Abstract
Thalassemia is common in Southeast Asia, where artemisinin derivatives are frequently used in the treatment of malaria. It has been previously reported that artemisinin derivatives can be concentrated by uninfected thalassemic erythrocytes in vitro but not by normal erythrocytes. As a follow-up to this report, we studied the antimalarial kinetics of intravascular artesunate (2.4 mg/kg of body weight) in 10 persons with normal hemoglobins and in 10 patients with thalassemia (2 with α-thalassemia type 1–hemoglobin Constant Spring and 8 with α-thalassemia type 1–α-thalassemia type 2). Concentrations of artesunate and its active metabolites in plasma were measured by bioassay and expressed relative to those of dihydroartemisinin, the major biologically active metabolite. Concentrations of intravascular artesunate in plasma peaked in both the normal individuals and the thalassemic individuals 15 min after injection (the first time point). Plasma drug concentrations at all time intervals, except that at 1 h, were significantly higher in thalassemic subjects than in normal subjects (P < 0.05). The area under the concentration-time curve was 9-fold higher (P < 0.001) and the volume of distribution at steady state was 15-fold lower (P < 0.001) in thalassemic than in normal subjects. In light of the potential neurotoxicity of artemisinin derivatives, these results suggest that thalassemic subjects may need a drug administration regimen different from that of normal patients.
Malarial infection is a major cause of morbidity and mortality worldwide. There are 100 to 200 million cases and 1 to 2 million deaths each year (13, 30). Traditionally, it has been treated with quinolines and antifolates. Unfortunately, the prevalence of multidrug-resistant Plasmodium falciparum is increasing both in Southeast Asia and Africa (13, 28). Artemisinin, a new class of endoperoxide antimalarial, has become a promising therapeutic option (6, 10, 17, 19). Artesunate and artemether, semisynthetic derivatives of artemisinin, are being used widely in China and Southeast Asia, where resistance to the classical antimalarials is common (14, 19).
Artemisinin derivatives are relatively ineffective in vitro against P. falciparum-infected erythrocytes from patients with the thalassemic disorders hemoglobin H and hemoglobin Constant Spring (Hb CS) (32). This lack of susceptibility is due to competition for uptake of drug by uninfected thalassemic erythrocytes (11). These results imply that thalassemic disease might modify the antimalarial properties of artemisinin derivatives and that normal dose regimens may need to be changed for these patients. The information about pharmacokinetics may serve to provide a rational basis for the initial adjustment of drug treatment of thalassemic patients. This may be of particular importance in areas of the world, such as Southeast Asia, where there are both high incidence of malaria infection and high frequencies of hemoglobinopathies (16). This study compares the pharmacokinetics of intravascular artesunate in 10 thalassemic subjects and 10 normal subjects by a bioassay.
MATERIALS AND METHODS
In vitro effects of artesunate.
Since thalassemic erythrocytes have a tendency to hemolyze, in vitro exposures of thalassemic erythrocytes to high concentrations of artesunate were performed before the human study could be considered safe enough to proceed. Blood was collected from 10 normal volunteers, 7 patients with α-thalassemia type 1–α-thalassemia type 2 (α-thal1–α-thal2), and 7 patients with α-thal1–Hb CS. The fresh whole blood samples were incubated at both room temperature and 37°C with various concentrations of artesunate (0 to 1 mM) for 0, 15, 30, 45, 60, 180, and 360 min. A complete evaluation of cells (erythrocyte count, hematocrit, hemoglobin type, leukocyte count, platelet count, and blood smear examination) was made, and blood indices were determined with a MaxM automatic counter (Coulter Corporation, Miami, Fla.). Released hemoglobin was quantitated with a kit from Sigma Diagnostics (St. Louis, Mo.).
In vivo antimalarial kinetics of artesunate.
This study was approved by the Ethics Committee of Mahidol University and by the Institutional Review Board of the University of Michigan. Ten healthy adult volunteers, two volunteers with α-thal1–Hb CS and eight volunteers with α-thal1–α-thal2 were admitted into the Hospital for Tropical Diseases, Mahidol University, for 6 h. All of them gave fully informed consent to participate in the study. None of them had a recent history of malaria infection or of taking other antimalarials or medications. None had received transfusions.
Artesunate (60 mg/vial, obtained from Guilin Pharmaceutical factory no. 2, Guangxi, China, and distributed by Atlantic Pharmaceutical Co. Ltd., Bangkok, Thailand) was administered intravascularly in the morning at a dose of 2.4 mg/kg of body weight. Blood samples (3 ml) were drawn aseptically into sterile anticoagulate tubes from the other arm at intervals of 0, 15, 30, 45, 60, 180, and 360 min after drug administration. Plasma was separated immediately and kept at −70°C. The assay was performed within 3 months after sample collection. Results of blood chemistry (liver function as well as renal function tests were included) and hematological examinations were analyzed before and 6 h after drug administration.
The bioassay was performed according to the method described previously (4, 15, 25), which is based on a principle similar to that of the microdilution technique for antimalarial sensitivity testing (7). The thawed plasma samples were first freed of immunoglobulin by incubation with Affi-Gel-bound protein A (Bio-Rad, Hercules, Calif.). The treated plasma was transferred into a 96-well plate, and twofold serial dilutions were made with fresh human sera. A parasitized-erythrocyte suspension was added, and then the plate was incubated at 37°C for 24 h in a candle jar. Tritiated hypoxanthine (Amersham, Arlington Heights, Ill.) was added, after which the plate was incubated for another 18 to 20 h. The infected cell suspension was harvested, and [3H]hypoxanthine incorporation was determined. Dihydroartemisinin, the principal artesunate metabolite, was used to set up the standard curve. Standard curves were prepared with normal, α-thal1–Hb CS, and α-thal1–α-thal2 plasma. For each plasma sample, the 50% inhibitory concentration was determined from the corresponding standard curve and used to calculate the plasma drug concentration.
Pharmacokinetic analysis.
The mean concentrations of artesunate in plasma from normal and thalassemic subjects were compared at each time point with SPSS for Windows, release 6.1.3, standard version (SPSS, Inc., Chicago, Ill.). Pharmacokinetic data for each subject were estimated by a noncompartment model with WinNonlin, standard edition, version 1.1 (SCI Software, Lexington, Ky.). Parameter estimates are also shown (see Table 2). Data are presented as means ± standard errors with 95% confidence intervals.
TABLE 2.
Pharmacokinetic data following a single intravenous injection of artesunate (2.4 mg/kg of body weight) into 10 normal individuals and 10 thalassemic individuals
| Subjects | Valuea for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (nmol/liter)b | Clast (nmol/liter)b | t1/2 (h)c | AUC (nmol/liter/h)b | λ (h)d | RT (min)e | CL (liter/h)f | Vss (liter/kg)f | |
| Normal | 306 ± 259 (94–913) | 2.5 ± 2.9 (0.5–9.6) | 1.37 ± 0.59 (1.04–2.06) | 147 ± 46.5 (95.2–215) | 0.43 ± 0.19 (0.34–0.67) | 33 ± 13 (17–57) | 38 ± 4.3 (25.3–56.5) | 48 ± 11 (12.4–95.9) |
| Thalassemic | 4,761 ± 2,561 (1,006–8,787) | 14.6 ± 6.46 (3.88–26.3) | 1.95 ± 0.82 (1.08–3.51) | 1,316 ± 613 (420–2,445) | 0.40 ± 0.14 (0.2–0.64) | 59 ± 21 (21–87) | 4.1 ± 0.54 (3–9) | 3.2 ± 0.78 (1.6–7.3) |
Data shown are means ± standard errors at 95% confidence intervals (ranges are in parentheses). Cmax, peak concentration in plasma; Clast, last concentration in plasma; AUC, area under the concentration-time curve; λ, terminal elimination rate constant; RT, residence time; CL, clearance.
P < 0.001.
P = 0.174.
P = 0.545.
P = 0.01.
P = 0.001.
RESULTS
In vitro effects of artesunate.
Normal and thalassemic erythrocytes were incubated with various concentrations of artesunate to determine whether the drug had any deleterious effect on thalassemic erythrocytes in vitro. No alterations in morphology or hemolysis were detected after the erythrocytes were incubated at concentrations as high as 1 mM for 6 h.
In vivo antimalarial kinetics of artesunate.
Characteristics and mean lab values for the thalassemic and normal volunteers are shown in Table 1. As expected, the thalassemic patients had lower levels of hemoglobin, hematocrits, and erythrocyte counts. No adverse effects or significant changes in lab values were seen either during or after the study.
TABLE 1.
Study subject characteristics
| Characteristica | Value for:
|
|
|---|---|---|
| Normal subjects | Thalassemic subjects | |
| No. studied | 10 | 10b |
| Male subjects (%) | 40 | 20 |
| Age (yr)c | 21.8 ± 1.7 (20–25) | 23.6 ± 3.3 (18–28) |
| Wt (kg)c | 50 ± 6.4 (43–52) | 53.4 ± 6.2 (45–57) |
| Hematologyc | ||
| Erythrocyte count (1012/liter) | 5.06 ± 0.45 (4.55–5.68) | 4 ± 0.4 (3.57–4.62) |
| Hemoglobin (g/dl) | 14.78 ± 1.02 (13.5–16) | 8 ± 0.3 (7.6–8.3) |
| Hematocrit (%) | 43.9 ± 5.02 (39.9–47.4) | 27 ± 0.9 (26.2–28.6) |
| CV (fl) | 86.86 ± 2.98 (81.6–88.7) | 68 ± 5.2 (62–73.3) |
| CH (pg) | 29.26 ± 0.69 (28.2–29.8) | 20 ± 1.6 (17.7–21.8) |
| CHC (g/dl) | 33.64 ± 0.54 (32.9–34.4) | 30 ± 1.3 (28.3–31.1) |
| Blood chemistryc | ||
| BUN (mg/dl) | 10.6 ± 2.61 (8–16) | 8.6 ± 2.3 (7–12) |
| Creatinine (mg/dl) | 1.02 ± 0.13 (0.9–1.2) | 0.72 ± 0.13 (0.6–0.9) |
| Total protein (g/dl) | 8.3 ± 0.46 (7.8–9) | 7.66 ± 0.54 (7–8.5) |
| Albumin (g/dl) | 4.9 ± 0.34 (4.5–5.1) | 4.66 ± 0.35 (4.3–5.1) |
| Globulin (g/dl) | 2.7 ± 0.44 (2.4–3.2) | 3.3 ± 0.5 (2.2–3.4) |
| Total bilirubin (mg/dl) | 0.9 ± 0.23 (0.7–1.2) | 1.82 ± 1.08 (1–3.2) |
| Direct bilirubin (mg/dl) | 0.28 ± 0.11 (0.1–0.4) | 0.32 ± 0.04 (0.3–0.4) |
| AST (U/liter) | 19.8 ± 2.49 (18–23) | 37.6 ± 3.9 (31–40) |
| ALT (U/liter) | 16.6 ± 9.04 (8–26) | 16.8 ± 2.95 (13–21) |
CV, corpuscular volume; CH, corpuscular hemoglobin; CHC, corpuscular hemoglobin concentration; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Two subjects had α-thal1–Hb CS, and eight subjects had α-thal1–α-thal2.
Data shown are means ± standard errors at 95% confidence intervals (ranges are in parentheses).
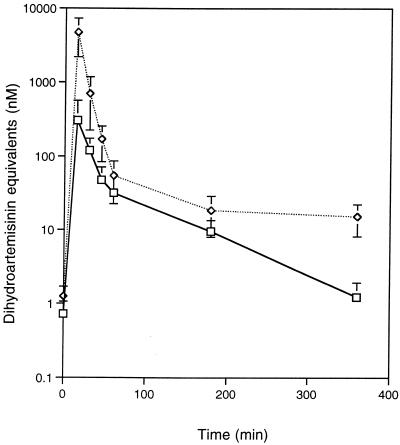
The time course for the disappearance of artesunate and its metabolites was calculated relative to that of dihydroartemisinin. We found no difference in drug concentrations when they were read from standard curves prepared from either normal or thalassemic sera. As shown in Fig. 1, no antimalarial activities were detected in the samples at 0 min of both normal and thalassemic subjects, indicating that none of them had received previous treatment. The concentrations of drug at all time points, except at 1 h, in the thalassemic plasma were significantly (P < 0.05) higher than those in normal plasma (Fig. 1). The concentration of artesunate in blood was still detectable 6 h after drug administration (14 ± 3 and 1.4 ± 0.28 nmol/liter in thalassemic and normal subjects, respectively).
FIG. 1.
Mean concentrations of drug in plasma samples, relative to those of dihydroartemisinin, after intravenous administration of artesunate (2.4 mg/kg of body weight) to normal (—□—) and thalassemic (⋯◊⋯) subjects. Error bars represent standard errors. Differences between concentrations are statistically significant (P < 0.02) at all time points except 0 and 60 min.
For both normal and thalassemic subjects, concentrations of artesunate in plasma peaked at the earliest time point after administration of drug (15 min). However, the peak concentrations in plasma, the last concentrations in plasma, the area under the concentration-time curve, and the mean residence time were significantly lower in normal subjects than in thalassemic subjects (P < 0.05). On the other hand, the volume of distribution at steady state (Vss) was significantly higher in normal than in thalassemic subjects (P < 0.001). No statistically significant differences were seen in the elimination half-lives (t1/2) or in terminal elimination rate constants between the two groups.
DISCUSSION
Artemisinin derivatives are novel antimalarial drugs that are being used increasingly, particularly in parts of the world where multidrug-resistant malaria is common. However, there is still relatively little information about their pharmacokinetic properties (19). This is partly due to difficulties in measuring these compounds in body fluids (9) as well as to the tight binding of drug to erythrocyte membranes (1) and plasma proteins (27, 31). The parent drug and its biologically active metabolite (dihydroartemisinin) can be separately measured by high-performance liquid chromatography with electrochemical detection (2, 8, 18, 21) or chemical derivatization (9, 26). However, these methods are difficult to set up. A simpler bioassay was developed (15) and has proven to be quite useful (25). Although the bioassay cannot discriminate the parent compound from its active metabolites, it can provide information that is sufficiently useful in designing dosing regimens. Comparisons of bioassay and high-performance liquid chromatography pharmacokinetic data, however, must be made cautiously (4).
There have been a few studies of the effects of malaria infection on antimalarial pharmacokinetics. Intravenous artesunate and its active metabolites (as measured by an immunoassay) were eliminated from the plasma of healthy volunteers with a t1/2 of 45 min (24). A similar t1/2 was found by the bioassay for children with acute falciparum infection, from whose plasma oral artesunate was eliminated with a t1/2 of 1 h (4). After administration of oral artemether, both artemether and dihydroartemisinin were found to reach higher peak concentrations and have longer t1/2s in malaria-infected patients than in healthy volunteers, although most of these differences were nonsignificant (22).
The present study confirms earlier reports that artesunate and its metabolites are eliminated from plasma rapidly. Time to highest antimalarial activity in plasma was seen at 15 min after drug administration, although this value may have been earlier since the first time point in this study was at 15 min. However, 15 min is consistent with results of previous studies of monkeys receiving intravenous artesunate (15). Our results showed that the mean antimalarial activity in plasma was still detectable and remained above the in vitro 50% inhibitory concentration (11) for at least 6 h after administration (14 ± 3 nmol/liter in thalassemic subjects).
Parasites interact with thalassemic erythrocytes differently than with normal erythrocytes (23, 29, 33, 34). It is interesting that artemisinin drugs also interact differently with the two types of erythrocytes.
Why are the concentrations of biologically active metabolites in plasma so much higher in thalassemic subjects than in normal individuals? The apparent Vss is 15-fold higher in normal subjects than in thalassemic subjects, suggesting that the drug becomes more widely distributed in the former than in the latter (3). It is unlikely that this is due to differences in levels of proteins in sera between thalassemic and normal subjects, since the standard concentration-response curves were identical for normal and thalassemic sera. The difference in Vsss may be related to the observation that the thalassemic erythrocytes take up much more artemisinin than normal erythrocytes in vitro (12). Thus, erythrocytes in the thalassemic patients might have taken up artesunate and its metabolites and then slowly released them into the plasma. Alternatively, thalassemic patients might metabolize artesunate differently than normal subjects, producing more of the active metabolites (such as dihydroartemisinin) and less of the inactive ones (such as deoxydihydroartemisinin).
High doses of artemisinin derivatives are neurotoxic in animals (5, 12) and may even be neurotoxic in humans (20). Thalassemic patients are likely to have higher concentrations of drug in plasma than normal patients but may also have lower concentrations of drug in other body tissue. Thus, thalassemic patients receiving the same drug doses as normal patients may be at either increased or decreased risk of neurotoxicity.
Further work is needed to explain the differences in pharmacokinetics between thalassemic and normal individuals. Knowledge of this difference and its causes may aid in designing appropriate drug dosage regimens, particularly in areas where there are both high incidence of malaria infection and high frequencies of abnormal hemoglobin genes.
ACKNOWLEDGMENTS
This work was carried out with financial support from the National Institutes of Health (grant 5 U01 AI35827).
We are grateful to the normal volunteers and thalassemic patients for their warm cooperation, to Paktiya Teja-Isavadharm for help in setting up the assay, and to Nick White for helpful discussions.
REFERENCES
- 1.Asawamahasakda W, Benakis A, Meshnick S R. The interaction of artemisinin with red cell membranes. J Lab Clin Med. 1994;123:757–762. [PubMed] [Google Scholar]
- 2.Benakis A, Paris M, Loutan L, Plessas C T, Plessas S T. Pharmacokinetics of artemisinin and artesunate after oral-administration in healthy-volunteers. Am J Trop Med Hyg. 1997;56:17–23. doi: 10.4269/ajtmh.1997.56.17. [DOI] [PubMed] [Google Scholar]
- 3.Benet L Z, Massoud N. Pharmacokinetics. In: Benet L Z, et al., editors. Pharmacokinetic basis for drug treatment. New York, N.Y: Raven Press; 1984. pp. 1–28. [Google Scholar]
- 4.Bethell D B, Tejaisavadharm P, Cao T P, Pham T T T, Ta T T M, Tran T N T, Nguyen T T H, Phuong P T, Kyle D, Day N P J, White N J. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:195–198. doi: 10.1016/s0035-9203(97)90222-4. [DOI] [PubMed] [Google Scholar]
- 5.Brewer T G, Grate S J, Peggins J O, Weina P J, Petras J M, Levine B S, Heiffer B M H, Schuster B G. Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg. 1994;51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- 6.Cumming J N, Ploypradith P, Posner G H. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol. 1997;37:253–299. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins R E, Canfield C J, Hayne J D, Chulay J. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duc D D, De Vries P J, Khanh N X, Binh L N, Kager P A, Von Boxtel C J. The pharmacokinetics of a single dose of artemisinin in healthy Vietnameses subjects. Am J Trop Med Hyg. 1994;51:785–790. doi: 10.4269/ajtmh.1994.51.785. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, G. 1994. Measurement of artemisinin and its derivatives in biological fluids. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):37–39. [DOI] [PubMed]
- 10.Hien T T, White N J. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 11.Kamchonwongpaisan S, Chandra-ngam G, Avery M A, Yuthavong Y. Resistance to artemisinin of malaria parasites (Plasmodium flaciparum) infecting α-thalassemic erythrocytes in vitro. Competition in drug accumulation with uninfected erythrocytes. J Clin Invest. 1994;93:467–473. doi: 10.1172/JCI116994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamchonwongpaisan S, McKeever P, Hossler P, Leung M Y, Ziffer H, Meshnick S R. Artemisinin neurotoxicity: neuropathology in rats and mechanistic studies in vitro. Am J Trop Med Hyg. 1997;56:7–12. doi: 10.4269/ajtmh.1997.56.7. [DOI] [PubMed] [Google Scholar]
- 13.Krogstad D J. Malaria as a reemerging disease. Epidemiol Rev. 1996;18:77–89. doi: 10.1093/oxfordjournals.epirev.a017918. [DOI] [PubMed] [Google Scholar]
- 14.Li, G. Q., X. B. Guo, L. C. Fu, L. H. X. Jian, and X. H. Wang. 1994. Clinical trials of artemisinin in the treatment of malaria in China. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):5–6. [DOI] [PubMed]
- 15.Li X, Rieckmann K. A bioassay for derivatives of qinghaosu (artemisinin) Trop Med Parasitol. 1992;43:195–196. [PubMed] [Google Scholar]
- 16.Livingstone F B. Frequencies of hemoglobin variants: thalassemia, the glucose-6-phosphate dehydrogenase deficiency, G6PD variants, and ovalocytosis in human populations. New York, N.Y: Oxford University Press; 1985. [Google Scholar]
- 17.Looareesuwan, S. 1994. Overview of clinical studies on artemisinin derivatives in Thailand. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):9–11. [DOI] [PubMed]
- 18.Melendez V, Peggins J O, Brewer T G, Theoharides A D. Determination of antimalarial arteether and its deethylated metabolite dihydroartemisinin in plasma by high performance liquid chromatography with reductive electro-chemical detection. J Pharm Sci. 1991;80:132–138. doi: 10.1002/jps.2600800209. [DOI] [PubMed] [Google Scholar]
- 19.Meshnick S M, Taylor T E, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller L G, Panosian C B. Ataxia and slurred speech after artesunate treatment for falciparum malaria. N Engl J Med. 1997;336:1328. doi: 10.1056/NEJM199705013361818. [DOI] [PubMed] [Google Scholar]
- 21.Navaratnam K, Mansor S M, Chin L K, Mordi M N, Asokan N, Bair N K. Determination of artemether and dihydroartemisinin in blood plasma by high performance liquid chromatography for application in clinical pharmacological studies. J Chromatogr B. 1995;669:289–294. doi: 10.1016/0378-4347(94)00109-i. [DOI] [PubMed] [Google Scholar]
- 22.Na-Bangchang K, Krabwang J, Thomas C G, Thanavibul A, Sukontason K, Ward S A, Edwards G. Pharmacokinetics of artemether after oral administration to healthy Thai males and patients with acute uncomplicated falciparum malaria. Br J Clin Pharmacol. 1994;37:249–253. doi: 10.1111/j.1365-2125.1994.tb04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senok A C, Li K, Nelson E A S, Yu L M, Tian L P, Oppenhenmer S J. Invasion and growth of Plasmodium falciparum is inhibited in fractionated thalassemic erythrocytes. Trans R Soc Trop Med Hyg. 1997;91:138–143. doi: 10.1016/s0035-9203(97)90200-5. [DOI] [PubMed] [Google Scholar]
- 24.Song Z Y, Zhao K C. Studies of qinghaosu and its active derivatives in biological materials and their pharmacokinetics. Proc Chin Acad Med Sci Peking Union Med Coll. 1989;4:229–234. [PubMed] [Google Scholar]
- 25.Teja-Isavadharm P, Nosten F, Kyle D E, Luxemburger C, Ter Kuile F, Peggins J O, Brewer T G, White N J. Comparative bioavailability of oral, rectal, and intramuscular artemether in healthy subjects: use of simultaneous measurement by high performance liquid chromatograpy and bioassay. Br J Clin Pharmacol. 1996;42:599–604. doi: 10.1111/j.1365-2125.1996.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 26.Titulaer H A C, Zuidema J, Kager P A, Wetsteyn J C F M, Lugt C H B, Merkus F W H M. The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to volunteers. J Pharm Pharmacol. 1990;42:810–813. doi: 10.1111/j.2042-7158.1990.tb07030.x. [DOI] [PubMed] [Google Scholar]
- 27.Wanwimolruk S, Edwards G, Ward S A, Breckenridge A M. The binding of the antimalarial arteether to plasma proteins in vitro. J Pharm Pharmacol. 1992;44:940–942. doi: 10.1111/j.2042-7158.1992.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 28.Wernsdorfer W H. Epidemiology of drug resistance in malaria. Acta Trop. 1994;56:143–156. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 29.Williams T N, Maitland K, Bennett S, Ganczakowski M, Peto T E A, Newbold C I, Bowden D K, Weatherall D J, Clegg J B. High incidence of malaria in α-thalassemic children. Nature. 1996;383:522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization, Malaria Unit. Global malaria control. Bull W H O. 1993;71:281–284. [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y Z, Aswamahasakda W, Meshnick S R. Alkylation of human albumin by the antimalarial artemisinin. Biochem Pharmacol. 1993;46:336–339. doi: 10.1016/0006-2952(93)90425-v. [DOI] [PubMed] [Google Scholar]
- 32.Yuthavong Y, Butthep P, Bunyaratvej A, Fucharoen S. Decreased sensitivity to artesunate and chloroquine of Plasmodium falciparum infecting hemoglobin H and/or hemoglobin Constant Spring erythrocytes. J Clin Invest. 1989;83:502–505. doi: 10.1172/JCI113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuthavong Y, Wilairat P. Protection against malaria by thalassemia and hemoglobin variants. Parasitol Today. 1993;9:241–245. doi: 10.1016/0169-4758(93)90065-n. [DOI] [PubMed] [Google Scholar]
- 34.Yuthavong Y, Wilairat P. Is the high incidence of malaria in α-thalassemic children evidence against the malaria hypothesis? Parasitol Today. 1997;13:207–208. doi: 10.1016/s0169-4758(97)01055-7. [DOI] [PubMed] [Google Scholar]