Key Points
-
•
Off-the-shelf FLT3 CAR_sIL-15 NK cells secrete IL-15, preferentially lyse human FLT3+ AML, and activate T cells.
-
•
FLT3 CAR_sIL-15 NK cells expressing soluble IL-15 prolong survival of FLT3+ AML–bearing mice without toxicity to normal hematopoiesis.
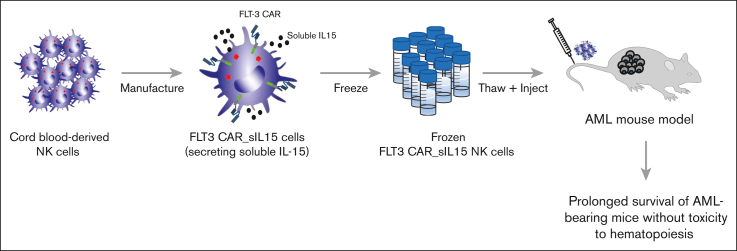
Visual Abstract
Abstract
The majority of patients with acute myeloid leukemia (AML) succumb to the disease or its complications, especially among older patients. Natural killer (NK) cells have been shown to have antileukemic activity in patients with AML; however, to our knowledge, primary NK cells armed with a chimeric antigen receptor (CAR) targeting antigens associated with AML as an “off-the-shelf” product for disease control have not been explored. We developed frozen, off-the-shelf allogeneic human NK cells engineered with a CAR recognizing FLT3 and secreting soluble interleukin-15 (IL-15) (FLT3 CAR_sIL-15 NK) to improve in vivo NK cell persistence and T-cell activation. FLT3 CAR_sIL-15 NK cells had higher cytotoxicity and interferon gamma secretion against FLT3+ AML cell lines when compared with activated NK cells lacking an FLT3 CAR or soluble IL-15. Frozen and thawed allogeneic FLT3 CAR_sIL-15 NK cells prolonged survival of both the MOLM-13 AML model as well as an orthotopic patient-derived xenograft AML model when compared with control NK cells. FLT3 CAR_sIL-15 NK cells showed no cytotoxicity against healthy blood mononuclear cells or hematopoietic stem cells. Collectively, our data suggest that FLT3 is an AML-associated antigen that can be targeted by frozen, allogeneic, off-the-shelf FLT3 CAR_sIL-15 NK cells that may provide a novel approach for the treatment of AML.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous clonal disorder derived from either a hematopoietic stem cell (HSC) or a lineage-specific progenitor cell. Initial standard of care with chemotherapy is able to induce complete remission in up to 80% of patients, but the conditions of most patients progress to relapsed or refractory disease with a 5-year survival rate of 27%.1,2 Thus, understanding the disease pathogenesis and evaluation of novel therapeutic approaches is an unmet need and has remained a therapeutic challenge. AML blasts that reside both in the bone marrow (BM) and the peripheral blood (PB) create an immunosuppressive tumor environment3,4 that suppresses the proliferation and activation of cytolytic effector immune cells. For example, natural killer (NK) cells that are part of the innate immune system are reduced in number and show low cytolytic activity in patients with AML when compared with NK cells from healthy donors.5
Further understanding of the immune system has resulted in the rapid development of targeted cellular therapies holding promising and remarkable therapeutic efficacy for patients with cancer. One such approach involves engineering immune cells to express a chimeric antigen receptor (CAR) to specifically recognize and target cell surface proteins on cancer cells. CAR T cells targeting antigens expressed on acute and chronic B-cell lymphocytic leukemia shed light on novel immune-based approaches for relapsed and/or refractory hematological malignancies following chemotherapy and HSC transplantation (HSCT).6, 7, 8, 9, 10 However, despite the clinical breakthroughs in CAR T-cell–based therapies for these and other lymphoid malignancies, several limitations remain, including cytokine release syndrome (CRS),11,12 neurotoxicity,13, 14, 15 and on-target off-tumor toxicity such as B-cell aplasia.11 Allogeneic CAR NK cells may provide an alternative to CAR T-cell therapies by providing a lower risk of CRS, tumor lysis syndrome, and graft-versus-host disease.16 Our work has focused on targeting the Feline McDonough Sarcoma (FMS)-like tyrosine kinase 3 (FLT3), a receptor tyrosine kinase whose surface expression is largely restricted to the CD34+CD38− fraction of HSCs.6,17,18 Mutations of FLT3 occur in ∼30% of AML19 and result in ligand-independent activation of the receptor as well as downstream signaling during the development of AML and is associated with poor prognosis.20 However, regardless of whether the AML blast expresses the wild-type or mutated form of the intracellular domain, the surface expression of FLT3 remains the same and can be targeted by FLT3 CAR T or CAR NK cells.
In this study, we engineered NK cells to express a specific anti–FLT3-CAR secreting soluble interleukin-15 (sIL-15), referred to as FLT3 CAR_sIL-15 NK, to further enhance the antitumor function of NK cells and CD8+ T cells, because these cytolytic cells express IL-15 receptors. The FLT3 CAR_sIL-15 NK cells were manufactured and frozen as an “off-the-shelf” allogeneic product, using our optimized platform for NK cell engineering, expansion, and freezing. Our data suggest that FLT3 CAR_sIL-15 NK cells represent a promising therapy for FLT3+ relapsed and/or refractory AML with limited toxicity to healthy HSCs.
Methods
Isolation and expansion of NK cells expressing FLT3 CAR
The NK-92 cell line21 was expanded and transduced with lentivirus as previously reported.6,22,23 Primary NK cell isolation from human umbilical cord blood and the expansion of the FLT3 CAR NK cells were performed as previously described.24 Transduction efficiency was assessed via flow cytometry (supplemental Methods).
Antitumor effect of FLT-3 CAR NK-92 and FLT3 CAR_sIL-15 NK cells in AML-bearing mice
NOD–SCID IL-2Rγnull (NSG) and NSG-SGM3 (NSGS) mice expressing human IL-3, granulocyte-macrophage colony–stimulating factor, and stem cell factor (The Jackson Laboratory) were housed at the City of Hope (COH) animal facility. These studies were approved by the COH Animal Care and Use Committee. For the FLT3 CAR NK-92 experiments, MOLM-13 expressing luciferase ZsGreen gene (MOLM-13_luc) was injected intravenously (IV) into NSG mice to establish a xenograft orthotopic AML model as previously reported, with some modifications.6 Nontransduced (NT), empty vector–transduced (EV), or FLT3 CAR NK-92 cells (1 × 107 cells, each), were injected on day 7 and day 14 after tumor engraftment. Mice were subjected to bioluminescence imaging following the manufacturer’s instructions (BLI, IVIS). For cord blood–derived FLT3 CAR_sIL-15 NK cell experiments, NSG mice were injected with MOLM-13_luc cells (1 × 104 cells per mouse). On day 5 and day 12 after engraftment, 1 × 107 FLT3 CAR_sIL-15 NK cells and 1 × 107 NK cells expressing sIL-15 alone (sIL-15 NK), both of which were derived from primary cord blood NK cells, were injected IV. Bioluminescence imaging was performed weekly using the Lago-X and was quantified using the Aura Imaging Software (Spectral Instruments Imaging).
In vivo stem cell engraftment
CD34+ cord blood HSCs (5 × 105 cells) were injected IV into NSGS mice. Four months later, mice were injected IV with 5 × 106 FLT3 CAR NK-92 cells or 5 × 106 EV NK-92 cells weekly (total 4 injections). After 1 month, mice were euthanized to quantify human CD34+ HSCs and their differentiation as measured based on the presence of mature lymphocytes and myeloid cells in the BM. For cord blood NK or CAR NK cells, this experiment was repeated with CD34+ cells from mobilized PB, and 3 × 105 cells were injected IV on day 1, followed by injection of 5 × 105 MOLM-13_luc cells on day 7, and treated with 1 × 107 FLT3 CAR_sIL-15 NK cells injected IV on days 14 and 21.
Statistical analysis
All statistical tests were conducted using GraphPad Prism and SAS. The standard Student t test was used to compare 2 independent groups for continuous end points, whereas one-way analysis of variance was used when ≥3 independent groups were compared. All tests were 2-sided, and P values were adjusted using the method of Holm if multiple comparisons existed. P < .05 was considered significant.
Results
Enhanced activity of FLT3 CAR NK-92 cells against FLT3+ AML
We assessed the expression of FLT3 on both human AML cell lines and primary AML cells. FLT3 was highly expressed on the AML cell lines MOLM-13, EOL-1, and MV4-11, whereas U937 lacked FLT3 expression (supplemental Figure 1A). We found that 22.7% (5 of 22) of the patient-derived primary AML samples demonstrated high expression of FLT3 (supplemental Figure 1B), whereas the remaining samples had a varied expression of FLT3 surface expression, as we have previously reported.6 We, first, expressed the FLT3 CAR construct in the cytolytic NK-92 cell line (FLT3 CAR NK-92), a large granular lymphocyte leukemic cell line, to test the efficacy by detecting green fluorescent protein using flow cytometry (supplemental Figure 1C-D). FLT3 CAR NK-92 cells demonstrated a significant increase in cytolytic activity and interferon gamma (IFN-γ) secretion against FLT3+ AML targets when compared with the EV NK-92 cells, and the enhanced killing was mediated by the CAR and not related to the nonspecific killing of NK-92 (supplemental Figure 2A-D). To evaluate the antitumor activity of FLT3 CAR NK-92 in vivo, mice were first inoculated with luciferase-expressing FLT3+ MOLM-13 AML cells via tail vein injection (day 0). Next, mice received 2 IV infusions of NT NK-92, EV NK-92, or FLT3 CAR NK-92 cells (1 × 107 cells) on days 7 and 14. Bioluminescence imaging showed a reduced tumor burden in mice injected with FLT3 CAR NK-92 cells compared to the mice injected with either NT NK-92 or EV NK-92 (Figure 1A), which also led to a significantly improved survival (Figure 1B).
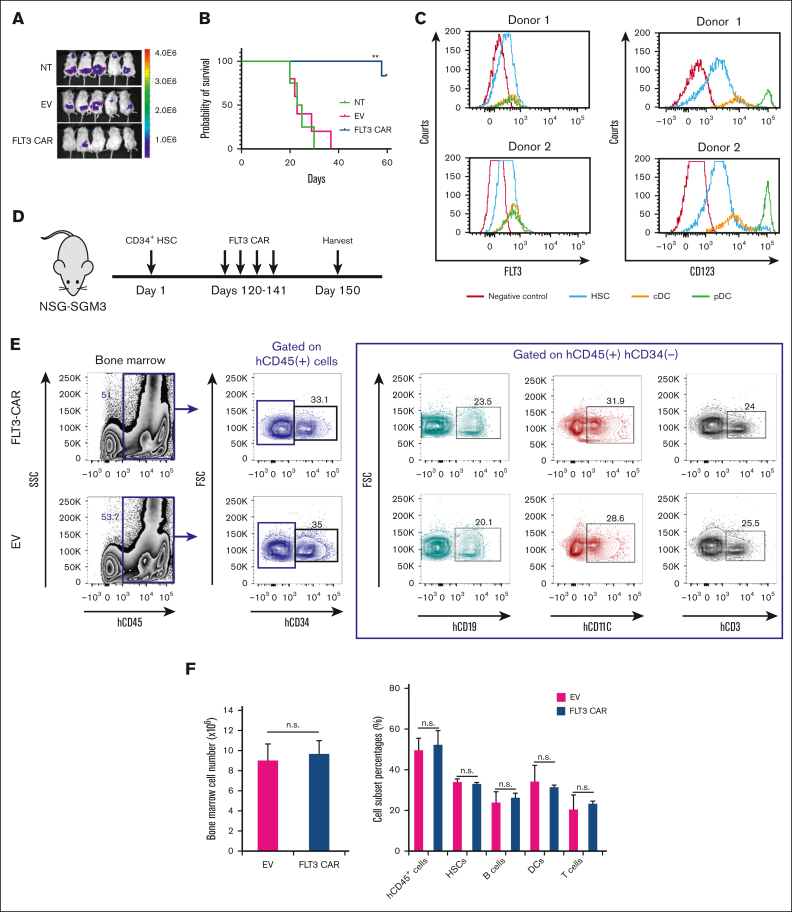
Figure 1.
FLT3 CAR NK-92 cells prolong the survival of mice that received human AML cell grafts but do not reject HSCs and their repopulation. (A) Bioluminescence imaging of NSG mice bearing human AML. NSG mice were inoculated with luciferase-expressing FLT3+ MOLM-13 AML cells via tail vein injection (day 0). Mice were injected on days 7 and 14 with 1 × 107 unmodified NK-92 cells (NT), EV NK-92 cells, or FLT3 CAR NK-92 cells, followed by imaging of mice on day 20. (B) Mice bearing MOLM-13 cells treated with unmodified NK-92 cells, EV NK-92 cells, or FLT3 CAR NK-92 cells. Overall survival was estimated using Kaplan-Meier survival curves (n = 5 for each group). (C) Expression of FLT3 and CD123 on the surface of CD34+ HSCs and DCs including the plasmacytoid DC (pDC) and the conventional DC (cDC). (D) Scheme of the in vivo experiment assessing the impact of FLT3 CAR NK-92 on CD34+ HSC and differentiated cells. On day 1, 5 × 105 human CD34+ HSCs were simultaneously IV injected into NSGS mice that express human IL-3, granulocyte-macrophage colony–stimulating factor, and stem cell factor. Four months later, mice were IV injected with either 5 × 106 FLT3 CAR NK92 cells or 5 × 106 EV NK92 cells weekly for 4 doses, and then mice were sacrificed on day 150 (n = 5 mice for each group). (E) At the time of euthanization, human CD34+ HSCs and their differentiation as measured based on the presence of mature lymphocytes and myeloid cells in the BM were assessed. Human CD45+ and CD34+ cells were counted as engrafted cells derived from human HSCs. A representative example of human CD45+CD34+, CD45+CD34−CD19+, CD45+CD34−CD11c+, and CD45+CD34−CD3+ from the BM is shown, defining human HSCs, B cells, DCs, and T cells, respectively. (F) Cumulative flow cytometric data are shown, demonstrating no difference in absolute cell number of total human CD45+ cells or the percentages of HSCs, B cells, DCs, or T cells as defined earlier, between mice infused with FLT3 CAR NK-92 cells and those with EV NK-92 cells. Data are presented as mean ± standard error of the mean (SEM). ∗∗P < .01. n.s., not significant.
To address potential hematopoietic toxicity, FLT3 CAR NK-92 cells were directed against healthy donor PB mononuclear cells (PBMCs), and no cytotoxicity was observed (supplemental Figure 2F). Because half of normal HSCs and dendritic cells (DCs) (conventional DCs or plasmacytoid DCs) express FLT3 (Figure 1C), we investigated the impact of FLT3 CAR NK-92 cells on healthy HSCs. NSGS mice received engraftment with cord blood CD34+ cells for 4 months, followed by treatment of either FLT3 CAR NK-92 cells or EV NK-92 cells. BM from these mice was collected 4 weeks after treatment (Figure 1D). The quantity of engrafted total human cells marked with hCD45+ and hCD34− was found to be similar between the EV NK-92 and the FLT3 CAR NK-92 cell group, and the number of CD34+ HSCs was also comparable (Figure 1E-F). We assessed the differentiation of human CD34+ cells in vivo and observed that FLT3 CAR NK-92 cells had no effect on the differentiation of healthy HSCs when compared with EV NK-92 cells (Figure 1E-F; supplemental Figure 3). These data suggest that FLT3 CAR NK-92 cells do not affect the capacity of engraftment and differentiation of HSCs.
Generation of primary FLT3 CAR NK cells from cord blood NK cells
Next, we investigated whether the function of primary cord blood NK cells transduced with an FLT3 CAR (FLT3 CAR NK) could efficiently kill FLT3+ AML cells, as was demonstrated for the FLT3 CAR NK-92 cell line. Studies with CAR T cells have demonstrated that the costimulatory domain represents an important factor that affects the functionality and persistence of the CAR T cells.25 In an attempt to improve the cytotoxicity of our CAR NK cells against FLT3+ AML, we generated 2 FLT3 CAR NK cell lines with different costimulatory domains, 1 with CD28/CD3ζ, and 1 with 2B4/CD3ζ, the latter of which was previously described in NK cells,26 and we used truncated CD19 (tCD19) as a marker to access the transduction efficiency (Figure 2A). Expressing these 2 constructs in cord blood NK cells showed more effective killing of FLT3+ MOLM-13 than that by the NK cells only expressing the tCD19 (tCD19 NK cells), with no significant difference observed for the 2 different costimulatory domains (Figure 2B).
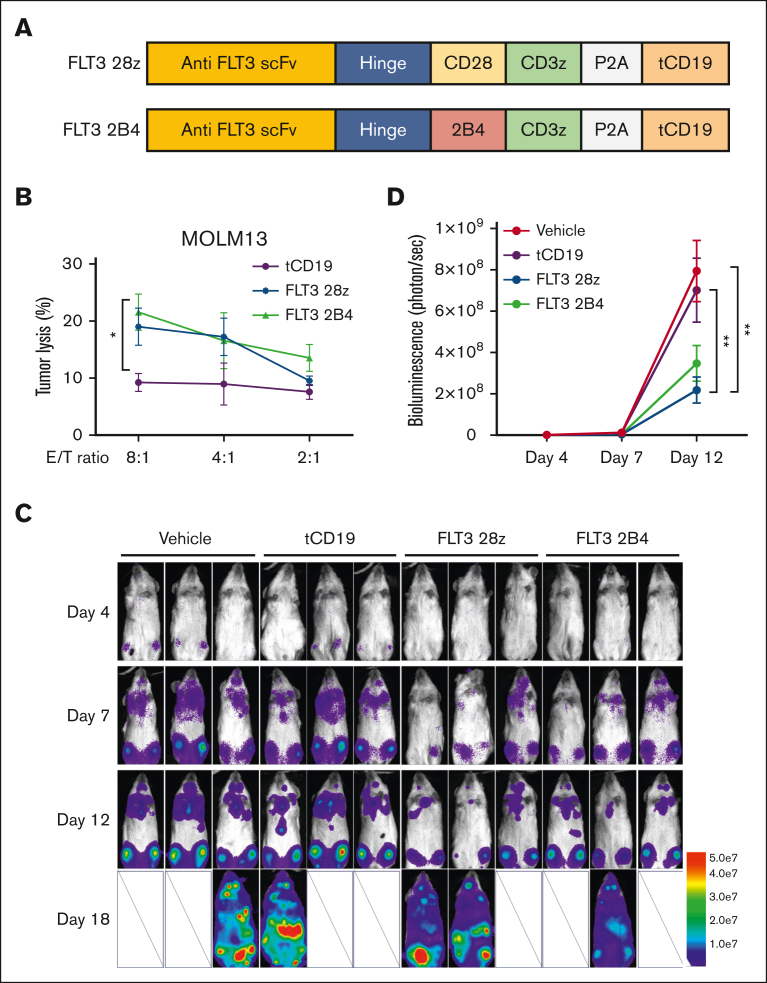
Figure 2.
Functional comparison of CD28 and 2B4 intracellular signaling domain of FLT3 CAR in primary cord blood NK cells. (A) Scheme of the FLT3 CAR constructs containing CD28 or 2B4 as the intracellular costimulatory domain. tCD19 is coexpressed to serve as a marker. (B) Functional assessment of the FLT3 CAR NK cells against FLT3+ MOLM-13 AML cells using a 4-hour Cr-51 release assay under the indicated effector-to-target (E:T) ratio. NK cells only expressing tCD19 serve as a control group (n = 3 donors). (C) Time-lapse luciferase imaging of AML mouse model established by an infusion of MOLM-13 AML cells expressing luciferase (MOLM-13_luc, 2 × 105 cells per mouse) with a single dose of indicated treatments, administered 2 days after tumor implantation (5 × 106 cells per dose). (D) Quantification of the ventral bioluminescence images (n = 3 mice). Data are shown as mean ± SEM. ∗P < .05;∗∗P < .01. scFv, single-chain variable fragment.
To test and compare the therapeutic efficacy of the 2 different FLT3 CAR NK cell lines with different costimulatory domains (CD28/CD3ζ and 2B4/CD3ζ) in vivo, we evaluated their killing ability in an AML xenograft mouse model. Two days after IV injection of the MOLM-13_luc cells, mice were treated with vehicle only, tCD19 NK cells, FLT3 CAR (CD28/CD3ζ) NK cells, or FLT3 CAR (2B4/CD3ζ) NK cells. Bioluminescence imaging showed a significant delay in tumor progression in mice treated with FLT3 CAR (CD28/CD3ζ) NK cells or FLT3 CAR (2B4/CD3ζ) NK cells when compared with the vehicle-treated or tCD19 NK cells (Figure 2C-D). Furthermore, there were no differences in the reduction of tumor burden between the FLT3 CAR NK cells expressing CD28/CD3ζ and those expressing 2B4/CD3ζ (Figure 2C-D), similar to the results observed in vitro.
In vivo persistence and paracrine activation of NK cells expressing different forms of IL-15
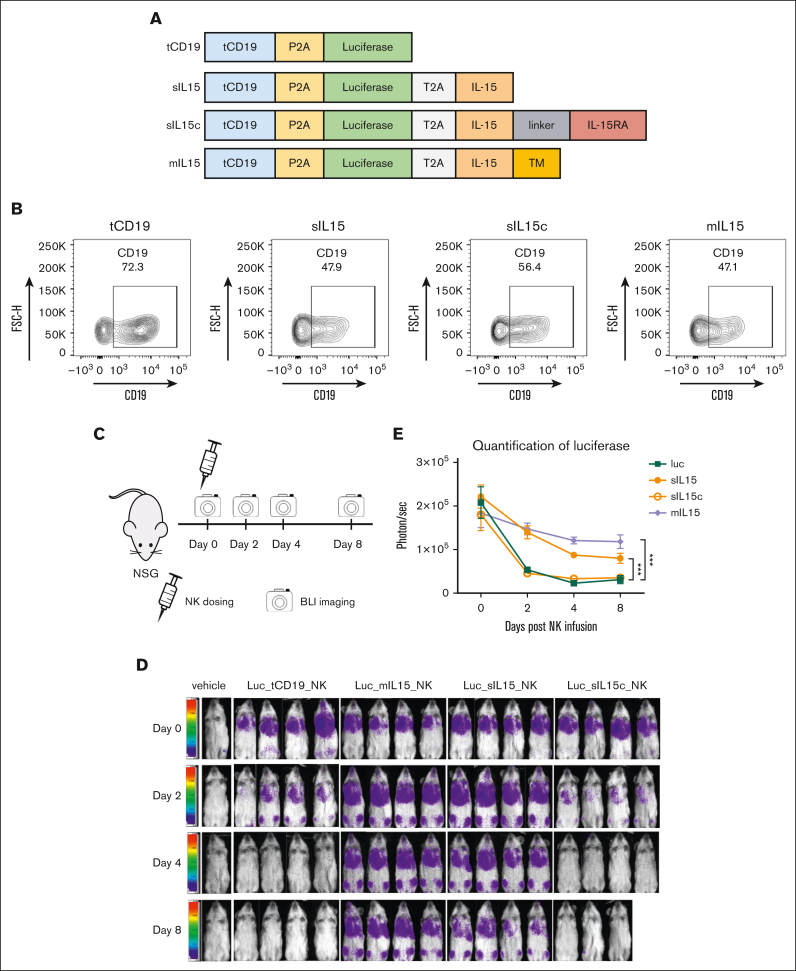
We previously identified IL-15 as the cytokine required for both mouse and human NK cell survival.27, 28, 29 To determine which form of IL-15 would be best suited to sustain FLT3 CAR NK cell in vivo, we constructed and expressed 3 different forms of IL-15 in NK cells: sIL-15, sIL-15/IL-15Rα (sIL-15c), and membrane-bound IL-15 (mIL-15), each with a luciferase enzyme. The transmembrane domain of mIL-15 was derived from platelet-derived growth factor receptor. tCD19 NK cells expressing a luciferase enzyme were used as control (Figure 3A). The transduction efficiency determined by the expression of tCD19 on NK cells was similar in all 4 groups (Figure 3B). The expression of IL-15 on mIL-15 NK cells was confirmed via flow cytometry (supplemental Figure 4A), and the secretion of sIL-15 and sIL-15c in the transduced NK cells was confirmed using enzyme-linked immunosorbent assay (supplemental Figure 4B-C). To assess the persistence of the different forms of IL-15 in vivo, a single dose of different luciferase-labeled NK cells was injected into NSG mice, showing initial homing to the lungs. After 8 days, the sIL-15 and mIL-15 NK groups had better persistence in comparison with the tCD19 and sIL-15c NK groups, in which a significant decrease in detection was observed (Figure 3C-E). We further stimulated NK cells expressing sIL-15 or mIL-15 with K562 cells and collected their conditioned media to stimulate healthy naïve T cells or NK cells. With sIL-15 NK cells, we observed a substantial increase in the mean fluorescence intensity of the activation markers CD69 and CD25 on NK cells and T cells, respectively, whereas supernatants from mIL-15 NK cells had a modest effect activating NK cells and no effect activating T cells, indicating that sIL-15 can stimulate neighboring immune effector cells in a paracrine manner not requiring cell-to-cell interaction (supplemental Figure 5A-B).
Figure 3.
Assessment of in vitro and in vivo persistence of different forms of IL-15 expressed in primary human cord blood NK cells. (A) Scheme of various constructs, each containing luciferase as well as tCD19 to serve as a parental control. Of the 4 constructs, 3 contain IL-15: sIL-15, sIL-15c, and mIL-15. The transgenes are separated by a P2A or T2A sequence, as indicated. (B) The transduction efficiency of each construct in NK cells was represented by the coexpressed tCD19. (C) A scheme of validating the persistence of the transduced NK cells in vivo. Human NK cells (4 × 106 cells per dose) that carry the luciferase gene with or without the various forms of IL-15 were administered into NSG mice via tail vein injection. NK persistence was monitored by bioluminescence imaging (BLI) (n = 4 mice per group). (D) Ventral BLI was performed on days 0, 2, 4, and 8 to monitor NK cell persistence. Due to an accident in operation, 1 mouse of sIL-15c was found dead before imagining on day 8. (E) Quantification of the ventral bioluminescence images. Data are shown as mean ± SEM; ∗∗∗P < .001. TM, transmembrane.
FLT3 CAR_sIL-15 NK cells eradicate FLT3+ AML cells
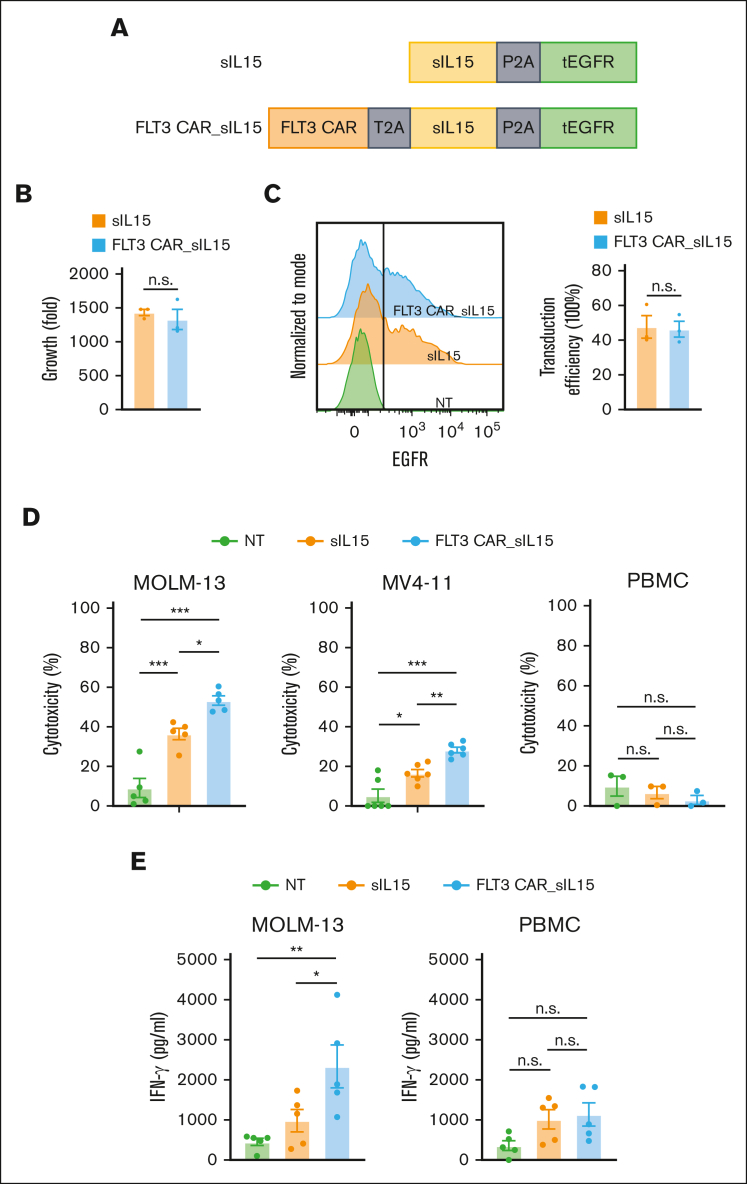
After determining that sIL-15 is suitable for our FLT3 CAR NK cells, we modified our previous constructs against FLT3 to contain the CD28/CD3ζ costimulatory domain, sIL-15, and truncate depidermal growth factor receptor (tEGFR) serving as a safety switch24 as well as a marker for measuring the transduction efficiency (Figure 4A). To assess the functionality of our FLT3 CAR_sIL-15 NK cells, we engineered and expanded our NK cells for 16 days (∼1500-fold), and no differences were observed in the expansion between sIL-15 NK cells and FLT3 CAR_sIL-15 NK cells (Figure 4B). The transduction efficiency was comparable between the sIL-15 NK cells and FLT3 CAR_sIL-15 NK cells (47.63% ± 11.27% and 46.33% ± 7.901%, respectively; Figure 4C), with a high purity of NK cells in each group (>97%). To assess the function and potential toxicity of FLT3 CAR_sIL-15 NK cells, we tested their ability to kill AML cells, MOLM-13 (FLT3+) and MV-411 (FLT3+) AML cell lines, and healthy donor PBMCs (FLT3−). FLT3 CAR_sIL-15 NK cells showed a significant increase in antitumor activity against FLT3+ targets when compared with sIL-15 NK cells and NT NK cells (Figure 4D, left and middle). Furthermore, each of the NK groups failed to demonstrate any cytotoxicity against healthy donor PBMCs (Figure 4D, right). Likewise, when cocultured with the FLT3+ AML cell line, FLT3 CAR_sIL-15 NK cells produced greater amounts of IFN-γ compared with NT NK cells (P < .01) or compared with sIL-15 NK cells (P < .05; Figure 4E, left). In addition, no difference in IFN-γ production was observed among the 3 groups against PBMCs (Figure 4E, right). We also assessed the cytotoxicity of FLT3 CAR_sIL-15 NK cells in comparison with that of FLT3 CAR NK cells without sIL-15 (FLT3 CAR) and NK cells expressing sIL-15 without FLT3 CAR (sIL-15), each targeting MOLM-13 cells. The data show that FLT3 CAR_sIL-15 NK cells had significantly enhanced antitumor efficacy compared with FLT3 CAR NK cells that lack sIL-15 and sIL-15 NK cells that lack FLT3 CAR, which suggests that sIL-15 has some effect on promoting FLT3 CAR NK cell function, at least in short-term cytotoxicity assays (supplemental Figure 5C-D). We also measured IL-15 production in a coculture system of MOLM-13 cells and NK cells. Our data show that compared with sIL-15 NK cells, FLT3 CAR_sIL-15 NK cells secreted significantly more IL-15 when cocultured with MOLM-13 cells (supplemental Figure 4B).
Figure 4.
FLT3 CAR_sIL-15 cord blood NK cells enhance cytotoxicity against FLT3+AML. (A) Scheme of FLT3 CAR_sIL-15 and its parental control (sIL-15) vector that expresses IL-15 but not the CAR. tEGFR is coexpressed to serve as a marker. (B) The expansion capacity of primary cord blood NK cells engineered with sIL-15 or FLT3 CAR_sIL-15 after 16 days of culture (n = 3 donors). (C) The transduction efficiencies of FLT3 CAR_sIL-15 NK cells and sIL-15 NK cells are determined based on the expression of tEGFR at the time of harvesting (day 16) (n = 3 donors). (D) The cytotoxicity of NT, sIL-15 NK, and FLT3 CAR_sIL-15 NK cells against FLT3+ AML cells (MOLM-13 or MV-411, n = 6 donors each) or FLT3-negative (FLT3−) cells (PBMC) (n = 3 donors), assessed via flow-based cytotoxicity assay at an E:T ratio of 4:1 for PBMCs and MV-411 and 2:1 for MOLM-13. (E) IFN-γ production of NT, sIL-15 NK, and FLT3 CAR_sIL-15 NK cells (n = 5 donors) against FLT3+ AML cells (MOLM-13) or FLT3− cells (PBMCs) at an E:T ratio of 4:1 for 18 hours and measured by enzyme-linked immunosorbent assay. Data are presented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Frozen FLT3 CAR_sIL-15 NK cells remain functional as an off-the-shelf product
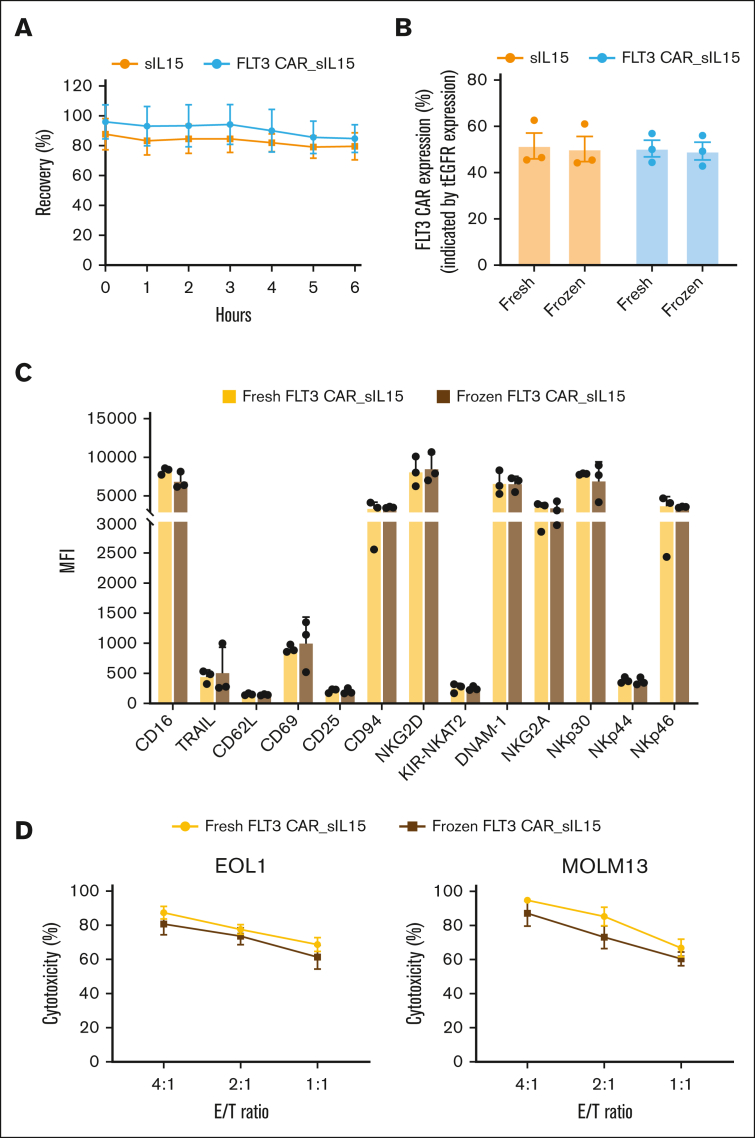
CAR NK cells may have an advantage over autologous CAR T-cell therapy because CAR NK cells can be produced as an allogeneic off-the-shelf product.30 We tested the functionality and phenotypes of the frozen FLT3 CAR_sIL-15 NK cell product, with the goal of making an off-the-shelf allogeneic product. Thawing of the cryovials of FLT3 CAR_sIL-15 NK cells and sIL-15 NK cells demonstrated >80% cell recovery of viable cells that were maintained for 6 hours in freezing medium at room temperature (Figure 5A). To assess the stability of the transgene expression, both FLT3 CAR_sIL-15 NK cells and sIL-15 NK cells maintained tEGFR expression at ∼50%, supporting the durability of NK cell transduction efficiency after thawing (Figure 5B). Examining the phenotype of the frozen sample, we assessed a panel of NK cell markers that demonstrated no significant differences between the phenotype of the fresh and that of the frozen product (Figure 5C; supplemental Figure 6A-B). Lastly, it was important to assess the cytotoxic potency of our frozen product in a flow cytometry assay against MOLM-13 and EOL-1 AML cell lines; no significant difference was observed in the cytotoxicity between the fresh and frozen product (Figure 5D). These results demonstrate that our FLT3 CAR_sIL-15 NK cells can be viably cryopreserved in liquid nitrogen and can be used as an off-the-shelf product without affecting their viability or potency.
Figure 5.
Off-the-shelf FLT3 CAR_sIL-15 cord blood NK cells retain transgene expression and cytolytic functions after 1 cycle of freeze-thaw. (A) sIL-15 NK and FLT3 CAR_sIL-15 NK cell numbers before freezing and 1 to 6 hours after thawing were compared. NK cells were thawed in the freezing medium at room temperature and the number of cells was determined using a Muse Cell Analyzer at the times indicated (n = 3 donors). (B) The transduction efficiency of sIL-15 NK or FLT3 CAR_sIL-15 NK cells before and after 1 week of freezing assessed by the expression of tEGFR (n = 3 donors). (C) Immunophenotypes of FLT3 CAR_sIL-15 NK cells before and after 1 week of freezing (n = 3 donors). Median mean fluorescence intensity (MFI) for each of the indicated cell surface markers is shown as a bar graph. (D) FLT3 CAR_sIL-15 NK cells after being frozen for 1 week were defrosted. The defrosted and freshly expanded FLT3 CAR_sIL-15 NK cells were cocultured with a FLT3+ AML cell line (EOL1 or MOLM-13) for 24 hours at the indicated E:T ratios, followed by cytolytic function testing by a flow cytometry-based assay (n = 3 donors). Data are shown as mean ± SEM. There are no statistically significant differences for any of the measurements taken between fresh cells and frozen cells transduced with the same construct in panels A-D.
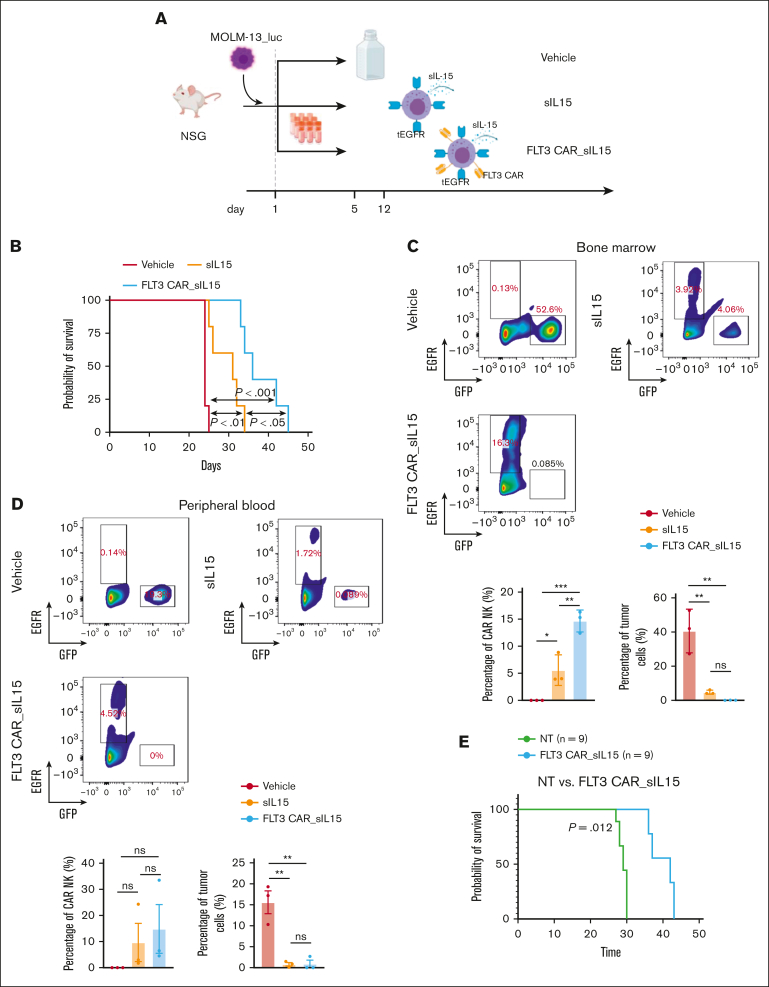
FLT3 CAR_sIL-15 NK cells demonstrate an antitumor activity against AML in vivo
To further demonstrate the potential therapeutic efficacy of our frozen FLT3 CAR_sIL-15 NK cells, we investigated their antitumor activity in an AML mouse model. Highly aggressive MOLM-13_luc AML cells were IV transplanted into NSG mice, followed by treatment with either a vehicle, sIL-15 NK cells or FLT3 CAR_sIL-15 NK cells (Figure 6A). The progression of leukemia was monitored over time via tumor bioluminescence (supplemental Figure 7A), and the mice treated with the sIL-15 NK cells (without the FLT3 CAR) showed a significant prolongation of survival compared with that of the vehicle group (Figure 6B). Furthermore, the infusion of FLT3 CAR_sIL-15 NK cells showed a significant prolongation of survival when compared with that in mice treated with either vehicle or sIL-15 NK cells, but all mice ultimately died of progressive leukemia (Figure 6B). To assess the persistence of the tumor burden or NK cells in vivo, we collected the BM and PB from mice on day 21. Our results indicate that, when compared with the vehicle, both FLT3 CAR_sIL-15 NK cells and sIL-15 NK cells were capable of significantly reducing FLT3 AML tumor cells in both the BM or PB (Figure 6C-D; supplemental Figure 7B-C). In addition, compared with sIL-15 NK cells, FLT3 CAR_sIL-15 NK cells were significantly increased in the BM (Figure 6C; supplemental Figure 7B) and showed a trend of increase in the PB, although the difference did not reach statistical significance (Figure 6D; supplemental Figure 7C). To ensure the reproducibility of our results, mice that received the FLT3(+) EOL-1 AML cell line grafts were treated with FLT3 CAR_sIL-15 NK cells and demonstrated enhanced tumor control when compared with such mice treated with vehicle or sIL-15 NK cells (supplemental Figure 8). In addition, a patient-derived xenograft model engrafted with primary AML blasts was established. The engraftment was confirmed via flow cytometry, 3 weeks after injection (supplemental Figure 9A-B). The data revealed a significant improvement in survival for the mice treated with FLT3 CAR_sIL-15 NK cells as compared with that for the vehicle-treated mice (Figure 6E). Data from these 3 mouse models demonstrate that our frozen and then thawed off-the-shelf FLT3 CAR_sIL-15 NK cells hold promise as a therapeutic tool for FLT3+ AML.
Figure 6.
Treatment of a human AML mouse model with off-the-shelf cord blood FLT3 CAR_sIL-15 NK cells. (A) The scheme for treating NSG mice, which received engraftment with the FLT3+ MOLM-13 AML cells, with off-the-shelf sIL-15 NK cells or FLT3 CAR_sIL-15 NK cells. MOLM-13 AML cells expressing luciferase (1 × 104 MOLM-13_luc_GFP cells) were injected into 12-week-old NSG mice on day 1, followed by an infusion of a corresponding treatment (1 × 107 NK cells per dose) on day 5 and day 12, via tail vein injection. (B) Survival analysis was estimated using the Kaplan-Meier method of the mice treated with vehicle (freezing buffer), sIL-15 NK cells, or FLT3 CAR_sIL-15 NK cells; n = 5 mice per group; P value was calculated using log-rank test. (C-D) Fluorescence-activated cell sorting analysis of the cells collected from the BM (C) or the PB (D) of the mice treated with vehicle (freezing buffer), sIL-15 NK cells, or FLT3 CAR_sIL-15 NK cells. The populations of tumor cells and NK cells were gated on GFP and EGFR, respectively; n = 4 mice per group. (E) Tumor-engrafted mice were treated with FLT3 CAR_s15 NK cells (1 × 107 cells per mouse) or vehicle on days 21 and 28. The survival was estimated using the Kaplan-Meier method (n = 9 per group). P value was calculated using log-rank test. The figure was generated with BioRender.com.
FLT3 CAR_sIL-15 NK cells show no toxicity against healthy HSCs and their differentiation in vivo
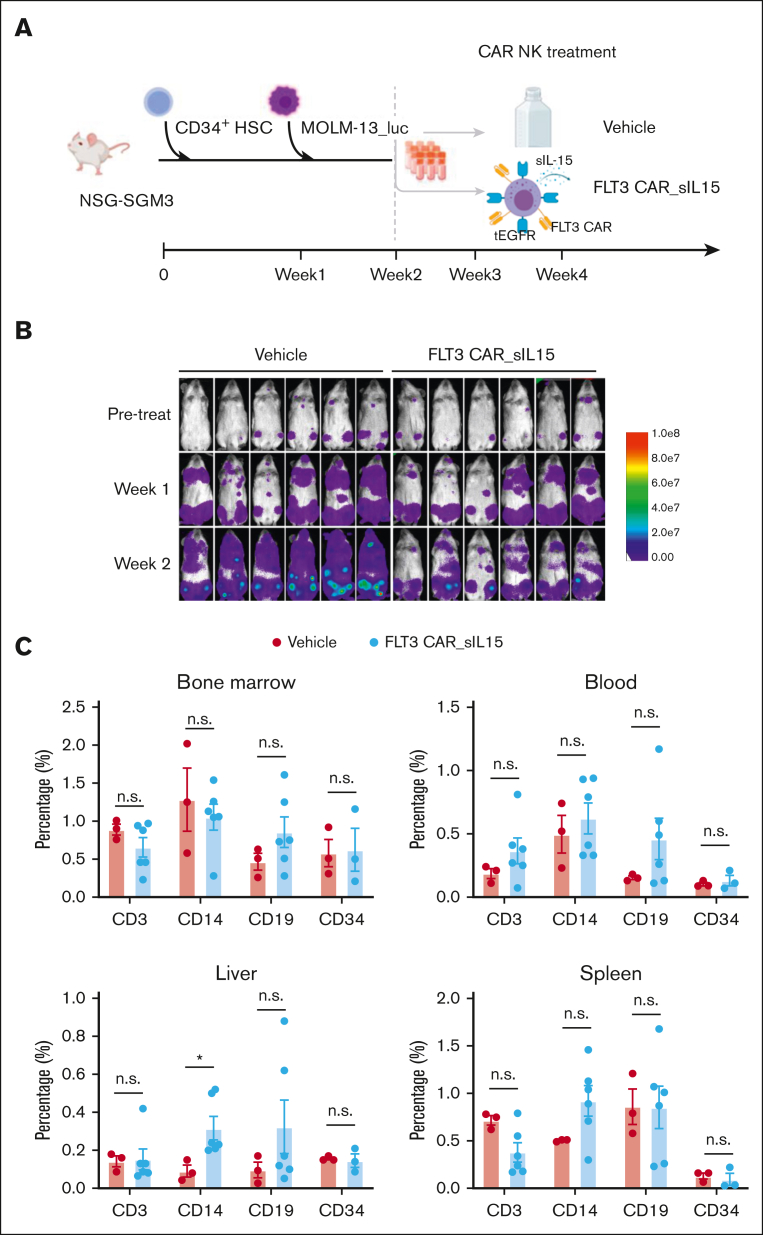
Previously, we demonstrated that FLT3 CAR NK-92 cells had no impact on HSCs differentiation in the absence of AML cells (Figure 1F). We extended this study to FLT3 CAR_sIL-15 NK cells generated from cord blood in the presence of AML. For this purpose, CD34+ HSCs from mobilized PB were engrafted into NSGS mice, followed by engraftment of the aggressive MOLM-13_luc AML cell line, and subsequently cord blood–derived FLT3 CAR_sIL-15 NK cells (Figure 7A). Of note, FLT3 CAR_sIL-15 NK cells and CD34+ HSCs were derived from different donors. The MOLM-13 tumor cells were engrafted to achieve complete activation of the FLT3 CAR_sIL-15 NK cells, mimicking the setting of future treatment planned for patients with FLT3+ AML. Tumor bioluminescence results showed that FLT3 CAR_sIL-15 NK cells significantly reduced the tumor burden compared with vehicle control (Figure 7B). Notably, no difference was observed in CD34+ HSC–derived immune cell populations between the vehicle- and FLT3 CAR_sIL-15 NK cell–treated groups in the BM, blood, liver, and spleen (Figure 7C; supplemental Figure 10), suggesting that the FLT3 CAR_sIL-15 NK cell treatment did not affect the differentiation of the normal HSCs. These results suggest the FLT3 CAR_sIL-15 NK cells have the potential to treat patients with FLT3+ AML without serious hematopoietic toxicity.
Figure 7.
Validation of FLT3 CAR_sIL-15 NK cell safety on human CD34 HSC cells in an AML mouse model. (A) Scheme of CD34+ HSC inoculation and FLT3 CAR_sIL-15 NK cell treatment. NSGS mice first received grafts of CD34+ HSCs (3 × 105 cells), followed 1 week later by receiving FLT3+ AML tumor cell grafts (5 × 105 MOLM-13_luc_GFP cells), which was followed by treatment with off-the-shelf frozen and then thawed (1 × 107 cells) FLT3 CAR_sIL-15 NK cells on days 14 and 21. Mice were euthanized on day 28 and human HSCs along with human lymphocyte subsets were isolated from the mice and subjected to Fluorescence-activated cell sorting analysis. (B) Time-lapsed luciferase images of the mice inoculated with CD34+ HSC and MOLM-13_luc_GFP AML cells and then treated with either vehicle control or FLT3 CAR_sIL-15 NK cells. (C) Fluorescence-activated cell sorting analysis of human B cells (CD19), T cells (CD3), HSCs (CD34), and monocytes (CD14) among the human CD45+ GFP− cells isolated from the BM, PB, liver, and spleen of NSGS mice that received engraftment with CD34+ HSCs (n = 3 or 6 mice per group). Percentages are calculated from cells gated on live human CD45+ GFP− single cells. Data are presented as mean ± SEM; ∗P < .05. The figure was generated with BioRender.com.
Discussion
Conventional chemotherapy and allogeneic HSCT have greatly improved the treatment of AML; however, challenges remain in treating relapsed and refractory disease as well as AML in older patients. Here, we developed an optimized platform for generating an off-the-shelf CAR NK cell product targeting FLT3 without affecting the functionality and viability of the viably frozen effector cells after thawing. FLT3 is an AML-associated antigen, and its mutations (eg, FLT3 internal tandem duplications) predict poor prognosis.31, 32, 33 FLT3 expression is found on the surface of nearly all leukemic blasts in ∼20% of patients with AML but not on the majority of healthy cells, including HSCs.34 We demonstrated that the FLT3 CAR_sIL-15 NK cells can specifically recognize FLT3+ AML cells in vitro and control the tumor burden in 3 different orthotopic xenograft mouse models of AML, with a significantly longer survival after only 2 or 4 infusions of FLT3 CAR_sIL-15 NK cells, compared with a similar treatment schedule with sIL-15 NK cells or vehicle control.
One limitation of NK cell therapy is the short lifespan of the infused NK cells in the absence of cytokines, such as IL-2 or IL-15, which may limit the therapeutic efficacy of CAR NK cell–based therapy. To overcome this obstacle, we incorporated IL-15 into our vector, because this cytokine reportedly is the survival factor for mouse and human NK cells.27,28 Unlike IL-2, IL-15 does not sustain the survival of regulatory T cells.35 For this reason, immune cells were previously genetically engineered to express different forms of IL-15.36, 37, 38 Although IL-15c is reportedly superior to IL-15 as an exogenously administered cytokine,39 our results demonstrate that engineered sIL-15c did not improve the persistence of cord blood NK cells in vivo. The reasons for this obversion are unclear but may be explained by the exhaustion of the NK cells during the ex vivo expansion process, with a much more potent immune stimulatory effect from IL-15c than that from IL-15 alone.39 Our selection of sIL-15 over mIL-15 was made because of a possible advantage of releasing sIL-15 into the tumor microenvironment. Theoretically, this could allow the activation of NT NK and CD8+ T cells in patients, thus amplifying the antitumor response. Therefore, although mIL-15 can only act upon cell-to-cell interaction, sIL-15 can act in both an autocrine and paracrine fashion to activate CAR NK cells as well as endogenous NK cells and CD8+ T cells, respectively. We validated the integration of sIL-15 in our FLT3 CAR construct and showed their prolongation of survival in vivo and the ability of the FLT3 CAR_sIL-15 NK cells to efficiently kill FLT3+ AML blasts both in vitro and in vivo. Of note, our group is applying sIL-15 engineered NK cells expressing PD-L1 to treat non–small cell lung cancer.40, 41, 42 In addition, we demonstrated that our frozen FLT3 CAR_sIL-15 NK cells are functional off-the-shelf and are stable for 6 hours after thawing at room temperature. The ability to cryopreserve cells viably and functionally ensures the practical deployment of FLT3 CAR_sIL-15 NK cells to treatment centers after they are introduced into the clinical setting.
AML is a severe and aggressive hematological malignancy with a 5-year overall survival rate of 27%.43 Older patients are often not eligible for HSCT because of complications such as severe graft-versus-host disease that can result in significant morbidity and mortality. CAR T-cell therapy is currently being explored in AML by targeting different antigens such as FLT3, CD123, CLL-1, CD33, and CD7, among others.44, 45, 46, 47, 48 One of the limitations of autologous CAR T-cell therapy has been CRS-based toxicity, neurotoxicity,13, 14, 15 and off-target toxicity.49 In contrast to T cells, NK cells have not been demonstrated to undergo clonal expansion after activation by specific tumor antigens despite having antileukemic activity in patients with AML.50,51 Furthermore, partially matched or mismatched allogeneic NK cells have been shown to be safe, with antitumor efficacy in patients with hematological malignancies.52 Thus, in contrast to autologous CAR T-cell therapy in AML, a frozen off-the-shelf allogeneic CAR NK cell can be immediately infused in patients with AML without the wait required to generate an autologous CAR T-cell product, thus minimizing the risk of relapse before the administration of a cell therapy product. Therefore, we, believe that CAR NK cells could offer an alternative approach to fit the role of non–cross resistant cellular therapy in AML, as demonstrated in a recent preclinical study targeting CD123.53 Our FLT3 CAR NK-92 data reported here are consistent with those of previous studies using NK-92 as a source for CAR NK cells.22,54,55 To our knowledge, the work presented in this study is the first to consider viably frozen, off-the-shelf FLT3 CAR_sIL-15 NK cells, generated from cord blood, secreting sIL-15 for the treatment of AML.
One of the major concerns of translating CAR cell therapies into the clinical applications is the on-target off-tumor effects. In this study, we show that FLT3 is only expressed on approximately half of the population of HSCs and DCs from healthy donors and is minimally or not expressed on other cell types. Consistent with data in humans, murine FLT3 is reportedly not expressed in self-renewing HSCs, and its expression is restricted to the multipotent stem cell stage and the lymphoid progenitor stage, when such cells are incapable of self-renewal.56 These data suggest that it is unlikely that FLT3 CAR NK cells would completely deplete HSCs and DCs should they be brought into the clinic. In support of this, our in vitro and in vivo experiments demonstrate that FLT3 CAR_sIL-15 NK cells do not eliminate PBMCs or CD34+ HSCs and their differentiated cell progeny. This could be attributed to the single-chain variable fragment itself, the costimulated domain selected for the study, or the differences between effector functions of T cells and NK cells. Unlike T cells, NK cell activity is regulated by the balance of a multitude of activation and inhibitory receptors.57 Our results are consistent with those of a similar study that showed that FLT3 CAR NK-92 had no cytotoxicity against mobilized human CD34+ cells.58 In addition, in a previous study, the 4G8 antibody, which recognizes FLT3, did not have any untoward effect on colony formation and was not cytotoxic against CD34 cells from the BM when administered alone.59 However, reports have shown that FLT3-CAR T cells can lower frequencies of CD34+ cells.60,61 Should unexpected toxicity be encountered, we incorporated tEGFR into our construct, which not only serves as a marker to detect FLT3 CAR expression but also acts as a safety switch to specifically eliminate CAR NK cells by administering the US Food and Drug Administration–approved anti-EGFR antibody, cetuximab.24
Given the results herein demonstrating that 2 or 4 sequential treatments of FLT3 CAR_sIL-15 NK cells can delay the progression of an aggressive AML in vivo, we believe it will be important to test this approach in combination with other non–cross resistant therapies. For example, the receptor tyrosine kinase inhibitors midostaurin and gilteritinib are US Food and Drug Administration–approved for the treatment of patients with AML with an activating mutation of FLT3.62,63 Because these 2 drugs increase the surface expression of FLT3,48 it will be important to assess whether FLT3 receptor tyrosine kinases can be combined with FLT3 CAR_sIL-15 NK cells to further delay the progression of AML. In addition, given the high surface density expression of both CD16 and NKG2D on the surface of the FLT3 CAR_sIL-15 NK cells, it will be reasonable to apply bispecific killer engagers to a second tumor-associated antigen on AML. Importantly, our AML mouse models used in this study are not models of minimal residual disease. The inability to detect disease before treatment can be because of the limitation of the imaging technology and/or a weak signal from the luciferase.
In conclusion, the data from this report suggest that FLT3 is an antigen associated with AML that is suitable for targeting by FLT3 CAR_sIL-15 NK cells, supporting the use of CAR NK cell therapy as an off-the-shelf product in the treatment of cancer. Additional regulatory work in this regard should allow us to pursue this cellular therapy in early phase clinical trials for patients with refractory or relapsed AML.
Conflict-of-interest disclosure: M.A.C. and J.Y. hold a patent as inventors of the FLT3 CAR and are cofounders of CytoImmune Therapeutics Inc. The remaining authors declare no competing financial interests.
Acknowledgments
Parts of this project were supported by a grant from the Gabrielle’s Angel Cancer Research Foundation and a grant from the National Institutes of Health (R01CA265095).
Authorship
Contribution: A.G.M. and K.-Y.T performed the experiments and wrote the manuscript; Z.L., Z.Z., H.C., L.T., T.L., A.A., and S.M. performed experiments; J.Z. performed the statistical analysis; C.-M.L. assisted with NK cell manufacturing; and M.A.C. and J.Y. designed the research project, revised the manuscript, and supervised the project in its entirety.
Footnotes
∗A.G.M., K.-Y.T., and Z.L. are joint first authors.
Data are available on request from the corresponding authors, Michael A. Caligiuri (mcaligiuri@coh.org) and Jianhua Yu (jiayu@coh.org).
The full-text version of this article contains a data supplement.
Contributor Information
Michael A. Caligiuri, Email: mcaligiuri@coh.org.
Jianhua Yu, Email: jiayu@coh.org.
Supplementary Material
References
- 1.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia. 2015;29(2):312–320. doi: 10.1038/leu.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70. doi: 10.1182/blood-2015-07-604546. [DOI] [PubMed] [Google Scholar]
- 3.Mussai F, De Santo C, Abu-Dayyeh I, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122(5):749–758. doi: 10.1182/blood-2013-01-480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isidori A, Salvestrini V, Ciciarello M, et al. The role of the immunosuppressive microenvironment in acute myeloid leukemia development and treatment. Expert Rev Hematol. 2014;7(6):807–818. doi: 10.1586/17474086.2014.958464. [DOI] [PubMed] [Google Scholar]
- 5.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Mao H, Zhang J, et al. Targeting FLT3 by chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Leukemia. 2017;31(8):1830–1834. doi: 10.1038/leu.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Q, Qian C, Xu N, et al. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14:82. doi: 10.1186/s13045-021-01092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Wang P, Li Z, He Y, Gan W, Jiang H. Anti-CLL1 chimeric antigen receptor T-cell therapy in children with relapsed/refractory acute myeloid leukemia. Clin Cancer Res. 2021;27(13):3549–3555. doi: 10.1158/1078-0432.CCR-20-4543. 2021. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q-s, Wang Y, Lv Hy, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13:168. doi: 10.1186/s13045-020-00998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappold I, Ziegler BL, Köhler I, et al. Functional and phenotypic characterization of cord blood and bone marrow subsets expressing FLT3 (CD135) receptor tyrosine kinase. Blood. 1997;90(1):111–125. [PubMed] [Google Scholar]
- 18.Xiao M, Oppenlander BK, Plunkett JM, Dooley DC. Expression of Flt3 and c-kit during growth and maturation of human CD34+CD38- cells. Exp Hematol. 1999;27(5):916–927. doi: 10.1016/s0301-472x(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 19.Antar A, Otrock ZK, El-Cheikh J, et al. Inhibition of FLT3 in AML: a focus on sorafenib. Bone Marrow Transplant. 2017;52(3):344–351. doi: 10.1038/bmt.2016.251. [DOI] [PubMed] [Google Scholar]
- 20.Sallmyr A, Fan J, Datta K, et al. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111(6):3173–3182. doi: 10.1182/blood-2007-05-092510. [DOI] [PubMed] [Google Scholar]
- 21.Gong J-H, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8(4):652–658. [PubMed] [Google Scholar]
- 22.Chu J, Deng Y, Benson DM, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Chu J, Keung Chan W, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng KY, Mansour AG, Zhu Z, et al. Off-the-shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology. 2022;162(4):1319–1333. doi: 10.1053/j.gastro.2021.12.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guedan S, Posey AD, Jr., Shaw C, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI insight. 2018;3(1):e96976. doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper MA, Bush JE, Fehniger TA, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 28.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99(5):937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma S, Caligiuri MA, Yu J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 2022;43(10):833–847. doi: 10.1016/j.it.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour AG, Teng K-Y, Lu T, Barr T, Yu J. Successes and Challenges of NK Immunotherapy: Breaking Tolerance to Cancer Resistance. Elsevier; 2021. CAR-NK cell immunotherapy: Development and challenges toward an off-the-shelf product; pp. 213–230. [Google Scholar]
- 31.Whitman SP, Maharry K, Radmacher MD, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116(18):3622–3626. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111(3):1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61(19):7233–7239. [PubMed] [Google Scholar]
- 34.Bochtler T, Fröhling S, Weichert W, et al. Evolution of a FLT3-TKD mutated subclone at meningeal relapse in acute promyelocytic leukemia. Cold Spring Harb Mol Case Stud. 2016;2(5):a001123. doi: 10.1101/mcs.a001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Lundqvist A. Immunomodulatory effects of IL-2 and IL-15; implications for cancer immunotherapy. Cancers. 2020;12:3586. doi: 10.3390/cancers12123586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imamura M, Shook D, Kamiya T, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124(7):1081–1088. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 37.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177(9):6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu T, Mansour AG, Ma R, et al. Abstract LB211: Tumor-reactive and anti-PD-L1 co-stimulated killer cells (TRACK-NK) for immunotherapy of non-small cell lung cancer. Cancer Res. 2022;82(suppl 12):LB211. [Google Scholar]
- 41.Villalona-Calero MA, Forman SJ, Palmer J, et al. A phase 1 trial of umbilical cord blood–derived tumor-reactive PD-L1+ natural killer cells engineered to express soluble IL-15 (TRACK-NK) in patients with non–small-cell lung cancer (NSCLC) refractory to PD-1/PD-L1 inhibitors. J Clin Oncol. 2023;41(suppl 16):TPS2665. [Google Scholar]
- 42.Dong W, Wu X, Ma S, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9(10):1422–1437. doi: 10.1158/2159-8290.CD-18-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119(1):34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes-Silva D, Atilla E, Atilla PA, et al. CD7 CAR T cells for the therapy of acute myeloid leukemia. Mol Ther. 2019;27(1):272–280. doi: 10.1016/j.ymthe.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arcangeli S, Rotiroti MC, Bardelli M, et al. Balance of anti-CD123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol Ther. 2017;25(8):1933–1945. doi: 10.1016/j.ymthe.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MY, Yu KR, Kenderian SS, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173(6):1439–1453.e19. doi: 10.1016/j.cell.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Chen S, Xiao W, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11:7. doi: 10.1186/s13045-017-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jetani H, García-Cadenas I, Nerreter T, et al. FLT3 inhibitor treatment increases FLT3 expression that exposes FLT3-ITD+ AML blasts to elimination by FLT3 CAR-T cells. Blood. 2018;132(suppl 1):903. [Google Scholar]
- 49.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggeri L, Mancusi A, Perruccio K, Burchielli E, Martelli MF, Velardi A. Natural killer cell alloreactivity for leukemia therapy. J Immunother. 2005;28(3):175–182. doi: 10.1097/01.cji.0000161395.88959.1f. [DOI] [PubMed] [Google Scholar]
- 51.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 52.Davis ZB, Felices M, Verneris MR, Miller JS. Natural killer cell adoptive transfer therapy: exploiting the first line of defense against cancer. Cancer J. 2015;21(6):486–491. doi: 10.1097/PPO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christodoulou I, Ho WJ, Marple A, et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J Immunother Cancer. 2021;9(12):e003894. doi: 10.1136/jitc-2021-003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Chu J, Keung Chan W, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Han J, Chu J, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7(19):27764–27777. doi: 10.18632/oncotarget.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD–specific STAT5 activation. Blood. 2009;114(24):5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oelsner S, Waldmann A, Billmeier A, et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int J Cancer. 2019;145(7):1935–1945. doi: 10.1002/ijc.32269. [DOI] [PubMed] [Google Scholar]
- 59.Hofmann M, Große-Hovest L, Nübling T, et al. Generation, selection and preclinical characterization of an Fc-optimized FLT3 antibody for the treatment of myeloid leukemia. Leukemia. 2012;26(6):1228–1237. doi: 10.1038/leu.2011.372. [DOI] [PubMed] [Google Scholar]
- 60.Shrestha E, Liang R, Sirochinsky C, Ben Jehuda R, Sandler V. Preclinical development of anti-FLT3 CAR-T therapy for the treatment of acute myeloid leukemia. Blood. 2020;136(suppl 1):4–5. [Google Scholar]
- 61.Jetani H, Garcia-Cadenas I, Nerreter T, et al. CAR T-cells targeting FLT3 have potent activity against FLT3− ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32(5):1168–1179. doi: 10.1038/s41375-018-0009-0. [DOI] [PubMed] [Google Scholar]
- 62.Lee LY, Hernandez D, Rajkhowa T, et al. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood. 2017;129(2):257–260. doi: 10.1182/blood-2016-10-745133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;129(26):3403–3406. doi: 10.1182/blood-2017-05-782292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.