1002/adhm.201500226. [DOI] [PubMed] [Google Scholar]
- 21.Sooryakumar D., Dexheimer T.S., Teicher B.A., Pommier Y. Molecular and cellular pharmacology of the novel noncamptothecin topoisomerase I inhibitor Genz-644282. Mol. Cancer Therapeut. 2011;10:1490. doi: 10.1158/1535-7163.MCT-10-1043. –9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki K., Rekhtman N., Quintanal-Villalonga Á., Rudin C.M. Neuroendocrine neoplasms of the lung and gastrointestinal system: convergent biology and a path to better therapies. Nat. Rev. Clin. Oncol. 2023;20 doi: 10.1038/s41571-022-00696-0. [DOI] [PubMed] [Google Scholar]
- 23.Huang X., Chisholm J., Zhuang J., Xiao Y., Duncan G., Chen X., Suk J.S., Hanes J. Protein nanocages that penetrate airway mucus and tumor tissue. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E6595. doi: 10.1073/pnas.1705407114. –E6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin R.J., Avery E., Xia C.Q. Predicting approximate clinically effective doses in oncology using preclinical efficacy and body surface area conversion: a retrospective analysis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.830972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study have been not deposited into a publicly available repository. All data are included in the article.