Abstract
Context
The American Thyroid Association risk stratification (ATA) and the American Joint Committee on Cancer Tumor Node Metastases (TNM) predict recurrence and mortality of differentiated thyroid cancer (DTC). BRAFV600E and TERT promoter mutations have been shown to correlate with the histopathological features and outcome of DTC. Our objectives were to study the correlation of these molecular markers with these clinicopathological-staging systems.
Patients and methods
We studied 296 unselected patients, 214 females and 82 males with a median age of 36 years (IQR 23.3-49.0). BRAFV600E and TERT promoter mutations were tested by PCR-based Sanger sequencing. Data were extracted from medical records and analysed using Chi-Square and Fisher Exact tests and Kaplan Meier analysis.
Results
Of 296 patients tested, 137 (46.3%) had BRAFV600E-positive tumors and 72 (24.3%) were positive for TERT promoter mutations. The BRAFV600E mutation did not correlate with the ATA and TNM staging, being non-significantly different in various stages of these systems and did not predict the development of persistent disease (PD) (P 0.12). Unlike BRAFV600E, TERT promoter mutations were more frequent in the ATA high-risk than in intermediate- or low-risk tumors (P 0.006) and in TNM stages III and IV than lower stages (P <0.0001). TERT promoter mutations also predicted the outcome, being present in 37.2% of patients with PD compared to only 15.4% in those without evidence of disease (P <0.0001). The same pattern was also seen when BRAFV600E and TERT promoter mutations were combined.
Conclusion
TERT promoter mutations alone or in combination with BRAFV600E mutation, but not BRAFV600E mutation alone, correlated well with the ATA and TNM staging and predicted development of PD, especially in higher stages of these systems.
Keywords: thyroid cancer, differentiated thyroid cancer, BRAFV600E , TERT promoter mutations, risk stratifications
1. Introduction
Differentiated thyroid cancer (DTC) has been increasingly diagnosed over the past four decades (1). This increase in incidence is largely attributable to improved diagnostic tools, particularly the widespread use of neck ultrasonography (1, 2). As a result, many small tumors with low risk of metastasis, recurrence, and mortality have been detected (3). This fact led to a more conservative approach to the management of DTC based on risk stratification (4, 5). Currently, several risk stratification systems are available for risk-based management planning (6–9). Two of the most widely used systems are the American Thyroid Association (ATA) and the American Joint Committee on Cancer Tumor Node and Metastasis (AJCC TNM) risk stratification systems (7, 8). The ATA system predicts risk of DTC recurrence, while the TNM system predicts risk of DTC-related mortality (6, 7, 9). Both systems have been well validated and are routinely used in clinical practice and research communications (9–11).
Advances in understanding the molecular pathogenesis of DTC have paralleled better understanding of its clinical behavior and outcome and the development of risk stratification systems (12–17). These advances in molecular genetics led to development of diagnostic tests and therapeutic agents for patients with thyroid nodules and advanced thyroid cancer, respectively (14, 18–20). The use of molecular markers for prognostication of DTC has also been studied but remains controversial and less mature than diagnostic and therapeutic advances (7, 21, 22). One of the earliest discoveries is the BRAFV600E mutation as a major oncogenic driver in papillary thyroid cancer (PTC) and to a lesser extent in poorly differentiated (PDTC) and anaplastic thyroid cancer (ATC) (15, 23, 24). Several studies have shown a strong association between BRAFV600E mutation and aggressive histopathological features of DTC (25–28). However, others questioned its prognostic value (29, 30). More recently, TERT promoter mutations (C250T and C228T) were discovered as strong oncogenic drivers in many types of thyroid cancer (31–33). They occur in approximately 10% of well-differentiated PTC but are increasingly commoner in the more aggressive types such as PDTC and ATC (13). Although these mutations are associated with aggressive histopathological features and worse outcome of DTC, especially when they co-occur with BRAFV600E or RAS mutations (34, 35), their use as prognostic markers is not yet widely accepted (7). The 2015 ATA guidelines acknowledge the potential prognostic value of BRAFV600E and TERT promoter mutations but do not fully endorse it or routinely recommend it (7). To further study the potential relationship between the clinicopathological staging systems and the driver mutations of DTC, we hypothesized that these mutations are more prevalent in higher ATA and TNM stages than the low-risk stages and that they may contribute further to risk stratification in different stages of these systems. For these reasons, we studied a cohort of patients with DTC in whom BRAFV600E and TERT promoter mutations have been tested and assessed their relationships with the ATA and TNM risk stratification systems. Specifically, we assessed the prevalence of BRAFV600E and TERT promoter mutations in different ATA and TNM stages and analysed their potential incremental prognostic value over these risk stratification systems.
2. Patients and methods
An Institutional Review Board (IRB) approval was obtained from the Office of Research Affairs, King Faisal Specialist Hospital and Research Centre, Riyadh Saudi Arabia (ORA # 2020-1514) with a waiver of consent to use archived Formalin Fixed Paraffin Embedded (FFPE) samples for mutation testing. We isolated tumor DNA, performed PCR and directly sequenced exon 15 of BRAF gene and the TERT promoter using the Dideoxy Chain Termination method. The DNA isolation, PCR primers and conditions, and the Sanger sequencing methods for BRAFV600E and TERT promoter mutations have been previously described (36–38). A total of 296 unselected DTC patients in whom BRAFV600E and TERT promoter mutations were available have been included in this study. Data on their demographics, histopathological data, ATA and TNM staging, management and outcome were obtained from their medical records. The outcome was assessed based on definitions included in the 2015 ATA guidelines (7). An excellent response (175 patients) was defined as absence of any evidence of disease with suppressed serum thyroglobulin (Tg) < 0.2 ng/dl and/or stimulated Tg < 1 ng/dl in the absence of Tg antibodies and negative imaging studies. Persistent disease included patients with structurally incomplete, biochemically incomplete and indeterminate response to therapy statuses as defined in the ATA guidelines for DTC (7).
2.1. Statistical methods
We expressed continuous variables as medians and interquartile ranges and categorical variables as rates, proportions and percentages. Fisher Exact and X2- tests were used for analysis of categorical variables and T test for continuous variables. Kaplan Meier survival analysis was used to analyse outcome over time stratified by ATA or TNM stages or presence or absence of BRAFV600E mutation and/or TERT promoter mutations. Disease-free survival is the time between the initial thyroid surgery and diagnosis of indeterminate, biochemically or structurally incomplete response (evidence of disease). A P value < 0.05 was considered significant.
3. Results
3.1. Clinicopathological characteristics
We studied 296 patients, 214 (73.3%) females, 82 (27.7%) males (F:M ratio 2.6:1) with a median age of 36 years (IQR 23.25-49 years). BRAFV600E mutation was significantly more prevalent in patients ≥ 55 years (29/45, 62%) than in those less than 55 years of age (109/251, 43.3%), P 0.03. Similarly, TERT promoter mutations were more prevalent in patients ≥ 55 years (28/45, 64.4%) than in those less than 55 years of age (43/251, 17%), P <0.0001. The histopathological characteristics, management and outcome are summarized in Table 1. The median follow up was 7.6 years (Interquartile range 5.25-10.1)
Table 1.
The clinical and pathological features, staging and outcome of 296 DTC patients.
| Characteristic | Median and IQR or No. (%) |
|---|---|
| Median age (IQR), years | 36 (23.25-49) |
| Sex F:M | 214:82 |
|
Tumor type
Classic papillary thyroid cancer (PTC) Follicular variant PTC Tall cell variant PTC Diffuse sclerosing PTC Follicular thyroid cancer Oncocytic (Hurthle) cell cancer |
181 (61) 62 (21) 34 (11.5) 8 (2.7) 7 (2.4) 4 (1.4) |
| Median Tumor size (range) cm | 2.5 (1.65-4.5) |
| Tumor multifocality | 188 (63.5) |
| Extra-thyroidal extension | 152 (51.4) |
| Lymphovascular invasion | 97 (32.8) |
| Lymph node metastases | 192 (64.9) |
| Distant metastases | 47 (15.9) |
|
ATA risk stratification
Low risk Intermediate Risk High risk |
96 (32.4) 125 (42.2) 75 (25.3 |
|
TNM stages
I II III IV |
99 (33.4) 150 (50.7) 27 (9.1) 20 (6.8) |
| Received radioactive iodine-131 | 259 (87.5%) |
| Median administered activity | 123 mCi (100-150) |
| Received additional therapies | 95 (32%) |
|
Outcome
No evidence of disease (Excellent response) Persistent disease (Indeterminate response, biochemically and structurally incomplete) |
175 (59) 121 (41) |
3.2. ATA and TNM risk stratification systems predict outcome of DTC
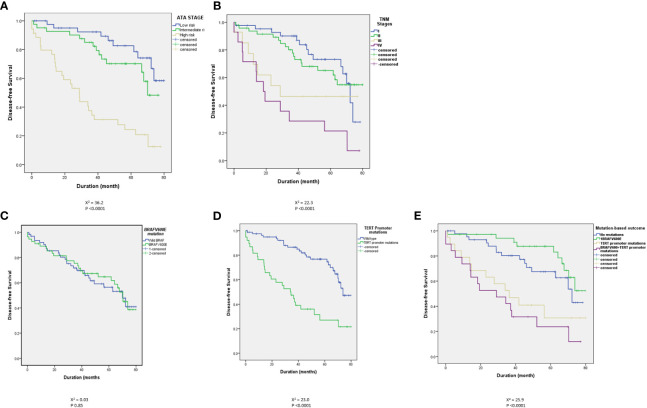
As demonstrated in many previous studies, in this study, the ATA and TNM risk stratification systems predict the outcome (Table 2 and Figure 1). Persistent disease increases from 19.8% in ATA low-risk to 72% in the high-risk classes (P<0.0001). Similarly, persistent disease increases from 31.3% in TNM stage I to 95% in stage IV (p <0.0001), (Table 2). Kaplan Meier analysis shows significant differences in the disease-free survival between different ATA and TNM stages (Figure 1). Of six patients who died due to DTC, five were ATA high grade and one ATA intermediate grade. Two were in TNM stage 2, two in stage 3 and two in stage 4.
Table 2.
Outcome of DTC in different ATA and TNM stages showing more persistent thyroid cancer in higher stages.
| ATA risk group | NED No. (%) |
PD No. (%) |
P value |
|---|---|---|---|
| Low risk | 77 (80.2) | 19 (19.8) | <0.0001 |
| Intermediate risk | 77 (64) | 48 (38.4) | |
| High risk | 21 (28.0) | 54 (72.0) | |
| TNM stages | |||
| I | 68 (68.7) | 31 (31.3) | <0.001 |
| II | 97 (64.7) | 53 (35.3) | |
| III | 9 (33.3) | 18 (66.7) | |
| IV | 1 (5) | 19 (95) | |
| Total | 175 (59) | 121 (41) | |
NED, no evidence of disease; PD, persistent disease.
Figure 1.
Kaplan Meier curves showing a clear and significant separation of disease-free survival (DFS) between different ATA stages (A) and different TNM stages (B). There was no statistically significant difference between BRAFV600E-positive and wild BRAF tumors (C) but there are statistically significant differences between TERT promoter mutation-positive tumors and tumors with wild type TERT promoter in the absence (D) or presence (E) of concomitant BRAFV600E mutation.
3.3. BRAFV600E mutation does not correlate with ATA and TNM staging
Of 296 patients tested, 137 (46.3%) had BRAFV600E-positive tumors while 159 patients (53.7%) had wild-BRAF tumors. BRAFV600E was positive in 92 of 181 (50.8%) conventional PTC, 18 of 62 (29%) follicular variant PTC (FVPTC), 25 of 34 (73.5%) tall cell varia PTC (TCPTC) and 2 of 8 (25%) diffuse sclerosing PTC (DSPTC). BRAFV600E mutation did not correlate with the ATA and TNM staging. As seen in Table 3, the rates of the mutation were not significantly different between low and higher stages. BRAFV600E also did not predict the outcome (Table 4 and Figure 1) with no difference in the rates of BRAFV600E mutation between those who achieved an excellent response (42.3%) and those who had a persistent disease (52.1%) (P 0.12), (Table 4). Six patients died of DTC in this cohort, four of them were BRAFV600E mutation-positive.
Table 3.
The prevalence of BRAFV600E and TERT promoter mutations in different ATA and TNM stages.
| Wild BRAF
No. (%) |
BRAFV600E
No. (%) |
P value | Wild TERT
No. (%) |
TERT promoter No. (%) |
P value | |
|---|---|---|---|---|---|---|
| ATA staging | ||||||
| Low risk | 54 (56.2) | 42 (43.8) | 0.33 | 74 (77.1) | 22 (22.9) | 0.007 |
| Intermediate risk | 61 (48.8) | 64 (51.2) | 103 (82.4) | 22 (17.6) | ||
| High risk | 44 (58.7) | 31 (41.3) | 47 (62.7) | 28 (37.3) | ||
| TNM staging | ||||||
| I | 55 (55.6) | 44 (44.4) | 0.41 | 96 (97.0) | 3 (3.0) | <0.001 |
| II | 77 (51.3) | 73 (48.7) | 104 (69.3) | 46 (30.7) | ||
| III | 18 (66.7) | 9 (33.3) | 19 (70.4) | 8 (29.6) | ||
| IV | 9 (45) | 11 (55) | 5 (25) | 15 (75) | ||
Table 4.
Outcome of DTC with respect to BRAFV600E, TERT promoter mutations or both.
| Total No. (%) |
NED No. (%) |
PD No. (%) |
P value | |
|---|---|---|---|---|
| BRAFV600E mutation | 137 (46.3) | 74/175 (42.3) | 63/121 (52.1) | 0.12 |
| TERT promoter mutations | 72 (24.3) | 27/175 (15.4) | 45/121 (37.2) | <0.0001 |
| BRAFV600E+TERT promoter mutations | 34 (11.5) | 8/175 (4.9) | 26/121 (21.5) | <0.0001 |
NED, no evidence of disease; PD, persistent disease.
3.4. TERT promoter mutations correlate with the ATA and TNM staging and predict outcome
TERT promoter mutations, C250T (9 tumors) and C228T (63 tumors) were found in tumors of 72 patients (24.3%). TERT promoter mutations were positive in 40/181 (22.1%) conventional PTC, 17/62 (27.4%) FVPTC, 9/34 (26.5%) TCPTC, 2/8 (25%) DSPTC, 3/7 (43%) follicular thyroid cancer (FTC) and ¼ (25%) Oncocytic thyroid cancer. Unlike BRAFV600E mutations, TERT promoter mutations were more frequent in the ATA high-risk (37.3%) than in intermediate- (17.6%) or low-risk tumors (22.9%) (P 0.007) (Table 3). More clearly is the higher prevalence of TERT promoter mutations in TNM stage IV (75%) than lower stages (Table 3). TERT promoter mutations also predicted the outcome, being present in 37.2% of patients with persistent disease compared to only 15.4% in those without evidence of disease (P <0.0001) (Table 4 and Figure 1). TERT promoter mutations were significantly more frequent in patients with structurally incomplete disease than other response to therapy status groups, being positive in only 19/175 (10.9%) in excellent response, 3/54 (5.6%) of indeterminate response, 3/14 (21.4%) of biochemically incomplete and 13/53 (24.5%) of structurally incomplete response (P <0.0001) (Table 5). Of six patients who died due to DTC, five (83%) were positive for TERT promoter mutations.
Table 5.
The outcome of 296 patients with DTC and its relationship to BRAFV600 and TERT promoter mutations.
| Excellent response No. (%) |
Indeterminate response No. (%) |
Biochemically incomplete No. (%) |
Structurally incomplete No. (%) |
P value | |
|---|---|---|---|---|---|
| BRAF Wild type | 101 (57.7) | 23 (42.6) | 5 (35.7) | 30 (56.6) | 0.12 |
| BRAFV600E | 74 (42.3) | 31 (57.4) | 9 (64.3) | 23 (43.4) | |
| TERT wild type | 148 (84.6) | 46 (85.2) | 5 (35.7) | 25 (47.2) | <0.0001 |
| TERT promoter mutated | 27 (15.4) | 8 (14.8) | 9 (64.3) | 28 (52.8) | |
| Total | 175 | 54 | 14 | 53 | 296 |
In a multivariate logistic regression model that included BRAFV600E mutation, TERT promoter mutations, age at diagnosis, tumor size, ATA stage and TNM stage, TERT promoter mutations remain a significant predictor of persistent disease (P 0.01, odds ratio 2.7, 95% CI 1.2-5.9).
3.5. Combination of BRAFV600E and TERT promoter mutations correlate with the ATA and TNM staging and predict outcome of DTC
The combination of BRAFV600E mutation and a TERT promoter mutation occurred in 34 cases (11.5%) and correlated well with high-risk ATA and higher TNM stages (Table 6). This combination occurred in 21.3% in high-risk ATA class compared to 8.8% and 7.3% in intermediate and low-risk stages, respectively (P 0.006, Table 6). It also occurred in 45% of TNM stage IV compared to 7.4%, 14.7% and 1% in stages III, II, and I, respectively (P < 0.0001) (Table 6). Of 34 cases that had this combination of BRAFV600E/mutated TERT, 26 (76.5%) continued to have persistent disease compared to only 8 (23.5%) in excellent response (P <0.0001) (Table 4). The percentages of patients with persistent disease increased progressively from 12.8% in patients with ATA low-risk and no mutations to 100% in patients with ATA high-risk with positive BRAFV600E/TERT promoter mutations (Table 7). Similarly, persistent disease increased progressively from 26.4% in stage I without BRAFV600E or TERT promoter mutations to 100% in stage IV disease with positive BRAFV600E/TERT promoter mutations (Table 7). In fact, all patients (100%) with BRAFV600E and TERT promoter mutation combination who are in ATA high-risk or TNM stage IV groups continued to have persistent disease (Table 7). The combination of TERT promoter/BRAFV600E mutations were significantly more frequent in patients with biochemically and structurally incomplete disease than other response to therapy status groups being positive in only 8/175 (4.6%) in excellent response, 5/54 (9.3%) of indeterminate response, 6/14 (42.9%) of biochemically incomplete and 15/53 (28.3%) of structurally incomplete response (P <0.0001).
Table 6.
Rates of different combinations of BRAFV600E and TERT promoter mutations in different ATA and TNM stages.
| Stage (Number of patients) | Wild BRAF/ wild TERT No. (%) |
BRAFV600E/ wild TERT No. (%) |
Wild BRAF/ Mutated TERT No. (%) |
BRAFV600E/ mutated TERT No. (%) |
P value |
|---|---|---|---|---|---|
| ATA staging | |||||
| Low-risk (96) | 39 (40.6) | 35 (36.5) | 15 (15.6) | 7 (7.3) | 0.006 |
| Intermediate risk (125) | 50 (40.0) | 53 (42.4) | 11 (8.8) | 11 (8.8) | |
| High-risk (75) | 32 (42.7) | 15 (20.0) | 12 (16) | 16 (21.3) | |
| TNM staging | |||||
| I (99) | 53 (53.5) | 43 (43.4) | 2 (2.0) | 1 (1.0) | <0.0001 |
| II (150) | 53 (35.3) | 51 (34.0) | 24 (16.0) | 22 (14.7) | |
| III (27) | 12 (44.4) | 7 (25.9) | 6 (22.2) | 2 (7.4) | |
| IV (20) | 3 (15.0) | 2 (10.0) | 6 (30) | 9 (45.0) | |
Table 7.
Number of cases with persistent disease/total number (%) in each ATA and TNM stage categorized by the presence and type of mutation.
| Stage | No mutations No. (%) |
BRAF only
No. (%) |
TERT only
No. (%) |
BRAF/TERT
No. (%) |
Total |
|---|---|---|---|---|---|
| ATA stages | P <0.0001 | ||||
| Low | 5/39 (12.8) | 9/35 (25.7) | 3/15 (20) | 2/7 (28.6) | 19/96 (19.8) |
| Intermediate | 16/50 (32.0) | 20/53 (37.7) | 4/11 (36.4) | 8/11 (72.7) | 48/125 (38.4) |
| High | 18/32 (56.2) | 8/15 (53.3) | 12/12 (100) | 16/16 (100) | 54/75 (72.0) |
| TNM stage | P <0.0001 | ||||
| I | 14/39 (26.4) | 16/43 (37.0) | 1/2 (50.0) | 0/1 (0) | 31/99 (31.3) |
| II | 15/53 (28.5) | 16/35 (31.4) | 7/24 (29.2) | 15/22 (68.5) | 53/150 (35.3) |
| III | 7/12 (58.3) | 4/7 (57.1) | 5/6 (83.3) | 2/2 (100) | 18/27 (66.7) |
| IV | 3/3 (100) | 1/2 (50.0) | 6/6 (100) | 9/9 (100) | 19/20 (95) |
4. Discussion
The ATA and TNM staging systems predict risk of recurrence and mortality, respectively (7–9). BRAFV600E and TERT promoter mutations have also been shown to predict risk of recurrence and mortality (26, 33, 39, 40). However, the relationship between these histopathological systems and molecular markers is not clear. In this study, we tried to analyse this potential relationship. Our findings confirm the previously shown high accuracy of the ATA and TNM staging systems in predicting the outcome (persistent disease) (7, 9) and lack of an association between BRAFV600E and these staging systems and the DTC outcome. However, the main finding of this study is the strong association between TERT promoter mutations and the ATA and TNM staging systems, and the high prognostic value of these mutations in isolation or in combination with BRAFV600E mutation in predicting the outcome of DTC, especially in the high ATA and TNM stages. The occurrence of these mutations in ATA high-risk or TNM stage IV tumors was associated with 100% chance of persistent disease. This suggests that TERT promoter mutations± BRAFV600E mutation identify a subgroup of patients in the high-risk ATA or TNM who have an extremely high risk of persistent disease. This group may need more proactive management and follow up approaches.
The association between TERT promoter mutations and aggressive histopathological features and outcome of DTC has been reported in several studies from different parts of the World. Similar to our study, a meta-analysis that included 11 studies and 3911 patients showed a graded risk of DTC based on the presence or absence of TERT promoter mutations and BRAFV600E mutation with the highest risk in DTC harboring both types of mutations followed by DTC with TERT mutation alone, BRAFV600E mutation and no mutation (41). In a more recent meta-analysis that included 51 studies with 11,382 patients from different populations, TERT promoter mutations were found in 10.9% of DTC in general and in 10.6% of PTC and 15.1% of FTC. In PTC, TERT promoter mutations were significantly associated with sex, age, tumor size, vascular invasion, extrathyroidal extension, lymph node and distant metastases, persistence/recurrence, and disease-specific mortality. Similarly, in FTC, TERT promoter mutations were significantly associated with age, distant metastases, advanced TNM stage, persistence/recurrence, and disease-specific mortality (42).
In another recent meta-analysis that looked at risk factors for development of radioiodine refractory thyroid cancer (RAIR), Luo Y. et al. included 13 studies with 1431 patients, of whom 603 were patients with RAIR. TERT and BRAFV600E mutations, extrathyroidal extensions and high-grade histopathological thyroid cancer subtypes were associated with increased risk of development of RAIR (43).
Over the past 3 decades, several staging systems have been proposed and validated (6, 8, 9). Although most of the old systems were designed to predict mortality of DTC, mortality is very low in DTC (2). On the other hand, persistent/recurrent DTC is common occurring in approximately 20-30% of patients (7, 44). Currently, the ATA risk stratification system, which encompasses several histopathological tumor features, is the most widely used system for predicting recurrence of DTC (7). It considers risk of DTC recurrence as a continuum but also classifies DTC into low-, intermediate- and high risk for recurrence (7, 45). Several studies have shown the robustness of this system for predicting recurrence and it is currently the most widely used system in clinical practice and research communication (10, 11, 46, 47). The AJCC TNM system is one of the mortality-predicting staging systems and is based on age and several histopathological features including tumor size, extrathyroidal invasion, lymph node and distant metastasis (8, 48). It has also been shown to be highly reliable in predicting cancer-specific mortality (8, 48).
The significant progress that took place in the field of molecular genetics of DTC was also translated in clinical practice to diagnostic tests for indeterminate thyroid nodules and therapeutic agents for progressive radioactive iodine refractory thyroid cancer (49–51). Due to conflicting studies and variable behavior of DTC carrying BRAFV600E or TERT promoter mutations, the use of these genetic markers in predicting the course and outcome of DTC remain controversial (7, 22, 52). In fact, the 2015 ATA thyroid cancer guidelines acknowledged the potential roles of these genetic markers for prognostication but did not fully endorse them as a basis for intensity of the management and follow up of patients with DTC (7).
Since the ATA and TNM staging systems are clinicopathological systems for predicting the outcome and BRAFV600E and TERT promoter mutations are potential predictors of outcome, we undertook this study to assess any potential relationship between these clinicopathological and molecular predictors of prognosis. Specifically, we aimed to study whether BRAFV600E and/or TERT promoter mutations may add incremental prognostic value to the ATA and TNM staging systems. Our results suggest that BRAFV600E does not correlate with the ATA or TNM staging systems and does not predict the outcome alone. However, TERT promoter mutations alone or in combination with BRAFV600E mutation have significant correlation with the ATA and TNM risk stratification systems and are predictive of disease-free survival and persistent/recurrent disease, especially in high-stage DTC. In patients with ATA high-risk group and TNM stage IV, the presence of TERT promoter alone or in combination with BRAFV600E predicts a very high probability of persistent disease. These results are in agreement with several studies that have shown a strong impact of TERT promoter mutations on the DTC behavior and outcome, especially when they co-occur with BRAFV600E mutation (33–36). However, our study also joins several previous studies that casted doubts on the prognostic role of BRAFV600E mutation alone (21, 29, 30, 39, 53). While there is no doubt about the strong oncogenic role of BRAFV600E, its final impact on the DTC behavior is probably influenced by other histopathological features and the stage of the disease (39, 53). The strong synergistic effect of TERT promoter mutations on tumors that also harbor BRAFV600E mutation is clear (34, 35) and it is possible that old studies that showed a strong prognostic impact of BRAFV600E mutation were enriched by then the unknown TERT promoter mutations. In other words, it is possible that studies that showed a strong prognostic role of BRAFV600E had high rates of TERT promoter mutations, which were not known to occur in DTC at the time of these old studies before 2013.
Our study has strengths and weaknesses. It included a good sample size from a single institution with uniform practice. However, the sample size is still relatively small for the study of an association. Reassuring in this study about the sample representation of DTC is the fact that the patients’ characteristics, the histopathological features, the rates of BRAFV600E and TERT promoter mutations and the distribution of patients between different ATA and TNM risk classes are the usual spectrum of DTC seen in most centers. The clinic pathological features are similar to a previous descriptive study in which we characterized DTC in Saudi population (54). Notably, the median age in our population (36 years) is younger than the median age of the SEER data (51 years) and the rate of distant metastases is high. These are similar in this study to our previous publication (54) and a more comprehensive recent study that looked at thyroid cancer in Saudi Arabia over the last 30 years (55). The rate of TERT promoter mutations is also relatively higher in our study than The Cancer Genome Atlas (TCGA) database but this latter contained only well differentiated PTC and our study contained a significant number of patients with tall cell subtype of PTC and other DTC types accounting for the relatively high TERT promoter mutation.
In summary, we have shown that BRAFV600E alone does not correlate with the widely used ATA and TNM staging systems while TERT promoter mutations alone or in combination with BRAFV600E do correlate with these systems and predict DTC outcome. Their presence in higher stages of these risk stratification systems is associated with a very high risk of persistent disease and probably worse outcome. Further studies with larger sample size, preferably multi institutional, are needed to assess the incremental prognostic value of these molecular markers over the current ATA and TNM risk stratification systems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Office of Research Affairs, King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because we only used archived paraffin block without any direct contact with the patients and the data were kept anonymous.
Author contributions
NM: Data curation, Writing – review & editing. KA: Data curation, Writing – review & editing. MA: Data curation, Methodology, Writing – review & editing. HA-H: Methodology, Writing – review & editing, Resources. AM: Data curation, Writing – review & editing. BA: Data curation, Methodology, Writing – review & editing. AA: Methodology, Conceptualization, Formal Analysis, Writing – original draft.
Acknowledgments
We would like to thank our colleagues in the Section of Endocrinology and Department of Molecular Oncology for their support.
Funding Statement
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol (2021) 9:225–34. doi: 10.1016/S2213-8587(21)00027-9 [DOI] [PubMed] [Google Scholar]
- 2. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3 [DOI] [PubMed] [Google Scholar]
- 3. Shih P, Nickel B, Degeling C, Thomas R, Brito JP, Mcleod DSA, et al. Terminology change for small low-risk papillary thyroid cancer as a response to overtreatment: Results from three Australian community juries. Thyroid (2021) 31:1067–75. doi: 10.1089/thy.2020.0694 [DOI] [PubMed] [Google Scholar]
- 4. Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur J Surg Oncol (2018) 44:307–15. doi: 10.1016/j.ejso.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 5. Smulever A, Pitoia F. Conservative management of low-risk papillary thyroid carcinoma: a review of the active surveillance experience. Thyroid Res (2023) 16:6. doi: 10.1186/s13044-023-00148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg (2007) 245:366–78. doi: 10.1097/01.sla.0000250445.92336.2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer: what changed and why? 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA: Mary Ann Liebert, Inc; (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: From detection to final follow-up. J Clin Endocrinol Metab (2019) 104:4087–100. doi: 10.1210/jc.2019-00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wijewardene A, Gill AJ, Gild M, Learoyd DL, Glover AR, Sywak M, et al. A retrospective cohort study with validation of predictors of differentiated thyroid cancer outcomes. Thyroid (2022) 32:1201–10. doi: 10.1089/thy.2021.0563 [DOI] [PubMed] [Google Scholar]
- 11. Wu J, Hu XY, Ghaznavi S, Kinnear S, Symonds CJ, Grundy P, et al. The prospective implementation of the 2015 ATA guidelines and modified ATA recurrence risk stratification system for treatment of differentiated thyroid cancer in a canadian tertiary care referral setting. Thyroid (2022) 32:1509–18. doi: 10.1089/thy.2022.0055 [DOI] [PubMed] [Google Scholar]
- 12. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer (2013) 13:184–99. doi: 10.1038/nrc3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cancer Genome Atlas Research, N . Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fagin JA, Wells SA, Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med (2016) 375:1054–67. doi: 10.1056/NEJMra1501993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest (2016) 126:1052–66. doi: 10.1172/JCI85271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riesco-Eizaguirre G, Santisteban P. ENDOCRINE TUMOURS: Advances in the molecular pathogenesis of thyroid cancer: lessons from the cancer genome. Eur J Endocrinol (2016) 175:R203–217. doi: 10.1530/EJE-16-0202 [DOI] [PubMed] [Google Scholar]
- 17. Volante M, Lam AK, Papotti M, Tallini G. Molecular pathology of poorly differentiated and anaplastic thyroid cancer: What do pathologists need to know? Endocr Pathol (2021) 32:63–76. doi: 10.1007/s12022-021-09665-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishino M. Molecular cytopathology for thyroid nodules: A review of methodology and test performance. Cancer Cytopathol (2016) 124:14–27. doi: 10.1002/cncy.21612 [DOI] [PubMed] [Google Scholar]
- 19. Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: Kinase inhibitors and beyond. Endocr Rev (2019) 40:1573–604. doi: 10.1210/er.2019-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernando J, Ros J, Arroyo A, Capdevila J. Clinical and translational challenges in thyroid cancer. Curr Med Chem (2020) 27:4806–22. doi: 10.2174/0929867327666200214125712 [DOI] [PubMed] [Google Scholar]
- 21. Tallini G, De Biase D, Durante C, Acquaviva G, Bisceglia M, Bruno R, et al. BRAF V600E and risk stratification of thyroid microcarcinoma: a multicenter pathological and clinical study. Mod Pathol (2015) 28:1343–59. doi: 10.1038/modpathol.2015.92 [DOI] [PubMed] [Google Scholar]
- 22. Censi S, Barollo S, Grespan E, Watutantrige-Fernando S, Manso J, Iacobone M, et al. Prognostic significance of TERT promoter and BRAF mutations in TIR-4 and TIR-5 thyroid cytology. Eur J Endocrinol (2019) 181:1–11. doi: 10.1530/EJE-19-0073 [DOI] [PubMed] [Google Scholar]
- 23. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst (2003) 95:625–7. doi: 10.1093/jnci/95.8.625 [DOI] [PubMed] [Google Scholar]
- 24. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res (2018) 24:3059–68. doi: 10.1158/1078-0432.CCR-18-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer (2005) 12:245–62. doi: 10.1677/erc.1.0978 [DOI] [PubMed] [Google Scholar]
- 26. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev (2007) 28:742–62. doi: 10.1210/er.2007-0007 [DOI] [PubMed] [Google Scholar]
- 27. Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Med (Baltimore) (2012) 91:274–86. doi: 10.1097/MD.0b013e31826a9c71 [DOI] [PubMed] [Google Scholar]
- 28. Pak K, Suh S, Kim SJ, Kim IJ. Prognostic value of genetic mutations in thyroid cancer: a meta-analysis. Thyroid (2015) 25:63–70. doi: 10.1089/thy.2014.0241 [DOI] [PubMed] [Google Scholar]
- 29. Soares P, Sobrinho-Simoes M. Cancer: Small papillary thyroid cancers–is BRAF of prognostic value? Nat Rev Endocrinol (2011) 7:9–10. doi: 10.1038/nrendo.2010.213 [DOI] [PubMed] [Google Scholar]
- 30. Zurnadzhy L, Bogdanova T, Rogounovitch TI, Ito M, Tronko M, Yamashita S, et al. The BRAF(V600E) mutation is not a risk factor for more aggressive tumor behavior in radiogenic and sporadic papillary thyroid carcinoma at a young age. Cancers (Basel) (2021) 13:6038. doi: 10.3389/fmed.2022.882727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab (2013) 98:E1562–1566. doi: 10.1210/jc.2013-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer (2013) 20:603–10. doi: 10.1530/ERC-13-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer (2016) 23:R143–155. doi: 10.1530/ERC-15-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol (2014) 32:2718–26. doi: 10.1200/JCO.2014.55.5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer (2016) 122:1370–9. doi: 10.1002/cncr.29934 [DOI] [PubMed] [Google Scholar]
- 36. Qasem E, Murugan AK, Al-Hindi H, Xing M, Almohanna M, Alswailem M, et al. TERT promoter mutations in thyroid cancer: a report from a Middle Eastern population. Endocr Relat Cancer (2015) 22:901–8. doi: 10.1530/ERC-15-0396 [DOI] [PubMed] [Google Scholar]
- 37. Murugan AK, Qasem E, Al-Hindi H, Shi Y, Alzahrani AS. Classical V600E and other non-hotspot BRAF mutations in adult differentiated thyroid cancer. J Transl Med (2016) 14:204. doi: 10.1186/s12967-016-0958-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alzahrani AS, Murugan AK, Qasem E, Alswailem M, Al-Hindi H, Shi Y. Single point mutations in pediatric differentiated thyroid cancer. Thyroid (2017) 27:189–96. doi: 10.1089/thy.2016.0339 [DOI] [PubMed] [Google Scholar]
- 39. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA (2013) 309:1493–501. doi: 10.1001/jama.2013.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol (2015) 33:42–50. doi: 10.1200/JCO.2014.56.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vuong HG, Altibi AMA, Duong UNP, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin Endocrinol (Oxf) (2017) 87:411–7. doi: 10.1111/cen.13413 [DOI] [PubMed] [Google Scholar]
- 42. Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: a systematic review and meta-analysis. Endocrine (2020) 67:44–57. doi: 10.1007/s12020-019-02117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo Y, Jiang H, Xu W, Wang X, Ma B, Liao T, et al. Clinical, pathological, and molecular characteristics correlating to the occurrence of radioiodine refractory differentiated thyroid carcinoma: A systematic review and meta-analysis. Front Oncol (2020) 10:549882. doi: 10.3389/fonc.2020.549882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg (2013) 37:376–84. doi: 10.1007/s00268-012-1835-2 [DOI] [PubMed] [Google Scholar]
- 45. American Thyroid Association Guidelines Taskforce on Thyroid, N., Differentiated Thyroid, C. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19:1167–214. doi: 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 46. Van Velsen EFS, Stegenga MT, Van Kemenade FJ, Kam BLR, Van Ginhoven TM, Visser WE, et al. Evaluating the 2015 American thyroid association risk stratification system in high-risk papillary and follicular thyroid cancer patients. Thyroid (2019) 29:1073–9. doi: 10.1089/thy.2019.0053 [DOI] [PubMed] [Google Scholar]
- 47. Grani G, Zatelli MC, Alfo M, Montesano T, Torlontano M, Morelli S, et al. Real-world performance of the American thyroid association risk estimates in predicting 1-year differentiated thyroid cancer outcomes: A prospective multicenter study of 2000 patients. Thyroid (2021) 31:264–71. doi: 10.1089/thy.2020.0272 [DOI] [PubMed] [Google Scholar]
- 48. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. Springer; (2017). [Google Scholar]
- 49. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet (2013) 381:1058–69. doi: 10.1016/S0140-6736(13)60109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boufraqech M, Nilubol N. Multi-omics signatures and translational potential to improve thyroid cancer patient outcome. Cancers (Basel) (2019) 11:1988. doi: 10.3390/cancers11121988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tirro E, Martorana F, Romano C, Vitale SR, Motta G, Di Gregorio S, et al. Molecular alterations in thyroid cancer: From bench to clinical practice. Genes (Basel) (2019) 10:709. doi: 10.3390/genes10090709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim D. Thyroid cancer: are molecular studies making any difference? J Laryngology Otology (2007) 121:917–26. doi: 10.1017/S0022215107009279 [DOI] [PubMed] [Google Scholar]
- 53. Li X, Kwon H. The impact of BRAF mutation on the recurrence of papillary thyroid carcinoma: A meta-analysis. Cancers (Basel) (2020) 12:2056. doi: 10.3390/cancers12082056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alzahrani AS, Alomar H, Alzahrani N. Thyroid cancer in Saudi Arabia: A histopathological and outcome study. Int J Endocrinol (2017) 2017:8423147. doi: 10.1155/2017/8423147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Flemban AF, Kabrah S, Alahmadi H, Alqurashi RK, Turaes AS, Almaghrabi R, et al. Patterns of thyroid cancer mortality and incidence in Saudi Arabia: A 30-year study. Diagnostics (Basel) (2022) 12:2716. doi: 10.3390/diagnostics12112716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.