Abstract
Oxygen stable isotopes (i.e., 16O, 17O, 18O) of nitrite (NO2−) are useful for investigating chemical processes and sources contributing to this important environmental contaminant and nutrient. However, it remains difficult to quantify the oxygen isotope compositions of NO2− due to the lack of internationally recognized NO2− reference materials with a well-known Δ(17O) value. Here we have adopted a combination of methodologies to develop a technique for measuring Δ(17O) of NO2− by reducing nitrate (NO3−) materials with internationally recognized Δ(17O) values to NO2− using activated cadmium catalyzed by chloride in a basic solution while conserving Δ(17O). The NO3− reference materials reduced to NO2− and sample NO2− unknowns are converted to N2O using sodium azide/acetic acid reagent and decomposed to O2 by passing through a heated gold tube and introduced into a continuous flow isotope ratio mass spectrometer for analysis at m/z 32, 33, and 34 for Δ(17O) quantification.
The adapted method involves the following main points:
-
•
NO3− reference materials with internationally recognized oxygen isotope composition are reduced to NO2− under high pH conditions that conserve Δ(17O) values.
-
•
The NO3− reference materials reduced to NO2− and sample NO2− with unknown Δ(17O) values are reduced to N2O using chemical methods involving sodium azide/acetic acid.
-
•
The product N2O is extracted, purified, decomposed to O2, and analyzed for its isotope composition using a continuous flow isotope ratio mass spectrometer for Δ(17O) quantification. The Δ(17O) of NO2− samples are calibrated with respect to the NO3− reference materials with known Δ(17O) values.
Method name: Triple Oxygen Stable Isotope Analysis of Nitrite Measured Using Continuous Flow Isotope Ratio Mass Spectrometry
Keywords: Isotope ratio mass spectrometry, Nitrate reduction, Nitrite, Chemical conversion, Mass-independence, Oxygen isotopes
Graphical abstract
Specifications table
| Subject area: | Earth and Planetary Sciences |
| More specific subject area: | Isotope Geochemistry |
| Name of your method: | Triple Oxygen Stable Isotope Analysis of Nitrite Measured Using Continuous Flow Isotope Ratio Mass Spectrometry |
| Name and reference of original method: | (1) M.R. McIlvin, M.A. Altabet, Chemical Conversion of Nitrate and Nitrite to Nitrous Oxide for Nitrogen and Oxygen Isotopic Analysis in Freshwater and Seawater, Anal. Chem. 77 (2005) 5589–5595. https://doi.org/10.1021/ac050528s. (2) E. Ryabenko, M.A. Altabet, D.W.R. Wallace, Effect of chloride on the chemical conversion of nitrate to nitrous oxide for δ15N analysis, Limnol. Oceanogr. Methods. 7 (2009) 545–552. https://doi.org/10.4319/lom.2009.7.545. (3) J. Kaiser, M.G. Hastings, B.Z. Houlton, T. Röckmann, D.M. Sigman, Triple Oxygen Isotope Analysis of Nitrate Using the Denitrifier Method and Thermal Decomposition of N2O, Anal. Chem. 79 (2007) 599–607. https://doi.org/10.1021/ac061022s. |
| Resource availability: | N/A |
Method details
Overview
The oxygen isotope composition (δ(18O) and Δ(17O)) of nitrite (NO2−) has numerous implications for environmental and geological sciences. For example, it has recently been shown to be a useful tool for evaluating nitrogen oxides (NOx = nitric oxide (NO) + nitrogen dioxide (NO2)) photochemical cycling from NO2 collected as NO2− on guaiacol/potassium hydroxide coated denuders from ambient, laboratory, and environmental chamber experiments [1], [2], [3]. Additionally, it could be a useful tool for investigating the impact of atmospheric deposition of nitrate (NO3−) on the biogeochemical cycling (e.g., denitrification) and reactive nitrogen processes occurring in ecosystems, contributing to a better understanding of nitrogen biogeochemical dynamics [4], [5], [6], [7]. However, there is currently no NO2− isotope reference material with an internationally accepted Δ(17O) value, limiting our ability to measure these values accurately in NO2− samples and across laboratories.
The following describes a method that can be used to quantify the triple oxygen stable isotope (16O, 17O, 18O) composition of nitrite in aqueous solutions (NO2−) in line with the “identical treatment principle” [8], intending to calibrate NO2− samples with NO2− reference materials. It is based on the sodium azide/acetic acid chemical method, originally designed for the 18O/16O and 15N/14N ratio determination of NO3− and NO2− in seawater and freshwater [9,10]. The presented method describes a procedure to reduce NO3− reference materials with known Δ(17O) values to NO2− adapted from previous methodology [9,10], with careful consideration of the potential impact of oxygen isotope reactions between NO2− and water, which is pH-dependent and rapid under acidic conditions erasing the original oxygen isotope composition of NO2− [11]. Once the NO3− reference materials are converted into NO2−, they can be used to quantify Δ(17O) of NO2− samples using a mixture of sodium azide in an acetic acid buffer that reduces samples to nitrous oxide (N2O). The product N2O is extracted, purified, and decomposed to nitrogen (N2) and oxygen (O2) gas, separated using gas chromatography, and analyzed at m/z 32, 33, and 34 for Δ(17O) determination using a continuous flow isotope ratio mass spectrometer (CF-IRMS). In a separate batch analysis, the generated N2O product from NO2− samples and reference materials are extracted, purified, and analyzed at m/z 44, 45, and 46 for δ(18O) determination.
Definitions—Isotope delta
Isotope composition is commonly expressed in delta notation (δ), which is quantified as the following (Eq. (1)):
| (1) |
where R is the ratio of the heavy isotope to the light isotope (e.g., 18O/16O; 17O/16O) in the sample and in internationally recognized isotope reference material, which is the Vienna Standard Mean Ocean Water (VSMOW) for oxygen. The oxygen isotope mass independence, a signature commonly used to trace ozone (O3) interactions, can be quantified as Δ(17O) using the linear definition with a mass-dependent coefficient of 0.52:
| (2) |
where λ is the mass-dependent relationship between δ(17O) and δ(18O). While λ can range from 0.5 to 0.531 [12], it is commonly set to 0.52, representing a reasonable average of oxygen mass-dependent relations observed in nature [13]. The linear Δ(17O) approximation with a coefficient of 0.52 is commonly used to describe large mass-independent effects such as those related to O3 reactions and is commonly used in the atmospheric chemistry community to track the influence of O3 oxidation on reactive components [14], [15], [16], [17]. However, other equations to calculate Δ(17O) exist that are used for more precise work involving oxygen fractionation [18], [19], [20], [21], [22]. Here the primary motivation of Δ(17O) quantification is for evaluating an atmospherically derived product (i.e., nitrogen dioxide collected as NO2−), such that the linear definition is used to be consistent with the stable isotope atmospheric chemistry community.
Reduction of nitrate to nitrite
The quantitative reduction of NO3− isotope reference materials (USGS34; USGS35) to NO2− is conducted using activated cadmium metal catalyzed using chloride in a basic (pH > 13) solution, adapted from a previously reported method [10]. Additional information about the USGS34 and USGS35 reference materials can be found in previous studies [23]. In the following methodology description, all utilized chemicals are reagent grade, and the solutions were made using ultra-high purity water (>18.2 MΩ).
-
1.
Four 50 mL centrifuge tubes (Falcon™ Conical Centrifuge Tubes) are separately labeled for two NO3− isotope reference materials (USGS34 & USGS35), one laboratory NO3− working material used as quality control (mix of 1:1 USGS34:USGS35), and one ultra-high purity MQ water (>18.2 MΩ) blank.
-
2.
The reagents used to reduce NO3− to NO2− are harmful and careful precautions should be taken, including using appropriate personal protective gear (i.e., lab coat, protective eyewear, and gloves) and conducting the work under a laboratory fume hood.
-
3.
Transfer 1 gm of cadmium powder (Alfa Aesar; 200 mesh) to each 50 mL centrifuge tube.
-
4.
Add 25 mL of 10 % hydrochloric acid (HCl; Fisher Chemical) in ultra-high purity water (w/v) to each centrifuge tube containing the cadmium powder.
-
5.
The centrifuge tubes are mixed using a vortex mixer (Fisher Scientific) for 5 min.
-
6.
The centrifuge tubes are centrifuged (Eppendorf Centrifuge 5810 R) for 5 min at 4000 rotations per minute (rpm).
-
7.
The 10 % HCl solutions are decanted and transferred to a waste container.
-
8.
The centrifuge tubes containing the cadmium powder are then rinsed with 20 mL of ultra-high purity water, centrifuged for 5 min at 4000 rpm, and decanted. This step is repeated three times. After the third rinse, the pH of each decanted solution is checked using pH strips to ensure that the last rinse is pH neutral. If the solution is still acidic, repeat the rinse step until the pH is neutral.
-
9.
The cadmium powder is now activated and ready to reduce the NO3− isotope reference materials.
-
10.
Transfer 35 mL of 50 μmol L−1 solution of the NO3− isotope reference materials and 35 mL of ultra-high purity water to their corresponding 50 mL centrifuge tubes with the activated cadmium powder.
-
11.
Add 5 mL of 10 M NaOH (Fisher Chemical) solution to each centrifuge tube, such that the pH will be greater than 13 in limit oxygen isotope exchange between NO2− and water.
-
12.
Add 12 g of sodium chloride (Macron Fine Chemicals) to each centrifuge tube.
-
13.
Place the centrifuge tubes on a platform shaker (New Brunswick Scientific Innova 2100) set at 180 rpm and allow them to shake overnight (i.e., at least 12 h).
-
14.
The centrifuge tubes are then centrifuged for 5 min at 4000 rpm. The solutions are transferred into 500 mL Nalgene bottles that have been leached with MQ water overnight, triple rinsed with MQ, and air-dried. These bottles are placed in a freezer set for −20 °C until subsequent isotope analysis. When stored at a pH > 13 and frozen when not in use, NO2− materials have been found to have a stable oxygen isotope composition (i.e., near negligible oxygen isotope exchange with water) for at least two years.
Conversion of nitrite to nitrous oxide
The quantitative reduction of NO2− to N2O is conducted using a chemical conversion technique involving sodium azide in an acetic acid buffer adapted from a previous method [9]. The conversion of NO2− to N2O is conducted within 20 mL borosilicate vials (DWK Life Sciences MicroLiter 20 mm Crimp Top Headspace Vials). The NO2− samples are converted to N2O in two separate batch analyses that are used to determine Δ(17O) and δ(18O) from O2 and N2O, respectively. The NO3− reference materials reduced to NO2− (USGS34; USGS35) are used for Δ(17O) calibration and NO2− reference salts (RSIL-N7373 and RSIL-N10219) are used for δ(18O) calibration of NO2− samples. Additional information about the RSIL NO2− reference materials, including δ(18O) values, blanks, NO2− oxygen isotope preservation, and contaminants, are available in a previously reported study [24].
-
1.
Before use, all glassware is acid-washed in a 5 % HCl in ultra-high purity water solution (v/v) overnight (i.e., at least 12 h), rinsed with ultra-high purity water, wrapped in aluminum foil, and ashed at 500 °C.
-
2.
Determine the number of vials needed for the NO2− samples, isotope reference materials, and blanks. We target a duplicate set of standards to be analyzed at the beginning and end of the batch analysis, and a single set of standards run intermittently every ten samples. Additionally, we analyze a sodium azide/acetic acid reagent blank at the start of each batch analysis.
-
3.
Rinse the vials three times with ultra-high purity water.
-
4.
Turn the vials over to drain.
-
5.While the vials are drying, prepare the sodium azide/acetic acid reagent:
-
a.Be sure to wear all appropriate personal protective gear for these steps (i.e., lab coat, protective eyewear, and gloves).
-
b.Ultimately, 2 mL of the sodium azide/acetic acid solution will be added to each vial. Determine the amount of the sodium azide/acetic acid solution that will be needed, and plan to make an extra 20 %. For example, for 50 vials, plan to prepare 120 mL of the sodium azide/acetic acid solution (50 vials × 2 mL/vial × 1.2 = 120 mL).
-
c.The sodium azide/acetic acid solution comprises equal parts (i.e., 1:1) of 2 M sodium azide and 40 % acetic acid. Calculate the volume of these two solutions that will be needed. For example, if 120 mL of the sodium azide/acetic acid solution is to be created, this is comprised of 60 mL of 2 M sodium azide and 60 mL of 40 % acetic acid. We note that 40 % acetic acid is used rather than 20 % acetic acid, as recommended in the original description of the sodium azide/acetic acid method [9]. This has been done to ensure that the NO2− contained in basic solutions is sufficiently acidified (pH < 5) to quantitatively convert NO2− to N2O. This amount of acetic acid is sufficient for converting NO2− contained in basic solutions (pH = 13) to N2O for injection volumes up to 10 mL. For larger injection volumes or higher pH solutions, the amount of acetic acid may need to be adjusted to ensure sufficient acidification of samples.
-
d.Based on the volume of 2 M sodium azide solution that is needed, calculate the amount (g) of sodium azide that you need to have 2 M. For example, if you need 60 mL of 2 M Sodium Azide: 2 M(moles/L) × (0.060) L × (65.01 g /mol) = 7.8 g of sodium azide.
-
e.Weigh out the amount of sodium azide (Fisher Chemical) needed and transfer it to a beaker large enough to accommodate the 2 M sodium azide solution.
-
f.Take the beaker containing the sodium azide under a laboratory fume hood. From this point forward, everything you do that involves sodium azide and acetic acid must be done under a laboratory fume hood. Anything that touches the solution must be thoroughly rinsed with water. Use another beaker (triple-rinsed with ultra-high purity water) to transfer the volume of MQ water needed to create the 2 M sodium azide solution into the beaker containing the previously weighed sodium azide. Mix the solution using a pre-cleaned stirring rod until the sodium azide solution fully dissolves.
-
g.Transfer the 2 M sodium azide solution into a 500 mL Dreshel bottle secured to a ring stand under the hood.
-
h.Prepare the volume of the 40 % acetic acid solution by diluting glacial acetic acid (Fisher Scientific) with ultra-high purity water. First, in a separate beaker, transfer the amount of ultra-high purity water needed for the dilution. Next, slowly transfer the amount of glacial acetic acid into the beaker.
-
i.Transfer the 40 % acetic acid solution to the 500 mL Dreshel bottle containing the 2 M sodium azide that is securely held to a ring stand.
-
j.Flush the sodium azide/acetic acid solution with nitrogen by connecting a tank or in-house line of N2 to the inlet of the Dreshel bottle.
-
k.Slowly and carefully begin flowing nitrogen and be sure that the solution begins to bubble without splashing.
-
l.Let the solution vigorously bubble with nitrogen for at least one hour to remove any potential N2O blank (Fig. 1A). While the solution is bubbling, steps (6–11) can be completed.
-
a.
-
6.
Label the bottom of the vials in such a manner as to designate which NO2− standards or samples, and the reagent blank will be transferred into each corresponding vial.
-
7.Dilute and transfer the sample and standards into separate 20 mL Crimp Top Headspace vials. All samples and standards are analyzed at a similar targeted NO2− concentration. This is important for accurate Δ(17O) and δ(18O) quantification due to NO2− and water oxygen isotope exchange during the reduction of NO2− to N2O using sodium azide/acetic acid that we find is dependent on NO2− concentration. We target to analyze all samples and standards at 20 μmol L−1. However, this is not possible for all samples, such that samples below 20 μmol L−1 are diluted to 5 μmol L−1 and calibrated to reference materials that are also diluted to 5 μmol L−1. Samples below 5 μmol L−1 are not recommended to be analyzed using this method for Δ(17O) because of relatively lower measurement precision concerns; however, low-concentration samples (e.g., as low as 2 μmol L−1) can be analyzed for δ(18O) with relatively high precision. The samples and standards are diluted to these targeted concentrations using 0.1 M NaOH solution.
-
a.Calculate the volume of the sample or standard needed for the isotope analysis. For Δ(17O), we target 35 nmol of NO2−, and for δ(18O), we target 10 nmol of NO2−, leading to the production of 35 and 10 nmol of N2O, respectively, due to the 1:1 combination of nitrite-N and azide-N.
-
b.Use a pipette and transfer the proper amount of sample or standard into each corresponding 20 mL Crimp Top Headspace vial. All NO2− samples, and standards must be in solutions with pH > 13 to prevent oxygen isotope exchange with water. Batches of the RSIL-N7373 and RSIL-N10219 reference materials and the NO3− reference materials reduced to NO2− are stored in 500 mL Nalgene bottles with pH > 13 and placed in a freezer when not in use. Previously, we observed significant oxygen isotope exchange for the RSIL-N7373 and RSIL-10219 standards when the pH was near 10. When stored at a pH > 13, the NO2− reference materials have been found to have a stable isotope composition (i.e., near negligible oxygen isotope exchange) for at least two years.
-
a.
-
8.
Next, use a pipette and transfer the proper amount of 0.1 M NaOH into each 20 mL vial to dilute the samples to their appropriate NO2− concentration targets of 20 or 5 μmol L−1.
-
9.
After transferring the sample and appropriately diluting the sample to targeted concentration brackets, cap the top of the vials with a rubber septum (Wheaton MicroLiter, Septa 20 mm Gray Butyl Stopper) (Fig. 1B).
-
10.
Once all samples have been transferred, place an aluminum crimp cap over the septa (Wheaton MicroLiter, seal 20 mm standard aluminum) and seal the caps manually or with an automated crimp-capper (Wheaton Crimpenstein) (Fig. 1C).
-
11.
Flush the headspace of each vial with helium for 10 min to remove any potential N2O and carbon dioxide (CO2), an isobaric influence, in the headspace (Fig. 1D).
-
12.
Transfer the vials under the hood where the sodium azide/acetic acid solution is bubbling.
-
13.
Turn off the flowing nitrogen to stop the bubbling of the sodium azide/acetic acid solution, and carefully remove the tubing attached to the inlet of the Dreshel bottle.
-
14.
Transfer the solution from the Dreshel bottle into a beaker secured to a ring stand.
-
15.
Transfer 2 mL of the sodium azide/acetic acid solution into each vial using a syringe. Before the injection, place an exit needle through the vial septa to limit back pressure and immediately remove the exit needle once the sodium azide/acetic acid injection is complete (Fig. 1E).
-
16.
Vigorously shake and let sit for at least 1 h.
-
17.
Create a 6 M sodium hydroxide (NaOH) mixed with 0.1 % phenolphthalein (Acros Organics) solution with enough volume to add 3 mL of 6 M NaOH + 0.1 % phenolphthalein into each vial.
-
18.
Add the 6 M NaOH + 0.1 % phenolphthalein reagent into each vial until neutralization is achieved (color should turn pink/purple, usually around 1–3 mL). Vigorously shake. This ends the reaction of NO2− to N2O using sodium azide/acetic acid (Fig. 1F).
-
19.
Transfer any extra sodium azide/acetic acid into its proper waste container.
Fig. 1.
Overview of preparing the sodium azide/acetic acid reagent for Δ(17O) and δ(18O) analysis of NO2−. (A) The sodium azide/acetic acid reagent is combined and flushed with nitrogen for at least one hour. (B) The NO2− standards and samples are transferred to 20 mL borosilicate vials. (C) The vials are capped with rubber septa and sealed with an aluminum crimp cap. (D) The headspace of the vials is flushed with helium for 10 min. (E) 2 mL of the sodium azide/acetic acid reagent is transferred to each vial. (F) After at least a one-hour reaction, 6 M NaOH and 0.1 % phenolphthalein solution are added to the vials to neutralize the solutions ending the reaction.
Δ(17O, NO2−) isotope analysis and data processing
The generated N2O from NO2− samples and reference materials are extracted, purified, and concentrated using an automated headspace extractor, decomposed to O2 by passing through a heated gold tube, and analyzed at m/z 32, 33, 34 for Δ(17O) determination using CF-IRMS. The measured Δ(17O, O2) are normalized to VSMOW using the NO3− isotope reference materials reduced to NO2− for Δ(17O, NO2−) determination. Measuring Δ(17O) from N2O decomposed to O2 using an automated headspace extraction, and CF-IRMS is a relatively routine methodology for numerous isotope ratio labs [14,22,25,26]. Here we highlight the general procedure and calibration scheme utilized for this type of measurement.
-
1.The generated N2O from the NO2− samples, reference materials (USGS34; USGS35), and blanks are extracted, purified, and concentrated utilizing a modified GasBench II (Thermo Scientific) [25,26].
-
a.N2O from the sample vials is purged with a helium carrier at 10–15 ml min−1 and is passed through a drierite and ascarite (II) trap to remove water and carbon dioxide. Next, the sample stream passes through a Supelco trap F hydrocarbon purge trap. The sample stream then passes through a Nafion dryer to further remove residual water.
-
b.The sample stream is preconcentrated by passing through a U-trap immersed in liquid nitrogen to trap N2O. The sample is then cyrofocused in a second U-trap immersed in liquid nitrogen. The concentrated N2O then passes through an additional Nafion dryer and then through a PoraPlot Q GC column to separate N2O.
-
a.
-
2.
The purified N2O is then passed through a gold tube heated to 770 °C to decompose N2O to N2 and O2. The generated N2 and O2 are separated using a Molsieve 5A GC column and introduced to the CF-IRMS for mass analysis at m/z 32, 33, and 34, corresponding to the molecular oxygen species 16O2, 16O17O, 16O18O, and 17O2 [22].
-
3.
The atomic δ(17O, O2) and δ(18O, O2) values are approximated by the molecular raw 33δ and 34δ values, measured by the CF-IRMS against an ultra-high purity O2 tank that is used as a working reference. The isobaric influence of 17O17O on the measure 34δ is negligibly small and is not considered [22]. Further, a “blank” correction is not conducted for the calibrations as the reagent blank associated with the NO2− reduction to N2O and further decomposed to O2 produces a peak that is below the limits of detection of our CF-IRMS (∼0.3 V × s). Compared to a typical sample O2 peak of 35 V × s for a 35 nmol of NO2− converted to O2, the reagent blank contribution is insignificant and contributes at most 0.8 % to the sample peak. However, we acknowledge that the lab reactive nitrogen (NO2−, NO3−, N2O) blank that includes all sources of possible contamination will likely vary from lab to lab and should be carefully quantified and appropriately corrected for if significant.
-
4.
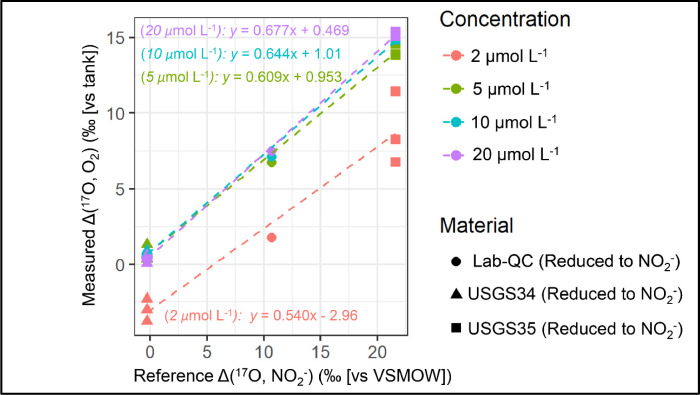
The measured atomic Δ(17O, O2) is then scale normalized to VSMOW by calibration to the NO3− reference materials reduced to NO2− with known Δ(17O) values to determine the Δ(17O) of NO2− samples (Fig. 2). We note that the NO3− reference materials are expected to undergo mass-dependent isotope fractionation associated with the reduction of NO3− to NO2− and the loss of one oxygen atom. The NO3− to NO2− reduction will impact the δ(18O) and δ(17O) values of the NO3− reference materials; however, assuming that this process is mass-dependent, the Δ(17O) of the NO3− reference materials reduced to NO2− is conserved (i.e., Δ(17O, NO3−) = Δ(17O, NO2−)).
-
5.
There is a strong dependence on the calibration of Δ(17O, O2 [vs tank]) to Δ(17O, NO2− [vs VSMOW]) with NO2− concentration that converges for higher concentrations (Fig. 2). Therefore, samples must be analyzed at the same concentration as the standards. Since the Δ(17O) of the water was the same for all batch analyses, deviations of the calibration slope from 1 measure the degree of oxygen isotope exchange between NO2− and water during the reduction of NO2− to N2O. For a target NO2− concentration of 2, 5, 10, and 20 μmol L−1, the calibration slopes were 0.540, 0.609, 0.644, 0.677, respectively, which indicated an increase in oxygen isotope exchange for lower NO2− concentrations (i.e., greater deviation of the slope away from 1). Little variation in the calibration slopes is observed between sample batches if reaction conditions, NO2− concentrations, and NO2− injection amounts are kept the same.
-
6.
After calibration, the long-term pooled standard deviations for Δ(17O) of the reduced NO3− reference materials in our laboratory are ±2.3 ‰, ±0.5 ‰, ±0.2 ‰, and ±0.2 ‰ for NO2− concentration targets of 2, 5, 10, and 20 μmol L−1, respectively. Since there is a strong dependence on the measurement precision with NO2− concentration, we recommend analyzing Δ(17O, NO2−) at as high of a concentration target as possible. However, in our experience, the Δ(17O, NO2−) precision tails off above 10–15 μmol L−1, such that analyzing Δ(17O, NO2−) at a NO2− concentration of 20 μmol L−1 should be an ideal target for generating the highest precision Δ(17O, NO2−) measurement as possible. The low slope and relatively low precision of standards analyzed at 2 μmol L−1, is why we recommend not analyzing low-concentration samples using the described protocol.
-
7.
Overall, the presented methodology allows for routine measurements of Δ(17O) of NO2− samples for relatively small amounts of samples (i.e., 35 nmol) for samples with a NO2− concentration above 5 μmol L−1.
Fig. 2.
The measured Δ(17O) values of O2 (relative to a reference O2 tank) produced from NO3− reference materials reduced to NO2− using activated cadmium catalyzed by chloride in a basic solution, converted to N2O using sodium azide/acetic acid reagent, and decomposed to O2 by passing through a heated gold tube versus the reference Δ(17O, NO2−) values (relative to VSMOW). Only the internationally recognized NO3− reference materials (USGS34 & USGS35) reduced to NO2− are used for the calibration. The Lab-QC (50:50 mix of USGS34:USGS35) is used to evaluate the system stability. The data is color-coded by the concentration and shade-coded by the NO2− reference material. There is a strong dependence of the Δ(17O) calibration on concentration, reflecting a range of oxygen isotope exchange with water during the NO2− reduction to N2O.
δ(18O, NO2−) isotope analysis and data processing
In a separate batch analysis, the generated N2O from NO2− samples and reference materials are extracted and purified using an automated headspace extractor and analyzed at m/z 44, 45, and 46 for δ(18O) determination from 46/44 using CF-IRMS. While the determination of the oxygen isotope composition of NO2− is the focus of the described protocol, the method also enables simultaneous measurement of δ(15N) from 45/44. The measured δ(18O, N2O) and δ(15N, N2O) are normalized to VSMOW and air, respectively, using NO2− isotope reference materials (RSIL-N7373; RSIL-10219). Measurement of δ(18O) and δ(15N) from N2O using an automated headspace extraction and CF-IRMS is a relatively routine methodology for numerous isotope ratio labs [9,[25], [26], [27]]. Here we highlight the general procedure and calibration scheme utilized for this type of measurement.
-
1.
Similar to the Δ(17O, NO2−) analysis, the generated N2O from the NO2− samples, reference materials (RSIL-N7373; RSIL-N10219), and blanks are extracted, purified, and concentrated using a modified headspace extractor (GasBench II) (see “Δ(17O, NO2−) Isotope Analysis and Data Processing”, Step 1 for additional details).
-
2.
The purified N2O is introduced into a CF-IRMS (Thermo Scientific Delta V Plus) for mass analysis at m/z 44, 45, and 46 for quantifying δ(15N) (from 45/44) and δ(18O) (from 46/44).
-
3.
Isobaric interferences for 17O are corrected in the m/z 45 signal (i.e., 15N14N16O, 14N217O) and for 17O and 15N in the m/z 46 signal (i.e., 14N218O, 15N14N17O, 15N216O) following previous suggestions [22]. The 17O isobaric corrections are based on the measured Δ(17O, NO2−) value and assume a λ = 0.52. This correction typically results in a 1–2 ‰ correction in δ(15N). The NO2− reference materials (RSIL-N7373 & RSIL-N10219) are used to generate a calibration for δ(15N) and δ(18O) of the NO2− unknowns.
-
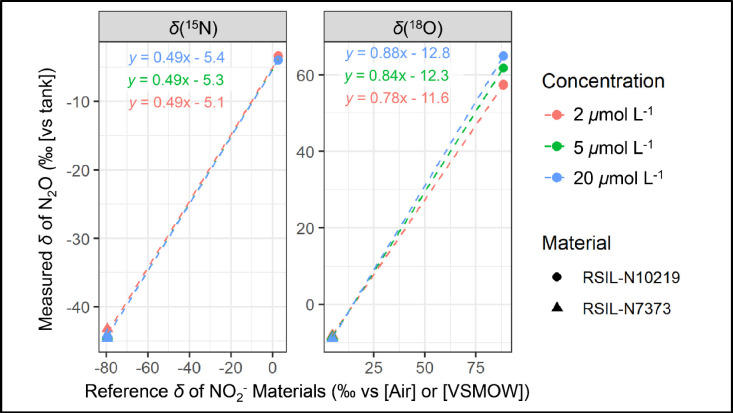
4.
An example of a typical δ(15N) and δ(18O) calibration using the RSIL-N7373 and RSIL-N10219 is shown in Fig. 3. The calibration slope for δ(15N) approaches the theoretical line of 0.5 (i.e., 1 nitrogen of the product N2O derives from the sample NO2− and the other nitrogen derives from the azide reagent) and is independent of NO2− concentration [9] (Fig. 3). The calibration slope for δ(18O) ranged from 0.78 to 0.88, indicating minor O isotope exchange of NO2− with water as the NO2− analyte is reduced to N2O (Fig. 3). The difference in the calibration δ(18O) slopes was observed depending on the concentration of the NO2− reference materials. In particular, the measured versus actual calibration slope decreased with lower concentrations reflecting increased oxygen isotope exchange with water. The δ(15N) calibrations provide direct evidence that the change in δ(18O) slope reflects O isotope exchange with water rather than a blank effect, as there was no dependence on the calibration slope for δ(15N) with concentration (Fig. 3). This finding is the reason why it is recommended that samples are calibrated with respect to standards at similar NO2− concentrations. For example, for a measured δ(18O) of 60 ‰, the δ(18O) calibration curves for 2, 5, and 20 μmol L−1 standards (Fig. 3) would result in a calibrated value of 91.8 ‰, 86.1 ‰, and 82.7 ‰. Thus, it is vital to concentration match samples and standards to reduce the potential impact of oxygen isotope exchange between NO2− and water on impacting the calibrated δ(18O) (and Δ(17O)) values. After calibration, the long-term pooled standard deviations for δ(15N) and δ(18O) of the NO2− reference materials in our laboratory are ±0.5 ‰ and ±0.8 ‰, respectively. There is not a significant difference in the isotope precision for δ(15N) and δ(18O) measurement as a function of NO2− concentration as observed for Δ(17O).
Fig. 3.
The measured δ(15N) and δ(18O) values of N2O (relative to a reference N2O tank) produced from NO2− reference materials (RSIL-N10219 and RSIL-N7373) converted to N2O using sodium azide/acetic acid reagent relative to the reference δ(15N) (relative to Air) and δ(18O) (relative to VSMOW). The data is color-coded by the concentration and shade-coded by the type of NO2− reference material. While there was no concentration dependence on the δ(15N) calibration, there was a strong concentration influence on the δ(18O) calibration, reflecting varying oxygen isotope exchange with water contribution during the nitrite reduction to N2O.
Method validation
Here we evaluate the described method for reducing NO3− reference materials (i.e., USGS34 & USGS35) to NO2− while preserving the original Δ(17O) value. We evaluate the method's ability to quantitively reduce NO3− to NO2− while limiting oxygen isotope exchange with water. The NO3− reference materials reduced to NO2− were calibrated with respect to NO2− reference materials (RISIL-N7373 and RISIL-N10219). Once we validated that the NO3− reference materials were quantitatively reduced to NO2− while conserving the Δ(17O) values, these materials were utilized to calibrate Δ(17O) and calculate δ(17O) of the NO2− reference materials (i.e., RSIL-7373 & RSIL-10219), which have been previously unknown. Additionally, the NO3− reference materials reduced to NO2− were used to calibrate Δ(17O) of atmospherically derived NO2− samples.
-
1.
After the NO3− reference materials were reduced to NO2−, a 2 mL aliquot from each vial was measured for the NO2− concentration using a standard colorimetric analysis (e.g., US EPA Methods 353.2) that is automated using a discrete analyzer (WESTCO Smartchem 200 Discrete Analyzer) as previously described [2]. Based on the measured [NO2−] and the expected [NO3−], the conversion efficiency was calculated and was always found to be greater than 95 %, indicating near-complete conversion. Further, our reagent “blank” following the reduction protocol has always been found to be below 1.0 μmol L−1.
-
2.
The described NO3− reduction to NO2− protocol calls for the reduction of an initial NO3− concentration of 50 μmol L−1. After reagent additions, the diluted NO3− concentration and thus the generated NO2− concentration is near 35 μmol L−1. The reagent blank associated with this conversion has always been found to be below the limit of detection of our analyzer of 1 μmol L−1, representing at most 3 % blank contribution. For analyzing Δ(17O, NO2−) samples, we always dilute the NO3− reduced reference materials from the initial 35 μmol L−1 batch of solutions to the appropriate concentration targets (e.g., 2, 5, and/or 20 μmol L−1) using 0.1 M NaOH, such that the reagent blank contribution should not increase even for when analyzing low concentration samples. In our experience, NO2− and NO3− reagent blanks can vary depending on the batch of reagents as well as reagent age, such that it is critical for users always to quantify the reagent blank to make appropriate blank corrections as necessary.
-
3.
The reduced NO3− reference materials were measured for their δ(15N) and δ(18O) values that were calibrated with respect to the NO2− reference materials (i.e., RSIL-N7373 & RSIL-N10219) (Table 1). The δ(15N) of the reduced NO3− was (−2.3 ± 0.2) ‰ and (2.8 ± 0.3) ‰, which were nearly identical to the reference values of −1.8 ‰ and 2.7 ‰ for USGS34 and USGS35, respectively (Table 1; [23]). We note that since δ(15N) is quantified by analyzing N2O at m/z 44, 45, and 46, appropriate corrections for 17O isobaric influences were conducted that include corrections for non-zero Δ17O that included the RSIL-N10219 and USGS35 reference materials. These corrections lead to an apparent offset of −0.5 ‰ for N-10219; and 1.0 ‰ for USGS35.
-
4.
The δ(18O) values of the NO3− reference materials reduced to NO2− using cadmium in a highly basic solution was (−21.4 ± 0.2) ‰ and (64.5 ± 0.5) ‰ for USGS34 and USGS35, respectively, which were calibrated with respect to the NO2− reference materials (RSIL-N7373 and RSIL-N7373). The NO3− reference materials reduced to NO2− were higher by (6.5 ± 0.2) ‰ and (7.0 ± 0.5) ‰ compared to the NO3− reference δ(18O) values (Table 1). Since the δ(17O) values of the NO2− reference materials (RSIL-N7373 and RSIL-N7373) are unknown, the NO3− reference materials reduced to NO2− could not be directly calibrated.
-
5.
During the reduction of NO3− to NO2−, there are a few possible fates for oxygen atoms that include transfer to the subsequent reactive nitrogen pool or loss as water and oxygen isotope exchange with water [11]. The loss of one oxygen atom during the reduction of NO3− to NO2− leads to isotope fractionation (ε(NO3− → NO2−)), such that the light isotopes of oxygen are preferentially lost, leaving the analyte pools progressive enriched in 18O (and 17O) as previously observed for NO3− and NO2− reduction to N2O [25]. This fractionation can explain the observed consistent shift of the measured δ(18O) values of the NO3- materials reduced to NO2− relative to their starting δ(18O) values.
-
6.
An additional complication when reducing NO3− to NO2− is the concern for oxygen isotope exchange between NO2− and water, which is rapid for low pH conditions [11], altering the NO2− isotope deltas. Oxygen isotope equilibrium between NO2− (solute) and water (solvent) would be expected to result in δ(18O, NO2−), which is about 14 ‰ higher than H2O. Assuming the δ(18O, H2O) of −6 ‰ (i.e., typical value for mid-latitudes) would indicate that NO2− oxygen isotope exchange with water would result in a δ(18O, NO2−) near 8 ‰.
-
7.
Thus, oxygen isotope exchange between NO2− and water would have been expected to cause the NO3− reference materials reduced to NO2− to have δ(18O) values that converge towards 8 ‰. However, the consistent δ(18O) offset between the reduced NO3− materials to NO2− and the reference δ(18O, NO3−) values of (6.5 ± 0.2) ‰ and (7.0 ± 0.5) ‰ for USGS34 and USGS35, respectively, indicates that oxygen isotope exchange between the product NO2− was non-existent. This is the result of conducting the NO3− reduction to NO2− in highly basic conditions (i.e., pH > 13). The consistent δ(18O) offset near 7.0 ‰ represents the oxygen isotope fractionation associated with NO3− reduction to NO2− using activated cadmium. Assuming the NO3− to NO2− reduction fractionation is mass-dependent with a λ near 0.52, the Δ(17O) values of the NO3− reference materials can reasonably be assumed to be conserved in the product NO2−. The NO3− reference materials reduced to NO2− can now be utilized to calibrate Δ(17O) of NO2− samples.
-
8.
Utilizing the Δ(17O) values of the reduced NO3− reference materials, the Δ(17O) of the NO2− reference materials were measured, and δ(17O) was calculated (Table 2). There is a 17O deficit for the RSIL-10219 sample (Δ(17O) = −12.9 ± 1.2 ‰), consistent with expectations as this material was prepared from non-mass-dependent labeled water (Icon Stable Isotopes) that, while enriched in 18O, was not proportionally enriched in 17O [11]. The low Δ(17O) value of the RSIL-10219 would indicate that previous studies that have utilized it for δ(15N) calibration would be slightly incorrect and lead to samples biased high in δ(15N) (approximately 0.5 to 1 ‰) if not appropriately accounted for the deficit in 17O.
-
9.
The reduced NO3− reference materials have been utilized to determine the Δ(17O) of NO2 (collected as NO2−) samples from environmental chamber experiments with measured values that range from 10.8 to 41.2 ‰ consistent with expectations for NO oxidation reactions involving ozone (O3terminal; Δ(17O) = (39 ± 2) ‰) and peroxy radicals (RO2/HO2; Δ(17O) = 0 ‰) [2].
Table 1.
Summary of the isotope deltas (δ(15N), δ(18O), and Δ(17O)) for the USGS34 and USGS35 NO3− reference materials, including their internationally recognized reference values and the measured and determined isotope deltas after reduction to NO2−.
| Isotope deltas Reference value (‰)a |
Isotope deltas After reduction to NO2− (‰)b |
||||
|---|---|---|---|---|---|
| Reference ID | δ(15N) | δ(18O) | Δ(17O) | δ(15N) | δ(18O) |
| USGS34 | −1.8 | −27.9 | −0.29 | −2.3 ± 0.2 | −21.4 ± 0.2 |
| USGS35 | 2.7 | 57.5 | 21.6 | 2.8 ± 0.3 | 64.5 ± 0.5 |
Reference values from [23].
Measured using the sodium azide/acetic acid method to convert NO2− materials to N2O, which were then analyzed using at CF-IRMS at m/z 44, 45, and 46 for δ(15N) and δ(18O) determination and normalized relative to NO2− reference materials (RSIL-N7373 and RSIL-N10219) with known δ(15N) and δ(18O) values.
Table 2.
Summary of the reference isotope deltas (δ(15N) and δ(18O)), measured Δ(17O), and calculated δ(17O) for the NO2− reference materials, including RSIL-N7373 and RSIL-N10219.
Method outlook
The described method and validation demonstrate the ability to quantify the triple oxygen isotope composition of NO2− by reducing NO3− reference materials with known Δ(17O) values under conditions that prevent oxygen isotope exchange between NO2− and water. The method can be used to accurately quantify Δ(17O) from NO2− materials (unknowns). Additionally, this method could quantify the Δ(17O) from NO3− materials (unknowns) by combining the NO3− reduction using cadmium with sodium azide/acetic acid reagent. This has the potential to replace the commonly utilized bacteria denitrifier method for Δ(17O) analysis of NO3−, which can be cumbersome and expensive to maintain.
CRediT authorship contribution statement
Wendell W. Walters: Conceptualization, Methodology, Investigation, Writing – original draft. Meredith G. Hastings: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Ocean and Atmospheric Administration (NOAA) Atmospheric Chemistry, Carbon Cycle, and Climate program (NOAA AC4 NA18OAR4310118). The authors would like to thank Ruby Ho for their laboratory assistance.
Footnotes
Related research article: Blum, D.E., Walters, W.W., Eris, G., Takeuchi, M., Huey, L.G., Tanner, D., Xu, W., Rivera-Rios, J.C., Liu, F., Ng, N.L., Hastings, M.G. Collection of Nitrogen Dioxide for Nitrogen and Oxygen Isotope Determination-Laboratory and Environmental Chamber Evaluation, Anal. Chem. 95 (2023) 3371–3378. https://doi.org/10.1021/acs.analchem.2c04672
Data availability
Data will be made available on request.
References
- 1.Walters W.W., Fang H., Michalski G. Summertime diurnal variations in the isotopic composition of atmospheric nitrogen dioxide at a small midwestern United States city. Atmos. Environ. 2018;179:1–11. doi: 10.1016/j.atmosenv.2018.01.047. [DOI] [Google Scholar]
- 2.Blum D.E., Walters W.W., Eris G., Takeuchi M., Huey L.G., Tanner D., Xu W., Rivera-Rios J.C., Liu F., Ng N.L., Hastings M.G. Collection of nitrogen dioxide for nitrogen and oxygen isotope determination-laboratory and environmental chamber evaluation. Anal. Chem. 2023;95:3371–3378. doi: 10.1021/acs.analchem.2c04672. [DOI] [PubMed] [Google Scholar]
- 3.Albertin S., Savarino J., Bekki S., Barbero A., Caillon N. Measurement report: nitrogen isotopes (δ15N) and first quantification of oxygen isotope anomalies (Δ17O, δ18O) in atmospheric nitrogen dioxide. Atmos. Chem. Phys. 2021;21:10477–10497. doi: 10.5194/acp-21-10477-2021. [DOI] [Google Scholar]
- 4.Clark S.C., Barnes R.T., Oleksy I.A., Baron J.S., Hastings M.G. Persistent nitrate in alpine waters with changing atmospheric deposition and warming trends. Environ. Sci. Technol. 2021;55:14946–14956. doi: 10.1021/acs.est.1c02515. [DOI] [PubMed] [Google Scholar]
- 5.Martin T.S., Primeau F., Casciotti K.L. Modeling oceanic nitrate and nitrite concentrations and isotopes using a 3-D inverse N cycle model. Biogeosciences. 2019;16:347–367. [Google Scholar]
- 6.Buchwald C., Casciotti K.L. Isotopic ratios of nitrite as tracers of the sources and age of oceanic nitrite. Nat. Geosci. 2013;6:308–313. doi: 10.1038/ngeo1745. [DOI] [Google Scholar]
- 7.Lewicka-Szczebak D., Jansen-Willems A., Müller C., Dyckmans J., Well R. Nitrite isotope characteristics and associated soil N transformations. Sci. Rep. 2021;11:5008. doi: 10.1038/s41598-021-83786-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner R.A., Brand W.A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 2001;15:501–519. doi: 10.1002/rcm.258. [DOI] [PubMed] [Google Scholar]
- 9.McIlvin M.R., Altabet M.A. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal. Chem. 2005;77:5589–5595. doi: 10.1021/ac050528s. [DOI] [PubMed] [Google Scholar]
- 10.Ryabenko E., Altabet M.A., Wallace D.W.R. Effect of chloride on the chemical conversion of nitrate to nitrous oxide for δ15N analysis. Limnol. Oceanogr. Methods. 2009;7:545–552. doi: 10.4319/lom.2009.7.545. [DOI] [Google Scholar]
- 11.Casciotti K.L., Böhlke J.K., McIlvin M.R., Mroczkowski S.J., Hannon J.E. Oxygen isotopes in nitrite: analysis, calibration, and equilibration. Anal. Chem. 2007;79:2427–2436. doi: 10.1021/ac061598h. [DOI] [PubMed] [Google Scholar]
- 12.Young E.D., Galy A., Nagahara H. Kinetic and equilibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance. Geochim. Cosmochim. Acta. 2002;66:1095–1104. doi: 10.1016/S0016-7037(01)00832-8. [DOI] [Google Scholar]
- 13.Walters W.W., Michalski G., Böhlke J.K., Alexander B., Savarino J., Thiemens M.H. Assessing the seasonal dynamics of nitrate and sulfate aerosols at the south pole utilizing stable isotopes. J. Geophys. Res. 2019;124:8161–8177. doi: 10.1029/2019JD030517. [DOI] [Google Scholar]
- 14.Kim H., Walters W.W., Bekker C., Murray L.T., Hastings M.G. Nitrate chemistry in the northeast US – part 2: oxygen isotopes reveal differences in particulate and gas-phase formation. Atmos. Chem. Phys. 2023;23:4203–4219. doi: 10.5194/acp-23-4203-2023. [DOI] [Google Scholar]
- 15.Michalski G., Scott Z., Kabiling M., Thiemens M.H. First measurements and modeling of Δ17O in atmospheric nitrate. Geophys. Res. Lett. 2003;30:1870. doi: 10.1029/2003GL017015. [DOI] [Google Scholar]
- 16.Alexander B., Sherwen T., Holmes C.D., Fisher J.A., Chen Q., Evans M.J., Kasibhatla P. Global inorganic nitrate production mechanisms: comparison of a global model with nitrate isotope observations. Atmos. Chem. Phys. 2020;20:3859–3877. doi: 10.5194/acp-20-3859-2020. [DOI] [Google Scholar]
- 17.Savarino J., Morin S., Erbland J., Grannec F., Patey M.D., Vicars W., Alexander B., Achterberg E.P. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl. Acad. Sci. U S A. 2013;110:17668–17673. doi: 10.1073/pnas.1216639110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters W.W., Michalski G. Theoretical calculation of oxygen equilibrium isotope fractionation factors involving various NOy molecules, OH, and H2O and its implications for isotope variations in atmospheric nitrate. Geochim. Cosmochim. Acta. 2016;191:89–101. doi: 10.1016/j.gca.2016.06.039. [DOI] [Google Scholar]
- 19.Barkan E., Luz B. High precision measurements of 17O/16O and 18O/16O ratios in H2O. Rapid Commun. Mass Spectrom. 2005;19:3737–3742. doi: 10.1002/rcm.2250. [DOI] [PubMed] [Google Scholar]
- 20.Surma J., Assonov S., Herwartz D., Voigt C., Staubwasser M. The evolution of 17O-excess in surface water of the arid environment during recharge and evaporation. Sci. Rep. 2018;8:4972. doi: 10.1038/s41598-018-23151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller M.F. Isotopic fractionation and the quantification of 17 O anomalies in the oxygen three-isotope system: an appraisal and geochemical significance. Geochim. Cosmochim. Acta. 2002;66:1881–1889. [Google Scholar]
- 22.Kaiser J., Hastings M.G., Houlton B.Z., Röckmann T., Sigman D.M. Triple oxygen isotope analysis of nitrate using the denitrifier method and thermal decomposition of N2O. Anal. Chem. 2007;79:599–607. doi: 10.1021/ac061022s. [DOI] [PubMed] [Google Scholar]
- 23.Böhlke J.K., Mroczkowski S.J., Coplen T.B. Oxygen isotopes in nitrate: new reference materials for 18O: 17O: 16O measurements and observations on nitrate-water equilibration. Rapid Commun. Mass Spectrom. 2003;17:1835–1846. doi: 10.1002/rcm.1123. [DOI] [PubMed] [Google Scholar]
- 24.Böhlke J.K., Smith R.L., Hannon J.E. Isotopic analysis of N and O in nitrite and nitrate by sequential selective bacterial reduction to N2O. Anal. Chem. 2007;79:5888–5895. doi: 10.1021/ac070176k. [DOI] [PubMed] [Google Scholar]
- 25.Casciotti K.L., Sigman D.M., Hastings M.G., Böhlke J.K., Hilkert A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 2002;74:4905–4912. doi: 10.1021/ac020113w. [DOI] [PubMed] [Google Scholar]
- 26.Sigman D.M., Casciotti K.L., Andreani M., Barford C., Galanter M., Böhlke J.K. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 2001;73:4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 27.Bekker C., Walters W.W., Murray L.T., Hastings M.G. Nitrate chemistry in the northeast US – part 1: nitrogen isotope seasonality tracks nitrate formation chemistry. Atmos. Chem. Phys. 2023;23:4185–4201. doi: 10.5194/acp-23-4185-2023. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.