Abstract
The spectrum and understanding of antibody-positive autoimmune encephalitis (AE) have expanded over the past few decades. In 2007, a rare subtype of AE known as anti-adenylate kinase 5 (AK5) encephalitis, was first reported. This disease is more common in elderly males, with limbic encephalitis as the core phenotype (characterized by subacute anterograde amnesia, sometimes with psychiatric symptoms, and rarely with seizures). Brain magnetic resonance imaging typically demonstrated initial temporal lobe T2/fluid-attenuated inversion recovery hyperintensities, and subsequent atrophy. No concomitant tumors have been found yet. AK5 antibody, targeting the intracellular antigen, is a biomarker for a non-paraneoplastic T-cell autoimmunity response, and can be detected in serum and cerebrospinal fluid using tissue-based and cell-based assays. Cytotoxic T-cell-mediating neuronal injury and loss play a pivotal role in the immunopathogenesis of anti-AK5 encephalitis. Patients mostly show poor response to immunotherapy and thus a poor prognosis in the long run. Herein, we review the literature and provide updated knowledge of this less-known entity, focusing on clinical characteristics, paraclinical findings, diagnosis process, and therapeutic approaches.
Keywords: Adenylate kinase 5 antibody, Autoimmune encephalitis, Limbic encephalitis, Immunotherapy
1. Introduction
Autoimmune encephalitis (AE) is an umbrella term for a heterogeneous group of non-infectious, immune-mediated inflammatory disorders of the central nervous system [1]. Symptoms typically include cognitive deficits, psychiatric and behavioral abnormalities, seizures, movement disorders, autonomic impairments, and disturbances of consciousness [2]. The majority of AE patients generate antibodies against neurons, generally divided into two groups depending on the cellular location of the antigens: intracellular or cell-surface antibodies, with antibodies targeting N-methyl-d-aspartate receptor (NMDAR) and leucine-rich glioma-inactivated 1 (LGI1) being the most common [3,4]. Over past two decades, AE researches have led to the discovery and characterization of a growing body of novel autoantibodies, adding further depth to the neuroimmunological spectrum [[4], [5], [6]]. In 2007, scholars from the University of Pennsylvania Hospital, Carolina Neurological Clinic, and Carmel Internal Medicine & Neurology, detected limbic encephalitis (LE) associated with autoantibodies against adenylate kinase 5 (AK5) in two patients [7]. AK5 antibody is a biomarker for a rare and severe autoimmune LE. Anti-AK5 encephalitis with LE as the core phenotype has unique characteristics. First, this rare intracellular antibody of this disorder is scarcely associated with tumors, similar to typical neuronal cell-surface antibody-related LE, such as anti-LGI1/contactin-associated protein-like 2 (CASPR2) encephalitis [8,9]. Second, unfavorable responses to immunotherapies are observed in most patients with LE and AK5 antibodies, analogous to those patients with intracellular antibody-related paraneoplastic LE, such as anti-Hu/Ma2 antibody and anti-kelch-like protein 11 (KLHL11) antibody [9,10]. Herein, we summarized the current knowledge on the clinical and paraclinical features, diagnosis process, and the management of anti-AK5 encephalitis.
We searched through PubMed for articles in English published between January 2007 and June 2022. The search terms were as follows: (adenylate kinase 5 OR AK5 OR adenylate kinase 5 antibody OR AK5 antibody OR anti-adenylate kinase 5 antibody OR anti-AK5 antibody) AND (encephalitis OR autoimmune encephalitis OR AE OR limbic encephalitis OR LE). All relevant articles were retrieved, and their references were searched to identify as many additional studies as possible. Based on 6 case series (29 patients), 2 individual case reports (2 patients), 31 patients with anti-AK5 encephalitis have been identified so far [7,9,[11], [12], [13], [14], [15]]. Only 1 patient whose critical data was missing was excluded. Finally, 30 patients with anti-AK5 encephalitis were included in our review (Supplementary Table 1). More than two-thirds of these cases were reported in France, followed by the United States and Germany.
2. Etiology and pathogenic mechanism
The exact causes of anti-AK5 encephalitis remain unknown. Only one patient reported prodromal upper respiratory tract infection [15]. No cancer or autoimmune comorbidities were identified. Human leukocyte antigen (HLA) genetic predispositions were described to be associated with the occurrence of the disease. In a recent study, 8/11 (72.7 %) patients with anti-AK5 encephalitis had HLA-II haplotypes DRB1*03:01-DQA1*05:01-DQB1*02:01, with 6/11 (54.5 %) having HLA-I haplotypes A1*01:01-B*08:01-C*07:01 as a part of the extended ancestral HLA haplotype 8.1, which suggested that genetic predisposition may play a role in the development of anti-AK5 encephalitis [9]. However, the HLA class II haplotype DQA1*05:01-DQB1*02:01-DRB1*03:01 has also been reported in glutamic acid decarboxylase 65 antibody associated neurological syndromes (stiff-person syndrome, cerebellar ataxia, and LE) [16] and the HLA class I haplotype A1*01:01-B*08:01-C*07:01 has not been described in other autoimmune diseases. Studies with larger cohorts are required to confirm these findings.
The precise mechanism underlying the development of anti-AK5 encephalitis remains unclear. AK5 reversibly catalyzes phosphate translation between adenine nucleotides and is related to various energetic signaling mechanisms [17]. AK5 is specifically expressed in the brain neuronal cytosolic fraction [11]. Unlike other typical neuronal cell-surface antibody-associated LE (i.e., anti-LGI1/CASPR2 encephalitis) for which responsible antibodies play pathogenic roles, no evidence of direct involvement of antibodies against AK5 has been demonstrated. Neuropathological analysis from previous studies revealed an expression of intense granzyme B, activated macrophages/microglia, and infiltration of immune cells in the brain parenchyma, predominated with T-cell (CD3+, CD4+, CD8+) [9,13,18], and these results resemble that observed in patients with paraneoplastic neurological syndrome with antibodies against intracellular antigens (e.g., Hu, Ma2, KLHL11), suggesting analogical pathological mechanism that cytotoxic T-cell-mediating immunological response plays a crucial role in the pathogenesis of anti-AK5 encephalitis [10,[19], [20], [21]]. Furthermore, similar to LE with antibodies targeting intracellular antigens, patients with anti-AK5 encephalitis showed poorer response to immunotherapy than those with antibodies targeting neuronal surface antigens (e.g., NMDAR, LGI1, CASPR2), thus also indicating different pathogenic mechanisms between these subtypes [10,11,[22], [23], [24], [25]].
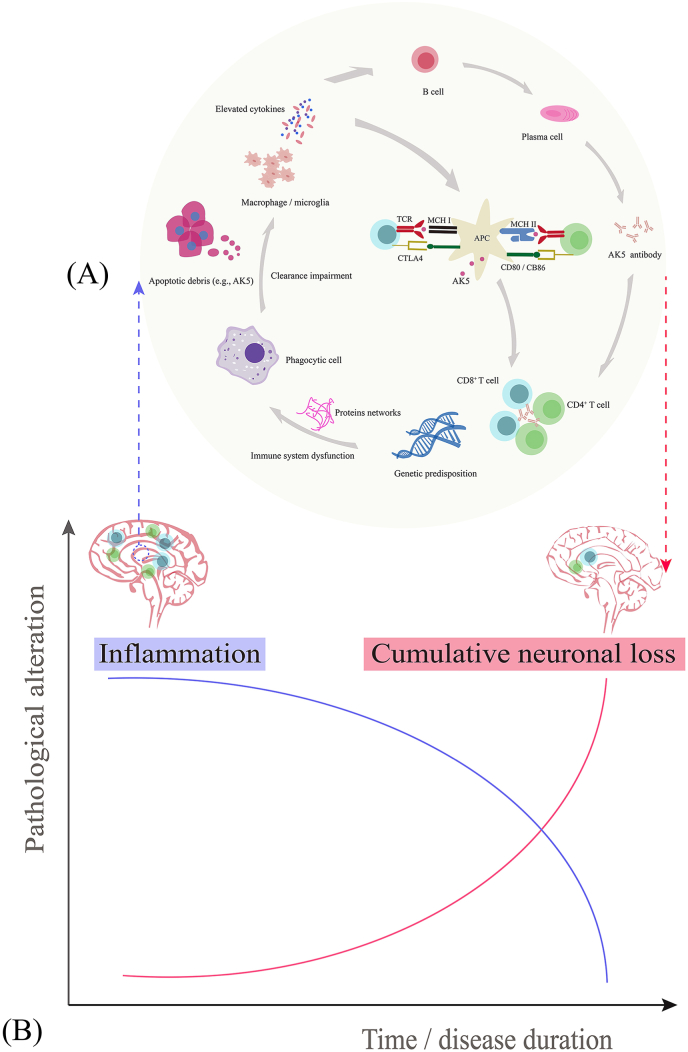
In some circumstances, immune system dysfunctions in individuals with genetic abnormalities predispose individuals to the occurrence of anti-AK5 encephalitis. Proteomic analysis revealed upregulated expression of multiple proteins in patients with anti-AK5 encephalitis. These proteins possibly form a complex interaction network that interferes with biological processes, including apoptosis signalling pathways as well as innate and adaptive immune responses [9]. The disintegration of neural apoptosis produces AK5-drived peptides [9]. Recently, it has been found that incomplete degradation or ineffective clearance of apoptotic cells activates phagocytic cells, such as dendritic cells and antigen-presenting cells. The subsequent immune pathways participate in innate/adaptive immune responses, including the release of cytokines as well as the recruitment and infiltration of autoreactive T cells (predominately CD4+, CD8+) [9]. In addition, macrophage/microglia may also be involved in the pathogenesis of the disease via the release of certain cytokines [9,11]. As a result, anti-AK5 encephalitis is initiated by neuroinflammatory injury and progresses into severe brain atrophy with cumulative irreversible neuronal loss in the chronic stage [9,11,18]. The possible pathophysiology of anti-AK5 encephalitis is shown in Fig. 1.
Fig. 1.
Schematic overview of the pathophysiological mechanism of anti-AK5 encephalitis. (A) In the context of genetic predisposition, immune system dysfunction induces complex protein networks that interfere with the apoptosis signaling pathway and innate and adaptive immune responses. The innate (phagocytic cells, cytokines, and macrophage/microglia) and adaptive (predominately CD4+, CD8+ T cells) immune responses strongly participate in the pathogenesis of anti-AK5 encephalitis. (B) Two-stage disease course in brain tissue damage consisting of severe inflammation at the active/early stage and irreversible cumulative neuronal loss at the progressive/advanced stage. Abbreviations: AK5, adenylate kinase 5; APC, antigen-presenting cell; CTLA4, cytotoxic T lymphocyte-associated antigen 4; MHC, major histocompatibility complex; TCR, T cell receptor.
3. Clinical features
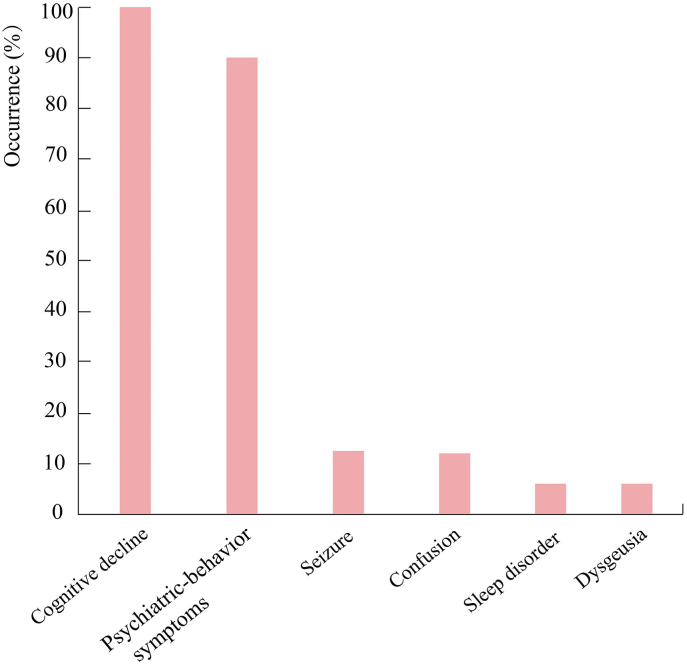
Most patients with anti-AK5 encephalitis were elderly men (21/30, 70 %) with a median age of 64 years (range: 48–94 years). The patients predominantly showed subacute onset (<12 weeks) of clinical symptoms. LE was observed in all patients with anti-AK5 encephalitis. Cognitive impairments, including anterograde amnesia, visuospatial disorientation, prosopagnosia, aphasia, executive dysfunctions, apraxia, and attention difficulty, were also observed in all patients (30/30, 100 %). In some severe cases (7/30), it gradually progresses to dementia. Psychiatric-behavior symptoms (27/30, 90 %), ranging from irritability, agitation, and aggressiveness to depression, anxiety, and psychosis with delusion and visual/olfactory hallucinations, were the second most common clinical characteristics and were observed in 27/30 (90 %) patients. Seizures appeared to be distinctly uncommon. Only 4/30 (13.3 %) patients experienced seizures (e.g., focal to bilateral tonic-clonic seizures) during disease course. Impaired consciousness was uncommon, and was only observed in 4/30 patients. In addition, other rare symptoms, such as sleep disorder (2/30) and dysgeusia (1/30), were also described. There were no significant differences in clinical features between the male group and female group. Moreover, none of the patients had tumors. Fig. 2 shows the common clinical symptoms of anti-AK5 encephalitis.
Fig. 2.
Summary of clinical symptoms for anti-AK 5 encephalitis. Abbreviations: AK5, adenylate kinase 5.
4. Paraclinical findings
4.1. Neuroimaging
Initial magnetic resonance imaging (MRI) revealed that 90 % (27/30) of the patients had temporal T2/fluid-attenuated inversion recovery (FLAIR) abnormalities. Of those, brain MRI mostly showed bilateral mesiotemporal lobe/hippocampi T2/FLAIR (26/30, 86.7 %) hyperintensities and sometimes extended to the insula or the frontal lobes. In addition, approximately one-third of patients (9/30, 30 %) had gadolinium-enhancing lesions in the temporal lobe (Fig. 3). Later in the disease course, 24/30 (80 %) patients underwent subsequent MRI, and the most common abnormalities were temporal lobe atrophy (21/24, 87.5 %), especially in the hippocampus, which may be linked to disease progression and longer disease duration. The median interval from temporal lobe hyperintensities to temporal lobe atrophy on MRI was six months (range: 2–54 months) in five patients.
Fig. 3.
Representative brain MRI of 3 patients with anti-AK5 encephalitis. (A) Brain MRI for patient 1 revealed mesiotemporal lobe FLAIR hyperintensities (A1) with contrast enhancement (A2), and atrophy after 3 months (A3). (B) Brain MRI for patient 2 revealed mesiotemporal lobe FLAIR hyperintensities (B1) with contrast enhancement (B2), and after progression to atrophy (B3). (C) Brain MRI for patient 3 revealed mesiotemporal lobe FLAIR hyperintensities (C1) with faint contrast enhancement (C2) that evolved to atrophy (C3). Reprinted from Ref 15. Copyright 2022, with permission from Elsevier. Abbreviations: AK5, adenylate kinase 5; FLAIR, fluid-attenuated inversion-recovery; MRI, magnetic resonance imaging.
4.2. Blood tests and cerebrospinal fluid analysis
Routine blood tests were generally unrevealing. All patients had abnormal changes in cerebrospinal fluid (CSF). Lymphocytic-predominant pleocytosis with a median of 22 cells/mL (range: 6–120 cells/mL) were observed in 23/30 patients and elevated protein levels with median of 65 mg/dL (range: 46–170 mg/dL) in 18/27 patients. Oligoclonal bands were found in 23/25 patients, and 7/9 patients had elevated immunoglobulin G (IgG) index (>0.7). Moreover, CSF tau proteins were overexpressed in 12/14 patients with a median of 934.5 pg/mL (range: 480–3024 pg/mL).
4.3. Antibody detection
Detection of antibodies against AK5 in serum and/or CSF is the confirmatory diagnostic test for anti-AK5 encephalitis. IgG1 was the most frequently discovered among all AK5 antibody IgG subclasses (IgG1-4) [9]. In order to improve diagnostic sensitivity and specificity for anti-AK5 encephalitis, a combination of the cell-based assay (CBA) and tissue-based assay (TBA) is recommended. The anti-AK5 encephalitis antibodies were detected by TBA for preliminary screening and were followed by CBA for confirmatory purposes [11,12]. One study revealed that the median titer of AK5 antibody by CBA in serum was 1:16000 (range: 1:100–1:16000), higher than that in CSF (median titer: 1:2560; range: 1:160–1:48,000) [11]. However, the alterations of antibody titer during the disease course (e.g., after immunotherapy) and their association with disease severity remain unclear. In general, CSF samples appear to provide superior sensitivity to serum for detecting AK5 antibody [15]. Considering the intrathecal synthesis of AK5 antibody, the specificity of the CSF antibody was relatively higher than that in serum [15]. Paired tests of serum and CSF samples are firstly suggested, and CSF is preferred if paired tests are unavailable.
5. Diagnostic work-up
The diagnosis of anti-AK5 encephalitis mainly refers to the 2016 diagnostic algorithm proposed by Graus et al. in the Lancet Neurology [4]. The anti-AK5 encephalitis should be considered in elderly male patients with subacute progressive anterograde amnesia, psychiatric symptoms (especially depression) and the absence of seizures, and radiologically unilateral or bilateral T2/FLAIR hyperintensity of the limbic lobe on MRI. In addition, the AK5 antibody testing for patients with LE should also be added in the rare scenario where routine common antibodies and tumor screening are negative. Considering the extremely low possibility of paraneoplastic cases based on the current data, tumor screening at initial diagnostic assessment seems sufficient in patients with anti-AK5 encephalitis.
Cognitive impairment is one of the typical clinical characteristics of anti-AK5 encephalitis and has been part of the diagnostic clue for this condition. Given anti-AK5 encephalitis is a rare cause of amnestic-behavioral clinical presentation, various mimics need to be fully considered as differential diagnoses, such as infectious, neoplastic, vascular and metabolic/toxic diseases, in particular neurodegenerative disorders (e.g., Alzheimer's disease [AD], Creuzfeldt-Jakob disease [CJD]) [9,26]. CSF biomarkers can be especially beneficial for distinguishing AD and anti-AK5 encephalitis [27]. Compared to patients with anti-AK5 encephalitis, patients with AD have elevated total-tau protein and phospho-tau protein level, but decreased amyloid β42/40 in CSF. The clinical diagnosis of CJD can be supported by typical clinical symptoms (cerebellar symptoms, visual disorders, myoclonus, and extrapyramidal symptoms), CSF analysis (elevated 14-3-3 protein and total-tau protein), MRI features (restricted diffusion and FLAIR hyperintensities in cortical regions and caudate nucleus) and electroencephalography findings (classic periodic sharp and slow wave complexes) [28]. In addition, other epidemiologically more prevalent LE phenotypic diseases should be excluded with caution (Table 1).
Table 1.
Comparison between anti-AK5 encephalitis and other antibody-positive CNS diseases commonly involving limbic system.
| Disease | Diagnostic biomarkers | Median age, year (range); female: male ratio | Common clinical features | Frequency of tumor (%); associated tumor types (%) | MRI findings | Preferred sample types; detection methodology |
|---|---|---|---|---|---|---|
| Anti-AK5 encephalitis [9,30] | Serum/CSF: AK5 antibody | 64 (48–94); 1:2.3 |
Cognitive impairment (100 %), psychiatric-behavioral disturbances (90 %), prosopagnosia (16.7 %), speech disorder (16.7 %) and seizure (13.3 %) | 0 | 90 % temporal T2/FLAIR hyperintensity | CSF; TBA (IFA) and CBA |
| Anti-LGI1 encephalitis [31,32] | Serum/CSF: LGI1 antibody | 54 (18–85); 1:2 |
Cognitive impairment 97 %), seizure (90 %) and behavioral disturbance (90 %) | <10 %; thymoma, SCLC | 75 % mesial temporal lobe T2/FLAIR hyperintensity | Serum; TBA (IFA) and CBA |
| Anti-CASPR2 Encephalitis [31,33] |
Serum/CSF: CASPR2 antibody | 65 (60–70);m ale predominance |
Cognitive impairment (80 %), seizure (50 %), CA (35 %), neuropathic pain (60 %), peripheral nerve hyperexcitability (55 %), insomnia (55 %) and autonomic dysfunction (45 %) | ∼20 %; mostly thymoma | 70 % normal, and 24 % bilateral medial temporal lobes T2/FLAIR hyperintensity | Serum; TBA (IFA) and CBA |
| Anti-AMPAR encephalitis [34] | Serum/CSF: AMPAR antibody | 57 (3–92); 1:2 | Cognitive impairment (82 %), psychiatric symptoms (80 %), altered state of consciousness (77 %), dyskinesia (38 %) and seizure (29 %) | ∼60 %; SCLC, thymoma and breast cancer |
60 % temporal lobe T2/FLAIR hyperintensity | CSF; TBA (IFA) and CBA |
| Anti-GABABR encephalitis [32] | Serum/CSF: GABABR Antibody |
55 (18–76); 1:2 | Seizure (93 %), cognitive impairment (82 %), psychiatric symptoms (77 %) and altered state of consciousness (51 %) | ∼50 %; SCLC | 30 % temporal lobe T2/FLAIR hyperintensity | CSF; TBA (IFA) and CBA |
| Anti-NMDAR encephalitis [35] | Serum/CSF: NMDAR antibody | 21 (<1–85); 4:1 | Psychiatric disorders (90 %), seizure (82 %), memory loss, motor disorders (75 % adults and 95 % children), speech disorders and central hypoventilation | ∼40 %; ovarian teratoma | 70 % normal and non-specific changes | CSF; TBA (IFA) and CBA |
| KLHL11-PNS [[36], [37], [38]] | Serum/CSF: KLHL11 antibody | 46 (9–79); predominantly males | Rhombencephalitis (70 %), cochleovestibulopathy (30 %; vertigo, sensorineural hearing loss and tinnitus) and LE (23 %) | >70 %; testicular germ cell tumor (78.7 %), teratoma (14.8 %) | T2/FLAIR hyperintensities in the temporal lobe, brainstem and cerebellum | Serum or CSF; TBA (IFA) and CBA |
| Ma2-PNS [20,30,36,[39], [40], [41]] | Serum/CSF: Ma and/or Ma2 antibody | Female: 64 (53–82); Male: 34 (22–70); 1:2 |
Diencephalitis, LE andbrainstem encephalitis | >90 %; testicular cancer (47 %), lung cancer (17 %), other cancers (36 %; mainly gastrointestinal tumors) | T2/FLAIR hyperintensities in the diencephalon, temporal lobe and brainstem | Serum; TBA (IFA) and western blot |
| Hu/ANNA-1-PNS [30,[40], [41], [42], [43]] | Serum/CSF: Hu/ANNA-1 antibody | 69 (36–76); slight female predominance | Rapid progressive cerebellar syndrome, brainstem encephalitis, LE and encephalomyelitis | >90 %; SCLC (75 %), NSCLC (10 %), extra-thoracic cancers (15 %) | T2/FLAIR Hyperintensities in temporal lobe and cerebellum | Serum; TBA (IFA) and western blot |
| Anti-mGluR5 encephalitis [44] | Serum/CSF: mGluR5 antibody | 29 (6–75); no predominance | Cognitive impairment (91 %), psychiatric and behavior disorder (91 %), sleep disturbance (64 %), seizure (55 %), decreased level of consciousness (55 %) and movement disorders (45 %) | ∼55 %; mostly Hodgkin lymphoma | 18 % temporal lobe T2/FLAIR hyperintensities | CSF; TBA (IFA) and CBA |
| GAD antibody-spectrum diseases [25,30,41,[45], [46], [47], [48]] | Serum: high titre of GAD65 antibody; CSF: GAD65 antibody |
60 (29–80); strong female predominance (>80 %) | Stiff-person syndrome spectrum, cerebellar syndrome, LE, and epilepsy | <10 %; SCLC, neuroendocrine tumor, thymoma, breast cancer, non-Hodgkin's lymphoma | Temporal lobe T2/FLAIR hyperintensities | Serum and CSF; radioimmunoassay and CBA |
Abbreviations: AK5, anti-adenylate kinase 5; AMPAR, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANNA-1, antineuronal nuclear antibody type 1; CA, cerebellar ataxia; CASPR2, contactin-associated protein-like 2; CBA, cell-based assay; CLL, chronic lymphocytic leukemia; CNS, central nervous system; CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; ELISA, enzyme-linked immunosorbent assay; FLAIR, fluid-attenuated inversion recovery; GABABR, gamma aminobutyric acid-B receptor; GAD, glutamic acid decarboxylase; IFA, indirect immunofluorescence assay; KLHL11, kelch-like protein 11; LE, limbic encephalitis; LGI1, leucine-rich glioma-inactivated 1; mGluR5, metabotropic glutamate receptor type 5; MRI, magnetic resonance imaging; NMDAR, N-methyl-d-aspartate receptor; NSCLC, non-small cell lung cancer; PCR, polymerase chain reaction; PNS, paraneoplastic neurological syndrome; SCLC, small cell lung cancer; TBA, tissue-based assay.
6. Management
Evidence-based guidelines for diagnosing and treating patients with anti-AK5 encephalitis are absent due to its rarity. Hitherto, the management regimens for anti-AK5 encephalitis are borrowed from guidelines of other common AE types, with immunosuppression as the main treatment choice [3]. Timely initiation of immunological therapy was crucial for reducing irreversible neuronal loss and severe neurological disability.
Approximately 93.3 % (28/30) of patients with available treatment profiles were administrated with immunotherapeutic agents. In nearly all cases (96.4 %, 27/28), first-line immunotherapies were administered at the early stage, with intravenous immunoglobulin (IVIG) (24/28; 0.4 g/kg per day for 5 days) being preferred, followed by intravenous methylprednisolone (IVMP) (22/28; 1000 mg for a 3–5 days session with a tapering scheme) and plasma exchange (PLEX) (3/28; 5–7 sessions over 7–14 days). Combined immunotherapies, including IVIG plus IVMP, or IVMP plus PLEX, were widely used, given its poor response to monotherapies. The optimal duration of corticosteroid treatment remains unknown, and 3–6 months may be reasonable [1,29].
Approximately 42.9 % (12/28) of patients were administrated with second-line immunotherapies, including cyclophosphamide (CTX) (10/28), rituximab (9/28), mycophenolate mofetil (1/28), and azathioprine (1/28). CTX targeting both T- and B-lymphocytes could be more appropriate given the cytotoxic T-cell-mediated pathogenesis of anti-AK5 encephalitis.
7. Clinical outcome and prognosis
Compared with other neuronal surface antibody-mediated LE, anti-AK5 encephalitis is associated with a higher refractory course, and most patients respond poorly to treatment even in combination with second-line immunotherapies. Progressive deterioration of neurological function (including mortality) occurred in 30 % (9/30) of patients, while only 46.7 % (14/30) stabilized, and only 23.3 % (7/30) improved. We compared the clinical and paraclinical findings in patients with different outcomes, aiming to identify the underlying risk factors of unsatisfactory prognosis. As a result, seizures were more common in the progressive decline group than the stabilization/improvement group (4/9 vs. 0/21, P = 0.005), while there were no significant differences in other clinical symptoms, CSF and MRI profiles (Table 2). Our findings indicated that the presence of seizures was associated with poor clinical outcome (higher modified Rankin Scale scores). Additionally, there were no differences in clinical outcomes (stabilization/improvement, progressive decline) between the patients with first-line immunotherapy and those with second-line immunotherapy (P = 0.221) (Table 2).
Table 2.
Comparison between the clinical and paraclinical findings in anti-AK5 encephalitis patients with different outcomes.
| Characteristics | N (%) |
aP value | |
|---|---|---|---|
| Stabilization/improvement (N = 21) | Progressive decline (N = 9) | ||
| Male | 13/21 (61.9) | 8/9 (88.9) | 0.210 |
| Cognitive deficit | 21/21 (100.0) | 9/9 (100.0) | 0.999 |
| Psychiatric-behavior change | 20/21 (95.2) | 7/9 (77.8) | 0.207 |
| Seizure | 0/21 (0.0) | 4/9 (44.4) | 0.005 |
| Altered level of consciousness | 1/21 (4.8) | 3/9 (33.3) | 0.069 |
| Bilateral temporal lobe T2/FLAIR hyperintensities | 19/21 (90.5) | 7/9 (77.8) | 0.563 |
| Pleocytosis | 16/21 (76.2) | 7/9 (77.8) | 0.999 |
| Positive oligoclonal bands in CSF | 18/19 (94.7) | 5/6 (83.3) | 0.430 |
| Elevated tau protein level in CSF | 7/9 (77.8) | 5/5 (100.0) | 0.505 |
| Immunotherapy | 0.221 | ||
| First-line immunotherapy | 9/20 (45.0) | 6/8 (75.0) | |
| Second-line immunotherapy | 11/20 (55.0) | 2/8 (25.0) | |
The definitions of outcomes (progressive decline, stabilization and improvement) in anti-AK5 encephalitis patients depend on inherent descriptions in referred literature.
Abbreviations: AK5, anti-adenylate kinase 5; CSF, cerebrospinal fluid; FLAIR, fluid-attenuated inversion recovery.
Fisher's exact test.
No relapses were reported during the recorded follow-up period (range: 1–72 months). Nearly two-thirds (19/30, 63.3 %) of anti-AK5 encephalitis patients needed assistance to run daily activities because of severe amnesia, aphasia, and spatial disorientation. Only 23.3 % (7/30) of the patients could perform daily activities unaided. Furthermore, 10 % (3/30) of patients died from severe neurological symptoms, such as generalized tonic-clonic seizures.
8. Conclusions and future directions
AK5 antibody has been recognized as a biomarker of a very rare and severe non-paraneoplastic LE. Anti-AK5 encephalitis should be highly suspected in elderly males with LE, predominately presenting with subacute anterograde amnesia with limbic system involvement on MRI. Paired serum and CSF antibody detection is the most important and accurate test for confirming anti-AK5 encephalitis. Although unfavorable outcomes are observed in most patients, early diagnosis and timely treatment for anti-AK5 encephalitis are still important. Additional cases and further investigations are required to better understand the nature of anti-AK5 encephalitis and develop novel therapies.
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China (grant number LQ23H090004).
Author contributions
Er-Chuang Li: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Qi-Lun Lai: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Meng-Ting Cai: Data curation, Formal analysis, Investigation, Writing – review & editing. Gao-Li Fang: Data curation, Formal analysis, Investigation, Writing – review & editing. Chun-Hong Shen: Data curation, Formal analysis, Investigation, Writing – review & editing. Mei-Ping Ding: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Validation, Writing – review & editing. Yin-Xi Zhang: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Handling Editor: Y Renaudineau
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2023.100218.
Contributor Information
Mei-Ping Ding, Email: dmp-neurology@zju.edu.cn.
Yin-Xi Zhang, Email: zyx-neurology@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Abbatemarco J.R., Yan C., Kunchok A., Rae-Grant A. Antibody-mediated autoimmune encephalitis: a practical approach. Cleve. Clin. J. Med. 2021;88:459–471. doi: 10.3949/ccjm.88a.20122. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y.X., Qiu W., Guan H.Z., Wu L.J., Ding M.P. Editorial: antibody-mediated autoimmune diseases of the CNS: challenges and approaches to diagnosis and management. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.844155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abboud H., Probasco J.C., Irani S., Ances B., Benavides D.R., Bradshaw M., Christo P.P., Dale R.C., Fernandez-Fournier M., Flanagan E.P., Gadoth A., George P., Grebenciucova E., Jammoul A., Lee S.T., Li Y., Matiello M., Morse A.M., Rae-Grant A., Rojas G., Rossman I., Schmitt S., Venkatesan A., Vernino S., Pittock S.J., Titulaer M.J. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J. Neurol. Neurosurg. Psychiatry. 2021;92:757–768. doi: 10.1136/jnnp-2020-325300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T., Cortese I., Dale R.C., Gelfand J.M., Geschwind M., Glaser C.A., Honnorat J., Höftberger R., Iizuka T., Irani S.R., Lancaster E., Leypoldt F., Prüss H., Rae-Grant A., Reindl M., Rosenfeld M.R., Rostásy K., Saiz A., Venkatesan A., Vincent A., Wandinger K.P., Waters P., Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/s1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bien C.G., Schulze-Bonhage A., Deckert M., Urbach H., Helmstaedter C., Grunwald T., Schaller C., Elger C.E. Limbic encephalitis not associated with neoplasm as a cause of temporal lobe epilepsy. Neurology. 2000;55:1823–1828. doi: 10.1212/wnl.55.12.1823. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe Y., Shimizu Y., Ooi S., Tanaka K., Inuzuka T., Nakashima K. Steroid-responsive limbic encephalitis. Intern. Med. 2003;42:428–432. doi: 10.2169/internalmedicine.42.428. [DOI] [PubMed] [Google Scholar]
- 7.Tüzün E., Rossi J.E., Karner S.F., Centurion A.F., Dalmau J. Adenylate kinase 5 autoimmunity in treatment refractory limbic encephalitis. J. Neuroimmunol. 2007;186:177–180. doi: 10.1016/j.jneuroim.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Sonderen A., Schreurs M.W., Wirtz P.W., Sillevis Smitt P.A., Titulaer M.J. From VGKC to LGI1 and Caspr2 encephalitis: the evolution of a disease entity over time. Autoimmun. Rev. 2016;15:970–974. doi: 10.1016/j.autrev.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Muñiz-Castrillo S., Hedou J.J., Ambati A., Jones D., Vogrig A., Pinto A.L., Benaiteau M., de Broucker T., Fechtenbaum L., Labauge P., Murnane M., Nocon C., Taifas I., Vialatte de Pémille C., Psimaras D., Joubert B., Dubois V., Wucher V., Desestret V., Mignot E., Honnorat J. Distinctive clinical presentation and pathogenic specificities of anti-AK5 encephalitis. Brain. 2021;144:2709–2721. doi: 10.1093/brain/awab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li E.C., Lai Q.L., Cai M.T., Zheng Y., Fang G.L., Fang W., Du B.Q., Shen C.H., Ding M.P., Zhang Y.X. Kelch-like protein 11 antibody-associated paraneoplastic neurological syndrome: a state-of-the-art review. Clin. Immunol. 2022;241 doi: 10.1016/j.clim.2022.109074. [DOI] [PubMed] [Google Scholar]
- 11.Do L.D., Chanson E., Desestret V., Joubert B., Ducray F., Brugière S., Couté Y., Formaglio M., Rogemond V., Thomas-Antérion C., Borrega L., Laurens B., Tison F., Curot J., De Brouker T., Lebrun-Frenay C., Delattre J.Y., Antoine J.C., Honnorat J. Characteristics in limbic encephalitis with anti-adenylate kinase 5 autoantibodies. Neurology. 2017;88:514–524. doi: 10.1212/wnl.0000000000003586. [DOI] [PubMed] [Google Scholar]
- 12.Bien C.I., Nehls F., Kollmar R., Weis M., Steinke W., Woermann F., Dalmau J., Bien C.G. Identification of adenylate kinase 5 antibodies during routine diagnostics in a tissue-based assay: three new cases and a review of the literature. J. Neuroimmunol. 2019;334 doi: 10.1016/j.jneuroim.2019.576975. [DOI] [PubMed] [Google Scholar]
- 13.Vicino A., Loser V., Salvioni Chiabotti P., Brouland J.P., Du Pasquier R. Anti-adenylate kinase 5 encephalitis with histologic evidence of CNS vasculitis. Neurol. Neuroimmunol. Neuroinflamm. 2021;8 doi: 10.1212/nxi.0000000000001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillaume C., Saguin E., Peroux E., Balcerac A., Ricard D. Anti-AK5 encephalitis: subacute anterograde amnesia is not the only clinical presentation. Acta Neurol. Belg. 2022:299–301. doi: 10.1007/s13760-021-01853-5. [DOI] [PubMed] [Google Scholar]
- 15.McKeon-Makki I., McKeon A., Yang B., Pittock S.J., Lopez-Chiriboga S., Komorowski L., Miske R., Zekeridou A. Adenylate kinase 5 (AK5) autoimmune encephalitis: clinical presentations and outcomes in three new patients. J. Neuroimmunol. 2022;367 doi: 10.1016/j.jneuroim.2022.577861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñiz-Castrillo S., Ambati A., Dubois V., Vogrig A., Joubert B., Rogemond V., Picard G., Lin L., Fabien N., Mignot E., Honnorat J. Primary DQ effect in the association between HLA and neurological syndromes with anti-GAD65 antibodies. J. Neurol. 2020;267:1906–1911. doi: 10.1007/s00415-020-09782-8. [DOI] [PubMed] [Google Scholar]
- 17.Ahn B., Chae Y.S., Lee S.K., Kim M., Kim H.S., Moon J.W., Park S.H. Identification of novel DNA hypermethylation of the adenylate kinase 5 promoter in colorectal adenocarcinoma. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-92147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng A.S., Kramer J., Centurion A., Dalmau J., Huang E., Cotter J.A., Geschwind M.D. Clinico-pathological correlation in adenylate kinase 5 autoimmune limbic encephalitis. J. Neuroimmunol. 2015;287:31–35. doi: 10.1016/j.jneuroim.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bien C.G., Vincent A., Barnett M.H., Becker A.J., Blümcke I., Graus F., Jellinger K.A., Reuss D.E., Ribalta T., Schlegel J., Sutton I., Lassmann H., Bauer J. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- 20.Dalmau J., Graus F., Villarejo A., Posner J.B., Blumenthal D., Thiessen B., Saiz A., Meneses P., Rosenfeld M.R. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 21.Rosenblum M.K. Paraneoplasia and autoimmunologic injury of the nervous system: the anti-Hu syndrome. Brain Pathol. 1993;3:199–212. doi: 10.1111/j.1750-3639.1993.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 22.Ariño H., Armangué T., Petit-Pedrol M., Sabater L., Martinez-Hernandez E., Hara M., Lancaster E., Saiz A., Dalmau J., Graus F. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87:759–765. doi: 10.1212/wnl.0000000000003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joubert B., Saint-Martin M., Noraz N., Picard G., Rogemond V., Ducray F., Desestret V., Psimaras D., Delattre J.Y., Antoine J.C., Honnorat J. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol. 2016;73:1115–1124. doi: 10.1001/jamaneurol.2016.1585. [DOI] [PubMed] [Google Scholar]
- 24.Seery N., Butzkueven H., O'Brien T.J., Monif M. Contemporary advances in anti-NMDAR antibody (Ab)-mediated encephalitis. Autoimmun. Rev. 2022;21 doi: 10.1016/j.autrev.2022.103057. [DOI] [PubMed] [Google Scholar]
- 25.Tsiortou P., Alexopoulos H., Dalakas M.C. GAD antibody-spectrum disorders: progress in clinical phenotypes, immunopathogenesis and therapeutic interventions. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillaume C., Saguin E., Peroux E., Balcerac A., Ricard D. Anti-AK5 encephalitis: subacute anterograde amnesia is not the only clinical presentation. Acta Neurol. Belg. 2023;123:299–301. doi: 10.1007/s13760-021-01853-5. [DOI] [PubMed] [Google Scholar]
- 27.Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., Cummings J., van der Flier W.M. Alzheimer's disease. Lancet. 2021;397:1577–1590. doi: 10.1016/s0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermann P., Appleby B., Brandel J.P., Caughey B., Collins S., Geschwind M.D., Green A., Haïk S., Kovacs G.G., Ladogana A., Llorens F., Mead S., Nishida N., Pal S., Parchi P., Pocchiari M., Satoh K., Zanusso G., Zerr I. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20:235–246. doi: 10.1016/s1474-4422(20)30477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abboud H., Probasco J., Irani S.R., Ances B., Benavides D.R., Bradshaw M., Christo P.P., Dale R.C., Fernandez-Fournier M., Flanagan E.P., Gadoth A., George P., Grebenciucova E., Jammoul A., Lee S.T., Li Y., Matiello M., Morse A.M., Rae-Grant A., Rojas G., Rossman I., Schmitt S., Venkatesan A., Vernino S., Pittock S.J., Titulaer M. Autoimmune encephalitis: proposed recommendations for symptomatic and long-term management. J. Neurol. Neurosurg. Psychiatry. 2021;92:897–907. doi: 10.1136/jnnp-2020-325302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bien C.G. Limbic encephalitis, handb. Clin. Neurol. 2022;187:467–487. doi: 10.1016/b978-0-12-823493-8.00024-9. [DOI] [PubMed] [Google Scholar]
- 31.van Sonderen A., Petit-Pedrol M., Dalmau J., Titulaer M.J. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. 2017;13:290–301. doi: 10.1038/nrneurol.2017.43. [DOI] [PubMed] [Google Scholar]
- 32.Ghimire P., Khanal U.P., Gajurel B.P., Karn R., Rajbhandari R., Paudel S., Gautam N., Ojha R. Anti-LGI1, anti-GABABR, and Anti-CASPR2 encephalitides in Asia: a systematic review. Brain Behav. 2020;10 doi: 10.1002/brb3.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Sonderen A., Ariño H., Petit-Pedrol M., Leypoldt F., Körtvélyessy P., Wandinger K.P., Lancaster E., Wirtz P.W., Schreurs M.W., Sillevis Smitt P.A., Graus F., Dalmau J., Titulaer M.J. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87:521–528. doi: 10.1212/wnl.0000000000002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T.Y., Cai M.T., Zheng Y., Lai Q.L., Shen C.H., Qiao S., Zhang Y.X. Anti-Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic acid receptor encephalitis: a review. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.652820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalmau J., Armangué T., Planagumà J., Radosevic M., Mannara F., Leypoldt F., Geis C., Lancaster E., Titulaer M.J., Rosenfeld M.R., Graus F. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045–1057. doi: 10.1016/s1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 36.Dubey D., Wilson M.R., Clarkson B., Giannini C., Gandhi M., Cheville J., Lennon V.A., Eggers S., Devine M.F., Mandel-Brehm C., Kryzer T., Hinson S.R., Khazaie K., Hales C., Kattah J., Pavelko K.D., Andrews P., Eaton J.E., Jitprapaikulsan J., Mills J.R., Flanagan E.P., Zekeridou A., Leibovich B., Fryer J., Torre M., Kaufman C., Thoreson J.B., Sagen J., Linnoila J.J., DeRisi J.L., Howe C.L., McKeon A., Pittock S.J. Expanded clinical phenotype, oncological associations, and immunopathologic insights of paraneoplastic kelch-like protein-11 encephalitis. JAMA Neurol. 2020;77:1420–1429. doi: 10.1001/jamaneurol.2020.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandel-Brehm C., Dubey D., Kryzer T.J., O'Donovan B.D., Tran B., Vazquez S.E., Sample H.A., Zorn K.C., Khan L.M., Bledsoe I.O., McKeon A., Pleasure S.J., Lennon V.A., DeRisi J.L., Wilson M.R., Pittock S.J. Kelch-like protein 11 antibodies in seminoma-associated paraneoplastic encephalitis. N. Engl. J. Med. 2019;381:47–54. doi: 10.1056/NEJMoa1816721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogrig A., Péricart S., Pinto A.L., Rogemond V., Muñiz-Castrillo S., Picard G., Selton M., Mittelbronn M., Lanoiselée H.M., Michenet P., Benaiteau M., Pariente J., Zéphir H., Giordana C., Montaut S., Salhi H., Bachoumas P., Montcuquet A., Letovanec I., Uro-Coste E., Honnorat J. Immunopathogenesis and proposed clinical score for identifying Kelch-like protein-11 encephalitis. Brain Commun. 2021;3 doi: 10.1093/braincomms/fcab185. fcab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mrabet S., Ben Achour N., Kraoua I., Benrhouma H., Klaa H., Rouissi A., Ben Ahmed M., Ben Youssef Turki I. Anti-Ma2-encephalitis in a 2 year-old child: a newly diagnosed case and literature review. Eur. J. Paediatr. Neurol. 2015;19:737–742. doi: 10.1016/j.ejpn.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Graus F., Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019;16:535–548. doi: 10.1038/s41571-019-0194-4. [DOI] [PubMed] [Google Scholar]
- 41.Budhram A., Yang L., Bhayana V., Mills J.R., Dubey D. Clinical sensitivity, specificity, and predictive value of neural antibody testing for autoimmune encephalitis. J. Appl. Lab. Med. 2022;7:350–356. doi: 10.1093/jalm/jfab127. [DOI] [PubMed] [Google Scholar]
- 42.Schwenkenbecher P., Chacko L.P., Wurster U., Pars K., Pul R., Sühs K.W., Stangel M., Skripuletz T. Intrathecal synthesis of anti-Hu antibodies distinguishes patients with paraneoplastic peripheral neuropathy and encephalitis. BMC Neurol. 2016;16:136. doi: 10.1186/s12883-016-0657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q., Michel K., Annahazi A., Demir I.E., Ceyhan G.O., Zeller F., Komorowski L., Stöcker W., Beyak M.J., Grundy D., Farrugia G., De Giorgio R., Schemann M. Anti-Hu antibodies activate enteric and sensory neurons. Sci. Rep. 2016;6 doi: 10.1038/srep38216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spatola M., Sabater L., Planagumà J., Martínez-Hernandez E., Armangué T., Prüss H., Iizuka T., Caparó Oblitas R.L., Antoine J.C., Li R., Heaney N., Tubridy N., Munteis Olivas E., Rosenfeld M.R., Graus F., Dalmau J. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology. 2018;90:e1964–e1972. doi: 10.1212/wnl.0000000000005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graus F., Saiz A., Dalmau J. GAD antibodies in neurological disorders - insights and challenges. Nat. Rev. Neurol. 2020;16:353–365. doi: 10.1038/s41582-020-0359-x. [DOI] [PubMed] [Google Scholar]
- 46.Ariño H., Höftberger R., Gresa-Arribas N., Martínez-Hernández E., Armangue T., Kruer M.C., Arpa J., Domingo J., Rojc B., Bataller L., Saiz A., Dalmau J., Graus F. Paraneoplastic neurological syndromes and glutamic acid decarboxylase antibodies. JAMA Neurol. 2015;72:874–881. doi: 10.1001/jamaneurol.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gresa-Arribas N., Ariño H., Martínez-Hernández E., Petit-Pedrol M., Sabater L., Saiz A., Dalmau J., Graus F. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dade M., Berzero G., Izquierdo C., Giry M., Benazra M., Delattre J.Y., Psimaras D., Alentorn A. Neurological syndromes associated with anti-GAD antibodies. Int. J. Mol. Sci. 2020;21:3701. doi: 10.3390/ijms21103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.