Abstract
Biofilms are aggregates of organized microbial growth that function as barriers and create a stable internal environment for cell survival. The bacteria in the biofilms exhibit characteristics that are quite different from the planktonic bacteria, such as strong resistance to antibiotics and other bactericides, getting out of host immunity, and developing in harsh environments, which all contribute to the persistent and intractable treatment. Hence, there is an urgent need to develop novel materials and strategies to combat biofilms. However, most of the reviews on anti-biofilms published in recent years are based on specific fields or materials. Microorganisms are ubiquitous, except in the context of medical and health issues; however, biofilms exert detrimental effects on the advancement and progress of various fields. Therefore, this review aims to provide a comprehensive summary of effective strategies and methodologies applicable across all industries. Firstly, the process of biofilms formation was introduced to enhance our comprehension of the “enemy”. Secondly, strategies to intervene in the important links of biofilms formation were discussed, taking timely action during the early weak stages of the “enemy”. Thirdly, treatment strategies for mature biofilms were summarized to deal with biofilms that break through the defense line. Finally, several substances with antibacterial properties were presented. The review concludes with the standpoint of the author about potential developments of anti-biofilms strategies. This review may help researchers quickly understand the research progress and challenges in the field of anti-biofilms to design more efficient methods and strategies to combat biofilms.
Keywords: Anti-biofilms, Strategies, Materials, Prevention biofilms, Treatment biofilms
Graphical abstract
1. Introduction
Biofilms are highly organized communities of microorganisms, including bacteria, fungi, and a mixture of strains. They represent a predominant form of microbial life that is ubiquitous in natural ecosystems. The formation of biofilms significantly enhances the survival efficiency of bacteria, rendering them 1000 times more resistant to pressures such as antibiotics, disinfectants, mechanical fluctuations, and physical scavenging compared to their planktonic counterparts [1,2]. For example, the biofilms of Y. pestis can enhance their transmission efficiency and withstand fluctuations in the external environment by regulating the feeding behavior of its host flea. This behavior is a consequence of evolutionary selection in response to environmental fluctuations and is referred to as “extended phenotypes” [3]. In addition, bacteria in biofilms can attain a competitive advantage by adopting a slow-growth strategy and occupying a favorable porous microenvironment that promotes the proliferation of diverse bacterial species [4]. Likewise, in iron-deficient environments, B. subtilis biofilms deviate from the conventional expansion mechanism by secreting pulcherriminic acid to chelate surrounding Fe3+ ions and resist the invasion of alien species [5]. The conclusion can be unequivocally inferred that biofilms significantly enhance the survival ability of planktonic cells during natural selection.
Biofilms have a pervasive impact on all aspects of human life and production. For instance, bacteria can develop highly resistant biofilms within the host organism and medical implant, resulting in chronic and persistent illnesses such as dental caries, sinusitis, osteomyelitis, endocarditis, and even fatality [[6], [7], [8], [9]]. In addition, the formation of biofilms in cooling and drinking water systems presents significant health concerns [10]. The presence of biofilms on metal surfaces can also lead to a reduction in the lifespan of equipment [11]. Finally, as a crucial component of the biosphere, biofilms play an indispensable role in the material cycling [12]. Therefore, uncontrolled biofilms may result in severe ecological consequences. Hence, the persistent contamination and infection resulting from biofilms should not be disregarded in the fields of clinical medicine, food industry, aquaculture, and other industries.
The conclusion is that, when confronted with such a formidable adversary, it is imperative to implement impeccable preventive and control measures. This review summarizes the limitations and challenges of current methods for anti-biofilms. The process of biofilms formation is initially introduced to enhance understanding of the “enemy” and develop more targeted strategies for defense and management. Secondly, measures to prevent biofilms formation and intervene in early-stage biofilms are discussed. Thirdly, strategies for controlling and treating mature biofilms are summarized (Fig. 1). The fourth aspect involves the comparison of materials possessing antimicrobial properties to enhance their application in the field of anti-biofilms. Finally, the review concludes by presenting the author's perspective regarding the future direction and expectations of research on anti-biofilms.
Fig. 1.
Schematic of anti-biofilms strategies. According to the growth cycle of biofilms, anti-biofilms strategies are divided into two aspects: preventing biofilms maturation and destroying mature biofilms. The prevention of biofilms maturation can be divided into “Inhibiting bacterial adhesion”, “Inhibiting ECM formation”, “Interfering with signaling between bacteria” and “Interfering with metabolism” according to the different stages of biofilms. Strategies to destroy mature biofilms include “Antibiotics and alternatives”, “Generating ROS”, “PTT”, “Nanomotors”, “Phages invasion” and “The role of probiotics”.
The present review provides a comprehensive perspective firmly rooted in the fundamental principles of anti-biofilms, distinguishing itself from other reviews that exclusively concentrate on specific fields or materials. It integrates knowledge from diverse disciplines, including molecular biology, microbiology, materials science, healthcare, and food safety, to analyze strategies for the prevention and control of biofilms. The present review may contribute to a more comprehensive comprehension of strategies targeting anti-biofilms and offer interdisciplinary solutions for addressing biofilms-related issues.
2. Formation of biofilms
A complete biofilms development cycle can be subdivided into 4 stages (Fig. 2). (1) Reversible attachment: planktonic bacteria attached to various environmental surfaces can return to the plankton state. (2) Irreversible attachment: bacteria form a highly organized multicellular community through extracellular polymeric substances (EPS) and extracellular matrix (ECM). By now the bacteria firmly attach to the carrier surface, and it is impossible to return to the single-cell floating state. Then bacteria gather together, grow and reproduce. (3) Maturation: the microbial community continues to grow and merge to form microcolonies with three-dimensional structures which usually look like a mushroom, besides, it can adapt its shape to better suit the surrounding environment. (4) “Seed dispersal” stage: in the late stage, with the increase of the biofilms volume, the bacteria in the inner layer of the biofilms may have growth retardation due to nutrient deficiency and accumulation of toxic metabolic waste. The biofilms undergo a spatial restructuring to enhance the living environment, resulting in the formation of a central cavity within the "mushroom" structure where free seed bacteria are released. Eventually, the top of the biofilms will burst open and disperse free seed bacteria to start new biofilms [13].
Fig. 2.
The formation of P. aeruginosa biofilms.
In this process, some important substances participate in the formation of biofilms. In the initial stage, bacteria rely on appendages such as flagellum, pili and fimbriae to swim to the surface and settle [14,15]. Then they secrete exopolysaccharides, extracellular DNA (eDNA) and proteins to bind with the surrounding bacteria. In addition, regulatory factors such as c-di-GMP play a crucial role in governing the population dynamics and persistence of biofilms during chronic infections. In a complete biofilms population, they also utilize the quorum sensing (QS) system to sense the surrounding environment to affect the reproduction and decomposition of biofilms [16].
The formation of biofilms facilitates the efficient exchange of genetic material among bacteria and their occupation of distinct ecological niches. Natural biofilms look like an intricate, diverse, and multicultural community akin to our cities [17].
3. Preventing biofilms maturation
3.1. Inhibiting bacterial adhesion
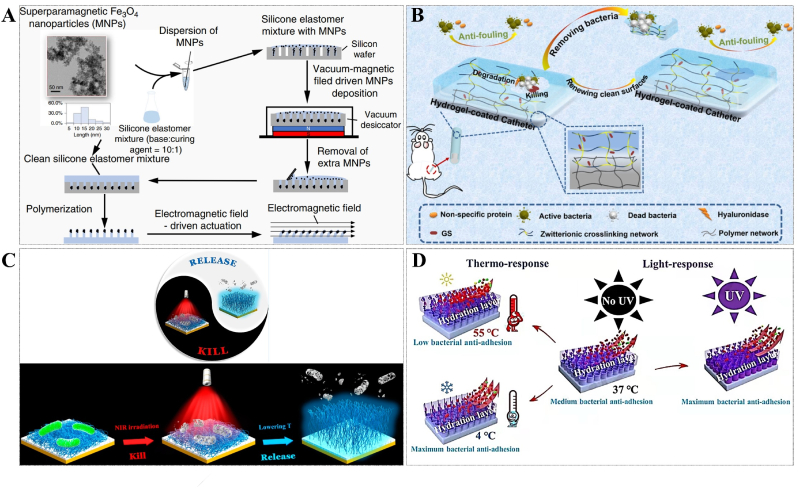
During the initial stage, bacteria exhibit a limited resistance level owing to the absence of protection provided by developed biofilms. The treatment of biofilms becomes significantly challenging once the bacteria enter the second stage and establish irreversible attachment. Therefore, inhibiting the initial bacterial adhesion is a key way to control the biofilms and avoid a larger crisis. The modification of surface coatings is a widely employed strategy for the prevention of infections associated with implants. Based on the characteristics of antibacterial and antifouling nano-topological structures on cicada and dragonfly wings, Yuan et al. developed a universal method for growing biological nano-daggers on various surfaces, endowing them with high sterilization efficiency [18]. In addition, inspired by the ciliary movement of human lung epithelial cells, which help remove foreign bodies and prevent infection, Gu et al. developed an antifouling surface based on active terrain. Under the action of an electromagnetic field, the micrometer-sized column on the surface of the material could move horizontally in a regular manner and prevent the formation of biofilms of morbific S. aureus, E. coli and P. aeruginosa in the catheter [19] (Fig. 3A). Besides, drawing inspiration from shark skin, Chung et al. designed a Sharklet AF™ surface that interfered with the formation of S. aureus biofilms in comparison to the control group [20].
Fig. 3.
(A) Schematic of the steps for active topography that mimics the ciliary movement of human lung epithelial cells. Reprinted from Ref. [19], Copyright (2020), with permission from Nature Portfolio. (B) Schematic representation of the zwitterionic hydrogel coatings of "Self-Defensive" triggered by acidic biofilms environment. Reprinted from Ref. [21], Copyright (2022), with permission from Amer Chemical Soc. (C) Schematic illustration of thermally triggered antibacterial and antifouling long-lasting coatings. Reprinted from Ref. [22], Copyright (2020), with permission from Amer Chemical Soc. (D) Schematic illustrating the mechanism of the coatings with anti-adhesive effect intelligently controlled by thermo and light. Reprinted from Ref. [23], Copyright (2021), with permission from Elsevier Science Sa.
Although antibacterial topological surfaces of antifouling coatings can kill harmful bacteria contact with the surfaces to prevent and reduce bacterial adhesion, their reuse efficiency is often compromised by the accumulation of dead cells and associated debris on the surfaces. Therefore, the presence of coatings that possess both dual antibacterial effectiveness and antifouling activities offers an additional advantage by inhibiting the adherence of deceased cells and debris [24]. Qu et al. developed an efficient smart antibacterial hybrid coating with photothermal bactericidal activity and bacteria release properties. This hybrid coating consists of two functional layers. One is a gold nanoparticle layer (GNPL) composed of gold based nanoparticles (GNPs) with photothermal bactericidal activity and unique micro-nano topography. It offers multiple bacterial contact sites and facilitates localized hyperthermia induced by near-infrared (NIR) radiation. The other layer is the vitamin C-degraded phase transitioned lysozyme film (PTLF) on the surface of GNPL, which acts as a contaminant release layer. The reusability of GNPL-PTLF coatings in multiple kill-release cycles is accompanied by the degradation of the PTLF layer, thereby severely limiting its service life. In addition, the renewal of the coating needs to rely on the role of external vitamin C, which has limitations in many applications [25]. This problem can be solved by using the bacteria's reaction to trigger the renewal of the coating. Zhang and her colleagues constructed a zwitterionic hydrogel coating with [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl) (SBMA) as the main component and hyaluronic acid (HA) as the crosslinker, as well as the gentamicin sulfate (GS) is grafted to HA by Schiff base bonds. Zwitterionic polymers are electrically neutral and form a barrier against protein adhesion. When a few bacteria escape the coating defenses and attach to the surface, these bacteria will form a weakly acidic microenvironment, resulting in the break of Schiff base bonds and the release of GS rapidly to kill the bacteria. At the same time, the hyaluronidase (HAase) secreted by these bacteria can rapidly degrade the surface and regenerate a new stain-resistant surface [21] (Fig. 3B).
However, the renewal of these two coating materials requires the sacrifice of part of the coating, which affects the long-term performance of the antibacterial coating. Wang and her colleagues developed a smart antibacterial surface with NIR-activated photothermal sterilization and heat-triggered bacterial release properties that can be reused without sacrificing coating materials. The surface deposited tannic acid (TA) and Fe3+, and subsequently fixed the poly(N-isopropyl acrylamide) (PNIPAAm) chain, a typical heat-responsive polymer whose solubility phase transition is reversible when the temperature exceeds its lower critical solution temperature (LCST). TA/Fe3+ complex is a typical metal ion-phenol network, which shows effective photothermal conversion properties under NIR to kill bacteria. Then the PNIPAAm can easily remove 90% of the attached bacteria from the surface by lowering the temperature [22] (Fig. 3C). However, the formation of such mixed surfaces requires complex equipment and procedures, which limits their practical application. In addition to bactericidal performance and coating renewal ability may be affected by the depth of NIR penetration.
Anti-biofilms are a long-term and unremitting enterprise, requiring antibacterial coatings to not only prevent bacterial attachment and destruction but also actively participate in the prevention of biofilms formation. Zou and his colleagues developed a bifunctional surface composed of an antifouling polymer and a natural anti-biofilms molecule. In this study, quercetin (Qe), a non-toxic flavonoid derived from plants, was employed as a bactericide and demonstrated its efficacy in inhibiting biofilms formation through multiple mechanisms including interference with QS, degradation of the ECM of biofilms, and inhibition of nucleic acid synthesis. In addition, 2-hydroxyethyl methacrylate (HEMA) imparts anti-adhesion properties to the surface and 3-(acrylamido)phenylboronic acid (APBA) provides a binding site for Qe by forming a borate bond. When a few bacteria break through the anti-adhesion layer to proliferate, the acid microenvironment triggers the subsequent release of Qe. Compared to the control group, this dual-functional surface has better anti-biofilms properties, which can guarantee at least 3 d of biofilms prevention without affecting the viability of normal mammalian cells [26].
In addition to considering the antibacterial effect of the coating, it is equally necessary to consider the potential immunological rejection when using it as a medical implant. To prevent overactivation of the immune system, Zwicker analyzed the possible inflammatory effects of macrophages after being exposed to surfaces coated with the antibacterial material poly (hexamethylene) biguanide hydrochloride (PHMB) and dead bacteria. The results showed that the PHMB coating resulted in a high reduction of viable bacteria, and there was no significant difference in cytokine secretion response, indicating good cytocompatibility [27].
Although various surface coatings against bacterial adhesion have been developed, there is still a great demand for antibacterial materials that can adapt to environmental changes such as temperature, pressure, or external wear and tear. Lin et al. integrated the photothermal response strategy with a composite surface, thereby imparting intelligent response capability to the coating for controlling anti-adhesion properties through both thermal and light responses. After 30 times of continuous wear under 27 kPa standard normal stress, the antibacterial rate of the surface against S. aureus and E. coli only decreased by 1.51%. The statement provides a solid basis for enhancing the durability of antibacterial materials in dynamic environments [23] (Fig. 3D). In addition, the antibacterial coating should react intelligently according to environmental changes and switch functions when necessary. Zhang designed and constructed a pH-responsive titanium-based implant of layer-by-layer surface with dual functions of antibacterial activity and osseointegration promotion. The negatively charged carboxyl group promotes the adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 cells in normal tissues and protects the normal cells from the toxic effects of quaternary ammonium salts (QAS) in the absence of biofilms adhesion on the surface. While the surface is covered by biofilms, anaerobic glycolysis in the anoxic internal environment generates an acidic microenvironment that alters the surface charge at the infected site, thereby triggering further antibacterial action of positively charged QAS. The experimental results of S. aureus infected mice confirmed the excellent antibacterial, anti-inflammatory, and bone integration properties of the coating. This adaptive antimicrobial coating strategy offers potential applications in preventing bacterial infections associated with titanium implants [28]. In order to facilitate a more comprehensive comparison of the performance of different coatings, we have compiled their antibacterial mechanisms, primary antibacterial materials, and lifespan in Table 1.
Table 1.
Comparison of several antibacterial coating materials.
| Coating type | Antibacterial mechanism | Major antibacterial material | lifespana | Reutilization | Biocompatibility | Application | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| Coating with positively-charged ZIF nano-dagger arrays | Electrostatic attraction, physically rupturing cell walls | – | ≥2 months | No | – | Surfaces of various materials, such as metal surfaces, poly(methyl methacrylate) (PMMA) plate, polypropylene fiber, silicone, glass, silicone rubber, and filter paper | – | [18] |
| Magnetically driven active topography | Physical disturbance | Fe3O4 nanoparticles | ≥30 d | Yes | Safety | Medical devices | – | [19] |
| Sharklet AF™ engineered topography | Engineered surface microtopographies | – | 21 d | No | – | Implantable device | – | [20] |
| Regenerable smart antibacterial surfaces | PTT | GNPL | – | Yes, ≥3 cycles | – | Silicon (Si), PDMS, and SS | The PTLF layer could be degraded by immersion in vitamin C solution to remove the killed bacteria. | [25] |
| “Self-defensive” antifouling zwitterionic hydrogel coatings | Hydration layer protection and drug sterilization | Zwitterionic poly sulfobetaine methacrylate (PSBMA) and GS | – | Yes | Safety | Medical devices | The coatings of the bacteria-adhering sites would be degraded by HAase secreted by these bacteria and peeled off to remove the bacteria. | [21] |
| Smart, photothermally activated, antibacterial surfaces | PTT | TA and Fe3+ ion | – | Yes, ≥3 cycles | – | Polydimethylsiloxane (PDMS), stainless steel (SS) surface | Releasing dead bacteria by a decrease in temperature. | [22] |
| Dual-functional anti-biofilms surface | Hydration layer protection, and natural anti-biofilms molecule interferes with biofilms formation | HEMA and Qe | 3 d | No | Safety | Implantable device | – | [26] |
| Smart titanium-containing composite material surface | Hydrated layer protection and electrostatic interaction | QAS and hydrophilic poly(2-hydroxyethyl methacrylate) (PHEMA) | – | Yes | Slight toxicity | Metal materials surfaces | Thermo and light-responsive surfaces can enhance bacterially anti-adhesive property. | [23] |
| Self-adaptive antibacterial and promoted osseointegration surface | Electrostatic interaction | QAS | – | No | Weak inflammatory response to tissues. | Orthopedic implant | Dual functions of antibacterial and promoted osseointegration. | [28] |
Lifespan refers to the length of time that a surface can effectively anti-bacteria and keep clean before biofilms emerge significantly. The lifespan can vary depending on factors such as the application scenarios, experimental operation, and environmental conditions.
3.2. Inhibiting ECM formation
The ECM is a powerful physical and metabolic barrier of biofilms, consisting of polysaccharides, lipids, eDNA, proteins, and so on, which can inactivate antibiotics, enhance the bacterial community, and improve the activity of the drug efflux pump. Dieltjens et al. showed that the inhibition of ECM could increase the susceptibility of bacteria to antibiotics and reduce bacterial adhesion, providing theoretical support for solving the problem of antimicrobial resistance by inhibiting ECM [29].
The eDNA is the predominant compound found in biofilms. Taking P. aeruginosa as an example, the formation of "mushroom" biofilms under glucose medium conditions follows a highly organized process. Firstly, the non-motile bacteria constitute the stalk of the mushroom, and subsequently, facilitated by fimbriae, a subset of these bacteria can migrate upwards to form the cap of the "mushroom". The eDNA, bound by fimbriae, can facilitate the aggregation of migrating bacteria along with the high concentration of eDNA outside the stalk. The bacteria subsequently undergo proliferation, leading to the formation of the mushroom cap [13]. Therefore, eDNA plays a crucial role in the formation and structural stability of biofilms. Brown's study found that the exogenous addition of deoxyribonuclease I (DNase I) or restriction enzymes could rapidly degrade eDNA, remove biofilms, and inhibit biofilms reconstitution within 48 h [30]. However, the stability and production cost of this natural enzyme affect its application. Therefore, some researchers have developed more economical and stable artificial simulated nucleases. Chen et al. developed a DNase analog artificial enzyme by adding a variety of cerium (Ce) (IV) complexes on the surface of colloidal magnetic “Fe3O4/SiO2 core/shell”, which exhibit the characteristics of stable, recyclable, and excellent biofilms removal efficiency [31]. Meanwhile, Hu et al. combined artificial enzyme and graphene oxide (GO) with photothermal therapy (PTT) to construct GO-based nitrilotriacetic acid-Ce (IV) composite (GO-NTA-Ce), which effectively eradicated biofilms infections of drug-resistant bacteria [32]. However, its practical application in vivo still needs to be evaluated due to the wide range of non-specific cleavage activity of nucleases.
Genetic and microscopic studies have shown that many different bacteria use proteins as a medium in biofilms formation [[33], [34], [35]]. The amyloid plays a crucial role in the formation of bacterial biofilms, contributing to the initial attachment of bacteria to surfaces, consolidation of bacterial clusters, and maintenance of biofilms structure. Additionally, they may contribute to biofilms resistance against environmental stresses such as antibiotics and immune response [36,37]. Among these, Csg A and Csg B are two critical participants in the synthesis of amyloid curli. Sergei Perov et al. identified structural similarities between fibrillar spine segments derived from Csg A and human pathogenic amyloid. Accordingly, they employed two D-enantiomeric peptides that were initially meant to interfere with Alzheimer's disease to prevent biofilms. It is wondrous that D-enantiomeric peptides prevented Csg A spine fibrillation and reduced biofilms formation of S. typhimurium [38]. This means that the prevention and control of biofilms can draw insights from the treatment strategies employed in Alzheimer's disease.
Additionally, lectin, a type of carbohydrate-binding protein with multiple glyco-binding sites, plays a pivotal role in the formation of biofilms. This process facilitates bacterial recognition and adhesion while promoting interconnections among bacteria. Among these, lectins Lec B and Lec A are essential compositions of virulence factors and biofilms of P. aeruginosa, significantly contributing to its drug resistance [13]. Therefore, the disruption of these proteins' function may potentially hinder the development of drug-resistant bacteria and impede the maturation process of biofilms. Sommer et al. developed a class of Lec B inhibitors with good selectivity and affinity, which effectively blocked the formation of P. aeruginosa biofilms in vitro. One of the inhibitors, C-glycoside 7, has an 80–90% inhibition rate on biofilms. Additionally, these inhibitors exhibited good oral bioavailability and excellent drug concentration in the urine and plasma of mice [39]. Besides, Wagner et al. created two diastereoisomeric galactose-derived epoxides to target Cys 62, a key cysteine residue in the carbohydrate-binding domain of Lec A, and produced a fluorescent derivative based on the epoxides for Lec A-specific labeling [40]. This enables the location of bacterial biofilms in infected hosts, making it possible to target pathogens and tissues for treatment. The combination of drugs or antibacterial materials with epoxides offers a promising avenue for advancing biofilms treatment. With the deepening of basic biofilms research, we need to explore more targeted strategies for biofilms treatment to cope with the coming crisis of antibiotic resistance.
3.3. Interfering with signaling between bacteria
QS refers to the communication process between cells, which allows bacteria to “communicate” and coordinate their work as a team, ultimately increasing the survival of the entire population in complex environments. In this process, many bacteria can synthesize and release signal molecules called autoinducers (AI). The concentration of AI increases with the accumulating of bacterial density, which can activate the expression of related genes and regulate the biological behavior of bacteria when it reaches certain concentrations [41]. This induction is commonly known as "cell density-dependent regulation of gene expression," as it exclusively occurs once the bacterial population surpasses a specific threshold.
Since animals lack QS systems, blocking QS to prevent the development of biofilms is a safe and viable tactic. To date, many compounds have been discovered to have superior QS system inhibitory action [[42], [43], [44]].
However, the complex and diverse signal regulation mechanisms of QS systems make it extremely difficult to inhibit biofilms with QS inhibitors (QSIs). According to the signal molecules and the sensing mechanism, QS can be divided into three types: oligopeptide QS system of Gram-positive bacteria, Lux I/R QS system of Gram-negative bacteria, and hybrid QS system [13]. For example, the bacterium P. aeruginosa possesses three QS systems, namely las, rhl, and pqs [45]. Among them, las and rhl systems belong to the Lux I/R system, which consists of 12-carbon long-chain acyl homoserine lactones (AHL) (N-3-oxododecanoyl-L-homoserine lactone, 3-O-C12-HSL) and 4-carbon short-chain AHL (N-butyryl-L-homoserine Lactone, C4-HSL), respectively. The molecules function as signaling molecules, with Las R and Rhl R proteins functioning as their respective receptor proteins. Besides, the pqs system utilizes 2-heptyl-3-hydroxy-quinolone as signaling molecules and Pqs R (Mvf R) as receptor proteins. These diverse and intricate QS systems not only facilitate the growth of bacterial biofilms but also pose significant challenges to biofilm management solely through QSIs. Therefore, multiple strategies often combine with QSI to enhance their effectiveness. The research conducted by Zou resulted in the development of an antibacterial material that utilizes acylase (ACY)'s capability to degrade AHL in Las and Rhl systems through amide bond cleavage, thereby establishing three layers of defense. The outermost layer of HA serves as the primary defense mechanism against initial bacterial adhesion, while the underlying ACY/quaternized chitosan (QCS) layer becomes exposed upon degradation of HA by attached bacteria. The QCS has the ability to eradicate bacteria, while ACY can disrupt the QS system. The material exhibited a remarkable 98% reduction in bacterial adhesion within a span of 4 h, while maintaining a biofilms reduction rate above 90% even after extending the culture time to 28 d [46]. Although this material has a specific and strong effect on bacteria and biofilms of P. aeruginosa, the inhibitory effect on Gram-positive bacteria remains to be evaluated.
Moreover, c-di-GMP is a novel second messenger widely distributed among bacteria. It is synthesized by diguanylate cyclase (DGC) enzymes containing the active GGDEF domain and degraded by phosphodiesterase (PDE) containing EAL or HD-GYP active domains [47]. Various metabolic pathways are regulated by controlling the synthesis and decomposition of c-di-GMP. The regulatory principle governing c-di-GMP in most bacteria is that at low concentrations, c-di-GMP enhances bacterial motility and the expression of toxins. While the high concentration of c-di-GMP promotes the expression of adhesion factors and the formation of biofilms. Moreover, the concentration of c-di-GMP within bacteria plays a crucial role in determining whether bacterial cells initiate biofilms formation or remain in planktonic mode as individual cells. Therefore, it represents a promising target for combating bacterial and biofilms infections [48]. Foletti et al. identified the proline-rich peptide Gup-Gup-Nap-Arg from a peptide library, which exhibits selective binding to c-di-GMP and inhibits the growth of P. aeruginosa biofilms [49]. However, Gram-positive bacteria exhibit significantly lower levels of GGDEF and EAL domains in comparison to their Gram-negative counterparts, implying that the inhibition of c-di-GMP has a diminished impact on Gram-positive bacteria.
3.4. Interfering with metabolism
When transitioning from planktonic cells to biofilms, bacteria may modulate various cellular metabolic pathways to adapt to a non-migratory colony life. These metabolic adaptations play a critical role in biofilms formation. For example, Gelinas' research showed that both S. aureus and E. faecalis require a number of purines to form biofilms, as evidenced by the upregulation of the “de novo purine biosynthesis pathway” during biofilms formation [50]. Additionally, Sinha's study revealed that the biofilms formation of P. aeruginosa relies on the ceramidase induced by host lipids to inhibit host wound healing [51]. These altered metabolic pathways could be the targets for the inhibition of biofilms in the future.
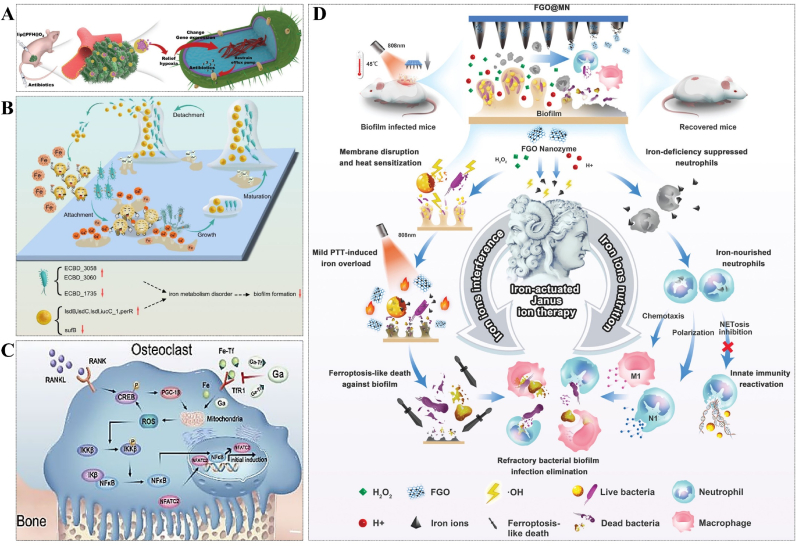
Additionally, the formation of biofilms disrupts the delicate balance between internal oxygen consumption and external oxygen supply in bacteria, leading to the creation of an anoxic microenvironment. This is a crucial factor contributing to the resistance exhibited by bacteria coated with biofilms. As a result, relieving hypoxia may be an effective strategy for combating antibiotic resistance in biofilms therapy. Ji's group used perfluorohexane (PFH) as an oxygen carrier in conjunction with liposomes and antibiotics to combat biofilms-related infections. The concentration of antibiotics decreased exponentially with the alleviation of hypoxia, which indicated that modulation of biofilms oxygen metabolism can enhance the bactericidal effect of antibiotics [52] (Fig. 4A).
Fig. 4.
(A) The schematic diagram of treatment of biofilms infection by relieving biofilms hypoxia environment. Reprinted from Ref. [52], Copyright (2020), with permission from Wiley. (B) Ga3+ interferes with biofilms formation by disturbing iron ion metabolism. Reprinted from Ref. [53], Copyright (2023), with permission from Springernature. (C) Ga3+ inhibits the NF-κB signaling pathway by targeting iron metabolism, then prevents osteoclast differentiation and osteolysis. Reprinted from Ref. [53], Copyright (2023), with permission from Springernature. (D) Schematic illustration of excess iron interfering with biofilms metabolism and activating immune cells. Reprinted from Ref. [54], Copyright (2022), with permission from Wiley-V C H Verlag Gmbh.
As one of the essential elements for the survival of most organisms on the Earth, iron ion also plays an important role in the formation of biofilms, which could be evidenced by the fact that siderophores mutant strains of P. aeruginosa can only produce flat biofilms rather than mature mushroom biofilms [13,55]. Additionally, to some extent, it has been demonstrated that biofilms can establish an effective defense against the neutrophils and other innate immune cells by competing with them to combine the iron ions. Therefore, it's an effective strategy to realize both biofilms therapy and immune response activation by regulating iron metabolism. Kaneko et al. used gallium (Ga), a non-redox simulant of iron with comparable ionic radius and charge density, to interfere with iron metabolism and biofilms formation, achieving some effect [56]. Similarly, Banin et al. reported that the desferrioxamine-Ga complex could isolate Fe3+ and release Ga3+ to compete for ferritin binding sites, effectively inhibiting the formation of bacterial biofilms through synergistic effects with gentamicin [57]. However, excess Ga enters the body and may cause damage to the kidneys, leading to kidney failure and even death. This greatly limits the application of Ga in anti-biofilms strategies. The recent evidence suggests that Ga3+ can effectively inhibit osteoclast activity [58,59]. After carefully considering the advantages and disadvantages, Ga3+ ventured into the realm of anti-biofilms with a fresh identity. The statement suggests that orthopedic patients utilizing biomedical implants will confront two significant therapeutic challenges: aseptic loosening caused by hyperactive osteoclastogenesis and bacterial infection facilitated by the formation of biofilms. Li et al. have successfully developed a biocompatible titanium alloy incorporated with Ga, which demonstrates remarkable capabilities in preventing biofilms formation and shows promising potential in mitigating the risk of aseptic loosening. The statement offers novel perspectives on addressing the clinical challenges associated with implant allocation [53] (Fig. 4B and C). Further investigation by Abouelhassan et al. found that halogenated phenazine antagonized methicillin-resistant S. aureus (MRSA) biofilms by influencing iron homeostasis and critical pathways of bacterial surface attachment [60].
In fact, elevated levels of iron ions can also hinder or disrupt the formation of biofilms. Zhu et al. proposed an innovative strategy called "iron-actuated Janus ion therapy (IJIT)" to effectively regulate iron metabolism in both biofilms and immune cells, aiming at combating biofilms formation. The biofilms microenvironmental responsive photothermal microneedle patch (FGO@MN) was developed by growing Fe3O4 nanoparticles (FNPs) on GO nanosheets and encapsulating them in methacrylated HA needle tips. The combination of GO and FNPs enhances the photothermal properties of the material, while FNPs with peroxidase (PODs) activity generate toxic hydroxyl radicals (·OH) to disrupt the heat shock response (HSR) system in the microenvironment of biofilms. These results allow mild PTT to destroy the biofilms and avoid thermal damage to normal cells. Disruption of the HSR system induces thermal sensitization in biofilms, leading to iron overload in bacterial cells and subsequent iron death. Additionally, this disruption promotes nourishment of surrounding neutrophils to inhibit biofilms. The experiments demonstrated the clearance effect of IJIT on biofilms infection after 15 d [54] (Fig. 4D).
All these phenomena indicate that the bacterial biofilms formation can be inhibited through interfering with bacterial metabolism, thereby presenting a novel approach to combat with biofilms.
4. Destroying mature biofilms
4.1. Chemical method
4.1.1. Antibiotics and alternatives
Antibiotics with strong and rapid antibacterial effect are widely employed as particular drugs for treating infectious diseases. Different kinds of antibiotics have different therapeutic mechanisms, which can be mainly divided into four categories: (1) interfering with the bacterial cell wall's production, such as penicillins and cephalosporins. (2) affecting the synthesis of bacterial proteins, such as tetracycline, aminoglycoside, macrolides, and chloramphenicol. (3) inhibiting the synthesis of bacterial nucleic acid, such as rifampicin. (4) damaging cell membrane, such as peptides.
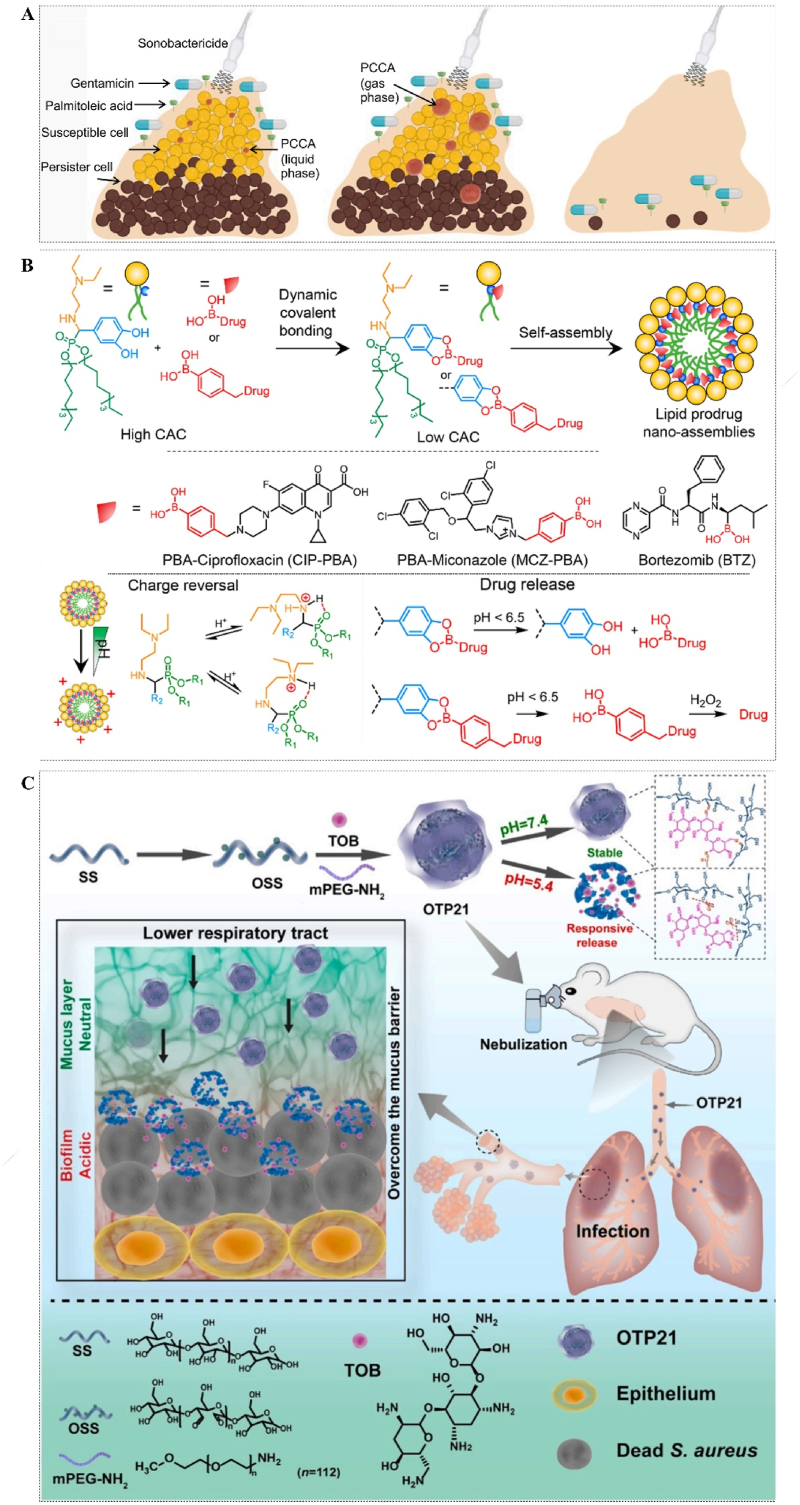
The biofilms that develop at the infection site, however, serve as a robust refuge for bacteria against most antimicrobial agents, thereby necessitating higher drug concentrations and potentially inducing toxic effects on normal cells. In Virginie's study, phase-change contrast agents (PCCA) were introduced to drug-exposed biofilms. The stable liquid PCCA can effectively penetrate the ECM of biofilms and expand into larger gases when exposed to ultrasound. These expanded gases then respond to ultrasonic oscillations, leading to the physical destruction of biofilms and enhanced drug penetration [61] (Fig. 5A). However, this approach may only be effective in addressing superficial biofilms infections of the body, while the treatment of deep tissue biofilms remains a significant challenge. The notion of enhancing drug concentrations as a means to achieve biofilms protection is evidently impractical. The utilization of prodrug nanocomponents holds significant promise in the precise targeting of diseased sites and the on-demand delivery of drugs. Liu et al. reported lipid prodrug nanoassemblies (LPNAs) synthesized by dynamic covalent boric acid between catechol on lipids and boric acid on drug molecules. The LPNAs undergo charge inversion in an acidic microenvironment and selectively release the drug, subsequently eradicating pathogens. Moreover, they exhibit superior efficacy compared to their unencapsulated counterparts in encapsulation and delivery tests involving three model drugs: ciprofloxacin (Cip), bortezomib, and miconazole [62] (Fig. 5B). In order to enhance the drug's efficiency further, they employed the same approach to synthesize antimicrobial hybrid amphiphile (aHA) utilizing DETA NONOate (nitric oxide, NO donor), phenylboronic acid-modified Cip, and 3,4-dihydroxybenaldehyde. The presence of NO can enhance the diffusion of biofilms by modulating the expression of secondary messenger c-di-GMP and bacterial PDE activity, thereby facilitating drug penetration within biofilms. Meanwhile, the aHA can undergo self-assembly into aggregates in aqueous solutions, exhibiting an impressive drug loading capacity of up to 73.8%. This remarkable attribute contributes significantly to its exceptional therapeutic efficacy against peritoneal and subcutaneous infections in mice [63]. Moreover, Ren designed and prepared OTP, a reactive drug-carrying nanoparticle system composed of cross-linked oxidized soluble starch (OSS) and tobramycin (TOB) through Schiff base reaction. Additionally, the super hydrophilic polymer mPEG-NH2 was modified to enhance its dispersion and penetration capabilities. The OTP comprises pH-sensitive imine bonds, enabling the release of TOBs upon activation by a slightly acidic biofilms environment, thereby enhancing drug delivery efficiency to the infected area. The hydrophilicity, small volume, and positive surface charge of OTP enhance its penetration efficiency and make it a potential treatment option for lower respiratory tract biofilms infections [64] (Fig. 5C).
Fig. 5.
(A) Schematic illustration of PCCA treating biofilms to promote drug penetration. Reprinted from Ref. [61], Copyright (2023), with permission from Cell Press. (B) Schematic diagram of the synthesis of prodrug nanocomponents (LPNAs) by dynamic covalent bonding. Reprinted from Ref. [62], Copyright (2023), with permission from Amer Chemical Soc. (C) Diagram of the synthesis process and mechanism of reactive drug-carrying nanoparticles (OTP). Reprinted from Ref. [64], Copyright (2022), with permission from Elsevier Sci Ltd.
Nevertheless, certain bacteria have developed corresponding defense mechanisms to counteract the invasion of antibiotics, including drug permeability disorders, active efflux mechanisms, alterations in drug target sites, and the production of drug-inactivating enzymes. Additionally, the formation of biofilms not only confers protection against drug invasion to bacteria but also facilitates the rapid transmission of resistance genes between bacterial populations. The treatment of biofilms infections with antibiotics poses a significant challenge, necessitating the exploration of alternative approaches.
Instead of antibiotics, antimicrobial peptides (AMPs) are gradually emerging as an alternative antimicrobial drug that does not easily induce drug resistance for the following reasons [65,66]: (1) The bactericidal mechanisms encompass various actions, such as disruption of bacterial cell membranes, modulation of bacterial cell membrane potential, and interference with internal biological processes within bacteria; (2) The broad-spectrum antibacterial activities of AMPs are not reliant on bacteria-specific receptors or enzymes, as they interact with bacterial cell membranes and other targets; (3) The adaptability of AMPs to various environments and targets is facilitated by their intricate structural composition and extensive sequence diversity; (4) The immune system response involves the synergistic interaction of AMPs with other immune cells and molecules to combat bacterial infections.
Engineering peptides based on the sequence of natural peptides have emerged as an excellent choice due to the inaccessibility of natural peptides. Riool et al. synthesized peptides SAAP-276 and SAAP-145 according to the amino acid sequence of human antibacterial peptide LL-37, which demonstrated an excellent inhibitory effect on drug-resistant bacteria and the formation of biofilms without inducing further drug resistance [67]. In addition, Pulido et al. synthesized engineering AMPs based on human RNase Ⅲ, showing great anti-biofilms properties against Gram-negative bacteria [68]. However, AMPs are easily degraded by bacterial and host proteases, so it is crucial to develop a promising drug delivery system to protect its activity. Klodzinska et al. coated the anti-biofilms peptide DJK-5 with a modified HA nanogel. The system showed excellent anti-biofilms properties and excellent biosafety in tests of intravenous and subcutaneous [69].
Essential oils (EOs), delicately crafted by nature, are a profound and treasured gift. They are organic plant extracts made from leaves, petals, roots, seeds, or stems, some of which contain over 300 distinct chemicals, such as alcohols, aldehydes, amides, amines, esters, ethers, heterocycles, ketones, phenols, and terpenes. Therefore, the antibacterial mechanisms of these substances are intricate and not easily susceptible to bacterial resistance. Due to their safety, potent antibacterial properties, and pleasant aroma, they are frequently utilized as natural alternative to chemical preservatives in the food and cosmetics industries. The major components, antibacterial properties, and anti-biofilms mechanisms of several EOs reported in the past two years have been summarized in Table 2 [[70], [71], [72], [73], [74], [75], [76], [77], [78]]. The quality of EOs, however, can be influenced by various environmental factors such as their geographical origin, sunlight exposure, duration of light exposure, and timing of harvest. Additionally, the extraction methods employed also impact the efficacy of these oils [[79], [80], [81]].
Table 2.
Comparison of antibacterial properties of EO.
| EOs | Source | Major Compounds | Pathogen Strains | Dosage/MIC (minimum inhibitory concentration) | Mechanism of anti-bacteria | Effects on biofilms/MBIC (minimal biofilms inhibitory concentration) | Mechanism of anti-biofilms | Safety | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| – | Clinopodium nepeta, Origanum vulgare and Foeniculum vulgare | piperitone oxide, estragole, and p-thymol, respectively | E. coli JM109, and its derived antibiotic-resistant cells | 0.3–0.966 μL/mL | methylation at both cytosine and adenine residues | – | – | – | [70] |
| Cedar EO | Cedar wood | e δ-cadinene (36.35%), (Z)-β-farnesene (13.8%), β-himachalene (9.4%), | M. luteus | MIC 90: 8.99 μL/mL | – | – | – | – | [71] |
| Cinnamon EO | – | cinnamaldehyde | Y. enterocolitica | 0.625 mg/mL | altering bacteria cell membrane structure, stability, and osmotic function | 0.078 mg/mL | interfering with the QS system by inhibiting AHL production | – | [72] |
| Clove essential oil (CEO) and Oregano essential oil (OEO) | – | eugenol and carvacrol, respectively | S. Derby | 0.8 mg/mL and 0.2 mg/mL, respectively | – | At 1/8 MIC, the inhibition rate of CEO and OEO on the biofilms formation was 90.29% and 48.79%. | suppressing the metabolic activity and the production of the extracellular polysaccharide | – | [73] |
| Composite EO | – | cinnamaldehyde, carvacrol, and eugenol | E. coli and S. aureus | 7.94 μL/mL | destroying bacteria cell wall and cell membrane integrity, and retarding the respiratory chain | The biofilms of S. aureus decreased by 11.19%, 15.32%, and 18.09%, and that of E. coli decreased by 14.25%, 21.72%, and 22.37% after exposure (0 h) to EOs at 0.5 MIC, 1 MIC, and 2 MIC, respectively. | – | – | [74] |
| J. intigrimma, J. roseae and J. gossypifolia EO | leaves of Jatropha | diterpenes | E. coli | 5 mg/mL, 5 mg/mL, and 2.5 mg/mL, respectively | – | 31.25 μg/mL, 250 and above 1000 μg/mL, respectively | disturbing adhesion proteins FimH | – | [75] |
| Propolis essential oil (PEO) | complex material that honey bees collect from resinous and balsamic material | β-himachalene (13.94%), α-curcumene (11.28%), α-bergamotene (4.5%) |
S. mutans | 0.625 μL/mL | – | 1/4 MIC or above can significantly reduce the biofilms biomasses compared to the control group (p < 0.05). | membrane permeability, releasing lactic dehydrogenase (LDH) and calcium ions; inhibiting bacterial proliferation; suppressing the activity of glucosyltransferases (GTFs) to reduce the production of extracellular polysaccharides and alleviate bacterial adherence | IC10 value of PEO on HOECs was 1.299 μL/mL, which was significantly higher than double the MIC. | [76] |
| Thymus x citriodorus EO | Thyme species | monoterpenes (72.6%), being geraniol (27.5%), 1,8-cineole (16.3%), and thymol (9.2%); | C. acnes and S. epidermidis | 0.06% | – | 0.12%–0.24% | – | affecting cellular viability in a dose-dependent manner. | [77] |
| Thymus zygis subsp. gracilis EO | Lamiaceae | p-cymene (25.98 ± 0.07%), thymol (22.64 ± 0.06%), carvacrol (21.28 ± 0.06%), and γ-terpinene (11.01 ± 0.03%) | L. monocytogenes | 0.02% (v/v) | – | 0.02% (v/v) | – | – | [78] |
4.1.2. Generating ROS
Reactive oxygen species (ROS) are chemically active atoms or groups containing oxygen, such as singlet oxygen (1O2), hydrogen peroxide (H2O2), ·OH, ozone (O3), NO, and so on. ROS can destroy the structure of the cell membrane by inducing unsaturated fatty acids in lipids to undergo peroxidation reactions, and further damage nucleic acids, proteins, as well as other biological macromolecules.
ROS can be produced in several methods to induce bactericidal and anti-biofilms effects. The process of photodynamic therapy (PDT) involves the excitation of a photosensitizer by laser irradiation at a specific wavelength, which subsequently transfers energy to surrounding oxygen molecules, leading to the generation of ROS. Chen et al. developed bimetallic metal-organic frameworks (MOFs) PCN-224 (Zr/Ti) that significantly stimulated the generation of ROS under visible light and exhibited effective antibacterial activity [82]. The anoxic microenvironment of the biofilms, however, can significantly diminish the efficacy of PDT. PODs-active nano-enzymes that catalyze the conversion of zymolyte into ROS in an oxygen-independent manner-are commonly utilized in anti-biofilms techniques. Dong's group designed a cascade reaction PODs nano-enzyme “CoPt@graphene (G) @glucose oxidase (GOx) (CoPt@G@GOx)”. The GOx enzyme oxidizes glucose to generate H2O2, which acts as a substrate for the POD mimicking "CoPt@G" catalyst, resulting in the production of highly reactive ·OH species. Additionally, the magnetic properties of "CoPt@G@Gox" facilitate the enrichment of nanoparticles (NPs), leading to approximately 6 × 104 times enhanced antibacterial activity in the presence of a magnetic field [83] (Fig. 6A). This strategy demonstrates a favorable efficacy in the management of oral diseases caused by biofilms. However, the presence of glucose as a vital energy source poses a new challenge in preventing the premature release of ROS during infection treatment. Here, Ji et al. constructed a targeted on-demand drug delivery system “AA@GS@HA-MNPs” by encapsulating ascorbic acid (AA) in graphene-mesoporous silica nanosheet (GS) and then modifying magnetic NPs (MNPs) and HA onto the surfaces of hybrids. Meanwhile, the MNPs were precoated with vancomycin. Upon arrival at the infected site, the HA coating of "AA@GS@HA-MNPs" is degraded by bacterial-secreted HAase, facilitating subsequent release of AA that can be catalyzed by MNPs to generate ·OH in situ. The combination of G's exceptional PTT properties with this system demonstrates a synergistic antibacterial and anti-biofilms activity against both Gram-positive and Gram-negative bacteria [84] (Fig. 6B).
Fig. 6.
(A) Anti-biofilms mechanism of cascade reaction hybrid PODs nano-enzyme (CoPt@G@GOx) for oral biofilms therapy. Reprinted from Ref. [83], Copyright (2022), with permission from Tsinghua Univ Press. (B) Schematic representation of synthesis process and mechanism that biofilms-induced on-demand ROS release delivery system "AA@GS@HA-MNPs". Reprinted from Ref. [84], Copyright (2016), with permission from Wiley-V C H Verlag Gmbh. (C) Schematic illustration for anti-biofilms therapy with Arg-CD-AcMH that cascade catalytic NO release. Reprinted from Ref. [85], Copyright (2022), with permission from Wiley-V C H Verlag Gmbh.
NO is an attractive antimicrobial agent that can both disperses bacterial biofilms and prevents them from developing drug resistance. Additionally, NO can accelerate wound healing by boosting the creation of collagen and the production of myofibroblast during skin reconstruction [[86], [87], [88]]. Therefore, it gradually occupies an important position in the infection treatment of biofilms. Taking the limited diffusion range and rapid degradation of NO into account, it is also imperative to devise diverse strategies for continuous NO delivery to the ECM. Jin-Wook's team has developed a “polyethyleneimine/diazoate-doped PLGA” that can bind to biofilms substrates through electrostatic interaction and promote NO delivery to wounds infected by MRSA biofilms. The release time of NO in the simulated wound fluid was prolonged by over 4 d, while the biofilms were dispersed in diabetic mice, resulting in accelerated wound healing compared to the control group on the 8th day post-injury [89]. To achieve a more precise release of NO at the site of wound infection, Shi and her colleagues opted for β-cyclodextrin (β-CD) modified by NO donor L-Arginine (L-Arg) and maltoheptaose (MH) modified by linear ferrocene terminated acetal as the framework molecules to synthesize amphipathic supramolecular Arg-CD-AcMH. Subsequently, glucoamylase (GA) and GOx were incorporated through electrostatic interaction. The NPs, upon reaching the surface of bacterial biofilms through hydration channels, encounter a weak acid environment (pH 5–6) that triggers the cleavage of the acetal bond and subsequent release of MH. Subsequently, MH undergoes hydrolysis by GA to generate glucose, which is then catalyzed by GOx to produce a significant amount of H2O2. This abundance of H2O2 activates L-Arg and leads to a substantial release of NO [85] (Fig. 6C). The sequential activation of ROS cascades enables precise localization of ROS release at the infection site, thereby minimizing damage to normal cells. Additionally, the utilization of NO disrupts biofilms formation and facilitates wound healing.
4.2. Physical method
4.2.1. PTT
The PTT technique induces local hyperthermia by employing non-invasive light irradiation and utilizing photothermal agents with high conversion efficiency. The bioactive substances, such as nucleic acids or proteins, are rendered inactive, compromising the integrity of bacteria and the structure of biofilms, thus augmenting the sterilization efficacy. It has benefits including tissue penetration, spatiotemporal controllability, and minimal invasiveness. Various materials such as Graphene, carbon quantum dots, metals, polydopamine (PDA), organic π-conjugated molecules and so on all exhibit certain PTT properties under NIR irradiation [[90], [91], [92], [93]]. The Food and Drug Administration (FDA) has granted clinical approval for the use of commonly employed molecules, such as indocyanine green (ICG) and methylene blue (MB).
The precise delivery of these PTT materials to the infected site poses a challenge. The PDA-ICG-NPs were prepared by Gao et al. using polyethylene glycol (PEG) and poly(beta-amino ester) (PAE), a pH-responsive mixed shell polymer, to encapsulate ICG-coated PDA. The presence of electrostatic interaction enables the transportation of PDA-ICG-NPs in the bloodstream, facilitating their targeted delivery, penetration, and accumulation on biofilms [94].
However, planar molecules like ICG exhibit strong face-to-face π-π stacking interactions in the aggregation state, leading to non-radiation decay and insufficient radiation decay. In order to effectively control molecular mobility in the aggregate/solution state and enhance the molar absorption rate, Tang's research team proposed an innovative concept for molecular design. The intramolecular excited state motion facilitated the successful synthesis of nanoparticles with excellent PTT characteristics induced by intramolecular motion (iMIPT) [95] (Fig. 7A–C). In subsequent studies, they prepared TN NPs with higher photothermal conversion properties based on iMIPT NPs, which successfully destroy mature S. aureus biofilms under NIR irradiation (808 nm, 1 W cm−1) without hazards in the dark [96] (Fig. 7D).
Fig. 7.
(A) Molecular structures of iMIPT nanoparticle precursor and two kinds of iMIPT nanoparticle. Reprinted from Ref. [95], Copyright (2019), with permission from Nature Portfolio. (B) 2TPE-NDTA-doped NP exhibits an outstanding iMIPT peculiarity. Reprinted from Ref. [95], Copyright (2019), with permission from Nature Portfolio. (C) Comparing the mechanisms of AIE and iMIPT. Reprinted from Ref. [95], Copyright (2019), with permission from Nature Portfolio. (D) An efficient anti-biofilms strategy for PTT based on the iMIPT mechanism. Reprinted from Ref. [96], Copyright (2021), with permission from Royal Soc Chemistry.
The use of PTT alone, however, requires temperatures above 70 °C, while excessively high temperatures can have detrimental effects on healthy tissue. In this case, Zhao et al. developed a cationic heat-sensitive liposome by coupling antibiotics with gold (Au) nanorods for the treatment of MRSA-induced osteomyelitis. The NPs with a positive charge can effectively penetrate the biofilms, enhancing their permeability and facilitating increased antibiotic access to bacteria. More than 80% of the antibiotics were rapidly released at a local temperature of 50 °C under 808 nm laser irradiation, resulting in the destruction of most of the biofilms [97] (Fig. 8A). Additionally, the combined application of PDT and PTT is frequently employed in numerous research studies for biofilms treatment. For instance, Yuan et al. proposed AI-MPDA, an integrated nanoplatform for light therapy comprising mesoporous polydopamine (MPDA), ICG, and L-Arg. The NO released by L-Arg combined with low-temperature PTT (≤45 °C) effectively eradicates bacteria and disrupts biological membrane structures within a few minutes of NIR irradiation [98] (Fig. 8B).
Fig. 8.
(A) Composition, structure, and mechanism of antibiotics and PTT combined antibacterial platform triggered by NIR. Reprinted from Ref. [97], Copyright (2023), with permission from Amer Chemical Soc. (B) AI-MPDA nanoparticles' biofilms removal mechanism is based on low-temperature PTT and NO-enhanced PDT. Reprinted from Ref. [98], Copyright (2020), with permission from Amer Chemical Soc. (C) Schematic diagram of the process for rapid biofilms elimination of Ti implants at mild temperatures assisted by NIR Ⅱ. Reprinted from Ref. [99], Copyright (2020), with permission from Elsevier Sci Ltd. (D) Schematic diagram of the synthesis process and mechanism of a cascade nanoreactor CPNC@GOx-Fe2+ with photothermal and wound protection properties. Reprinted from Ref. [100], Copyright (2023), with permission from Wiley-V C H Verlag Gmbh.
The light undergoes gradual attenuation as it propagates through human tissues due to internal dispersion, reflection, and absorption. Therefore, the applicability of NIR-Ⅰ (808 nm) is limited in comparison to NIR-Ⅱ (1000–1700 nm). Chu's team has developed a NIR-II PTT anti-biofilms system consisting of curcumin (Cur) and co-doped rare earth ions Ho, Yb, and F titanium dioxide (TiO2) NRs. This system overcomes the limitation that TiO2 cannot be activated by NIR-II light and enhances the photocatalytic activity of TiO2. Cur endows the material with QS inhibition and anti-inflammatory properties. The system effectively prevents mild implant infection in the absence of light irradiation and efficiently eradicates biofilms at 45 °C under NIR-II irradiation. After penetrating 1.2 cm of pork, the power output of the 808 nm laser decreases to less than 0.03 W, while the power output of the 1060 nm laser remains approximately at 0.05 W even after penetrating through a thickness of 2.2 cm [99] (Fig. 8C).
The utilization of appropriate dressings is essential in addition to PTT and other treatment methods for effective eradication of wound biofilms, ensuring protection against secondary injury caused by residual bacteria. For this purpose, Chen et al. developed a chitosan-modified palladium nano-cube (CPNC) based photothermal cascade nanoreactor (CPNC@GOx-Fe2+) incorporating GOx and Fe2+, which effectively catalyzes the oxidation of glucose to generate highly reactive ·OH. Besides, the photothermal characteristic of CPNC had a synergistic bacteriostatic effect on S. mutans. At the same time, the continuous generation of ·OH initiates the radical polymerization of poly(ethylene glycol) diacrylate (PEGDA), leading to the in situ formation of hydrogels, which create a moist and sterile environment for wound healing. The proposed approach presents a promising solution for the treatment of open wound infections. However, its application is restricted to superficial bacterial biofilms infections due to the limited tissue penetration ability of NIR [100] (Fig. 8D).
4.2.2. Nanomotors
In the past few years, nanomotors have been used to destroy bacterial biofilms due to their active motion and high permeability. Among them, magnetic nanomotors based on Fe3O4 have been widely used to artificially destroy the membrane, which significantly improves the penetration ability and bactericidal effect of drugs on biofilms. However, the development of MNPs with targeted mobility is challenging. Stanton et al. utilized the non-pathogenic magnetotactic bacteria (MSR-1) and drug-loaded mesoporous silica microtubules to construct biological hybrids that could be directed into mature E. coli biofilms. Then the acidic microenvironment of the biofilms could trigger the release of the antibiotic Cip, ensuring efficient drug delivery [101].
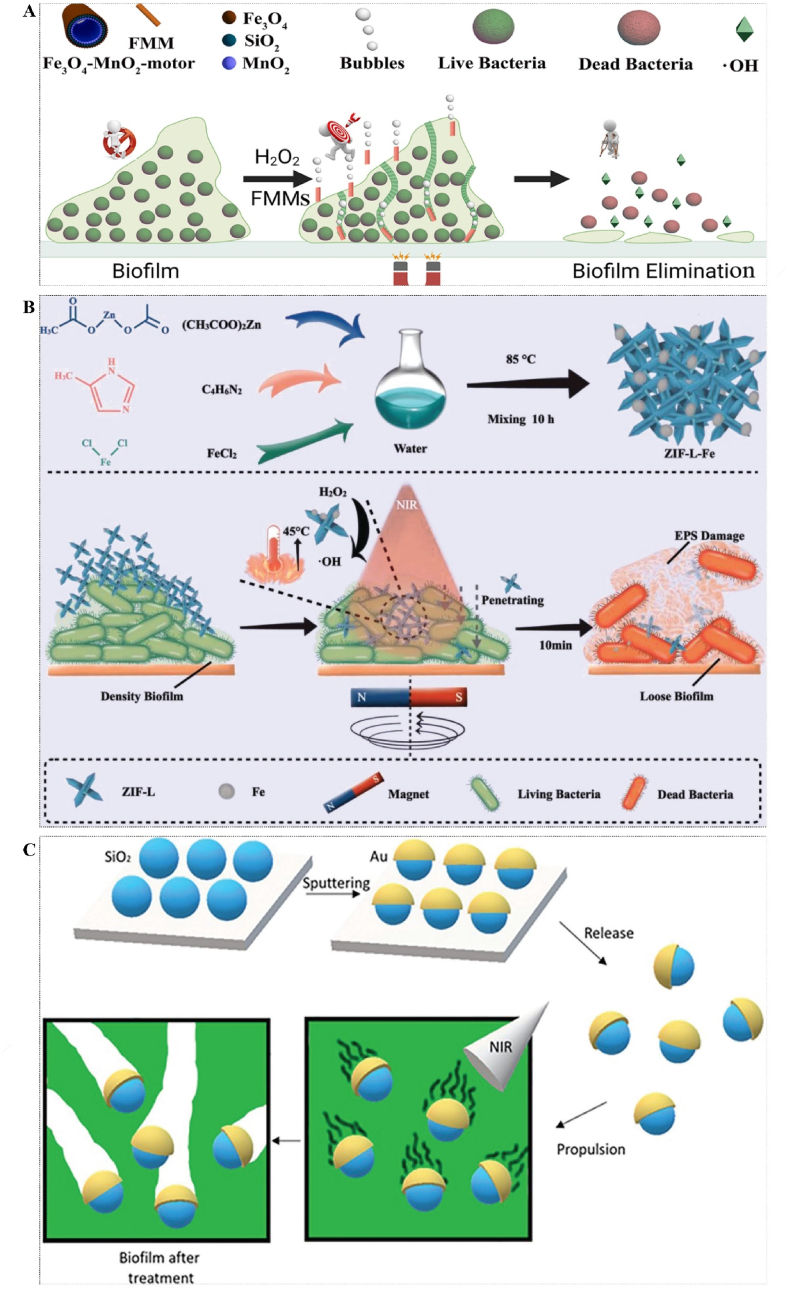
In addition, the magnetically driving NPs to drill biofilms still have many defects, such as slow motion and uncontrolled direction. Therefore, Ji et al. developed a magnetically controlled multifunctional micromotor system by using MNPs and mesoporous silica with MnO2 as catalysts and H2O2 as fuels. When MnO2 contact with H2O2 in the mesopore, a huge number of oxygen microbubbles could be immediately released, acting as a powerful propellant to propel the NPs move through the biofilms. Meanwhile, H2O2 was transformed into a highly toxic ·OH to kill bacteria due to the presence of magnetic Fe3O4. This strategy combines powerful mechanical damage, ROS, and precise magnetic guidance to achieve a short time of sterilization and anti-biofilms effect [102] (Fig. 9A).
Fig. 9.
(A) Schematic of a multifunctional nanomotor driven by magnetic and dye. Reprinted from Ref. [102], Copyright (2022), with permission from Elsevier. (B) Synthesis process and mechanism of magnetic cloud bomb. Reprinted from Ref. [103], Copyright (2023), with permission from Wiley-V C H Verlag Gmbh. (C) Schematic diagram of the mechanism of a self-propelled mesoporous SiO2/Au nanomotor driven by NIR light. Reprinted from Ref. [104], Copyright (2023), with permission from Wiley.
Still, the destruction effect of the biofilms is also influenced by the nanomotors' structure and dispersion. Sharp-shaped NPs are more likely to breach bacterial membranes and cause cytoplasmic leakage compared to blunt particles. Inspired by traditional cloud bombs, Qian et al. have modified the structure of zeolitic imidazolate framework (ZIF) from a sheet-like form to a flower-like configuration with sharp edges, known as ZIF-L-Fe. This modification facilitates enhanced penetration of biofilms through the application of magnetic force and promotes improved dispersion of Fe NPs. Additionally, it possesses PTT and PDT properties, enabling the eradication of MRSA at low temperatures (42–45 °C) while effectively catalyzing H2O2. In vitro or in vivo experiments conducted on mice skin wounds demonstrated that the synthesized MNPs exhibited potent antimicrobial effects against MRSA and biofilms [103] (Fig. 9B).
Although magnetically driven nanoparticles offer an ideal means of physically destroying biofilms, the loading and regulation of the magnetic field pose significant challenges that are constrained by various factors, including material limitations and biofilms location. Maric initially proposed a self-propelled nanomotor composed of mesoporous SiO2/Au, which is driven by NIR light. In this investigation, mesoporous silica nanoparticles were coated with a thin layer of Au to create asymmetric nanomotors. The mesoporous SiO2/Au nanomotors exhibited remarkable self-propulsion performance, ranging from a few μm/s to approximately 100 μm/s, which could be finely tuned by adjusting the laser power. Additionally, in conjunction with the PTT effect of Au, the SiO2/Au nanomotors exhibit enhanced penetration through the ECM and dispersion of biofilms. Following laser irradiation for 30 s and 3 min, there was a respective increase in the eradication rate of biofilms by 20% and 71%, respectively [104] (Fig. 9C). The light-driven nanomotor exhibits superior remote controllability compared to magnetically driven nanomotors. However, it is imperative to consider the targeting of biofilms and potential damage caused by light and heat to normal tissues.
4.3. Biological method
4.3.1. Phages invasion
Phages are frequently recommended as an alternative to antibiotics for treating biofilms since they are affordable, targeted, and do not disrupt the normal microbiota [105]. Some phages also possess the ability to degrade polysaccharides and rapidly destroy the integrity of the membrane [106,107]. Significantly, Cano et al. demonstrated the effectiveness of phages in the clinical treatment of recurrent Klebsiella pneumoniae infection on the false knee by applying the intravenous phage therapy. After treatment, the local infection symptoms were dramatically reduced, and the false knee function gradually improved without related adverse events [108]. This is a significant attempt of phages to move from the experimental to the clinical stage. Engineering bacteriophages may be promising candidates for the inhibition of pathogenic bacteria and biofilms in the future [109,110].
The early stage of phage therapy, however, involves extensive preparatory work that necessitates time and precise laboratory technical support, thereby augmenting the intricacy of treatment. In addition, the human immune system may elicit an immunological response to the phages, thereby diminishing their therapeutic efficacy and potentially inducing allergic reactions. Further investigations are warranted to substantiate the long-term effectiveness and safety of phage therapy.
4.3.2. The role of probiotics
Interestingly, not all biofilms exert a negative impact on human production and life. For instance, some biofilms play an essential role in delaying food spoilage, inhibiting toxic microorganisms, optimizing fermentation, and improving the yield and quality of food fermentation. Moreover, E. faecium and P. pentosaceus isolated from fermented fish and chickens have potential antibacterial and anti-biofilms effects on foodborne pathogenic bacteria, including B. cereus, S. enterica and E. coli [111]. Besides, T. halophilus, which is used to season salty fermented dishes, also possesses anti-biofilms activity against S. typhimurium and S. aureus [112]. These aforementioned methods employ beneficial bacteria to fight harmful bacteria, exhibiting excellent potential in the food industry. More importantly, this method has little environmental pollution, lasting effects, low cost, safety for people and livestock, and is not easy to produce resistance.
5. Raw materials with bactericidal and anti-biofilms potential
In addition to antibiotics, certain materials are extensively utilized in the field of antibacterial and anti-biofilms applications owing to their inherent properties. Here, we provide a comprehensive overview of the antimicrobial mechanisms and diverse applications associated with these commonly employed materials (Table 3).
Table 3.
Antimicrobial mechanism and application of common materials.
| Material | Antibacterial mechanism | Application | Bacterial type | Antibacterial adjuvant and antibacterial mechanism | Biocompatibility | Ref. |
|---|---|---|---|---|---|---|
| Au | Physical destruction of the biofilms by light-driven motion | – | P. aeruginosa | – | – | [104] |
| Au nanorods | PTT | Ablation infected osteomyelitis | MRSA | Cip (antibiotic) | Cell viability was >80%. | [97] |
| Au NPs | Treatment of subcutaneous abscess | S. aureus | PHMB (electrostatic interaction) | Cell viability was >90%. | [113] | |
| Fe2+ | ·OH is produced through the Fenton or Fenton-like reaction | Protecting tooth extraction wound | S. mutans | CPNC (PTT) | No significant cytotoxicity, and hemolysis rates <5%. |
[100] |
| Fe3O4 | Magnetically driven physical destruction | Treatment of skin and soft tissue infection | MRSA | ZIF (physical destruction of the biofilms by a flower-like structure with sharp edges) | Cell viability was >90%. | [103] |
| Elimination of microchannels biofilms | S. aureus, MRSA and P. aeruginosa | H2O2 (dye drives physical destruction of the biofilms) | No significant cytotoxicity. | [102] | ||
| POD-like activity catalyze the generation of·OH | Wounds and subcutaneous implant-associated biofilms infection treatment | S. aureus and E. coli | GO (PTT) | No significant cytotoxicity. | [54] | |
| Ga3+ | Interfering with Fe3+ metabolism of bacteria or biofilms | Orthopedic implants Ti–Ga alloys | S. aureus and E. coli | – | No significant cytotoxicity. | [53] |
| Pt | POD-like activity catalyze the generation of ·OH | Therapy of biofilms-induced periodontitis | S. aureus | – | Cell viability was >90%, and hemolysis rates were <5%. | [114] |
| Ce (IV) | DNase activity, degrades eDNA | Treatment of subcutaneous abscess | S. aureus | GO (PTT) | Cell viability was >80%. | [32] |
| Snitrosoglutathione (GSNO) | Release NO due to GSNO could be disrupted by heat, light, and some metal ions | Therapy of infected wounds | E. coli and S. aureus | – | Cell viability was >90%. | [88] |
| L-Arg | Release NO due to oxidization of H2O2 | Treatment of infected wounds. | E. coli and S. aureus | – | – | [85] |
| NONOate | Release NO in an acidic microenvironment | Alleviate inflammation in a subcutaneous infection | S. aureus | Cip (antibiotic) | No significant cytotoxicity. | [63] |
| QSC | Electrostatic interaction | Coating | P. aeruginosa | ACY (QSI) | Cell viability was >95%. | [46] |
| QAS | Electrostatic interaction | Coating applied on the surface of orthopedic implant materials | E. coli and S. aureus | – | Hemolysis rates <0.1%. | [28] |
6. Conclusions and future perspectives
Biofilms are bacterial populations surrounded by ECM that operate as a barrier against external antimicrobial agents. Developing new antibacterial drugs and strategies has become an urgent demand. We summarize the limitations and development prospects of the above strategies (Table 4).
Table 4.
Limitations and prospects of anti-biofilms strategies.
| Strategies | Advantages | Disadvantages | Application |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Superficial wound | Deep tissue | Medical implant | Food packaging | Food preservation | Instrument and equipment | Environmental governance | |||||
| Preventing biofilms maturation | Inhibiting bacterial adhesion | Tackling the root of problems | Limited permanence; immunological rejection; uncontrollable and non-selective | ✓ | ✓ | ✓ | |||||
| Inhibiting ECM formation | Cutting the key link | In addition to eDNA, other EPS lack sufficient research; safety and efficacy need to be evaluated; highly specific and may not be effective for other biofilms | ✓ | ✓ | ✓ | ||||||
| Interfering with signaling between bacteria | Cutting the key link; less toxic side effects | Effective for specific biofilms; complex signal transduction mechanism; dose assessment is difficult | ✓ | ✓ | ✓ | ✓ | |||||
| Interfering with metabolism | Cutting the key link | Affect normal metabolism in the body; toxic and side effects need to be evaluated | ✓ | ✓ | ✓ | ✓ | |||||
| Destroying mature biofilms | Chemical methods | Antibiotics and alternatives | Usual strategy; clear mechanism | Antimicrobial resistance; drugs are difficult to penetrate biofilms | ✓ | ✓ | ✓ | ✓ | |||
| ROS | Broad spectrum | Non-specific cell killer; affects wound healing; limits the application of ROS by the anaerobic environment in biofilms; instability | ✓ | ✓ | ✓ | ✓ | |||||
| Physical methods | PTT | Long-range control; broad spectrum | Damage to normal tissue; limited depth of treatment | ✓ | ✓ | ✓ | ✓ | ||||
| Nanomotors | Broad spectrum; improves the penetration ability of drugs | The speed and direction of MNPs are not controllable | ✓ | ✓ | ✓ | ||||||
| Biological method | Phages invasion | High efficiency; specificity; self-adaptability; non-toxic | Complex preparation work; induce anaphylaxis | ✓ | ✓ | ✓ | |||||
| The role of probiotics | Mutual restraint, security, stability | Slow effect | ✓ | ✓ | |||||||
In the future, the development trend of controlling bacterial biofilms will mainly focus on the following aspects:
-
(1)
Study of the composition and structure of biofilms: In-depth understanding of the composition and structure of bacterial biofilms is essential for controlling their development. Future research will focus on exploring molecular-level insights and the application of surface nanotechnology to further understand and intervene in the formation process of bacterial biofilms.
-
(2)
Development of novel antibiotics and antimicrobials: Currently, there is an increasing number of bacteria that are becoming resistant to traditional antibiotics. Therefore, future research should focus on developing new natural or synthetic antibiotics and antimicrobials to effectively combat bacterial biofilms.
-
(3)
Research and application of new sterilization techniques: In addition to traditional antimicrobial drugs, future research should further explore and apply innovative sterilization techniques and methods in nanotechnology and bioengineering.
-
(4)
Pay close attention to bacterial biofilms in the other fields: Although bacterial biofilms are often considered as pathogens, they also pose a significant potential menace in certain fields. For example, the presence of biofilms adhered to the surfaces of food processing equipment and certain foods poses challenges in terms of cleaning, thereby elevating the potential risks associated with food safety. While the biofilms are also adhered to the instrument's surface, thereby reducing its lifespan. Additionally, the presence of biofilms in sewage poses a significant challenge to wastewater treatment processes. Furthermore, the proliferation of biofilms in livestock and feeding environments has a significant impact on the quality and cost of meat. In the future, research and exploration of controlling biofilms in other fields should be concerned.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Financial support was provided by the National Natural Science Foundation of China (21877083, 31901045, 31901056, 32171452), Jiangsu Specially-Appointed Professor (06200048, 06200053), Excellent Youth Foundation of Jiangsu Scientific Committee (BK20220060).
Contributor Information
Haiwei Ji, Email: jihaiwei64@ntu.edu.cn.
Yuling Qin, Email: ylqin@ntu.edu.cn.
Li Wu, Email: wuli8686@ntu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Alonso V.P.P., Goncalves M.P.M.B.B., de Brito F.A.E., Barboza G.R., Rocha L.d.O., Silva N.C.C. Dry surface biofilms in the food processing industry: an overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods. Compr. Rev. Food Sci. F. 2023;22(1):688–713. doi: 10.1111/1541-4337.13089. 10.1111/1541-4337.13089. [DOI] [PubMed] [Google Scholar]
- 2.Khalid S.J., Ain Q., Khan S.J., Jalil A., Siddiqui M.F., Ahmad T., Badshah M., Adnan F. Targeting Acyl Homoserine Lactones (AHLs) by the quorum quenching bacterial strains to control biofilm formation in Pseudomonas aeruginosa. Saudi J. Biol. Sci. 2022;29(3):1673–1682. doi: 10.1016/j.sjbs.2021.10.064. 10.1016/j.sjbs.2021.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui Y.J., Schmid B.V., Cao H.L., Dai X., Du Z.M., Easterday W.R., Fang H.H., Guo C.Y., Huang S.Q., Liu W.B., Qi Z.Z., Song Y.J., Tian H.Y., Wang M., Wu Y.R., Xu B., Yang C., Yang J., Yang X.W., Zhang Q.W., Jakobsen K.S., Zhang Y.J., Stenseth N.C., Yang R.F. Evolutionary selection of biofilm-mediated extended phenotypes in Yersinia pestis in response to a fluctuating environment. Nat. Commun. 2020;11(1):281. doi: 10.1038/s41467-019-14099-w. 10.1038/s41467-019-14099-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyte K.Z., Tabuteau H., Gaffney E.A., Foster K.R., Durham W.M. Microbial competition in porous environments can select against rapid biofilm growth. P. Natl. Acad. Sci. U. S. A. 2017;114(2):E161–E170. doi: 10.1073/pnas.1525228113. 10.1073/pnas.1525228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnaouteli S., Matoz-Fernandez D.A., Porter M., Kalamara M., Abbott J., MacPhee C.E., Davidson F.A., Stanley-wall N.R. Pulcherrimin formation controls growth arrest of the Bacillus subtilis biofilm. P. Natl. Acad. Sci. U. S. A. 2019;116(27):13553–13562. doi: 10.1073/pnas.1903982116. 10.1073/pnas.1903982116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Z., Liu Y., Anniwaer A., You Y., Guo J., Tang Y., Fu L., Yi L., Huang C. Rational designs of biomaterials for combating oral biofilm infections. Adv. Mater. 2023 doi: 10.1002/adma.202305633. [DOI] [PubMed] [Google Scholar]
- 7.Thapa R.K., Kim J.O., Kim J. Antimicrobial strategies for topical biofilm-based wound infections: past, present, and future. J. Pharm. Invest. 2023 10.1007/s40005-023-00628-9. [Google Scholar]
- 8.Sharma S., Mohler J., Mahajan S.D., Schwartz S.A., Bruggemann L., Aalinkeel R. Microbial biofilm: a review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms. 2023;11(6):1614. doi: 10.3390/microorganisms11061614. 10.3390/microorganisms11061614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almasri D., Dahman Y. Prosthetic joint infections: biofilm formation, management, and the potential of mesoporous bioactive glass as a new treatment option. Pharmaceutics. 2023;15(5):1401. doi: 10.3390/pharmaceutics15051401. 10.3390/pharmaceutics15051401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gholipour S., Shamsizadeh Z., Gwenzi W., Nikaeen M. The bacterial biofilm resistome in drinking water distribution systems: a systematic review. Chemosphere. 2023;329 doi: 10.1016/j.chemosphere.2023.138642. [DOI] [PubMed] [Google Scholar]
- 11.Yang H., Xu Z., Xu Z., Li Y. Mini-review of biofilm interactions with surface materials in industrial piping system. Membranes. 2023;13(2):125. doi: 10.3390/membranes13020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X.W., Kang F.X., Qu X.L., Fu H.Y., Alvarez P.J.J., Tao S., Zhu D.Q. Role of extracellular polymeric substances in microbial reduction of arsenate to arsenite by escherichia coli and Bacillus subtilis. Environ. Sci. Technol. 2020;54(10):6185–6193. doi: 10.1021/acs.est.0c01186. 10.1021/acs.est.0c01186. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X.D., Shi W.Y. first ed. People's Medical Publishing House; China: 2012. Microbial Biofilms and Infection. [Google Scholar]
- 14.Cordero M., Mitarai N., Jauffred L. Motility mediates satellite formation in confined biofilms. ISME J. 2023 doi: 10.1038/s41396-023-01494-x. 10.1038/s41396-023-01494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Blumenfeld R., Feng X.-Q., Weitz D.A. ?Phase transitions? in bacteria-from structural transitions in free living bacteria to phenotypic transitions in bacteria within biofilms. Phys. Life Rev. 2022;43:98–138. doi: 10.1016/j.plrev.2022.09.004. 10.1016/j.plrev.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Cendra M.d.M., Torrents E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021;49 doi: 10.1016/j.biotechadv.2021.107734. [DOI] [PubMed] [Google Scholar]
- 17.Paula A.J., Hwang G., Koo H. Dynamics of bacterial population growth in biofilms resemble spatial and structural aspects of urbanization. Nat. Commun. 2020;11(1):1354. doi: 10.1038/s41467-020-15165-4. 10.1038/s41467-020-15165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Y., Zhang Y.G. Enhanced biomimic bactericidal surfaces by coating with positively-charged ZIF nano-dagger arrays. Nanomed.-Nanotechnol. 2017;13(7):2199–2207. doi: 10.1016/j.nano.2017.06.003. 10.1016/j.nano.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Gu H., Lee S.W., Carnicelli J., Zhang T., Ren D.C. Magnetically driven active topography for long-term biofilm control. Nat. Commun. 2020;11(1):2211. doi: 10.1038/s41467-020-16055-5. 10.1038/s41467-020-16055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung K.K., Schumacher J.F., Sampson E.M., Burne R.A., Antonelli P.J., Brennana A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases. 2007;2(2):89–94. doi: 10.1116/1.2751405. 10.1116/1.2751405. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Wu M., Peng P., Liu J., Lu J., Qian S., Feng J. "Self-defensive" antifouling zwitterionic hydrogel coatings on polymeric substrates. ACS Appl. Mater. Interfaces. 2022:56097–56109. doi: 10.1021/acsami.2c17272. 10.1021/acsami.2c17272. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y.R., Wei T., Qu Y.C., Zhou Y., Zheng Y.J., Huang C.B., Zhang Y.X., Yu Q., Chen H. Smart, photothermally activated, antibacterial surfaces with thermally triggered bacteria-releasing properties. ACS Appl. Mater. Interfaces. 2020;12(19):21283–21291. doi: 10.1021/acsami.9b17581. 10.1021/acsami.9b17581. [DOI] [PubMed] [Google Scholar]
- 23.Lin J., Hu J., Wang W., Liu K., Zhou C., Liu Z., Kong S., Lin S., Deng Y., Guo Z. Thermo and light-responsive strategies of smart titanium-containing composite material surface for enhancing bacterially anti-adhesive property. Chem. Eng. J. 2021;407 doi: 10.1016/j.cej.2020.125783. [DOI] [Google Scholar]
- 24.Wei T., Tang Z.C., Yu Q., Chen H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces. 2017;9(43):37511–37523. doi: 10.1021/acsami.7b13565. 10.1021/acsami.7b13565. [DOI] [PubMed] [Google Scholar]
- 25.Qu Y.C., Wei T., Zhao J., Jiang S.B., Yang P., Yu Q., Chen H. Regenerable smart antibacterial surfaces: full removal of killed bacteria via a sequential degradable layer. J. Mater. Chem. B. 2018;6(23):3946–3955. doi: 10.1039/c8tb01122b. 10.1039/c8tb01122b. [DOI] [PubMed] [Google Scholar]
- 26.Zou Y., Lu K.Y., Lin Y.C., Wu Y., Wang Y.R., Li L.H.Z., Huang C.B., Zhang Y.X., Brash J.L., Chen H., Yu Q. Dual-functional surfaces based on an antifouling polymer and a natural antibiofilm molecule: prevention of biofilm formation without using biocides. ACS Appl. Mater. Interfaces. 2021;13(38):45191–45200. doi: 10.1021/acsami.1c10747. 10.1021/acsami.1c10747. [DOI] [PubMed] [Google Scholar]
- 27.Zwicker P., Schmidt T., Hornschuh M., Lode H., Kramer A., Mueller G. In vitro response of THP-1 derived macrophages to antimicrobially effective PHMB-coated Ti6Al4V alloy implant material with and without contamination with S. epidermidis and P. aeruginosa. Biomater. Res. 2022;26(1):1. doi: 10.1186/s40824-021-00247-1. 10.1186/s40824-021-00247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F., Hu Q., Wei Y., Meng W., Wang R., Liu J., Nie Y., Luo R., Wang Y., Shen B. Surface modification of titanium implants by pH-responsive coating designed for self-adaptive antibacterial and promoted osseointegration. Chem. Eng. J. 2022;435 doi: 10.1016/j.cej.2022.134802. [DOI] [Google Scholar]
- 29.Dieltjens L., Appermans K., Lissens M., Lories B., Kim W., Van der Eycken E.V., Foster K.R., Steenackers H.P. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 2020;11(1):107. doi: 10.1038/s41467-019-13660-x. 10.1038/s41467-019-13660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown H.L., Hanman K., Reuter M., Betts R.P., van Vliet A.H.M. Campylobacter jejuni biofilms contain extracellular DNA and are sensitive to DNase I treatment. Front. Microbiol. 2015;6:699. doi: 10.3389/fmicb.2015.00699. 10.3389/fmicb.2015.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z.W., Ji H.W., Liu C.Q., Bing W., Wang Z.Z., Qu X.G. A multinuclear metal complex based DNase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angew. Chem., Int. Ed. 2016;55(36):10732–10736. doi: 10.1002/anie.201605296. 10.1002/anie.201605296. [DOI] [PubMed] [Google Scholar]
- 32.Hu H.L., Kang X.X., Shan Z.H., Yang X.X., Bing W., Wu L., Ge H.Y., Ji H.W. A DNase-mimetic artificial enzyme for the eradication of drug-resistant bacterial biofilm infections. Nanoscale. 2022;14(7):2676–2685. doi: 10.1039/d1nr07629a. 10.1039/d1nr07629a. [DOI] [PubMed] [Google Scholar]
- 33.Rahman M.A., Amirkhani A., Chowdhury D., Mempin M., Molloy M.P., Deva A.K., Vickery K., Hu H. Proteome of Staphylococcus aureus biofilm changes significantly with aging. Int. J. Mol. Sci. 2022;23(12):6415. doi: 10.3390/ijms23126415. 10.3390/ijms23126415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danforth D.R., Melloni M., Tristano J., Mintz K.P. Contribution of adhesion proteins to Aggregatibacter actinomycetemcomitans biofilm formation. Mol. Oral Microbiol. 2021;36(4):243–253. doi: 10.1111/omi.12346. 10.1111/omi.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matilla-Cuenca L., Taglialegna A., Gil C., Toledo-Arana A., Lasa I., Valle J. Bacterial biofilm functionalization through Bap amyloid engineering. Npj Biofilms Microbi. 2022;8(1):62. doi: 10.1038/s41522-022-00324-w. 10.1038/s41522-022-00324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbey U., Andreasen M. Functional amyloids from bacterial biofilms - structural properties and interaction partners. Chem. Sci. 2022;13(22):6457–6477. doi: 10.1039/d2sc00645f. 10.1039/d2sc00645f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salinas N., Povolotsky T.L., Landau M., Kolodkin-Gal I. Emerging roles of functional bacterial amyloids in gene regulation, toxicity, and immunomodulation. Microbiol. Mol. Biol. R. 2021;85(1) doi: 10.1128/mmbr.00062-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perov S., Lidor O., Salinas N., Golan N., Tayeb-Fligelman E., Deshmukh M., Willbold D., Landau M. Structural insights into curli CsgA cross-beta fibril architecture inspire repurposing of anti-amyloid compounds as anti-biofilm agents. PLoS Pathog. 2019;15(8) doi: 10.1371/journal.ppat.1007978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer R., Wagner S., Rox K., Varrot A., Hauck D., Wamhoff E.C., Schreiber J., Ryckmans T., Brunner T., Rademacher C., Hartmann R.W., Bronstrup M., Imberty A., Titz A. Glycomimetic, Orally bioavailable lecB inhibitors block biofilm formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018;140(7):2537–2545. doi: 10.1021/jacs.7b11133. 10.1021/jacs.7b11133. [DOI] [PubMed] [Google Scholar]
- 40.Wagner S., Hauck D., Hoffmann M., Sommer R., Joachim I., Muller R., Imberty A., Varrot A., Titz A. Covalent lectin inhibition and application in bacterial biofilm imaging. Angew. Chem., Int. Ed. 2017;56(52):16559–16564. doi: 10.1002/anie.201709368. 10.1002/anie.201709368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav J., Das S., Singh S., Jyoti A., Srivastava V.K., Sharma V., Kumar S., Kumar S., Kaushik S. Deciphering the role of S-adenosyl homocysteine nucleosidase in quorum sensing mediated biofilm formation. Curr. Protein Pept. Sci. 2022;23(4):211–225. doi: 10.2174/1389203723666220519152507. 10.2174/1389203723666220519152507. [DOI] [PubMed] [Google Scholar]
- 42.Coelho P., Oliveira J., Fernandes I., Araujo P., Pereira A.R., Gameiro P., Bessa L.J. Pyranoanthocyanins interfering with the quorum sensing of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Mol. Sci. 2021;22(16):8559. doi: 10.3390/ijms22168559. 10.3390/ijms22168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang D., Hao S., Zhao L., Shi F., Ye G., Zou Y., Song X., Li L., Yin Z., He X., Feng S., Chen H., Zhang Y., Gao Y., Li Y., Tang H. Paeonol attenuates quorum-sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.692474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh V.K., Mishra A., Jha B. 3-benzyl-hexahydro-pyrrolo 1,2a pyrazine-1,4-dione extracted from exiguobacterium indicum showed anti-biofilm activity against Pseudomonas aeruginosa by attenuating quorum sensing. Front. Microbiol. 2019;10:1269. doi: 10.3389/fmicb.2019.01269. 10.3389/fmicb.2019.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q., Mao S., Wang H., Ye X. The molecular architecture of Pseudomonas aeruginosa quorum-sensing inhibitors. Mar. Drugs. 2022;20(8):488. doi: 10.3390/md20080488. 10.3390/md20080488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou Y., Liu C.X., Zhang H.X., Wu Y., Lin Y.C., Cheng J.J., Lu K.Y., Li L.H.Z., Zhang Y.X., Chen H., Yu Q. Three lines of defense: a multifunctional coating with anti-adhesion, bacteria-killing and anti-quorum sensing properties for preventing biofilm formation of Pseudomonas aeruginosa. Acta Biomater. 2022;151:254–263. doi: 10.1016/j.actbio.2022.08.008. 10.1016/j.actbio.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee P., Sahoo P.K., Sheenu, Adhikary A., Ruhal R., Jain D. Molecular and structural facets of c-di-GMP signalling associated with biofilm formation in Pseudomonas aeruginosa. Mol. Aspects Med. 2021;81 doi: 10.1016/j.mam.2021.101001. [DOI] [PubMed] [Google Scholar]
- 48.Liu X.B., Cao B., Yang L., Gu J.D. Biofilm control by interfering with c-di-GMP metabolism and signaling. Biotechnol. Adv. 2022;56 doi: 10.1016/j.biotechadv.2022.107915. [DOI] [PubMed] [Google Scholar]
- 49.Foletti C., Kramer R.A., Mauser H., Jenal U., Bleicher K.H., Wennemers H. Functionalized proline-rich peptides bind the bacterial second messenger c-di-GMP. Angew. Chem., Int. Ed. 2018;57(26):7729–7733. doi: 10.1002/anie.201801845. 10.1002/anie.201801845. [DOI] [PubMed] [Google Scholar]
- 50.Gelinas M., Museau L., Milot A., Beauregard P.B. The de novo purine biosynthesis pathway is the only commonly regulated cellular pathway during biofilm formation in TSB-based medium in Staphylococcus aureus and Enterococcus faecalis. Microbiol. Spectr. 2021;9(3) doi: 10.1128/Spectrum.00804-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha M., Ghosh N., Wijesinghe D.S., Mathew-Steiner S.S., Das A., Singh K., Masry M.E., Khanna S., Inoue H., Yamazaki K., Kawada M., Gordillo G.M., Roy S., Sen C.K. Pseudomonas aeruginosa theft biofilm require host lipids of cutaneous wound. Ann. Surg. 2021:E634–E647. doi: 10.1097/SLA.0000000000005252. 10.1097/sla.0000000000005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu D.F., Zou L.Y., Yu W.J., Jia F., Han H.J., Yao K., Jin Q., Ji J. Relief of biofilm hypoxia using an oxygen nanocarrier: a new paradigm for enhanced antibiotic therapy. Adv. Sci. 2020;7(12) doi: 10.1002/advs.202000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li F.P., Huang K., Wang J.B., Yuan K., Yang Y.Q., Liu Y.H., Zhou X.H., Kong K.Y., Yang T., He J., Liu C.J., Ao H.Y., Liu F.X., Liu Q., Tang T.T., Yang S.B. A dual functional Ti-Ga alloy: inhibiting biofilm formation and osteoclastogenesis differentiation via disturbing iron metabolism. Biomater. Res. 2023;27(1):24. doi: 10.1186/s40824-023-00362-1. 10.1186/s40824-023-00362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu W., Mei J., Zhang X., Zhou J., Xu D., Su Z., Fang S., Wang J., Zhang X., Zhu C. Photothermal nanozyme-based microneedle patch against refractory bacterial biofilm infection via iron-actuated janus ion therapy. Adv. Mater. 2022;34(51) doi: 10.1002/adma.202207961. 10.1002/adma.202207961. [DOI] [PubMed] [Google Scholar]
- 55.Oliveira F., Rohde H., Vilanova M., Cerca N. The emerging role of iron acquisition in biofilm-associated infections. Trends Microbiol. 2021;29(9):772–775. doi: 10.1016/j.tim.2021.02.009. 10.1016/j.tim.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Kaneko Y., Thoendel M., Olakanmi O., Britigan B.E., Singh P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 2007;117(4):877–888. doi: 10.1172/JCI30783. 10.1172/jci30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banin E., Lozinski A., Brady K.M., Berenshtein E., Butterfield P.W., Moshe M., Chevion M., Greenberg E.P., Banin E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. P. Natl. Acad. Sci. U. S. A. 2008;105(43):16761–16766. doi: 10.1073/pnas.0808608105. 10.1073/pnas.0808608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y.Q., Mei Y.X., Song Y.S., Bachus C., Sun C.X., Sheshbaradaran H., Glogauer M. AP-002: a novel inhibitor of osteoclast differentiation and function without disruption of osteogenesis. Eur. J. Pharmacol. 2020;889 doi: 10.1016/j.ejphar.2020.173613. [DOI] [PubMed] [Google Scholar]
- 59.Geljic I.S., Melis N., Boukhechba F., Schaub S., Mellier C., Janvier P., Laugier J.P., Bouler J.M., Verron E., Scimeca J.C. Gallium enhances reconstructive properties of a calcium phosphate bone biomaterial. J. Tissue. Eng. Regen. M. 2018;12(2):E854–E866. doi: 10.1002/term.2396. 10.1002/term.2396. [DOI] [PubMed] [Google Scholar]
- 60.Abouelhassan Y., Zhang Y.P., Jin S.G., Huigens R.W. Transcript profiling of MRSA biofilms treated with a halogenated phenazine eradicating agent: a platform for defining cellular targets and pathways critical to biofilm survival. Angew. Chem., Int. Ed. 2018;57(47):15523–15528. doi: 10.1002/anie.201809785. 10.1002/anie.201809785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papadopoulou V., Sidders A.E., Lu K.-Y., Velez A.Z., Durham P.G., Bui D.T., Angeles-Solano M., Dayton P.A., Rowe S.E. Overcoming biological barriers to improve treatment of a Staphylococcus aureus wound infection. Cell Chem. Biol. 2023;30(5):513–526.e5. doi: 10.1016/j.chembiol.2023.04.009. 10.1016/j.chembiol.2023.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding Y.X., Hu X.W., Piao Y.Z., Huang R., Xie L.P., Yan X.J., Sun H., Li Y.F., Shi L.Q., Liu Y. Lipid prodrug nanoassemblies via dynamic covalent boronates. ACS Nano. 2023 doi: 10.1021/acsnano.2c12233. 10.1021/acsnano.2c12233. [DOI] [PubMed] [Google Scholar]
- 63.Zhan Y.Z., Hu X.W., Li Y.F., Wang Y.R., Chen H., Omolo C.A., Govender T., Li H.P., Huang F., Shi L.Q., Hu X.L., Liu Y. Antimicrobial hybrid amphiphile via dynamic covalent bonds enables bacterial biofilm dispersal and bacteria eradication. Adv. Funct. Mater. 2023;33(23) 10.1002/adfm.202214299. [Google Scholar]
- 64.Ren H.M., Han L., Zhang L.J., Zhao Y.Q., Lei C.Y., Xiu Z.P., Zhao N.A., Yu B.R., Zhou F., Duan S., Xu F.J. Inhalable responsive polysaccharide-based antibiotic delivery nanoparticles to overcome mucus barrier for lung infection treatment. Nano Today. 2022;44 doi: 10.1016/j.nantod.2022.101489. [DOI] [Google Scholar]
- 65.Rima M., Rima M., Fajloun Z., Sabatier J.-M., Bechinger B., Naas T. Antimicrobial peptides: a potent alternative to antibiotics. Antibiotics-Basel. 2021;10(9):1095. doi: 10.3390/antibiotics10091095. 10.3390/antibiotics10091095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X., Zuo S., Wang B., Zhang K., Wang Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules. 2022;27(9):2675. doi: 10.3390/molecules27092675. 10.3390/molecules27092675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riool M., de Breij A., de Boer L., Kwakman P.H.S., Cordfunke R.A., Cohen O., Malanovic N., Emanuel N., Lohner K., Drijfhout J.W., Nibbering P.H., Zaat S.A.J. Controlled release of LL-37-derived synthetic antimicrobial and anti-biofilm peptides SAAP-145 and SAAP-276 prevents experimental biomaterial-associated Staphylococcus aureus infection. Adv. Funct. Mater. 2017;27(20) doi: 10.1002/adfm.201606623. [DOI] [Google Scholar]
- 68.Pulido D., Prats-Ejarque G., Villalba C., Albacar M., Moussaoui M., Andreu D., Volkmer R., Torrent M., Boix E. Positional scanning library applied to the human eosinophil cationic protein/RNase3 N-terminus reveals novel and potent anti-biofilm peptides. Eur. J. Med. Chem. 2018;152:590–599. doi: 10.1016/j.ejmech.2018.05.012. 10.1016/j.ejmech.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Klodzinska S.N., Pletzer D., Rahanjam N., Rades T., Hancock R.E.W., Nielsen H.M. Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5. Nanomed.-Nanotechnol. 2019;20 doi: 10.1016/j.nano.2019.10202266. [DOI] [PubMed] [Google Scholar]
- 70.D'Aquila P., Paparazzo E., Crudo M., Bonacci S., Procopio A., Passarino G., Bellizzi D. Antibacterial activity and epigenetic remodeling of essential oils from Calabrian aromatic plants. Nutrients. 2022;14(2):391. doi: 10.3390/nu14020391. 10.3390/nu14020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kacaniova M., Galovicova L., Valkova V., Duranova H., Stefanikova J., Cmikova N., Vukic M., Vukovic N.L., Kowalczewski P.L. Chemical composition, antioxidant, in vitro and in situ antimicrobial, antibiofilm, and anti-insect activity of Cedar atlantica essential oil. Plants-Basel. 2022;11(3):358. doi: 10.3390/plants11030358. 10.3390/plants11030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao C., Tian L., Lu J.X., Zhang S.Y., Wu D.Y., Xiao P., Gong G.L. Cinnamaldehyde reduces Yersinia enterocolitica activity and biofilm formation by altering membrane permeability and signalling. Food Biosci. 2023;53 doi: 10.1016/j.fbio.2023.102791. [DOI] [Google Scholar]
- 73.Liu Y.E., Yan Y.Q., Dong P.C., Ni L.X., Luo X., Zhang Y.M., Zhu L.X. Inhibitory effects of clove and oregano essential oils on biofilm formation of Salmonella Derby isolated from beef processing plant. LWT--Food Sci. Technol. 2022;162 doi: 10.1016/j.lwt.2022.113486. [DOI] [Google Scholar]
- 74.Hu J.J., Zhu H.X., Feng Y.W., Yu M.J., Xu Y.Q., Zhao Y.D., Zheng B., Lin J.H., Miao W.H., Zhou R.S., Cullen P.J. Emulsions containing composite (clove, oregano, and cinnamon) essential oils: phase inversion preparation, physicochemical properties and antibacterial mechanism. Food Chem. 2023;421 doi: 10.1016/j.foodchem.2023.136201. [DOI] [PubMed] [Google Scholar]
- 75.El-Din M.I.G., Youssef F.S., Altyar A.E., Ashour M.L. GC/MS analyses of the essential oils obtained from different Jatropha Species, their discrimination using chemometric analysis and assessment of their antibacterial and anti-biofilm activities. Plants-Basel. 2022;11(9):1268. doi: 10.3390/plants11091268. 10.3390/plants11091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan J., Yuan W.Q., Guo Y.Y., Wu Q., Wang F., Xuan H.Z. Anti-biofilm activities of Chinese Poplar Propolis essential oil against Streptococcus mutans. Nutrients. 2022;14(16):3290. doi: 10.3390/nu14163290. 10.3390/nu14163290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliveira A.S., Rolo J., Gaspar C., Cavaleiro C., Salgueiro L., Palmeira-de-Oliveira R., Ferraz C., Coelho S., Pastorinho M.R., Sousa A.C., Teixeira J.P., Martinez-de-Oliveira J., Palmeira-de-Oliveira A. Chemical characterization and bioactive potential of Thymus x citriodorus (Pers.) Schreb. preparations for anti-acne applications: antimicrobial, anti-biofilm, anti-inflammatory and safety profiles. J. Ethnopharmacol. 2022;287 doi: 10.1016/j.jep.2021.114935. [DOI] [PubMed] [Google Scholar]
- 78.Bouymajane A., Filali F.R., Ed-Dra A., Aazza M., Nalbone L., Giarratana F., Alibrando F., Miceli N., Mondello L., Cacciola F. Chemical profile, antibacterial, antioxidant, and anisakicidal activities of Thymus zygis subsp. gracilis essential oil and its effect against Listeria monocytogenes. Int. J. Food Microbiol. 2022;383 doi: 10.1016/j.ijfoodmicro.2022.109960. [DOI] [PubMed] [Google Scholar]
- 79.Khalid K.A. Evaluation of leaf, flower and peel bitter orange essential oils and their constituents in response to various planting locations of Egypt. J. Essent. Oil Bear. Pl. 2022;25(2):208–218. 10.1080/0972060x.2022.2055435. [Google Scholar]
- 80.Khalid K.A.A., Abd-Rabbu H.S.S., Wahba H.E.E. Harvest seasons induced variations in the compositions of gold crest and sodom apple essential oils. J. Essent. Oil Bear. Pl. 2022;25(5):1159–1168. 10.1080/0972060x.2022.2153627. [Google Scholar]
- 81.Silveira R.M., Carvalho A.F.U., Bunger M.O., Gomes M.M., da Costa I.R. How much do the environmental conditions interfere with the essential oils of Eugenia spp. L. (Myrtaceae)? J. Braz. Chem. Soc. 2022;33(3):274. 10.21577/0103-5053.20210146. [Google Scholar]
- 82.Chen M., Long Z., Dong R.H., Wang L., Zhang J.J., Li S.X., Zhao X.H., Hou X.D., Shao H.W., Jiang X.Y. Titanium incorporation into Zr-porphyrinic metal-organic frameworks with enhanced antibacterial activity against multidrug-resistant pathogens. Small. 2020;16(7) doi: 10.1002/smll.201906240. [DOI] [PubMed] [Google Scholar]
- 83.Dong Q., Li Z.Q., Xu J.Q., Yuan Q., Chen L., Chen Z. Versatile graphitic nanozymes for magneto actuated cascade reaction-enhanced treatment of S. mutans biofilms. Nano Res. 2022;15(11):9800–9808. 10.1007/s12274-022-4258-x. [Google Scholar]
- 84.Ji H.W., Dong K., Yan Z.Q., Ding C., Chen Z.W., Ren J.S., Qu X.G. Bacterial hyaluronidase self-triggered prodrug release for chemo-photothermal synergistic treatment of bacterial infection. Small. 2016;12(45):6200–6206. doi: 10.1002/smll.201601729. 10.1002/smll.201601729. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y.T., Cao Y.F., Cheng J., Yu W.W., Liu M.S., Yin J.J., Huang C.S., Liang X.Q., Zhou H.C., Liu H.B., Yang Z., Fang Y., Wei H., Zhao G.H. Construction of self-activated nanoreactors for cascade catalytic anti-biofilm therapy based on H2O2 self-generation and switch-on NO release. Adv. Funct. Mater. 2022;32(20) doi: 10.1002/adfm.202111148. [DOI] [Google Scholar]
- 86.Cai Y.-M., Zhang Y.-D., Yang L. NO donors and NO delivery methods for controlling biofilms in chronic lung infections. Appl. Microbiol. Biotechnol. 2021;105(10):3931–3954. doi: 10.1007/s00253-021-11274-2. 10.1007/s00253-021-11274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang T.-Y., Zhu X.-Y., Wu F.G. Antibacterial gas therapy: strategies, advances, and prospects. Bioact. Mater. 2023;23:129–155. doi: 10.1016/j.bioactmat.2022.10.008. 10.1016/j.bioactmat.2022.10.008.63M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li N., Li N., Qiu W.W., Wang Q., Jia J., Wang X.L., Yu J.Y., Li X.R., Li F.X., Wu D.Q. Phenylalanine-based poly(ester urea)s composite films with nitric oxide-releasing capability for anti-biofilm and infected wound healing applications. J. Colloid Interface Sci. 2022;607:1849–1863. doi: 10.1016/j.jcis.2021.10.016. 10.1016/j.jcis.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 89.Hasan N., Cao J., Lee J., Naeem M., Hlaing S.P., Kim J., Jung Y., Lee B.-L., Yoo J.-W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mat. Sci. Eng. C-Mater. 2019;103 doi: 10.1016/j.msec.2019.109741. [DOI] [PubMed] [Google Scholar]
- 90.Lv Z.Q., He S.J., Wang Y.F., Zhu X.Y. Noble metal nanomaterials for NIR-triggered photothermal therapy in cancer. Adv. Healthcare Mater. 2021;10(6) doi: 10.1002/adhm.202001806. [DOI] [PubMed] [Google Scholar]
- 91.Lu J.H., Cai L.L., Dai Y., Liu Y.W., Zuo F.M., Ni C., Shi M.L., Li J.J. Polydopamine-based nanoparticles for photothermal therapy/chemotherapy and their synergistic therapy with autophagy inhibitor to promote antitumor treatment. Chem. Rec. 2021;21(4):781–796. doi: 10.1002/tcr.202000170. 10.1002/tcr.202000170. [DOI] [PubMed] [Google Scholar]
- 92.Dash B.S., Das S., Chen J.P. Photosensitizer-functionalized nanocomposites for light-activated cancer theranostics. Int. J. Mol. Sci. 2021;22(13):6658. doi: 10.3390/ijms22136658. 10.3390/ijms22136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lagos K.J., Buzza H.H., Bagnato V.S., Romero M.P. Carbon-based materials in photodynamic and photothermal therapies applied to tumor destruction. Int. J. Mol. Sci. 2022;23(1):22. doi: 10.3390/ijms23010022. 10.3390/ijms23010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao R., Su L., Yu T., Liu J., van der Mei H.C., Ren Y., Chen G., Shi L., Peterson B.W., Busscher H.J. Encapsulation of photothermal nanoparticles in stealth and pH-responsive micelles for eradication of infectious biofilms in vitro and in vivo. Nanomaterials. 2021;11(12):3180. doi: 10.3390/nano11123180. 10.3390/nano11123180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Z., Chen C., Wu W., Wang F., Du L., Zhang X., Xiong Y., He X., Cai Y., Kwok R.T.K., Lam J.W.Y., Gao X., Sun P., Phillips D.L., Ding D., Tang B.Z. Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles. Nat. Commun. 2019;10:768. doi: 10.1038/s41467-019-08722-z. 10.1038/s41467-019-08722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He W., Wang Z., Bai H., Zhao Z., Kwok R.T.K., Lam J.W.Y., Tang B.Z. Highly efficient photothermal nanoparticles for the rapid eradication of bacterial biofilms. Nanoscale. 2021;13(32):13610–13616. doi: 10.1039/d1nr03471e. 10.1039/d1nr03471e. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Z., Zhang Y., Cheng Y., Li J., Wang W., Huang S., Ma X., Zhang X. Thermosensitive nanotherapeutics for localized photothermal ablation of MRSA-infected osteomyelitis combined with chemotherapy. ACS Appl. Mater. Interfaces. 2023;15(10):12842–12854. doi: 10.1021/acsami.2c23312. 10.1021/acsami.2c23312. [DOI] [PubMed] [Google Scholar]
- 98.Yuan Z., Lin C.C., He Y., Tao B.L., Chen M.W., Zhang J.X., Liu P., Cai K.Y. Near-Infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination. ACS Nano. 2020;14(3):3546–3562. doi: 10.1021/acsnano.9b09871. 10.1021/acsnano.9b09871. [DOI] [PubMed] [Google Scholar]
- 99.Zhang G., Yang Y., Shi J., Yao X., Chen W., Wei X., Zhang X., Chu P.K. Near-infrared light II - assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120634. [DOI] [PubMed] [Google Scholar]
- 100.Chen L., Peng M.N., Zhou J.A., Hu X.W., Piao Y.Z., Li H.P., Hu R.D., Li Y.F., Shi L.Q., Liu Y. Supramolecular photothermal cascade nano-reactor enables photothermal effect, cascade reaction, and in situ hydrogelation for biofilm-associated tooth-extraction wound healing. Adv. Mater. 2023;35(31) doi: 10.1002/adma.202301664. [DOI] [PubMed] [Google Scholar]
- 101.Stanton M.M., Park B.W., Vilele D., Bente K., Faivre D., Sitti M., Sanchez S. Magnetotactic bacteria powered biohybrids target E. coli biofilms. ACS Nano. 2017;11(10):9968–9978. doi: 10.1021/acsnano.7b04128. 10.1021/acsnano.7b04128. [DOI] [PubMed] [Google Scholar]
- 102.Ji H.W., Hu H.L., Tang Q., Kang X.X., Liu X.D., Zhao L.F., Jing R.R., Wu M.M., Li G., Zhou X.B., Liu J.X., Wang Q., Cong H., Wu L., Qin Y.L. Precisely controlled and deeply penetrated micro-nano hybrid multifunctional motors with enhanced antibacterial activity against refractory biofilm infections. J. Hazard Mater. 2022;436 doi: 10.1016/j.jhazmat.2022.129210. [DOI] [PubMed] [Google Scholar]
- 103.Qian Y., Zhang L., Li N., Dong F., Yang Z., Dong H., Wang Y., Wang M. A magnetic cloud bomb for effective biofilm eradication. Adv. Funct. Mater. 2023;33(15) doi: 10.1002/adfm.202214330. [DOI] [Google Scholar]
- 104.Maric T., Lovind A., Zhang Z., Geng J., Boisen A. Near-Infrared light-driven mesoporous SiO2/Au nanomotors for eradication of Pseudomonas aeruginosa biofilm. Adv. Healthcare Mater. 2023;12(13) doi: 10.1002/adhm.202203018. 10.1002/adhm.202203018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang C., Yu X., Guo W., Guo C., Guo X., Li Q., Zhu Y. Bacteriophage-mediated control of biofilm: a promising new dawn for the future. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.825828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaszowska M., Majkowska-Skrobek G., Markwitz P., Lood C., Jachymek W., Maciejewska A., Lukasiewicz J., Drulis-Kawa Z. The mutation in wbaP cps gene cluster selected by phage-borne depolymerase abolishes capsule production and diminishes the virulence of klebsiella pneumoniae. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zurabov F., Zhilenkov E. Characterization of four virulent Klebsiella pneumoniae bacteriophages, and evaluation of their potential use in complex phage preparation. Virol. J. 2021;18(1):9. doi: 10.1186/s12985-020-01485-w. 10.1186/s12985-020-01485-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cano E.J., Caflisch K.M., Bollyky P.L., Van Belleghem J.D., Patel R., Fackler J., Brownstein M.J., Horne B.A., Biswas B., Henry M., Malagon F., Lewallen D.G., Suh G.A. Phage therapy for limb-threatening prosthetic knee klebsiella pneumoniae infection: case report and in vitro characterization of anti-biofilm activity. Clin. Infect. Dis. 2021;73(1):E144–E151. doi: 10.1093/cid/ciaa705. 10.1093/cid/ciaa705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun R., Yu P., Zuo P., Villagran D., Mathieu J., Alvarez P.J.J. Biofilm control in flow-through systems using polyvalent phages delivered by peptide-modified M13 coliphages with enhanced polysaccharide affinity. Environ. Sci. Technol. 2022 doi: 10.1021/acs.est.2c06561. 10.1021/acs.est.2c06561. [DOI] [PubMed] [Google Scholar]
- 110.Tang M., Huang Z., Zhang X., Kong J., Zhou B., Han Y., Zhang Y., Chen L., Zhou T. Phage resistance formation and fitness costs of hypervirulent Klebsiella pneumoniae mediated by K2 capsule-specific phage and the corresponding mechanisms. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1156292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tatsaporn T., Kornkanok K. Using potential lactic acid bacteria biofilms and their compounds to control biofilms of foodborne pathogens. Biotechnol. Rep. 2020;26 doi: 10.1016/j.btre.2020.e00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yao S.J., Hao L.Y., Zhou R.Q., Jin Y., Huang J., Wu C.D. Formation of biofilm by tetragenococcus halophilus benefited stress tolerance and anti-biofilm activity against S. aureus and S. Typhimurium. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.819302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He X., Dai L., Ye L., Sun X., Enoch O., Hu R., Zan X., Lin F., Shen J. A vehicle-free antimicrobial polymer hybrid gold nanoparticle as synergistically therapeutic platforms for Staphylococcus aureus infected wound healing. Adv. Sci. 2022;9(14) doi: 10.1002/advs.202105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu Y., Cheng Y., Tan L., Liu X.M., Li Z.Y., Zheng Y.F., Wu T., Liang Y.Q., Cui Z.D., Zhu S.L., Wu S.L. Theory-screened MOF-based single-atom catalysts for facile and effective therapy of biofilm-induced periodontitis. Chem. Eng. J. 2022;431(4) doi: 10.1016/j.cej.2021.133279. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.