Abstract
Purpose
Patient-derived tumor cells can be a powerful resource for studying pathophysiological mechanisms and developing robust strategies for precision medicine. However, establishing organoids from patient-derived cells is challenging because of limited access to tissue specimens. Therefore, we aimed to establish organoids from malignant ascites and pleural effusions.
Materials and Methods
Ascitic or pleural fluid from pancreatic, gastric, and breast cancer patients was collected and concentrated to culture tumor cells ex vivo. Organoids were considered to be successfully cultured when maintained for five or more passages. Immunohistochemical staining was performed to compare the molecular features, and drug sensitivity was assayed to analyze the clinical responses of original patients.
Results
We collected 70 fluid samples from 58 patients (pancreatic cancer, n=39; gastric cancer, n=21; and breast cancer, n=10). The overall success rate was 40%; however, it differed with types of malignancy, with pancreatic, gastric, and breast cancers showing 48.7%, 33.3%, and 20%, respectively. Cytopathological results significantly differed between successful and failed cases (p=0.014). Immunohistochemical staining of breast cancer organoids showed molecular features identical to those of tumor tissues. In drug sensitivity assays, pancreatic cancer organoids recapitulated the clinical responses of the original patients.
Conclusion
Tumor organoids established from malignant ascites or pleural effusion of pancreatic, gastric, and breast cancers reflect the molecular characteristics and drug sensitivity profiles. Our organoid platform could be used as a testbed for patients with pleural and peritoneal metastases to guide precision oncology and drug discovery.
Keywords: Organoids, Pancreatic neoplasms, Stomach neoplasms, Breast neoplasms, Ascites, Pleural effusion
Introduction
Malignant ascites and pleural effusion arise from metastatic tumors in the peritoneal and pleural cavities. Peritoneal metastasis frequently occurs in gastrointestinal cancers (e.g., stomach, colon, and pancreas) while pleural metastasis occurs in lung and breast cancers [1–3]. The pathophysiological mechanism of fluid accumulation in pleural/peritoneal metastases is complex and multifactorial [4,5]. Nevertheless, free-floating tumor cells in the fluid can often be detected by cytopathological analyses, such as cytospin or liquid-based cytology, which reflects the aggressive nature of metastatic tumor. Metastases to these sites are associated with grave prognoses owing to inadequate responses to standard chemotherapy, which eventually causes organ dysfunction and death [6,7]. Therefore, strategies to overcome this clinical limitation are urgently required.
An organoid is a three-dimensional (3D) culture system that efficiently recapitulates in vivo aspects of a tissue or organ [8]. Expanded to cancer, a patient’s tumor cells obtained from surgical or biopsy specimens can be grown in 3D structures that systematically recapitulate molecular characteristics of the original tumor [9–12]. Organoids can be established in many different types of human cancers, and drug sensitivity profiles are highly correlated with the patient’s clinical response [13–15]. Therefore, organoids can serve as a powerful resource to study the pathophysiological mechanisms of the disease itself, as well as for developing robust therapeutic strategies.
Most tumor organoids are derived from the cells of tissue specimens. However, obtaining tissues may not be feasible in patients with metastases because it requires invasive procedures. Occasionally, the tumor may be located in deep anatomical sites that are inaccessible for biopsy. Owing to these limitations, establishing tumor organoids in patients with metastatic cancers has been challenging, even though this group has the highest research priority. In contrast, free-floating tumor cells are readily accessible in patients with pleural/peritoneal metastases through minimally invasive needle aspiration techniques. As tumor cells in malignant ascites and pleural effusion reflect the metastatic tumor burden, they can be utilized for investigational purposes through ex vivo cultures [16,17].
Previous studies have shown that tumor organoids could be established from ascites or pleural fluid of pancreatic, gastric, and breast cancer patients [18–20]. However, the success rates of organoid culture from these fluid samples have not been thoroughly examined. Also, whether these fluid-derived organoids could reflect the therapeutic responses of the matching patients have not been studied.
In this study, we demonstrated that tumor organoids can be established from malignant ascites or pleural effusion from pancreatic, gastric, and breast cancers using a refined protocol for organoids development from tissues. We described the success rates of long-term cultures based on clinical and cytologic factors and compared drug sensitivity profiles of tumor organoids to that of patient’s clinical response.
Materials and Methods
1. Sample collection and establishment of organoids
Malignant ascites or pleural effusions were collected during paracentesis or thoracentesis performed during standard care for patients with pancreatic, gastric, or breast cancer at the National Cancer Center (Goyang, Republic of Korea). Briefly, 50–100 mL of fluid samples were collected in sterile containers, centrifuged at 1,500 ×g for 10 minutes, and washed to concentrate the tumor cells. After additional refining steps and cell counting, 1×105 cells were embedded in 40 μL of Matrigel (Corning Inc., Corning, NY) and seeded in each well of a 24-well cell culture plate. After the Matrigel was solidified, 500 μL medium supplemented with defined growth factors (S1 Table) was added to each well and grown under standard culture conditions (37°C, 5% CO2). Patient sample collection and experimental procedures were reviewed and approved by the Institutional Review Board of the National Cancer Center (IRB Nos. NCC2019-0034, NCC2021-0232, NCC2020-0299, and NCC2020-0337).
2. Maintenance of organoid culture
Growth media were changed twice a week, and the organoids were subcultured when they reached a predefined size depending on the morphology and growth rates. The organoids were considered to be successfully cultured when they were maintained for five or more passages. During subculture at passages 1 and 5, the organoids were cryopreserved and analyzed for short tandem repeats and mycoplasma for quality control.
3. Drug sensitivity assay
Pancreatic cancer organoids were seeded in a 384-well plate with 500 cells embedded in 20 μL of 10% Matrigel in each well. After three days, drugs were added to each well and maintained in culture for five days. Cell viability was assessed using a CellTiter-Glo 3D Cell Viability Assay (Promega, Madison, WI) following the manufacturer’s protocol. The drugs used in the assays were 5-fluorouracil (#HY-90006, MedChemExpress, Monmouth Junction, NJ), oxaliplatin (#HY-17371, MedChemExpress), irinotecan (Onyvide, provided by Ipsen, Paris, France), gemcitabine (Gemcit, provided by Dong-A ST, Seoul, Korea), nab-paclitaxel (Abraxane, provided by Cellgene, Summit, NJ), erlotinib (#HY-50896, MedChemExpress), epirubicin (#HY-13624A, MedChemExpress), cisplatin (Cisplan inj, provided by Dong-A ST), and carboplatin (#17393, MedChemExpress).
4. Droplet digital PCR for KRAS mutations
PCR reaction was performed using QX200 Droplet Digital PCR (ddPCR) System (Bio-Rad, Hercules, CA) with mixture KRAS G12D or G12V mutation detection reagent (Bio-Rad) which includes wild-type probe labeled with HEX dye and mutant probe with FAM dye, ddPCR supermix for probes (Bio-Rad), and 2 μL DNA template in final volume of 20 μL with DEPC-treated water. Positive controls for G12D or G12V mutation were CCRF-CEM and CFPAC-1 cell line.
5. Statistical analysis
Statistical analyses were performed using R (ver. 4.2.0, R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism (ver. 9.4.1, GraphPad Software, San Diego, CA). Descriptive statistics are reported as numbers and percentages of patients. Continuous variables were compared using the t test, and categorical variables were analyzed using the chi-square or Fisher’s exact test.
Results
1. Patients’ characteristics
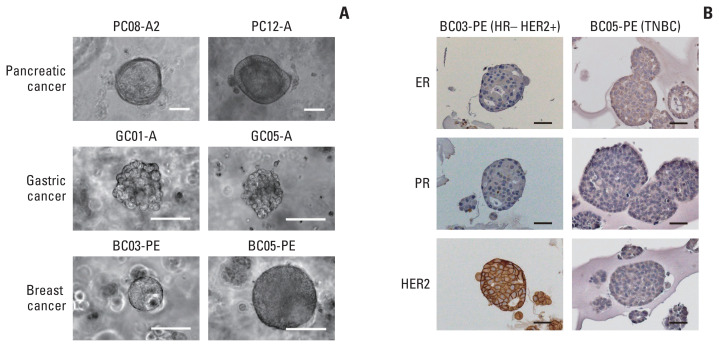
In this study, 70 fluid samples were collected from malignant ascites or pleural effusion of 58 patients; 39 were from pancreatic cancer, 21 from gastric cancer, and 10 from breast cancer (Table 1, S1–S3 Tables). As the proportion of tumor cells and their biological characteristics vary among different samples, organoids were not formed in many cases. In addition, in some cases, where 3D structures of organoids were formed at the initial passage, maintaining them in subcultures was unsuccessful. We found that tumor organoids subcultured for five or more passages could be expanded and maintained as a long-term culture. Therefore, we defined our success of organoid culture as the ability to maintain them for five or more passages (Fig. 1A, S4–S6 Figs.).
Table 1.
Patient characteristics
| Total | Success | Fail | p-value | |
|---|---|---|---|---|
| No. (%) | 70 | 28 (40.0) | 42 (60.0) | |
| Age (yr), median (range) | 63 (34–83) | 63 (34–78) | 64 (37–83) | 0.351 |
| Sex | ||||
| Male | 32 | 13 (40.6) | 19 (59.4) | > 0.99 |
| Female | 38 | 15 (39.5) | 23 (60.5) | |
| Cancer type | ||||
| Pancreas | 39 | 19 (48.7) | 20 (51.3) | 0.193 |
| Gastric | 21 | 7 (33.3) | 14 (66.7) | |
| Breast | 10 | 2 (20.0) | 8 (80.0) | |
| Sample type | ||||
| Ascites | 53 | 21 (39.6) | 32 (60.4) | > 0.99 |
| Pleural effusion | 17 | 7 (41.2) | 10 (58.8) | |
| Cytopathology | ||||
| Positive | 35 | 17 (48.4) | 18 (51.6) | 0.014 |
| Negative | 32 | 8 (25.0) | 24 (75.0) | |
| N/A | 3 | 3 (100) | 0 | |
Values are presented as number (%) unless otherwise indicated. N/A, not available.
Fig. 1.
(A) Representative microscopic images of organoids derived from malignant ascites or pleural effusion of pancreatic, gastric, and breast cancer patients. Scale bars=100 μm. (B) Immunohistochemical staining of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) in breast cancer organoids. TNBC, triple-negative breast cancer. Scale bars=50 μm.
The overall success rate of organoid culture (maintained for at least five passages) was 40.0%; however, it differed depending on the type of malignancy, with pancreatic, gastric, and breast cancers showing 48.7%, 33.3%, and 20.0% success rates, respectively. More samples were collected from ascitic fluid (n=53) than from pleural effusion (n=17), which was attributed to the type of primary cancer. Cytopathology with either cytospin or liquid-based cytology was applied for ascitic and pleural fluids in parallel to detect the presence of malignant cells. Thirty-five patients were positive in cytopathology, with a success rate of 48.4%. However, 24 patients were negative for cytopathology, with a success rate of 25.0%. Unlike the positive predictive value, the negative predictive value of cytopathology test was high (75%). Statistical analysis with Fisher’s exact test also showed that the cytopathology results significantly differed between the successful and failed cases (p=0.014).
2. Pancreatic cancer organoids
A total of 39 samples were collected from patients with pancreatic cancer, and 19 samples were successfully cultured (Table 2, S2 Table). These organoids were stained positive for cytokeratin 19 (CK19), supporting their origin of pancreatic tumor cells (S4 Fig.) [21]. As pancreatic cancer frequently metastasizes to the peritoneal cavity, most fluid samples were collected from malignant ascites (n=33) and six were from pleural effusion. The rate of successful organoid culture was not statistically different between the ascitic and pleural fluids. In total, 18 samples were positive, and 18 were negative for cytopathology. Cytopathology was not tested in three cases. The prediction rate for successful organoid culture was 50% and 61.1% in cytopathology-positive and -negative cases, respectively. The negative and positive prediction rates for cytopathology were neither high enough nor statistically significant.
Table 2.
Patient characteristics of pancreatic cancer
| Total | Success | Fail | p-value | |
|---|---|---|---|---|
| No. (%) | 39 | 19 (48.7) | 20 (51.3) | |
| Age (yr), median (range) | 65 (38–80) | 64 (51–78) | 66 (38–80) | 0.965 |
| Sample type | ||||
| Ascites | 33 | 15 (45.5) | 18 (54.5) | 0.408 |
| Pleural effusion | 6 | 4 (66.7) | 2 (33.3) | |
| Cytopathology | ||||
| Positive | 18 | 9 (50.0) | 9 (50.0) | 0.195 |
| Negative | 18 | 7 (38.9) | 11 (61.1) | |
| N/A | 3 | 3 (100) | 0 | |
Values are presented as number (%) unless otherwise indicated. N/A, not available.
3. Gastric cancer organoids
Twenty-one samples were collected from gastric cancer patients (Table 3, S3 Table). The majority of samples (n=20) were from ascites with six successful cases, and one successful case was from pleural effusion. Cytopathology results were positive for 12 cases, and six were successfully cultured. In contrast, only one out of nine cytopathology-negative cases was successful. The prediction rate for successful organoid culture was 50% for cytopathology-positive cases. For cytology-negative cases, the negative prediction rate was 88.9%. Nevertheless, the difference was not statistically significant, probably because of the small number of cases in this dataset. Regarding tumor differentiation, most cases (n=20) were from poorly differentiated adenocarcinomas, indicating that this subtype shows highly aggressive behavior to cause pleural/peritoneal metastases. Six poorly differentiated and one moderately differentiated carcinoma cells were successfully cultured. CDX2 and CK19 are protein markers that are expressed in a subset of gastric epithelial cells, and our gastric cancer organoids were also positively stained for CDX2 and/or CK19 (S5 Fig.) [21]. Human epidermal growth factor receptor 2 (HER2) is a druggable target in metastatic gastric cancer, and one case was positive for HER2 staining, which was unsuccessful in the organoid culture. The low number of HER2-positive cases reflects the characteristics of the original tumor, as most poorly differentiated gastric cancers are negative for HER2 expression [22]. The number of prior lines of systemic therapy did not differ between the successful and unsuccessful cases.
Table 3.
Patient characteristics of gastric cancer
| Total | Success | Fail | p-value | |
|---|---|---|---|---|
| No. (%) | 21 (100) | 7 (33.3) | 14 (66.7) | |
| Age (yr), median (range) | 59 (34–83) | 54 (34–66) | 60 (37–83) | 0.156 |
| Sample type | ||||
| Ascites | 20 | 6 (30.0) | 14 (70.0) | 0.333 |
| Pleural effusion | 1 | 1 (100) | 0 | |
| Cytopathology | ||||
| Positive | 12 | 6 (50.0) | 6 (50.0) | 0.161 |
| Negative | 9 | 1 (11.1) | 8 (88.9) | |
| Tumor differentiation | ||||
| Moderate | 1 | 1 (100) | 0 | 0.333 |
| Poor | 20 | 6 (30.0) | 14 (70.0) | |
| HER2 expression | ||||
| Negative | 20 | 7 (35.0) | 13 (65.0) | > 0.99 |
| Positive | 1 | 0 | 1 (100) | |
| Prior lines of therapy | ||||
| 0 or 1 | 8 | 3 (37.5) | 5 (62.5) | > 0.99 |
| 2 or more | 13 | 4 (30.8) | 9 (69.2) | |
Values are presented as number (%) unless otherwise indicated. HER2, human epidermal growth factor receptor 2.
4. Breast cancer organoids
Ten pleural fluid samples were collected from breast cancer patients (Table 4, S7 Table). Five were positive for cytopathology; two were successfully cultured, and none of the cytopathology-negative cases were successful. Among the clinical subtypes of breast cancer, two were positive for hormone receptor (HR), three were HR+/HER2+, one was HER2+, and four were triple-negative breast cancer (TNBC). One case of HER2+ breast cancer and another case of TNBC were successfully cultured. Clinically, the Ki67 index of tumor tissues is used to classify the subtype of breast cancer with a cutoff of 14% [23]. Seven cases had a Ki67 index > 14%, and two were successfully cultured. Both of the successfully cultured organoids were positively stained for mammaglobin, which is the tissue-specific marker for breast epithelial cells (S6 Fig.) [21].
Table 4.
Patient characteristics of breast cancer
| Total | Success | Fail | p-value | |
|---|---|---|---|---|
| No. (%) | 10 | 2 (20.0) | 8 (80.0) | |
| Age (yr), median (range) | 59 (47–72) | 47 (47–47) | 61 (54–72) | < 0.001 |
| Sample type | ||||
| Pleural effusion | 10 | 2 (20.0) | 8 (80.0) | |
| Cytopathology | ||||
| Positive | 5 | 2 (40.0) | 3 (60.0) | 0.444 |
| Negative | 5 | 0 | 5 (100) | |
| Clinical subtype | ||||
| HR+ | 2 | 0 | 2 (100) | 0.289 |
| HR+ HER2+ | 3 | 0 | 3 (100) | |
| HER2+ | 1 | 1 (100) | 0 | |
| TNBC | 4 | 1 (25.0) | 3 (75.0) | |
| Ki67 index (%) | ||||
| < 14 | 2 | 0 | 2 (100) | > 0.99 |
| ≥14 | 7 | 2 (28.6) | 5 (71.4) | |
| N/A | 1 | 0 | 1 (100) | |
Values are presented as number (%) unless otherwise indicated. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; N/A, not available; TNBC, triple-negative breast cancer.
5. Organoids retain their molecular and histologic characteristics
To assess whether organoids derived from ascites or pleural effusion recapitulate the molecular characteristics of the primary tumor, we performed immunohistochemical staining of breast cancer organoids for estrogen receptor (ER), progesterone receptor (PR), and HER2. For breast cancer, two organoids were successfully cultured; one was HR−/HER2+, and the other was TNBC. Immunohistochemical staining showed that the organoid in the HR−/HER+ case was positive for HER2 and negative for both ER and PR. In addition, TNBC cells were negative for all three proteins (Fig. 1B).
In pancreatic cancer, KRAS mutation is the most common oncogenic alteration found in more than 90% of the samples [24]. Therefore, we compared the mutation profiles of KRAS from patients’ plasma circulating tumor DNA and their organoids by ddPCR in seven cases. Although the detailed mutation profiles varied by each patient, the organoids derived from their ascites or pleural effusion also retained the driver oncogenic alterations (S8 Table). We also compared the mutational status of the KRAS oncogene in a pancreatic cancer patient’s ascitic fluid collected serially (PC14-A1 at baseline, and PC14-A2 at 1-month post-chemotherapy with gemcitabine and nab-paclitaxel), and the tumor organoids derived from them. Although the fractional abundance was lower with the ascitic fluid, both the ascites and organoid samples revealed the presence of KRAS G12V, showing that oncogenic driver alterations are consistent and confirming that the organoids were derived from the metastatic tumor cells in the peritoneal cavity. The morphologies of the two organoids that were derived at different time points from the same patient were also comparable (S9 Fig.).
6. Ex vivo drug sensitivity profiles of organoids recapitulate patients’ responses to chemotherapeutic agents
Patients with malignant ascites or pleural effusion are candidates for palliative chemotherapy, and tumor organoids derived from these samples can be utilized to test drug response to plan for personalized treatment strategies. In addition, organoids derived at this time point could better reflect the molecular characteristics of metastatic tumors than do the primary tissues. The feasibility of serial sample acquisition is another advantage of malignant fluid-derived organoids. Therefore, dynamic changes can also be monitored in organoids derived from sequential samples. Based on these facts, we performed drug sensitivity assays using pancreatic cancer organoids.
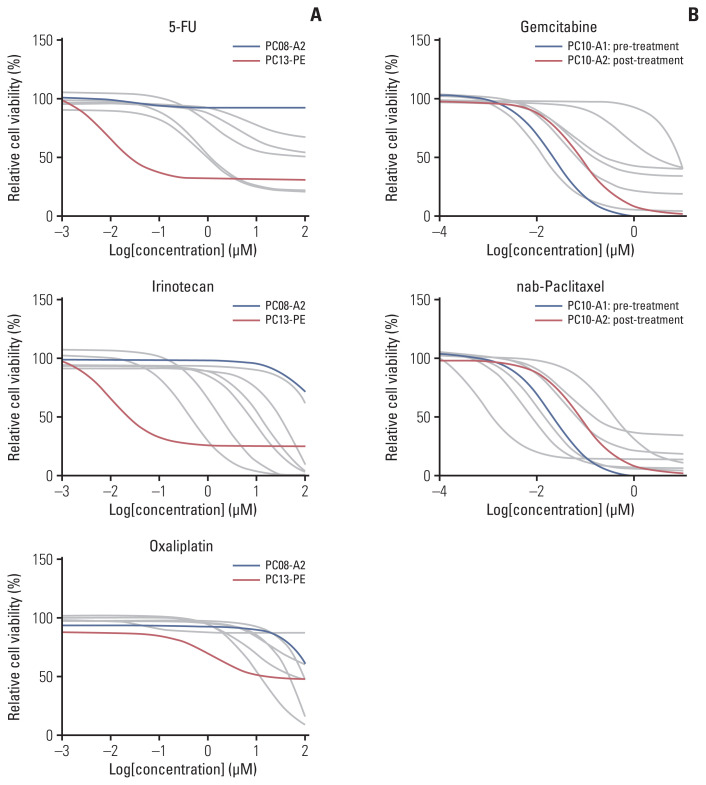
We performed sensitivity assays with ten drugs, six of which are used in standard regimens for pancreatic cancer (5-fluorouracil, oxaliplatin, irinotecan, gemcitabine, nab-paclitaxel, and erlotinib) and three that are not routinely used but considered potential therapeutic agents (epirubicin, cisplatin, and carboplatin) [25–27]. Eight pancreatic cancer organoids derived from five patients were selected (S10 Fig.). As the patients were treated with FOLRINOX (5-fluorouracil, irinotecan, and oxaliplatin) and/or gemcitabine with nab-paclitaxel regimens, the drug response profiles (area under the dose-response curve) of organoids were compared with therapeutic responses of the original patients (S11 Table, S10 Fig.).
In the test results of 5-fluorouracil, irinotecan, and oxaliplatin, organoids derived from a patient with a good clinical response to FOLFIRINOX (PC13-PE, progression-free survival of 6.5 months) showed a relatively better sensitivity profile than the case with a poor outcome (PC08-A2, progression-free survival of 1.8 months) (Fig. 2A). Organoids were derived from sequential samples obtained from the same patient. In the case (PC10) with organoids derived from ascites before (PC10-A1) and after (PC10-A2) chemotherapy with gemcitabine and nab-paclitaxel, the dose-response curves of both agents showed a right shift in the post-chemotherapy samples (Fig. 2B). The patient’s best response was a stable disease that lasted six months, and organoid from the post-chemotherapy sample (PC10-A2) was considered to reflect acquired drug resistance profiles to gemcitabine and nab-paclitaxel. These data show that the drug responses tested in organoids correlate well with the patient’s clinical responses to matching agents.
Fig. 2.
Drug response curves for 5-fluorouracil (5-FU), irinotecan, oxaliplatin (A), and gemcitabine and nab-paclitaxel (B) in pancreatic cancer organoids. The data presented in the plot are the average of four replicate measurements of each sample.
Discussion
This study demonstrated that organoids can be established from malignant ascites or pleural effusion from patients with advanced stages of pancreatic, gastric, and breast cancers. Metastasis to the pleural/peritoneal cavity is a poor prognostic factor for these malignancies [1,2,7,16,17]. As the clinical benefits of conventional therapies are limited, novel approaches are required to overcome this hurdle. Organoids retain the molecular and histological features of primary tumor tissues and reflect drug sensitivity profiles [9–15,28,29]. Compared to obtaining tumor tissues, collecting ascitic or pleural fluid samples has added benefits. First, it is less invasive because it can be achieved using ultrasound-guided needle aspiration. This is a considerable advantage for cancers located in deep anatomical locations, such as pancreatic cancer. Second, serial sample acquisition allows the collection of tumor cells during treatment course that can best recapitulate the biology of the patient’s status. Since pleural/peritoneal metastases poorly respond to chemotherapy, and aggravation of these lesions eventually leads to organ dysfunction and death, establishing a testing platform of drug response targeting tumors from these sites is imperative.
In parallel with the organoid culture, all fluid samples were tested for cytopathology using either cytospin or liquid-based cytology. The overall positive predictive value (PPV) was 48.4% and the negative predictive value (NPV) was 75.0% for cytopathology. The PPV was similar among different malignancies, with 50% for pancreatic and gastric cancers and 40% for breast cancer. However, the NPV was 54.5% for pancreatic cancer, 88.9% for gastric cancer, and 100% for breast cancers. The patient-derived organoid (PDO) establishment rate highly correlates with the tumor cellularity in the original specimen [13]. Counting the number of tumor cells in ascites or pleural fluid is challenging. However, cases that were negative for cytopathology probably contained a low number of tumor cells, which led to a significant difference in the success rates between cytopathology-positive and cytopathology-negative cases. However, this differed among cancer types as the cytopathology-negative samples mostly failed in gastric and breast cancers, but approximately half of the cytopathology-negative cases were successfully cultured in pancreatic cancer. This might be because of the relatively high aggressive nature of peritoneal metastatic cells in pancreatic cancer, and a lower number of cells might be required to form organoids [30]. In line with this, cytopathology could help in candidate selection for PDO establishment in gastric and breast cancer, but not as much in pancreatic cancer.
Li et al. [19] also cultured malignant ascites-derived organoids (MADO) from gastric cancer patients. They showed molecular and histological characteristics similar to those of ascitic tumor cells. The reported success rate was 92% (11 out of 12 cases), which was higher than our results. However, their definition of successful organoid culture was not precisely described in the paper, and a direct comparison between studies should be cautiously performed. Moreover, their optimized MADO method required supernatant of patient-derived ascitic fluid in the culture medium. From our perspective, this method is not sustainable for maintaining organoids in long-term culture as the patients’ fluid samples cannot be obtained repeatedly. In contrast, our study used only defined growth factors for the culture medium, which allows maintenance without requiring patient-derived fluid samples.
PDOs from tumor tissues recapitulate drug responses in patients [13,14]. Furthermore, the establishment of biobanks for these organoids has been demonstrated for drug screening purpose and personalized therapies [9,10]. Our results are in accordance with these, as PDOs derived from malignant ascites showed drug sensitivity profiles similar as the original patient’s clinical responses. As pleural/peritoneal metastases are poorly sensitive to standard therapeutic options, these data indicate the importance of organoids as a test bed for personalized therapy and novel drug discovery, particularly for recalcitrant cancers.
This study had several limitations. Most importantly, the number of patients included in this study was small. Statistical tests for clinicopathologic features that predict successful culture showed that only cytopathology results were significant. Nevertheless, larger cohorts might be necessary to identify predictive factors for successful establishment of organoids. Second, the success rate varied among the cancer subtypes. These numbers are lower than the reported values when organoids are established from tumor tissue. However, this might be because of the small number of tumor cells, as cells in the ascitic or pleural fluid are relatively sparse compared with those in surgical or biopsy specimens. This was reflected in the cytology results, as the tumor cells were not visible in approximately 46% of the fluid samples. Third, the clinical subtypes of each cancer were skewed toward more aggressive features. For example, gastric cancer mainly consisted of poorly differentiated adenocarcinoma with only one HER2+ case. Four of the ten breast cancer samples were TNBC. The proportions of these clinical subtypes differ from those of primary tumors [31,32]. They are enriched with more aggressive tumors, indicating that these tumors are more prone to metastasis into the pleural/peritoneal cavity.
Despite these limitations, organoids established from malignant ascites or pleural effusions of pancreatic, gastric, and breast cancers provide a robust platform for drug sensitivity assays and molecular profiling, which could guide precision oncology and drug discovery. Furthermore, this approach could be extended to other malignancies, such as colon or ovarian cancers, and could provide novel perspectives that can supplement tissue-based organoid research.
Acknowledgments
This work was supported by grants from the National Cancer Center [Grant No. 2010120] and the National Research Foundation of Korea (NRF), funded by the Korean government (MSIT) [Grant No. 2020M3A9A5036362]. We thank Hyun-Jin Kim and Young Hwa Kang for their support.
Footnotes
Ethical Statement
This study had been authorized by the Institutional Review Boards of the National Cancer Center approved the study protocol (NCC2019-0034, NCC2021-0232, NCC2020-0299, and NCC2020-0337). Informed consent was obtained from all participants.
Author Contributions
Conceived and designed the analysis: Choi W, Kim YH, Kong SY.
Contributed data or analysis tools: Choi W, Kim YH, Woo SM, Yu Y, Lee MR, Lee WJ, Chun JW, Sim SH, Chae H, Shim H, Lee SK, Kong SY.
Performed the analysis: Choi W, Kim YH, Kong SY.
Wrote the paper: Choi W, Kim YH, Lee KS, Kong SY.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Rijken A, Lurvink RJ, Luyer MD, Nieuwenhuijzen GA, van Erning FN, van Sandick JW, et al. The burden of peritoneal metastases from gastric cancer: a systematic review on the incidence, risk factors and survival. J Clin Med. 2021;10:4882. doi: 10.3390/jcm10214882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rijken A, Bakkers C, van Erning FN, van der Geest LG, de Vos-Geelen J, Besselink MG, et al. Incidence, treatment, and survival of synchronous peritoneal metastases in pancreatic cancer: update of a nationwide cohort. Pancreas. 2021;50:827–33. doi: 10.1097/MPA.0000000000001857. [DOI] [PubMed] [Google Scholar]
- 3.Gayen S. Malignant pleural effusion: presentation, diagnosis, and management. Am J Med. 2022;135:1188–92. doi: 10.1016/j.amjmed.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Sun F, Feng M, Guan W. Mechanisms of peritoneal dissemination in gastric cancer. Oncol Lett. 2017;14:6991–8. doi: 10.3892/ol.2017.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avula LR, Hagerty B, Alewine C. Molecular mediators of peritoneal metastasis in pancreatic cancer. Cancer Metastasis Rev. 2020;39:1223–43. doi: 10.1007/s10555-020-09924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarema R, Ohorchak M, Hyrya P, Kovalchuk Y, Safiyan V, Karelin I, et al. Gastric cancer with peritoneal metastases: efficiency of standard treatment methods. World J Gastrointest Oncol. 2020;12:569–81. doi: 10.4251/wjgo.v12.i5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IH. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: a population-based study. Pancreas. 2013;42:72–5. doi: 10.1097/MPA.0b013e31825abf8c. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–84. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–86. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Yan HH, Siu HC, Law S, Ho SL, Yue SS, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–97. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci U S A. 2019;116:26580–90. doi: 10.1073/pnas.1911273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SI, Jeon AR, Kim MK, Lee YS, Im JE, Koh JW, et al. Development of patient-derived preclinical platform for metastatic pancreatic cancer: PDOX and a subsequent organoid model system using percutaneous biopsy samples. Front Oncol. 2019;9:875. doi: 10.3389/fonc.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–6. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019;11:eaay2574. doi: 10.1126/scitranslmed.aay2574. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, et al. Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv Sci (Weinh) 2021;8:e2101176. doi: 10.1002/advs.202101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng LN, Wen F, Xu P, Zhang S. Prognostic significance of malignant ascites in gastric cancer patients with peritoneal metastasis: a systemic review and meta-analysis. World J Clin Cases. 2019;7:3247–58. doi: 10.12998/wjcc.v7.i20.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baretti M, Pulluri B, Tsai HL, Blackford AL, Wolfgang CL, Laheru D, et al. The significance of ascites in patients with pancreatic ductal adenocarcinoma: a case-control study. Pancreas. 2019;48:585–9. doi: 10.1097/MPA.0000000000001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai S, McOlash L, Palen K, Johnson B, Duris C, Yang Q, et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 2018;18:335. doi: 10.1186/s12885-018-4238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Xu H, Zhang L, Song L, Feng D, Peng X, et al. Malignant ascites-derived organoid (MADO) cultures for gastric cancer in vitro modelling and drug screening. J Cancer Res Clin Oncol. 2019;145:2637–47. doi: 10.1007/s00432-019-03004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan B, Zhao D, Liu Y, Li N, Song C, Li N, et al. Breast cancer organoids from malignant pleural effusion-derived tumor cells as an individualized medicine platform. In Vitro Cell Dev Biol Anim. 2021;57:510–8. doi: 10.1007/s11626-021-00563-9. [DOI] [PubMed] [Google Scholar]
- 21.Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas: a tool for pathology. J Pathol. 2008;216:387–93. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- 22.Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 23.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32:185–203. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 26.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 28.Mo S, Tang P, Luo W, Zhang L, Li Y, Hu X, et al. Patient-derived organoids from colorectal cancer with paired liver metastasis reveal tumor heterogeneity and predict response to chemotherapy. Adv Sci (Weinh) 2022;9:e2204097. doi: 10.1002/advs.202204097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26:17–26. doi: 10.1016/j.stem.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260–5. doi: 10.1016/j.gie.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P. Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US From 2010 to 2016. JAMA Netw Open. 2020;3:e2013226. doi: 10.1001/jamanetworkopen.2020.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng F, Liu J, Wang F, Zheng G, Wang Q, Liu S, et al. Prognostic value of differentiation status in gastric cancer. BMC Cancer. 2018;18:865. doi: 10.1186/s12885-018-4780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.