Summary
Fish require abundant nutrients to generate a large number of eggs for spawning. Based on the evolutionary conservation of human FBN2 and its C-terminal placensin-like sequences in fish, we identified a peptide hormone gonacin (GONAdal Cell placensIN) and found its high expression in early-stage germ cells in the ovary and testis of zebrafish. We demonstrated that gonacin is essential for food intake, glucose release, and ovarian development in zebrafish. Similar expression patterns and functions of gonacin were also demonstrated in rainbow trout. Gonacin represents the first hormone secreted by germ cells with endocrine functions in vertebrates, bridging the energy homeostasis and reproduction.
Subject areas: Natural sciences, Biological sciences, Endocrinology, Female reproductive endocrinology, Male reproductive endocrinology
Graphical abstract

Highlights
-
•
Gonacin is cleaved from Fbn2 gene and mainly secreted by germ cells of gonads
-
•
Gonacin stimulates glucose secretion by the liver and stimulates the appetite
-
•
Gonacin stimulates oocyte maturation and vitellogenin expression in zebrafish
-
•
Gonacin is a gonadal hormone with both metabolic and gonadal functions in zebrafish
Natural sciences; Biological sciences; Endocrinology; Female reproductive endocrinology; Male reproductive endocrinology
Introduction
Most fish release thousands to millions of eggs, scattering them in the water where the male fish fertilize them. Thus, fish need to invest large energy resources for oocyte derivation. During the fish reproductive cycle, ovaries develop continuously to produce large numbers of female germ cells. For example, the gonadosomatic index of female rainbow trout increases sharply from 0.2% to 11% during 6 months of the breeding cycle.1 Energy resources come from feeding, and food is converted into energy through a variety of metabolic pathways such as glucose generation, which are utilized for diverse physiological activities, including gonadal development, to maintain the survival and reproduction of the individual. Hormonal regulation of the close connection between energy balance and reproduction has been the subject of considerable attention.2,3,4 Here, we identified a novel peptide hormone secreted from the gonads in fish, capable of bridging metabolic homeostasis and reproduction.
Recent studies demonstrated that the C-terminus of the human FBN1 gene encodes a glucogenic and orexigenic protein hormone asprosin5,6,7 and its deletion is responsible for the anorexic behavior and defective glucose secretion in patients with neonatal progeroid syndrome.6,8 In addition, human FBN2 encodes a placenta-secreted glucogenic hormone placensin9, capable of regulating metabolic and placental functions. Placensin represents a reproductive organ-secreted metabolic hormone. Serum placensin showed stage-dependent increases during human pregnancy with further elevations in patients with gestational diabetes mellitus.9 Zebrafish represent a valuable model to investigate vertebrate physiology.10,11 Based on the evolutionary conservation of the C-terminal region of FBN2, we identified another glucogenic and orexigenic hormone produced by gonadal germ cells of zebrafish and named it gonacin (GONAdal Cell placensIN). Gonacin represents the first gonadal germ cell endocrine hormone with metabolic and gonadal regulatory functions.
Results
Gonacin, the C-terminal cleavage product of profibrillin-2 in zebrafish is specifically expressed in gonadal germ cells
Like most mammals, three different Fbns have been identified in zebrafish in the GenBank database. Although two Fbn2-like sequences in the zebrafish genome were named as Fbn2a and Fbn2b, phylogenetic analysis revealed that zebrafish Fbn2a and Fbn2b are clustered into the subgroups Fbn2 and Fbn3, respectively (Figure 1A). Thus, we named Fbn2a as Fbn2 and Fbn2b as Fbn3 in zebrafish. Gene synteny analysis further confirmed that zebrafish Fbn2 and Fbn3 share a high degree of synteny with human Fbn2 and Fbn3, respectively (Figure 1B). Although a conserved furin cleavage site could be found at C-terminal sequences of all three human FBN genes (Figure S1) and zebrafish Fbn1 and 3, a furin cut site was not found in the same position of zebrafish Fbn2. To further investigate whether zebrafish Fbn2 has other furin cut sites, we performed RACE (Rapid Amplification of cDNA Ends) assay. A novel splicing form of fbn2 with an upstream consensus furin cleavage site at the C-terminal end was identified in zebrafish. A 319 aa (amino acids) asprosin/placensin-like peptide was predicted to be cut at the C-terminal region of Fbn2 (Figure 1C). These results indicated that zebrafish fbn2 lost the downstream furin cut site but evolved an upstream furin cleavage site. We further analyzed the sequence of fbn2 in other fish species. Most fishes have canonical furin cleavage sites as mammalian Fbn2 (Figure S2A). Although the placensin-like polypeptide derived from the zebrafish Fbn2 is longer than mammalian homologues, sequence comparison of the corresponding sequences of human and mouse placensin with zebrafish showed 46% and 45% sequence identity, respectively, suggesting the core sequence of zebrafish gonacin is still conserved as compared with mammalian counterparts. In addition, this polypeptide showed 38% sequence identity compared with human asprosin. Interestingly, like zebrafish, an upstream furin cleavage site was found in several cyprinid fish including common carp (Cyprinus carpio), a native cavefish (Onychostoma macrolepis), and tiger barb (Puntigrus tetrazona) (Figure S2B), suggesting that a longer form of gonacin could be derived using upstream furin cleavage sites in cyprinid fish.
Figure 1.
Phylogenic comparison of FBN genes and identification of gonacin in zebrafish gonads
(A) Phylogenic tracing of three FBN genes in diverse vertebrates showing three zebrafish paralogs (red dots).
(B) Syntenic regions of FBN2 and FBN3 genes in human and zebrafish chromosomes.
(C) Discovery of a consensus furin cleavage site upstream of placensin-like sequences in zebrafish Fbn2. Furin cleavage sites were marked by red boxes. The meaning of the number on the right side is the number of amino acids from the translation starting site.
(D) Expression of fbn2 transcripts was mainly detected in the gonad tissues of in adult zebrafish as detected by RT-PCR and electrophoresis (upper panel) as well as real-time qPCR (lower panel) in different tissues of adult zebrafish.
(E) Expression of gonacin protein was detected by Western blotting in zebrafish ovary and testis. Tissue extracts were subjected to immunoblotting using gonacin antibodies. M: marker protein.
(F) Expression of gonacin protein was detected by fluorescence immunostaining in the testis of adult zebrafish. Signals for DAPI (a), gonacin (b), phalloidin (c), and all three (d). (e) shows the signal of the box area indicated in (d). Signals for DAPI (f), GFP driven by the piwi promoter (g), gonacin (h), and all three (i). Signals for DPI (j), gonacin (k), Vasa (L), and all three (n). gonacin (green) and Vasa (red) are localized in germ cells. (g) The co-localization of gonacin (red) and GFP driven by the piwi promoter (green) in spermatogonia cells. DAPI (blue) was used as a nuclear counterstaining and phalloidin (red) was used as an F-actin counterstaining. Scale bar: 25 μm (a, b, c, and d), 10 μm (e, f, g, h, i, j, k, l, m, and n).
(G) Expression of gonacin protein was detected by fluorescence immunostaining in zebrafish ovary. Expression of gonacin in the ovary of zebrafish at 35 dpf (a, b, c, d, e, and f) and 90 dpf (g, h, i, j, k, and l). (b) and (h) show amplified areas in (a) and (g), respectively. Signals for gonacin (green, d and j) and Vasa (red, e and k) were in germ cells. DAPI (blue) was used as a nuclear counterstaining (c and i) and all three (f and l). Scale bar: 25 μm (a and g), 20 μm (c, d, e, f, i, j, k, and l), 10 μm (b and h).
We examined the fbn2 mRNA expression profile across zebrafish tissues using RT-PCR followed by electrophoresis and found that fbn2 mRNA is predominantly expressed in the ovary and testis (Figure 1D). Real-time qPCR analysis confirmed the highest fbn2 mRNA expression in ovary and testis across zebrafish tissues (Figure 1D). We further checked whether fbn2 mRNA is expressed in germ or somatic cells in gonads. In the ovary, real-time qPCR showed that fbn2 mRNA is specifically expressed in oocytes but not in follicular somatic cells (Figure S3A). Spatial expression of fbn2 mRNA in ovary and testis was further investigated using in situ hybridization and fbn2 transcripts were found in the ooplasm of early-stage oocytes and early-stage sperm cells (Figure S3B). The specific expression of fbn2 in early-stage oocytes was further supported by a recent study based on the single-cell RNA sequencing in zebrafish ovaries12 (Figure S3C).
To investigate whether a placensin-like peptide of zebrafish Fbn2 could be cleaved in zebrafish, furin enzyme expression was investigated by RT-PCR. Two Furin gene (furina and furinb) transcripts are ubiquitously expressed in gonadal and other tissues examined in zebrafish (Figure S4). We then generated polyclonal antibodies against the predicted placensin-like peptide at the C-terminal end of zebrafish Fbn2. The specificity of this antibody to C-end of Fbn2 was validated by using recombinant proteins predicted from zebrafish C-terminal sequence of Fbn1/2/3 (Figure S5). Two N-glycosylation sites were predicted in this peptide (Figure S6), with the predicted size of placensin-like peptide around 40-kDa (N-linked glycosylation sites estimated to be 2.5 kDa per site). Using this antibody, a band at the corresponding M.W. was detected by immunoblotting in the ovary and testis of adult zebrafish (Figure 1E). Several higher M.W. bands found in immunoblotting could represent dimers or nonspecific bands. Cell types expressing gonacin proteins in the ovary and testis were further detected by fluorescence immunostaining. Strong signals could be found in ovarian and testicular germ cells of zebrafish (Figures 1F and 1G). Localization of gonacin in germ cells was confirmed by Vasa (Ddx4, germ cell marker) antibodies13 and using piwi1 transgenic fish with GFP (Green Fluorescent Protein) marking early-stage germ cells14 (Figures 1F and 1G). In addition, the expression of gonacin during the early development of zebrafish was studied by immunostaining, and gonacin signals were found in primordial germ cells of 7, 8, and 9 dpf (days post fertilization) embryos (Figure S7). Due to its high expression in germ cells at different developmental stages, we named this peptide derived from zebrafish Fbn2 gene as gonacin.
Gonacin stimulates glucose secretion by the liver of zebrafish
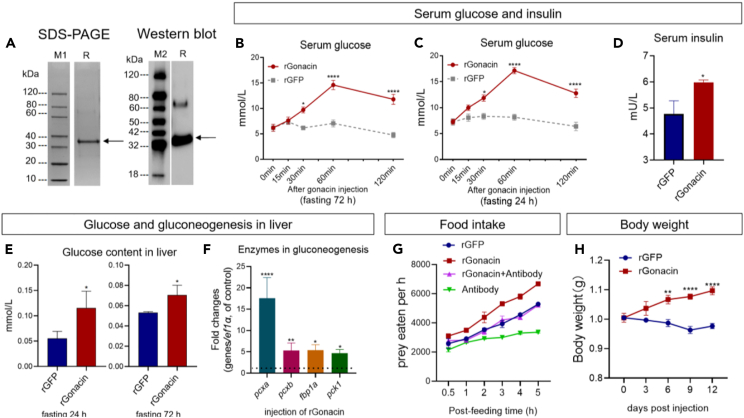
Both asprosin and placensin have glucogenic actions.5,6,7,9 To study the function of gonacin in zebrafish, we generated Escherichia coli-derived gonacin with His tags appended at its N-terminus. Recombinant gonacin was purified using a Nickel column, followed by electrophoresis and immunoblotting (Figure 2A, ∼35 kDa band). The molecular weight of bacterially generated gonacin was lower than that detected in zebrafish tissues; the difference is consistent with the glycosylation of native gonacin.
Figure 2.
Glucogenic and orexigenic activities of gonacin in zebrafish
(A) Characterization of recombinant zebrafish gonacin protein (rGonacin). Coomassie blue staining (left panel) and immunoblotting (right panel) of bacteria-derived recombinant gonacin. M1, M2: marker proteins, R: rGonacin.
(B and C) Serum glucose was measured at indicated times after a single 4 μg dose of intraperitoneal injection of rGonacin or recombinant GFP protein (rGFP, using as the control) into zebrafish that had been subjected to a 72 h fast (B) and 24 h fast (C) before injection. ∗, p < 0.05; ∗∗∗∗, p < 0.0001, compared with control (n = 8 in each group). two-way ANOVA with Bonferroni post-test was used to calculate the p value.
(D) Serum insulin levels were measured at 1 h after a single dose of intraperitoneal injection of rGonacin (4 μg) or rGFP in zebrafish (n = 3 in each group). ∗, p < 0.05; Unpaired Student’s t test was used to calculate the p value.
(E) Liver glucose content was measured by ELISA at 1 h after a single intraperitoneal injection of rGonacin (4 μg) or rGFP (control) into zebrafish that had been subjected to a 72 h fast (left panel) and 24 h fast (right panel) prior to injection (n = 6 in each group). ∗, p < 0.05, compared with control. Unpaired Student’s t test was used to calculate the p value.
(F) The expression of glucogenesis enzymes including fbp1a, pck1, pcxa, and pcxb in the liver after a single intraperitoneal injection of rGonacin (4 μg) or rGFP (control) in vivo. The columns represent fold changes of the relative mRNA levels in the treated group over its respective GFP injection controls (mean ± SEM) ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; n = 5. Unpaired Student’s t test was used to calculate the p value.
(G) Food intake was measured at indicated times after a single 4 μg dose of intraperitoneal injection of rGonacin (4 μg) or GFP (control), 4 μL anti-gonacin antibodies (Antibody) or 4 μg rGonacin together with 4 μL antibody (rGonacin+Antibody) in zebrafish that had been subjected to an 18 h fast before injection (n = 6 in each group).
(H) Body weight was measured at indicated times after 4 times of intraperitoneal injections of recombinant gonacin or GFP (control) at 3 days intervals in zebrafish. ∗∗, p < 0.01; ∗∗∗∗, p < 0.0001. two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
We studied whether gonacin has glucogenic actions in zebrafish. Acute responses to gonacin were investigated following a single intraperitoneal injection of recombinant zebrafish gonacin (rGonacin) in zebrafish that had been subjected to a 72 h or 24 h fasting with serum glucose levels measured at 0, 15, 30, 60, 120 min post-injection. Gonacin led to a time-dependent increase in serum glucose levels (Figures 2B and 2C). In contrast, serum glucose levels were not changed after the injection of recombinant GFP (rGFP) in the control group (Figures 2B and 2C). We also monitored serum insulin levels and found increases in serum insulin at 60 min after rGonacin injection (Figure 2D). To confirm the liver as the site of gonacin action, the glucose content in the liver was assessed after the injection of rGonacin and increased hepatic glucose content was found (Figure 2E). Levels of several key enzymes in gluconeogenesis including fructose-1,6-bisphosphatase 1a (fbp1a), phosphoenolpyruvate carboxykinase 1 (pck1), pyruvate carboxylase a (pcxa) and pyruvate carboxylase a (pcxb) were increased in the liver after rGonacin injection for 60 min as detected by real-time qPCR (Figure 2F). We further used immunostaining to confirm the increase of Pcxb protein expression in the liver after rGonacin injection for 15 min (Figure S8). Since rGonacin has His tags appended at its N-terminus, immunostaining was performed using His antibody after the IP injection of rGonacin in zebrafish. We found strong signals in the liver but not the heart after the injection of rGonacin at 15 min post-injection (Figure S9), suggesting that gonacin can be retained by the liver and likely acting on hepatic receptors in vivo.
Gonacin stimulates appetite in zebrafish
Asprosin has been demonstrated to promote food intake.5,7,15 To study whether gonacin has orexigenic actions in zebrafish, we administered a single dose of rGonacin or rGFP intraperitoneally to adult zebrafish. rGonacin-injected zebrafish displayed greater food intake than rGFP-injected zebrafish over the next 5 h (Figure 2G). Treatment with the gonacin-specific polyclonal antibodies completely neutralized gonacin-induced increase of food intake (Figure 2G). Using these antibodies alone, basal food intake could also be significantly decreased compared to the rGFP injection group (Figure 2G), suggesting the suppressive effect of the antibodies on the endogenous actions of gonacin. To understand chronic responses to rGonacin, zebrafish were IP injected with rGonacin or rGFP for 4 times at three-day intervals. There was a significant increase in body weight when the zebrafish were fed the normal chow (Figure 2H). These results demonstrated the orexigenic actions of gonacin in zebrafish.
Gonacin stimulates oocyte maturation and vitellogenin expression in zebrafish
The observed preferential expression of gonacin in gonads of zebrafish suggests gonacin likely regulates gonadal development. Ovarian follicles were incubated with rGonacin in vitro. Oocyte maturation as reflected by germinal vesicle breakdown (GVBD)16 could be strongly induced by treatment with rGonacin in a dose- and time-dependent manner, compared with positive controls using 17α, 20β-Dihydroxy-4-pregnen-3-one (DHP)16 (Figures 3A, 3B, and 3C). The morphology and histology of ovarian follicles after treatment with rGonacin and DHP were shown in Figure 3A. The action of gonacin on oocyte maturation could be completely blocked by gonacin-specific antibodies (Figure 3D). We have demonstrated that glucose can induce oocyte maturation through the pentose phosphate pathway (PPP) pathway in zebrafish.17 Gonacin-induced oocyte maturation could be totally blocked by diphenyleneiodonium (DPI), an inhibitor of the PPP pathway (Figure 3E). These results suggest that gonacin can induce oocyte maturation by regulating glucose metabolism. Since the liver is a target of gonacin action, we further monitored the expression of eight vitellogenin (vtg) genes in the liver and found increases of four vtgs including vtg1, vtg5, vtg6 and vtg7 at 15 min after rGonacin injection (Figure 3F). These results suggest that gonacin can stimulate vitellogenin expression in the liver of zebrafish.
Figure 3.
Promotion of oocyte maturation and vitellogenesis by gonacin in zebrafish
(A) The morphology (upper panel) and histology (lower panel) of ovarian follicles after treatment with rGonacin (100 μg/mL) for 4 h, using DHP (5 ng/ml) as a positive control. Scale bar: 200 μm.
(B) Increased rate of oocyte maturation following treatment with rGonacin at different doses for 16 h. DHP (5 ng/ml) treatment group was used as a positive control. ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001, one-way ANOVA followed by Fisher’s least significant difference test was used to calculate the p value.
(C) Effects of treatment with rGonacin (100 μg/mL) on the rate of oocyte maturation at indicated time points. ∗, p < 0.05; ∗∗∗∗, p < 0.0001, two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
(D) Effects of treatment with gonacin antibodies for 16 h on the rate of rGonacin-induced oocyte maturation. ∗∗∗∗, p < 0.0001, one-way ANOVA followed by Fisher’s least significant difference test was used to calculate the p value.
(E) Blockage of rGonacin-induced oocyte maturation by co-treatment with a PPP inhibitor (DPI) for 16 h. ∗∗∗∗, p < 0.0001, one-way ANOVA followed by Fisher’s least significant difference test was used to calculate the p value.
(F) The expression of vtg1-8 in liver after a single intraperitoneal injection of rGonacin (4 μg) or rGFP (control) in vivo. The columns represent fold changes of relative mRNA levels in the treated group over its respective GFP injection controls (mean ± SEM) ∗, p < 0.05; ∗∗, p < 0.01; n = 4. Unpaired Student’s t test was used to calculate the p value.
Larvae lethality phenotype of gonacin null zebrafish and its rescue by overexpressing gonacin
Using the CRISPR-Cas9-mediated gene knockout technique,18 we established a gonacin knockout zebrafish line by deleting one bp in exon 60 (Figures 4A and 4B). This deletion resulted in a frameshift and the ablation of the gonacin-coding region in the Fbn2 gene. We found all gonacin homozygous knockout zebrafish died between 6 and 18 dpf and no homozygous mutants could be found at 18 dpf, unlike the high survival rates in wild type (WT) and heterozygous groups (Figure 4C). To demonstrate the larvae lethality was due to the absence of gonacin, we constructed a gonacin-overexpression zebrafish model and found the larvae lethality phenotype of mutants could be rescued by overexpression of gonacin as assessed at 18 dpf (Figure 4C). We then analyzed the cause of larvae lethality in gonacin mutants. Since the zebrafish larvae initiate food intake at 5–6 dpf,19 it is possible that the larvae lethality is due to defects in food intake in gonacin mutant. We assessed food intake at 7 dpf and found that food intake was not affected in gonacin mutants (Figure 4D). However, we found some foods were retained in the intestine of gonacin mutant at 7 dpf, whereas no foods were found in intestine of heterozygous, WT and the mutant rescued by overexpression of gonacin at 5 h after feeding, suggesting that the digestive function, likely food transit, was affected in the absence of gonacin (Figure 4E). Consistently, we also found expression of gonacin in intestine of WT larvae but not in gonacin null larvae (Figure S7). Also, expression of two key gluconeogenic enzymes (pck1 and pcxa) was decreased in liver of gonacin mutants compared to the WT and rescue groups as detected by real-time qPCR (Figure 4F). Thus, the observed larvae lethality might be due to digestive and gluconeogenesis dysfunction in gonacin null zebrafish.
Figure 4.
Overexpression of gonacin rescued the larvae lethality phenotype of gonacin null zebrafish
(A) Location of the CRISPR/Cas9 binding sites on first exon encoding gonacin of zebrafish to generate gonacin null fish.
(B) Sequencing results from the wildtype (WT, gonacin+/+), heterozygous (gonacin+/−), and homozygous (gonacin−/−) zebrafish.
(C) Percentages of WT, heterozygous, and homozygous fish during early development from 4 to 18 dpf of zebrafish (n = 20 in each day) (Left panel). Percentages of WT, heterozygous and homozygous fish after overexpression of gonacin in gonacin null zebrafish at 18 dpf (n = 20 in each group) (Right panel).
(D) Food intake was assessed by feeding food with luminal tracer in the larvae of WT (+/+) and gonacin mutant fish. a) Individual exhibiting the signal of luminal tracer in the intestine of WT, gonacin heterozygous (+/−) and gonacin homozygous (−/−) fish (7 dpf) after 30 min post feeding. b) Quantitative analysis of luminal tracer signals in the intestine of WT, gonacin+/− and gonacin−/− at 7 dpf at 30 min post-feeding. Scale bar: 0.5 mm.
(E) Intestinal transit of food was assessed in zebrafish larvae of WT, gonacin mutants, and gonacin mutants rescued by overexpression of gonacin. a) Individuals exhibiting luminal tracer signals in the intestine of different groups. Rescue: gonacin homozygous rescued by overexpression of gonacin. All zebrafish larvae (7 dpf) were monitored at 5 h after feeding. b) Quantitative analysis of luminal tracer signals in the intestine of different groups. ∗∗, p < 0.01; ∗∗∗∗, p < 0.0001; n = 8. One-way ANOVA followed by Fisher’s least significant difference test was used to calculate the p value. Scale bar: 0.5 mm.
(F) The expression of glucogenesis enzymes (pck1 and pcxa) in the liver of WT, gonacin mutants, and gonacin mutants rescued by overexpression of gonacin. ∗, p < 0.05; ∗∗, p < 0.01; n = 5. Unpaired Student’s t test was used to calculate the p value. One-way ANOVA followed by Fisher’s least significant difference test was used to calculate the p value.
Conditional knockout of gonacin in germ cells led to defects in appetite control, glucose secretion, and ovarian development in zebrafish
Due to the larvae lethality phenotype in gonacin mutant zebrafish, we generated a conditional knockout (CKO) mutant fish with gonacin gene deletion in germ cells. A CRISPR/Cas9 vector system was employed for germ cell-specific gene disruption in zebrafish (Figures 5Aa and S10). The specific gene knockout in gonads was assessed by genomic sequencing, which showed that the mutation in gonacin sequence was only induced in gonads but not in other tissues examined (Figure S11). Western blot analysis further confirmed that gene expression level of gonacin was dramatically decreased in testis and ovary of CKO compared to the WT (Figure 5Ab).
Figure 5.
Conditional knockout of gonacin in germ cells led to defects in appetite, serum glucose level, and ovarian development in zebrafish
(A) Conditional knockout (CKO) of gonacin in germ cells of zebrafish. a) Construction of a plasmid for targeted knockout of gonacin in germ cells of zebrafish. b) The expression of gonacin was detected in the testis and ovary of wild type (WT) but negligible in CKO at 80 dpf, using β-actin as an internal control.
(B) Embryo survival of WT and CKO zebrafish.
(C) Assessment of the growth of zebrafish after CKO of gonacin. a) The morphology of WT and CKO zebrafish at 45 dpf. b) Body weight (left panel) and body length (right panel) of WT and CKO zebrafish at 45 dpf. ∗∗, p < 0.01; ∗∗∗, p < 0.001, compared with WT (n = 21). c) The morphology of WT and CKO zebrafish at 90 dpf. d) Body weight (left panel) and body length (right panel) of WT and CKO zebrafish at 90 dpf. ∗∗∗∗, p < 0.0001, compared with WT (n = 7). Scale bar: 0.5 cm.
(D) Assessment on the food intake of zebrafish after CKO of gonacin. The food intake was assessed in WT and CKO zebrafish at 40 dpf (panel a, n = 15 in each group) and 100 dpf (panel b, n = 15 in each group). ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001, two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
(E) Lower serum glucose level in CKO fish. Adult WT or CKO zebrafish (120 dpf) were subjected to 24 h fasting before serum glucose measurement. ∗, p < 0.05; n = 5. Unpaired Student’s t test was used to calculate the p value.
(F) Fertility and gonadal development after CKO of gonacin in zebrafish at 100 dpf. a) Number of embryos produced in WT and CKO female zebrafish after crossing with WT males. ∗∗∗∗, p < 0.0001 (n = 7 in each group). b) Morphology of ovaries dissected from WT and CKO female zebrafish (Left panel: dashed areas denote the ovary, scale bar: 1 cm. Right panel: ovaries isolated and dispersed, scale bar: 1.5 mm). c) Gonadosomatic Index (GSI) of WT and CKO female zebrafish. ∗∗∗, p < 0.001, compared with WT female zebrafish (n = 7 in each group). d) Histology of ovaries from WT and CKO female zebrafish. e) Percentage of different stage follicles in WT and CKO female zebrafish. ∗∗, p < 0.01; ∗∗∗∗, p < 0.0001, compared with WT female zebrafish. The data were derived from 3 individual zebrafish. PG, primary growth stage; PV, pre-vitellogenic stage; EV, early-vitellogenic stage; MV, mid-vitellogenic stage; FG, fully grown stage follicles. Scale bar: 100 μm.
We found the survival rate and embryonic development of gonacin CKO zebrafish was not different from that of WT as assessed at 9 dpf (Figure 5B), but the growth of gonacin CKO zebrafish was significantly retarded as compared to the WT. At 45 dpf and 90 dpf, the body weight and body length of gonacin CKO zebrafish were significantly lower than those in their age-matched WT zebrafish (Figure 5C). Furthermore, food intake was significantly decreased in gonacin CKO zebrafish compared with their age-matched WT counterparts at 40 dpf and 100 dpf, respectively (Figure 5D). We further monitored basal serum glucose levels in adult fish and found lower glucose levels in CKO fish as compared with WT animals after 24 h fasting (Figure 5E).
To investigate the effect of CKO on fertility, mating between gonacin CKO adult females and WT males did not produce any offspring at 100 dpf (Fig. 5Fa), indicating gonacin CKO adult females were infertile. To further analyze the causation of fertility defects observed in female gonacin CKO zebrafish, we examined morphology and histology of adult fish ovaries. The ovary size was significantly decreased in the CKO mutants at 100 dpf (Figure 5Fb). Also, a significant decrease in the gonadosomatic index (GSI) was found in the CKO mutant females (Figure 5 Dc). Histological analyses for the ovaries of CKO and WT fish at 100 dpf further showed that the proportion of primary growth (PG) stage and pre-vitellogenic (PV) stage was increased, but that for early-vitellogenic (EV) and mid-vitellogenic (MV) stage was decreased. Also, no fully grown (FG) stage follicles were found in CKO mutant ovaries (Figures 5Fd and 5Fe).
In males, we found mating of gonacin CKO adult male zebrafish with WT females produced the normal number of offspring, the survival rate of embryos was normal suggesting sperm quality is unaffected (Figure S12A). Testes from WT and CKO adults were morphologically similar, but the size of testis in CKO is smaller than the WT (Figure S12B). GSI was slightly decreased in CKO compared to WT without showing statistical significance (Figure S12C). Histological analysis from H&E staining revealed that all stages of spermatogenesis could be found in the testes of both WT and CKO (Figure S12D). These findings suggest that spermatogenesis is only partially affected.
Overexpression of gonacin in transgenic zebrafish enhances body growth, serum glucose, ovarian development, and food intake
To further analyze the role of gonacin in zebrafish, we generated four different gonacin transgenic zebrafish lines using the Tol2 system,20 one is for whole body overexpression (Tg(CMV:gonacin)), another for whole body inducible overexpression (Tg(hsp70:gonacin)) while two others are for germ cell-specific overexpression (Tg(piwil1:gonacin) and Tg(vasa:gonacin)).
In the Tg(CMV:gonacin) fish line (Figure 6A), the gonacin mRNA was increased in this transgenic zebrafish (Figure 6B). The food intake was increased in this transgenic fish at 50 dpf (Figure 6C). The growth of Tg(CMV:gonacin) zebrafish was enhanced as compared to the WT. At 50 dpf (Figures 6D and 6E), the body weight and body length of Tg(CMV:gonacin) were significantly higher than those in their age-matched WT zebrafish. These findings indicate that growth and food intake could be enhanced by gonacin in zebrafish.
Figure 6.
Overexpression of gonacin in the whole body could enhance the body growth, serum glucose, and food intake of zebrafish
(A) Overexpression of gonacin in whole body by the establishment of gonacin transgenic zebrafish driven by CMV promoter.
(B) The expression of gonacin was detected in the embryos of wild type (WT) and Tg(CMV:gonacin) at 8 dpf, using ef1a as an internal control. ∗∗∗, p < 0.001; Unpaired Student’s t test was used to calculate the p value.
(C) Assessment of the food intake in Tg(CMV:gonacin). The food intake was assessed in WT and Tg(CMV:gonacin) at 55 dpf (panel a, n = 15 in each group). ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
(D) The morphology of WT and Tg(CMV:gonacin) zebrafish at 50 dpf. Scale bar: 0.5 cm.
(E) Body weight (left panel) and body length (right panel) of WT and Tg(CMV:gonacin) at 50 dpf. ∗∗, p < 0.01; ∗∗∗, p < 0.001 compared with WT (n = 10).
(F) Overexpression of gonacin in whole body by the establishment of gonacin transgenic zebrafish driven by hsp70 promoter.
(G) The expression of gonacin was detected in the ovary of wild type (WT) and Tg(hsp70:gonacin) at 70 dpf after after 40°C heat shock for 1 h (n = 5 in each group), using ef1a as an internal control. ∗∗, p < 0.01; Unpaired Student’s t test was used to calculate the p value.
(H) Assessment of the food intake in Tg(hsp70:gonacin). The food intake was assessed in WT and Tg(hsp70:gonacin) at 70 dpf (panel a, n = 15 in each group). ∗, p < 0.05; ∗∗∗∗, p < 0.0001, two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
(I) Assessment of the blood glucose in Tg(hsp70:gonacin). The blood glucose was assessed in WT and Tg(hsp70:gonacin) at 70 dpf after 40°C heat shock for 1 h (n = 5 in each group). ∗∗, p < 0.01; Unpaired Student’s t test was used to calculate the p value.
(J) The expression of glucogenesis enzymes including pck1, pcxa, pcxb, and fbp1b, in the liver in WT and Tg(hsp70:gonacin) at 70 dpf after 40°C heat shock for 1 h (n = 5 in each group). The columns represent fold changes of the relative mRNA levels in the Tg(hsp70:gonacin) group over its respective WT controls (mean ± SEM) ∗, p < 0.05; ∗∗, p < 0.01; n = 5. Unpaired Student’s t test was used to calculate the p value.
To further confirm the essential role of gonacin on food intake and glucose secretion, we constructed an inducible transgenic fish line (Tg(hsp70:gonacin)) driven by the heat shock protein 70 (hsp70) promoter (Figure 6F). The gonacin mRNA was increased in this transgenic zebrafish as assessed in the ovary at 70 dpf after from 28 to 40OC (heat shock) for 1 h (Figure 6G). The food intake and serum glucose levels were increased after heat shock for 1 h (Figures 6H and 6I). Levels of several key enzymes in gluconeogenesis including pck1, pcxa, pcxb, and fbp1b were increased in the liver after heat shock for 1 h. as detected by real-time qPCR (Figure 6J). These findings indicate that food intake, serum glucose level, and gluconeogenesis could be enhanced by transient overexpression of gonacin in zebrafish.
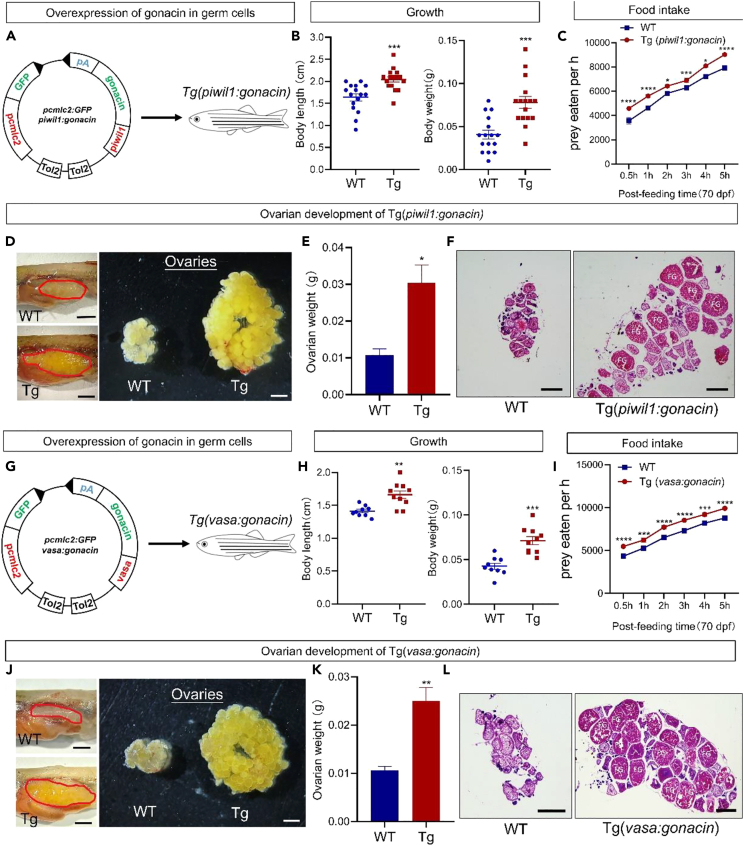
Gonacin is highly expressed in germ cells. In order to check the effects of gonacin overexpression in germ cells, we further established Tg(piwil1:gonacin) and Tg(vasa:gonacin) fish lines. In the Tg(piwil1:gonacin) fish line (Figure 7A), the body weight and body length of transgenic fish were higher than those in WT fish at 50 dpf (Figure 7B). Food intake was increased in this transgenic fish at 70 dpf (Figure 7C). Also, the ovary size and weight were significantly increased in the Tg(piwil1:gonacin) at 50 dpf (Figures 7D and 7E). Histological analyses at 50 dpf further showed that FG stage follicles were found in transgenic but not in WT ovaries (Figure 7F). Likewise, in the Tg(vasa:gonacin) fish line (Figure 7G), body weight and body length of transgenic fish were higher than those in WT fish at 50 dpf (Figure 7H). Also, food intake was increased at 70 dpf (Figure 7I). The ovary size and weight was increased in the Tg(vasa:gonacin) at 50 dpf (Figures 7J and 7K). Histological analyses at 50 dpf further showed that FG stage follicles were found in transgenic but not in WT ovaries (Figure 7L). These findings indicate that body growth, ovarian development, and food intake could be enhanced by overexpression of gonacin in germ cells of zebrafish.
Figure 7.
Overexpression of gonacin in germ cells of zebrafish enhanced body growth, gonadal development, and food intake
(A) Establishment of Tg(piwil1:gonacin) transgenic zebrafish.
(B) Body weight (left panel) and body length (right panel) of WT and Tg(piwil1:gonacin) at 50 dpf. ∗∗∗, p < 0.001 compared with WT (n = 15).
(C) Assessment of food intake in Tg(piwil1:gonacin). The food intake was assessed in WT and Tg(piwil1:gonacin) at 70 dpf (panel a, n = 15 in each group). ∗, p < 0.05; ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001, two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
(D) Morphology of ovaries dissected from WT and Tg(piwil1:gonacin) female zebrafish (Left panel: dashed areas denote the ovary, scale bar: 0.5 cm. Right panel: ovaries isolated and dispersed, scale bar: 1.5 mm).
(E) Ovarian weights of WT and Tg(piwil1:gonacin) zebrafish at 50 dpf. ∗, p < 0.05, compared with WT counterparts (n = 5 in each group).
(F) Histology of ovaries from WT and Tg(piwil1:gonacin) female zebrafish at 50 dpf.
(G) Establishment of Tg(vasa:gonacin) transgenic zebrafish. Scale bar: 500 μm.
(H) Body weight (left panel) and body length (right panel) of WT and Tg(vasa:gonacin) zebrafish at 50 dpf. ∗∗, p < 0.01; ∗∗∗, p < 0.001 compared with WT (n = 10).
(I) Assessment of food intake in Tg(vasa:gonacin). Food intake was assessed in WT and Tg(vasa:gonacin) at 70 dpf (panel a, n = 15 in each group). ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001, two-way ANOVA with Sidak’s multiple comparisons test was used to calculate the p value.
(J) Morphology of ovaries dissected from WT and Tg(vasa:gonacin) female zebrafish (Left panel: dashed areas denote the ovary, scale bar: 0.5 cm. Right panel: ovaries isolated and dispersed, scale bar: 1.5 mm).
(K) Ovary weight of WT and Tg(vasa:gonacin) female zebrafish at 50 dpf. ∗∗, p < 0.01, compared with WT females (n = 5 in each group).
(L) Histology of ovaries from WT and Tg(vasa:gonacin) female zebrafish at 50 dpf. FG, fully grown stage follicles. Scale bar: 500 μm.
Identification of gonacin in rainbow trout
We further investigated the roles of gonacin in another fish species. Rainbow trout (Oncorhynchus mykiss) belongs to the Salmonidae family, distinct from the Cyprinidae family to which zebrafish belong. In rainbow trout, gonacin sequence was found in the C-terminal end of Fbn2 with a canonical furin cleavage position, leading to a predicted gonacin with similar sizes (136 amino acid residues) as human asprosin and placensin (140 and 133 amino acid residues, respectively) (Figure 8A). RT-PCR and real-time qPCR results showed that fbn2 mRNA is also highly expressed in gonads of adult rainbow trout (Figure 8B). We generated polyclonal antibodies against rainbow trout gonacin for immunostaining and strong signals in germ cells could be observed in ovaries and testes of rainbow trout at 8 mpf (months post-fertilization) (Figure 8C). The co-localization of gonacin and Vasa (a germ cell marker) further confirmed the expression of gonacin in germ cells of gonads in rainbow trouts (Figure 8C). We also generated recombinant rainbow trout gonacin protein using the E. coli system. Purified gonacin of 18 kDa was demonstrated by electrophoresis and immunoblotting (Figure 8D). After the IP administration of this protein into rainbow trout at 3 mpf, blood glucose levels were increased compared to the rGFP-injected group (Figure 8E). Furthermore, as compared to GFP-injected group, we found gonacin-injected rainbow trout displayed greater food intake, but gonacin antibodies decreased basal food intake and neutralized gonacin-induced increases of food intake (Figure 8F). Consistent with findings in zebrafish, rainbow trout gonad derived-gonacin with a canonical molecular weight is a glucogenic and orexigenic hormone.
Figure 8.
Identification of gonacin in rainbow trout exhibiting gonadal expression and showing glucogenic and orexigenic activities
(A) Identification of gonacin in the C-terminal end of rainbow trout Fbn2 with a canonical furin cleavage position.
(B) Expression of rainbow trout fbn2 mRNA in different adult tissues detected by RT-PCR followed by electrophoresis (upper panel) and real-time PCR (lower panel).
(C) Expression of rainbow trout gonacin in ovary and testis detected by immunostaining. Localization of gonacin signal (Green, upper panel) and co-localization of gonacin (Green) and Vasa (Red) signal (lower panel) in ovary and testis of rainbow trout at 8 months post-fertilization.
(D) Characterization of recombinant rainbow trout gonacin protein (rGonacin). Coomassie blue staining and immunoblotting of bacteria-derived rGonacin. M1, M2: marker proteins; R, rGonacin.
(E) Serum glucose levels were measured after an intraperitoneal injection of rGonacin in vivo. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001, compared with control (n = 7 in each group). two-way ANOVA with Bonferroni post-test was used to calculate the p value.
(F) Food intake was measured at indicated time points after injection of a single dose of rGFP, 5 μg rGonacin or 4 μL anti-gonacin antibodies or following co-injection of 5 μg rGonacin and 4 μL anti-gonacin.
Discussion
Fish require abundant energy to generate a large number of eggs for spawning. Here, we identified a gonadal hormone with both metabolic and gonadal functions in fish. A structural protein Fbn2 encodes a peptide hormone that could be derived following furin cleavage of the C-terminal region of profibrillin-2. We named the present hormone gonacin based on the original naming for asprosin to describe the unique function of asprosin distinct from FBN-derived structural proteins and based on its tissue origin (white adipose tissue). The naming of placensin also follows the same rule (placenta tissue). Gonacin was named after its gonadal origin, as another caudamin hormone recently proposed.21 Using five transgenic fish models (whole-body knockout, germ cell-specific knockout, whole-body inducible overexpression, and two germ cell-specific overexpression), we provided evidence to support the essential roles of gonacin in fish survival and reproduction. Gonacin is secreted by gonadal germ cells and acts as a paracrine hormone to promote ovarian development. Furthermore, gonacin is secreted into the general circulation as an endocrine hormone to stimulate food intake, food transit, hepatic glucose secretion and gluconeogenesis. Thus, gonacin plays essential roles in promoting ovarian development and maintaining metabolic homeostasis in the coordination of both the survival of the individual and propagation of the species (Figure 9).
Figure 9.
Schematic illustration of the roles of gonacin in the regulation of appetite, glucose release, food transit and ovarian development
Gonacin is a C-terminal cleavage product of profibrillin-2 and mainly secreted from the early-stage germ cells of fish, capable of promoting ovarian development. Gonacin can also traffic to the liver, brain and intestine to stimulate glucose release, food intake, and food transit respectively.
To maintain the survival and reproduction of any organism, the individual needs to strictly regulate homeostasis among gonadal development, feeding, and glucose metabolism. A variety of hormones in key tissues such as the pancreas, intestines, and adipose tissues can sense the body’s energy and nutrient needs, including incretin secreted by intestines,22 leptin secreted by adipose tissues,23 and kisspeptins produced from the hypothalamus.24 Acting coordinately, these hormones regulate feeding, glucose metabolism and reproduction. As an endocrine organ of the body, the ovary can also secrete a variety of hormones including steroid hormones and growth factors. However, no factor from the ovary was found to promote energy metabolism. Here, we found gonacin secreted from ovaries can promote oocyte maturation, feeding, and glucose metabolism. In addition, the gonads are made up of somatic cells and germ cells, and most hormones are derived from gonadal somatic cells rather than germ cells. Although the role of germ cells in regulating ovarian development itself has received considerable attention,25,26 whether they play a role in the regulation of feeding and glucose metabolism remains unclear. Until now, very few secreted factors were identified in germ cells. Some members of the BMP family such as Gdf9 and Bmp15 are secreted from oocyte and play paracrine roles in regulating oogenesis.27 In fish models, we found that gonacin is predominantly expressed in the early-stage germ cells in ovaries and testes. During gonadal development and regeneration, these germ cells can proliferate and derive large numbers of germ cells in fish.28 Therefore, with the proliferation of early stage germ cells during gonadal development, gonacin secreted from these cells traffics to the brain and liver to promote food intake and glucose release. Thus, gonacin represents the first hormone secreted by gonadal germ cells with endocrine functions.
To study the physiological roles of gonacin, we established gonacin gene knockout zebrafish models using the CRISPR/Cas9 system. All larvae of gonacin null zebrafish died after 8 dpf and the observed larvae lethality might be due to digestive and gluconeogenesis dysfunctions in gonacin null zebrafish. We further overexpressed gonacin in gonacin null fish and found the larvae lethality could be rescued, demonstrating the essential role of gonacin during larva development. We also performed a conditional knockout (CKO) of gonacin in germ cells of zebrafish. Body growth, food intake, glucose secretion, and ovarian development were affected in this mutant. These findings are consistent with our results showing that recombinant gonacin could promote food intake, glucose secretion, and ovarian development in zebrafish and rainbow trout. Recombinant gonacin protein also stimulated oocyte maturation in zebrafish, consistent with defective ovarian folliculogenesis in CKO fish. These findings suggest that gonacin is involved in the regulation of different aspects of oogenesis in zebrafish. Oogenesis including vitellogenesis and oocyte maturation needs much energy, gonacin secreted from female germ cells might be an important factor to support oogenesis by regulating energy metabolism. However, the testis development and male fertility were minimally affected in CKO fish. Differences between the phenotypes in the ovary and testis of gonacin CKO zebrafish might be due to the different energy requirements of gonads. The development of oocytes is more sensitive to energy homeostasis than sperm. Compared to the sperm, oocytes need to store a lot of nutrients and energy for embryo development. For example, the energetic investment in gonads relative to soma was estimated at 11% in males but 67% in females during sexual maturation in the brown trout.29 After conditional knockout of gonacin, the energy homeostasis is affected, leading to defects in oogenesis but not in spermatogenesis. Observed differences in phenotypes between male and female gonacin null fish could also due to divergent compensatory roles of Fbn1 and/or Fbn3.
In mammals, placensin is exclusively expressed in human placenta but not in the placenta of rodents,9 likely a primate innovation for the unique human placental physiology. In fish, gonacin is almost exclusively expressed in female and male germ cells. All these changes could evolve through mutations in the promoter regions of FBN2 genes without coding variations affecting protein functions. Like the action of asprosin, both gonacin and placensin can be released into the circulation and have glucogenic actions through regulating gluconeogenesis in liver. The orexigenic activities of gonacin were found in both zebrafish and rainbow trout, which is consistent with the findings in mammals that asprosin can also stimulate appetite.5,15 In mammals, eggs ovulated and fertilized by sperm in the oviduct. After implantation, embryos are grown within mother’s womb. The growth of embryos depends on the support of placenta. Placensin is secreted from the placenta to maintain metabolic homeostasis during human pregnancy. In fish, large number of eggs needs energy. With the development of gonads, increased gonacin secretion from increasing number of germ cells could stimulate appetite and glucose release. In humans, FBN1 is expressed highly in placenta, ovary, and digestive tract, which may derive asprosin to act similarly as c-terminal product of fbn2 in zebrafish.
Although Fbn2 gene was mainly expressed in gonads, we also found its expression in the intestine of larva zebrafish. The important role of gonacin during larva development is confirmed by the lethality of global gonacin deletion as compared with the survival of fish with the conditional deletion of gonacin in gonadal cells. Although hypophagia was not found in whole body gonacin knock out larvae zebrafish, we found hypophagia in adults of gonacin germ-cell conditional knockout fish. We cannot rule out age-dependent effects of this hormone in the regulation of food intake. In addition, early lethality of gonacin knockout might also be due to fibrillin2 dysfunctions, leading to defective connective tissues. Gonacin could be retained by the liver and likely acted on hepatic receptors in vivo. Zebrafish gonacin is larger in size than gonacin found in the rainbow trout and most other fish species, containing the canonical furin cleavage site. However, gonacin from both zebrafish and rainbow trout have similar bioactivity, suggesting the extreme C-terminal sequences after the canonical furin cleavage site are important for receptor interactions.
In summary, we identified a hormone cleaved from the C-terminal of Fbn2 protein in zebrafish. Like asprosin and placensin, gonacin is the cleavage product of a structural protein profibrillin mediated by a unique enzyme cleavage system.21 Gonacin can promote food intake, glucose release and oocyte maturation, bridging the energy homeostasis and reproduction.
Limitations of the study
Although our work shows the high expression of Gonacin in germ cells of gonads and the essential role of Gonacin in food intake, glucose release, and ovarian development, which were demonstrated in zebrafish and rainbow trout, whether it is conserved in other fish species needs to be further addressed. In this study, we found gonacin can traffic to the liver and likely acted on hepatic receptors in vivo. In mammals, the olfactory receptor OLFR734 was proposed as a receptor for asprosin and asprosin promotes hepatic glucose production, appetite, and male fertility via OLFR734.30,31,32 Considering the evolutionary similarity between gonacin and asprosin, it is possible that gonacin can also stimulate glucose production through a homolog of OLFR734 in zebrafish. However, OLFR734 ortholog could not be found in fish, suggesting that other uncharacterized receptors might mediate the action of gonacin in fish, an interesting future research direction.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Polyclonal Anti-β-actin | Cell Signaling Technology | Cat# 4967; RRID: AB_10695744 |
| Mouse Monoclonal Anti-GFP | Proteintech | Cat# 66002-1-Ig; RRID:AB_11182611 |
| Rabbit Polyclonal Anti-Vasa | Abcam | Cat# ab209710 |
| Mouse Monoclonal Anti-PCB | Santa Cruz Biotechnology | Cat# sc-271493; RRID:AB_10649369 |
| HRP Conjugated Goat Anti-mouse IgG (H + L) Secondary Antibody | Cell Signaling Technology | Cat# 7076; RRID:AB_330924 |
| HRP Conjugated Goat Anti-rabbit IgG (H + L) Secondary Antibody | Cell Signaling Technology | Cat# 7074; RRID:AB_2099233 |
| Alexa 488 Conjugated Goat Anti-rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody | Thermo Fisher | Cat# A-11008; RRID:AB_143165 |
| Alexa 488 Conjugated Goat Anti-mouse IgG (H + L) Cross-Adsorbed Secondary Antibody | Thermo Fisher | Cat# A-11001; RRID:AB_2534069 |
| Alexa 594 Conjugated Goat Anti-rabbit IgG (H + L) Secondary Antibody | Thermo Fisher | Cat# A-21207; RRID:AB_141637 |
| Bacterial and virus strains | ||
| E.coli DH5α Competent Cells | Takara Bio | Cat# 9057 |
| Chemicals, peptides, and recombinant proteins | ||
| TRIzol | Invitrogen | Cat# 15596026 |
| Leibovitz (L-15) culture medium | Gibco | Cat# 11415064 |
| Penicillin-Streptomycin | Gibco | Cat# 15140148 |
| Propidium iodide | Thermo Fisher | Cat# P3566 |
| Endonucleases | NEB | N/A |
| LR Clonase™ II Plus enzyme | Invitrogen | Cat# 12538200 |
| Recombinant GFP protein | BIOSS | Cat# bs-33009P |
| 4′-6-diamidino-2-phenylindole | 4′-6-diamidino-2-phenylindole | Cat# 62248 |
| Alexa Fluor 555-Phalloidin | UElandy | Cat# YP0060L |
| Tissue-Tek® O.C.T. Compound | Sakura | Cat# 4583 |
| Clarity Western ECL Substrate | BioRad | Cat# 1705061 |
| Rainbow trout gonacin recombinant protein | Genscript | N/A |
| Zebrafish gonacin recombinant protein | Genscript | N/A |
| Critical commercial assays | ||
| PrimeScript™ RT reagent Kit | Takara Bio | Cat# RR047A |
| Alexa Fluor™ 488 Tyramide SuperBoost™ Kit, Goat Anti-Rabbit IgG | Invitrogen | Cat# B40943 |
| SYBR Green real-time PCR master mix kit | Toyobo | Cat# QPK-201 |
| DIG RNA Labeling Kit | Roche | Cat# 11175025910 |
| Guide-it Recombinant Cas9 | Clontech | Cat# 632641 |
| yellow-green fluorescent polystyrene microspheres | Yuan Biotechnology | Cat# YMFlo |
| Experimental models: Organisms/strains | ||
| Zebrafish: AB strain | China Zebrafish Resource Center | N/A |
| Zerafish: Tg(piwil1:EGFP-UTRnanos3) | China Zebrafish Resource Center | N/A |
| Recombinant DNA | ||
| Primer: fbn2 forward: GCTGCTCTAACACACAGGGT | This paper | N/A |
| Primer: fbn2 reverse: GAGGGTCTGAGTCTTGAGCG | This paper | N/A |
| Primer: pck1 forward: GCGCTAAGCTGCCCAAAATC | This paper | N/A |
| Primer: pck1 reverse: GATTAACGTGTGTGTTGCGTG | This paper | N/A |
| Primer: pcxa forward: AGTGGAGAGGGCATGGGTAT | This paper | N/A |
| Primer: pcxa reverse: TACTGGTGGGATGACCGGAT | This paper | N/A |
| Primer: pcxb forward: TAACCGGATCAGGCCTCTCA | This paper | N/A |
| Primer: pcxb reverse: GGTGGGCTGAACGACAAAAC | This paper | N/A |
| Primer: gck forward: CACCGCTGACCTGCTATGAT | This paper | N/A |
| Primer: gck reverse: AGTCGGCCACTTCACATACG | This paper | N/A |
| Primer: fbp1a forward: ACTGCCATCGTTGTAGAGCC | This paper | N/A |
| Primer: fbp1a reverse: TCTTTTTCTGACGGCTCGCT | This paper | N/A |
| Primer: fbp1b forward: CCACAGGACAGGGAGTCAAC | This paper | N/A |
| Primer: fbp1b reverse: TACATCATCAGGGGAGCCCA | This paper | N/A |
| Primer: ef1α forward: AGCGCAATCAGCCTGAGAGGTA | This paper | N/A |
| Primer: ef1α reverse: GCTGGACAAGCTGAAGGCTGAG | This paper | N/A |
| Primer: ef1α forward: GGCTGACTGTGCTGTGCTGATTG | This paper | N/A |
| Primer: ef1α reverse: CTTGTCGGTGGGACGGCTAGG | This paper | N/A |
| Primer: piwi1 forward: CCGCTCGAGCAAAGAACGAACACCT | This paper | N/A |
| Primer: piwi1 reverse: TCCCCCGGGACCGGTGCTTTACAAAT | This paper | N/A |
| Primer: fbn2 forward: TCAGACCTAACACCGTGTGG | This paper | N/A |
| Primer: fbn2 reverse: TGTTGTGTGTAACCCTGTGG | This paper | N/A |
| Primer: fbn2 forward: TGTTTGTCAGACCTAACACCGTG | This paper | N/A |
| Primer: fbn2 reverse: GATCTGCAGCGTGTACTGTCTC | This paper | N/A |
| Primer: fbn2 rainbow trout forward: GGAAACGCAGGGAAACAACC | This paper | N/A |
| Primer: fbn2 rainbow trout reverse: TTGGGCTGCCCTAGAGAGAT | This paper | N/A |
| Primer: fbn2 rainbow trout forward: AGCGCAATCAGCCTGAGAGGTA | This paper | N/A |
| Primer: fbn2 rainbow trout reverse: GCTGGACAAGCTGAAGGCTGAG | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.0 | Graphpad Software | https://www.graphpad.com/ |
Resource availability
Lead contact
Further information and requests for resources and materials may be directed to and will be fulfilled by the lead contact, Dr. Jianzhen Li (lijianzhen@nwnu.edu.cn).
Materials availability
This study did not generate new unique reagents or materials.
Experimental model and study participant details
The wide-type (WT) zebrafish of the AB strain and Tg(piwil1:EGFP-UTRnanos3) were purchased in China Zebrafish Resource Center (Wuhan, China). Zebrafish were maintained in the aquarium system (28 ± 1°C; photoperiod of 14 h light and 10 h darkness). The fish were fed three to four times daily with newly hatched brine shrimp (Brine Shrimp Direct, USA). Rainbow trout were obtained from the National salmon and trout breeding farm in Linxia City, Gansu province, China. The different gender and developmental stages of fish used in this study were described in the results section. All fish experiments were conducted in accordance to the regulations of the Animal Experimentation Ethics Committee of Northwest Normal University.
Method details
Chemicals and reagents
Most chemicals were purchased from Sigma (USA). Leibovitz (L-15) culture medium and Penicillin-Streptomycin were obtained from GIBCO (USA). Propidium iodide (PI) was from Invitrogen (USA). The antibodies for β-actin, HRP-labeled goat anti-mouse IgG, goat anti-rabbit IgG were purchased from Cell Signaling Technology (USA). Endonucleases for plasmid construction were purchased from NEB (England). LR Clonase II Plus enzyme for Gateway Cloning, DAPI, and phalloidin were purchased from Thermo Fisher (USA). Recombinant GFP protein was from BIOSS (China). The antibodies for goat anti-rabbit Alexa 488/598, the Alexa Fluor 488 Tyramide SuperBoost Kit and 4′-6-diamidino-2-phenylindole (DAPI) were from Thermo Fisher (USA). The antibodies for GFP were from Proteintech (China). The antibodies for Vasa were from Abcam (USA). The phalloidin-Alexa Fluor 555 was from UElandy (China). Tissue-Tek O.C.T. Compound was from Sakura (Japan).
RNA extraction, RT-PCR and real-time quantitative PCR
Total RNA was isolated from tissues of zebrafish and rainbow trout by extraction with TRIzol reagents (Invitrogen, USA). A total of 1 μg of RNA template was used for reverse transcription and cDNA synthesis using a first-strand cDNA synthesis kit (Takara, Japan). The primers used are listed in Table S1. Each 20 μL amplification reaction contained 10 μL of SYBR Green real-time PCR master mix (Toyobo, Japan), plus 0.5 mM each of forward and reverse primers, and 2 μL of cDNA template. The qPCR was performed on a ABI real-time system (ABI, USA).
In situ hybridization
cDNAs of C-terminal of zebrafish fbn2 were amplified and RNA probes of sense and antisense fbn2 were synthesized using the DIG RNA Labeling Kit (Roche, USA). Primers for probe synthesis are listed in Table S1. The hybridization process was performed as described.33 Briefly, after deparaffinization, gonad sections were prehybridized for over 30 min before incubated with hybridization buffer containing 150 ng DIG-labeled probes at 42°C for 16 h. Then, slides were washed in a graded series of saline sodium citrate (SSC, twice for 2X, once for 1X, 0.5X and 0.1X) at 55°C for 15 to 30 min each. Hybridization signals were detected using anti-DIG conjugated with alkaline phosphatase and the NBT/BCIP stock solution purchased from Roche. Subsequently, slides were dehydrated, mounted and photographed using the Carl Zeiss Axiophot 2 Upright Microscope (Zeiss, Germany).
Expression and purification of recombinant gonacin proteins
For studies in zebrafish, we produced the long c-terminal peptide (at the non-canonical furin cleavage site) of zebrafish Fbn2. For studies in rainbow trout, we produced a short c-terminal peptide based on the canonical cleavage found in mammalian FBN2. The zebrafish and rainbow trout gonacin cDNA fragments corresponding to the mature polypeptide were synthesized and cloned into the pET30a expression vector. The gonacin recombinant protein was expressed and purified from the E.coli bacterial system (Genscript, China). To verify antibody specificity for gonacin, recombinant proteins corresponding to C-terminal fragments of zebrafish Fbn1 (asprosin-like) and Fbn3 were also generated.
Antibody production and Western blot
Gonacin polyclonal antibodies were produced as described.33 Pcxb antibodies were purchased from Santa Cruz Biotechnology. For Western blot analysis, tissue lysates were separated by 10% SDS-PAGE gels and transferred to PVDF membranes followed by incubation with the primary antibody at 4°C overnight and secondary antibodies at room temperature for 2 h. Membranes were incubated with Western ECL Substrate (BioRad, USA) and bands were visualized using the BioRad ChemDoc Imaging System. The images were then analyzed using ImageJ software (National Institutes of Health, USA).
Immunohistochemistry
Fish were euthanized by an overdose of Tricaine, followed by submersion in ice water for 10 min. To obtain gonads, fish were then decapitated, cut open along the ventral midline, and fixed overnight in 4% paraformaldehyde (PFA)/1× Phosphate Buffered Saline (PBS). To obtain 1-9 dpf larvae, the larvae were fixed directly in 4% PFA overnight. The immunohistochemistry was performed as before.34 Briefly, for whole-mount fluorescence immunostaining of the fish gonads and larvae, the samples were dehydrated in gradient methanol after fixation and cooling in 100% methanol to −20°C for at least 2 h. For larvae, removal of pigmentation was performed in 3% H2O2/0.5% KOH for 1 h followed by permeabilisation in proteinase K (10 μg/ml) for 10 min. The samples were blocked for 2 h at room temperature in antibody blocking solution and placed in anti-gonacin (1:100) in blocking solution overnight at 4°C. Gonads were then washed 4 × 15 min plus 2 × 30 min in PBT and blocked in antibody blocking solution for 2 h at room temperature before overnight incubation in anti-rabbit-Alexa488 (1:200) in antibody blocking solution at 4°C. Confocal images were photographed under a confocal microscope (Leica SP8, Germany). For fluorescence immunostaining of the gonadal sections, the samples were embedded in a mixture of O.C.T. Compound and water at a ratio of 1:1 and sectioned at 7 μm thickness with a freezing microtome (Leica). For anti-gonacin staining, the sections were washed with PBT (1×PBS/0.1% Tween) for three times, samples were blocked for 2 h at room temperature in an antibody blocking solution. Antibody against zebrafish Gonacin (1:100) was applied to the slides overnight at 4°C. The samples were washed, incubated with goat anti-rabbit Alexa 488 (1:200) at room temperature, and then mounted and counterstained with 4′-6-diamidino-2-phenylindole (DAPI) and phalloidin-Alexa Fluor 555. For anti-Gonacin and anti-Vasa staining, the anti-gonacin staining was performed according to instructions of Alexa Fluor 488 Tyramide SuperBoost Kit. For anit-gonacin and piwil-GFP staining, antibody against gonacin (1:100) and GFP (1:300) was applied to the slides overnight at 4°C. The samples were washed, incubated with goat anti-rabbit Alexa 488 (1:200; Invitrogen, USA) and goat anti-mouse Alexa 598. Signals were visualized and photographed by confocal microscope (Leica SP8, Germany). Signals were photographed under an inverted fluorescent microscope (Leica DM6 B, Germany).
Ovarian follicle separation and monitoring oocyte maturation
Ovaries from 10 to 20 adult female zebrafish were removed and mixed together after anesthetization on ice followed by decapitation. The follicles at fully grown (FG, >650 μm in diameter) stage were isolated in a 90-mm dish containing 60% Leibovitz’s L-15 medium. About 30 follicles/well at FG stages were gently distributed and incubated in the 24-well culture plates (Corning, USA) at 28°C. The follicles that had undergone GVBD, a visible morphological marker for oocyte maturation, were counted after treatment. Each group had four replicate wells and each experiment was repeated at least three times.
Intraperitoneal injection of gonacin
Intraperitoneal injection was performed according to the procedure of our previous study.35 After fasting and anesthetization in cold water (around 15°C), adult zebrafish were quickly placed on an agar gel plate. A microinjection system (WPI, USA) was used. Recombinant zebrafish gonacin protein (the long c-terminal peptide after the non-canonical furin cleavage site of zebrafish Fbn2, 1 mg/ml, 4 μL/fish) or anti-gonacin antibodies was injected into the midline between the pelvic fins. After fasting and anesthetization in cold water (around 4°C), rainbow trout at 3 months post fertilization were placed on wet sponge. A microsyringe (Hamilton, China) was used for injection. Recombinant rainbow trout gonacin protein (short c-terminal peptide based on the furin cleavage site found in mammalian Fbn2, 1 mg/ml, 5 μL/fish) or anti-gonacin antibodies was injected into the midline between the pelvic fins. After injection, the fish were immediately transferred back to the aquarium for recovery.
Zebrafish transgenesis and gene editing
Gonacin coding sequence with signal peptide sequence of zebrafish glucagon 1 (ATGATGGTAAGGATCTATGCTTTCATTGGACTTTTACTTCTCATCCTGATCCAGAGCAGCCTGCAG) was synthesized and cloned into pTol2-EGFP vector with CMV promoter by Genscript (China). The Tol2-gonacin-EGFP construct was used for producing transgenic zebrafish lines. The Tol2-gonacin-EGFP plasmids DNA (CMV:gonacin, hsp70:gonacin, piwil1:gonacin and vasa:gonacin plasmids, 50 ng/μL) and 35 ng/μL transposase mRNA were co-injected into one-cell stage zebrafish embryos produced by crossing gonacin heterozygous mutant according to established procedures.36 Injected embryos were screened for GFP expression between 48 and 72 hpf. GFP-positive individuals were raised, and genotypes were confirmed by PCR and sequencing. Primers used were listed in Table S1.
The zebrafish gonacin mutant was generated by the CRISPR/Cas9-induced gene knockout method. Targeted gene knockout by CRISPR/Cas9 was performed according to a previous study.37 sgRNA (seed sequence is TAATACGACTCACTATAGGGAGAACGACTGCGATGACGTTTTAGAGCTAGAA) for zebrafish gonacin was synthesized by Genscript (China). Cas9 protein was purchased from Takara (Japan). The F0 mutants were produced by injecting fertilized wild type (WT) eggs at the one cell stage with a mixture of gRNAs and Cas9 protein. Mutations were detected by PCR genotyping and sequencing. The mosaic F0 mutants were reared to adulthood and males carrying the desired mutation were mated with WT females to produce the heterozygous F1 population (+/−). Adult F1 mutants carrying the same mutation genotype were mated to produce the F2 population, which included some individuals bearing the homozygous gonacin (−/−) genotype.
Conditional knockout of gonacin in germ cells in zebrafish using a vector system
The strategy for conditional knockout of gonacin in germ cells was performed according to a published protocol.38 Tol2kit system was a gift from Prof. Koichi Kawakami at National Institute of Genetics in Japan and Prof. Kristen M Kwan at University of Utah in USA.20 The strategy for plasmid construction was summarized in Figure S9. The primers used were listed in Table S1. Zebrafish piwil1 promoter was amplified from zebrafish genomic DNA. Firstly, PCR products was cloned into pTol2-EGFP vector. The green fluorescence driven by piwi1 promoter could be observed in primordial germ cells during early development (Figure S9), suggesting the specificity of this piwi1 promoter. The piwi1 promoter was then cloned into p5E-MCS. The gonacin gRNA double-strand DNA was synthesized in vitro and cloned into the destination vector pDestTol2CG2-U6 (Addgene, USA). Four vectors including a destination vector (pDestTol2CG2-U6:gonacin gRNA), a 5′ entry vector (p5E-piwil1), a middle entry vector (pME-Cas9), and a 3′ entry vector (p3E-polyA) were recombined by Gateway reaction according to manufacturer’s protocol. Correct recombinant of plasmids was checked by SmaI and NdeI digestion and sequencing. The final engineered vector has three key features: 1) a zebrafish U6-3 promoter to drive the expression of a gRNA scaffold targeted for exon 60 of zebrafish fbn2 gene; 2) a zebrafish piwi1 promoter to drive the expression of a Cas9 scaffold.39 The spatial-temporal expression of this piwi promoter was assessed by replacing the Cas9 scaffold with a sequence encoding GFP. Green fluorescence could be observed in primordial germ cells during early development and early-stage germ cells in adult zebrafish (Figure S13), and 3) GFP expression under the control of the heart-specific cmlc2 promoter, which serves as a transgene marker.20 A total of 20 pg plasmid DNA and 20 pg Tol2 mRNA were injected into single-cell embryos. After microinjection, the embryos were cultured in the E3 medium at 28.5°C. Using embryos without green fluorescence as the control group, embryos with green fluorescencethe in heart were selected and raised to adulthood. To evaluate gene targeting, gonads, muscle, brain, heart, and liver of zebrafish were collected for sequencing. DNA sequencing with two peaks suggest the mutation was induced by the CRISPR/Cas9.
Histological analysis
The fish were sampled at different time points. They were anesthetized on ice and immediately fixed in 4% paraformaldehyde solution for at least 24 h followed by dehydration and embedding in paraffin. The histological analysis was performed as described previously.35 The samples were sectioned at 6 μm thickness using the Leica microtome (Leica Microsystems, Wetzlar, Germany). The sections were deparaffinized, hydrated, stained with hematoxylin and eosin (H&E), and mounted with neutral balsam for examination with Leica microscope (Leica, Germany).
Food intake
Food intake in adults: measures to quantify the food intake of adult zebrafish and young rainbow trout were described previously with slight modifications.40 Briefly, at least six fish with similar body weights in each group were stocked in a 2-L tank. Brine shrimps (1500 shrimp/100 mg BW) were added to the tanks at normal feeding time (10:00 h). The number of brine shrimps remaining in the tank was counted at 10, 30, 60, 90, 120, and 180 min after feeding. The total number of brine shrimps in the tank was calculated according to the counting number of the 25-mL aliquot water.
Food intake in larva: Fluorescent microsphere larval food was made as described.41 100 mg Larval fish food (Larval AP100, Zeigler Bros, USA) was mixed thoroughly with yellow-green fluorescent polystyrene microspheres (Yuan Biotechnology, China, 1.0% solution). Working in dim light, the slurry was layered thinly on a microscope slide, air-dried, scraped off, crushed into a fine powder, and stored at 4°C until use. Swimming larvae (7 dpf) were placed in wells in a 6-well tissue culture plate in 5 mL of E3 solution containing 2 mg of fluorescent microsphere food mixture per well. The number of larvae per well ranged from 15 to 20 to ensure that the larval density was similar between experiments and treatment conditions. At the end of the feeding period, the larvae were anesthetized with 0.02% tricaine, transferred to narrow molded troughs in an agar plate, mounted laterally and embedded in 3% methylcellulose containing tricaine before scoring based on the amount of fluorescent food in the gut. The images were then analyzed using ImageJ software (National Institutes of Health, USA).
Serum glucose and insulin measurements
To examine the effect of gonacin on serum glucose levels in adult zebrafish and young rainbow trout, intraperitoneal injections of 4-5 μL rGonacin or rGFP were performed. The zebrafish or rainbow trout were fasted and weighed. Cold water anesthesia was performed before the injection, the temperature was decreased slowly at a rate of around 5°C/minute. Blood collection was performed using the method described previously with slight modifications.40 Briefly, the fish were placed into the ice water until anesthetized. Fish were cut between the anal fin to induce bleeding before blood aspiration with a glass capillary tube (0.7 mm in diameter). The fish recovered from the anesthesia upon entering the warm tank water. The serum insulin level was measured by a fish insulin ELISA kit (Affandi-e, China) following the manufacturer’s manual. The serum glucose level was measured by OneTouch UltraEasy Glucometer (JOHNSON, USA) at different time points after injection.
Quantification and statistical analysis
All results are presented as mean ± SEM. p values were calculated by unpaired Student’s t test or by one-way or two-way ANOVA. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001 and ∗∗∗∗, p < 0.0001. The statistical tests were performed using the GraphPad InStat software (GraphPad Software, USA).
Acknowledgments
This research work was supported by the National Natural Science Foundation of China (32060170) and the Natural Science Foundation of Gansu Province (22JR5RA148).
Author contributions
Conceptualization and design: JW Hsueh, JZ Li.
Methodology: YX Hu, SY Zhao, ZQ Liu, Tao Kang, JZ Li.
Investigation: YX Hu, SY Zhao, ZQ Liu, Tao Kang, JZ Li.
Supervision: JW Hsueh, JZ Li.
Writing—original draft: JW Hsueh, JZ Li.
Writing—review & editing: JW Hsueh, JZ Li.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: September 28, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108065.
Contributor Information
Aaron J.W. Hsueh, Email: aaron.hsueh@stanford.edu.
Jianzhen Li, Email: lijianzhen@nwnu.edu.cn.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Kobayashi T., Fushiki S., Sakai N., Hara A., Amano M., Aida K., Nakamura M., Nagahama Y. Oogenesis and changes in the levels of reproductive hormones in triploid female rainbow trout. Fish. Sci. 1998;64:206–215. [Google Scholar]
- 2.Schneider J.E. Energy balance and reproduction. Physiol. Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Navarro V.M. Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020;16:407–420. doi: 10.1038/s41574-020-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Fernandez R., Martini A.C., Navarro V.M., Castellano J.M., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol. Cell. Endocrinol. 2006;254–255:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Duerrschmid C., He Y., Wang C., Li C., Bournat J.C., Romere C., Saha P.K., Lee M.E., Phillips K.J., Jain M., et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017;23:1444–1453. doi: 10.1038/nm.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romere C., Duerrschmid C., Bournat J., Constable P., Jain M., Xia F., Saha P.K., Del Solar M., Zhu B., York B., et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165:566–579. doi: 10.1016/j.cell.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra I., Duerrschmid C., Ku Z., He Y., Xie W., Silva E.S., Hoffman J., Xin W., Zhang N., Xu Y., et al. Asprosin-neutralizing antibodies as a treatment for metabolic syndrome. Elife. 2021;10 doi: 10.7554/eLife.63784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann J.G., Xie W., Chopra A.R. Energy regulation mechanism and therapeutic potential of asprosin. Diabetes. 2020;69:559–566. doi: 10.2337/dbi19-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y., He J.H., Hu L.L., Jiang L.L., Fang L., Yao G.D., Wang S.J., Yang Q., Guo Y., Liu L., et al. Placensin is a glucogenic hormone secreted by human placenta. EMBO Rep. 2020;21 doi: 10.15252/embr.201949530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briggs J.P. The zebrafish: a new model organism for integrative physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R3–R9. doi: 10.1152/ajpregu.00589.2001. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Ge W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Mol. Cell. Endocrinol. 2020;507 doi: 10.1016/j.mce.2020.110778. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Kossack M.E., McFaul M.E., Christensen L.N., Siebert S., Wyatt S.R., Kamei C.N., Horst S., Arroyo N., Drummond I.A., et al. Single-cell transcriptome reveals insights into the development and function of the zebrafish ovary. Elife. 2022;11 doi: 10.7554/eLife.76014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houwing S., Kamminga L.M., Berezikov E., Cronembold D., Girard A., van den Elst H., Filippov D.V., Blaser H., Raz E., Moens C.B., et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Ye D., Zhu L., Zhang Q., Xiong F., Wang H., Wang X., He M., Zhu Z., Sun Y. Abundance of early embryonic primordial germ cells promotes zebrafish female differentiation as revealed by lifetime labeling of germline. Mar. Biotechnol. 2019;21:217–228. doi: 10.1007/s10126-019-09874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra I., Xie W.R., Bournat J.C., He Y., Wang C., Silva E.S., Liu H., Ku Z., Chen Y., Erokwu B.O., et al. Protein tyrosine phosphatase receptor delta serves as the orexigenic asprosin receptor. Cell Metabol. 2022;34:549–563.e8. doi: 10.1016/j.cmet.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagahama Y., Yamashita M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008;50(Suppl 1):S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 17.Kang T., Zhao S., Shi L., Li J. Glucose metabolism is required for oocyte maturation of zebrafish. Biochem. Biophys. Res. Commun. 2021;559:191–196. doi: 10.1016/j.bbrc.2021.04.059. [DOI] [PubMed] [Google Scholar]
- 18.Irion U., Krauss J., Nüsslein-Volhard C. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development. 2014;141:4827–4830. doi: 10.1242/dev.115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez R.E., Galitan L., Cameron J., Goodwin N., Ramakrishnan L. Delay of initial feeding of zebrafish larvae until 8 days postfertilization has no impact on survival or growth through the juvenile stage. Zebrafish. 2018;15:515–518. doi: 10.1089/zeb.2018.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan K.M., Fujimoto E., Grabher C., Mangum B.D., Hardy M.E., Campbell D.S., Parant J.M., Yost H.J., Kanki J.P., Chien C.B. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dynam. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 21.Basu B., Jain M., Chopra A.R. Caudamins, a new subclass of protein hormones. Trends Endocrinol. Metabol. 2021;32:1007–1014. doi: 10.1016/j.tem.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drucker D.J. The biology of incretin hormones. Cell Metabol. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Spicer L.J. Leptin: a possible metabolic signal affecting reproduction. Domest. Anim. Endocrinol. 2001;21:251–270. doi: 10.1016/s0739-7240(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 24.Castellano J.M., Roa J., Luque R.M., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. KiSS-1/kisspeptins and the metabolic control of reproduction: physiologic roles and putative physiopathological implications. Peptides. 2009;30:139–145. doi: 10.1016/j.peptides.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Gilchrist R.B., Lane M., Thompson J.G. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T., Sato T., Yamamoto Y., Watakabe I., Ohkawa Y., Suyama M., Kobayashi S., Tanaka M. foxl3 is a germ cell-intrinsic factor involved in sperm-egg fate decision in medaka. Science. 2015;349:328–331. doi: 10.1126/science.aaa2657. [DOI] [PubMed] [Google Scholar]
- 27.Otsuka F., McTavish K.J., Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011;78:9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Z., Mao X., Luo L. Germline stem cells drive ovary regeneration in zebrafish. Cell Rep. 2019;26:1709–1717.e3. doi: 10.1016/j.celrep.2019.01.061. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson N., Jonsson B. Energy allocation in polymorphic Brown Trout. Funct. Ecol. 1997;11:310–317. [Google Scholar]
- 30.Li E., Shan H., Chen L., Long A., Zhang Y., Liu Y., Jia L., Wei F., Han J., Li T., et al. OLFR734 Mediates Glucose Metabolism as a Receptor of Asprosin. Cell Metabol. 2019;30:319–328.e8. doi: 10.1016/j.cmet.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Long A., Chen L., Jia L., Wang Y. The Asprosin-OLFR734 module regulates appetitive behaviors. Cell Discov. 2020;6:19. doi: 10.1038/s41421-020-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei F., Long A., Wang Y. The Asprosin-OLFR734 hormonal signaling axis modulates male fertility. Cell Discov. 2019;5:55. doi: 10.1038/s41421-019-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Liu Z., Wang D., Cheng C.H.K. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biol. Reprod. 2011;84:476–486. doi: 10.1095/biolreprod.110.086363. [DOI] [PubMed] [Google Scholar]
- 34.Tanimoto R., Sekii K., Morohaku K., Li J., Pepin D., Obata Y. Blocking Estrogen-Induced AMH Expression Is Crucial for Normal Follicle Formation. Development. 2021;148 doi: 10.1242/dev.197459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z., Niu C., Li J. Pyroptosis is involved in ovulation of zebrafish. Cell Discov. 2021;7:40. doi: 10.1038/s41421-021-00263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suster M.L., Kikuta H., Urasaki A., Asakawa K., Kawakami K. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol. Biol. 2009;561:41–63. doi: 10.1007/978-1-60327-019-9_3. [DOI] [PubMed] [Google Scholar]
- 37.Chu L., Li J., Liu Y., Hu W., Cheng C.H.K. Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Mol. Endocrinol. 2014;28:1785–1795. doi: 10.1210/me.2014-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ablain J., Durand E.M., Yang S., Zhou Y., Zon L.I. A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Dev. Cell. 2015;32:756–764. doi: 10.1016/j.devcel.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leu D.H., Draper B.W. The ziwi promoter drives germline-specific gene expression in zebrafish. Dev. Dynam. 2010;239:2714–2721. doi: 10.1002/dvdy.22404. [DOI] [PubMed] [Google Scholar]
- 40.Pedroso G.L., Hammes T.O., Escobar T.D.C., Fracasso L.B., Forgiarini L.F., da Silveira T.R. Blood collection for biochemical analysis in adult zebrafish. J. Vis. Exp. 2012 doi: 10.3791/3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Field H.A., Kelley K.A., Martell L., Goldstein A.M., Serluca F.C. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neuro Gastroenterol. Motil. 2009;21:304–312. doi: 10.1111/j.1365-2982.2008.01234.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.