Highlights
-
•
The largest meta-analysis of dietary inflammatory index among cancer survivors.
-
•
Inclusion of three outcomes, 13 studies, and 14,920 adult cancer survivors.
-
•
Inflammatory diet can increase all-cause mortality by 34% among cancer survivors.
-
•
Only post-diagnosis diet had a significant association with all-cause mortality.
Keywords: Dietary inflammatory index, Cancer recurrence, All-cause mortality, Cancer mortality, Meta-analysis, Cancer survivors
Abstract
Cancer remains the second leading cause of death globally. Chronic inflammatory environments promote the growth of tumors, and the intake of certain food items can increase systemic inflammation. This study examined the relationship between the inflammatory potential of diet, measured by the Dietary Inflammatory Index (DII), and recurrence, all-cause, and cancer-specific mortality among cancer survivors. Web of Science, Medline, CINHAL, and PsycINFO databases were searched in April 2022. Two independent reviewers screened all searches. Of the 1,443 studies, 13 studies involving 14,920 cancer survivors passed all the screening stages. Three studies reported cancer recurrence, 12 reported all-cause mortality, and six reported cancer-specific mortality. Seven studies calculated DII from pre-diagnosis diets, five from post-diagnosis diets, and one from both pre-and post-diagnosis diets. A random-effects model meta-analysis showed that high DII was not associated with an increased risk of recurrence (HR = 1.09, 95 % CI = 0.77, 1.54, n = 4) and all-cause (HR = 1.08, 95 % CI = 0.99, 1.19, n = 14) and cancer-specific mortality (H = 1.07, 95 % CI = 0.92, 1.25, n = 6). Analysis by the timing of dietary assessment showed that only post-diagnosis DII was associated with an increased risk of all-cause mortality (HR = 1.34, 95 % CI = 1.05, 1.72, n = 6) by 34 %; however, cancer type did not modify these associations. The quality of the study assessed using the Newcastle Ottawa Scale indicated all but one studies were good. The risk of all-cause mortality among cancer survivors could be reduced by consuming more anti-inflammatory diets after cancer diagnosis.
Graphical abstract
Introduction
Cancer is currently the second leading cause of death in the United States (US), with 1958,310 new cancer cases and 609,820 cancer deaths estimated in 2023 [1]. The link between cancer and diet has been well established, with large prospective studies [2,3] demonstrating a significant association between specific dietary components and cancer risk. For example, the European Prospective Investigation into Cancer and Nutrition (EPIC) study measured the food consumption of over 500,000 individuals in ten European countries and demonstrated that doubling fiber intake from average consumption levels could significantly halve the risk of colorectal cancer (RR = 0.5) [2]. A meta-analysis of 14 cohort studies showed that women who consumed highest levels of saturated fat were 1.19 times more likely to develop breast cancer, compared with those who ate lowest levels [3]. Another large meta-analysis of cohort studies also showed that diet quality, as measured by various dietary quality indices, was inversely associated with cancer incidence or mortality, reporting an 11–16 % reduced risk of cancer incidence among participants without cancer at baseline, while a 12–23 % decreased risk of all-cause mortality or an 11–25 % decreased risk of cancer-specific mortality among cancer survivors [4].
Inflammation can play a substantial role in cancer development and progression [5]. Chronic inflammation promotes immunosuppression primarily through the action of immature myeloid-derived suppressor cells [6]. The suppression of the innate and adaptive immune system leads to pro-cancer inflammatory environments that promote the formation and growth of tumors, while preventing effective anti-tumor responses [6]. Nutrition can modulate inflammation, as evidenced by decreasing inflammatory biomarkers, such as high-sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor alpha, with the intake of certain food items, such tomatoes, walnuts, garlic powder and flaxseed flour [7,8]. Thus, there has been growing interest in the interplay between diet, inflammation, and cancer risks.
The Dietary Inflammatory Index (DII) was developed in 2004 by Cavicchia's team at the University of South Carolina's Arnold School of Public Health to quantify inflammatory potential of diet, with the first version of the DII published in 2009 [9]. The association between consumption levels of relevant dietary factors (i.e., energy, 32 nutrients, four food products, four spices, and caffeine) and the levels of inflammatory biomarkers (i.e., inflammatory weight) was found through the scoring of 927 peer-reviewed articles published in biomedical journals up to 2007. The DII was calculated as a sum of multiplications of the dietary inflammatory weight of those 42 dietary parameters mentioned times the intake [10]. The second version of the DII developed by Shivappa et al. in 20148 marked a major improvement in its reliability by including 1943 publications published up to December 2010 and determined 45 main food parameters most relevant to inflammation. It also used 11 large dietary data sets from diverse global locations to develop a composite database, accounting for the diversity of diet and reflecting dietary norms to which reported dietary intake could be compared [8].
The effects of diet-associated inflammation on health outcomes, such as cardiovascular diseases and cancers, have been accumulated using the original and the revised versions of DII. Consequently, meta-analyses were conducted to report a robust association between a high DII category and an increased risk for cancer incidence including breast cancer [11], [12], [13], colorectal cancer [14], [15], [16], esophageal cancer [17], gastric cancer [18], gynecological cancers [19], ovarian cancer [20], pancreatic cancer [21], prostate cancer [22,23], urologic cancer [24], and upper aerodigestive tract cancers [25,26], while only one meta-analysis was conducted for cancer mortality [27], reporting individuals in the highest category of DII score having an increased risk of cancer mortality (RR 1.67; 95 % CI 1.13, 2.48) [27]. However, this meta-analysis included only two publications, one [28] of which examined cancer mortality among prostate cancer survivors, suggesting that there is a need to update the meta-analysis on cancer mortality among survivors. The current meta-analysis aimed to determine the strength of associations between inflammatory potential of diet, measured by the DII, and cancer outcomes (i.e., recurrence, all-cause mortality, and cancer-specific mortality) among cancer survivors.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to structure the current study. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; University of York; York, United Kingdom) in August 2022 (CRD42022350719).
Literature search
The initial group of potential sources was accumulated through searches of the Web of Science, Medline, CINHAL, and PsycINFO databases on April 15, 2022. Keywords and extenders — such as inflammation, dietary inflammatory index, diet inflammatory potential, DII, cancer, malignancy, carcinogenesis, recurrence, mortality, death, survival, and prognosis — were used to collect as many publications pertaining to DII and cancer outcomes as possible. The search was limited to articles published in peer-reviewed journals after January 1, 2009, as DII was first published in 2009 [9]. The reference lists of the included studies and prior meta-analyses and review articles were reviewed to identify additional publications that could be eligible. The full search strategies are shown in Supplementary Table S1.
Eligibility criteria
All initial search were exported to Covidence® (Covidence, Inc.; Melbourne, Australia) [29] to be screened for eligibility with streamlined features. Screening was based on its compatibility with the pre-established Participants, Exposure, Comparison, Outcome, and Study design (PECOS) framework, shown in Supplemental Table S2, which guided study eligibility. The population consisted of adult cancer survivors (age ≥ 18 years) with an exposure of dietary inflammatory potential calculated through the DII. Comparisons were made between the highest category of DII score, indicating the most pro-inflammatory diet, and the lowest category, indicating the most anti-inflammatory diet. The outcomes of cancer recurrence, all-cause mortality, and cancer-specific mortality through a hazard ratio (HR) or risk ratio (RR) with a 95 % confidence interval (CI) were evaluated. Study designs included cohort studies with follow-up evaluations. Initial screenings were performed by reviewing titles and abstracts. After this stage, the remaining articles were subject to a full-text review to determine its eligibility. Each source was reviewed by two blinded reviewers, and any conflicts were resolved through discussions to reach a consensus.
Quality assessment
The methodological quality of included studies was evaluated using the modified Newcastle-Ottawa Scale for cohort studies [30]. This scale examines the quality of cohort studies based on three main domains: (1) the adequacy of the recruitment/selection of study participants, (2) comparability of comparison groups, and (3) ascertainment of outcomes. A study receives a star for meeting a specific criterion within these domains. The total score for this assessment ranges from 0 to 9 stars, with a score of 7+ stars indicative of “good quality”, and a score of ≤3 stars indicative of “poor quality.”
Data extraction
Data extracted from each study were as follows: (1) Study characteristics: title, first author, year of publication, country of study, study name, study design, sample size, and follow-up periods, (2) Population characteristics: age, race/ethnicity, sex, smoking status, body mass index (BMI), and cancer type, (3) Exposure: the timing of dietary assessment regarding cancer diagnosis (pre- or post-diagnosis), dietary assessment methods, DII version, the number of food parameters included in DII calculation, and supplement use, (4) Comparison: high versus low DII categories or continuous comparison, and (5) Outcome: recurrence and mortality (all-cause and cancer-specific), RR/HR (i.e., RR/HRHighVs.Low) and 95 % CI, and the covariates included in the multivariable model.
Data synthesis & statistical analysis
Meta-analysis was conducted using a random-effects model with Bayesian method comparing the highest DII category (indicating the most inflammatory potential) versus lowest DII category. A Bayesian method is the preferred approach when the number of studies included in the meta-analysis is small, as it reflects uncertainty in the estimation of between-study heterogeneity [31]. Two studies [32,33] reported HRs comparing the lowest versus the highest DII categories (i.e., HRLowVs.High), therefore, appropriate mathematical conversions were conducted before including them in the analysis. When a study reported both results using DII with and without supplement use, the result with supplement use was included in the meta-analysis as more studies included supplement use in the calculation of DII. The analysis was stratified by outcomes, the timing of dietary assessment (pre-or post-diagnosis), and cancer type in order to determine whether any of these factors had a significant modifying effect on cancer outcomes, in conjunction with DII score. Heterogeneity between studies was assessed using Q tests and I2 tests, while a sensitivity analysis was conducted by excluding one study at a time to determine if the results were sensitive to the influence of a single study. Publication bias was assessed with funnel plots and Egger's tests when more than ten studies were included in a meta-analysis [34]. Statistical analyses were conducted using Stata v. 17 (StataCorp LLC; College Station, TX) and the metafor package [35] of R software R (R Core Team; Vienna, Austria). A two-tailed P ≤ 0.05 was considered statistically significant.
Results
Search results
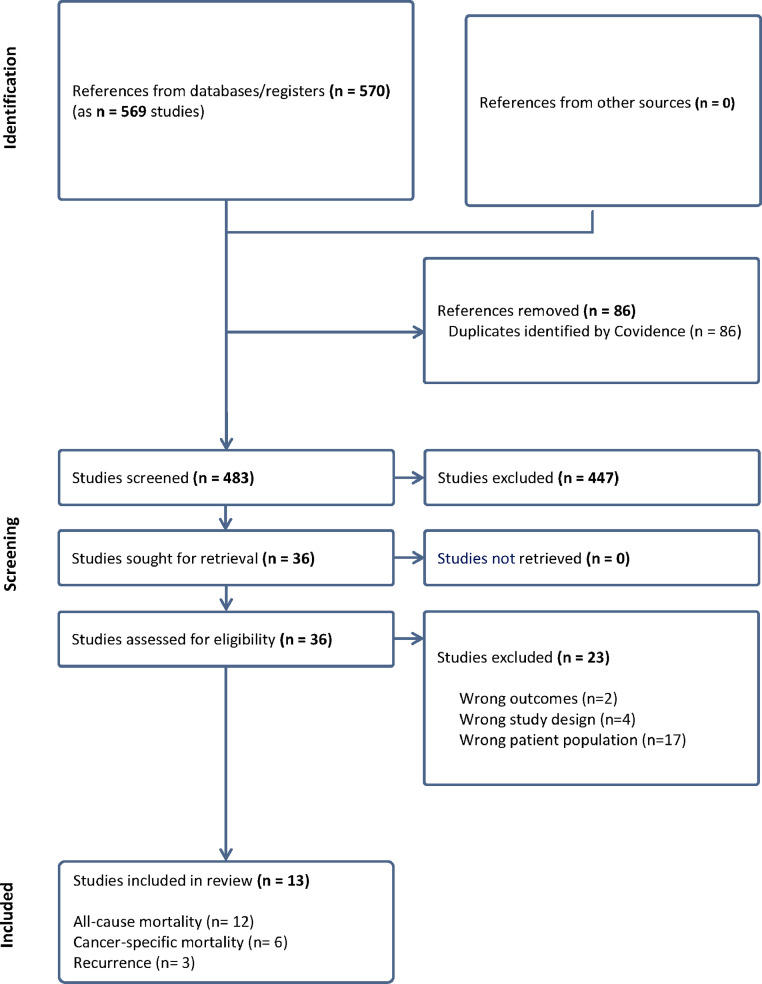
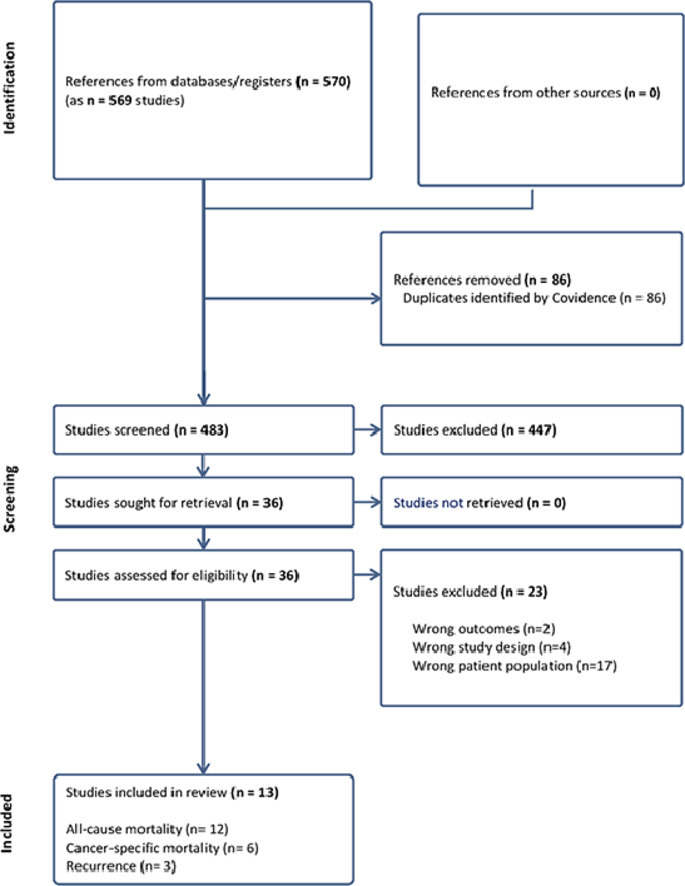
The search of the Web of Science, Medline, CINHAL, and PsycINFO databases yielded 569 unique studies, of which 86 were duplicates and automatically removed by Covidence®, leaving 483 to be screened for titles and abstracts. Out of the 483 studies, 447 were found to be irrelevant, leaving 36 for to be screened in full for eligibility. Out of these studies, 23 were excluded, leaving 13 studies for data extraction as shown in the PRISMA flowchart (Fig. 1). The reasons for exclusion are presented in Supplementary Table S3.
Fig. 1.
PRISMA Flow Chart.
Characteristics of included studies
The relevant characteristics of each study are presented in Table 1. Five studies were conducted in the US [32,33,[36], [37], [38]], two studies in Italy [28,39], two studies in Australia [40,41], and one each in Poland [42], Netherlands [43], Korea [44], and Germany [45]. The 13 studies were all cohort studies, with follow-up periods ranging from 1 year [42] to 14.6 years [38] after diagnosis. Twelve, six, and three studies reported the relationship between DII and all-cause mortality [28,32,33,[37], [38], [39], [40], [41], [42], [43], [44], [45]], cancer-specific mortality [28,32,33,[38], [39], [40]], and recurrence [36,43,44], respectively. Five studies [32,36,42,43,45] examined colorectal cancer, four [33,38,39,44] breast cancer, two [37,40] ovarian cancer, one [41] on endometrial cancer, and one [28] prostate cancer. The sample size varied from 463 colorectal cancer survivors [32] to 2150 breast cancer survivors [33], both from Women's Health Initiative (WHI) study, leading to a total sample size of 14,920 cancer survivors in the current study. The proportion of female participants varied from 0 % in a prostate cancer study [28] to 100 % in women's cancer studies [33,[37], [38], [39], [40], [41],44] and a colorectal study from WHI [32].
Table 1.
Characteristics of included studies (N = 13) examining the association between Dietary Inflammatory Index score and outcomes in cancer survivors.
| First author Year Country |
Source population Study design Cancer type Sample size Follow-up (Mean; years) |
Age (Mean; years) Race (% White) Sex (% female) BMI (kg/m2) Current smokers (%) |
Dietary assessment tool Timing |
DII version # Parameters Inclusion of supplements Comparison |
Outcomes reported (number of cases) Ascertainment methods |
Multivariable adjusted HR/RR (95 % CI) |
Covariates included in the model | Study quality score |
|---|---|---|---|---|---|---|---|---|
| Pre-diagnosis diet | ||||||||
| Galas 2014 [42] Poland |
Polish cohort study Prospective cohort Colorectal cancer 689 1–5 years after diagnosis |
58.0 100 % white 43.3 % female 69.5 % BMI ≥ 25 25.1 % |
SFFQ (148 items) Asked about 5 years prior to symptoms |
DII 2009 23 No supplements High vs. low, Continuous |
All-cause mortality (309) Vital records |
All-cause mortality High vs. low: Without distant metastases: 0.76 (0.55, 1.08) With distant metastases: 1.06 (0.76, 1.48) Continuous: Without distant metastases: 0.98 (0.92, 1.05) With distant metastases: 1.003 (0.93 1.08) |
Age, smoking status, marital status, overweight or obesity, calendar year when surgery was performed, surgery type, cancer site, chemotherapy after surgery, and radiotherapy after surgery | 8 |
| Zucchetto 2016 [28] Italy |
Italian case-control study Retrospective cohort Prostate cancer 726 Median 12.7 years after diagnosis |
Median 66 100 % White 100 % male Median 26.4 <15 cig/da = 10% >15 cig/day = 9.5 % |
FFQ (78 items) Two years before diagnosis |
DII 2014 32 No supplements T3 vs. T1 |
All-cause mortality (244) Regional health system data and population-based cancer registry Cancer mortality (76) Regional health system data and population-based cancer registry |
All-cause mortality 1.25 (0.86, 1.83) Cancer mortality 1.42 (0.73, 2.76) |
Area of residences, calendar period of cancer diagnosis, age at diagnosis, education, smoking habits, abdominal obesity, alcohol intake, energy intake, and Gleason score | 8 |
| Sardo Molmenti 2017 [36] USA |
WBF and UDCA Phase III clinical trials Prospective cohort Colorectal cancer 1727 WBF: 3.1 years UCDA: 3.2 years after diagnosis |
65.9 ± 8.6 94.8 % white 32 % female 28 ± 4.8 12.7 % |
FFQ (113 items) At baseline |
DII 2014 27 Including supplements T3 vs. T1 |
Cancer recurrence (780) Colonoscopy |
Cancer recurrence 0.95 (0.74, 1.22) |
Age, sex, waist circumference, smoking, aspirin use, and moderate-vigorous physical activity | 8 |
| Zucchetto 2017 [39] Italy |
Italian case-control study Retrospective cohort Breast cancer 1453 Median 12.6 years after diagnosis |
Range 23–74 100 % White 100 % female 43.8 % BMI ≥ 25 <15 cig/d = 12.5 % >15 cig/d = 7.4 % |
FFQ (78 items) Two years before diagnosis |
DII 2014 31 No supplements T3 vs. T1 |
All-cause mortality (503) Population-based cancer registry Cancer mortality (398) Population-based cancer registry |
All-cause mortality 1.00 (0.78–1.28) Cancer mortality 0.97 (0.73–1.27) |
Area of residence, calendar year of diagnosis, age at diagnosis, education, menopausal status, smoking habits, BMI, total energy intake, hormone receptor status, and TNM tumor stage | 8 |
| Peres 2019 [37] USA |
AACES Retrospective cohort Ovarian cancer 490 Median 3.5 years after diagnosis |
Median 57 (20–79) 100 % Black 100 % female 84.5 % BMI ≥ 25 12 % |
FFQ (110 items) 1 year before diagnosis |
E-DII 2014 27 Both Q4 vs. Q1, Continuous |
All-cause mortality (223) Cancer registries and annual contact |
All-cause mortality Including supplements Q4 vs Q1: 1.35 (0.90, 2.03) Per 1 unit: 1.06 (0.99, 1.13) Excluding supplements Q4 vs Q1: 1.28 (0.85, 1.92) Per 1 unit: 1.03 (0.95, 1.11) |
Age at diagnosis, site, stage at diagnosis, smoking status, BMI, comorbidities, household income, and any supplement use within the year prior to diagnosis | 8 |
| Nagle 2019 [40] Australia |
AOCS Retrospective cohort Ovarian cancer 857 5.9 ± 3.9 years after diagnosis |
59.4 100 % white 100 % female 55.7 % BMI ≥ 25 16.2 % |
FFQ (139 items) At study enrollment |
DII 2014 31 Both (Data for excluding supplements were not shown) Q4 vs. Q1, Continuous |
All-cause mortality (592) Medical records and NDI Cancer mortality (541) Medical records and NDI |
All-cause mortality Q4 vs. Q1: 1.04 (0.81, 1.33) Per z score: 0.99 (0.91, 1.08) Cancer mortality Q4 vs. Q1: 1.03 (0.79, 1.33) Per z score: 0.98 (0.90, 1.08) |
Age, total energy intake, histological subtype, FIGO stage, tumor grade, residual disease, comorbidity, and smoking | 6 |
| Nagle 2020 [41] Australia |
ANECS Retrospective cohort Endometrial cancer 1251 Median 7.2 years after start of primary treatment |
Range 18–79 100 % White 100 % female 75.7% BMI ≥ 25 NR |
FFQ (139 items) At baseline |
E-DII 2014 31 Both (Data for including supplements were not shown) Q4 vs. Q1 |
All-cause mortality (160) Medical records and NDI Cancer mortality (110) Medical records and NDI |
All-cause mortality 0.88 (0.55, 1.41) Cancer mortality No association (Results not shown) |
Age, total energy, education, age at menarche, parity, diabetes status, oral contraceptive use, menopausal status, menopausal hormone therapy, and supplement use | 7 |
| Pre- & Post-diagnosis diet | ||||||||
| Wesselink 2021 [43] Netherlands |
COLON Prospective cohort Colorectal cancer 1334 for mortality 1242 for recurrence Median 3.2 years for recurrence Median 4.8 years for all-cause mortality |
66.2 100 % White 36 % female 26.0 11 % |
FFQ (204 items) At diagnosis and at 6 months after diagnosis |
ADII 2014 28 NR T3 vs. T1 |
Recurrence (228) Dutch Cancer Registry All-cause mortality (279) Municipal Personal Record Database |
Recurrence Pre-diagnosis: 0.98 (0.94, 1.04) Post-diagnosis: 0.96 (0.91, 1.02) All-cause mortality Pre-diagnosis: 1.03 (0.98, 1.07) Post-diagnosis: 1.00 (0.95, 1.05) |
Age, sex, and stage of disease | 8 |
| Post-diagnosis diet | ||||||||
| Jang 2018 [44] Korea |
Korean clinic Prospective cohort Breast cancer 511 213 months |
51.9 ± 10.7 100 % Korean 100 % female 24.7 % BMI ≥ 25 NR |
24-hour recall 5.4 months after breast cancer surgery |
DII 2014 34 NR T3 vs. T1 |
Cancer recurrence (88) Medical records All-cause mortality (44) Medical records and histopathology reports |
Cancer recurrence 2.35 (1.17, 4.71) All-cause mortality 3.05 (1.08, 8.83) |
Age, BMI, postmenopausal status, subtype, histologic grade, tumor size, lymph node metastasis, AJCC stage, treatment, and energy intake | 9 |
| Zheng 2018 [33] USA |
WHI Prospective cohort Breast cancer 2150 Median 13.3 years after diagnosis |
66.2 (range: 50–79) 86 % White 100 % female Mean 27.0–29.6 5.2 % |
FFQ (120 items) Average 1.5 years after diagnosis |
E-DII 2014 32 Including supplements Q1 vs. Q4 |
All-cause mortality (580) Annual clinic visits and mailings; If NDI was not available, death certificates were used Cancer mortality (212) Autopsy and medical records; If NDI was not available, death certificates were used |
All-cause mortality 0.82 (0.63, 1.05) Cancer mortality 0.96 (0.62, 1.49) |
WHI component, smoking status at baseline, income levels, cancer stage, education, years from cancer diagnosis to FFQ, baseline physical activity in MET-h/week, total energy intake per day, BMI at baseline, hormone replacement use status at baseline, and the covariate of time-dependent status before and after post-diagnosis FFQ | 9 |
| Ratjen 2019 [45] Germany |
Biobank PopGen Prospective cohort Colorectal cancer 1404 Median 7 years after dietary assessment |
Median 69 100 % White 44 % female Median 26.2 9 % |
FFQ (112 items) Median of 6 years after diagnosis |
E-DII 2014 27 NR Q4 vs. Q1, Continuous |
All-cause mortality (204) Population registries |
All-cause mortality Q4 vs Q1: 1.36 (0.88, 2.09) Per 1-unit: 1.08 (0.97, 1.20) |
Sex, age at diet assessment, BMI, physical activity, survival time from colorectal cancer diagnosis until diet assessment, tumor location, occurrence of metastases, occurrence of other cancer, type of therapy, current stoma, smoking status, alcohol intake, (time X age), (time X BMI), and (time X metastases) | 8 |
| Zheng 2020 [32] USA |
WHI Prospective cohort Colorectal cancer 463 Median 11.6 years after diagnosis |
67.8 83 % White 100 % female Mean 27.4–30.0 7.3 % |
FFQ (120 items) Average 1.7 years after diagnosis |
E-DII 2014 31 Both T1 vs. T3 |
All-cause mortality (162) Annual clinic visits and mailings; If NDI was not available, death certificates were used Cancer mortality (77) Autopsy and medical records; If NDI was not available, death certificates were used |
All-cause mortality Including supplements: 0.49 (0.31, 0.79) Excluding supplements: 0.72 (0.46, 1.12) Cancer mortality Including supplements: 0.58 (0.28, 1.22) Excluding supplements: 0.75 (0.36, 1.57) |
Age group at baseline, race/ethnicity, smoking status at baseline, income levels, cancer stage, education, years from cancer diagnosis to FFQ, baseline physical activity in MET-h/week, total energy intake per day, BMI at baseline, cancer differentiation grading, and the covariate of time-dependent status before and after postdiagnosis FFQ | 9 |
| Wang 2020 [38] USA |
PLCO Prospective cohort Breast cancer 1064 Median of 14.6 (10.5, 16.8) years after diagnosis |
65.3 91.2 % white 100 % female 55.6 % BMI ≥ 26 39.3 % |
FFQ (124 items) Median 3 years after randomization |
E-DII 2014 37 Including supplements T3 vs. T1, Continuous |
All-cause mortality (296) Annual contact, medical records, and NDI Cancer Mortality (100) Autopsy and medical records |
All-cause mortality T3 vs. T1: 1.34 (1.01, 1.81) Per z score: 1.06 (1.00, 1.13) Cancer mortality T3 vs. T1: 1.47 (0.89, 2.43) Per z score: 1.10 (1.00, 1.22) |
Total energy intake, BMI, trial arm, race, marital status, income, educational level, smoking status, hormone replacement therapy, history of diabetes, physical activity, stage, estrogen receptor status, and progesterone receptor status | 9 |
AACES: African American Cancer Epidemiology Study, ADII: Adapted Dietary Inflammatory Index, AJCC: American Joint Committee on Cancer, ANECS: Australian National Endometrial Cancer Study Group, AOCS: Australian Ovarian Cancer Study, BMI: Body Mass Index, CI: confidence interval, COLON: Colorectal cancer: Longitudinal, Observational study, DII: Dietary Inflammatory Index; E-DII: Energy-adjusted Dietary Inflammatory Index, ER: estrogen receptor, FIGO: International Federation of Obstetricians and Gynecologists, FFQ: food frequency questionnaire, HR: hazard ratio, NR: not reported, PACE: Pacing, graded Activity, and Cognitive behavior therapy; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, PR: progesterone receptor, UCDA: Ursodeoxycholic Acid Phase III clinical trials, WHI: Women's Health Initiative, WBF: Wheat Bran Fiber Clinical Trial.
Dietary inflammatory index
Dietary intakes were assessed using food frequency questionnaires (FFQs), with the exception of one study [44], which used a 24 h dietary recall method. All studies compared DII using a dichotomous comparison (e.g., high vs. low, tertile 3 [T3] vs. tertile 1 [T1], and quartile 4 [Q4] vs. quartile 1 [Q1]) while five studies [37,38,40,42,45] additionally compared continuous DII scores. Seven studies [28,36,37,[39], [40], [41], [42]] evaluated pre-diagnosis DII, five studies [32,33,38,44,45] examined post-diagnosis DII, and only one study [43] assessed both pre-and post-diagnosis DIIs. Different versions of the DII were utilized among the studies, including the original 2009 version of the DII [42], and the updated 2014 version [8,28,36,[39], [40], [41],44], including the energy-adjusted DII (E-DII) [32,33,37,38,45] and the Adapted DII (ADII) [43]. While E-DII adjusts for energy intake and uses intakes of food parameters per 1000 kcal of energy amount, ADII is similar to E-DII but limits to 29 dietary parameters [46]. The number of food parameters included in DII varied from 23 in Galas and colleague's cohort study [42] in Poland to 37 in Wang and colleague's Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial [38] in the US.
Main outcomes
Three studies examined cancer recurrence [36,43,44], and 12 studies examined all-cause mortality [28,32,33,[37], [38], [39], [40], [41], [42], [43], [44], [45]], while 7 studies examined cancer-specific mortality [28,32,33,[38], [39], [40], [41]]. Among the population of 3632 cancer survivors where cancer recurrence data were available, there were 1096 cancer recurrences. All-cause mortality data were collected for 13,193 individuals with cancer, and 3596 deaths from any causes were reported. Cancer-specific deaths occurred in 1514 out of 8518 cancer survivors. Cancer recurrence was ascertained through colonoscopy examination [36], medical records [44], or cancer registry [43]. Deaths were confirmed through annual contacts [32,33,37,38], medical records [32,33,38,40,41,44], population-based cancer registry [28,37,39,43,45], or linking to either the National Death Index [32,33,38,40,41] or government vital status data [42,43]. Cause of death was determined by referencing National death index [32,33,40,41], cancer registry [28,39], or medical records [32,33,38,40,41].
Quality of included studies
The result of the quality assessment of selected studies is reported in Table 1. All but one study [40] had a quality score of ≥7 stars, indicating “good quality.” Detailed scoring was displayed in Supplementary Table S4.
Effects on cancer recurrence
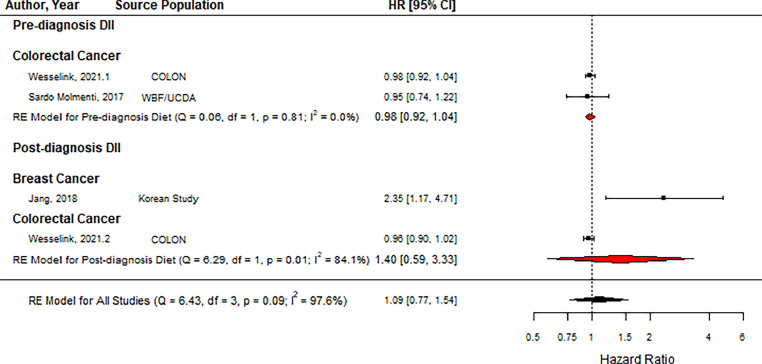
As shown in Fig. 2, DII was not associated with an increased risk of cancer recurrence (HR = 1.09, 95 % CI = 0.77, 1.54, n = 4). Subgroup analysis also showed that neither pre-diagnosis DII [36,43,44] nor post-diagnosis DII was associated with cancer recurrence (Pre-diagnosis: HR = 0.98, 95 % CI = 0.92, 1.04, n = 2; Post-diagnosis: HR = 1.40, 95 % CI = 0.59, 3.33, n = 2).
Fig. 2.
Forest plot showing pooled hazard ratios using high vs. low DII score comparison for recurrence. Black squares and horizontal lines represent a hazard ratio (HR) and a 95 % confidence interval (CI) for each study. The dotted vertical line indicates the line of no effect. The red diamond represents the pooled HR with its 95 % CI for pre-and post-diagnosis DIIs. The black diamond represents the pooled HR with its 95 % CI. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Abbreviations: COLON: Colorectal cancer, Observational, Longitudinal study, WBF: Wheat Bran Fiber Clinical Trial, UCDA: Ursodeoxycholic Acid Phase III trials, DII: dietary inflammatory index.
Effects on all-cause mortality
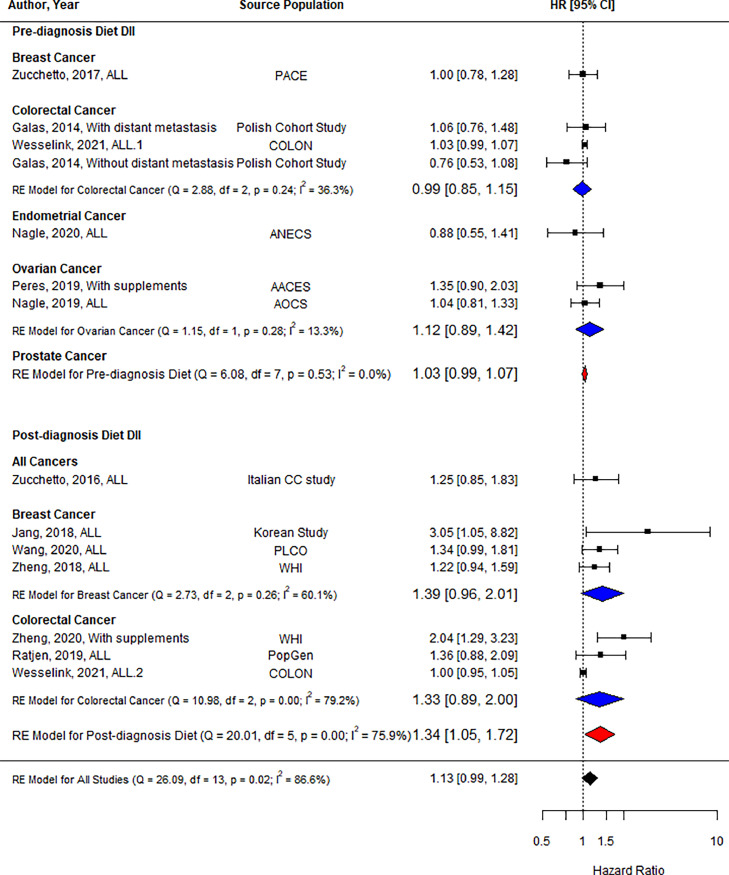
Twelve studies reported all-cause mortality as an outcome [28,32,33,[37], [38], [39], [40], [41], [42], [43], [44], [45]], with one study [42] reporting results separately for individuals with and without distant metastasis and another study [43] reporting results for both pre-and post-diagnosis DII. Analysis of the high/low comparisons in Fig. 3 showed no association between DII and all-cause mortality (HR = 1.13, 95 % CI = 0.99, 1.28, n = 14). When including only studies using pre-diagnosis DII, there was no association with all-cause mortality (HR = 1.03, 95 % CI = 0.99, 1.07, n = 8), while post-diagnosis DII demonstrated an association between the highest DII category and an increased risk of all-cause mortality by 34 % (HR = 1.34, 95 % CI = 1.05, 1.72, n = 6). Subgroup analysis indicated that the association was not modified by cancer type.
Fig. 3.
Forest plot showing pooled hazard ratios using high vs. low DII score comparison for all-cause mortality. Black squares and horizontal lines represent a hazard ratio (HR) and a 95 % confidence interval (CI) for each study. The dotted vertical line indicates the line of no effect. The blue diamond represents the pooled HR with its 95 % CI for each cancer if there is more than one study. The red diamond represents the pooled HR with its 95 % CI for pre-and post-diagnosis DIIs. The black diamond represents the pooled HR with its 95 % CI. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Abbreviations: AACES: African American Cancer Epidemiology Study, AOCS: Australian Ovarian Cancer Study, CC: case-control study, COLON: Colorectal cancer, Observational, Longitudinal study, ANECS: Australian National Endometrial Cancer Study, DII: dietary inflammatory index, PLCO: Prostate, Lung, Colorectal and Ovarian cancer screening trial, WHI: Women's Health Initiative.
As shown in Supplementary Fig. 1, analysis of continuous comparisons showed no significant increase for all-cause mortality with 1-unit increase in DII score e (HR = 1.03, 95 % CI = 1.00, 1.06, n = 6). However, 1-unit increase in post-diagnosis DII score was associated with a 7 % increased risk of all-cause mortality (HR = 1.07, 95 % CI = 1.01, 1.13, n = 2).
Effects on cancer-specific mortality
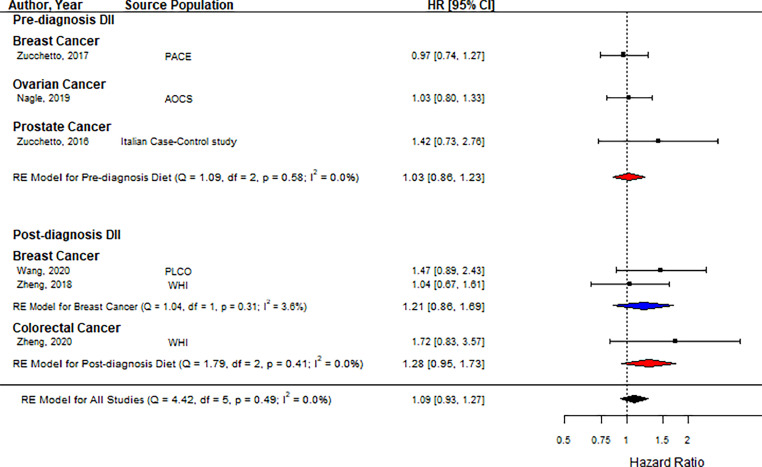
There were six studies reporting cancer-specific mortality as an outcome [28,32,33,[38], [39], [40]]. Analysis of the high/low comparison found no association between DII score and cancer mortality as shown in Fig. 4 (HR = 1.09, 95 % CI = 0.93, 1.27, n = 6). Stratified analysis by the timing of dietary assessment showed that both pre-diagnosis DII (HR = 1.03, 95 % CI = 0.86, 1.23, n = 3) and post-diagnosis DII (HR= 1.28, 95 % CI = 0.95, 1.73, n = 3) were not associated with an increased risk of cancer-specific mortality. Subgroup analysis by cancer type indicated that the association was not modified by cancer type.
Fig. 4.
Forest plot showing pooled hazard ratios using high vs. low DII score comparison for cancer-specific mortality. Black squares and horizontal lines represent a hazard ratio (HR) and a 95 % confidence interval (CI) for each study. The dotted vertical line indicates the line of no effect. The blue diamond represents the pooled HR with its 95 % CI for each cancer if there is more than one study. The red diamond represents the pooled HR with its 95 % CI for pre-and post-diagnosis DIIs. The black diamond represents the pooled HR with its 95 % CI. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Abbreviations: AOCS: Australian Ovarian Cancer Study, DII: dietary inflammatory index, PLCO: Prostate, Lung, Colorectal and Ovarian cancer screening trial, WHI: Women's Health Initiative.
There was no association between continuous values of DII and cancer-specific mortality, as shown in Supplementary Fig. 1 (HR = 1.04, 95 % CI = 0.93, 1.16, n = 2), including pre-and post-diagnosis DII together.
Sensitivity analysis and publication bias
Sensitivity analyses for all-cause mortality with pre-and post-diagnosis DII showed that the results were robust (see Supplementary Figs. 2 & 3). Publication bias was accessed for all-cause mortality as the number of studies included in the meta-analysis was greater than 10. The funnel plot (see Supplementary Fig. 4) indicates noteworthy evidence of publication bias with the p-value of 0.034 from Egger's test. When the publication bias was further examined for pre-diagnosis DII and post-diagnosis DII separately, funnel plots show non-symmetry for post-diagnosis DII only (p < 0.0001) and symmetry for pre-diagnosis DII (p = 0.997).
Discussion
This meta-analysis identified and analyzed 13 studies that examined the relationship between DII score and cancer outcomes among 14,920 cancer survivors. The results from the meta-analysis demonstrate that individuals in the highest post-diagnosis DII category, indicating a pro-inflammatory diet, had a 34 % increased risk of all-cause mortality but no increased risk for recurrence and cancer-specific mortality. No associations were observed from pre-diagnosis DII comparisons. These findings suggest that consuming a more anti-inflammatory diet particularly after cancer diagnosis can lower the risk of all-cause mortality among cancer survivors.
The DII consists of a maximum of 45 food parameters with a dietary inflammatory weight; A negative weight denotes anti-inflammatory qualities while a positive weight indicates pro-inflammatory qualities. In order to promote more anti-inflammatory diets after cancer diagnosis, healthcare providers should encourage cancer survivors to consume more of the food parameters with a negative inflammatory weight as well as to avoid or to decrease intake of foods with a positive inflammatory weight. Examples of some parameters with a negative weight would be β-carotene, fiber, garlic, ginger, magnesium, n-3 fatty acids, turmeric, vitamin C, vitamin D, green/black tea, and flavones [8]. Examples of parameters that have a positive inflammatory weight include vitamin B12, cholesterol, iron, saturated fat, and trans-fat [8]. This could translate to adding more fruits, vegetables, nuts, whole grains, and fish to the diet, while cutting down on red meat, processed food, and fast food. These adjustments to diet after cancer diagnosis could consequently increase diet quality, which have been shown to decrease the risk of all-cause mortality among cancer survivors [4,47,48]. The reason for this may be because a pro-inflammatory diet, indicated by a high DII score, promotes pro-cancer inflammatory environments and suppression of the innate and adaptive immune system, which prevents effective anti-tumor responses and facilitates the growth of a tumor that has been formed [6]. Also, inflammation has been shown to mediate other chronic diseases such as cardiovascular disease, chronic obstructive pulmonary disease, and diabetes [49], the leading causes of death in the US. Therefore, anti-inflammatory diet can lower the risk for these diseases, reducing the risk for all-cause mortality.
Our finding that post-diagnosis diet has a more significant impact on cancer outcomes than pre-diagnosis diet is consistent with previous findings in breast cancer survivors, reporting post-diagnosis diet quality rather than pre-diagnosis diet quality being associated with better survival [48]. Thus, it may be advantageous to promote anti-inflammatory diets to cancer survivors after diagnosis throughout cancer survivorship for better prognosis. An Italian cross-sectional study [50] conducted on breast cancer survivors found that after diagnosis, patients were more receptive to adopting beneficial lifestyle changes to improve their health, emphasizing the importance of the patient to physician relationship to develop tailored nutrition counselling and intervention programs [50]. As collaborative communication improves the patient-physician relationship [51] as well as patient's adherence to the intervention [52] and their treatment outcomes [53], more training of health providers in communication skills is warranted.
There are numerous other factors that may influence dietary intake, including age, sex, education, income, and sociocultural factors [54], [55], [56]. Socioeconomic status (SES), in particular, has been identified as a significant obstacle to a healthy diet. A cross-sectional analysis of US adult cancer survivors showed that quality of diet was poorer for individuals of lower SES compared to those of higher SES [57]. However, nutrition knowledge and beliefs can act as an effect modifier [58], indicating the importance of nutrition education in some groups of cancer survivors. The reasons for poorer quality of diet stem from a lack of access to grocery stores offering wide selections of healthy foods, food insecurity due to lower income, and a lack of education on healthy eating [59]. The culmination of all these factors may drive people of lower SES to consume more fast food or processed food, which may have higher inflammatory potential. Socioeconomic investment and education initiatives among cancer survivors, similar to ones in the general population such as the US Department of Agriculture's Supplemental Nutrition Assistance Program - Education [60] and the Expanded Food and Nutrition Education Program [61], could be an avenue to improve diet and health in cancer survivors at risk for food insecurity.
Post-diagnosis DII scores were not found to be significantly associated with cancer-specific mortality. This finding conflicts with results from a prior meta-analysis [27], which found a significantly increased cancer mortality with higher DII score. However, it is important to note that the other meta-analysis [27] included only two studies (one from prostate survivors and one from a large cancer-free, population-based cohort), and that their population was not exclusive to cancer survivors. Therefore, we conducted this meta-analysis by including six studies on cancer-specific mortality among cancer survivors. However, the result suggests that an updated meta-analysis is warranted as more studies among cancer survivors are published in the future.
Variations in the results of individual studies may be attributed to differences in the characteristics of included studies. The population for each cohort was drawn from different countries, which have different dietary norms that could have some effects on DII score. Additionally, the study included various types of cancers, although some cancers may be impacted more by dietary factors than others. All but one study [44] used FFQs to assess dietary intake. While the FFQ is commonly used in epidemiologic studies due to its convenience, it is prone to recall bias as it requires individuals to recall their usual frequency of consumption of different foods in the past [62]. Different numbers of food items were assessed by the FFQ ranging from 78 to 204, leading to a different number of DII parameters in each study ranging from 23 to 37, which could also lead to greater variation in the study results. Only one study used a 24 h recall to assess dietary intake, which is typically a detailed and accurate representation of short-term dietary intake but may not be representative of habitual intake [62]. The timepoints for dietary assessment differed, with some targeting for pre-diagnosis diets and the others targeting for post-diagnosis diets. Other factors, such as cancer treatment or tobacco usage, may have also had different impacts on the results, but not all studies collected these variables and included in the multivariable analysis. Different versions of the DII were utilized among the studies, which may also account for some variations in the results as well.
In addition to heterogeneity among studies mentioned above, this meta-analysis has several limitations that are important to note. The first limitation is that the number of studies included in the meta-analysis is small for certain outcomes such as cancer-specific mortality and recurrence and certain cancer types such as prostate, ovarian, or endometrial cancer, so meta-analysis was either less precise or not feasible. To better estimate the uncertainty of between-study heterogeneity in a small meta-analysis [31], we used the Bayesian random effects model. The small number of studies included in a meta-analysis also prevented assessing the precise publication bias, which may exist due to the fact that studies with negative results or failure to reject the null hypothesis are less likely to be published [63]. Second, although multivariable-adjusted results were utilized for the meta-analysis, it is still impossible to rule out all potential confounding effects, as the covariates included in the analysis varied from study to study. For example, obesity status, the significant risk factor for inflammation, was not included in all studies; only ten studies included obesity with a different variable. Seven studies [32,33,[37], [38], [39],44,45] included BMI, each one used overweight/obese status [42], abdominal obesity [28], or waist circumference [36], respectively. Any residual confounding by obesity could influence the outcome of the current study. In addition, there are many variables aside from dietary inflammatory potential that may affect outcomes among cancer survivors. Third, the majority of the included studies were from the US or Europe, so the cohorts were predominantly white populations. This raises concerns about the generalizability of the study findings to different racial/ethnic populations with diverse cultures, lifestyles, and dietary habits. Fourth, the analysis took data from observational cohort studies, so the strength of the evidence is not as strong as if randomized control trials were included. However, it should be noted that the methodological qualities of included studies were mostly high.
Limitations aside, this meta-analysis also has several strengths worth noting. To the best of our knowledge, this is the largest meta-analysis conducted among populations of cancer survivors. The analysis consists of 13 studies, each with a distinct study population, with high methodological quality scores when assessed by the Newcastle Ottawa Scale for cohort studies. This suggests that the chance of bias or errors in the studies were minimized throughout the study design and analysis phases. Lastly, all studies, with the exception of one, used the DII developed by the same research group, which establishes consistency in assessing DII throughout the studies.
Conclusions
The findings from the current analysis indicate that an anti-inflammatory diet following cancer diagnosis, can decrease the risk of all-cause mortality. This result supports the need for initiatives to educate and inform individuals with cancer about the potential risks of dietary inflammatory potential and encourage them to make healthier food choices. The recommendation of decreasing the consumption of pro-inflammatory food components, while increasing the consumption of anti-inflammatory food components, should be included with other lifestyle recommendations, such as smoking cessation and participation in physical activity. These findings also warrant further research on cancer recurrence and cancer-specific mortality among cancer survivors in order to clarify the relationship between dietary inflammation and those outcomes. As more studies are published, future meta-analyses will have greater statistical power and will be able to give a more precise estimate of DII impacts on cancer outcomes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Declaration of generative AI in scientific writing
We did not use AI or AI-assisted technology in the writing process of this manuscript.
Data availability
The data generated in this study for the meta-analyses are available upon request from the corresponding author. The data generated in this study, apart from the data used for the meta-analyses, are available within the article.
CRediT authorship contribution statement
Eric Han: Conceptualization, Validation, Investigation, Data curation, Writing – original draft. Eunkyung Lee: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. Brian Sukhu: Validation, Data curation, Writing – review & editing. Jeanette Garcia: Writing – review & editing. Humberto López Castillo: Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgements
None.
Footnotes
Funding: None; Registration: PROSPERO; registration number: 42022350719.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101798.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Reedy J., Krebs-Smith S.M., Miller P.E., et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014;144(6):881–889. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd N.F., Stone J., Vogt K.N., Connelly B.S., Martin L.J., Minkin S. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br. J. Cancer. 2003;89(9):1672–1685. doi: 10.1038/sj.bjc.6601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morze J., Danielewicz A., Hoffmann G., Schwingshackl L. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: a second update of a systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2020;120(12):1998–2031. doi: 10.1016/j.jand.2020.08.076. e1915. [DOI] [PubMed] [Google Scholar]
- 5.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baniyash M., Sade-Feldman M., Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol. Immunother. 2014;63(1):11–20. doi: 10.1007/s00262-013-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galland L. Diet and inflammation. Nutr. Clin. Pract. 2010;25(6):634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 8.Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hebert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavicchia P.P., Steck S.E., Hurley T.G., et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity c-reactive protein. J. Nutr. 2009;139(12):2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hébert J.R., Shivappa N., Wirth M.D., Hussey J.R., Hurley T.G. Perspective: the dietary inflammatory index (dii)—lessons learned, improvements made, and future directions. Adv. Nutr. 2019;10(2):185–195. doi: 10.1093/advances/nmy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Liu C., Zhou C., et al. Meta-analysis of the association between the dietary inflammatory index (dii) and breast cancer risk. Eur. J. Clin. Nutr. 2019;73(4):509–517. doi: 10.1038/s41430-018-0196-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Gao Y., Wei N., Du K., Jia Q. Strong association between the dietary inflammatory index(dii) and breast cancer: a systematic review and meta-analysis. Aging (Albany NY) 2021;13(9):13039–13047. doi: 10.18632/aging.202985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayati Z., Jafarabadi M.A., Pirouzpanah S. Dietary inflammatory index and breast cancer risk: an updated meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022;76(8):1073–1087. doi: 10.1038/s41430-021-01039-5. [DOI] [PubMed] [Google Scholar]
- 14.Shivappa N., Godos J., Hébert J.R., et al. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients. 2017;9(9) doi: 10.3390/nu9091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C., Wang W., Zhang D. Association between dietary inflammation index and the risk of colorectal cancer: a meta-analysis. Nutr. Cancer. 2018;70(1):14–22. doi: 10.1080/01635581.2017.1374418. [DOI] [PubMed] [Google Scholar]
- 16.Syed Soffian S.S., Mohammed Nawi A., Hod R., et al. Meta-analysis of the association between dietary inflammatory index (dii) and colorectal cancer. Nutrients. 2022;14(8) doi: 10.3390/nu14081555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q.J., Ou L., Li K., Ou F.R. Meta-analysis of the relationship between dietary inflammatory index and esophageal cancer risk. Medicine (Baltimore). 2020;99(49):e23539. doi: 10.1097/md.0000000000023539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y., Jiao H., Qu L., Liu H. Positive association between dietary inflammatory index and gastric cancer risk: a systematic review and meta-analysis. Nutr. Cancer. 2020;72(8):1290–1296. doi: 10.1080/01635581.2019.1679197. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z.Y., Gao X.P., Zhu S., et al. Dietary inflammatory index and risk of gynecological cancers: a systematic review and meta-analysis of observational studies. J. Gynecol. Oncol. 2019;30(3):e23. doi: 10.3802/jgo.2019.30.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Ma J., Jin Y., Cheng S., Huang S., Wang Y. Dietary inflammatory index and ovarian cancer risk: a meta-analysis. Nutr. Cancer. 2022;74(3):796–805. doi: 10.1080/01635581.2021.1931366. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z., Hong Y., Cheng Y. Dietary inflammatory index and pancreatic cancer risk: a systematic review and dose-response meta-analysis. Public Health Nutr. 2021;24(18):6427–6435. doi: 10.1017/s1368980021001579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohseni R., Abbasi S., Mohseni F., Rahimi F., Alizadeh S. Association between dietary inflammatory index and the risk of prostate cancer: a meta-analysis. Nutr. Cancer. 2019;71(3):359–366. doi: 10.1080/01635581.2018.1516787. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y., Li Q., Xu X. Dietary inflammatory index and the risk of prostate cancer: a dose-response meta-analysis. Eur. J. Clin. Nutr. 2020;74(7):1001–1008. doi: 10.1038/s41430-019-0500-3. [DOI] [PubMed] [Google Scholar]
- 24.Lu D.L., Ren Z.J., Zhang Q., et al. Meta-analysis of the association between the inflammatory potential of diet and urologic cancer risk. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua R., Liang G., Yang F. Meta-analysis of the association between dietary inflammatory index (dii) and upper aerodigestive tract cancer risk. Medicine (Baltimore). 2020;99(17):e19879. doi: 10.1097/md.0000000000019879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J., Ling Y., Mi S., et al. Association between dietary inflammatory index and upper aerodigestive tract cancer risk: a systematic review and dose-response meta-analysis. Oral Oncol. 2020;103 doi: 10.1016/j.oraloncology.2020.104587. [DOI] [PubMed] [Google Scholar]
- 27.Fowler M.E., Akinyemiju T.F. Meta-analysis of the association between dietary inflammatory index (dii) and cancer outcomes. Int. J. Cancer. 2017;141(11):2215–2227. doi: 10.1002/ijc.30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zucchetto A., Gini A., Shivappa N., et al. Dietary inflammatory index and prostate cancer survival. Int. J. Cancer. 2016;139(11):2398–2404. doi: 10.1002/ijc.30208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veritas Health Innovation; Melbourne, Australia: 2022. Covidence Systematic Review Software. [Google Scholar]
- 30.Wells, G., Shea, B., O'Connell, D., et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses; 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 15, 2021.
- 31.Deeks J.J., Higgins J.P.T., Altman D.G., Higgins J.P.T., Thomas J., Chandler J., et al., editors. Cochrane Handbook For Systematic Reviews of Interventions Version 6.3 (updated february 2022) 2022. Chapter 10: analysing data and undertaking meta-analyses. Cochrane. [Google Scholar]
- 32.Zheng J., Tabung F.K., Zhang J., et al. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the women's health initiative. Eur. J. Nutr. 2020;59(3):965–977. doi: 10.1007/s00394-019-01956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J., Tabung F.K., Zhang J., et al. Association between post-cancer diagnosis dietary inflammatory potential and mortality among invasive breast cancer survivors in the women's health initiative. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. cosponsored by the Am. Soc. Prevent. Oncol. 2018;27(4):454–463. doi: 10.1158/1055-9965.EPI-17-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalton J.E., Bolen S.D., Mascha E.J. Publication bias: the elephant in the review. Anesth. Analg. 2016;123(4):812–813. doi: 10.1213/ane.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viechtbauer W. Conducting meta-analyses in r with the metafor package. J. Stat. Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 36.Sardo Molmenti C.L., Steck S.E., Thomson C.A., et al. Dietary inflammatory index and risk of colorectal adenoma recurrence: a pooled analysis. Nutr. Cancer. 2017;69(2):238–247. doi: 10.1080/01635581.2017.1263752. [DOI] [PubMed] [Google Scholar]
- 37.Peres L.C., Hebert J.R., Qin B., et al. Prediagnostic proinflammatory dietary potential is associated with all-cause mortality among african-american women with high-grade serous ovarian carcinoma. J. Nutr. 2019;149(9):1606–1616. doi: 10.1093/jn/nxz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K., Sun J.Z., Wu Q.X., et al. Long-term anti-inflammatory diet in relation to improved breast cancer prognosis: a prospective cohort study. NPJ Breast Cancer. 2020;6:36. doi: 10.1038/s41523-020-00179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zucchetto A., Serraino D., Shivappa N., et al. Dietary inflammatory index before diagnosis and survival in an italian cohort of women with breast cancer. Br. J. Nutr. 2017;117(10):1456–1462. doi: 10.1017/s0007114517001258. [DOI] [PubMed] [Google Scholar]
- 40.Nagle C.M., Ibiebele T., Shivappa N., et al. The association between the inflammatory potential of diet and risk of developing, and survival following, a diagnosis of ovarian cancer. Eur. J. Nutr. 2019;58(4):1747–1756. doi: 10.1007/s00394-018-1779-x. [DOI] [PubMed] [Google Scholar]
- 41.Nagle C.M., Ibiebele T., Shivappa N., et al. Dietary inflammatory index, risk and survival among women with endometrial cancer. Cancer Causes Control: CCC. 2020;31(2):203–207. doi: 10.1007/s10552-019-01257-0. [DOI] [PubMed] [Google Scholar]
- 42.Galas A., Kulig J. Low-grade dietary-related inflammation and survival after colorectal cancer surgery. J. Cancer Res. Clin. Oncol. 2014;140(9):1517–1525. doi: 10.1007/s00432-014-1711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wesselink E., Staritsky L.E., van Zutphen M., et al. The association between the adapted dietary inflammatory index and colorectal cancer recurrence and all-cause mortality. Clin. Nutr. 2021;40(6):4436–4443. doi: 10.1016/j.clnu.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Jang H., Chung M.S., Kang S.S., Park Y. Association between the dietary inflammatory index and risk for cancer recurrence and mortality among patients with breast cancer. Nutrients. 2018;10(8) doi: 10.3390/nu10081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratjen I., Shivappa N., Schafmayer C., et al. Association between the dietary inflammatory index and all-cause mortality in colorectal cancer long-term survivors. Int. J. Cancer. 2019;144(6):1292–1301. doi: 10.1002/ijc.31919. [DOI] [PubMed] [Google Scholar]
- 46.van Woudenbergh G.J., Theofylaktopoulou D., Kuijsten A., et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the cohort study on diabetes and atherosclerosis maastricht (codam) and the hoorn study. Am. J. Clin. Nutr. 2013;98(6):1533–1542. doi: 10.3945/ajcn.112.056333. [DOI] [PubMed] [Google Scholar]
- 47.Park S.Y., Kang M., Shvetsov Y.B., et al. Diet quality and all-cause and cancer-specific mortality in cancer survivors and non-cancer individuals: the multiethnic cohort study. Eur. J. Nutr. 2022;61(2):925–933. doi: 10.1007/s00394-021-02700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee E., Kady V., Han E., Montan K., Normuminova M., Rovito M.J. Healthy eating and mortality among breast cancer survivors: a systematic review and meta-analysis of cohort studies. Int. J. Environ. Res. Public Health. 2022;19(13) doi: 10.3390/ijerph19137579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pahwa R., Goyal A., Jialal I. Statpearls. Treasure Island (FL); 2022. Chronic inflammation. [Google Scholar]
- 50.Caprara G., Tieri M., Fabi A., et al. Results of the echo (eating habits changes in oncologic patients) survey: an Italian cross-sectional multicentric study to explore dietary changes and dietary supplement use, in breast cancer survivors. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.705927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honavar S.G. Patient-physician relationship - communication is the key. Indian J. Ophthalmol. 2018;66(11):1527–1528. doi: 10.4103/ijo.IJO_1760_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zolnierek K.B., Dimatteo M.R. Physician communication and patient adherence to treatment: a meta-analysis. Med. Care. 2009;47(8):826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavakoly Sany S.B., Behzhad F., Ferns G., Peyman N. Communication skills training for physicians improves health literacy and medical outcomes among patients with hypertension: a randomized controlled trial. BMC Health Serv. Res. 2020;20(1):60. doi: 10.1186/s12913-020-4901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thiele S., Mensink G.B.M., Beitz R. Determinants of diet quality. Public Health Nutr. 2004;7(1):29–37. doi: 10.1079/Phn2003516. [DOI] [PubMed] [Google Scholar]
- 55.Deroover K., Bucher T., Vandelanotte C., de Vries H., Duncan M.J. Practical nutrition knowledge mediates the relationship between sociodemographic characteristics and diet quality in adults: a cross-sectional analysis. Am. J. Health Promot. 2020;34(1):59–62. doi: 10.1177/0890117119878074. [DOI] [PubMed] [Google Scholar]
- 56.Hiza H.A., Casavale K.O., Guenther P.M., Davis C.A. Diet quality of americans differs by age, sex, race/ethnicity, income, and education level. J. Acad. Nutr. Diet. 2013;113(2):297–306. doi: 10.1016/j.jand.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Lee E., Zhu J., Velazquez J., et al. Evaluation of diet quality among American adult cancer survivors: results from 2005 to 2016 national health and nutrition examination survey. J. Acad. Nutr. Diet. 2020 doi: 10.1016/j.jand.2020.08.086. [DOI] [PubMed] [Google Scholar]
- 58.Beydoun M.A., Wang Y. Do nutrition knowledge and beliefs modify the association of socio-economic factors and diet quality among us adults? Prev. Med. 2008;46(2):145–153. doi: 10.1016/j.ypmed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 59.Grimm K.A., Moore L.V., Scanlon K.S., Centers for Disease, C, Prevention Access to healthier food retailers - united states, 2011. MMWR. 2013;62(3):20–26. Suppl. [PubMed] [Google Scholar]
- 60.Rivera R.L., Maulding M.K., Eicher-Miller H.A. Effect of supplemental nutrition assistance program–education (snap-ed) on food security and dietary outcomes. Nutr. Rev. 2019;77(12):903–921. doi: 10.1093/nutrit/nuz013. [DOI] [PubMed] [Google Scholar]
- 61.Gills S.M.H., Auld G., Hess A., Guenther P.M., Baker S.S. Positive change in healthy eating scores among adults with low income after expanded food and nutrition education program participation. J. Nutr. Educ. Behav. 2021;53(6):503–510. doi: 10.1016/j.jneb.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Kipnis V., Midthune D., Freedman L., et al. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002;5(6A):915–923. doi: 10.1079/PHN2002383. [DOI] [PubMed] [Google Scholar]
- 63.Sharma H., Verma S. Is positive publication bias really a bias, or an intentionally created discrimination toward negative results? Saudi J. Anaesth. 2019;13(4):352–355. doi: 10.4103/sja.SJA_124_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study for the meta-analyses are available upon request from the corresponding author. The data generated in this study, apart from the data used for the meta-analyses, are available within the article.