Abstract
Keverprazan, a novel potassium‐competitive acid blocker, was approved for treating acid‐related diseases. This study aimed to analyze the safety, pharmacokinetics (PKs) and pharmacodynamics (PDs) of multiple doses of keverprazan. This was a randomized, positive‐/placebo‐controlled, phase Ic trial. Twenty‐six healthy adults were randomized to receive 20 mg/day keverprazan (n = 8), 40 mg/day keverprazan (n = 8), placebo (n = 6), or 20 mg/day vonoprazan (n = 4) for 7 days. Safety, PK and PD assessments were conducted. In the keverprazan, vonoprazan, and placebo groups, adverse events (AEs) were reported in nine (56.25%), two (50.00%), and three (50.00%) subjects, respectively. AEs were mild except a moderate abdominal pain leading to withdraw. No serious AEs occurred. The plasma concentration‐time profiles of keverprazan showed rapid absorption (median time to maximum plasma concentration of 1.25–3.0 h). The terminal half‐life was 6.23 and 7.01 h for keverprazan 20 and 40 mg groups on day 7. The maximum plasma concentration was 43.1 and 93.2 ng/mL, respectively. There was no apparent accumulation of keverprazan and the major metabolite after 7‐day administration. The intragastric pH greater than 5 holding time ratios (HTRs) over 24 h postdose increased from 79.1%, 84.4%, and 84.5% on day 1 to 99.0%, 97.4%, and 100.0% on day 7 in the vonoprazan 20 mg and keverprazan 20 and 40 mg groups, respectively. The intragastric pH greater than 5 HTR of keverprazan reached a plateau at 20 mg. Keverprazan is well‐tolerable. A steady‐state in exposure was generally reached after 7 days of treatment. A dose of 20 mg/day keverprazan can elicit a significant, stable, and long‐lasting gastric acid inhibition effect.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Potassium‐competitive acid blockers (P‐CABs) act competitively and reversibly block the potassium ions from binding to the proton pump, and thus represent novel alternatives to proton pump inhibitors for managing the acid‐related diseases. Keverprazan is a novel P‐CAB designed based on the structure of vonoprazan, and was approved by China National Products Administration for the treatment of reflux esophagitis or duodenal ulcer in February 2023. There is no report on the pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of keverprazan.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study aimed to analyze the safety, tolerability, PKs, and PDs of multiple oral doses of keverprazan in healthy adult participants.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Keverprazan is well‐tolerable. A steady‐state in exposure was generally reached after 7 days of treatment. A dose of 20 mg/day keverprazan can elicit a significant, stable, and long‐lasting gastric acid inhibition effect.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study used vonoprazan as the positive drug control to analyze the PKs and PDs of multiple doses of keverprazan. The results could help improve our understanding of keverprazan for its clinical application in patients with acid‐related diseases.
INTRODUCTION
Acid‐related diseases (ARDs) are a group of diseases related to gastric acid secretion or sensitivity and mainly include gastroesophageal reflux disease (GERD), gastric and duodenal ulcers, and nonsteroidal anti‐inflammatory drug‐associated peptic ulcer disease. 1 , 2 , 3 , 4 ARDs have significant impacts on patient quality of life because of the high prevalence rates and chronic nature. 1 , 2 , 3 , 4 , 5
The introduction of proton pump inhibitors (PPIs) has significantly improved the management of ARDs, as PPIs irreversibly inhibit the H+/K+‐ATPase, the final step of gastric acid secretion, therefore increasing intragastric pH and alleviating acidity‐related symptoms. 6 Unfortunately, PPIs are prodrugs that require activation in an acid environment, and they must be taken before a meal. Furthermore, their efficacy at night is limited, 6 , 7 , 8 and a significant proportion of patients with GERD (about 40%) fail to respond to PPIs. 9 The nondurable and unstable efficacy can be explained, at least in part, by the relatively short half‐life of PPIs. 9
Potassium‐competitive acid blockers (P‐CABs) are showing promise in clinical practice, and offering potential benefits over traditional PPIs in the management of ARDs. 10 , 11 , 12 Contrary to PPIs, P‐CABs are not prodrugs that require acid activation and are unaffected by meals. 13 , 14 In addition, they display more favorable pharmacokinetics (PKs) than PPIs. 15 Vonoprazan, a P‐CAB currently approved for treating reflux esophagitis in Japan and China, demonstrates enhanced durability and stability in its efficacy when compared to a conventional PPI, lansoprazan. 16 Vonoprazan has also shown superior results in healing and maintaining the erosive esophagitis, especially in severe cases. 17 This aspect is crucial for patients suffering from this condition as it contributes to the alleviation of symptoms and potentially prevents complications. Furthermore, a systematic review involving thousands of participants demonstrated vonoprazan's superiority over PPIs in eradicating H. pylori and treating erosive esophagitis and comparable performance for other ARDs. 18 These collective findings underscore the potential of P‐CABs in the clinical management of ARDs.
Keverprazan is a novel P‐CAB designed based on the structure of vonoprazan by exploring its structure–activity relationships and demonstrates better water solubility compared with vonoprazan at the same doses. 19 Keverprazan has a high distribution in the stomach. A preclinical study indicated that the acid suppression effect of keverprazan was comparable with vonoprazan. 20 A completed clinical trial (yet unpublished data; ChiCTR2100050171) reported that a single dose of 20 mg keverprazan showed similar time to reach the peak concentration (T max; 1.50 vs. 1.50 h) and elimination half‐life (t 1/2; 6.75 vs. 5.8 h) to 20 mg vonoprazan, as reported in a previous trial. 16 Furthermore, the percentages of time of intragastric pH greater than 3 (pH >3 holding‐time ratio [HTR]), pH greater than 4 (pH >4 HTR), and pH greater than 5 (pH >5 HTR) over 24 h after a single dose of 20 mg keverprazan were above 80%, and the night‐time intragastric pH greater than 3, pH greater than 4, and pH greater than 5 HTRs were above 95%. The effect of inhibiting gastric acid secretion of keverprazan was stable, durable, and superior to lansoprazole 30 mg. Still, the PK and pharmacodynamic (PD) results of multiple doses of keverprazan remained unreported.
Therefore, this study aimed to analyze the safety, tolerability, PK, and PD characteristics of multiple oral doses of keverprazan in healthy adult participants. The results could help improve our understanding of keverprazan for its clinical application in patients with ARDs.
METHODS
Study design
This randomized, double‐blind, positive‐/placebo‐controlled, phase Ic clinical trial was conducted to analyze the safety, tolerability, PK, and PD of multiple oral doses of keverprazan in healthy Chinese subjects. Considering that lansoprazole was used as the positive control drug in the previous single ascending‐dose study of keverprazan (#ChiCTR2100050171), the results indicated that keverprazan produced longer half‐life, and stable and lasting inhibition efficacy of intragastric acidity when compared with lansoprazole (S. Zhou, L. Xie, C. Zhou, Y. Zhao, L. Wang, S. Ding, J. Chen, B. Zhu, M. Su, and F. Shao, unpublished data). Meanwhile, keverprazan was designed based on the structure of vonoprazan (a P‐CAB). To obtain the PK and PD properties of keverprazan compared to P‐CABs, vonoprazan was selected as the positive control drug in this multiple‐dose study. This study was performed at the Phase I Clinical Trial Unit, the First Affiliated Hospital with Nanjing Medical University (Nanjing, China). It was conducted in compliance with the Good Clinical Practice Guideline, the ethical principles stated in the Declaration of Helsinki, and local applicable regulations or laws. The study protocol, amendments, informed consent forms, and other study‐related documents were reviewed and approved by the Medical Ethics Committee of the hospital. This study was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn/searchproj.aspx; #ChiCTR2100050136). All participants provided written informed consent prior to any study procedure.
Subjects
Healthy male and female Chinese subjects aged 18–45 years, with body mass index of 18.00–26.00 kg/m2 and body weight greater than or equal to 50 kg for men and greater than or equal to 45 kg for women were eligible to participate in this study. Their health status was determined based on medical history, physical examination, laboratory tests, and 12‐lead electrocardiogram (ECG). In addition, they were required to take effective contraceptive measures within 3 months after the first dosing. Women of childbearing age had to get negative serum pregnancy tests at screening, on the day prior to dosing, and discharge.
Subjects were excluded if they had any medical history or diseases which may affect the safety or PK assessments, such as hepatic or renal dysfunction, gastrointestinal/rectal bleeding, gastric or duodenal ulcer, persistent nausea, inflammatory bowel disease, gastrointestinal tract surgeries affecting gastric acid secretion, difficulty in emptying, or urinary tract obstruction. Other exclusion criteria included known allergy or hypersensitivity to any components of the study drug, vonoprazan, or PPIs, positive test results for HBsAg, hepatitis C virus, or HIV antibodies, family history of long QT syndrome, ingestion of food and beverages known to induce or inhibit hepatic drug metabolism within 48 h before dosing, ingestion of food and beverages known to induce or inhibit hepatic drug metabolism (such as CYP3A4) within 48 h before dosing, administration of any over‐the‐counter medication within 2 weeks or any prescription within 4 weeks before dosing, smoking more than 10 cigarettes/day or alcohol abuse within 3 months before the study, donation, or loss of 400 mL or more blood volume within 3 months prior to screening, participation in any clinical trials within 3 months before the study, or illegal drug use or abuse within 12 months prior to the study.
Study flow
A total of 26 participants were randomly assigned to two cohorts (20 and 40 mg), and each cohort comprised 13 subjects. The eligible subjects were sequentially block‐randomized to keverprazan, matching placebo or 20 mg of vonoprazan (Takeda Pharmaceutical Company Limited) at a ratio of 8:3:2 under fasting conditions. The dose of the study drug was escalated to 40 mg only if the safety, tolerability, PK and PD data of the 20 mg group were considered acceptable after evaluation.
Subjects were screened from day ‐14 to ‐3 and admitted to the Phase I Clinical Unit for baseline procedures on day ‐2, 2 days prior to drug administration. On the day of baseline PD evaluation (day ‐1), all subjects were requested to maintain the same time schedule during the study (eating, drinking water, and work and rest). After more than 10 h overnight fast, the corresponding drug were administered to subjects at 8:00 a.m. with 250 mL of water for 7 consecutive days. Specific volume of water and standard meals were given at designated times postdose. Subjects were not allowed to lie down within 2 h after dosing, except assessments were required. After completion of the PK and safety assessments on the morning of day 9, subjects were discharged and returned to the Phase I Clinical Unit on day 15–17 for follow‐up.
PK assessments
Sample collection for PK analysis
Blood samples for PK analysis were collected within 0.5 h before drug administration on the first day, 0.25, 0.5, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, and 24 h (i.e., before drug administration on day 2) after drug administration on day 1, within 0.5 h before drug administration on days 4, 5, and 6, within 0.5 h before drug administration on day 7, and 0.25, 0.5, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h after drug administration on day 7. At each blood sampling point, 4 mL of blood was collected into tubes containing K2EDTA and centrifuged at 1500 g for 10 min at 4°C. Then, plasma was stored at −70°C until analysis. Plasma concentrations of keverprazan, its main metabolite M9, and vonoprazan were analyzed using high performance liquid chromatography‐tandem mass spectrometry methods. The methods were validated by Beijing Scinovo Laboratories Ltd. in accordance with the method validation guidance of National Medical Products Administration. The standard curves were linear over the concentration ranges of 0.05–5000 ng/mL for keverprazan, 0.15–15,000 ng/mL for M9, and 0.25–40 ng/mL for vonoprazan in plasma, respectively.
PK analysis
PK parameters were calculated with noncompartmental methods using Phoenix WinNonlin software (version 8.0; Pharsight Corporation). The peak plasma concentration after administration on day 1 (C max), steady‐state trough concentration (C SS(min)), peak steady‐state concentration (C SS(max)), time to reach the minimum concentration (Tmin), and T max were taken directly from the observed data. The average value of the steady‐state concentration (C AVSS) was calculated as area under the curve (AUC)ss/τ (τ = 24). The degree of fluctuation was calculated as (C SS(max)−C SS(min))/C AVSS. The t 1/2 values were calculated as ln2/λ z using the best fit mode, where λ z was the terminal elimination rate constant. The area under the concentration‐time curve from time zero to the last measurable timepoint (AUC0–t ) was estimated using the linear trapezoidal method and AUC from time zero to infinity (AUC0–∞) was calculated as AUC0–t + C t/λ z, where C t was the last measured concentration. Apparent total body clearance (CL/F) after oral dosing and apparent volume of distribution in terminal phase were estimated as dose/AUC0–∞ and CL/λ z, respectively. R AUC0–24h and R Cmax were calculated as AUCss/AUC0–24h, day1 and C SS(max)/C max, day1, respectively.
PD assessments
PD analysis was based on intragastric pH monitoring which was performed continuously for 24 h on day ‐1 (baseline), day 1, and day 7 using an ambulatory pH monitor (Ohmega, MMS). According to the PH change, a disposable single‐use pH probe connected with the monitor was inserted intranasally into the stomach and fixed at about 10 cm below the lower esophageal sphincter. Before insertion, the electrode of probe was activated in buffer solution (pH 7) and calibrated with standard buffers at pH 7.01 and pH 1.07 at room temperature. Intragastric pH was automatically recorded every 1 s (1 Hz) and the recorded data (the average pH in each 1‐min interval) were uploaded to a dedicated computer after completing the monitoring.
For PD analysis, the intragastric pH greater than 3, pH greater than 4, and pH greater than 5 HTRs over the 24 h postdose on day 1 and day 7 were calculated. Additionally, the night‐time pH HTRs were calculated and defined as the percentage of time pH greater than 3, pH greater than 4, and pH greater than 5 during the period 12–24 h postdose on day 1 and day 7.
Safety monitoring
Safety monitoring included adverse events (AEs), vital signs, physical examination, laboratory tests (blood routine, urine routine, blood biochemistry, coagulation tests, urinary β2‐microglobulin, serum human chorionic gonadotropin, ECG, fundus examination, and concomitant treatment). All participants underwent safety assessments from baseline to 48 h after the last administration, and were followed for safety during 8–10 days after the last administration. In cases where abnormal and clinically significant results were found, we closely monitored the subjects until the observed AEs returned to normal, baseline level, or a stable state.
All AEs were coded based on their System Organ Classes and Preferred Terms in the Medical Dictionary for Regulatory Activities (version 20.0). Treatment‐related adverse events (TRAEs) were defined as AEs that started or worsened after the first administration of the study drug.
Statistical analysis
The safety analysis set included all subjects who received at least one of the study drugs. Descriptive statistics using mean and standard deviation (SD) or number and percentage were performed in safety assessments and subject demographics.
The PK analysis set comprised subjects who received at least one of the study drugs and provided sufficient data to calculate greater than or equal to one reliable PK parameter(s). The PK parameters were calculated based on the PK analysis set and were expressed as mean and SD or median and range (T max). The PD analysis set comprised subjects who received at least one of the study drugs and who had sufficient intragastric pH data. Mean intragastric pH greater than 3, pH greater than 4, and pH greater than 5 HTRs over 24 h, as well as the mean night‐time pH greater than 3, pH greater than 4, and pH greater than 5 HTRs on day 1 and day 7 were calculated using descriptive statistics in PD analysis set. Furthermore, correlations of the HTR values with C max and AUC0–24h on day 1 and day 7 were plotted to analyze the PK/PD relationships.
RESULTS
Characteristics of the participants
A total of 26 healthy Chinese subjects were enrolled and randomized to receive keverprazan 20 mg/day (n = 8) or 40 mg/day (n = 8), matching placebo (n = 6), or vonoprazan 20 mg/day (n = 4). One participant in the keverprazan 20 mg group withdrew the study on day 6 and did not receive the last dose due to an AE. The other subjects completed the study according to protocol. All 26 subjects were included in the safety and PD analysis, and 20 of them who received keverprazan or vonoprazan were included in the PK analysis (Figure 1). The demographic characteristics among groups were balanced. Two participants each in the keverprazan 40 mg and placebo groups had a surgical history. There were no clinically significant differences in demographic characteristics among the groups (Table 1).
FIGURE 1.
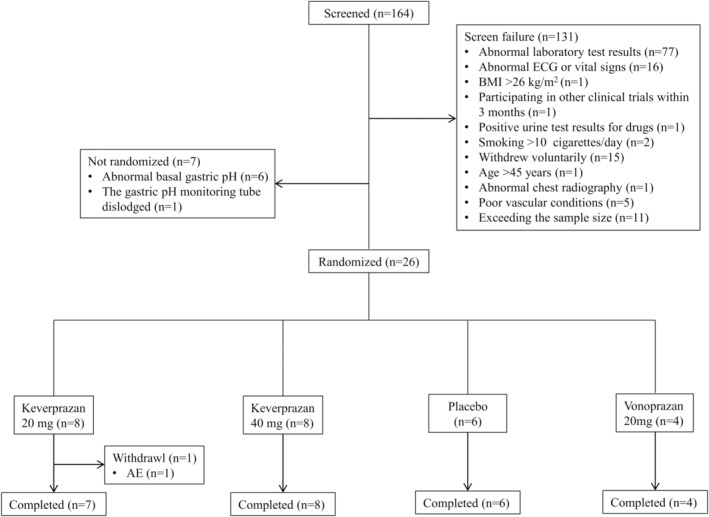
Enrollment flowchart. AE, adverse event; BMI, body mass index; ECG, echocardiogram.
TABLE 1.
Baseline subject demographic characteristics.
| Characteristics | Keverprazan 20 mg | Keverprazan 40 mg | Vonoprazan 20 mg | Placebo |
|---|---|---|---|---|
| N | 8 | 8 | 4 | 6 |
| Age, years | 22.63 ± 4.17 | 28.88 ± 4.22 | 26.00 ± 4.08 | 30.67 ± 2.07 |
| Gender, male | 4 (50.0%) | 4 (50.0%) | 2 (50.0%) | 2 (33.3%) |
| Height, cm | 167.15 ± 6.81 | 165.20 ± 7.09 | 165.03 ± 10.92 | 166.27 ± 11.63 |
| Weight, kg | 64.57 ± 7.51 | 60.18 ± 7.59 | 60.50 ± 9.13 | 60.18 ± 8.85 |
| BMI, kg/m2 | 23.10 ± 2.28 | 22.04 ± 2.28 | 22.12 ± 1.12 | 21.70 ± 1.43 |
| Surgical history | 0 | 2 (25.0%) | 0 | 2 (33.3%) |
Abbreviation: BMI, body mass index.
Safety and tolerability
Keverprazan appeared to be safe and well‐tolerated, with no deaths or serious adverse events (SAEs) (Table 2). Only one (12.50%) subject in the keverprazan 20 mg group withdrew from the study due to abdominal pain on day 6, which was moderate and relieved after taking levofloxacin 0.5 g once daily for 2 days.
TABLE 2.
Safety profile.
| AE | Keverprazan 20 mg | Keverprazan 40 mg | Vonoprazan 20 mg | Placebo |
|---|---|---|---|---|
| N | 8 | 8 | 4 | 6 |
| Any | 3 (37.50%) | 6 (75.00%) | 2 (50.00%) | 3 (50.00%) |
| TRAE | 2 (25.00%) | 2 (25.00%) | 2 (50.00%) | 3 (50.00%) |
| SAE | 0 | 0 | 0 | 0 |
| AE leading to study discontinuation | 1 (12.50%) | 0 | 0 | 0 |
Abbreviations: AE, adverse event; SAE, severe adverse event; TRAE: treatment‐related adverse event.
A total of 36 AEs occurred in 14 (53.85%) subjects during the trial, including 22 AEs in nine (56.25%) subjects in the keverprazan group, six AEs in two (50%) subjects in the vonoprazan group, and eight AEs in three (50%) subjects in the placebo group. Among them, 19 AEs reported by nine subjects were considered to be possibly, probably, or definitely related to the study drug. Except the aforementioned moderate abdominal pain, all the other AEs were mild and recovered to normal upon the completion of the trial without any intervention or dose reduction or discontinuation. The preferred terms of the AEs are listed in Table S1.
PK parameters
Figure 2 shows the mean concentration‐time curves for keverprazan, M9, and vonoprazan after multiple oral doses of keverprazan 20/40 mg or vonoprazan 20 mg. The corresponding PK parameters are summarized in Table 3. Following administration of multiple oral doses of keverprazan 20 or 40 mg, the plasma concentration‐time profiles of keverprazan showed rapid absorption. The plasma concentrations of keverprazan peaked between 1 and 3 h postdose, and then declined in a monophasic manner with a mean t 1/2 of 6.23–7.01 h. The steady‐state conditions were achieved after 1 week of daily dosing of keverprazan or vonoprazan as indicated by the trough concentrations (Figure S1). Keverprazan 20/40 mg groups revealed similar T max (1.25–3 vs. 1.5–2 h), similar t 1/2 (6.23–7.01 vs. 6.27–6.52 h), higher C max (43.1–93.2 vs. 28.8–59.3 ng/mL), and higher AUC (361–1131 vs. 230–512 h ng/mL) on day 7 compared with those on day 1 (Table 3). The higher C max and AUC in keverprazan 20 mg group compared with vonoprazan 20 mg group was observed on day 1 and day 7. The major metabolite of keverprazan in plasma was M9, demonstrating a mean exposure ratio of M9 to the parent drug in plasma of 7.65–7.76 on day 1 and 4.99–5.63 on day 7 in the keverprazan 20/40 mg groups, respectively. R AUC0–24h and R Cmax of keverprazan and M9 indicated no apparent accumulation in human bodies after consecutive dosing for 7 days.
FIGURE 2.
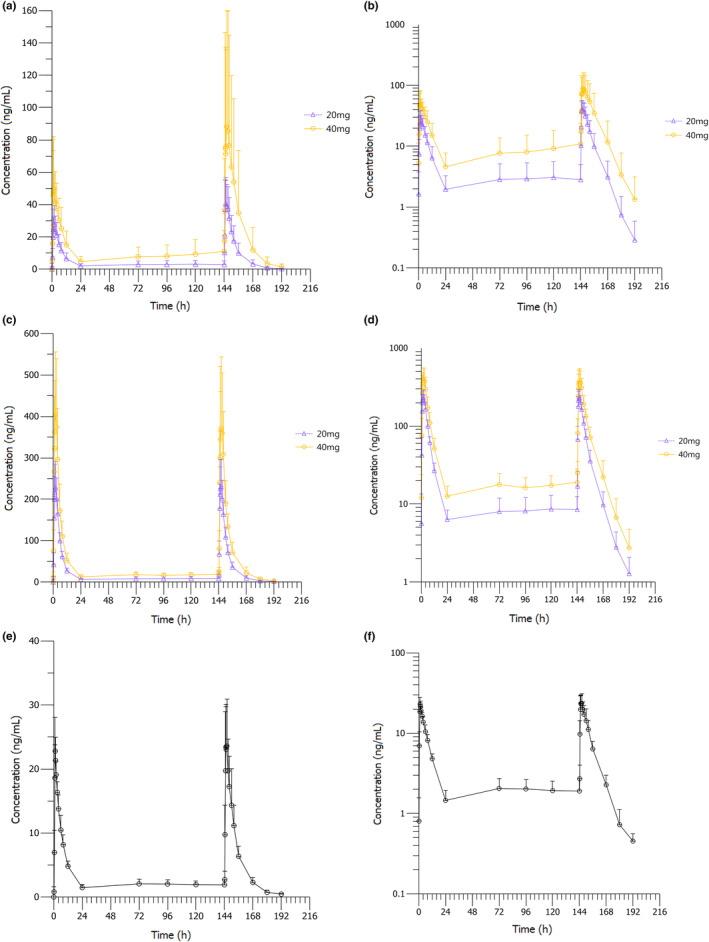
Mean concentration‐time curves of keverprazan, M9, and vonoprazan in the plasma after multiple oral doses of keverprazan 20/40 mg or vonoprazan 20 mg. (a) Keverprazan on a linear scale. (b) Keverprazan on a semilogarithmic scale. (c) M9 on a linear scale. (d) M9 on a semilogarithmic scale. (e) Vonoprazan 20 mg on a linear scale. (f) Vonoprazan 20 mg on a semilogarithmic scale.
TABLE 3.
PK parameters of keverprazan, M9, and vonoprazan on day 1 and day 7 upon consecutive drug administration for 7 days.
| Parameters | Keverprazan | M9 | Vonoprazan | ||
|---|---|---|---|---|---|
| 20 mg | 40 mg | 20 mg | 40 mg | 20 mg | |
| N | 8 | 8 | 8 | 8 | 4 |
| Day 1 | |||||
| t 1/2, h | 6.27 ± 1.21 | 6.52 ± 1.10 | 4.77 ± 0.798 | 5.21 ± 1.00 | 6.47 ± 1.17 |
| T max, h | 2.00 (1.25, 2.00) | 1.50 (1.00, 3.00) | 2.00 (1.50, 3.00) | 2.00 (2.00, 3.00) | 1.25 (1.25, 1.50) |
| C max, ng/mL | 28.8 ± 11.8 | 59.3 ± 31.5 | 238 ± 65.3 | 419 ± 170 | 23.2 ± 4.63 |
| AUC0–24h, h·ng/mL | 230 ± 100 | 467 ± 235 | 1462 ± 297 | 2634 ± 903 | 168 ± 21.9 |
| AUC0–∞, h·ng/mL | 249 ± 114 | 512 ± 268 | 1507 ± 302 | 2732 ± 905 | 182 ± 26.1 |
| V z/F, L | 849 ± 390 | 967 ± 707 | — | — | 1030 ± 158 |
| CL/F, L/h | 96.1 ± 45.4 | 101 ± 59.5 | — | — | 112 ± 17.4 |
| AUC0–t_M9/AUC0–t_ keverprazan | — | — | 7.76 ± 4.50 | 7.65 ± 5.89 | — |
| Day 7 | |||||
| t 1/2, h | 6.23 ± 0.776 | 7.01 ± 1.10 | 7.21 ± 0.828 | 7.32 ± 0.918 | 7.71 ± 1.70 |
| T max, h | 1.25 (1.00, 3.00) | 3.00 (1.00, 3.00) | 2.00 (1.25, 2.00) | 2.00 (1.50, 3.00) | 1.63 (1.25, 3.00) |
| C max, ng/mL | 43.1 ± 14.9 | 93.2 ± 71.2 | 232 ± 67.0 | 392 ± 163 | 27.2 ± 6.59 |
| T min, h | 10.3 ± 12.8 | 9.00 ± 12.4 | 3.43 ± 9.07 | 6.0 ± 11.1 | 6.00 ± 12.0 |
| C min, ng/mL | 2.94 ± 2.39 | 10.7 ± 13.0 | 8.80 ± 4.09 | 18.5 ± 9.60 | 1.89 ± 0.811 |
| C AVSS | 15.0 ± 7.19 | 41.6 ± 40.4 | 67.9 ± 15.3 | 121 ± 35.4 | 9.02 ± 2.20 |
| AUC0–24h, h·ng/mL | 361 ± 173 | 998 ± 971 | 1631 ± 366 | 2915 ± 850 | 216 ± 52.8 |
| AUC0–t , h·ng/mL | 390 ± 198 | 1117 ± 1116 | 1730 ± 398 | 3145 ± 905 | 239 ± 60.4 |
| AUC0–∞, h·ng/mL | 393 ± 201 | 1131 ± 1137 | 1744 ± 405 | 3175 ± 915 | 244 ± 62.4 |
| V z/F, L | 609 ± 333 | 656 ± 428 | — | — | 1056 ± 251 |
| CLss/F, L/h | 69.5 ± 39.3 | 66.3 ± 40.3 | — | — | 96.9 ± 24.8 |
| DF, % | 287 ± 56.1 | 230 ± 59.5 | 327 ± 60.3 | 302 ± 76.7 | 287 ± 68.8 |
| R AUC0–24h | 1.51 ± 0.447 | 1.94 ± 0.849 | 1.10 ± 0.104 | 1.13 ± 0.171 | 1.28 ± 0.194 |
| R Cmax | 1.51 ± 0.637 | 1.59 ± 0.684 | 0.970 ± 0.0539 | 0.935 ± 0.0917 | 1.17 ± 0.0581 |
| AUC0‐t_M9/AUC0‐t_keverprazan | — | — | 5.63 ± 3.60 | 4.99 ± 3.69 | — |
Abbreviations: AUC, area under the concentration curve; C AVSS, average value of the steady‐state concentration; CL/F, apparent total body clearance; CLss/F, oral clearance; C max, peak concentration; C min, trough concentration; DF, degree of fluctuation; R, accumulation ratio; t 1/2, elimination half‐life; T max, time to reach C max; T min, time to reach C min; V z/F, volume of distribution.
PD parameters
Figure 3 shows the 24‐h mean intragastric pH‐time curves on day 1 and day 7 after administering 20 mg keverprazan, 40 mg keverprazan, 20 mg vonoprazan, and placebo. During baseline, the intragastric pH was influenced by diet and sharply increased after meals between 4 h and 10 h, then gradually decreased. After administration of keverprazan 20/40 mg and vonoprazan 20 mg on day 1, the intragastric pH values rose substantially and rapidly, which were significantly higher than the placebo group. The pH values remained relatively constantly high over 24 h in the keverprazan groups but showed more variations in the vonoprazan group during 14–18 h. On day 7, the pH remained relatively constantly high over 24 h in the keverprazan and vonoprazan groups.
FIGURE 3.
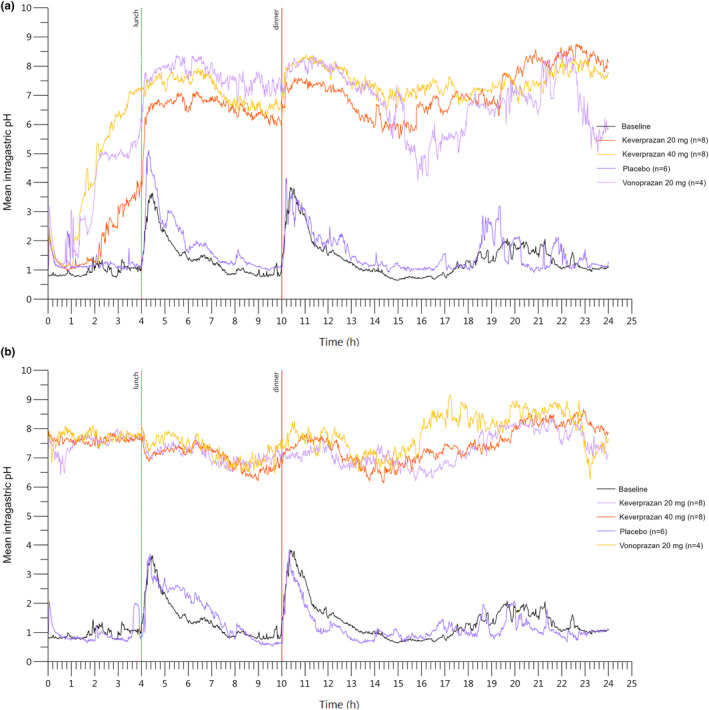
Mean intragastric pH‐time curves on day 1 (a) and day 7 (b) after the administration of 20 mg keverprazan, 40 mg keverprazan, 20 mg vonoprazan, or placebo.
Table 4 shows the intragastric pH greater than 3, pH greater than 4, and pH greater than 5 HTRs over the 24 h postdose and during the night‐time period on day 1 and day 7 after the administration of 20 mg keverprazan, 40 mg keverprazan, 20 mg vonoprazan, and placebo. The intragastric pH greater than 3, pH greater than 4, and pH greater than 5 HTRs were similar (near or above 80%) among the keverprazan 20/40 mg and vonoprazan 20 mg groups on day 1, which were all increased to similar levels (above 95%) on day 7. In particular, the intragastric pH greater than 5 HTRs in the treatments of placebo, 20 mg vonoprazan, and 20/40 mg keverprazan groups were 3.1 ± 2.3%, 79.1% ± 15.4%, 84.4% ± 2.8%, and 84.5% ± 19.8% on day 1, respectively, and the corresponding values were 3.2% ± 3.6%, 99.0% ± 1.8%, 97.4% ± 6.0%, and 100.0% ± 0.0% on day 7, respectively. Notably, the intragastric pH greater than 5 HTRs during night‐time were numerically higher in the keverprazan 20/40 mg groups compared with the vonoprazan 20 mg group on day 1 (99.7% ± 0.6% vs. 93.6% ± 17.9% vs. 82.4% ± 22.4%). Nevertheless, the differences seemed to disappear on day 7 (95.3% ± 11.4% vs. 99.9% ± 0.0% vs. 98.1% ± 3.7%).
TABLE 4.
Mean intragastric pH >3, >4, and >5 HTRs (%) during the 24 h and during the night‐time period (12–24 h postdose) following multiple oral doses of keverprazan 20/40 mg, vonoprazan 20 mg, or placebo on day 1 and day 7 in healthy Chinese subjects.
| Parameters | Day 1 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 6) | Vonoprazan 20 mg (n = 4) | Keverprazan 20 mg (n = 8) | Keverprazan 40 mg (n = 8) | Placebo (n = 6) | Vonoprazan 20 mg (n = 4) | Keverprazan 20 mg (n = 8) | Keverprazan 40 mg (n = 8) | |
| 24 h pH >3 HTR % | 9.4 ± 2.7 | 85.6 ± 9.2 | 85.5 ± 3.0 | 87.6 ± 12.5 | 7.6 ± 6.2 | 99.6 ± 0.7 | 99.6 ± 1.1 | 100.0 ± 0.0 |
| 24 h pH >4 HTR % | 5.6 ± 2.4 | 82.2 ± 12.6 | 85.0 ± 3.0 | 85.8 ± 16.9 | 4.5 ± 4.3 | 99.4 ± 1.2 | 98.3 ± 4.3 | 100.0 ± 0.0 |
| 24 h pH >5 HTR % | 3.1 ± 2.3 | 79.1 ± 15.4 | 84.4 ± 2.8 | 84.5 ± 19.8 | 3.2 ± 3.6 | 99.0 ± 1.8 | 97.4 ± 6.0 | 100.0 ± 0.0 |
| Night‐time pH >3 HTR % | 6.0 ± 5.6 | 93.7 ± 7.4 | 99.9 ± 0.0 | 97.8 ± 6.2 | 2.7 ± 4.3 | 99.2 ± 1.4 | 99.2 ± 1.9 | 99.9 ± 0.0 |
| Night‐time pH >4 HTR % | 3.9 ± 4.7 | 87.9 ± 15.7 | 99.9 ± 0.0 | 95.2 ± 13.5 | 1.5 ± 2.7 | 98.8 ± 2.3 | 96.9 ± 8.1 | 99.9 ± 0.0 |
| Night‐time pH >5 HTR % | 2.2 ± 3.7 | 82.4 ± 22.4 | 99.7 ± 0.6 | 93.6 ± 17.9 | 0.9 ± 1.9 | 98.1 ± 3.7 | 95.3 ± 11.4 | 99.9 ± 0.0 |
Abbreviation: HTRs, holding time ratios.
PK‐PD relationships
The relationship between intragastric pH greater than 5 HTRs over the 24 h postdose and the plasma exposure (AUC0–24h and C max) of keverprazan on day 1 and day 7 were plotted (Figure S2). The intragastric pH greater than 5 HTRs did not increase with dosage and reached a plateau at the 20 mg dose group.
DISCUSSION
This study first analyzed the safety, tolerability, PK, and PD characteristics of multiple oral doses of keverprazan in healthy adult participants. The results suggest that keverprazan is tolerable. A dose of 20 mg keverprazan can elicit a significant, stable, and long‐lasting gastric acid inhibition effect. Steady‐state conditions were achieved after 1 week of daily dosing.
In the keverprazan group, all AEs were mild, except for one case of moderate abdominal pain, of which the exact nature was unknown. The occurrence rate of TRAEs was relatively low, no SAEs occurred, and only one participant dropped out due to AEs. These results indicate that the doses of 20 and 40 mg of keverprazan were safe and tolerable. Moreover, the categories of the AEs for keverprazan were similar to other P‐CABs. 11 , 12 , 16 , 17 , 21 , 22 , 23 , 24 , 25 , 26 , 27 No new safety signals appeared. The small number of participants might preclude the observation of rare AEs. Still, a phase II trial in patients with duodenal ulcers suggests the favorable safety profile of keverprazan. 28
The results of the primary PK parameters of 20 or 40 mg keverprazan on day 1 in this study were consistent with those in the single‐dose phase Ia trial (yet unpublished, ChiCTR2100050171), with C max of 28.8 versus 31.7 ng/mL, T max of 2.00 versus 1.50 h, and t 1/2 of 6.27 versus 6.75 h for 20 mg keverprazan, and C max of 59.3 versus 56.1 ng/mL, T max of 1.50 versus 1.63 h, and t 1/2 of 6.52 versus 7.17 h for 40 mg keverprazan. The T max and t 1/2 observed on day 1 were generally similar to those of 20 mg vonoprazan in the present study (T max of 1.25 h and t 1/2 of 6.47 h) and 20/40 mg vonoprazan in a previous report (T max of 1.5 vs. 1.5 h and t 1/2 of 5.8 vs. 6.7 h for 20 vs. 40 mg groups, respectively). 16 Upon drug administration for 7 consecutive days, the plasma concentrations of keverprazan and vonoprazan reached a steady state. Keverprazan 20 mg group had higher C max (43.1 vs. 27.2 ng/mL), AUC0–t (390 vs. 239 h·ng/mL), AUC0–∞ (393 vs. 244 h·ng/mL), and lower CL/F (69.5 vs. 96.9 L/h) compared with the vonoprazan 20 mg group after multiple doses on day 7. In particular, the t 1/2 of 20 mg keverprazan (6.23 h) was significantly longer than those of PPIs (0.5–2 h) 29 and tegoprazan 100 mg (3.7 h), 10 indicating that keverprazan may have higher exposure and longer effect time than PPIs and previous P‐CABs.
A significant acid inhibition effect was elicited 1–2 h after a single dose of 20 or 40 mg keverprazan. It was observed in this study that the intragastric pH values of the vonoprazan group dropped 16–18 h after drug administration on day 1, indicating a weakening fluctuation in its acid inhibition effect, consistent with a trial of vonoprazan. 27 In contrast, the intragastric pH values of the 20/40 mg keverprazan groups remained at a relatively high level, suggesting a more stable efficacy than vonoprazan. It is consistent with the findings of the single‐dose trial and might be attributed to a more stable and durable efficacy of keverprazan due to its higher exposure, lower clearance.
Moreover, the intragastric pH greater than 3, pH greater than 4, and pH greater than 5 HTRs during 24 h postdose on day 1 were all above 80%, and the corresponding values during night‐time on day 1 were all above 90%. These results of night‐time HTRs on day 1 are numerically better than that of the positive control group. Upon consecutive drug administration for 7 days, the acid inhibition effect of keverprazan reached a steady‐state, and further increases in HTRs with the increase in drug exposure were not observed, indicating that the existence of a ceiling effect in the increase in the intragastric pH values and a plateau effect at the threshold of a dosage of 20 mg. The data also support further research on determining the clinical dosage based on the dosage of 20 mg. In addition, the acid inhibition effect of 20 and 40 mg keverprazan was similar to that of 20 mg vonoprazan on day 7 (i.e., when vonoprazan also achieved a steady‐state). These results support the efficacy of P‐CABs for avoiding nocturnal acid breakthrough which was often observed with PPIs when the intragastric pH naturally decreases and PPIs become less activated. 6 , 7 , 8 These results are of clinical significance because nocturnal acid breakthroughs in patients with ARDs are a significant cause of decreased sleep quality and quality of life. 1 , 2 , 3 , 4
In healthy participants, differences were observed in the PK parameters after multiple doses on day 7 between 20 mg keverprazan and 20 mg vonoprazan, including higher C max and AUC, lower CL/F, and numerically higher intragastric pH greater than 5 HTR of keverprazan during night‐time on day 1. Whether these differences between the two drugs in the actual clinical outcomes of patients with ARDs exist requires further research.
In conclusion, a dose of 20 mg keverprazan is tolerable and can elicit a significant, stable, and long‐lasting gastric acid inhibition effect. A steady‐state in PKs and PDs was generally reached after 7 days of treatment. The results suggest that the efficacy and safety of keverprazan for treating ARDs are worth further exploration.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. S.Z., L.X., C.Z., and F.S. designed the research. S.Z., L.X., C.Z., J.C., S.D., B.Z., M.S., and F.S. performed the research. S.Z., L.W., M.S., and F.S. analyzed the data.
FUNDING INFORMATION
This study was funded by Jiangsu Carephar Pharmaceutical Co., Ltd.
CONFLICT OF INTEREST STATEMENT
M.S. is the employee of Jiangsu Carephar Pharmaceutical Co., Ltd. All other authors declared no competing interests for this work.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors would like to thank the subjects and their families for their contributions to this clinical study.
Zhou S, Xie L, Zhou C, et al. Keverprazan, a novel potassium‐competitive acid blocker: Multiple oral doses safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy subjects. Clin Transl Sci. 2023;16:1911‐1922. doi: 10.1111/cts.13598
Contributor Information
Mei Su, Email: sumei@carephar.com.
Feng Shao, Email: jsphshaofeng@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kellerman R, Kintanar T. Gastroesophageal reflux disease. Prim Care. 2017;44(4):561‐573. [DOI] [PubMed] [Google Scholar]
- 2. Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):267‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390(10094):613‐624. [DOI] [PubMed] [Google Scholar]
- 4. Kavitt RT, Lipowska AM, Anyane‐Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132(4):447‐456. [DOI] [PubMed] [Google Scholar]
- 5. Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani‐Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro‐oesophageal reflux symptoms: a meta‐analysis. Gut. 2018;67(3):430‐440. [DOI] [PubMed] [Google Scholar]
- 6. Perry IE, Sonu I, Scarpignato C, Akiyama J, Hongo M, Vega KJ. Potential proton pump inhibitor‐related adverse effects. Ann N Y Acad Sci. 2020;1481(1):43‐58. [DOI] [PubMed] [Google Scholar]
- 7. Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long‐term proton pump inhibitor use. J Neurogastroenterol Motil. 2018;24(2):182‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed A, Clarke JO. Proton pump inhibitors (PPI). In: StatPearls [Internet]. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 9. Scarpignato C, Hongo M, Wu JCY, et al. Pharmacologic treatment of GERD: where we are now, and where are we going? Ann N Y Acad Sci. 2020;1482(1):193‐212. [DOI] [PubMed] [Google Scholar]
- 10. Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ‐12420), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50(7):751‐759. [DOI] [PubMed] [Google Scholar]
- 11. Rawla P, Sunkara T, Ofosu A, Gaduputi V. Potassium‐competitive acid blockers – are they the next generation of proton pump inhibitors? World J Gastrointest Pharmacol Ther. 2018;9(7):63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oshima T, Miwa H. Potent potassium‐competitive acid blockers: a new era for the treatment of acid‐related diseases. J Neurogastroenterol Motil. 2018;24(3):334‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han S, Choi HY, Kim YH, et al. Effect of food on the pharmacokinetics and pharmacodynamics of a single oral dose of tegoprazan. Clin Ther. 2021;43(8):1371‐1380. [DOI] [PubMed] [Google Scholar]
- 14. Yoon DY, Sunwoo J, Shin N, et al. Effect of meal timing on pharmacokinetics and pharmacodynamics of tegoprazan in healthy male volunteers. Clin Transl Sci. 2021;14(3):934‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sunwoo J, Oh J, Moon SJ, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2018;48(2):206‐218. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK‐438 (vonoprazan), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laine L, DeVault K, Katz P, et al. Vonoprazan versus lansoprazole for healing and maintenance of healing of erosive esophagitis: a randomized trial. Gastroenterology. 2023;164(1):61‐71. [DOI] [PubMed] [Google Scholar]
- 18. Simadibrata DM, Syam AF, Lee YY. A comparison of efficacy and safety of potassium‐competitive acid blocker and proton pump inhibitor in gastric acid‐related diseases: a systematic review and meta‐analysis. J Gastroenterol Hepatol. 2022;37(12):2217‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bin Shu YZ, Cai M, Shao Q, Yuan Y, Liu J, Qiao H. Embryo‐fetal developmental toxicity and toxicokinetics of carenoprazan hydrochloride in rabbits. Chin J Pharmacol Toxicol. 2020;34(7):502‐510. [Google Scholar]
- 20. Li CY, Su M, Yan YY, et al. KFP‐H008 blocks gastric acid secretion through inhibiting H(+)‐K(+)‐ATPase. Eur J Pharmacol. 2017;810:112‐119. [DOI] [PubMed] [Google Scholar]
- 21. He J, Cao G, Yu J, et al. Safety, tolerability and pharmacokinetics of single ascending and multiple oral doses of tegoprazan in healthy Chinese subjects. Clin Drug Investig. 2021;41(1):89‐97. [DOI] [PubMed] [Google Scholar]
- 22. Bunchorntavakul C, Buranathawornsom A. Randomized clinical trial: 7‐day vonoprazan‐based versus 14‐day omeprazole‐based triple therapy for Helicobacter pylori . J Gastroenterol Hepatol. 2021;36(12):3308‐3313. [DOI] [PubMed] [Google Scholar]
- 23. Echizen H. The first‐in‐class potassium‐competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet. 2016;55(4):409‐418. [DOI] [PubMed] [Google Scholar]
- 24. Kawai D, Takenaka R, Ishiguro M, et al. Vonoprazan versus lansoprazole in the treatment of artificial gastric ulcers after endoscopic submucossal dissection: a randomized, open‐label trial. BMC Gastroenterol. 2021;21(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laine L, Sharma P, Mulford DJ, et al. Pharmacodynamics and pharmacokinetics of the potassium‐competitive acid blocker Vonoprazan and the proton pump inhibitor lansoprazole in US subjects. Am J Gastroenterol. 2022;117(7):1158‐1161. [DOI] [PubMed] [Google Scholar]
- 26. Mulford DJ, Leifke E, Hibberd M, Howden CW. The effect of food on the pharmacokinetics of the potassium‐competitive acid blocker vonoprazan. Clin Pharmacol Drug Dev. 2022;11(2):278‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakurai Y, Mori Y, Okamoto H, et al. Acid‐inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects – a randomised open‐label cross‐over study. Aliment Pharmacol Ther. 2015;42(6):719‐730. [DOI] [PubMed] [Google Scholar]
- 28. Tan ND, Liu XW, Liu CX, et al. Efficacy of keverprazan for duodenal ulcer: a phase II randomized, double‐blind, parallel‐controlled trial. J Gastroenterol Hepatol. 2022;37(11):2060‐2066. [DOI] [PubMed] [Google Scholar]
- 29. Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19(1):25‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


