Abstract
A series of styrene-butadiene di-block copolymers with high cis-1,4 unit content (greater than 92%) polybutadiene (PB) and isotactic-rich polystyrene (PS) (mmmm > 65%) was synthesized using α-diimine nickel catalysts (Ni-diimine). Four different Ni-diimine catalysts were synthesized via a complexing reaction between nickel (II) naphthenate and laboratory-made α-diimine ligands L1, L2, L3 and L4, which have different steric volume structures. The results indicate that the Ni-diimine catalyst prepared using the L4 ligand with a higher steric volume can effectively initiate the block polymerization of butadiene and styrene, and the resulting polymer has distinguished cis-1,4 structure unit PB and high isotactic-selective PS block. Differential scanning calorimetry and electrochemical performance tests show that these block copolymers with cis-1,4-regulated and isotactic-selective polymerization have advantages in terms of high-temperature and low-temperature resistance as well as corrosion resistance. Therefore, these copolymers are expected to be widely used in some harsh industrial environments.
Keywords: α-diimine nickel; cis-1,4 unit; isotactic; di-block; styrene; butadiene
1. Introduction
Styrene-butadiene block or styrene-butadiene-styrene tri-block copolymers (PS-b-PB or SBS) are important thermoplastic elastomers in various industrial fields owing to their excellent properties [1–5]. Many literature reports have shown that the low-temperature resistance and mechanism performance of butadiene rubber or styrene-butadiene block copolymers can be significantly improved by increasing the cis-1,4 structure unit content in the butadiene block [6–8]. Meanwhile, the stereoselectivity of polystyrene (PS) block also significantly influences the combined properties of these copolymers, and a high tacticity degree (including isotactic and syndiotactic degree) results in a higher melting point, high tensile modulus, excellent chemical resistance and other desirable properties [9,10].
At present, the methods for synthesizing styrene-butadiene block copolymers include anionic polymerization [11], ring-opening metathesis polymerization (ROMP) [12], and ternary rare earth-catalysed coordination polymerization [13], etc. Among them, the polymers obtained by anionic polymerization and ROMP have cis-1,4 structure content of less than 50% [9]. In ternary rare earth-catalysed coordination polymerization, neodymium carboxylate and neodymium phosphonate are usually used as the main catalysts [14,15], and high cis-1,4-selectivity (greater than 96%) can be obtained. However, the styrene block in the resulting copolymers generally exhibit atactic sequences, and the monomer conversion rate is very low [13,16].
In recent years, a series of metallocene rare earth catalysts has been applied in the preparation of styrene-butadiene block copolymers by many scholars [3,17]. Cui et al. [18] and Hou et al. [19], respectively, used a linked-half-sandwich lutetium-bis(allyl) complex and half-sandwich metallocene scandium catalysts to efficiently synthesize styrene-butadiene block copolymers with high cis-1,4 unit content and pure syndiotactic selectivity. However, the high cost of the co-catalyst [Ph3C][B(C6F5)4] has seriously limited their application. Titanocene and methylaluminoxane catalyst systems can also be used in the synthesis of styrene-butadiene block copolymers. The resulting copolymers exhibit syndiotactic styrene content of 95%, and the cis-1,4 structure content of the butadiene is higher than 70%. However, the conversion rate of monomer styrene and butadiene is only about 20% [20].
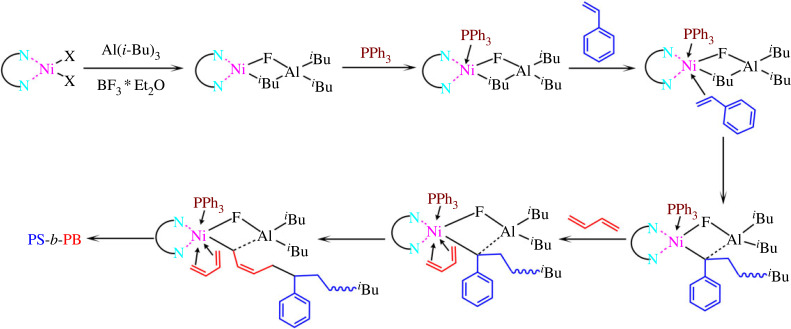
In this article, α-diimine nickel complexes with different structures were synthesized and combined with alkyl aluminium, boron trifluoride ether and triphenyl phosphine to form a catalytic system. This catalytic system was applied to the synthesis of styrene-butadiene block copolymers. Block copolymers with high cis-1,4 structure content and rich isotactic styrene block copolymers were prepared. Moreover, the influence of the ligand structure on catalytic activity and the spatial structure of the polymer was studied. The structure of α-diimine and the synthesis process of the copolymer are shown in scheme 1.
Scheme 1.
(a) Synthesis route of Ni-diimine; (b) synthesis route of PS-b-PB via Ni-diimine catalysts.
2. Experimental
2.1. Materials
1,3-Butadiene (Bd, 1.9 M in hexane) in a ChemSeal bottle was purchased from Energy China and used as received. Styrene (St, 99%) was obtained from Macklin China. The styrene was purified by distillation over calcium hydride with dilution to 2 M in hexane. 2,3-Butanedione (98%), benzil (98%), 2,6-dimethylaniline (99%), 2,6-diisopropylaniline (96%) and diisobutylaluminium hydride (Al(i-Bu)3, 1 M in n-hexane) were obtained from Energy China. Boron trifluoride etherate (B, 98%), trifluoroacetic acid (TFA, 99%), triphenyl phosphine (PPh3, 97%) and 2,6-di-tert-butyl-p-cresol (Antiager 264, 97%) were purchased from Macklin China and used as received. Nickel (II) naphthenate (Ni, 5 wt %) was supplied by Meryer China and diluted to 0.025 M with cyclohexane. Toluene, cyclohexane (analytically pure reagent (AR)), hexane (AR), methanol (AR), ethanol (AR) and butanone were purchased from Sinopharm Chemical Reagent Co., Ltd. and used as received.
2.2. Synthesis of α-diimine ligands
The synthesis routes of the α-diimine ligands are schematically illustrated in scheme 1 [21]. A detailed synthesis procedure of ligand L4 is described as a typical example. First, 5.26 g (25 mmol) benzil, 8.86 g (50 mmol) 2,6-diisopropylaniline, 0.46 g trifluoroacetic acid and 40 ml toluene were added to a 100 ml round-bottom flask. This mixture was heated to 130°C and stirred for 24 h. Then, the solution was transferred to a low-temperature environment of −15°C, and brown-yellow crystals were precipitated. The resulting product was washed with ice methanol and dried under vacuum at 40°C until a constant-weight product was obtained. The obtained solid yellow product was denoted ligand L4. The process yield was 95.2%. 1H nuclear magnetic resonance (NMR) (600 MHz, CDCl3): δ 7.3–8.17(m, 5H), δ 6.91–7.22(m, 3H), δ 2.90(m, 1H), δ 1.07(m, 12H). A series of ligands (L1, L2, L3 and L4, see scheme 1 and table 2 for more detail) was synthesized with the same synthesis process. The detailed synthesis procedures of L1, L2 and L3 are shown in the electronic supplementary material, S1. L1 : 1H NMR(600 MHz, CDCl3): δ 7.07(d, 2H), δ 6.94(t, 1H), δ 2.04(d, 9H)–L2: 1H NMR(600 MHz, CDCl3): δ 7.16(d, 2H), δ 7.09(m, 1H), δ 2.71(m, 2H), δ 2.07(s, 3H), δ 1.18(m, 12H); L3: 1H NMR(600 MHz, CDCl3): δ 7.16(d, 2H), δ 7.09(m, 1H), δ 2.71(m, 2H), δ 2.07(s, 3H), δ 1.18(m, 12H).
Table 2.
Synthesis of PS-b-PBs via four different Ni-diimine catalystsa.
| entry | catalyst | convb | convb Bd (%) | St contc | Mdn × 104 g mol−1 | Mw/Mdn | cis-1,4e | mmmmf |
|---|---|---|---|---|---|---|---|---|
| St (%) | (%) | (%) | (%) | |||||
| 1 | Ni-diimine-I | 22.8 | 36.5 | 19.4 | 5.0 | 3.36 | 70.3 | 19.2 |
| 2 | Ni-diimine-II | 45.3 | 53.2 | 24.5 | 7.5 | 2.35 | 80.5 | 24.6 |
| 3 | Ni-diimine-III | 60.5 | 68.3 | 28.3 | 8.1 | 2.87 | 90.3 | 53.6 |
| 4 | Ni-diimine-IV | 96.5 | 82.1 | 29.9 | 13.8 | 1.59 | 95.2 | 65.3 |
aFirst step, [Ni] = 0.01 mmol, catalyst ratio: [Ni]/[St]/[Al]/[B]/[PPh3] = 1 : 25 : 20 : 30 : 1, [St]/[Ni] = 400, second step, [Bd]/[Ni] = 2000.
bConversion of monomer in each steps.
cDetermined by the 1H NMR spectrum.
dMeasured by SEC-MALLS.
eDetermined by the 1H NMR and 13C NMR spectrum.
fDetermined by 13C NMR.
2.3. Synthesis of α-diimine nickel catalysts
The synthesis routes of the α-diimine nickel catalysts (Ni-diimine-I, Ni-diimine-II, Ni-diimine-III and Ni-diimine-IV) are shown in scheme 1. A detailed synthesis procedure of Ni-diimine-IV is provided as a typical example. First, 2.64 g (5 mmol) L4, 5.87 g (5 mmol) nickel naphthenate and 38 ml cyclohexane were added to a 100 ml round-bottom flask. The reaction was carried out at room temperature for 3 h to obtain a green transparent solution. Next, the catalyst solution was transferred to a constant-temperature environment at 5°C for static storage until yellow-green crystals were precipitated. After filtration, the crystals were transferred to an oven at 70°C and dried to a constant weight. Finally, the resulting product was dissolved in cyclohexane to form a stable complex solution with a concentration of 0.025 M.
2.4. Synthesis of styrene-butadiene di-block copolymer using α-diimine nickel catalysts
All synthesis operations were conducted under a dry argon atmosphere. The detailed polymerization procedure of PS-b-PB-2 (table 1) is described as a typical example. Ni-diimine-IV solution (Ni, 0.01 mmol, 0.1 M in cyclohexane), styrene (St, 0.25 mmol, 2 M in cyclohexane), Al(i-Bu)3 (Al, 0.2 mmol, 1 M in n-hexane) and boron trifluoride etherate (B, 0.3 mmol, [B]/[Al] = 1.5) were sequentially injected into a Schlenk tube with a rubber septum by a syringe. This mixture was aged at 50°C under continuous stirring. After 15 min, triphenyl phosphine (PPh3, 0.01 mmol) was injected, and the mixture was aged for another 15 min. After ageing, a reddish brown transparent catalyst solution was obtained.
Table 1.
Synthesis of PS-b-PB via Ni-diimine-IVa.
| entry | monomer | convb (%) | St contc (%) | Mdn × 104 g mol−1 | Mw/Mdn | cis-1,4e (%) | mmmmf (%) |
|---|---|---|---|---|---|---|---|
| 1 | St | 96.5 | 100 | 4.3 | 1.32 | — | 65.2 |
| 2 | Bd | 82.1 | 29.9 | 13.8 | 1.59 | 95.2 | 65.3 |
a1, 2 correspond to the first, second polymerization steps. First step, [Ni] = 0.01 mmol, Catalyst ratio: [Ni]/[St]/[Al]/[B]/[PPh3] = 1 : 10 : 20 : 30 : 1, [St]/[Ni] = 400, second step, [Bd]/[Ni] = 2000.
bConversion of monomer in each polymerization steps.
cDetermined by 1H NMR.
dDetermined by SEC-MALLS.
eDetermined by 1H NMR and 13C NMR.
fDetermined by 13C NMR.
Next, the styrene solution (2 mmol, 2 M in hexane, [St]/[Ni] = 400) was injected into the Schlenk tube with the preformed catalyst solution using a syringe. Polymerization was carried out at 50°C for 3 h. Then, butadiene solution (Bd, 10 mmol, 1.9 M in n-hexane, [Bd]/[Nd] = 2000) was added. This polymerization was carried out at 50°C for 3 h under stirring, followed by quenching with ethanol containing antiager 264 (1 wt %) as a stabilizer. The resulting product was precipitated in ethanol and repeatedly washed with ethanol, then extracted with butanone and n-hexane three times each. Finally, the product was dried under vacuum at 55°C until a white solid with a constant weight was obtained.
2.5. Characterization
The Fourier transform-infrared (FT-IR) spectra of the α-diimine ligands and resulting polymers were measured by a Bruker VERTEX70 spectrophotometer. 1H NMR and 13C NMR spectra were recorded by a Bruker 400 MHz instrument using CDCl3 as the solvent. For the polybutadiene (PB) and PS-b-PB polymers, the ratio of 1,4- and 1,2-unit content was determined by 1H NMR, and the ratio of cis-1,4 and trans-1,4 unit content of PB was determined by 13C NMR. In addition, the ratio of the PS and PB blocks in the copolymer was also determined by 1H NMR according to a previously published method. The number-average molecular weights (Mn) and dispersity (Mw/Mn) of the polymers were measured by exclusion chromatography (DAWN EOS) and a size exclusion chromatography-multi-angle laser light scatter instrument (SEC-MALLS; Wyatt Technology). To perform this analysis, the polymer samples were dissolved in tetrahydrofuran with a concentration of 5.0 mg ml−1, and the eluent flow rate was 0.5 ml min−1. Catalyst morphology was observed using a Zeiss Sigma 300 scanning electron microscope. X-ray photoelectron spectroscopy (XPS) was performed to measure the composition of the α-diimine nickel and nickel (II) naphthenate complexes with a Thermo Scientific ESCALAB Xi+. Differential scanning calorimetry (DSC) curves were collected on a METTLER TOLEDO DSC3 instrument. A 10 mg sample was scanned at a scan rate of 5°C min−1 from −150°C to 150°C or 50°C to 300°C. Nyquist plots and potentiodynamic polarization curves were obtained with a ParStat 3000A electrochemical workstation (Princeton, USA). A three-electrode cell was used, and the working, counter, and reference electrodes were samples coated on a stainless copper substrate (1 cm2), a platinum plate and saturated calomel, respectively. The electrolyte was 3.5 wt % sodium chloride solution. Electrochemical tests were carried out on the samples in their original states for the blank and samples in the frequency range of 10−2 to 105 Hz and the potential range of −0.5 to 1.5 V (1 mV S−1), using the polymer coating on the copper matrix as the working electrode, the platinum sheet as the counter electrode, and the saturated calomel as the reference electrode.
3. Results and discussion
3.1. Synthesis of α-diimine ligand and α-diimine nickel catalysts
The α-diimine ligands were prepared by a ketoamine condensation reaction, as shown in scheme 1a. The FT-IR and 1H NMR spectra of α-diimine ligands L1, L2, L3 and L4 are shown in the electronic supplementary material, figures S1–S8. The α-diimine nickel catalysts were prepared using the α-diimine ligands and nickel naphthenate, as shown in scheme 1b. The FT-IR spectra of ligand L4 and α-diimine nickel catalyst Ni-diimine-IV are shown in figure 1. In the FT-IR spectrum of L4, a C = N stretching vibration absorption peak is observed near 1624 cm−1. The FT-IR spectrum of the catalyst shows C = N stretching vibrations in the low-frequency direction [22,23]. This shows the coordination between the nitrogen atom in the ligand and the central metal ion, indicating the formation of the α-diimine catalyst.
Figure 1.
FT-IR of α-diimine ligand L4 and Ni-diimine-IV.
The morphology structure of the Ni-diamine-IV nickel catalysts was observed by scanning electron microscopy (SEM). As shown in figure 2a,b, the catalyst is a monodisperse irregular particle, approximately 500 µm in length and 140 µm in width. It can be seen from figure 2c, the Ni-diamine-IV catalyst is lamellar in arrangement, which is owing to the π-π stacking interactions between the benzene rings in the catalysts structure, and the forming stable π-π coplanar aggregates allows the catalyst crystals to grow in a layer growth pattern [24]. In addition, the catalyst was analysed using SEM-energy dispersive spectroscopy, and four elements had been detected, which, respectively, were Ni, N, C and O (figure 2d–g). The results also demonstrated that the Ni-diamine-IV catalysts were prepared successfully.
Figure 2.
(a) and (b) SEM image of the Ni-diimine-IV crystal morphology; (c) local magnification image of (b); (d), (e), (f), (g) distribution of Ni, N, C and O elements in the selected area of (c).
XPS was used to further study the composition and structure of the α-diimine nickel catalyst as shown in the electronic supplementary material, figure S9(a). C, N, O and Ni signals were detected at 284.8, 398.7, 531.1 and 856.1 eV in the full survey spectrum of the α-diimine nickel catalyst. The high-resolution Ni 2p spectrum (electronic supplementary material, figure S9(b)) is divided into four peaks. The satellite peaks are located at 861.2 and 879.1 eV, and two strong peaks corresponding to Ni 2p3/2 and Ni 2p1/2 are observed at binding energies of 856.3 and 873.9 eV, respectively. These peaks correspond to Ni2+. These XPS results further demonstrate the coordination of the α-diimine ligand with nickel [25].
3.2. Synthesis of styrene-butadiene di-block copolymer using α-diimine ligand nickel catalysts
3.2.1. Synthesis of styrene-butadiene di-block copolymer using α-diimine nickel catalysts
Owing to the different polymerization activities of styrene and butadiene monomers, the traditional Zigler-Natta nickel system catalyst has high catalytic activity and spatial stereoselectivity for 1,3-butadiene but low polymerization activity for styrene. Thus, it is difficult to obtain block copolymers with high styrene content. To solve this problem, Liu et al. [26] used n-butyllithium to initiate styrene and obtain the macromolecular alkylation reagent PSLi, which was then combined with nickel naphthenate to achieve copolymerization with butadiene. However, the St block of the PS-b-PB obtained by this method presented atactic sequence, with extremely low stereoselectivity. Therefore, in this work, α-diimine and nickel naphthenate were complexed to obtain the α-diimine nickel catalyst, which was combined with Al(i-Bu)3/BF3*Et2O/PPh3 to achieve the highly efficient polymerization of styrene with highly spatially stereoselective butadiene. Finally, PS-b-PB block copolymer was obtained.
Ni-diimine-IV was aged with Al(i-Bu)3, BF3*Et2O, and PPh3 to create the complex catalyst system. This system was used to initiate the copolymerization of styrene and butadiene. Although the α-diimine nickel catalyst has certain catalytic activity for both styrene and butadiene monomers, its catalytic activity for butadiene is obviously higher than that for styrene. Therefore, the polymerization of styrene is carried out first in this work, and the catalytic active centre after homopolymerization of styrene still maintains a high catalytic activity for the second monomer butadiene, as shown in table 1. The Ni-diimine-IV catalyst system exhibited high catalytic activity and strong stereoselectivity to both monomers. Therefore, the resulting PS-b-PB polymer with a distinguished cis-1,4 structure unit polybutadiene (cis-1,4 > 95%) and high isotactic-selective PS block (mmmm > 65%) was obtained.
The 1H NMR spectrum, SEC curve, FT-IR spectrum and 13C NMR spectrum of the di-block copolymer synthesized with the Ni-diimine-IV catalyst are shown in figure 3. As shown in figure 3a, the chemical shifts of the polymer between 6.25 and 7.25 ppm correspond to the benzene rings of the PS block, and the chemical shift peaks at 5.40 ppm and 5.05 ppm correspond to the 1,4- and 1,2- microstructure peaks of the PB block [27,28]. Integrating the chemical shift peaks of the 1H NMR spectrum shows that the styrene content in the polymer chains is 29.9% [19,29]. Figure 3b shows the step-by-step SEC curves of PS and PS-b-PB . The Mn of the product obtained after styrene polymerization is only 4.3 × 104 g mol−1. By contrast, the Mn of PS-b-PB (obtained after the addition of butadiene monomer) is higher and a single peak is maintained, demonstrating that PS and PB homopolymers do not exist in the PS-b-PB block copolymer.
Figure 3.
(a) 1H NMR spectra, (b) SEC curve of the polymers in each polymerization steps of PS-b-PB, (c) FT-IR and (d) 13C NMR spectrum of PS-b-PB via Ni-diimine-IV catalysts.
The FT-IR spectrum in figure 3c shows absorption peaks at 699 cm−1 and 1493 cm−1 that correspond to the out-of-plane deformation vibration and skeleton vibration of the protons on the benzene ring in the PS block of the polymer. The absorption peaks at 740 cm−1, 966 cm−1 and 911 cm−1, respectively, correspond to the out-of-plane protons in the cis-1,4, trans-1,4, and 1,2- structural units of the PB block of the polymer. These results show that the PS-b-PB copolymer contains high cis-1,4 unit content (cis-1,4 > 95%) [19]. The 13C NMR spectrum in figure 3d shows chemical shifts at 27.4 ppm, 32.6 ppm, 34.2 ppm and 144.9–146.4 ppm corresponding to cis-1,4, trans-1,4, 1,2-, and PS microstructure, respectively [26,30,31]. These results demonstrate that the PS-b-PB copolymer is cis-1,4-regulated and isotactic-selective (mmmm > 65%), which is consistent with the FT-IR results.
3.2.2. Steric volume effects of α-diimine nickel catalysts
α-diimine nickel catalysts with different steric volumes were synthesized by the same process to catalyse the block copolymerization of styrene and butadiene. As shown in table 2, with increasing α-diimine nickel catalyst steric volume, the catalytic activity towards the two monomers improves. At the same time, the cis-1,4 unit content stereoselectivity in the PB block and the isotacticity of the PS block significantly increase with increasing steric volume. Compared with the methyl group, the rigid benzene ring structure in the Ni-diimine-III and Ni-diimine-IV catalysts increases their axial steric hindrance and inhibits the rotation of the CAr-N bond to a certain extent. Thus, the stability and activity of the catalytic active centre formed by these catalysts are improved [32]. Meanwhile, the steric hindrance of the substituent group on the N-aryl group improves the molecular weight and the stereoselectivity of the resulting PS-b-PB copolymer [33,34]. Therefore, the di-block copolymer prepared with the Ni-diimine-IV catalyst achieves distinguished stereoselectivity with higher cis-1,4 unit content in the PB block and higher isotacticity in the PS block.
3.2.3. Effect of the monomer feed ratio of St/Ni
As shown in table 3, a series of PS-b-PBs with different styrene contents were synthesized by changing the amount of styrene added in the first step of the polymerization, and the actual percentage of the PS component in copolymer could be effectively controlled using this Ni-diimine-IV/Al(i-Bu)3/BF3*Et2O/PPh3 catalytic system. Moreover, these block copolymers obtained also have high cis-1,4-regulated and isotactic-selectivity.
Table 3.
Effect of St/Ni ratios in the synthesis of PS-b-PBa.
| entry | St/Ni | Bd/Ni | St contb (%) | cis-1,4c (%) | mmmmd (%) |
|---|---|---|---|---|---|
| 5 | 100 | 2000 | 10.4 | 93.8 | 50.1 |
| 6 | 200 | 2000 | 21.3 | 94.1 | 58.1 |
| 7 | 400 | 2000 | 29.9 | 95.2 | 65.2 |
| 8 | 800 | 2000 | 35.7 | 94.3 | 64.9 |
aFirst step, [Ni] = 0.01 mmol, catalyst ratio: [Ni]/[St]/[Al]/[B]/[PPh3] = 1 : 25 : 20 : 30 : 1.
bDetermined by the 1H NMR spectrum.
cDetermined by the 1H NMR and 13C NMR spectrum.
dDetermined by 13C NMR.
3.2.4. Proposed mechanism
The proposed mechanism of the block polymerization of butadiene and styrene with the α-diimine nickel catalyst is shown in scheme 2, based on the literature reports [35–38]. First, Ni-diimine-IV is reduced by Al(i-Bu)3 and combines with BF3*Et2O to form a stable Ni-Al bimetallic active centre. However, the catalytic system has no catalytic activity for styrene and butadiene in this form. When PPh3([PPh3]/[Ni] = 1) is added, the heteroatom P changes the electron density of the catalytic active centre, which improves the catalytic activity of the system to styrene and butadiene, and also improves the stability of the catalytic active centre. Simultaneously, the steric volume regulation of the catalyst effectively enhances the catalytic activity and stereoselectivity towards the resulting PS-b-PB copolymer.
Scheme 2.
Synthesis mechanism of PB-b-PS via Ni-diimine catalysts.
3.3. Performance of styrene-butadiene di-block copolymer via Ni-diimine catalysts
3.3.1. Thermal properties
The DSC curve of the PB-b-PS sample (entry 4) is shown in figure 4. As shown in figure 4a, this di-block copolymer with high cis-1,4 unit content (95.2%) has a Tg of −101.1°C in the low-temperature range, which is very close to the Tg of neodymium-based butadiene rubber [18]. The high-temperature section of the curve shown in figure 4b demonstrates that the softening temperature of styrene in the polymer is 101.5°C, and multiple melting peaks appear at 180–240°C. These peaks are close to the isotactic styrene melting peaks reported in the literature [39]. These data further prove that the di-block PB-b-PS copolymers containing both high cis-1,4 unit content (greater than 95%) and isotactic-rich PS (mmmm > 65%) styrene block were successfully synthesized with the α-diimine nickel catalyst.
Figure 4.
DSC curve of PS-b-PB via α-diimine nickel catalysts: (a) Tg of PB blocks and PS blocks; and (b) Tm of PS blocks.
3.3.2. Electrochemical performance
The corrosion resistance of different polymer coatings was evaluated by Nyquist plot tests and Tafel curves, as shown in figure 5. It shows that as for the PS-b-PB coating with high styrene content and high cis-1,4 content (entry 4), the PS-b-PB coating with low styrene content and high cis-1,4 content (entry 2), the PS-b-PB coating with low styrene content and low cis-1,4 content (entry 1), and the pure PB coating with low cis-1,4 content (PB; the synthesis process of PB is reported in the electronic supplementary material, S4), the styrene content and cis-1,4 content determine the impedance of the PS-b-PB coatings, as shown in figure 5a and the electronic supplementary material, table S1. Pure PB (prepared without PS block) has the smallest impedance arc. Higher styrene content and cis-1,4 content lead to a larger impedance arc. The pure PB coating has the smallest impedance arc and therefore the lowest impedance value. According to figure 5b and the electronic supplementary material, table S2, Icorr (self-corrosion current) decreases with increasing styrene and cis-1,4 content [40,41,42]. This further proves that higher styrene content and cis-1,4 content in the PS-b-PB polymer coatings lead to better corrosion resistance, which is consistent with the results of the Nyquist plots.
Figure 5.
Nyquist plots (a) and Tafel curves (b) of different polymer coatings.
4. Conclusion
A series of α-diimine nickel complexes with different steric structures were synthesized. Then, these Ni-diimine complexes were aged with Al(i-Bu)3, BF3*Et2O and PPh3 to prepare nickel-based catalytic systems, which were successfully used in the copolymerization of styrene and butadiene. The result shows that the Ni-diimine prepared using a ligand with a larger steric volume has higher catalytic activity and can be used to prepare PS-b-PB copolymers with better cis-1,4 (greater than 95%) and isotactic (mmmm > 65%) stereoselectivity. In addition, DSC and electrochemical performance tests show that these block copolymers with cis-1,4-regulated PB and isotactic-riched PS have excellent performance in terms of high-temperature and low-temperature resistance as well as corrosion resistance. Therefore, these copolymers are expected to be widely used in some harsh industrial environments.
Acknowledgements
The authors cordially thank the Shaanxi University of Technology and Northwestern Polytechnical University for providing various characterization facilities.
Contributor Information
Jie Liu, Email: liujie509_1982@126.com.
Xin Min, Email: 15991672082@163.com.
Ethics
This article does not present research with ethical considerations.
Data accessibility
The data are also provided in the electronic supplementary material [43].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
N.Z.: investigation, methodology, writing—original draft; J.L.: formal analysis, investigation; Z.L.: formal analysis; J.Lu.: data curation; Y.N.: methodology; X.M.: conceptualization, funding acquisition.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 52003148), the Key Research and Development Project of Shaanxi Province (grant no. 2023-YBGY-475), Key Scientific Research Project of Education Department of Shaanxi Province (grant no. 22JS003), Scientific and technological research projects of Jiangxi Education Department (grant no. GJJ201838), Scientific Research Fund of Jiangxi Provincial Education Department (grant no. GJJ151327), the key cultivation project funds of Shaanxi University of Technology (grant no. SLGKYXM2201) and the start-up funds of Shaanxi University of Technology (grant no. SLGRCQD2313).
References
- 1.Lin F, Wang MY, Pan YP, Tang T, Cui DM, Liu B. 2017. Sequence and regularity controlled coordination copolymerization of butadiene and styrene: strategy and mechanism. Macromolecules 50, 849-856. ( 10.1021/acs.macromol.6b02413) [DOI] [Google Scholar]
- 2.Zhang S, et al. 2016. Engineering oriented hierarchical lamellar structures in SBS/PS blends via a pressure-induced flow field. RSC Adv. 6, 21 546-21 554. ( 10.1039/C5RA26979B) [DOI] [Google Scholar]
- 3.Vasilenko IV, Yeong HY, Delgado M, Ouardad S, Peruch F, Voit B, Ganachaud F, Kostjuket SV. 2015. A catalyst platform for unique cationic (co)polymerization in aqueous emulsion. Angew. Chem. Int. Ed. 54, 12 728-12 732. ( 10.1002/anie.201501157) [DOI] [PubMed] [Google Scholar]
- 4.Avci H, et al. 2020. Flexible poly(styrene-ethylene-butadiene-styrene) hybrid nanofibers for bioengineering and water filtration applications. J. Appl. Polym. Sci. 137, 49184. ( 10.1002/app.49184) [DOI] [Google Scholar]
- 5.Lee I, Bates FS. 2013. Synthesis, structure, and properties of alternating and random poly(styrene-b-butadiene) multiblock copolymers. Macromolecules 46, 4529-4539. ( 10.1021/ma400479b) [DOI] [Google Scholar]
- 6.Liu XF, Zhou T, Liu YC, Zhang AM, Yuan CY, Zhang WD. 2015. Cross-linking process of cis-polybutadiene rubber with peroxides studied by two-dimensional infrared correlation spectroscopy: a detailed tracking. RSC Adv. 5, 10 231-10 242. ( 10.1039/c4ra13502d) [DOI] [Google Scholar]
- 7.Wu CJ, Liu B, Lin F, Wang MY, Cui DM. 2017. cis-1,4-Selective copolymerization of ethylene and butadiene: a compromise between two mechanisms. Angew. Chem. Int. Ed. 56, 6975-6979. ( 10.1002/anie.201702128) [DOI] [PubMed] [Google Scholar]
- 8.Friebe L, Nuyken O, Windisch H, Obrecht W. 2003. In situ preparation of a compatibilized poly(cis-1,4-butadiene)/poly(ε-caprolactone) blend. Macromol. Mater. Eng. 288, 484-494. ( 10.1002/mame.200390045) [DOI] [Google Scholar]
- 9.Lanzi M, Paganin L, Di-Nicola FP, Trombini C. 2015. Effects of polar additives on the anionic polymerization of 1,3-butadiene and styrene. J. Polym. Res. 22, 208. ( 10.1007/s10965-015-0854-8) [DOI] [Google Scholar]
- 10.Pan YP, Rong WF, Jian ZB, Cui DM. 2012. Ligands dominate highly syndioselective polymerization of styrene by using constrained-geometry-configuration rare-earth metal precursors. Macromolecules 45, 1248-1253. ( 10.1021/ma202558g) [DOI] [Google Scholar]
- 11.Kim DH, Park SS, Park SH, Jeon JY, Kim HB, Lee Y. 2017. Preparation of polystyrene-polyolefin multiblock copolymers by sequential coordination and anionic polymerization. RSC Adv. 7, 5948-5956. ( 10.1039/C6RA25848D) [DOI] [Google Scholar]
- 12.Myers SB, Register RA. 2008. Block copolymers synthesized by ROMP-to-anionic polymerization transformation. Macromolecules 41, 5283-5288. ( 10.1021/ma800844g) [DOI] [Google Scholar]
- 13.Zhu H, Wu YX, Zhao JW, Guo QL, Huang QG, Wu GY. 2007. Styrene-butadiene block copolymer with high cis-1,4 microstructure. J. Appl. Polym. Sci. 106, 103-109. ( 10.1002/app.26528) [DOI] [Google Scholar]
- 14.Friebe L, Müller JM, Nuyken O, Obrecht W. 2007. Molar mass control by diethyl zinc in the polymerization of butadiene initiated by the ternary catalyst system neodymium versatate/ diisobutylaluminum hydride/ethylaluminum sesquichloride. J. Macromol. Sci. A 43, 11-22. ( 10.1080/10601320500405786) [DOI] [Google Scholar]
- 15.Tang ZW, Liang A, Liang HD, Zhao JW, Xu L, Zhang J. 2019. Reversible coordinative chain transfer polymerization of butadiene using a neodymium phosphonate catalyst. Macromol. Res. 27, 789-794. ( 10.1007/s13233-019-7105-5) [DOI] [Google Scholar]
- 16.Liu J, Min X, Zhu XZ, Wang ZC, Wang T, Fan XD. 2019. A new synthesis strategy on styrene-butadiene di-block copolymer containing high cis-1,4 unit via transfer of anionic to coordination polymerization. Polymers 11, 195. ( 10.3390/polym11020195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaita S, Hou ZM, Wakatsuki Y. 2001. Random- and block-copolymerization of 1,3-butadiene with styrene based on the stereospecific living system: (C5Me5)2Sm(μ-Me)2AlMe2/Al(i-Bu)3/ [Ph3C][B(C6F5)4]1. Macromolecules 34, 1539-1541. ( 10.1021/ma001609n) [DOI] [Google Scholar]
- 18.Jian ZB, Tang SJ, Cui DM. 2010. A lutetium allyl complex that bears a pyridyl-functionalized cyclopentadienyl ligand: dual catalysis on highly syndiospecific and cis-1,4-selective (co)polymerizations of styrene and butadiene. Chem. Eur. J. 16, 14 007-14 015. ( 10.1002/chem.201001634) [DOI] [PubMed] [Google Scholar]
- 19.Guo F, Meng R, Li Y, Hou ZM. 2015. Highly cis-1,4-selective terpolymerization of 1,3-butadiene and isoprene with styrene by a C5H5-ligated scandium catalyst. Polymer 76, 159-167. ( 10.1016/j.polymer.2015.08.060) [DOI] [Google Scholar]
- 20.Ban HT, Kase T, Kawabe M, Miyazawa A, Ishihara T, Hagihara H, Tsunogae Y, Murata M, Shiono T. 2006. A new approach to styrenic thermoplastic elastomers: synthesis and characterization of crystalline styrene-butadiene-styrene triblock copolymers. Macromolecules 39, 171-176. ( 10.1021/ma051576h) [DOI] [Google Scholar]
- 21.Gates D, Svejda S, Onate E, Killian C, Johnson L, White P, Brookhart M. 2000. Synthesis of branched polyethylene pressure (α-diimine)nickel(II) catalysts: influence of temperature, ethylene pressure, and ligand structure on polymer properties. Macromolecules 33, 2320-2334. ( 10.1021/ma991234+) [DOI] [Google Scholar]
- 22.Hu YM, Zhang CQ, Liu XG, Gao KK, Cao YM, Zhang CY, Zhang XQ. 2014. Methylaluminoxane-activated neodymium chloride tributylphosphate catalyst for isoprene polymerization. J. Appl. Polym. Sci. 131, 40153. ( 10.1002/app.40153) [DOI] [Google Scholar]
- 23.Li D, Ma FM, Guo LJ, Huang J, Zhang Y, Li F, Li CQ. 2021. Polynuclear (α-diimine) nickel(II) complex as catalyst for ethylene oligomerization. Appl. Organomet. Chem. 36, 6509. ( 10.1002/aoc.6509) [DOI] [Google Scholar]
- 24.Li GY, Zhang B, Yan J, Wang ZG. 2014. Micro- and mesoporous poly(schiff-base)s constructed from different building blocks and their adsorption behaviors towards organic vapors and CO2 gas. J. Mater. Chem. A 2, 18 881-18 888. ( 10.1039/C4TA04429K) [DOI] [Google Scholar]
- 25.Liang HY, Lin JH, Jia HN, Chen SL, Qi JL, Cao J, Lin TS, Fei WD, Feng JC. 2018. Hierarchical NiCo-LDH@NiOOH core-shell heterostructure on carbon fiber cloth as battery-like electrode for supercapacitor. J. Power Sources 378, 248-254. ( 10.1016/j.jpowsour.2017.12.046) [DOI] [Google Scholar]
- 26.Liu J, Zheng N, Min X, Liu JH, Li ZZ, Ji XH. 2021. Synthesis of butadiene/isoprene-styrene di-block copolymer with high cis-1,4 unit content based on a neodymium phosphate ester. RSC Adv. 11, 37 436-37 442. ( 10.1039/D1RA06923C) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L, Zhang KY, Nishiura M, Hou ZM. 2011. Chain-shuttling polymerization at two different scandium sites: regio- and stereospecific ‘One-Pot’ block copolymerization of styrene, isoprene, and butadiene. Angew. Chem. Int. Ed. 50, 12 012-12 015. ( 10.1002/anie.201104011) [DOI] [PubMed] [Google Scholar]
- 28.Ren CY, Li GL, Dong WM, Jiang LS, Zhang XQ, Wang FS. 2007. Soluble neodymium chloride 2-ethylhexanol complex as a highly active catalyst for controlled isoprene polymerization. Polymer 48, 2470-2474. ( 10.1016/j.polymer.2007.02.027) [DOI] [Google Scholar]
- 29.Lv K, Cui DM. 2010. CCC-pincer bis(carbene) lanthanide dibromides. Catalysis on highly cis-1,4-selective polymerization of isoprene and active species. Organometallics 29, 2987-2993. ( 10.1021/om1002039) [DOI] [Google Scholar]
- 30.Yang MJ, Cha CX, Shen ZQ. 1990. Polymerization of styrene by rare earth coordination catalystst. Polym. J. 22, 919-923. ( 10.1295/polymj.22.919) [DOI] [Google Scholar]
- 31.Ishihara N, Seimiya T, Kuramoto M, Uoi M. 1986. Crystalline syndiotactic polystyrene. Macromolecules 19, 2464-2465. ( 10.1021/ma00163a027) [DOI] [Google Scholar]
- 32.Liu FS, et al. 2009. Thermostable α-diimine nickel(II) catalyst for ethylene polymerization: effects of the substituted backbone structure on catalytic properties and branching structure of polyethylene. Macromolecules 42, 7789-7796. ( 10.1021/ma9013466) [DOI] [Google Scholar]
- 33.Johnson LK, Killian CM, Brookhart M. 1995. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-Olefins. J. Am. Chem. Soc. 117, 6414-6415. ( 10.1021/ja00128a054) [DOI] [Google Scholar]
- 34.Ye ZB, Xu LX, Dong ZM, Xiang P. 2013. Designing polyethylenes of complex chain architectures via Pd-diimine-catalyzed ‘living’ ethylenepolymerization. Chem. Commun. 49, 6235-6255. ( 10.1039/C3CC42517G) [DOI] [PubMed] [Google Scholar]
- 35.Coutinho FMB, Rocha TCJ, Mello IL, Nunes DSS, Soares BG, Costa MAS. 2005. Effect of electron donors on 1,3-butadiene polymerization by a Ziegler-Natta catalyst based on neodymium. J. Appl. Polym. Sci. 98, 2539-2543. ( 10.1002/app.22391) [DOI] [Google Scholar]
- 36.Ricci G, Sommazzi A, Masi F, Ricci M, Boglia A, Leone G. 2010. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coordin. Chem. Rev. 254, 661-676. ( 10.1016/j.ccr.2009.09.023) [DOI] [Google Scholar]
- 37.Mu HL, Li P, Song DP, Li YS. 2015. Neutral nickel catalysts for olefin homo- and copolymerization: relationships between catalyst structures and catalytic properties. Chem. Rev. 115, 12 091-12 137. ( 10.1021/cr500370f) [DOI] [PubMed] [Google Scholar]
- 38.Quisenberry KT, Smith JD, Voehler M, Stec DF, Hanusa TP, Brennessel WW. 2005. Trimethylsilylated allyl complexes of nickel. The stabilized bis(π-allyl)nickel complex [η3-1,3-(SiMe3)2C3H3]2Ni and its mono(π-allyl)NiX (X=Br, I) derivatives. J. Am. Chem. Soc. 127, 4376-4387. ( 10.1021/ja044308s) [DOI] [PubMed] [Google Scholar]
- 39.Li BT, Liu WH, Wu YX. 2012. Synthesis of long-chain branched isotactic-rich polystyrene via cationic polymerization. Polymer 53, 3194-3202. ( 10.1016/j.polymer.2012.04.030) [DOI] [Google Scholar]
- 40.Kumar MP, Singh MP, Srivastava C. 2015. Electrochemical behavior of Zn-graphene composite coatings. RSC Adv. 32, 25 603-25 608. ( 10.1039/C5RA02898A) [DOI] [Google Scholar]
- 41.Liu J, Zheng N, Li ZL, Liu Z, Wang GQ, Gui LS, Lin J. 2022. Fast self-healing and antifouling polyurethane/fluorinated polysiloxane-microcapsules-silica composite material. Adv. Compos. Hybrid Mater. 5, 1899-1909. ( 10.1007/s42114-022-00515-1) [DOI] [Google Scholar]
- 42.Zheng N, Liu J, Wang GQ, Yao P, Dang LH, Liu Z, Lu JF, Li WG. 2023. Robust UV/moisture dual curable PDMS-microcapsule-silica functional material for self-healing, antifouling, and antibacterial applications. Nano Res. 16, 7810-7819. ( 10.1007/s12274-023-5563-8) [DOI] [Google Scholar]
- 43.Zheng N, Liu J, Li Z, Lu J, Ni Y, Min X. 2023. Synthesis of block copolymer with cis-1,4-polybutadiene and isotactic-rich polystyrene using α-diimine nickel catalysts. Figshare. ( 10.6084/m9.figshare.c.6875398) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zheng N, Liu J, Li Z, Lu J, Ni Y, Min X. 2023. Synthesis of block copolymer with cis-1,4-polybutadiene and isotactic-rich polystyrene using α-diimine nickel catalysts. Figshare. ( 10.6084/m9.figshare.c.6875398) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are also provided in the electronic supplementary material [43].