Abstract
Atopic dermatitis (AD) is a common inflammatory skin disorder characterized by recurrent eczematous lesions and intense itching. The disorder affects people of all ages and ethnicities, has a substantial psychosocial impact on patients and relatives, and is the leading cause of the global burden of skin diseases. Atopic dermatitis is associated with an increased risk of multiple comorbidities, including food allergies, asthma, allergic rhinitis, and mental health disorders. The pathophysiology is complex and involves a strong genetic predisposition, epidermal dysfunction, and T-cell-driven inflammation. Although type-2 mechanisms are dominant, there is increasing evidence that the disorder involves multiple immune pathways. Until recently, the management of AD rested mainly on the judicious use of emollients, topical steroids, and topical calcineurin inhibitors in the majority of patients, and systemic immunosuppressants were advocated in severely diseased patients. However, in the last few years, new therapeutic strategies have been designed and developed to target the various steps in the chain of molecular events that lead to the AD phenotype. This review highlights the recent advancements in the management of AD.
Key words: Janus kinase inhibitors, alopecia areata, baricitinib, atopic dermatitis, eczema
Introduction
Atopic dermatitis (AD) is a common, chronic, relapsing, inflammatory skin disease that primarily affects young children. Atopy is defined as an inherited tendency to produce immunoglobulin (Ig) E antibodies in response to minute amounts of common environmental proteins such as pollen, house dust mites, and food allergens. Dermatitis derives from the Greek derma, which means skin, and itis, which means inflammation.1 Dermatitis and eczema are often used synonymously, although the term eczema is sometimes reserved for the acute manifestation of the disease (from Greek, ekzema, to boil over); here, no distinction is made. About 80% of disease cases typically start in infancy or childhood, with the remainder developing during adulthood. The disease displays high heterogeneity in its natural course, and individual trajectories are unpredictable.2 The onset of AD most commonly occurs between 3 and 6 months of age, with approximately 60% of children with AD presenting symptoms in the first 12 months.3
Consensus guidelines indicate that AD is characterized by essential features such as pruritus and eczema (acute, subacute, or chronic), with eczema lesions displaying typical morphology or age-specific patterns and having a chronic or relapsing history. The presence of these essential features in combination with an early age of onset, atopy, and xerosis supports a diagnosis of AD.4 Current evidence suggests that AD is a primary skin barrier defect that facilitates the development of other atopic conditions. In fact, AD is often the initial step in the “atopic march” (the sequential development of allergic disease manifestations during early childhood), which leads to asthma and/or allergic rhinitis in the majority of afflicted patients.5 Early AD may also be a causative factor in the development of food allergy.6 Furthermore, sleep deprivation in AD patients owing to pruritus increases the risk of developing attention-deficit/hyperactivity disorder, anxiety, and depression. 7 Based on the varying and alternating disease symptoms, four clinical phenotypes of AD were recently identified. These are, namely, early-onset transient, early-onset persistent, late-onset, and infrequent AD.8 AD can also be classified based on age, phenotype, and cytokine profile, such as pediatric and adult AD, extrinsic and intrinsic AD, and acute and chronic AD.9
The prevalence of AD varies widely across the globe due to regional, country-specific, age group, and data-capturing methodological differences, affecting 0.2% to 36% of the pediatric population (ages <18 years).10Whereas the point prevalence in children varies from 2.7% to 20.1% across countries, it ranges from 2.1% to 4.9% in adults.11 The high prevalence, high patient/caregiver burden, and increased healthcare utilization highlight the considerable public health burden associated with AD (Figures 1 and 2).12 The causes of atopic dermatitis are complex and multifactorial. There is a strong genetic component, with evidence for multiple mechanisms of genetic risk. Loss-of-function mutations in the gene encoding filaggrin (FLG) are the most strongly and consistently reported genetic variants, supporting a key role for the skin barrier, as filaggrin as a major structural protein in the epidermis. Many cohort studies on FLG mutations in AD have revealed that approximately 25-50% of AD patients harbor filaggrin mutations as a predisposing factor.13 Although genetics are clearly important in atopic dermatitis, the increasing global prevalence of the disorder highlights the role of environmental factors.
Some maternal exposures during pregnancy may predispose to an increased risk of AD in childhood, including maternal stress, cigarette smoke, antibiotic exposure, alcohol consumption, omega-3 long-chain polyunsaturated fatty acids, and probiotics. Skin exposures to irritants, pruritogens, and early life exposure to dirt and pathogens (hygiene hypothesis), including bacterial endotoxins, helminths, herpesvirus, farm animals, dogs, unpasteurized milk, etc., as well as outdoor and indoor air pollution and ultraviolet (UV) radiation exposure, increase the risk of AD.
Therapeutic strategies in atopic dermatitis
Currently, no complete cure is available for AD, and the only way to manage the disease is through palliative treatment. There are various substances to treat AD, from topical treatments and immunosuppressive agents to biologics, which are detailed below. The supportive therapies are aimed at avoiding secondary infections and improving skin integrity.14 Even though a guidelinebased treatment strategy is still not available for controlling AD, efficient clinical management usually involves basic management and step-up treatment (Figure 3).4
Education
For optimal disease management, patients and/or their caregivers should be educated about the chronic nature of the disease, the need for continued adherence to proper skin care practices, and the appropriate use and application of topical therapies. Poor treatment outcomes are often related to poor adherence, especially to topical therapies, resulting from irrational fears about adverse effects and insufficient information.15 It has been found that time invested in discussing these concerns and training patients and caregivers has a significant impact on the outcomes of the disease. For better understanding, patients may also receive detailed instructions/information on proper drug usage, skincare, and flare prevention.16
Figure 1.
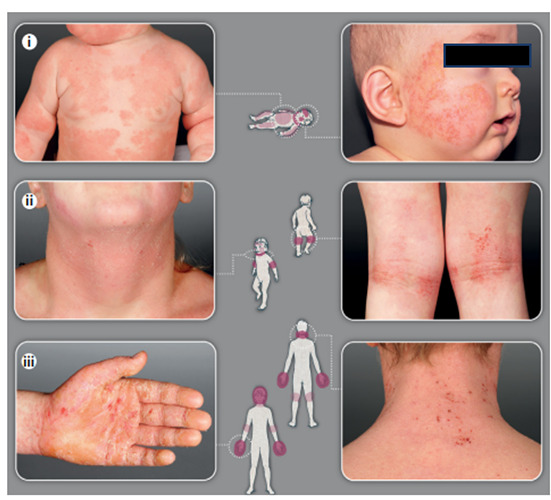
Typical clinical appearance and location of atopic dermatitis in infants.
Figure 2.
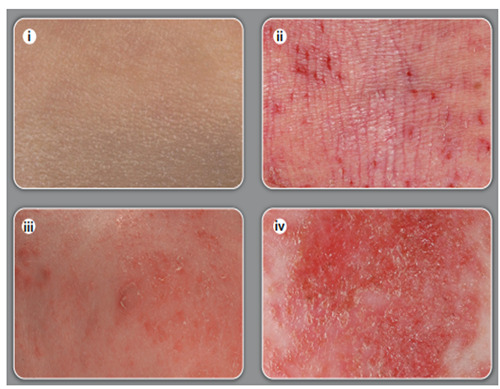
Close-up view of skin.
Figure 3.
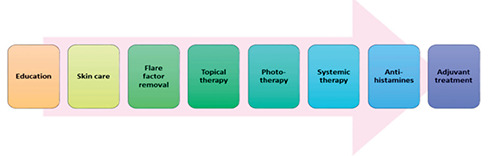
Treatment pattern of atopic dermatitis.
Skincare
A key feature of AD management is appropriate daily skin care. Although the frequency of bathing is somewhat controversial, most experts suggest daily bathing. Bathing once or twice daily (depending on the severity of AD) in warm water for 10-15 minutes is recommended to help hydrate and cleanse the skin, assist in the debridement of infected skin, and improve the penetration of topical therapies.17 A generous application of moisturizers or emollients is highly recommended for patients suffering from mild, moderate, or severe AD. Some evidence suggests that the persistent use of moisturizers from birth is a fruitful method to prevent the onset of AD in high-risk infants.18 This helps in controlling flare-ups and diminishing the associated pruritus and xerosis, hence reducing the need to use topical steroid preparations.19 Moisturization reduces trans-epidermal water loss, thus maintaining the skin moisture required for proper repair of the damaged skin barrier. These moisturizers may be delivered in the form of cream, lotion, or ointments, depending on the patient’s preference, site of application, climate, and extent of skin dryness.20
Topical anti-inflammatory therapies
Topical corticosteroids are the first-line pharmacologic treatment for AD. These agents effectively control atopic fares through their anti-inflammatory, antiproliferative, and immunosuppressive actions. Inappropriate use can cause local side effects, including skin atrophy, purpura, striae, telangiectasias, dyspigmentation, and facial acneiform changes.21 Systemic side effects from topical corticosteroids are considered very uncommon but can include hypothalamic-pituitary-adrenal suppression and growth retardation. 22 The corticosteroids were categorized by Niedner into category I (mild) to group IV (very potent) based on their potency.23
Mild and moderate corticosteroid creams are reserved for children, while adults can be treated with stronger preparations. Mild and moderate corticosteroids should be used chiefly for treating eczema on body sites where the skin is thin, notably in the face, axillae, groins, and anogenital area, whereas strong corticosteroids should be used for treating eczema on the rest of the body. Although only a small subset (those with severe disease) requires potent preparations, very strong preparations are rarely needed.1
On the other hand, tacrolimus and pimecrolimus are two nonsteroidal topical calcineurin inhibitors (TCIs) that do not have the long-term harmful effects of topical corticosteroids (TCSs) and can also safeguard the skin barrier weakened by prolonged use of topical steroids. However, these TCIs may cause momentary itching or burning sensations at the site of application.18While studies have suggested that 0.1% tacrolimus ointment in combination with TCSs is effective and safe for long-term treatment of moderate to severe AD in both children and adults, 1% pimecrolimus ointment is considered effective and safe for mild to moderate AD in both adults and children.24
Phototherapy
UV radiations act as antimicrobials and lessen Staphylococcus aureus colonization on atopic skin. UVA1 treatment has also revealed the immunosuppression of interleukin (IL)-5, IL-13, and IL-31.25 Recent research supports the importance of vitamin D during phototherapy in healing atopic lesions.26 Phototherapy for two weeks exhibited a marked change in the balance of vitamin D, with an enlarged level of serum calcidiol contributing to an effective cure for AD lesions. Reduction and functional loss of dendritic cells in the skin’s epidermal and dermal regions help immunosuppression through the source of UV light.27
Treatment of skin infections
Chronic AD conditions can lead to other complications such as bacterial, fungal, and viral superinfections. It is especially well established that AD skin lesions are very susceptible to colonization by S. aureus, which can further worsen the itch by inducing pruritus. Hence, short-term use of topical and/or antimicrobial therapies is considered the second line of treatment strategy since continued use of such medications may lead to antibacterial resistance. 28 While cephalosporin is an efficient antibiotic, the antiseptic properties of bleach also help to control S. aureus infections in AD patients; however, some clinicians prefer to also prescribe antibiotics with anti-inflammatory properties, such as doxycycline and co-trimoxazole. Superinfections caused by viruses such as the herpes simplex virus are often treated with antivirals such as acyclovir or its derivatives. Fungal infections such as those caused by M. furfur can be treated with antifungals containing azole agents either topically or systemically.18
Although first-generation antihistamines (e.g., hydroxyzine, diphenhydramine, and chlorpheniramine) do not directly affect the itching associated with AD, the sedative effects of these agents have been found to help improve sleep in patients with AD. However, these agents have been found to reduce rapid eye movement- sleep, impair learning, and reduce work efficiency and, therefore, are not routinely recommended for patients with AD. Non-sedating second-generation antihistamines appear to provide modest benefit in AD patients with allergic triggers, and, hence, a therapeutic trial of these agents may be considered in certain clinical situations.29
Systemic immunosuppressant treatments
Systemic corticosteroids should only be used in exceptional cases for short-term flare treatment or when starting another systemic therapy. The most commonly used conventional systemic treatments for atopic dermatitis are ciclosporin, methotrexate, azathioprine, and mycophenolate mofetil. However, robust long-term data on the effects of these drugs in atopic dermatitis are largely missing, and most trials remain placebo-controlled with no headto- head comparisons.30 Except for ciclosporin, which is approved for the treatment of patients aged 16 years and older in at least 15 European countries, Australia, and Japan, the use of these repurposed legacy immunosuppressants in atopic dermatitis is considered off-label.30 Ciclosporin has the most robust efficacy and safety data from randomized controlled trials, with a mean improvement in clinical severity scores of 55% from baseline after 6–8 weeks of treatment.31 It has a rapid onset of action, but end-organ toxicity and the cumulative risk of malignancy restrict long-term use. Therefore, most guidelines recommend continuous use for no longer than 1-2 years.32 Although robust long-term data are scant, azathioprine and methotrexate appear to be effective and fairly safe off-label treatments for severe atopic dermatitis, even in children. 33 Both drugs work slowly, with maximum benefits appearing after 4-8 weeks for azathioprine and 8-12 weeks for methotrexate. A small placebo-controlled trial showed a 37% improvement in mean disease activity with azathioprine compared with 20% for placebo at week 12.34
Emerging therapeutic biologics for atopic dermatitis
A summary of various therapeutic biologics and small molecules used to treat AD is reported in Figure 4.
Interleukin-4 and interleukin -13 inhibitors
Dupilumab is a monoclonal antibody that targets the IL-4 receptor a subunit, eventually blocking the signaling of IL-4 and IL-13, the two cytokines that play a key role in AD. It is a fully human, IgG4-based monoclonal antibody produced by the application of VelociGene technology.35 The efficacy of dupilumab in treating AD is supported by robust clinical data. Pruritus is significantly reduced by week 4, and improvement in the investigator global assessment scale is noticed by 12 weeks.36 The reduction in clinical severity was sustained throughout the study period and was associated with a high degree of patient satisfaction. A significant decrease in the incidence of skin infections in users of dupilumab was noted in the drug trials.37 This has been attributed to the positive effects of dupilumab on epidermal barrier function. A recent meta-analysis concluded that dupilumab is more efficacious in the initial 4 months of therapy in controlling adult AD than methotrexate or azathioprine and is comparable to cyclosporine given at a higher dose of more than 3 mg/kg/day.38
Advantages of dupilumab include a lack of need for close laboratory monitoring and an established long-term efficacy for over 52 weeks in conventional doses and for up to 148 weeks in adults who were put on a 300 mg weekly dose.39 In 2016, the Food and Drug Administration (FDA) granted dupilumab “breakthrough therapy” designation for the treatment of severe AD in children 6 months to 11 years of age, and later, in March 2019, the FDA approved the drug for the treatment of adolescents.40
Tralokinumab targets IL-13, a cytokine that is preferentially expressed in keratinocytes. Upregulation of IL-13 has been consistently documented in the lesional skin of AD patients. A phase 2a trial showed promising results in adults aged 12 years and older. Tralokinumab decreased the pruritus score within a week, and the improvement in the disease burden was sustained till the end of the study period.39
Lebrikizumab is a more selective inhibitor of IL-13, and the results from recently published trials are convincing.41 The data is shown in Table 1.
Treatment of atopic dermatitis: beyond targeting of interleukin-4/ interleukin-13
Nemolizumab is a human monoclonal antibody targeting IL- 31α subunit, which contributes to AD pathogenesis. IL-31 is responsible for causing pruritus in AD. It is expressed mainly by T helper (Th) 2 lymphocytes and functionally targets a variety of immune cells, such as basophils, eosinophils, and monocytes, along with epithelial cells and keratinocytes. Clinical trials have shown that nemolizumab has good efficacy in reducing pruritus and skin inflammation.42
Ustekinumab is a human IgG1 monoclonal antibody that inhibits IL-12 and IL-23 by blocking their common subunit p40 and thus significantly reduces Th1 and Th17/Th22 responses. It is commonly used to treat psoriasis by reducing skin inflammation. However, some phase II clinical trials show great efficacy of ustekinumab in the treatment of moderate to severe AD patients.43
Etokimab is a human IgG1 monoclonal antibody that inhibits IL-33, an important player in AD pathogenesis. IL-33 contributes to inflammation in AD through the IL-33/ST2 signaling pathway, thus initiating innate and adaptive type 2 responses. A phase II clinical trial with etokimab revealed that it effectively blocked IL- 33, which acted upstream of the inflammatory signaling cascade and hence modulated neutrophil migration directly and indirectly through inhibitory effects on the IL-8 pathway.44
Tezepelumab is an IgG2λ human monoclonal antibody targeting thymic stromal lymphopoietin (TSLP). Tezepelumab binds to the TSLP receptor and prevents the interaction with its receptor, thereby blocking all downstream inflammatory pathways. Phase II clinical trials with tezepelumab showed limited efficacy and statistically insignificant clinical improvement. Further studies with longer treatment periods may be required for better treatment effects.45 Omalizumab is a recombinant humanized monoclonal antibody that affects IgE antibodies. In a case study conducted by Holm and colleagues, out of nine omalizumab-treated AD patients, 50% revealed an excellent response, 12.5% displayed a moderate response, and 37.5% presented no effect to the treatment. 46
Table 1.
New therapeutic options in the future management of atopic dermatitis.
| No | Name | Target | Route of administration |
|---|---|---|---|
| 1 | Dupilumab | IL-4 α receptor | Subcutaneous |
| 2 | Tralokinumab | IL-13 | Subcutaneous |
| 3 | Lebrikizumab | IL-13 | Subcutaneous |
| 4 | Nemolizumab | IL-31 receptor A | Subcutaneous |
| 5 | Fezakinumab | IL-22 | Subcutaneous |
| 6 | Abrocitinib | JAK 1 | Oral |
| 7 | Upadacitinib | JAK 1 | Oral |
| 8 | Baricitinib | JAK 1 and 2 | Oral |
| 9 | Tofacitinib | JAK 1 and 3 | Oral, topical (ointment) |
| 10 | Ruxolitinib | JAK 1 and 2 | Topical (cream) |
| 11 | Deglocitinib | JAK 1, 2, 3 and TYK 2 | Topical (ointment) |
| 12 | Crisaborole | PDE 4 | Topical (ointment) |
| 13 | Tapinarof | Aryl hydrocarbon receptor | Topical (cream) |
IL, interleukin; JAK, Janus kinase; PDE, phosphodiesterase; TYK, tyrosine kinase.
Janus kinase inhibitors’ role in the management of atopic dermatitis
Baricitinib is a first-generation Janus kinase (JAK) 1/2 inhibitor. It has already been approved for the treatment of other inflammatory diseases, such as rheumatoid arthritis. Clinical trials of baricitinib for treating AD showed promising results in reducing AD clinical signs and relieving pruritus.47
Abrocitinib is a second-generation small molecule inhibitor that selectively inhibits JAK1 associated with receptor chains, which dimerizes on receptor activation to form receptor complexes. Selective inhibition of JAK1 helps regulate a wide range of inflammatory cytokines without causing adverse effects of JAK2 inhibition such as neutropenia and anemia. Phase II clinical trials with abrocitinib have shown promising results with rapid improvement of clinical symptoms of AD and pruritus and very low rates of adverse effects.48
Upadacitinib is a selective JAK1 inhibitor that was FDAapproved in 2019 for the treatment of rheumatoid arthritis. However, currently, phase III clinical trials are ongoing for the potential use of upadacitinib for treating moderate to severe AD. Phase II trials have shown promising results with good tolerability and low adverse effects, with an improvement in AD symptoms from the first week of treatment. The study also indicated good clinical efficacy endpoints and a significant reduction in pruritus.49
Gusacitinib is the first dual JAK/tyrosine kinase (SYK) inhibitor to undergo clinical trials in AD patients. Dual inhibition of JAK and SYK can widen the range of targeted cytokines and increase the clinical efficacy of JAK inhibition by resolving different AD subtypes. Clinical trials with gusacitinib have indicated good tolerability and a significant reduction in pruritus and inflammatory lesion count.50
The most prevalent (2-5% occurrence rate) treatment-emergent adverse events from oral JAK inhibitor use in AD were nausea, upper respiratory tract infection, headache, herpes zoster, herpes simplex, acne, increased blood creatine phosphokinase levels, and decreased platelet counts. Topical JAK inhibitors were not associated with systemic effects. All studies reported that JAK inhibitors were well tolerated in patients with AD in comparison with the control group. While the use of JAK inhibitors in patients suffering from AD is very promising, trials reported to date are of short duration (maximum 16 weeks), and more information on the long-term safety of these novel agents is required.51
Phosphodiesterase inhibitors’ role in atopic dermatitis management
Crisaborole is a low-molecular-weight molecule that permeates the skin and is well tolerated in adults and children with AD.52 It is an inhibitor of phosphodiesterase (PDE) 4, a cyclic adenosine monophosphate-degrading enzyme expressed in monocytes, lymphocytes, endothelial cells, and smooth muscle cells. Crisaborole may have a broader range of activity than topical calcineurin inhibitors. Improvement in disease severity was seen as early as day 8 of therapy with crisaborole. Application site pain following crisaborole was uncommon (4.4%). However, in a subsequent report, application site pain developed in 5 out of 10 adults who applied crisaborole over their faces.52
Figure 4.
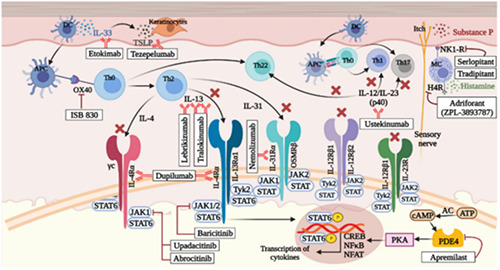
A summary of various therapeutic biologics and small molecules used to treat atopic dermatitis.
Apremilast is an oral PDE inhibitor that failed to show a clinically meaningful response in a recently conducted phase II trial in AD patients when used at a dose of 30 mg twice a day. Apremilast may be more effective in chronic AD, where Th1 and Th17 cells play a significant role.53
Tapinorof is an aryl hydrocarbon receptor modulator. It also has antioxidant activity. In a study done on patients aged 12 years and above, improvement was seen in 52% of patients using 1% tapinorof cream twice a day in comparison to 24% in the group applying vehicle alone.54,55
Conclusions
Despite being the most common among non-fatal skin diseases, the pathophysiology of AD is still poorly understood. Perspectives on this burdensome disease are continuously evolving with the advancement of our knowledge about the underlying molecular mechanisms.
It is quite evident that AD is not just a skin condition but extends beyond damaged sleep, disturbed social performances, and disruption to the overall development of the individual.
There have been noteworthy changes in the treatment landscape for AD in recent years. Dupilumab has been approved by the US FDA for use in children down to 6 years of age. Topical therapeutic options that have shown satisfactory results in children include deglocitinib, a pan-JAK inhibitor, and crisaborole, a PDE 4 inhibitor. Oral JAK inhibitors have shown promising results in studies done on adults with AD and will represent valuable additions to the treatment options for moderate to severe AD in the near future.
Funding Statement
Funding: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy 2014;2014:354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol 2021;126: 417-28.e2. [DOI] [PubMed] [Google Scholar]
- 3.Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol 1994;30:35-9. [DOI] [PubMed] [Google Scholar]
- 4.Eichenfeld LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014;70:338-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003;112:S118-27. [DOI] [PubMed] [Google Scholar]
- 6.Tsakok T, Marrs T, Mohsin M, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol 2016;137:1071-8. [DOI] [PubMed] [Google Scholar]
- 7.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013;131:428-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang EJ, Sekhon S, Sanchez IM, et al. Recent developments in atopic dermatitis. Pediatrics 2018;142:e20181102. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima S, Nomura T, Common J, Kabashima K. Insights into atopic dermatitis gained from genetically defined mouse models. J Allergy Clin Immunol 2019;143:13-25. [DOI] [PubMed] [Google Scholar]
- 10.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015;66:8-16. [DOI] [PubMed] [Google Scholar]
- 11.Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018;73:1284-93. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin 2017;35:283-9. [DOI] [PubMed] [Google Scholar]
- 13.Sandilands A, Terron-Kwiatkowski A, Hull PR, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 2007;39:650-4. [DOI] [PubMed] [Google Scholar]
- 14.Saini S, Pansare M. New insights and treatments in atopic dermatitis. Pediatr Clin North Am 2019;66:1021-33. [DOI] [PubMed] [Google Scholar]
- 15.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387:1109-22. [DOI] [PubMed] [Google Scholar]
- 16.Mandlik DS, Mandlik SK. Atopic dermatitis: new insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol Immunotoxicol 2021;43:105-25. [DOI] [PubMed] [Google Scholar]
- 17.Gittler JK, Wang JF, Orlow SJ. Bathing and associated treatments in atopic dermatitis. Am J Clin Dermatol 2017;18:45-57. [DOI] [PubMed] [Google Scholar]
- 18.Maliyar K, Sibbald C, Pope E, Sibbald RG. Diagnosis and management of atopic dermatitis: a review. Adv Skin Wound Care 2018;31:538-50. [DOI] [PubMed] [Google Scholar]
- 19.Sher LG, Chang J, Patel IB, et al. Relieving the pruritus of atopic dermatitis: a meta-analysis. Acta Derm Venereol 2012;92:455-61. [DOI] [PubMed] [Google Scholar]
- 20.Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol 1997;14:321-4. [DOI] [PubMed] [Google Scholar]
- 21.Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf 2015;38:493-509. [DOI] [PubMed] [Google Scholar]
- 22.Levin E, Gupta R, Butler D, et al. Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat 2014;25:501-6. [DOI] [PubMed] [Google Scholar]
- 23.Niedner R. Therapy with systemic glucocorticoids. Hautarzt 2001;52:1062-71. [Article in German]. [DOI] [PubMed] [Google Scholar]
- 24.Lübbe J, Friedlander SF, Cribier B, et al. Safety, efficacy, and dosage of 1% pimecrolimus cream for the treatment of atopic dermatitis in daily practice. Am J Clin Dermatol 2006;7:121-31. [DOI] [PubMed] [Google Scholar]
- 25.Dotterud LK, Wilsgaard T, Vorland LH, Falk ES. The effect of UVB radiation on skin microbiota in patients with atopic dermatitis and healthy controls. Int J Circumpolar Health 2008;67:254-60. [DOI] [PubMed] [Google Scholar]
- 26.Vähävihu K, Ylianttila L, Salmelin R, et al. Heliotherapy improves vitamin D balance and atopic dermatitis. Br J Dermatol 2008;158:1323-8. [DOI] [PubMed] [Google Scholar]
- 27.Grabbe J, Welker P, Humke S, et al. High-dose ultraviolet A1 (UVA1), but not UVA/UVB therapy, decreases IgE binding cells in lesional skin of patients with atopic eczema. J Invest Dermatol 1996;107:419-22. [DOI] [PubMed] [Google Scholar]
- 28.Huang JT, Abrams M, Tlougan B, et al. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123:808-14. [DOI] [PubMed] [Google Scholar]
- 29.Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol 2018;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014;133:429-38. [DOI] [PubMed] [Google Scholar]
- 31.Seger EW, Wechter T, Strowd L, Feldman SR. Relative efficacy of systemic treatments for atopic dermatitis. J Am Acad Dermatol 2019;80:411-6.e4. [DOI] [PubMed] [Google Scholar]
- 32.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet 2006;367:839-46. [DOI] [PubMed] [Google Scholar]
- 33.Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol 2017;77:623-33. [DOI] [PubMed] [Google Scholar]
- 34.Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit Type 2 inflammation. Allergy 2020;75:1188-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335-48. [DOI] [PubMed] [Google Scholar]
- 36.Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol 2020;21:567-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichenfield LF, Bieber T, Beck LA, et al. Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol 2019;20:443-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol 2020;156:659-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL- 13 mAb. J Allergy Clin Immunol 2019;143:135-41. [DOI] [PubMed] [Google Scholar]
- 40.Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020;83:1282-93. [DOI] [PubMed] [Google Scholar]
- 41.Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2B randomized clinical trial. JAMA Dermatol 2020;156:411-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deleanu D, Nedelea I. Biological therapies for atopic dermatitis: an update. Exp Ther Med 2018;17:1061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khattri S, Brunner P, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol 2017;26:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YL, Gutowska-Owsiak D, Hardman CS, et al. Proof-ofconcept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci Transl Med 2019;11:eaax2945. [DOI] [PubMed] [Google Scholar]
- 45.Parnes JR, Sullivan JT, Chen L, Dias C. Pharmacokinetics, safety, and tolerability of tezepelumab (AMG 157) in healthy and atopic dermatitis adult subjects. Clin Pharmacol Ther 2019;106:441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holm JG, Thomsen SF. Omalizumab for atopic dermatitis: evidence for and against its use. G Ital Deramatol Venereol 2019;154:480-7. [DOI] [PubMed] [Google Scholar]
- 47.Nezamololama N, Fieldhouse K, Metzger K, Gooderham M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020;9:2020-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-tosevere atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020;396:255-66. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol 2020;21:783-98. [DOI] [PubMed] [Google Scholar]
- 50.Pavel AB, Song T, Kim HJ, et al. Oral janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J Allergy Clin Immunol 2019;144:1011-24. [DOI] [PubMed] [Google Scholar]
- 51.Wood H, Chandler A, Nezamololama N, et al. Safety of janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol 2022;61:746-54. [DOI] [PubMed] [Google Scholar]
- 52.Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016;75:494-503.e6. [DOI] [PubMed] [Google Scholar]
- 53.Pao-Ling Lin C, Gordon S, Her MJ, Rosmarin D. A retrospective study: application site pain with the use of crisaborole, a topical phosphodiesterase 4 inhibitor. J Am Acad Dermatol 2019;80:1451-3. [DOI] [PubMed] [Google Scholar]
- 54.Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol 2019;139:1063-72. [DOI] [PubMed] [Google Scholar]
- 55.Peppers J, Paller AS, Maeda-Chubachi T, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol 2019;80:89-98.e3. [DOI] [PubMed] [Google Scholar]


