Abstract
Background
While antiretroviral therapy (ART) has improved outcomes for people with HIV (PWH), brain dysfunction is still evident. Immune activation and inflammation remain elevated in PWH receiving ART, thereby contributing to morbidity and mortality. Previous studies demonstrated reduced functional and structural changes in PWH; however, underlying mechanisms remain elusive.
Methods
Our cohort consisted of PWH with ART adherence and viral suppression ( < 50 copies/mL; N = 173). Measurements included immune cell markers of overall immune health (CD4/CD8 T-cell ratio) and myeloid inflammation (CD16+ monocytes), plasma markers of inflammatory status (soluble CD163 and CD14), and structural and functional neuroimaging (volume and cerebral blood flow [CBF], respectively).
Results
Decreased CD4/CD8 ratios correlated with reduced brain volume, and higher levels of inflammatory CD16+ monocytes were associated with reduced brain volume in total cortex and gray matter. An increase in plasma soluble CD14—a marker of acute peripheral inflammation attributed to circulating microbial products—was associated with reduced CBF within the frontal, parietal, temporal, and occipital cortices and total gray matter.
Conclusions
CD4/CD8 ratio and number of CD16+ monocytes, which are chronic immune cell markers, are associated with volumetric loss in the brain. Additionally, this study shows a potential new association between plasma soluble CD14 and CBF.
Keywords: HIV, brain, inflammation, neuroimaging
With the advent of antiretroviral therapy (ART), mortality for people with HIV (PWH) has greatly decreased. Several potential drivers of chronic inflammation in PWH have been identified, including low-level residual HIV expression during suppressive ART, microbial dysbiosis and translocation, coinfections, and lifestyle factors. Despite viral suppression, an estimated 15% to 50% of PWH will experience neurocognitive impairment after HIV infection, which presents in graded levels of severity, with the most prevalent being asymptomatic impairment in the post-ART era [1]. Persistence of neurocognitive impairment in PWH may reflect several underlying factors: chronic low-grade inflammation, initial insult during untreated viremia leading to legacy effects, deterioration or senescence of the immune system, persistent proviral DNA, or low-level viral replication in the brain [2–4]. In ART-adherent, virologically controlled cases of HIV, questions remain concerning the connection between peripheral immune activation and brain structure and function.
Peripheral immune cells, such as T lymphocytes and monocytes, provide insight into the function, efficacy, and priming of the immune system in PWH and the chronic effects of HIV infection. The CD4 T-lymphocyte count has been used to assess HIV infection, and it defines the threshold of progression to AIDS. The CD4/CD8 ratio has recently become a strong measure for assessing health risks in the now ART-adherent, virally controlled HIV population [5, 6]. A CD4/CD8 ratio >0.8 is generally considered immunologically healthy, with the ratio normally falling between 1.5 and 2.5 and with variation based on sex, genetic differences, age, and immunologic challenges. In PWH, the CD4/CD8 ratio is associated with immunosenescence and immune exhaustion in young adults, and the inversion of the CD4/CD8 ratio (<0.8) is associated with increased non-AIDS morbidity and mortality [5, 7, 8]. Additionally, the CD4/CD8 ratio is representative of residual viral replication, correlative to the development of neurocognitive disorders [9, 10], and independently associated with age-related disease in well-controlled cases of HIV [5, 11].
Monocytes and macrophages are long-lived innate myeloid cells that are responsible for pathogen recognition, cell debris clearance, and inflammatory regulation in the body. Monocytes are a heterogenic cell population that are differentiated by CD14 and CD16 expression, with the latter associated with a mature and more inflammatory monocyte. CD16+ monocytes are an important pool of cells that can be infected with HIV. CD16+ monocytes are substantially increased in PWH, are not susceptible to cytopathic effects of HIV, express higher levels of the HIV coreceptor CCR5, preferentially transmigrate across the blood-brain barrier, and sustain HIV infection and replication despite successful ART intervention. Even with ART, monocytes can continue to harbor HIV DNA, serving as a reservoir for HIV persistence and latent infection; in particular, CD16+ monocytes have higher HIV viral DNA as compared with CD16– monocytes [12]. CD16+ monocytes have been well characterized as contributing to HIV pathogenesis in the brain and neuropsychological performance [12]; however, in well-controlled cases of HIV, the role of peripheral CD16+ monocyte expansion with regard to volumetric changes in the brain remains less defined. In addition, there are well-documented sex differences in monocyte phenotypes in HIV-uninfected populations, yet studies are limited in PWH.
In addition to the peripheral leukocyte changes seen in PWH, immunologic plasma biomarkers provide an important measure of immune function. Soluble CD163 (sCD163) is generated solely from the shedding of CD163 from circulating monocytes and serves as a well-established biomarker of peripheral monocyte activation [13]. CD14 is considered a monocyte differentiation marker, and soluble CD14 (sCD14) acts as a sequestering coreceptor for lipopolysaccharide in circulation. As sCD14 has been characterized as an acute immune plasma protein, higher plasma levels of sCD14 in PWH are considered indicative of lipopolysaccharide exposure, likely from microbial translocation into the circulation [13]. Plasma sCD163 and sCD14 are also predictors of all-cause mortality in PWH [14, 15]. Increased plasma sCD163 and sCD14 have been consistently associated with an increased risk of developing neurocognitive impairment in PWH [16, 17]. Residual immune activation and inflammation differ between men and women. sCD163 levels are elevated while sCD14 is decreased in women vs men [18, 19]. sCD14 was recently associated with reduced frontal and temporal cortical volume in women with HIV and with evidence of brain white matter injury in PWH [20, 21]. Despite these studies, there remain few studies investigating the relationship between sCD14 and sCD163 and changes in brain structure and function in PWH.
Evaluation of brain changes with structural and perfusion neuroimaging allows for an understanding of pathophysiologic changes seen in the brain [22–25]. Here, we use noninvasive neuroimaging to identify global changes in brain volumes and differences in cerebral blood flow (CBF). Changes in brain volume in the cortical and subcortical gray regions are observed shortly after primary HIV infection, with reduced neuronal injury following intervention with ART [26–32]. However, several other factors may contribute to continued progressive brain volume loss, such as low viral persistence, inflammation, and accelerated aging. CBF provides a sensitive functional measure of brain dysfunction by identifying cortical and gray matter areas that have impaired perfusion in PWH [22]. Reduced cortical perfusion has been observed in several studies that compared PWH with individuals who were seronegative [22, 23, 33]. Yet, studies identifying peripheral correlates to changes in brain structure and perfusion are lacking in the field of HIV.
Since persistent inflammation can drive or exacerbate neurodegeneration in the brain, we aim to better understand the interplay between peripheral immune dysfunction and structural and functional changes in the brain. In this study, we evaluated immune function using flow cytometry and enzyme-linked immunosorbent assays and utilized neuroimaging to assess brain volume and CBF in a large cohort of patients who were ART adherent and had virologically controlled HIV (<50 copies of HIV RNA/mL). These relationships were evaluated in PWH by sex. Previous studies have shown that immune markers and CBF vary by sex. In addition, we looked at cardiovascular disease risk here since it is associated with HIV, inflammation, and CBF [34].
METHODS
Study Cohort
Participants were recruited from the Infectious Disease Clinic at Washington University in St Louis (WUSTL) and the WUSTL AIDS Clinical Trial Unit. This study included 173 participants with HIV-1 infection that was virologically well controlled with ART (<50 copies of HIV RNA per milliliter). EDTA plasma was collected from all 173 participants. Peripheral blood mononuclear cells (PBMCs) were available in a subset of 126 participants, brain volumes in 154, and CBF measurements in 148. The study was approved by the Institutional Review Board at WUSTL (201805047 and 201508002), and all participants gave written informed consent. Either self-report or medical record confirmation was used to determine the duration of infection and ART treatment.
Neuroimaging Protocol
Participants underwent magnetic resonance imaging on a Siemens 3.0-T Trio scanner with a 12-channel head coil. The neuroimaging protocol included T1-weighted magnetization-prepared rapid gradient echo (repetition time/echo time = 2400/3.2 ms, spatial resolution = 1 × 1 × 1 mm, matrix size = 256 × 256 × 176). Pseudo-continuous arterial spin labeling (pCASL) was acquired to estimate regional baseline CBF (repetition time/echo time = 3500/9.0 ms, label duration = 1500 ms, postlabeling delay = 1200 ms, spatial resolution = 3.4 × 3.4 × 5 mm, matrix size = 64 × 64 × 22). Two pCASL runs were acquired per session, with participants in a wakeful state and visually fixated on a crosshair target.
Images were preprocessed with a custom pipeline incorporating tools from the FMRIB Software Library [35]. T1-weighted scans were skull stripped and linearly, then nonlinearly, registered to the standard Montreal Neurological Institute (MNI152) brain template. Native-space T1 scans were segmented with FreeSurfer version 5.3 and regional gray matter volumes calculated. Cortical and total gray matter volumes were Z-transformed (ie, expressed in standard deviations above or below the sample mean).
pCASL equilibrium magnetization (M0) volumes were linearly registered to participant T1-weighted images. pCASL frames were motion corrected, and any tag-control pairs with >0.5-mm misalignment were excluded. Perfusion weighting was obtained by pairwise subtraction of the tag and control frames and converted to quantitative CBF in units of milliliters of blood per 100 g of tissue per minute by application of the standard 1-compartment model, per consensus guidelines for clinical populations [36]. CBF was averaged between the 2 runs, and mean values were taken for total gray matter and from cortical and subcortical FreeSurfer regions of interest.
PBMC Isolation and Flow Cytometry for T-Cell and Monocyte Subpopulations
EDTA-anticoagulated blood, collected at the time of neuroimaging, was used to isolate PBMCs by using the standard Ficoll separation method. For flow cytometry phenotypic analyses in a subset of PWH (n = 125), cryopreserved PBMCs were quickly thawed, washed, and stained with an antibody cocktail (Supplementary Table 1). All antibodies were titrated to determine optimal concentrations. Antibody capture beads (UltraComp eBeads; Invitrogen) were used for single-color compensation controls for each reagent used. Cells were analyzed with FlowJo version 10.7. CD3+ T lymphocytes were selected and gated for CD8+ and CD4+ cells. Monocyte subsets were distinguished as CD14+ CD16– classical monocytes and patrolling/inflammatory CD16+ monocytes.
Plasma Inflammatory Markers
EDTA plasma was used for enzyme-linked immunoassay quantitation of sCD14 (pg/mL; R&D Systems) and sCD163 (ng/mL; Tecan), according to the manufacturer’s instructions. Measurements were run in duplicate with appropriate experimental controls and accepted with a percentage coefficient of variation ≤25%; samples were repeated if the cutoff was exceeded.
Cardiovascular Risk
Cardiovascular disease risk factors were assessed per the 10-year cardiovascular disease risk score from the Framingham Heart Study (framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/). This calculator includes the following factors: age, sex, hypertension treatment history, current smoking status, history of diabetes mellitus, systolic blood pressure, and total and HDL cholesterol. Cardiovascular risk scores were available for 141 participants.
Statistical Methods
Plasma markers, immune cell populations, brain volumes, and CBF were compared between male and female participants by a 2-tailed unpaired t test with a designated alpha of 0.05. Mean values for measured variables by sex were reported with the resulting P value. Plasma markers, immune cell populations, brain volumes, and CBF were compared across the entire cohort by Spearman rank correlation. For any plasma or immune markers that indicated a sex difference, subsequent multiple regression analyses were conducted that included the plasma or immune marker, sex, and a marker × sex interaction term as independent variables and brain volumes and CBF as dependent variables. Finally, relationships among plasma markers, immune cell populations, brain volumes or CBF, and the Framingham cardiovascular disease risk score were compared by Spearman rank correlation. Comparisons between immune cell populations and brain volumes were made with a significance threshold of α = 0.05, and 95% CIs were calculated. For comparisons of plasma biomarkers and CBF, α was set to 0.01 to account for 5 comparisons, and 99% CIs were calculated. Correlations were graphed on dot plots with the linear regression of the correlation. Prism version 9.0 (GraphPad Software) was used for statistical analyses.
RESULTS
Demographics and HIV Clinical Variables
PWH included in the study were adherent to an effective ART regimen and had a plasma viral load <50 copies/mL at the time of the study. Demographic information and relevant HIV metrics are reported in Table 1. The mean age of participants was 54 years (range, 23–75). The cohort was 77.5% male (n = 134) and 22.5% female (n = 39). The self-identified racial breakdown was 65.9% African American (n = 114), 31.8% Caucasian (n = 55), and 2.3% other racial identities (n = 4). Of participants with CD4 nadir, the median was 98 cells/μL (IQR, 20–238; n = 89), and the current median was 609 cells/μL (IQR, 448–864; n = 169). Of PWH who reported the duration of HIV infection, the median was 237 months (IQR, 119–312; n = 155). The median time receiving ART was 180 months (IQR, 96–252; n = 160).
Table 1.
Demographics and Clinical Data (N = 173)
| No. (%) or Median (IQR) | |
|---|---|
| Age, y | 54 (23–75)a |
| Race: African American | 114 (65.9) |
| Education, y | 13 (8–20)a |
| Sex: male | 134 (77.5) |
| Hepatitis C infection | 23 (13.5) |
| 10-y Framingham risk score (n = 141) | 14.35 (6.53–22.28) |
| CNS penetration effectiveness (n = 158) | 7 (6–8.75) |
| CD4 count, cells/μL | |
| Nadir (n = 89) | 98 (20–238) |
| Most recent (n = 169) | 609 (448–864) |
| Duration, mo | |
| HIV (n = 155) | 237 (119–312) |
| ART (n = 160) | 180 (96–252) |
| Regimen containing | |
| NRTI | 164 (95.3) |
| NNRTI | 70 (40.7) |
| Protease inhibitor | 40 (23.3) |
| INSTI | 101 (58.7) |
| Fusion | 1 (0.58) |
| Substance use | |
| Cocaine | 22 (12.9) |
| Amphetamine | 8 (4.7) |
| Methamphetamine | 7 (4.1) |
| THC | 73 (42.7) |
| MTD | 2 (1.2) |
| Opiates | 9 (5.3) |
| Benzodiazepine | 20 (11.7) |
| TCA | 16 (9.4) |
| Smoking/chew | 78 (45.6) |
Abbreviations: ART, antiretroviral therapy; CNS, central nervous system; INSTI, integrase strand transfer inhibitor; MTD, methadone; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; TCA, tricyclic antidepressant; THC, tetrahydrocannabinol.
Mean (range).
Sex-Based Differences in the Cohort
To compare potential sex differences within this cohort, measurements of immune markers, brain volume, and CBF were compared between male and female participants (Table 2). We observed no significant differences in T lymphocytes, including the CD4/CD8 ratio, monocyte populations, and plasma sCD14, between men and women with HIV. Women showed significantly elevated plasma sCD163 (P = .046) as compared with men. There were no significant sex differences in brain volume. Women showed increased CBF in the frontal (P = .029), parietal (P = .035), and occipital (P = .035) regions and gray matter (P = .035).
Table 2.
Sex Differences in Immunologic and Neuroimaging Variables
| Mean | |||
|---|---|---|---|
| Male (n = 134) | Female (n = 39) | P Valuea | |
| Age, y | 54.3 | 52.2 | … |
| Race: African American, No. (%) | 83 (62) | 31 (79) | —b |
| 10-Y Framingham risk score | 17.33 | 11.4 | .0048 |
| Duration, mo | |||
| HIV | 221.7 | 211.1 | … |
| ART | 175.7 | 169.7 | … |
| CD4/CD8 lymphocyte ratio | 0.763 | 0.622 | … |
| Monocytes, % | |||
| CD14–CD16+ | 8.51 | 8.15 | … |
| CD14+CD16+ | 6.16 | 5.40 | … |
| Total CD16+ | 13.6 | 13.0 | … |
| CD14+CD16− | 85.1 | 86.4 | … |
| Soluble, ng/mL | |||
| CD14 | 1682 | 1719 | … |
| CD163 | 936 | 1156 | .047 |
| Volume | |||
| Cortex | –0.0446 | 0.0716 | … |
| Total gray matter | –0.0254 | 0.0138 | … |
| Cerebral blood flow | |||
| Frontal | 59.3 | 68.9 | .029 |
| Parietal | 60.7 | 70.2 | .035 |
| Temporal | 55.2 | 62.4 | .059c |
| Occipital | 60.8 | 70.4 | .036 |
| Gray matter | 56.9 | 65.8 | .035 |
Abbreviation: ART, antiretroviral therapy.
Based on unpaired 2-tailed t test. Cells without data indicate P values that were not significant.
Not applicable.
Trending toward significance.
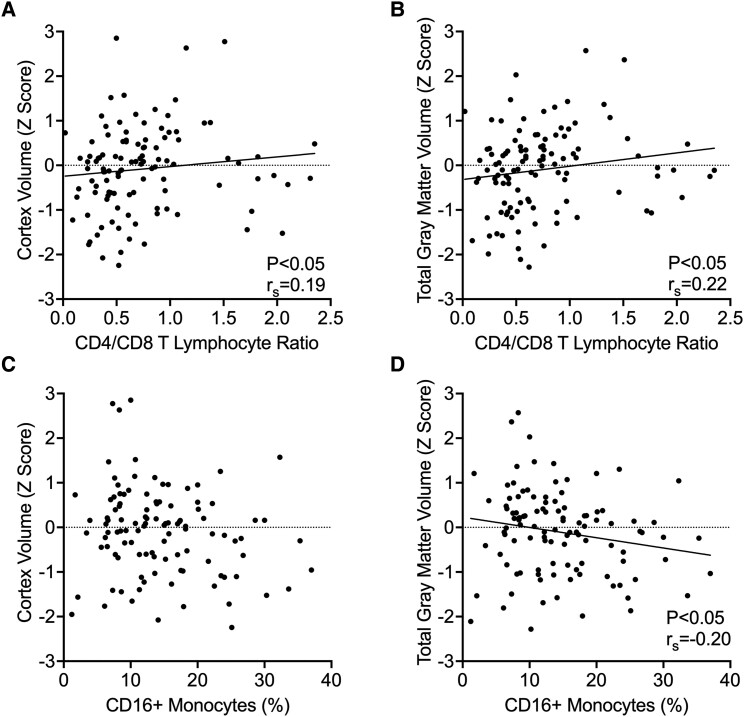
Lower CD4/CD8 T-Lymphocyte Ratio Is Associated With Reduced Brain Volume in PWH
In the cohort, 66.1% of participants with PBMCs available had a CD4/CD8 ratio <0.8 (n = 78). We correlated the CD4/CD8 ratio to the total cortex and total gray matter volume. A lower CD4/CD8 T-lymphocyte ratio was associated with reduced total cortex volume (Figure 1A: P = .04, rs = 0.19). Similarly, a lower CD4/CD8 T-lymphocyte ratio was associated with reduced total gray matter volume (Figure 1B: P = .02, rs = 0.22). There were no significant associations between CD4/CD8 ratio and CBF.
Figure 1.
Lower CD4/CD8 ratio and higher CD16+ inflammatory monocytes correlate with lower brain volume. The CD4/CD8 ratio of T lymphocytes was compared with total cortex and gray matter volumes in people with HIV. A, Cortex volume was positively correlated with CD4/CD8 ratio (P = .04, rs = 0.19). B, Total gray matter volume was positively correlated with CD4/CD8 ratio (P = .02, rs = 0.21). C, Cortex volume did not correlate with the percentage of CD16+ inflammatory monocytes (not significant). D, Total gray matter volume was negatively correlated with the CD16+ inflammatory monocytes (P = .03, rs = −0.20). For associations with significant Spearman correlations, the trend is represented as a linear regression trendline.
Elevated CD16+ Inflammatory Monocytes Are Associated With Reduced Cortex and Gray Matter Volume in PWH
Regarding peripheral monocytes, there was no observed association between CD16+ inflammatory monocytes and cortex volume (Figure 1C). However, increased CD16+ monocytes were associated with reduced total gray matter volume (Figure 1D: P = .03, rs = –0.20). No significant associations were seen between CD16+ monocyte expansion and CBF. Neither were any associations seen with CD14+ CD16– classical monocytes.
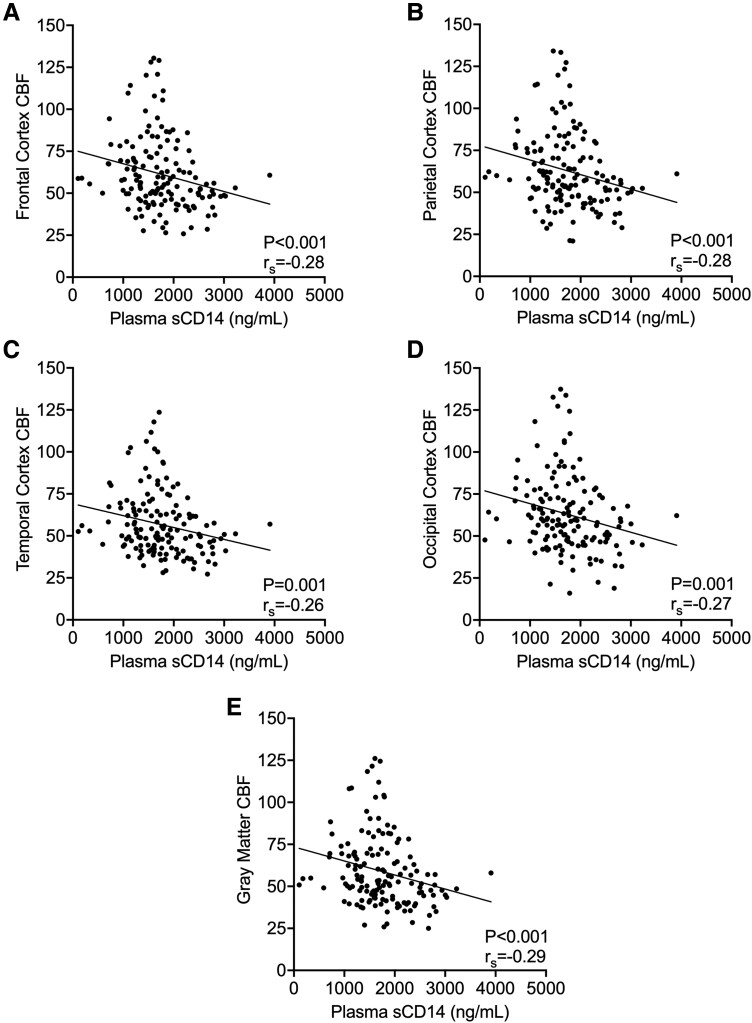
Elevated Plasma sCD14 Correlates With Reduced CBF
Higher plasma sCD14 was associated with reduced CBF within all cortical regions: frontal (Figure 2A: P = .0007, rs = 0.28), parietal (Figure 2B: P = .0007, rs = −0.28), temporal (Figure 2C: P = .0014, rs = −0.26), and occipital (Figure 2D: P = .0012, rs = −0.27). Similarly, higher plasma sCD14 was associated with reduced CBF in total gray matter (Figure 2E: P = .0005, rs = −0.29).
Figure 2.
Elevated plasma sCD14 correlates with reduced CBF. Plasma sCD14 was compared with the CBF of frontal, parietal, temporal, and occipital cortical regions and gray matter. A–E, CBF measures of the frontal, parietal, temporal, and occipital cortex and gray matter were all negatively correlated with the plasma sCD14. For associations with significant Spearman correlations, the trend is represented as a linear regression trendline. CBF, cerebral blood flow; sCD14, soluble CD14.
We did not observe associations between plasma sCD163 and CBF in cortical regions or total gray matter. There was no significant association between brain volumes and plasma markers sCD14 and sCD163. Follow-up regression analyses including sex and an sCD163 × sex interaction term, due to the observed sex difference in sCD163 concentrations, also did not reveal any significant interactions between sex and sCD163 on brain volumes or CBF.
Increased Cardiovascular Risk Correlates With Elevated sCD14 and Reduced CBF
Of the examined plasma markers and immune cell populations, only higher plasma sCD14 was significantly correlated with increased cardiovascular risk (P = .003, rs = 0.25). Higher cardiovascular risk was also significantly associated with reduced CBF across all regions, including the frontal lobe (P < .001, rs = −0.55), parietal lobe (P < .001, rs = −0.57), temporal lobe (P < .001, rs = −0.55), occipital lobe (P < .001, rs = −0.56), and total gray matter (P < .001, rs = −0.56), as well as smaller cortex volume (P = .0002, rs = −0.37) and gray matter volume (P = .00001, rs = −0.39).
DISCUSSION
Using peripheral blood, we quantified differences in T-lymphocyte, monocyte, and immunologic biomarkers in a cohort with well-controlled HIV. Additionally, we evaluated brain structure and function through noninvasive neuroimaging. We identified associations between markers of chronic immune dysregulation (low CD4/CD8 ratio and elevated CD16+ monocytes) and decreased brain volume and between sCD14 and reduced CBF in cortical regions and total gray matter. This study provided an important addition to the interplay between peripheral immune dysfunction and central nervous system neuroimaging changes seen in PWH.
Through this study, we expanded on the paradigm of chronic and acute processes in the peripheral immune system and the central nervous system. The CD4/CD8 ratio is a marker of general immune health, with a lower ratio indicative of poor immune status, and data support a threshold value of 0.8 as being more strongly associated with adverse outcomes [8]. Our recent study showed that structural brain aging is an indicator of cognitive function and reflects HIV serostatus rather than current viral load [22]. Here, we correlated chronic immune dysfunction to brain volumetrics.
Our results suggest at least 2 immunologic mechanisms underlying brain volumetric loss over the course of HIV infection. The CD4/CD8 ratio is representative of T-lymphocyte immune function; however, the ratio could be driven either by the loss of CD4 T lymphocytes or from a reactive expansion of CD8 T lymphocytes. CD4 T-lymphocyte loss could suggest an initial hit from untreated HIV or from progression to end-stage disease. Conversely, CD8 T-lymphocyte expansion remains persistent for years following the initiation of ART and viral suppression [37]. Expanded CD8 T lymphocytes in PWH who are chronically infected tend to be more mature in phenotype with evidence of immune exhaustion, leading to impaired effector and antiviral function, or immunosenescence, potentiating a proinflammatory response [37]. A recent study on early HIV in a cohort of mostly men examined the longitudinal pattern of the CD4/CD8 ratio and its relationship with neurocognitive and motor performance [38]. It demonstrated that the trajectory of the CD4/CD8 ratio was independently associated with motor/psychomotor speed performance. It also suggested that the CD4/CD8 ratio provides unique information beyond CD4 T-cell count alone into potential mechanisms of HIV neuropathogenesis and that levels of immune suppression (CD4 count) and immune activation/exhaustion (CD8 count) are necessary for neurocognitive impairment. Our data add to this by demonstrating that an inverted ratio due to immune dysfunction is associated with brain atrophy.
An increase in CD16+ monocytes has been shown to correlate with HIV pathophysiology in the brain [12, 39]. The association of a high percentage of inflammatory CD16+ monocytes and reduced brain volume could be indicative of ongoing inflammatory processes in the brain, infiltration of peripheral monocytes into the brain, or potential reseeding of the brain viral reservoir. Alternatively, structural changes in the brain may partly result from a legacy effect of early tissue damage with irreversible cell death [22]. Overall, these data suggest that a loss of immune health and monocyte phenotypic expansion are associated with brain volume loss.
Plasma sCD14 is a dynamic marker of the immune response, as sCD14 is implicated in innate immune cell regulation, microbial translocation, and endothelial activation. An increase in sCD14 in the plasma is indicative of systemic immune activation. However, the relationship between sCD14 and brain vascular physiology in PWH remains unclear. Prior studies have shown that CBF is reduced during HIV infection [23, 40] and that CBF depends on age and current viral load and is improved by medication adherence [22]. We observed that higher plasma sCD14 was associated with reduced CBF in all cortical regions in PWH. These associations show that markers of more dynamic processes, such as acute immune sensing and microbial translocation, are associated with diminished CBF. We show a strong association between the plasma sCD14 and CBF across all cortical regions and total gray matter.
Since sex differences in immune markers and CBF are observed in the general population, potential sex-based differences within our cohort of PWH were examined. We found that women have significantly higher plasma sCD163, which has been reported in other clinical cohorts comparing men and women with HIV [18]. Women also showed higher CBF than men in this cohort, which is in line with previously reported data indicating that women in the general population have higher overall CBF vs men [41].
This study has several limitations in our assessment of immunologic marker associations with brain structure and function. First, this study consists solely of well-controlled cases of HIV and lacks a comparative cohort of uninfected individuals. Second, an abnormal CD4/CD8 ratio has been associated with cytomegalovirus (CMV) infection [42]. As CMV data were not available for all individuals in the cohort, we cannot rule out a possible effect of coinfection with CMV on a low CD4/CD8 ratio. In our statistical models, we did not examine more complex models, including 10-year Framingham risk and immune markers on brain volumes and CBF, since we lacked 10-year Framingham risk in about 30 participants. Future analyses with more individuals could be performed. Additionally, we are unable to determine the independent contribution of 2 important variables: HIV status and long-term use of antiretrovirals. Last, this study is cross-sectional in design, providing only a singular time point for participants in the cohort, and does not include neurocognitive testing.
This study sought to determine if there is continued inflammation within virologically well-controlled cases of HIV and whether this contributes to the observed differences seen with neuroimaging. This study advances our understanding of brain changes and inflammation in chronic HIV. As this study consists of a cohort with well-controlled HIV, these data demonstrate the critical role that peripheral cellular response plays in sustained brain damage and dysfunction despite viral suppression. We show strong correlational evidence of chronic immune dysfunction and activation implicated in HIV-associated brain structural changes and pathogenesis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Tricia H Burdo, Department of Microbiology, Immunology, and Inflammation, Center for Neurovirology and Gene Editing, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania.
Jake A Robinson, Department of Microbiology, Immunology, and Inflammation, Center for Neurovirology and Gene Editing, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania.
Sarah Cooley, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Mandy D Smith, Department of Microbiology, Immunology, and Inflammation, Center for Neurovirology and Gene Editing, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania.
Jacqueline Flynn, Department of Microbiology, Immunology, and Inflammation, Center for Neurovirology and Gene Editing, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania.
Kalen J Petersen, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Brittany Nelson, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Elizabeth Westerhaus, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Julie Wisch, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Beau M Ances, Department of Neurology, Washington University in St Louis, St Louis, Missouri, USA.
Notes
Availability of data and materials. All relevant raw data will be freely available upon a reasonable request. No uniquely generated resources, such as strains, tools, chemical compounds, antibodies, cell lines, or mutant lines, were produced here.
Authors’ contributions. Conceptualization: T. H. B., B. M. A. Investigation: M. D. S., J. F., K. J. P., B. N., S. C., E. W., J. W. Analysis: J. A. R., K. J. P., S. C., J. W. Writing–original draft: J. A. R., T. H. B. Writing–review and editing: B. M. A., K. J. P., S. C. Manuscript review: all authors. Visualization: J. A. R., T. H. B. Funding acquisition: B. M. A., T. H. B.
Financial support. This work was supported by the National Institutes of Health (R01NR012907, R01NR012657, R01 NR014449, R01 MH118031 to B. M. A.; R01 DA054009, R01 MH118031 S1 to B. M. A. and T. H. B.; T32 MH079785 to T. H. B. supporting J. A. R.; F32 MH129151 to K. J. P.). This work was also supported by the generous support of the Barnes-Jewish Hospital, the Washington University Institute of Clinical and Translational Sciences Foundation (UL1 TR000448), and the Hope Center for Neurological Disorders.
References
- 1. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gabuzda D, Jamieson BD, Collman RG, et al. Pathogenesis of aging and age-related comorbidities in people with HIV: highlights from the HIV ACTION workshop. Pathog Immun 2020; 5:143–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McArthur JC, Johnson TP. Chronic inflammation mediates brain injury in HIV infection: relevance for cure strategies. Curr Opin Neurol 2020; 33:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandera A, Taramasso L, Bozzi G, et al. HIV-associated neurocognitive impairment in the modern ART era: are we close to discovering reliable biomarkers in the setting of virological suppression? Front Aging Neurosci 2019; 11:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McBride JA, Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell US about HIV and health? PLoS Pathog 2017; 13:e1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. J Infect Dis 2015; 211:1726–34. [DOI] [PubMed] [Google Scholar]
- 7. Sainz T, Serrano-Villar S, Diaz L, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013; 27:1513–6. [DOI] [PubMed] [Google Scholar]
- 8. Aksak-Was BJ, Kowalska JD, Zabek P, et al. Immune restoration affects 10-year survival in people living with HIV/AIDS. HIV Med 2023; 24:325–34. [DOI] [PubMed] [Google Scholar]
- 9. Vassallo M, Durant J, Lebrun-Frenay C, et al. Virologically suppressed patients with asymptomatic and symptomatic HIV-associated neurocognitive disorders do not display the same pattern of immune activation. HIV Med 2015; 16:431–40. [DOI] [PubMed] [Google Scholar]
- 10. Vassallo M, Fabre R, Durant J, et al. A decreasing CD4/CD8 ratio over time and lower CSF-penetrating antiretroviral regimens are associated with a higher risk of neurocognitive deterioration, independently of viral replication. J Neurovirol 2017; 23:216–25. [DOI] [PubMed] [Google Scholar]
- 11. Riddler SA, Aga E, Bosch RJ, et al. Continued slow decay of the residual plasma viremia level in HIV-1–infected adults receiving long-term antiretroviral therapy. J Infect Dis 2016; 213:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veenhuis RT, Williams DW, Shirk EN, et al. Higher circulating intermediate monocytes are associated with cognitive function in women with HIV. JCI Insight 2021; 6:e146215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knudsen TB, Ertner G, Petersen J, et al. Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1–infected individuals. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 15. Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imp BM, Rubin LH, Tien PC, et al. Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 2017; 215:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012; 11:867–75. [DOI] [PubMed] [Google Scholar]
- 20. Kamkwalala AR, Wang X, Maki PM, et al. Brief report: higher peripheral monocyte activation markers are associated with smaller frontal and temporal cortical volumes in women with HIV. J Acquir Immune Defic Syndr 2020; 84:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang K, Premeaux TA, Cobigo Y, et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS 2020; 34:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen KJ, Metcalf N, Cooley S, et al. Accelerated brain aging and cerebral blood flow reduction in persons with human immunodeficiency virus. Clin Infect Dis 2021; 73:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology 2009; 73:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nir TM, Fouche JP, Ananworanich J, et al. Association of immunosuppression and viral load with subcortical brain volume in an international sample of people living with HIV. JAMA Netw Open 2021; 4:e2031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012; 59:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanford R, Ances BM, Meyerhoff DJ, et al. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis 2018; 67:1697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanford R, Fellows LK, Ances BM, Collins DL. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA Neurol 2018; 75:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooley SA, Navid J, Wisch JK, et al. Relationships between viral load, neuroimaging, and NP in persons living with HIV. J Acquir Immune Defic Syndr 2021; 87:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanford R, Fernandez Cruz AL, Scott SC, et al. Regionally specific brain volumetric and cortical thickness changes in HIV-infected patients in the HAART era. J Acquir Immune Defic Syndr 2017; 74:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ortega M, Heaps JM, Joska J, et al. HIV clades B and C are associated with reduced brain volumetrics. J Neurovirol 2013; 19:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guha A, Brier MR, Ortega M, Westerhaus E, Nelson B, Ances BM. Topographies of cortical and subcortical volume loss in HIV and aging in the cART era. J Acquir Immune Defic Syndr 2016; 73:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology 2013; 80:1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strain JF, Cooley S, Kilgore C, et al. The structural and functional correlates of frailty in persons living with HIV. Clin Infect Dis 2022; 75:1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glans M, Cooley SA, Vaida F, et al. Effects of Framingham 10-year cardiovascular risk score and viral load on brain integrity in persons with HIV. J Acquir Immune Defic Syndr 2022; 90:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(suppl 1):S208–19. [DOI] [PubMed] [Google Scholar]
- 36. Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer's disease. J Alzheimers Dis 2010; 20:871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mudd JC, Lederman MM. CD8 T cell persistence in treated HIV infection. Curr Opin HIV AIDS 2014; 9:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le LT, Price RW, Gisslen M, et al. Correlation between CD4/CD8 ratio and neurocognitive performance during early HIV infection. HIV Med 2022; 24:442–52. [DOI] [PubMed] [Google Scholar]
- 39. Burdo TH, Soulas C, Orzechowski K, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010; 6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Towgood KJ, Pitkanen M, Kulasegaram R, et al. Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Hum Brain Mapp 2013; 34:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alisch JSR, Khattar N, Kim RW, et al. Sex and age-related differences in cerebral blood flow investigated using pseudo-continuous arterial spin labeling magnetic resonance imaging. Aging (Albany NY) 2021; 13:4911–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith DM, Nakazawa M, Freeman ML, et al. Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis 2016; 63:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.