Graphical abstract
Keywords: Ion mobility, Tandem mass spectrometry, LC-MS/MS, Differential mobility spectrometry, Steroids
Highlights
-
•
Measurements of many steroids are routinely used for clinical diagnosis.
-
•
Specific steroid measurements are challenging due to similarities amongst steroids.
-
•
DMS improves the specificity of traditional LC-MS/MS techniques.
-
•
DMS improves the S/N and reduces interferences.
-
•
Validation of cortisol/cortisone in urine demonstrates the viability of DMS.
Abstract
Introduction
Steroid measurements are important for diagnosis and monitoring of many conditions and treatment regiments; however, due to structural and chemical similarities amongst steroids, these analyses are challenging, even for highly specific techniques such as liquid chromatography-tandem mass spectrometry (LC-MS/MS). Differential mobility spectrometry (DMS) has the potential to improve these analyses by providing an orthogonal and complementary separation technique.
Methods
Initially, the potential for DMS to improve signal-to-noise ratio (S/N) and reduce interference was tested by comparing chromatograms acquired with and without DMS when performing measurements of six different steroids. Subsequently, a full clinical validation of cortisol and cortisone in urine was performed with the LC-DMS-MS/MS method.
Results and Discussion
DMS significantly reduced interferences observed in the chromatograms and boosted S/N by between 1.6 and 13.8 times. Additionally, DMS improved the agreement between quantifier/qualifier fragment ion results for cortisol and cortisone as indicated by the increase in R2 from approximately 0.81 to 0.98. All validation studies met acceptance criteria and we observed exceptional analytical performance in terms of precision, with % CVs less than 8%.
Conclusions
DMS improved the specificity of the steroid measurements by reducing interferences and improving S/N. The validation studies prove that these benefits did not come at the expense of other aspects of analytical performance. This study indicates that DMS has the potential to benefit not just clinical measurements of challenging analytes, but many clinical LC-MS/MS analyses.
1. Introduction
Steroids are a class of biomolecules derived from cholesterol that are hydrophobic and exhibit similar chemical properties as lipids. Steroids have diverse and critical biological functions, serving as hormones to regulate metabolism, sexual development, fertility, blood pressure, salt retention, gastrointestinal processes, and growth; therefore, measurements of steroids are clinically useful for diagnosing and monitoring of a plethora of conditions [1], [2]. However, due to the shared cholesterol backbone structure, there is a large amount of structural and chemical similarity among steroids, leading to a tendency for interference and cross-reactivity [2], [3], [4]. Consequently, achieving highly specific measurements of steroids is challenging.
Historically, steroids measurements have been largely performed by immunoassays, but these tests suffered from inadequate specificity, with cross-reactivity and interference from other steroids too common [2], [3], [4]. Today, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is widely recognized as the preferred platform for steroid measurement due to its superior analytical specificity and sensitivity [3], [5]. Yet, steroid analyses using LC-MS/MS are not without challenges. Circulating levels of steroids are often low, and steroids are difficult to ionize, making sensitivity a challenge [6]. The aforementioned structural and chemical similarities make steroids difficult to isolate using standard sample preparation and chromatographic techniques [7], [8]. Additionally, there is a high prevalence of isomers and many steroids produce shared product ions when fragmented in the mass spectrometer [8], [9]. Hence, LC-MS/MS still struggles with completely eliminating steroid measurement interferences.
Ion mobility is an emerging technique in clinical laboratories that separates gas phase ions based on their mobility in the presence of a buffer gas and an electric field; therefore, the mechanism of separation is charge, size, and shape [10], [11], [12], [13]. As a result, ion mobility has the potential to complement established LC-MS/MS techniques and further enhance the specificity of measurements for technically challenging analytes, such as steroids. Numerous studies have demonstrated successful separations of steroid isomers that are indistinguishable by mass spectrometry alone and difficult to separate chromatographically [10]. In these studies, cation adducts [14], [15], [16], [17], [18], [19], [20], [21] or derivatization [22], [23], [24] have been utilized to enhance the separation of steroid isomers. Additionally, collision cross sections (CCS) have shown great potential for adding an additional level of specificity and confidence in steroid measurements [10], [15], [20], [23], [24], [25], [26].
However, despite the potential benefits outlined in these studies, ion mobility is not widely adopted for the routine analysis of low levels of steroids in biological matrices, such as those performed in clinical laboratories. One notable exception is the work of Guddat et al, which demonstrated improved signal-to-noise ratio (S/N) and specificity when using LC- high field asymmetric ion mobility spectrometry (FAIMS)-MS/MS compared to analyses without FAIMS [27]. Another study by Arthur and coworkers developed a high-throughput method for measuring sodium adducts of steroid metabolites in urine using LC-FAIMS-MS [28]. Expanding on this previous work, we present the results of our analyses using a commercially available differential mobility spectrometry (DMS) device for LC-MS/MS measurements of cortisol, cortisone, 24,25-dihydroxyvitamin D, androstenedione (ANST), and 17-hydroxyprogesterone (OHPG) in clinical samples. The chromatograms presented here clearly demonstrate the sensitivity and specificity benefits of using DMS when performing high-throughput analysis of clinical specimens. Additionally, a full validation of cortisol and cortisone measurements in urine was performed to demonstrate the robustness, reproducibility, and suitability of the technology for routine clinical testing.
2. Materials and methods
The Mayo Clinic Institutional Review Board (IRB)/Ethics Committee ruled that approval was not required for this study. Patient samples were deidentified prior to use in this study.
2.1. Chemicals and reagents
LC-MS grade Methanol (MeOH), acetonitrile (ACN), isopropyl alcohol (IPA), formic acid, and ammonium formate, ammonium acetate, hexane, methylene chloride, were purchased from MilliporeSigma (Burlington, MA). Hydrochloric acid (HCl) and acetone were purchased from Fisher Scientific (Hampton, NH).
2.2. Measurements of androstenedione and 17-Hydroxyprogesterone in serum
2.2.1. Preparation of androstenedione and 17-Hydroxyprogesterone Calibrators, QC, and samples
This method has been described previously; thus, it is described here briefly with method changes [29]. OHPG (catalog#-H-085) and ANST (catalog#-A-075) calibration materials were purchased from Cerilliant (Round Rock, TX). Calibration material was diluted and spiked into 1 % BSA to yield calibration levels of 16, 40, 200, 1000, 2500, and 4000 ng/dL. Four levels of quality controls samples (QC) are produced by spiking calibration material into charcoal stripped human serum (SeraCare Life Sciences, Milford, MA, catalog#-HS-230) to produce final concentrations of 60, 100, 700, and 2,000 ng/dL. Isotopically labeled ANST was purchased from IsoSciences (catalog#- S14216) and isotopically labeled OHPG was purchased from Cerilliant (catalog#-H-096). These solutions were used to create a working internal standard (IS) solution of 30 ng/mL by diluting in 70 % methanol. Calibrators, QC, and samples were prepared for analysis by placing 100 μL into a 96 well plate followed by the addition of 25 μL of IS solution and 370 μL of acetonitrile. Samples were then vortexed for 30 sec and centrifuged for 15 min at 3000 × g.
2.2.2. LC-MS/MS analysis of androstenedione and 17-Hydroxyprogesterone
The supernatant (50 μL) of the sample preparation process outlined above was injected for LC-MS/MS analysis using a Thermo Scientific (Waltham, MA) TLX-2 system. The LC method is shown in Table S1 and the mobile phases are shown in Table S2. We purified samples by using a 4x3 mm C18 Security Guard Cartridge (Phenomenex catalog#-AJO-4287, Torrance, CA) followed by separation on a 50 × 3.0 mm Accucore RP-MS column (ThermoFisher Scientific catalog #-17626–053030). The LC eluates underwent mass spectrometry analysis using a Sciex 6500 + mass spectrometer equipped with a SelexION DMS system. All compounds were optimized by infusion of target compounds and using a tee to simulate LC elution conditions. First, separation voltage (SV) was set to 3875 V and the software ramping function was used to determine the optimum compensation voltage and DMS offset parameters. The SelexION has five options for resolution that range in specification of full width at half max of the compensation voltage transmission window from 0.6 ± 0.2 (high resolution) to 3.7 ± 0.4 (open resolution). Higher resolution comes at the expense of transmission, and it was determined that open or low resolution was appropriate for the applications shown here. The MS/MS parameters with and without SelexION are shown in Table S3. Source parameters for SelexION versus no SelexION can be seen in Tables S4 and S5, respectively.
2.3. Measurement of 24, 25 dihydroxyvitamin D in serum
2.3.1. Preparation of 24, 25 dihydroxyvitamin D Calibrators, QC, and samples
This method was previously published [30] and is described here only briefly. Calibration material for 24, 25 dihydroxyvitamin D2 and D3 was purchased from Medical Isotopes, Inc. (Pelham, NH, catalog#-18821 and 18866). Calibrators were prepared by spiking both D2 and D3 into vitamin D depleted serum purchased from SeraCare Life Sciences (catalog#-502079-1L) to yield final concentrations of 0.1, 0.5, 1, 5,10, and 25 ng/mL. Quality control samples were prepared by spiking materials in the same manner as calibrators at concentrations of 0.75, 7.5, and 20 ng/mL. 24,25 dihydroxyvitamin D2-d3 was purchased from IsoSciences (Ambler, PA, catalog#-13043) and 24,25 dihydroxyvitamin D3-d6 was purchased from Toronto Research Chemicals (North York, ON, Canada, catalog#-D455087). The labeled materials were used to make a working internal standard with a concentration of 25 ng/mL for both D2 and D3 in 70 % MeOH.
When preparing samples, 500 μL was placed into a glass tube followed by the addition of 50 μL of the working IS solution. The solution was then vortexed and incubated for 15 min. Next, 0.5 mL of 0.2 M HCl was added. 24,25 dihydroxyvitamin D was then enriched using solid phase extraction (SPE) with cartridges purchased from Agilent (Santa Clara, CA, catalog#-1210C18OHT) and positive pressure displacement. Cartridges were preconditioned with 2.5 mL of MeOH and then 2.5 mL of water followed by the addition of the sample. Cartridges were washed with 5 mL of 70 % MeOH and then 2 mL of 90:10 hexane:methylene chloride, and then sample was eluted with 5 mL of 90:10 hexane:isopropanol. This solution was then dried at 45 °C and extracts were reconstituted in 0.002 % 4-Phenyl-1,2,4-triazoline-3,5-dione (MilliporeSigma) in ACN to be derivatized while drying at 60 °C. The final derivatized product was then reconstituted in 150 μL of 70 % MeOH.
2.3.2. LC-MS/MS analysis of 24, 25 dihydroxyvitamin D
Instrumentation used was as described in section 2.2.2. This method did not use TurboFlow online purification. The injection volume was 50 μL. Mobile phase A was 1 mM Ammonium Acetate in water and mobile phase B was 100 % methanol. The analytical column was a 2.1x50 mm Agilent (Santa Clara, CA) XDB-C8 (catalog#- 971700–906). The LC method can be seen in Table S6. The mass spectrometry parameters are shown in Table S7 and source conditions with the SelexION are in Table S8 and without are in Table S9.
2.4. Measurement of cortisol and cortisone in urine
2.4.1. Preparation of cortisol and cortisone calibrators, QC, and samples
This method was originally published by Taylor et al. in 2002 [31] and changes to the method are outlined here. Cortisol (catalog#-C-106) and cortisone (catalog#-C-130) calibration materials were purchased from Cerilliant. Calibration levels were 0.08, 0.25, 1, 4, 10, and 20 μg/dL in stripped urine purchased from BioChemed Services (Winchester, VA). QC samples were also prepared by spiking calibration material into stripped urine at concentrations of 0.16, 1.5, 4, and 10 μg/dL. Deuterated ISs were purchased from IsoSciences and used at a working concentration of 0.03 μg/mL cortisol and 0.06 ug/mL cortisone. Samples were prepared for analysis by first putting 100 μL urine into a 96 well plate followed by the addition of 50 μL of working IS solution and vortex mixing.
2.4.2. LC-MS/MS analysis of cortisol and cortisone
The solution described in the previous section was then injected directly (30 μL) for online purification and LC-MS/MS analysis. Instrumentation used was as described in section 2.2.2. The LC method is shown in Table S10 and mobile phases can be seen in Table S11. The parameters for mass spectrometry analysis are shown in Table S12 and source conditions with the SelexION are in Table S13 and without are in Table S14.
2.4.3. Validation of cortisol and cortisone in urine using LC-DMS-MS/MS
This method was validated in accordance with Clinical & Laboratory Standards Institute (CLSI) guidelines. Intraassay and interassay imprecision were tested using 20 replicate measurements of five pools with concentrations starting at the lower limit of quantitation (LLOQ) and spanning the analytical measuring range (AMR). The acceptance criterium for precision was less than 20 % CV at the LLOQ and less than 10 % for the other levels. The limit of the blank (LOB) was established by 20 replicate measurements of extracted blank matrix across 10 days using the calculation: LOB = Mean of blank + unidirectional Z-score for 95 % probability (=1.65) × SD of the blank. The limit of detection (LOD) was established by spiking concentrations above the upper 95 % boundary of the LOB (again 20 replicates over 10 days) using the corresponding calculation: LOD = LOB + unidirectional Zscore for 95 % probability (=1.65) × SD of the low-sample pool. Only the primary fragment ion transition was used for LOD/LOB studies. To be considered acceptable, the LOD had to be less than the LLOQ.
Linearity across the analytical measuring range was confirmed by serial dilutions of five patient samples and subsequent linear regression analysis had to yield a slope of 1 ± 0.1 and a R2 ≥ 0.98 to be considered acceptable. Carryover was monitored by injecting a blank sample after highest calibrator during each analysis and the resulting calculated concentration had to be less than the LLOQ to be acceptable. Accuracy was confirmed by comparing results with an established LC-MS/MS test using the same extraction protocol, but without the use of DMS. Results were compared using Passing-Bablock regression analysis and the acceptance criteria were a slope of 1 ± 0.1 and a r ≥ 0.98.
The potential impact of ion suppression or enhancement was assessed using ten randomly selected patient samples with low cortisol and cortisone concentration. Post-extraction, cortisol and cortisone were spiked into the reconstitution solvent at three levels across the AMR. The resulting signal was compared to spikes in reconstitution solvent in the absence of the matrix to yield percent suppression/enhancement. Interference from common drugs and steroids was also tested and is discussed further in the Results and Discussion section below. The resulting concentration from analysis had to be less than the LLOQ to not be considered an interference.
2.5. Data processing
Following acquisition, all data files were further processed and integrated in Analyst version 1.7.2. S/N was determined in Analyst by defining a 0.5 min noise region with minimal interfering signals. The same noise region was used for the data generated with and without the SelexION. The integrated signal was then automatically divided by calculated noise to determine the S/N. Data were exported to Microsoft Excel for further processing and plotting. Passing-Bablock regression analysis was performed using Analyse-it (Leeds, UK).
3. Results and discussion
The primary aim of this study was to assess the potential of using DMS to enhance the S/N and reduce interferences when performing quantitative analysis of steroids. To achieve this, samples were prepared and injected consecutively on the same LC-MS/MS system, with and without the SelexION DMS system. The only changes made between the two datasets were the optimized parameters for source conditions, depending on whether the SelexION was installed or removed.
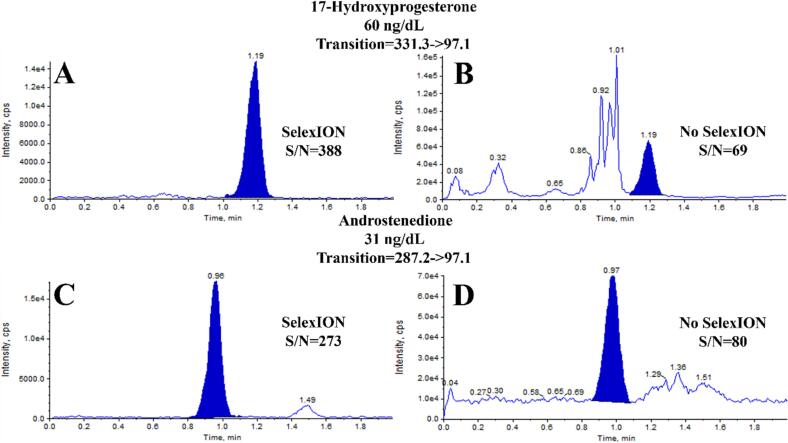
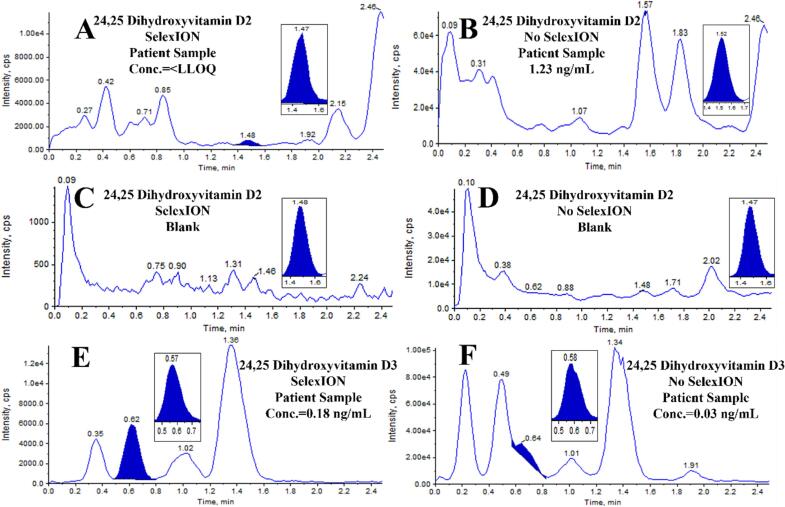
The first comparison focused on the measurement of OHPG and ANST in serum. The chromatograms in Fig. 1 demonstrate the differences when using or not using the SelexION device. In the case of OHPG (Fig. 1A & B) and ANST (Fig. 1C & D), using the DMS system resulted in a reduction in both signal and noise, as well as potential interferences. Despite the drop in signal, there was a disproportionately larger decrease in noise and interferences, leading to an approximate 6-fold improvement in S/N. A similar comparison was made for 24,25-dihydroxyvitamin D2 (Fig. 2A–D) and 24,25-dihydroxyvitamin D3 (Fig. 2E & F). Without DMS, a chromatographic peak corresponding to the retention time of the IS was observed for 24,25-dihydroxyvitamin D2 (Fig. 2B). However, when the DMS device was used, this signal was eliminated (Fig. 2A). For reference, extracted blanks are shown in Fig. 2C & D. Given the high prevalence of patient samples with undetectable levels of 24,25-dihydroxyvitamin D2 in the normal population and the inability of that signal to be detected when utilizing an additional level of separation, it is likely that this peak was an interference and would be falsely integrated as 24,25 dihydroxyvitamin D2 if DMS was not used, providing evidence of the specificity advantages offered by the DMS technology. Regarding 24,25-dihydroxyvitamin D3, it was not possible to integrate the chromatographic peak, which would have resulted in an inability to report a test result without DMS (Fig. 2F). However, with the DMS system, a clear and detectable signal with sufficient S/N was achieved (Fig. 2E), enabling reporting of the test result.
Fig. 1.
Comparison of chromatograms from measurements of OHPG and ANST performed with and without the use of SelexION DMS. A) OHPG with SelexION, B) OHPG without SelexION, C) ANST with SelexION, D) ANST without SelexION.
Fig. 2.
Comparison of chromatograms from measurements of 24,25 dihydroxyvitamin D2 (transition 586.35->298.2) and D3 (transition 574.35->298.2) performed with and without the use of SelexION DMS. For retention time reference, the internal standard signal is inset. A) D2 in patient sample with SelexION, B) D2 in patient sample without SelexION, C) D2 extracted blank with SelexION D) D2 Extracted blank without SelexION E) D3 in patient sample with SelexION, F) D3 in patient sample without SelexION.
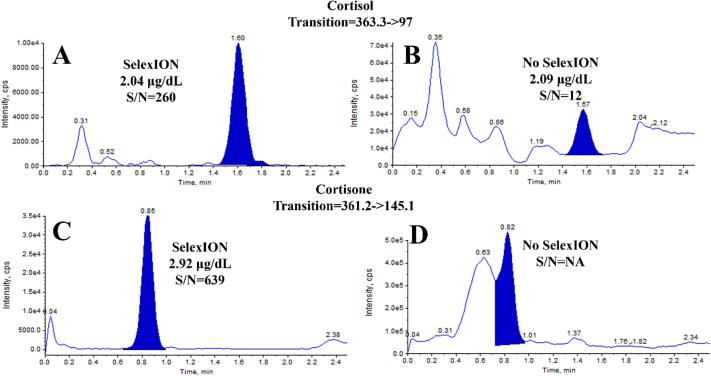
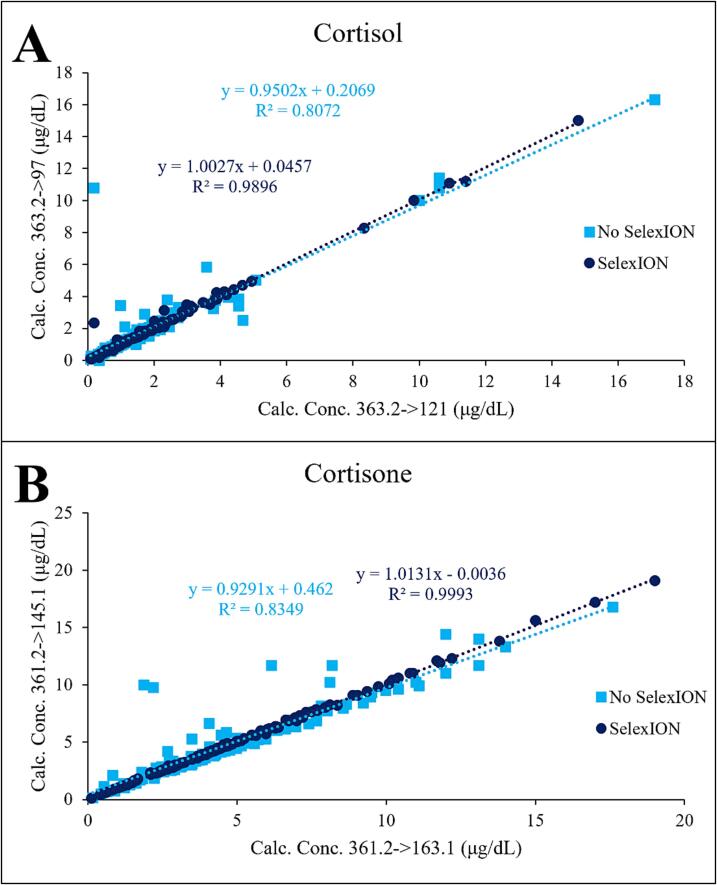
The final comparison was conducted on cortisol (Fig. 3A & B) and cortisone (Fig. 3C & D) in urine testing. Similar to the previously discussed analytes, these chromatograms show a significant increase in S/N for cortisol (20x) and the elimination of a major interference for cortisone. Additionally, the use of DMS for cortisol and cortisone provides another advantage, as depicted in Fig. 4, which shows a linear regression comparison of the calculated concentration results from the qualifier/confirmatory fragment ion and the quantifier/primary fragment. The SelexION device improved the agreement, as indicated by both the slope and the increase in R2. This improvement holds considerable significance since many tests require the fragment ion results to be within 20 %. Therefore, enhancing this agreement instills greater confidence in the analysis and enables a larger number of sample results to be provided to patients and clinicians. Table 1 presents an overall comparison of the average increase in S/N for all patient samples tested. With the use of the SelexION DMS device, the average S/N improvements ranged from 1.6 to 13.8 when compared to not using DMS.
Fig. 3.
Comparison of chromatograms from measurements of cortisol and cortisone performed with and without the use of SelexION DMS. A) cortisol with SelexION, B) cortisol without SelexION, C) cortisone with SelexION, D) cortisone without SelexION.
Fig. 4.
Linear regression comparison of the calculated concentration results from the qualifier/confirmatory fragment ion and the quantifier/primary fragment for (A) cortisol and (B) cortisone.
Table 1.
Average S/N increase when using SelexION DMS compared to not using SelexION DMS. The S/N of the chromatogram with the SelexION was divided by the S/N of the chromatogram without the SelexION to yield a S/N fold improvement for each patient sample. These results were then averaged. The number of patient samples with results within the AMR are also shown.
| Analyte | ANST | OHPG | D2 | D3 | Cortisol | Cortisone |
|---|---|---|---|---|---|---|
| Number of Patient Samples | 18 | 18 | 16 | 32 | 130 | 129 |
| Ave. Fold S/N Increase with SelexION | 2.4 | 6.7 | 2.2 | 1.6 | 13.8 | 7.7 |
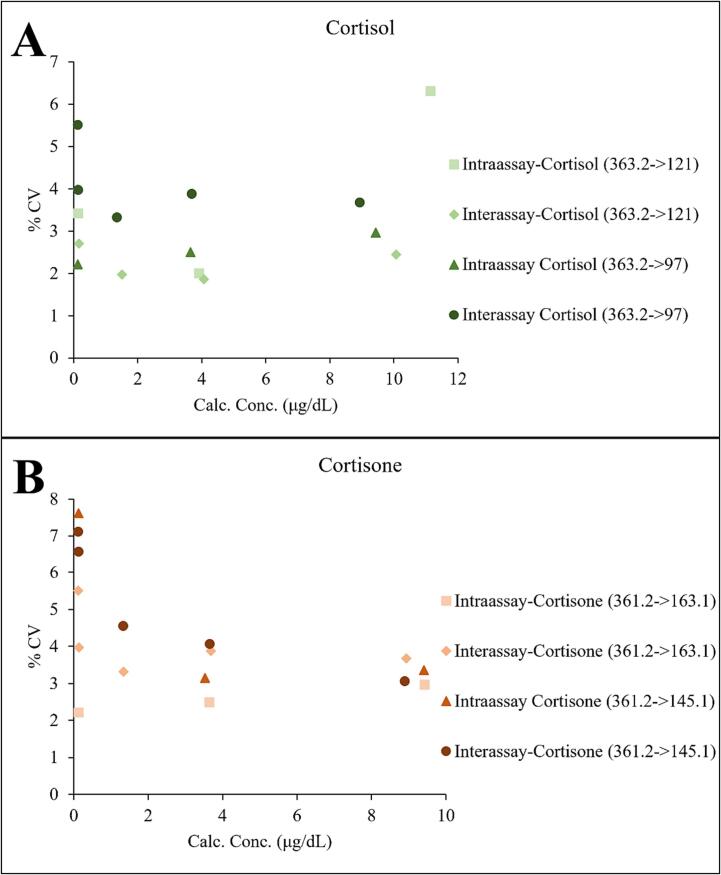
Given the apparent benefits from the use of the SelexION DMS device in terms of reducing interferences and improving S/N, we elected to fully validate the technology for the measurement of cortisol and cortisone in urine. The precision from 20 replicate measurements was excellent, with the %CV being below 8 % at all levels (Fig. 5). The calculated LOB and LOD for cortisol were 0.009 μg/dL and 0.047 μg/dL, respectively. For cortisone, the LOB was 0.017 μg/dL and the LOD was 0.026 μg/dL. The LOD met acceptance criteria for both analytes. Linear regression analysis of the linearity study for cortisol is shown in Fig. S1, and for cortisone it is shown in Fig. S2. The acceptance criteria were met for all linearity studies. The observed carryover after the highest calibrator was less than 30 % of the LLOQ, meeting the acceptance criteria.
Fig. 5.
Intra and interassay precision of replicate measurements of (A) cortisol and (B) cortisone in urine (n = 20). Data from both transitions for each analyte are shown.
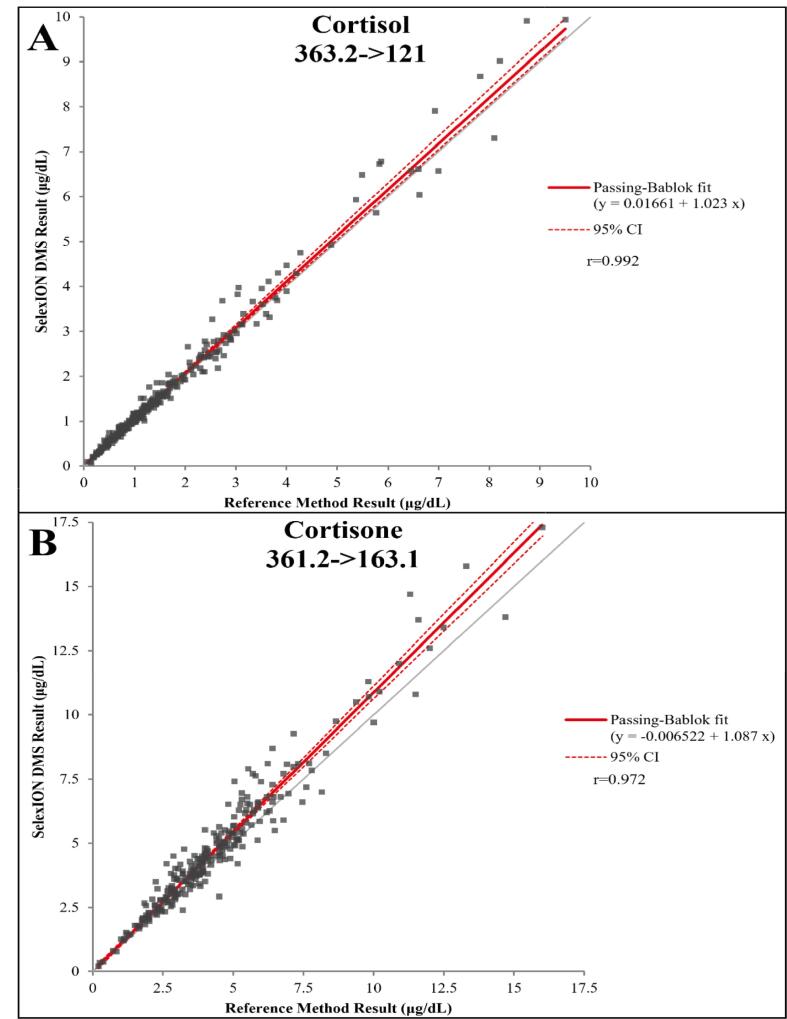
Passing-Bablok regression analysis for assessment of cortisol and cortisone accuracy can be seen in Fig. 6 for the primary/quantifier fragment ion transitions and Fig. S3 for the secondary/qualifier fragment ion transitions. For this analysis, samples with obvious interferences observed in the chromatograms, or a greater than 20 % difference between the ion transitions, were removed for the reference method to minimize the effects of the readily apparent limitations on the comparison. After the removal of samples with interference and those outside the AMR, 267 samples for cortisol and 261 samples for cortisone were included in the Passing-Bablok regression analysis. The results from all four Passing-Bablok comparisons exceeded the acceptance criteria for r and slope and indicated strong agreement. Based on the ionization suppression/enhancement study, cortisol exhibited 1.4 % suppression, and cortisone exhibited 0.7 % suppression after IS correction, suggesting that matrix effects are minimal when performing these analyses. Additionally, the compounds shown in Table S15 were tested as possible interferents at the shown concentrations, totaling 78 interference studies. No interferences were observed.
Fig. 6.
Passing-Bablok regression analysis comparing reference method results to SelexION DMS Results for (A) cortisol and (B) cortisone. The primary fragment ion transition comparison is shown. N = 267 for cortisol and 261 for cortisone.
This study shows that the use of DMS enhances the specificity of the analysis without sacrificing other aspects of analytical performance. However, a limitation of this study was that full validation was only conducted for two compounds, due to resource and time constraints. Moving forward, we intend to assess the potential of DMS to improve analyses of additional compounds through small studies and additional validations. A potential benefit that was not addressed in this study, but is a future goal, is the potential for DMS to improve throughput by eliminating the need for long, complicated chromatographic methods. Instead, it would enable shorter separation and run times. It is conceivable that using DMS will provide benefits for most analytes, not just interference-prone and challenging analytes, as was the focus of this study.
4. Conclusions
This study provided further evidence that DMS is a powerful complement to established LC-MS/MS instrumentation, improving the specificity of steroid analyses, which are notoriously prone to interference and cross-reactivity. For the six steroids tested, DMS reduced the prevalence of interference and improved the S/N of the analysis. Validation studies performed for cortisol and cortisone in urine demonstrated exceptional analytical performance when DMS was utilized, as all validation studies met acceptance criteria. Of note, the %CVs observed were all less than 10 % and the calculated concentration results when using DMS showed excellent agreement with a reference method. Based on the results of this investigation, DMS has the potential to greatly benefit not just clinical measurements of challenging analytes, but also most clinical LC-MS/MS analyses.
CRediT authorship contribution statement
Yubo Chai: Validation, Formal analysis, Writing – review & editing. Stefan K.G. Grebe: Supervision, Writing – review & editing. Anthony Maus: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
N/A
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2023.10.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Holst J.P., Soldin O.P., Guo T., Soldin S.J. Steroid hormones: relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004;24(1):105–118. doi: 10.1016/j.cll.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew R. Clinical measurement of steroid metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2001;15(1):1–16. doi: 10.1053/beem.2001.0116. [DOI] [PubMed] [Google Scholar]
- 3.Taylor A.E., Keevil B., Huhtaniemi I.T. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015;173(2):D1–D12. doi: 10.1530/EJE-15-0338. [DOI] [PubMed] [Google Scholar]
- 4.Krasowski M.D., Drees D., Morris C.S., Maakestad J., Blau J.L., Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin. Pathol. 2014;14(1):33. doi: 10.1186/1472-6890-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casals G., Costa R.F., Rull E.U., Escobar-Morreale H.F., Argente J., Sesmilo G., Biagetti B. Recommendations for the measurement of sexual steroids in clinical practice. A position statement of SEQCML/SEEN/SEEP. Adv. Lab. Med. Av. En Med. Lab. 2023;4(1):52–60. doi: 10.1515/almed-2023-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosner W., Hankinson S.E., Sluss P.M., Vesper H.W., Wierman M.E. Challenges to the measurement of estradiol: an endocrine society position statement. J. Clin. Endocrinol. Metab. 2013;98(4):1376–1387. doi: 10.1210/jc.2012-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finšgar M., Perva-Uzunalić A., Behr H., Ledinek N., Knez Ž., Novak Z. An improved reversed-phase high-performance liquid chromatography method for the analysis of related substances of prednisolone in active ingredient. ACS Omega. 2020;5(14):7987–8000. doi: 10.1021/acsomega.0c00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matysik S., Liebisch G. Quantification of steroid hormones in human serum by liquid chromatography-high resolution tandem mass spectrometry. J. Chromatogr. A. 2017;1526:112–118. doi: 10.1016/j.chroma.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Kushnir M.M., Song B., Yang E., Frank E.L. Development and clinical evaluation of a high-throughput LC–MS/MS Assay for Vitamin B6 in human plasma and serum. J. Appl. Lab. Med. 2021;6(3):702–714. doi: 10.1093/jalm/jfaa166. [DOI] [PubMed] [Google Scholar]
- 10.Neal S.P., Wilson K.M., Velosa D.C., Chouinard C.D. Targeted Glucocorticoid Analysis Using Ion Mobility-Mass Spectrometry (IM-MS) J. Mass Spectrom. Adv. Clin. Lab. 2022;24:50–56. doi: 10.1016/j.jmsacl.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rister A.L., Dodds E.D. Steroid analysis by ion mobility spectrometry. Steroids. 2020;153 doi: 10.1016/j.steroids.2019.108531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouinard C.D., Wei M.S., Beekman C.R., Kemperman R.H.J., Yost R.A. Ion mobility in clinical analysis: current progress and future perspectives. Clin. Chem. 2016;62(1):124–133. doi: 10.1373/clinchem.2015.238840. [DOI] [PubMed] [Google Scholar]
- 13.Hooshfar S., Tchu S., Yun C., Lynch K.L. Development of a high-throughput differential mobility separation-tandem mass spectrometry (DMS-MS/MS) method for clinical urine drug testing. J. Mass Spectrom. Adv. Clin. Lab. 2022;23:50–57. doi: 10.1016/j.jmsacl.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chouinard C.D., Beekman C.R., Kemperman R.H.J., King H.M., Yost R.A. Ion mobility-mass spectrometry separation of steroid structural isomers and epimers. Int. J. Ion Mobil. Spectrom. 2017;20(1):31–39. doi: 10.1007/s12127-016-0213-4. [DOI] [Google Scholar]
- 15.Chouinard C.D., Cruzeiro V.W.D., Roitberg A.E., Yost R.A. Experimental and theoretical investigation of sodiated multimers of steroid epimers with ion mobility-mass spectrometry. J. Am. Soc. Mass Spectrom. 2017;28(2):323–331. doi: 10.1007/s13361-016-1525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei M.S., Kemperman R.H.J., Palumbo M.A., Yost R.A. Separation of structurally similar anabolic steroids as cation adducts in FAIMS-MS. J. Am. Soc. Mass Spectrom. 2020;31(2):355–365. doi: 10.1021/jasms.9b00127. [DOI] [PubMed] [Google Scholar]
- 17.Oranzi N.R., Kemperman R.H.J., Wei M.S., Petkovska V.I., Granato S.W., Rochon B., Kaszycki J., La Rotta A., Jeanne Dit Fouque K., Fernandez-Lima F., Yost R.A. Measuring the integrity of gas-phase conformers of sodiated 25-hydroxyvitamin D3 by drift tube, traveling wave, trapped, and high-field asymmetric ion mobility. Anal. Chem. 2019;91(6):4092–4099. doi: 10.1021/acs.analchem.8b05723. [DOI] [PubMed] [Google Scholar]
- 18.Delvaux A., Rathahao-Paris E., Alves S. An emerging powerful technique for distinguishing isomers: trapped ion mobility spectrometry time-of-flight mass spectrometry for rapid characterization of estrogen isomers. Rapid Commun. Mass Spectrom. 2020;34(24):e8928. doi: 10.1002/rcm.8928. [DOI] [PubMed] [Google Scholar]
- 19.Cole R.B., Bayat P., Murray J.S., Albers C., Brombach D. “Conformation Pinning” by Anion Attachment Enabling Separation of Isomeric Steroid Monomers by Ion Mobility Spectrometry. J. Mass Spectrom. 2020;55(12):e4657. [Google Scholar]
- 20.Chouinard C.D., Cruzeiro V.W.D., Kemperman R.H.J., Oranzi N.R., Roitberg A.E., Yost R.A. Cation-dependent conformations in 25-hydroxyvitamin D3-cation adducts measured by ion mobility-mass spectrometry and theoretical modeling. Int. J. Mass Spectrom. 2018;432:1–8. doi: 10.1016/j.ijms.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rister A.L., Martin T.L., Dodds E.D. Formation of multimeric steroid metal adducts and implications for isomer mixture separation by traveling wave ion mobility spectrometry. J. Mass Spectrom. 2019;54(5):429–436. doi: 10.1002/jms.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahonen L., Fasciotti M., Gennäs G.B.a., Kotiaho T., Daroda R.J., Eberlin M., Kostiainen R. Separation of steroid isomers by ion mobility mass spectrometry. J. Chromatogr. A. 2013;1310:133–137. doi: 10.1016/j.chroma.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 23.Maddox S.W., Fraser Caris R.H., Baker K.L., Burkus-Matesevac A., Peverati R., Chouinard C.D. Ozone-Induced Cleavage of Endocyclic C═C Double Bonds within Steroid Epimers Produces Unique Gas-Phase Conformations. J. Am. Soc. Mass Spectrom. 2020;31(2):411–417. doi: 10.1021/jasms.9b00058. [DOI] [PubMed] [Google Scholar]
- 24.Maddox S.W., Olsen S.S.H., Velosa D.C., Burkus-Matesevac A., Peverati R., Chouinard C.D. Improved identification of isomeric steroids using the Paternò-Büchi reaction with ion mobility-mass spectrometry. J. Am. Soc. Mass Spectrom. 2020;31(10):2086–2092. doi: 10.1021/jasms.0c00215. [DOI] [PubMed] [Google Scholar]
- 25.Graton J., Hernández-Mesa M., Normand S., Dervilly G., Le Questel J.-Y., Le Bizec B. Characterization of steroids through collision cross sections: contribution of quantum chemistry calculations. Anal. Chem. 2020;92(8):6034–6042. doi: 10.1021/acs.analchem.0c00357. [DOI] [PubMed] [Google Scholar]
- 26.May J.C., Morris C.B., McLean J.A. Ion mobility collision cross section compendium. Anal. Chem. 2017;89(2):1032–1044. doi: 10.1021/acs.analchem.6b04905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guddat S., Thevis M., Kapron J., Thomas A., Schänzer W. Application of FAIMS to anabolic androgenic steroids in sport drug testing. Drug Test. Anal. 2009;1(11–12):545–553. doi: 10.1002/dta.73. [DOI] [PubMed] [Google Scholar]
- 28.Arthur K.L., Turner M.A., Brailsford A.D., Kicman A.T., Cowan D.A., Reynolds J.C., Creaser C.S. Rapid analysis of anabolic steroid metabolites in urine by combining field asymmetric waveform ion mobility spectrometry with liquid chromatography and mass spectrometry. Anal. Chem. 2017;89(14):7431–7437. doi: 10.1021/acs.analchem.7b00940. [DOI] [PubMed] [Google Scholar]
- 29.Singh, R. J. Quantitation of 17-OH-Progesterone (OHPG) for Diagnosis of Congenital Adrenal Hyperplasia (CAH). In Clinical Applications of Mass Spectrometry: Methods and Protocols; Garg, U., Hammett-Stabler, C. A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2010; pp 271–277. https://doi.org/10.1007/978-1-60761-459-3_25. [DOI] [PubMed]
- 30.Ketha H., Kumar R., Singh R.J. LC-MS/MS for Identifying Patients with CYP24A1 Mutations. Clin. Chem. 2016;62(1):236–242. doi: 10.1373/clinchem.2015.244459. [DOI] [PubMed] [Google Scholar]
- 31.Taylor R.L., Machacek D., Singh R.J. Validation of a High-Throughput Liquid Chromatography-Tandem Mass Spectrometry Method for Urinary Cortisol and Cortisone. Clin. Chem. 2002;48(9):1511–1519. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.