Abstract
Neurodegenerative tauopathies are caused by the transition of tau protein from a monomer to a toxic aggregate. They include Alzheimer disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Pick disease (PiD). We have previously proposed that tau monomer exists in two conformational ensembles: an inert form (Mi), which does not self-assemble, and seed-competent form (Ms), which self-assembles and templates ordered assembly growth. We proposed that cis/trans isomerization of tau at P301, the site of dominant disease-associated S/L missense mutations, might underlie the transition of wild-type tau to a seed-competent state. Consequently, we created monoclonal antibodies using non-natural antigens consisting of fluorinated proline (P∗) at the analogous P270 in repeat 1 (R1), biased toward the trans-configuration at either the R1/R2 (TENLKHQP∗GGGKVQIINKK) or the R1/R3 (TENLKHQP∗GGGKVQIVYK) interfaces. Two antibodies, MD2.2 and MD3.1, efficiently immunoprecipitated soluble seeds from AD and PSP but not CBD or PiD brain samples. The antibodies efficiently stained brain samples of AD, PSP, and PiD, but not CBD. They did not immunoprecipitate or immunostain tau from the control brain. Creation of potent anti-seed antibodies based on the trans-proline epitope implicates local unfolding around P301 in pathogenesis. MD2.2 and MD3.1 may also be useful for therapy and diagnosis.
Keywords: tau, prion, conformation, conformation-specific antibody, trans-proline, amyloid
Deposition of tau in ordered assemblies underlies Alzheimer disease (AD) and related neurodegenerative tauopathies, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Pick disease (PiD) (1). These disorders are associated with distinct insoluble tau assembly structures that have been resolved by cryogenic electron microscopy (cryo-EM) (2). Considerable experimental data support the hypothesis that tau pathology progresses via trans-cellular propagation of protein aggregates in which tau assemblies form in one cell, are released into the extracellular space, and are taken up by neighboring cells where they act as templates to convert native tau into a growing aggregate (3, 4). This process, termed “seeding,” is readily replicated in simple cell models based on the expression of full-length tau (5) or tau repeat domain (RD) (6) fused to fluorescent proteins. When complementary fluorescent protein fusions are co-expressed in “biosensor” cells, aggregation is easily detected by fluorescence resonance energy transfer (FRET) (6, 7). Biosensor cells are useful to characterize tau seeding activity in mouse models and human tissues and detect tau seeds prior to the development of neurofibrillary tau pathology in humans (8, 9) and mice (6, 10).
The tau RD (amino acids 244–378) forms the backbone of amyloid cores in pathological tau deposits (11, 12). Distinct conformations of the RD underlie the seeding and strain identity of pathological tau (13, 14). Strains, by definition, propagate faithfully in vivo and cause distinct patterns of pathology. We have previously propagated tau strains derived from distinct tauopathies in cultured biosensor cells. These create unique patterns of neuropathology, rates of progression, and regional involvement upon inoculation into a transgenic mouse model (15, 16, 17). Others, by inoculating insoluble material from tauopathy brains, have also produced different patterns of pathology in mouse models (18, 19, 20). Subsequently, cryo-EM has resolved in atomic detail tau assemblies from different tauopathies and documented distinct conformations of the RD (21). The simplest interpretation of these data is that individual tauopathies are caused by unique, disease-specific tau assemblies (17).
The mechanistic origins of tauopathy are mysterious and likely diverse. We have previously concluded that tau monomer exists in two general conformational ensembles, an inert form (Mi) that does not readily self-assemble or act as a template, and a seed-competent form (Ms) that can self-assemble or trigger further refolding and assembly of monomers into oligomers (13). We have described methods to produce Ms in vitro (22). Ms has myriad sub-structures, as isomers isolated from the human brain or cell lines or encode distinct strains (14). Ms is the earliest form of seed-competent tau we have detected in a mouse model, preceding the formation of larger assemblies (10), and we have linked the formation of certain forms of Ms to changes in local tau structure (13, 22). Specifically, based on cross-linking mass spectrometry, molecular dynamic modeling, and biochemical studies, we have proposed that cis-trans proline isomerization at P301 underlies the formation of Ms in certain cases (13, 23, 24), with amyloid-forming motifs VQIINK and VQIVYK relatively more exposed in seed-competent (Ms) versus inert (Mi) tau. We have observed that a fluorinated trans-proline isomer (25, 26, 27) at residue 270/301 (depending on 3R versus 4R tau) favors an “open” conformation in vitro that is predicted to expose these amyloidogenic motifs and promote aggregation (23).
To test these ideas further, we have now used linear peptide antigens with a synthetic fluorinated trans-proline residue to produce monoclonal antibodies against this region of tau, employing fluorinated proline analogs to mimic misfolded pathogenic species (Fig. 1). We employed a novel screening and selection protocol to enrich seed specificity. We selected two novel monoclonal antibodies produced by this method, MD2.2 and MD3.1, and have characterized their binding characteristics in vitro with recombinant protein, brain lysates, and immunohistochemistry.
Figure 1.
Peptide antigens for the MD antibody series. The MD2.2 and MD3.1 epitopes (gray) comprise amino acids 263 to 281 (4R) and 263 to 311Δ275 to 305 (3R) of the tau sequence, respectively. Both include a fluorinated proline (N-Boc-trans-4-fluoro-L-proline) at residue 270, shifting the equilibrium in favor of the trans-proline conformation.
Results
MD2.2 and MD3.1 each bind FL tau and peptide antigens
MD2.2 and MD3.1 were raised against 263 to 281 (R1R2) and 263 to 311Δ275 to 305 (R1R3) tau sequences, respectively, with fluorinated proline at residue 270 position (Fig. 1). After their original selection based on binding AD seeds, we compared the binding characteristics of MD2.2 and MD3.1 to HJ8.5, a high-affinity antibody that binds the N-terminus of tau (28).
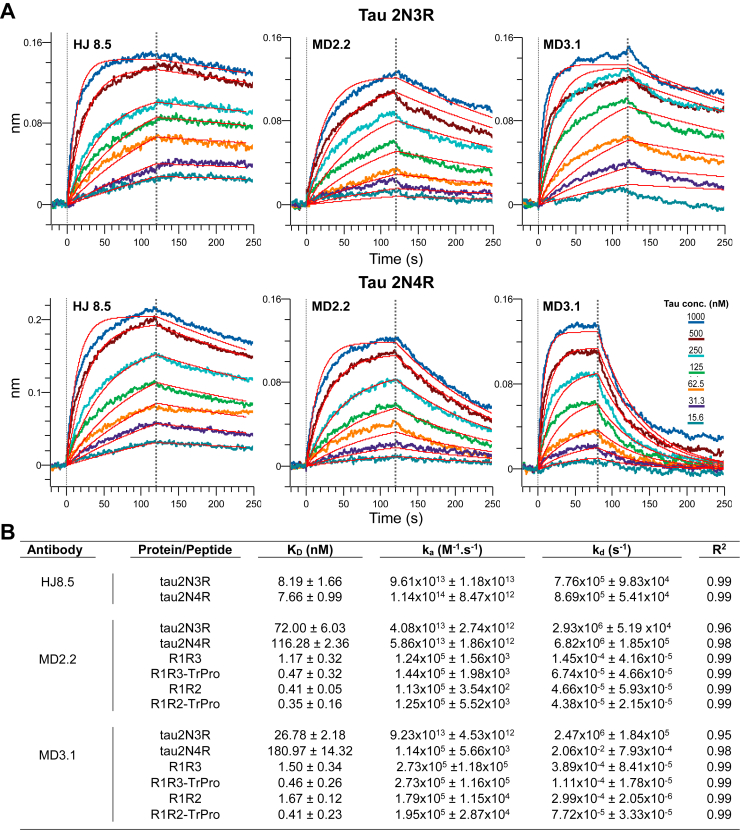
We first used surface interferometry to determine the affinity of HJ8.5, MD2.2, and MD3.1 for recombinant full-length (FL) tau (3R and 4R). HJ8.5 bound both tau isoforms with similar affinity in the low nanomolar range. MD2.2 and MD3.1 each bound 3R tau with slightly higher affinity than 4R tau, and with a KD ∼5 to 10× greater than HJ8.5 (i.e., lower affinity) (Fig. 2). We next checked binding to short peptides: R1R2, trans-P-R1R2, R1R3, and trans-P-R1R3. MD2.2 and MD3.1 each bound them with ∼10x higher affinity than FL protein (Fig. 2B). Consistent with the antigens used to create them, MD2.2 and MD3.1 bound the trans-P-R1R2 and trans-P-R1R3 peptides with KD two- to three-fold lower than that for trans-P-R1R2 and trans-P-R1R3 peptides (Figs. 2B and S1). HJ8.5, which binds the N-terminus of tau, did not bind any of the peptides (Fig. S1). Taken together, our data indicated MD2.2 and MD3.1 each had higher affinity for trans-P configurations while still binding FL tau isoforms. Because there was no difference in 3R versus 4R tau binding, the antibodies apparently bound epitopes common to both isoforms.
Figure 2.
Binding properties to recombinant tau and peptide antigens.A, kinetic traces for full-length 2N3R (upper panel) and 2N4R (bottom panel) recombinant tau. The kinetic traces are fitted to 1:1 kinetics. For MD2.2 and MD3.1, which target the repeat domain containing multiple similar PGGG-containing repeats, the curves show fast-on, fast-off kinetics with a poorer fit of 1:1 kinetics. B, KD, ka, kd, and R2 for HJ8.5, MD2.2, and MD3.1 were calculated based on a grouped curve fit of seven tau concentrations with 1:1 binding parameters. Data for HJ8.5 binding with various peptides is not shown (see Fig. S1).
Immunoprecipitation of tau versus tau seeds from AD versus control brain
We next tested the binding properties of MD2.2 and MD3.1 for seeds from the control versus AD brain (3R/4R tau). We prepared soluble homogenates of frozen frontal cortex from three healthy control and three AD brains. We used HJ8.5, MD2.2, and MD3.1 to immunoprecipitate (IP) samples. MD2.2 and MD3.1 did not bind detectable tau from control brains, whereas HJ8.5 bound nearly all of it (Fig. 3, A, C, and E). When MD2.2 and MD3.1 were used to IP AD brain we detected a very faint signal, whereas HJ8.5 immunoprecipitated most soluble protein. Using tau RD(P301S) v2H biosensor cells (7), we detected no seeding activity in the control brain homogenates (Fig. 3, B, D, and F). We detected robust seeding activity in all three AD brains, observed in the supernatant fractions of the control IPs using non-specific mouse IgG (Fig. 3, H, J, and L). In contrast to the Western blots, MD2.2 and MD3.1 immunoprecipitated virtually all detectable AD seeds (Fig. 3, H, J, and L). This contrasted with HJ8.5, which precipitated nearly all total soluble tau but left significant seeding activity in the supernatant in all cases. The MD2.2 and MD3.1 antibodies therefore bound a small subset of soluble tau that was absent from control brains and accounted for essentially all seed-competent tau in AD brains.
Figure 3.
MD2.2 and MD3.1 bind soluble tau AD seeds. Western blots of total tau in the IP and supernatant fractions from immunoprecipitation of three control brains (A, C, and E) and three AD brains (G, I, and K) indicated that MD2.2 and MD3.1 bound no tau from control brains and only a very small fraction of tau from AD brains. By contrast, HJ8.5 bound nearly all tau from all brains. Seeding assays of the IP and supernatant fractions showed that nearly all seeding activity from AD brains was in the IP fractions of MD2.2 and MD3.1, whereas much was left in the supernatant by HJ8.5 (H, J, and L). No significant seeding activity was detected in control brains (B, D, and F). Input was the pre-IP total protein for each brain; tau−/− negative control was the equivalent total protein from a tau−/− mouse brain, tau rec indicates recombinant tau monomer (2N4R). Non-specific mouse IgG was the negative control for immunoprecipitation. Error bars represent the S.E.M. Each assay was performed in technical triplicate.
MD2.2 and MD3.1 discriminate seeds from different tauopathies
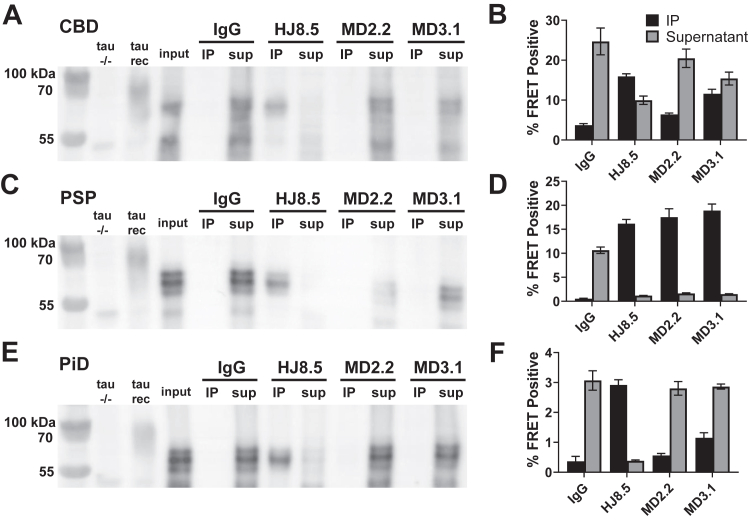
Tau assembly structures vary among tauopathies. To test the specificity of MD2.2 and MD3.1 for AD versus other tauopathies, we prepared soluble homogenates of frozen frontal cortex from corticobasal degeneration (CBD, 4R tau), progressive supranuclear palsy (PSP, 4R tau), and Pick disease (PiD, 3R tau) brains. We detected no tau by WB in the IP fractions of MD2.2 and MD3.1, whereas HJ8.5 immunoprecipitated virtually all detectable tau (Fig. 4, A, C, and E). All three tauopathy brains exhibited tau seeding in v2H biosensors (Fig. 4, B, D, and F). MD2.2 left most seeding from CBD and PiD brains in the supernatant, while binding PSP seeds with high efficiency. MD3.1 also bound seeds well from the PSP brain, but with lower efficiency for CBD and PiD. HJ8.5 failed to IP CBD seeds efficiently but did so for PiD and PSP. In summary MD2.2 and MD3.1 efficiently bound tau-associated seeds from AD and PSP, but less so from CBD and PiD.
Figure 4.
MD2.2 and MD3.1 exhibit specificity for tauopathy seeds. IP of brain protein from the non-AD tauopathies CBD (A), PSP (C), and Pick disease (E) demonstrated undetectable total tau by MD2.2 and MD3.1 versus HJ8.5, which precipitated most tau. Seeding assays revealed significant seeds from CBD (B) and PiD (F) in the supernatant after IP with MD2.2 and MD3.1. Conversely, nearly all PSP seeds were bound by MD2.2 and MD3.1 (D). Input was the pre-IP total protein for each brain; tau−/− negative control was the equivalent total protein from a tau−/− mouse brain, tau mono indicates recombinant tau monomer (2N4R). Non-specific mouse IgG was the negative control for immunoprecipitation. Error bars represent the S.E.M. Each assay was performed in technical triplicate.
MD2.2 and MD3.1 discriminate tauopathies in situ
We stained adjacent sections prepared from tissue microarray (TMA) blocks with MD2.2, MD3.1, HJ8.5, and AT8, the standard phospho-tau antibody used to identify pathological tau (29). MD2.2 and MD3.1 stained tau in AD, PSP, and PiD but not CBD or brains containing α-synuclein or TDP-43 pathology (Figure 5, Figure 6, Figure 7). In AD, MD2.2 and MD3.1 preferentially stained perinuclear and cell body tau inclusions (Fig. 5A; expanded in Fig. S2), particularly in brains with lighter AT8 staining. In contrast, AT8 exhibited more diffuse staining throughout the neuropil, and HJ8.5 featured high background staining with poor signal-to-noise ratio (Fig. 5A). In PSP brains, MD2.2 and MD3.1 stained only small, granular perinuclear rings of accumulated tau and not tangles or tufted astrocytes like AT8 (Fig. 5B, expanded in Fig. S2). Overall the staining patterns for MD2.2 and MD3.1 revealed a small fraction of total AT8 signal in AD and an extremely small fraction in PSP. In CBD brain sections, we observed no staining by either antibody (Fig. 6A). In PiD sections, MD2.2 and MD3.1 exhibited some Pick body labeling, albeit much less than either AT8 or HJ8.5 (Fig. 6B). Neither MD3.1 or MD2.2 stained other neurodegenerative disease brain samples without tau pathology, an MSA brain featuring α-synuclein aggregates, or an FTLD brain featuring TDP-43 aggregates (Fig. 7). Taken together, the staining characteristics of MD2.2 and MD3.1 reflected specificity for different tau assembly structures, as distinct from AT8.
Figure 5.
MD2.2 and MD3.1 staining of AD and PSP brains. Representative images of tissue microarray IHC staining of fixed frontal cortex of eight AD brains (A) and eight PSP brains (B) showed less total tau staining on average by MD2.2 and MD3.1 than by HJ8.5, and a distinct pattern of tau aggregates versus AT8, with perinuclear granular bodies noted particularly in PSP (arrowheads). Scale bar = 50 μm.
Figure 6.
MD2.2 and MD3.1 staining of CBD and PiD brains. Representative images of tissue microarray IHC staining of fixed frontal cortex of eight CBD brains (A) and eight Pick disease brains (B). CBD brain exhibited no significant tau staining above background. In PiD there was faint labeling of Pick bodies (arrowheads). This contrasted with staining by AT8 and HJ8.5. Scale bar = 50 μm.
Figure 7.
MD2.2 and MD3.1 staining of AD and PSP versus non-tauopathy brains. Immunohistochemistry of brains with AT8(+) pathology indicated more MD2.2 and MD3.1 staining in AD and PSP than in CBD and PiD. The Braak II sections are from a Lewy body dementia brain with AD NFTs only in limbic tissue and exhibited staining with MD2.2 and MD3.1. In non-tauopathy MSA or FTLD-TDP brains, there was no staining. Scale bar = 50 μm.
Differential binding based on strain-dependent epitope exposure
MD2.2 and MD3.1 are strain-specific, efficiently binding seeds of AD and PSP but not CBD or PiD. This could reflect distinct proteolytic processing or post-translational modifications. Alternatively, we hypothesized that the epitopes were differentially exposed. Cryo-EM has demonstrated distinct conformations of the amyloid cores from these four tauopathies, composed of beta sheets of two (AD, PiD), three (CBD), or four (PSP) layers (21). Consequently, we used published cryo-EM structures coupled with molecular dynamic modeling (Rosetta) of the unresolved epitopes to consider these binding characteristics. With an ab initio protocol to build trimers of the fibril cores, we modeled the flanking (“fuzzy coat”) regions of the N- and C-termini to include the full 2N4R tau sequence (Fig. 8). We then computed solvent accessible surface area (SASA) for the R1R2 epitope (residues 263–280) in each published disease-associated structure, with HJ8.5 as a control (Fig. 8). Unsurprisingly, the model predicted the HJ8.5 epitope, far removed from the fibril cores, to be highly accessible. This was consistent with its ability to bind both tau monomer and assemblies. For the AD assembly, the R1R2 epitope was predicted to be highly exposed, possibly explaining the efficacy of antibodies in binding AD seeds. By contrast, the R1R2 epitope, and VQIINK amyloid motif, were predicted to be more buried in CBD. In PSP, the modeling predicted higher solvent accessibility of the epitope, although not as much as in AD (Fig. 8). Although it is not possible to structurally resolve highly mobile flanking sequence structure(s) within fibrils, the modeling and IP/seeding data were consistent with the idea that epitope accessibility might determine antibody avidity, at least for AD seeds versus others.
Figure 8.
Modeling of full-length tau fibrils in AD, PSP, and CBD. Rosetta was used to create ab initio models of published core structures within the context of FL tau to study the “fuzzy coat.” A, schematic illustration of model generation. B, schematic representation of full-length tau and repeat domain of tau highlighting the binding epitopes for HJ8.5, MD2.2, and MD3.1 antibodies. The repeat domains are colored red (R1), green (R2), blue (R3), and magenta (R4). The N- and C-termini of tau are colored grey. C, distinct ensemble representation of antibody binding regions R1R2 (MD2.2), C-term of R1R2 (VQIINK) and HJ8.5 using SASA values. AD, CBD and PSP SASA distributions are colored purple, yellow, and cyan, respectively. Note the strong SASA score for AD fibrils bound by both MD2.2 and MD3.1. PSP exhibits a small degree of predicted accessibility versus CBD. HJ8.5 is predicted to bind all structures equally.
Discussion
This study tested a prediction based on earlier work which indicated that tau monomer exists in distinct conformational ensembles, Mi versus Ms, discriminated by local folding patterns that differentially expose motifs required for self-assembly. Specifically, we have proposed that Ms, a trans-proline configuration of P301, unfolds a local hairpin to expose the critical aggregation motifs VQIINK or VQIVYK (13). To test this idea, we used an immunogen with a non-natural trans-proline residue to vaccinate mice and derived two monoclonal antibodies: MD2.2 directed at a trans-P-R2R3 epitope (4R), and MD3.1 directed at a trans-P-R1R3 epitope (3R). These antibodies had noteworthy characteristics. Both bound recombinant FL tau monomer in vitro, based on surface interferometry. However, when used to IP tau derived from control brains they did not bind detectable tau, and with tauopathy brains, they precipitated minimal detectable tau versus a control N-terminal antibody (HJ8.5). They efficiently depleted seeds from AD and PSP lysates in a biosensor assay, while they bound those from CBD or PiD less efficiently. Both antibodies efficiently stained AD brain, but not CBD, with less efficient staining of PiD and PSP. Neither antibody stained control brain. Taken together with earlier studies, these data are consistent with the idea that local folding exposes unique epitopes in tau that enable aggregation, but that these events are disease (or strain)-specific.
Soluble seed-competent tau is a small component of tauopathy brain tau
The differential binding of MD2.2 and MD3.1 to seed-competent versus inert tau in the brain revealed relative amounts of these species. While the antibodies had a relatively high affinity for recombinant tau monomer in vitro, they failed to immunoprecipitate tau monomer from the control brain. We hypothesized that the inert recombinant protein (Mi) was relatively flexible, and might transiently adopt a diversity of conformations; that Mi within the control brain was more rigidly folded or otherwise restricted to conformations that mask epitope accessibility; or a combination of both. The differential seed binding was striking, with virtually all seeding activity immunoprecipitated from AD and PSP brain lysates but not CBD or PSP. Even with complete precipitation of seeds, only minimal tau monomer was detected on Western blot (and no evidence of higher-order species). Thus, compared to total tau (as detected by HJ8.5 immunoprecipitation), seed-competent tau (whether monomer or assembly) appeared to represent a tiny fraction of the soluble protein. This may explain why antibodies that bind all forms of tau, such as HJ8.5, have failed to efficiently reduce pathology after peripheral administration—most tau bound would represent non-pathological species.
Diagnostics and therapeutics
Anti-tau antibodies have enabled the pathological characterization of tauopathies, and anti-tau immunotherapy is a promising therapeutic strategy (30, 31). Four anti-tau monoclonal antibody therapies, gosuranemab, tilavonemab, semorinemab, and zagotenemab, target epitopes N-terminal to the RD and have all failed in phase II clinical trials for either AD or PSP (32, 33, 34, 35, 36, 37, 38). We note in this study that HJ8.5, the mouse version of tilavonemab, failed to efficiently IP all tau seeds compared to MD3.1 and MD2.2, despite efficient binding of full-length tau. As tau seeds likely must include the repeat domain, it is possible that proteolytic processing could remove N-terminal or C-terminal epitopes (25, 39, 40, 41), rendering an N-terminal antibody less effective.
We note that a humanized monoclonal antibody, E2814, also targets a repeat domain epitope (HVPPGG) present in both R2 and R4 and reduced tau deposition in vivo (42). The epitope is fairly similar to the HQPGG sequence in R1 that was contained in the antigens used to create MD2.2 and MD3.1. The active immunotherapy AADvac1 also uses an epitope similar to those for MD2.2 and MD3.1 but with a proline deletion rather than a synthetic trans-proline. This could have a similar effect on the secondary structure of the antigen. Importantly, despite multiple early anti-tau vaccine failures, upcoming clinical trials will answer questions about the efficacy and clinical utility of targeting conformation-specific RD epitopes. In addition to their potential therapeutic value, the specificity of these antibodies for tau seeds may facilitate their use as diagnostics to detect pathological species in biofluids.
Experimental procedures
Brain tissue
All human tissues used in these experiments were derived from autopsy subjects. Since for research purposes deceased people are not considered human subjects, these studies were considered exempt from human subjects research regulations and did not require IRB approval. We identified cases from the brain bank of the Alzheimer’s Disease Center at UT Southwestern, selecting those with a single histopathological diagnosis of either AD, PSP, CBD, or PiD based on standard neuropathological evaluation, and excluding mixed pathologies. Negative control brains derived from cases of multiple system atrophy due to α-synuclein accumulation, and frontotemporal lobar degeneration due to TDP-43 accumulation. We chose eight cases per disease for formalin-fixed, paraffin-embedded (FFPE) tissue and four cases per disease for frozen tissue. We included frozen frontal cortex from three healthy control brains, free of any detectable neuropathology. We used cores of middle frontal gyrus from each formalin-fixed brain for TMA construction (below), and frozen samples of frontal gyri for soluble protein brain extracts. We performed a detergent-free homogenization of all frozen brain samples in 10% w/vol of 1× TBS with protease inhibitor cocktail (Roche) by mechanical disruption at 4 °C followed by intermittent water bath sonication (Qsonica), for 5 min total “on” time at an amplitude of 65. We centrifuged homogenates at 21,000g for 15 min at 4 °C to remove cellular debris and determined protein concentrations by BCA assay (ThermoFisher).
Generation of MD series of antibodies
We produced a series of novel monoclonal anti-tau antibodies (MD series) with a contract research organization (CRO, Genscript). The CRO synthesized linear peptides with sequences corresponding to amino acids 263 to 281 (R1R2) and 263 to 311Δ275 to 305 (R1R3) of tau (assuming 2N4R, 441 aa) for the MD2 and MD3 antigens, respectively, substituting N-Boc-trans-4-fluoro-L-proline at residue 270, and conjugated them to keyhole limpet hemocyanin (KLH). Peptides containing a 4R fluorinated proline were used because the modification shifts the preceding backbone torsional angles toward the trans conformation (26, 43), which we and others have shown shifts proteins and peptides into more aggregation-prone conformations (23, 44, 45). We reasoned that producing antibodies that recognize these aggregation-prone epitopes might mimic more closely pathogenic conformations observed in the disease.
The CRO provided antisera from inoculated mice for screening with an immunoprecipitation (IP)-seeding assay. We incubated supersaturating concentrations of antisera with Dynabeads Protein A (ThermoFisher) and used them to IP tau from 10 μg total soluble protein from an AD brain. We eluted protein in low pH elution buffer (Pierce) equivalent to the starting IP/supernatant volume and neutralized with 1:10 1 M Tris pH 8.5. We measured the tau seeding activity in equivalent volumes of IP eluent and supernatant with a cell-based tau seeding assay (below). We identified the mice with antisera most efficient in immunoprecipitating AD tau seeds, and the CRO used these mice for thymus cell fusion to produce hybridoma clones. We analyzed supernatants from parental clones and subsequently subclones as above, including IP of seeds from non-AD tauopathies, and selected two hybridomas for detailed analysis: MD2.2 and MD3.1.
Surface interferometry
We measured the kinetics of antibody association and dissociation using surface interferometry (Octet Red 384, FortéBio). To study antibody binding to peptides, we first loaded pre-equilibrated streptavidin biosensor probes with 400 nM of biotinylated peptides in 1× kinetics buffer (Sartorius) for 120 s, before a 180 s association and 180 s dissociation step. The peptides consisted of: R1/R2, R1/R2-transPro and R1/R3 and R1/R3-transPro. We next tested binding to antibodies by incubation with serially diluted preparations of N-terminal antibody HJ8.5 (28), MD2.2, and MD3.1.
To measure binding to recombinant full-length (FL) tau monomer (tau 2N4R) and truncated tau monomer without the second repeat (tau 2N3R), we loaded the pre-equilibrated AMC biosensors (in 1× kinetics buffer from Sartorius) with 100 nM HJ8.5, MD2.2, and MD3.1 antibodies for 120 s, before a 120 s association step (except MD3.1) and 180 s dissociation step, with serial dilutions of 2N4R and 2N3R tau monomer proteins. MD3.1 showed a drift after 80 s of association (possibly due to the dissociation of immobilized antibody from biosensors), hence, we carried out the binding studies of MD3.1 with 80 s association and 180 s dissociation.
Finally, we used Octet System Data Analysis Software (FortéBio) to fit the kinetic curves to equations based on 1:1 kinetics and calculate KD, ka (on), and kd (off). We performed double referencing (sample and biosensor) for all our measurements to background subtract nonspecific binding.
Immunoprecipitation
50 μl of Dynabeads Protein A (ThermoFisher) were washed per the manufacturer protocol and incubated with 10 μg of monoclonal antibodies for 1 h at room temperature. We then added washed beads to 10 μg total soluble protein diluted in binding buffer (PBS + 0.02% Tween 20). We collected supernatants eluted captured proteins in low pH elution buffer (Pierce), and neutralized the buffer with 1:10 1 M Tris pH 8.5 with a final volume equivalent to the supernatant.
Western blotting
We performed SDS-PAGE on IP and supernatant samples equivalent to 2 μg total starting protein with 4 to 12% Bis-Tris gels. We transferred protein to a PVDF membrane, blocked with 5% dry milk, and probed with a 1:2000 dilution of anti-tau rabbit polyclonal antibody, followed by a goat anti-rabbit secondary, and developed the blots with the ECL Ultra detection kit (ThermoFisher). For unprocessed gel images, please see Figs. S3 and S4.
Tau seeding assay
We performed tau seeding assays using the tau RD P301S v2H HEK biosensor cell line as previously described (7). For each brain, we determined the optimal total mass to use for tau seeding assays using a serial dilution dose–response curve to ensure the assay was well within the linear range in each case. We incubated the equivalent volume of IP and supernatant with Lipofectamine 2000 transfection reagent and added them to three wells per sample of biosensor cells in a 96-well plate. We collected cells after 48 h and fixed them in 2% PFA for 10 min before measuring % FRET by flow cytometry as previously described (46).
Tissue microarrays
We constructed a TMA containing samples from each tauopathy case, including cores of FFPE middle frontal gyrus and cerebellum (as negative control) from eight brains per disease using an Epredia 3DHistech TMA Master II (Richard-Allan Scientific, Kalamazoo, MI) robotic tissue micorarrayer and 1.6 mm diameter cores. Donor core sites were determined from overlays of H&E-stained scout sections of each donor block, prepared on an Aperio CS2 (Leica Biosystems) whole slide imager. We mounted 5 μm sections from the TMA block on glass slides and performed immunohistochemical staining via a Bond III (Leica) automated immunostaining platform with optimal dilutions of each primary antibody determined by an initial serial dilution to minimize background while retaining foreground staining in sections with pathology. Dilutions were 1:200 AT8 (ThermoFisher), 1:16,000 MD2.2, 1:4000 MD3.1, 1:32,000 HJ8.5. We captured high-resolution images of whole slides with an optical scanner and selected qualitatively representative fields from each stained tissue core.
Modeling of flanking sequences of amyloid fibrils
To model the full 2N4R tau sequence in the context of cryo-EM assemblies we employed a comparative procedure in Rosetta (47) leveraging the AD, PSP, and CBD fibril conformations as templates (PDBids: 5O3L, 6TJO and 7P65). Fragments for full-length 2N4R tau were built using the fragment picker tool and 500 models of FL tau monomers were built for AD, PSP and CBD using the homology modeling module (47). Individual monomers were assembled into trimers using structural alignment to the cryo-EM template and trimeric assemblies were scored according to their predicted stability (Fig. S5). The lowest scoring models were subsequently minimized using constraints derived from the trimeric fibril core template. Next, to evaluate the exposure of the different epitopes (HJ8.5, residues 25–30; R1R2, residues 263–280), we calculated the per-residue SASA using the SasaCalc class in PyRosetta version across the AD, PSP and CBD trimeric ensembles (48). SASA was calculated with 1.4 Å probe radius. SASA distributions for the AD, PSP, and CBD trimeric ensembles were calculated for the R1R2 (MD2.2), C-term of R1R2 (VQIINK), and HJ8.5 epitopes. The data were plotted with the seaborn library and matplotlib in python3.7 modules. For the MD2.2 epitope, we also computed the SASA distributions for a fraction of the sequence to identify the minimal sequence that distinguishes PSP from CBD SASA in the MD2.2 epitope. For simplicity, we focused on the MD2.2 epitope in the context of 4R tau, which is present in all disease-associated assemblies.
Data availability
Data relevant to the manuscript is available online: https://zenodo.org/record/8206697.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by NIH ADC grant AG012300, the McCune Foundation, and the Winspear Center for Research on the Neuropathology of Alzheimer Disease.
Author contributions
B. D. H., L. A. J., M. I. D., and A. G. conceptualization; R. S., T. Y., B. D. H., and A. G. formal analysis; R. S., T. Y., B. D. H., J. D. B., and A. G. methodology; M. I. D., B. D. H., and A. G. writing–original draft; B. D. H., C. L. W., L. A. J., and A. G. writing–review and editing; P. S. resources; M. I. D. supervision; M. I. D., L.A.J., and C.L.W. funding.
Funding and additional information
This work was supported by BDH: NIH R25NS098987-01, Alzheimer’s Association Grant AACSF-591977( M. I. D., L. A. J., C. L. W.) and NIH RF1AG065407 and the Chan-Zuckerberg Initiative (C. L. W.)
Reviewed by members of the JBC Editorial Board. Edited by Elizabeth J. Coulson
Supporting information
BLI kinetics curves of antibody binding to various R1R2/R1R3 tau peptides.A, kinetic traces for R1R2 (upper panel) and R1R2 trans-proline (bottom panel) peptides. The kinetic traces are fitted to 1:1 kinetics. B, (A) Kinetic traces for R1R3 (upper panel) and R1R3 trans-proline (bottom panel) peptides. The kinetic traces are fitted to 1:1 kinetics. Whereas both MD2.2 and MD3.1 bound peptides, we observed no significant binding with HJ8.5.
Expanded high-magnification images of MD2.2 and MD3.1-positive perinuclear inclusions in AD and PSP brain tissue. Scale bar = 20 μm.
Unprocessed images of western blots used inFigure 3.
Unprocessed images of western blots used inFigure 4.
Energy distribution plot of assembled AD, CBD and PSP models in fibril form. Energy distribution of ensembles produced for AD, CBD and PSP trimers containing full length 2N4R tau. The energies are shown as Rosetta Energy Units (REU).
References
- 1.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 2.Scheres S.H., Zhang W., Falcon B., Goedert M. Cryo-EM structures of tau filaments. Curr. Opin. Struct. Biol. 2020;64:17–25. doi: 10.1016/j.sbi.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons G.S., Lee V.M.Y., Trojanowski J.Q. Mechanisms of cell-to-cell transmission of pathological tau: a review. JAMA Neurol. 2019;76:101–108. doi: 10.1001/jamaneurol.2018.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goedert M., Eisenberg D.S., Crowther R.A. Propagation of tau aggregates and neurodegeneration. Annu. Rev. Neurosci. 2017;40:189–210. doi: 10.1146/annurev-neuro-072116-031153. [DOI] [PubMed] [Google Scholar]
- 5.Frost B., Jacks R.L., Diamond M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes B.B., Furman J.L., Mahan T.E., Yamasaki T.R., Mirbaha H., Eades W.C., et al. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4376–4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hitt B.D., Vaquer-Alicea J., Manon V.A., Beaver J.D., Kashmer O.M., Garcia J.N., et al. Ultrasensitive tau biosensor cells detect no seeding in Alzheimer's disease CSF. Acta Neuropathol. Commun. 2021;9:99. doi: 10.1186/s40478-021-01185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furman J.L., Vaquer-Alicea J., White C.L.,, 3rd, Cairns N.J., Nelson P.T., Diamond M.I. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 2017;133:91–100. doi: 10.1007/s00401-016-1644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stopschinski B.E., Del Tredici K., Estill-Terpack S.J., Ghebremdehin E., Yu F.F., Braak H., et al. Anatomic survey of seeding in Alzheimer's disease brains reveals unexpected patterns. Acta Neuropathol. Commun. 2021;9:164. doi: 10.1186/s40478-021-01255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirbaha H., Chen D., Mullapudi V., Terpack S.J., White C.L., Joachimiak L.A., et al. Seed-competent tau monomer initiates pathology in PS19 tauopathy mice. bioRxiv. 2022 doi: 10.1101/2022.01.03.474806. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowther T., Goedert M., Wischik C.M. The repeat region of microtubule-associated protein tau forms part of the core of the paired helical filament of Alzheimer's disease. Ann. Med. 1989;21:127–132. doi: 10.3109/07853898909149199. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick A.W.P., Falcon B., He S., Murzin A.G., Murshudov G., Garringer H.J., et al. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirbaha H., Chen D., Morazova O.A., Ruff K.M., Sharma A.M., Liu X., et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife. 2018;7 doi: 10.7554/eLife.36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A.M., Thomas T.L., Woodard D.R., Kashmer O.M., Diamond M.I. Tau monomer encodes strains. Elife. 2018;7 doi: 10.7554/eLife.37813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman S.K., Sanders D.W., Thomas T.L., Ruchinskas A.J., Vaquer-Alicea J., Sharma A.M., et al. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron. 2016;92:796–812. doi: 10.1016/j.neuron.2016.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders D.W., Kaufman S.K., DeVos S.L., Sharma A.M., Mirbaha H., Li A., et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaquer-Alicea J., Diamond M.I., Joachimiak L.A. Tau strains shape disease. Acta Neuropathol. 2021;142:57–71. doi: 10.1007/s00401-021-02301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boluda S., Iba M., Zhang B., Raible K.M., Lee V.M., Trojanowski J.Q. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer's disease or corticobasal degeneration brains. Acta Neuropathol. 2015;129:221–237. doi: 10.1007/s00401-014-1373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavaguera F., Akatsu H., Fraser G., Crowther R.A., Frank S., Hench J., et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y., Zhang W., Yang Y., Murzin A.G., Falcon B., Kotecha A., et al. Structure-based classification of tauopathies. Nature. 2021;598:359–363. doi: 10.1038/s41586-021-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou Z., Chen D., Ryder B.D., Joachimiak L.A. Biophysical properties of a tau seed. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-93093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D., Drombosky K.W., Hou Z., Sari L., Kashmer O.M., Ryder B.D., et al. Tau local structure shields an amyloid-forming motif and controls aggregation propensity. Nat. Commun. 2019;10:2493. doi: 10.1038/s41467-019-10355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drombosky K.W., Chen D., Woodard D., Sari L., Lin M., Diamond M.I., et al. Native tau structure is disrupted by disease-associated mutations that promote aggregation. bioRxiv. 2018 doi: 10.1101/330266. [preprint] [DOI] [Google Scholar]
- 25.Blennow K., Chen C., Cicognola C., Wildsmith K.R., Manser P.T., Bohorquez S.M.S., et al. Cerebrospinal fluid tau fragment correlates with tau PET: a candidate biomarker for tangle pathology. Brain. 2020;143:650–660. doi: 10.1093/brain/awz346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondal R., Agbaria M., Nairoukh Z. Fluorinated rings: conformation and application. Chemistry. 2021;27:7193–7213. doi: 10.1002/chem.202005425. [DOI] [PubMed] [Google Scholar]
- 27.Muralidharan A., Schmidt J.R., Yethiraj A. Solvation induced ring Puckering effect in fluorinated prolines and its inclusion in classical force fields. J. Phys. Chem. B. 2020;124:5899–5906. doi: 10.1021/acs.jpcb.0c04312. [DOI] [PubMed] [Google Scholar]
- 28.Yanamandra K., Kfoury N., Jiang H., Mahan T.E., Ma S., Maloney S.E., et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goedert M., Jakes R., Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 30.Colin M., Dujardin S., Schraen-Maschke S., Meno-Tetang G., Duyckaerts C., Courade J.P., et al. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 2020;139:3–25. doi: 10.1007/s00401-019-02087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak P., Kontsekova E., Zilka N., Novak M. Ten years of tau-targeted immunotherapy: path walked and the roads ahead. Front. Neurosci. 2018;12:798. doi: 10.3389/fnins.2018.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boxer A.L., Qureshi I., Ahlijanian M., Grundman M., Golbe L.I., Litvan I., et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial the. Lancet Neurol. 2019;18:549–558. doi: 10.1016/S1474-4422(19)30139-5. [DOI] [PubMed] [Google Scholar]
- 33.Dam T., Boxer A.L., Golbe L.I., Hoglinger G.U., Morris H.R., Litvan I., et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: a phase 2, randomized, placebo-controlled trial. Nat. Med. 2021;27:1451–1457. doi: 10.1038/s41591-021-01455-x. [DOI] [PubMed] [Google Scholar]
- 34.Hoglinger G.U., Litvan I., Mendonca N., Wang D., Zheng H., Rendenbach-Mueller B., et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial the. Lancet Neurol. 2021;20:182–192. doi: 10.1016/S1474-4422(20)30489-0. [DOI] [PubMed] [Google Scholar]
- 35.Ayalon G., Lee S.H., Adolfsson O., Foo-Atkins C., Atwal J.K., Blendstrup M., et al. Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer's disease. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb2639. [DOI] [PubMed] [Google Scholar]
- 36.Vaz M., Silvestre S. Alzheimer's disease: recent treatment strategies. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173554. [DOI] [PubMed] [Google Scholar]
- 37.Mullard A. Anti-tau antibody failures stack up. Nat. Rev. Drug Discov. 2021;20:888. doi: 10.1038/d41573-021-00187-4. [DOI] [PubMed] [Google Scholar]
- 38.Mullard A. Failure of first anti-tau antibody in Alzheimer disease highlights risks of history repeating. Nat. Rev. Drug Discov. 2021;20:3–5. doi: 10.1038/d41573-020-00217-7. [DOI] [PubMed] [Google Scholar]
- 39.Binder L.I., Guillozet-Bongaarts A.L., Garcia-Sierra F., Berry R.W. Tau, tangles, and Alzheimer's disease. Biochim. Biophys. Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Cicognola C., Brinkmalm G., Wahlgren J., Portelius E., Gobom J., Cullen N.C., et al. Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer's disease. Acta Neuropathol. 2019;137:279–296. doi: 10.1007/s00401-018-1948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato C., Barthelemy N.R., Mawuenyega K.G., Patterson B.W., Gordon B.A., Jockel-Balsarotti J., et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;98:861–864. doi: 10.1016/j.neuron.2018.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts M., Sevastou I., Imaizumi Y., Mistry K., Talma S., Dey M., et al. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease. Acta Neuropathol. Commun. 2020;8:13. doi: 10.1186/s40478-020-0884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newberry R.W., Raines R.T. 4-Fluoroprolines: conformational analysis and effects on the stability and folding of peptides and proteins. Top. Heterocycl. Chem. 2017;48:1–25. doi: 10.1007/7081_2015_196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torbeev V.Y., Hilvert D. Both the cis-trans equilibrium and isomerization dynamics of a single proline amide modulate beta2-microglobulin amyloid assembly. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20051–20056. doi: 10.1073/pnas.1310414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eberhardt E.S., Panisik N., Jr., Raines R.T. Inductive effects on the energetics of prolyl peptide Bond isomerization: implications for collagen folding and stability. J. Am. Chem. Soc. 1996;118:12261–12266. doi: 10.1021/ja9623119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furman J.L., Holmes B.B., Diamond M.I. Sensitive detection of proteopathic seeding activity with FRET flow cytometry. J. Vis. Exp. 2015 doi: 10.3791/53205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Y., DiMaio F., Wang R.Y., Kim D., Miles C., Brunette T., et al. High-resolution comparative modeling with RosettaCM. Structure. 2013;21:1735–1742. doi: 10.1016/j.str.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhury S., Lyskov S., Gray J.J. PyRosetta: a script-based interface for implementing molecular modeling algorithms using Rosetta. Bioinformatics. 2010;26:689–691. doi: 10.1093/bioinformatics/btq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BLI kinetics curves of antibody binding to various R1R2/R1R3 tau peptides.A, kinetic traces for R1R2 (upper panel) and R1R2 trans-proline (bottom panel) peptides. The kinetic traces are fitted to 1:1 kinetics. B, (A) Kinetic traces for R1R3 (upper panel) and R1R3 trans-proline (bottom panel) peptides. The kinetic traces are fitted to 1:1 kinetics. Whereas both MD2.2 and MD3.1 bound peptides, we observed no significant binding with HJ8.5.
Expanded high-magnification images of MD2.2 and MD3.1-positive perinuclear inclusions in AD and PSP brain tissue. Scale bar = 20 μm.
Unprocessed images of western blots used inFigure 3.
Unprocessed images of western blots used inFigure 4.
Energy distribution plot of assembled AD, CBD and PSP models in fibril form. Energy distribution of ensembles produced for AD, CBD and PSP trimers containing full length 2N4R tau. The energies are shown as Rosetta Energy Units (REU).
Data Availability Statement
Data relevant to the manuscript is available online: https://zenodo.org/record/8206697.