Abstract
Background and purpose
Non-ossifying fibroma (NOF) is one of the most commonly seen benign bone tumours. Although renowned for their benign behaviour and tendency for spontaneous healing, these tumours can occasionally exhibit an aggressive course. Few published papers have focused on the treatment options of symptomatic NOFs.
The aim of this case report is to discuss the clinical presentation of a painful and unusually aggressive multiple NOF of the distal tibia in a female adolescent patient.
Case presentation
The case of a 17-year-old female patient who was complaining of a painful swollen right lower leg for the past few months. The symptoms became gradually worse, preventing her from sporting activities and becoming more and more debilitating. The patient was diagnosed with a particularly aggressive multiple non-ossifying fibroma of the distal tibia and fibula. She was treated with lesion curettage, bone grafting and external fixation with good clinical and radiological outcomes.
Conclusion
Non-ossifying fibroma is a benign lesion that only requires observation in most of the cases. However, symptomatic lesions with aggressive behaviour or complicated with pathologic fracture may warrant surgical intervention.
Keywords: Non-ossifying fibroma, Multiple, Aggressive, Surgery, External fixation
Highlights
-
•
Non-ossifying fibromas (NOFs) are common benign tumours.
-
•
Multiple localizations are usually seen in syndromic NOFs.
-
•
Treatment options in symptomatic NOF are still controversial.
-
•
Curettage and bone grafting is the gold standard in aggressive or complicated NOFs
1. Introduction
The non-ossifying fibroma (NOF) is among the most common benign bone tumours (Baumhoer et al., 2020; Stacy and Dixon, 2007).
The exact incidence of NOFs is unknown. It has been estimated that approximately 30 % of all children have one or more undetected lesions (Emori et al., 2022; Herget et al., 2016).
While the fibrous cortical defect (FCD) typically remains confined to the bone cortex, the NOF is larger in size, with variable endo medullary extension (Baumhoer et al., 2020; Mankin et al., 2009; Betsy et al., 2004; Bowers et al., 2013).
Due to their typical radiological appearance, most NOFs can be diagnosed on plain radiology (Rammanohar et al., 2021; Błaż et al., 2011).
Clinically, the NOF is usually asymptomatic (Emori et al., 2022; Mankin et al., 2009). However, in larger lesions, localized swelling, pain or even pathological fractures in rare aggressive cases can occur (Baumhoer et al., 2020; Mankin et al., 2009; Shimal et al., 2010).
Overall, the prognosis of NOFs is good (Herget et al., 2016) with a tendency for spontaneous healing of the lesion which seems to disappear within few years (Baumhoer et al., 2020; Betsy et al., 2004; Sakamoto et al., 2017).
Even though NOFs can be found in most long bones, their commonest locations are about the knee joint, most commonly at the distal femoral metaphysis, followed by the distal tibial metaphysis (Hetts et al., 2007; Glockenberg et al., 1997; Reynolds et al., 2018). The mandible is another classic location mostly reported in several case reports (Bowers et al., 2013; Khandaitkar et al., 2023).
Multiple non-ossifying fibromas (MNOFs) are of common occurrence. They may be isolated and usually symmetrical, or in association with other skeletal and extra-skeletal abnormalities forming the group of syndromic MNOFs (Corsi et al., 2017; Dorfman and Czerniak, 1999). Syndromic MNOFs are often asymmetrical and are seen in association of diverse developmental syndromes, such as type I neurofibromatosis and Jaffé-Campanacci syndrome (Corsi et al., 2017).
Although most of the authors agree that asymptomatic NOFs don't require any further actions, the management of painful and large aggressive lesions is less consensual ranging from simple observation to various surgical techniques (Reynolds et al., 2018).
In this case report we describe a case of non-syndromic MNOF with a peculiar aggressive course.
2. Case description
2.1. History
We report the case of a 17-year-old female patient with no previous medical or surgical history, who presented to the Orthopaedic outpatient clinic with the main complaint of a painful swollen right lower leg. She was a middle-distance runner at the level of high school competition. Her symptoms and signs started a year ago when the patient started feeling a swelling of her distal right leg, gradually increasing in size, with no reported history of trauma or other general symptoms. Few months ago, she started feeling pain especially at the end of her athletic training sessions. Over the time, the pain worsened and was reportedly present even at rest. She was forced to stop all sporting activities.
2.2. Clinical evaluation
On clinical examination, she was a healthy-looking young patient. She was walking with no obvious limp but the right single-leg stance was slightly unstable. On inspection, there was an obvious swelling localized at the supra malleolar region. This swelling was ill-defined, hard to the touch, fixed and painful on palpation. The overlying skin was normal with no inflammation signs. The right knee and ankle were stable with full range of motion. The remainder of the physical examination didn't show any abnormalities, especially skin inspection, that didn't reveal any “café au lait” spots.
2.3. Imaging
An antero-posterior and lateral radiographic views of the affected leg were requested and revealed a well circumscribed metaphyso-diaphyseal radio lucent lesion about 3 cm above the tibiotalar joint and extending about 7 cm proximally. This lesion was multiloculated, filling the entire medullary canal and expanding the cortices that looked thin without being ruptured. There was no obvious periosteal reaction seen. A similar 2 cm lucent lesion was visible on the adjacent fibular diaphysis (Fig. 1).
Fig. 1.
Plain X-ray of the ankle (a: anteroposterior and b: lateral) showing a well circumscribed metaphyso-diaphyseal radio lucent multiloculated lesion, filling the entire medullary canal and expanding the cortices. No obvious periosteal reaction was seen. A similar lesion is visible on the adjacent fibular diaphysis (arrow head).
MRI examination revealed a metaphyseal/diaphyseal tumour located in the distal tibia, measuring 36 mm × 42 mm × 77 mm (width × length × height), with a predominantly fibrous component. This mass was multiloculated, in hypointense signal on T1 and heterogenous hyperintense signal on T2 weighted images. It enhanced heterogeneously after gadolinium injection. The lesion expanded the cortices circumferentially, with no detectable surrounding soft tissues infiltration. A second process of similar appearance was found on the adjacent distal fibula, measuring 7 mm × 12 mm × 22 mm (width × length × height) (Fig. 2).
Fig. 2.
On MRI, a metaphyseal/diaphyseal tumour with a predominantly fibrous component can be seen. This mass was expanding the cortices circumferentially, with no detectable invasion of the surrounding soft tissues. A second process of similar appearance was found on the distal fibula.
2.4. Surgical management
A biopsy was carried on and a specimen made of friable, light brownish materiel was sent through for histo-pathological examination. On histology, it was a benign giant-cell-rich tumour (Fig. 3) which morphological features were suggestive of the diagnosis of either a NOF or a solid variant of an aneurismal bone cyst. The diagnosis of a giant cell tumour was ruled out mainly because of the young age of the patient.
Fig. 3.

Mesenchymal tumour made of multinucleated giant-cells (arrow heads) mixed with a spindle cell component. No atypia nor mitosis were seen. (HE X40).
The patient was operated under general anaesthesia. First, autologous cancellous bone graft was harvested from the posterior-superior iliac crests. The patient was then turned into the supine position and an anterolateral approach was used. A large cortical window was opened allowing to reach all the cavities of the tumour for a thorough curettage. The residual cavity was filled up with a mixture of autologous bone graft and bone chips. Finally, the cortical window was replaced. Since the fibular lesion was readily accessible, the same approach was used to expose and locate it using fluoroscopic guidance. After a thorough curettage, it was found that only a thin posterior cortex was left and decision was made to perform an en bloc tumour resection of the tumour while staying above the inferior tibiofibular joint ligaments' insertion to avoid compromising the syndesmosis stability.
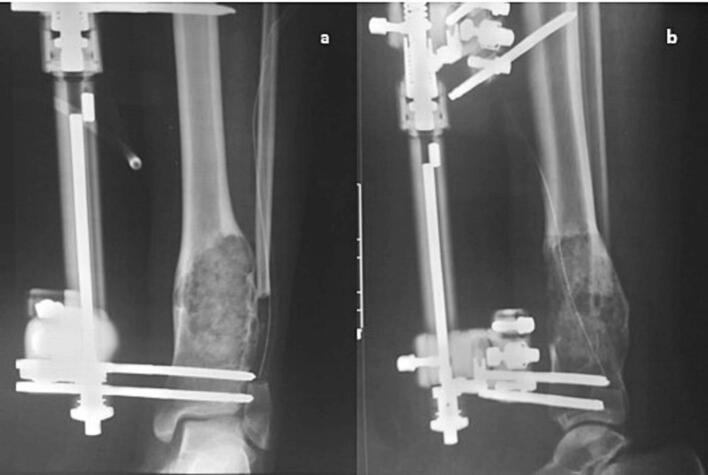
A tibio-tibial monoplane external fixator was finally applied to prevent any pathological fractures (Fig. 4).
Fig. 4.
Immediate antero-posterior (a) and lateral (b) post operative x-ray views showing the bone graft with the spanning external fixator.
The final histology report revealed a NOF.
2.5. Outcomes
The external fixator was removed after six weeks and the patient resumed partial weight bearing. The patient was seen at three months, six months and yearly thereafter with serial control x-rays showing a good graft integration with progressive filling of the cavity (Fig. 5).
Fig. 5.

At five years follow-up, control x-ray confirms the full integration of the bone graft with complete filling of the residual cavity with a healed NOF. No signs of syndesmotic instability such as medial clear space widening or arthritic changes were visible with a congruent ankle mortise.
At five years post operative, the patient is pain free and resumed her running activity at a university competitive level.
3. Discussion
Non-ossifying fibromas (NOFs) are benign fibrous bone lesions commonly encountered in the metaphyseal region of skeletally immature long bones of the lower limbs closest to the most fertile growth plates (Baumhoer et al., 2020).
Even though benign, these lesions are the most common cause of referral to orthopaedic oncology clinics (Stacy and Dixon, 2007).
Owing to their typical radiological appearance, most NOFs can be diagnosed based solely on plain radiology with no need for histological confirmation (“No touch lesion”) (Emori et al., 2022; Herget et al., 2016; Betsy et al., 2004). NOFs most commonly appear on plain x-ray as a well-defined, polycyclic lesion with an eccentric location. The overlying cortex may appear thin and expanded (Rammanohar et al., 2021; Błaż et al., 2011). In our case, although NOF was highly suspected, other differential diagnoses such as aneurysmal bone cyst in its solid variant couldn't be ruled out.
Overall, the prognosis of NOFs is good (Herget et al., 2016) with a tendency for self-limitation of the lesion which seems to spontaneously disappear around the age of 20 to 25 (Baumhoer et al., 2020; Betsy et al., 2004; Sakamoto et al., 2017). Ritschl et al. described a stereotyped progression of the NOF in four staged from a simple lytic lesion (stage A) to a healed completely sclerotic lesion (stage D) (Ritschl et al., 1988). Our case corresponds to the Stage B according to the Ritschl radiologic classification.
As a matter of fact, classically, NOFs were considered to be more of a developmental bone defect rather than a real neoplasm (Sakamoto et al., 2017; Hetts et al., 2007). This idea was recently challenged by Baumhoer et al. and later by Bobée et al. who found an RAS-MAPK activation by somatic mutations in NOFs indicating that these actually should be considered as true neoplasms that are part of the broad RASopathy group of tumours (Baumhoer et al., 2019; Bovée and Hogendoorn, 2019).
Multiple non-ossifying fibromas (MNOFs) are not uncommon representing less than 5 % of all NOF (Emori et al., 2022). They may be isolated and usually symmetrical, or in association with other skeletal and extra-skeletal abnormalities forming the group of syndromic MNOFs (Corsi et al., 2017; Dorfman and Czerniak, 1999). Syndromic MNOFs are often asymmetrical and are seen in association of diverse developmental syndromes, such as type I neurofibromatosis and Jaffé-Campanacci syndrome (Corsi et al., 2017).
Most of the time, the NOF is of incidental diagnosis (Emori et al., 2022; Mankin et al., 2009). Symptomatic NOFs are uncommon (Bowers et al., 2013; Glockenberg et al., 1997). It has been reported that in larger lesions, localized swelling and pain can be reported especially during athletic activities (Herget et al., 2016). Pathological or stress fractures in rare aggressive cases can be seen (Baumhoer et al., 2020; Mankin et al., 2009; Shimal et al., 2010). Shimal et al., over a period of 18 years, reported five cases of skeletally immature patients presenting with a concomitant NOF and a stress fracture (Shimal et al., 2010). He emphasizes that the onset of the symptoms is typically insidious as is the case in our patient. Causes of the pain in our patient can either be the size and the aggressiveness of the lesion or a possible undiagnosed stress fracture.
Very few published studies focused on the treatment of patients with NOFs, either conservatively or operatively (Reynolds et al., 2018). The widely accepted attitude towards asymptomatic NOFs is a “wait and see” philosophy (Bowers et al., 2013; Glockenberg et al., 1997). Some authors adopt the same attitude even in the presence of a pathologic fracture because of the tendency of these lesions to heal spontaneously (Hudson et al., 1993; Easley and Kneisl, 1997).
Andrecchio et al. as well as other authors recommend a more interventional management when dealing with symptomatic NOFs (pain, fracture…) (Andreacchio et al., 2018) Surgical management classically consists in a curettage and bone grafting. Arata et al. (Arata et al., 1981) in a review of 23 cases of pathological fractures through a NOF concluded that a tumour occupying more than 50 % of the medullary canal or extending more than 33 mm in height are of high risk of fracture and “should be monitored closely”.
In our case, the size of the lesion filling almost the entire medullary canal, its multiplicity, the associated pain and its impact on the sporting activity of the patient were the reasons for indicating the surgical management.
Together with the classic curettage and bone grafting (either autologous or heterologous), other surgical techniques have been described such as percutaneous cryoablation and calcium sulphate grafts (Andreacchio et al., 2018; Ngo et al., 2015). In our case, because of the circumferential thinning of the distal tibial cortices, we opted for an external fixation bridging the lytic lesion to forestall any possible pathologic fracture. This method is not unanimously adapted as some authors prefer using bridging locking plates, meanwhile others like Andrecchio et al. (Andreacchio et al., 2018) didn't use any fixation devices after treating with curettage and calcium phosphate graft nine patients with NOFs. Matsubara et al. treated two patients with curettage and external fixation with good results (Matsubara and Tsuchiya, 2019).
Regardless of the treatment modality, all the reported lesions healed and no definite malignant transformation has ever been reported in the literature. In fact, few cases of concomitant coexistence of NOFs with malignant bone tumours have been published. Kyriakos et al. concluded that these were only “a chance occurrence” and rejected the previous reports of malignant transformation because they lacked convincing radiologic or histopathologic evidence of a pre-existent benign fibrous lesion (Kyriakos and Murphy, 1981).
4. Conclusion
Non-ossifying fibromas (NOFs) are benign bone tumours of common occurrence. With their tendency to spontaneous healing, therapeutic abstention and simple observation are sufficient in most of the cases. Arata's criteria can be helpful in detecting the rare aggressive cases where surgery can be indicated. Symptomatic NOFs causing pain and discomfort should be considered for surgical management consisting in a curettage associated with bone grafting especially in large defects. The prognosis is generally good functional results and no risk of malignant transformation.
Consent
A written consent was obtained from the patient for her personal anonymized data to be published.
Ethics declaration
Ethical approval was granted from the “Ethics Committee “of the MTM hospital; Tunisia.
CRediT authorship contribution statement
Walid Bouaicha: Writing – original draft, Formal analysis, Conceptualization. Mohamed Jlidi: Writing – original draft, Conceptualization. Salwa Nechi: Writing – review & editing, Investigation, Formal analysis, Conceptualization. Mouldi Lammouchi: Writing – original draft, Conceptualization. Siwar Sbaihi: Writing – review & editing, Investigation. Selim Daas: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare no known competing financial interest.
Data availability
Data will be made available on request.
References
- Andreacchio A., Alberghina F., Testa G., Canavese F. Surgical treatment for symptomatic non-ossifying fibromas of the lower extremity with calcium sulfate grafts in skeletally immature patients. Eur. J. Orthop. Surg. Traumatol. 2018;28(2):291–297. doi: 10.1007/s00590-017-2028-3. [DOI] [PubMed] [Google Scholar]
- Arata M.A., Peterson H.A., Dahlin D.C. Pathological fractures through non-ossifying fibromas. Review of the Mayo Clinic experience. J. Bone Joint Surg. Am. 1981;63(6):980–988. [PubMed] [Google Scholar]
- Baumhoer D., Kovac M., Sperveslage J., Ameline B., Strobl A.C., Krause A., Trautmann M., Wardelmann E., Nathrath M., Höller S., et al. Activating mutations in the MAP-kinase pathway define non-ossifying fibroma of bone. J. Pathol. 2019;248(1):116:22. doi: 10.1002/path.5216. [DOI] [PubMed] [Google Scholar]
- Baumhoer D., Rogozhin D.V. In: WHO Classification of Tumours of Soft Tissue and Bone. 5th, editor. IARC Press; Lyon: 2020. Non-ossifying fibroma; pp. 447–448. [Google Scholar]
- Betsy M., Kupersmith L.M., Springfield D.S. Metaphyseal fibrous defects. J. Am. Acad. Orthop. Surg. 2004;12(2):89–95. doi: 10.5435/00124635-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Błaż M., Palczewski P., Swiątkowski J., Gołębiowski M. Cortical fibrous defects and non-ossifying fibromas in children and young adults: the analysis of radiological features in 28 cases and a review of literature. Pol. J. Radiol. 2011;76(4):32–39. [PMC free article] [PubMed] [Google Scholar]
- Bovée J.V., Hogendoorn P.C. Non-ossifying fibroma: a RAS-MAPK driven benign bone neoplasm. J. Pathol. 2019;248(2):127–130. doi: 10.1002/path.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers L.M., Cohen D.M., Bhattacharyya I., Pettigrew J.C., Jr., Stavropoulos M.F. The non-ossifying fi broma: a case report and review of the literature. Head Neck Pathol. 2013;7(2):203–210. doi: 10.1007/s12105-012-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A., Remoli C., Riminucci M., Ippolito E., Dimitriou J. A unique case of multiple non-ossifying fibromas with polyostotic monomelic distribution and aggressive clinical course. Skelet. Radiol. 2017;46(2):233–236. doi: 10.1007/s00256-016-2523-3. [DOI] [PubMed] [Google Scholar]
- Dorfman H.D., Czerniak B. Bone tumors. St Louis, MO, Mosby, 1998. Hum. Pathol. 1999;30:1269. [Google Scholar]
- Easley M.E., Kneisl J.S. Pathologic fractures through nonossifying fi bromas: is prophylactic treatment warranted. J. Pediatr. Orthop. 1997;17:808–813. [PubMed] [Google Scholar]
- Emori M., Tsuchie H., Teramoto A., et al. Non-ossifying fibromas and fibrous cortical defects around the knee - an epidemiologic survey in a Japanese pediatric population. BMC Musculoskelet. Disord. 2022;23(1):378 doi: 10.1186/s12891-022-05330-9. Published 2022 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockenberg A., Sobel E., Noël J.F., Nonossifyingvfi broma. Four cases and review of the literature. J. Am. Podiatr. Med. Assoc. 1997;87(2):66–69. doi: 10.7547/87507315-87-2-66. [DOI] [PubMed] [Google Scholar]
- Herget G.W., Mauer D., Krauß T., et al. Non-ossifying fibroma: natural history with an emphasis on a stage-related growth, fracture risk and the need for follow-up. BMC Musculoskelet. Disord. 2016;17:147. doi: 10.1186/s12891-016-1004-0. Published 2016 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetts S.W., Hilchey S.D., Wilson R., Franc B. Case 110: nonossifying fibroma. Radiology. 2007;243:288–292. doi: 10.1148/radiol.2431040427. [PMID: 17392261 DOI:v10.1148/radiol.2431040427] [DOI] [PubMed] [Google Scholar]
- Hudson T.M., Stiles R.G., Monson D.K. Fibrous lesions of bone. Radiol. Clin. N. Am. 1993;31(2):279–297. [PubMed] [Google Scholar]
- Khandaitkar S., Lamba G., Kolte V., Shenoi R., Shukla D. Non-ossifying fibroma of mandible in a four-year-old girl: a case report. Cureus. 2023;15(3) doi: 10.7759/cureus.36470. Published 2023 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakos M., Murphy W.A. Concurrence of metaphyseal fibrous defect and osteosarcoma. Skelet. Radiol. 1981;6:179–186. doi: 10.1007/BF00347184. [DOI] [PubMed] [Google Scholar]
- Mankin H.J., Trahan C.A., Fondren G., Mankin C.J. Non-ossifying fibroma, fibrous cortical defect and Jaffe-Campanacci syndrome: a biologic and clinical review. Chir. Organi Mov. 2009;93(1):1–7. doi: 10.1007/s12306-009-0016-4. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Tsuchiya H. Treatment of bone tumor using external fixator. J. Orthop. Sci. 2019;24(1):1–8. doi: 10.1016/j.jos.2018.06.022. [DOI] [PubMed] [Google Scholar]
- Ngo T.H., Bize P., Letovanec I., Cherix S., Choong P.F., Rüdiger H.A. Percutaneous cryoablation for a symptomatic non-ossifying fibroma. A case report. Diagn. Interv. Imag. 2015;96(1):107–109. doi: 10.1016/j.diii.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Rammanohar J., Zhang C., Thahir A., Krkovic M. Imaging of non-ossifying fibromas: a case series. Cureus. 2021;13(3) doi: 10.7759/cureus.14102. Published 2021 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T.J., Coviello J.P., Costello M. Clinical reasoning in the face of uncertainty: conservative physical therapy management of a teenage athlete diagnosed with a proximal humeral non-ossifying fibroma. Int. J. Sports Phys. Ther. 2018;13(6):1049–1060. [PMC free article] [PubMed] [Google Scholar]
- Ritschl P., Karnel F., Hajek P. Fibrous metaphyseal defects–determination of their origin and natural history using a radiomorphological study. Skelet. Radiol. 1988;17(1):8–15. doi: 10.1007/BF00361448. [DOI] [PubMed] [Google Scholar]
- Sakamoto A., Arai R., Okamoto T., Matsuda S. Non-ossifying fibromas: case series, including in uncommon upper extremity sites. World J. Orthop. 2017;8(7):561–566. doi: 10.5312/wjo.v8.i7.561. Published 2017 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimal A., Davies A.M., James S.L., Grimer R.J. Fatigue type stress fractures of the lower limb associated with fibrous cortical defects/non-ossifying fibromas in the skeletally immature. Clin. Radiol. 2010;65(5):382–386. doi: 10.1016/j.crad.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Stacy G.S., Dixon L.B. Pitfalls in MR image interpretation prompting referrals to an orthopedic oncology clinic. Radiographics. 2007;27(3):805–826. doi: 10.1148/rg.273065031. (discussion 827–808) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.