Abstract
Background
Respiratory syncytial virus (RSV) causes significant disease burden in older adults. MVA-BN-RSV is a novel poxvirus-vectored vaccine encoding internal and external RSV proteins.
Methods
In a phase 2a randomized double-blind, placebo-controlled trial, healthy participants aged 18 to 50 years received MVA-BN-RSV or placebo, then were challenged 4 weeks later with RSV-A Memphis 37b. Viral load was assessed from nasal washes. RSV symptoms were collected. Antibody titers and cellular markers were assessed before and after vaccination and challenge.
Results
After receiving MVA-BN-RSV or placebo, 31 and 32 participants, respectively, were challenged. Viral load areas under the curve from nasal washes were lower (P = .017) for MVA-BN-RSV (median = 0.00) than placebo (median = 49.05). Total symptom scores also were lower (median = 2.50 and 27.00, respectively; P = .004). Vaccine efficacy against symptomatic, laboratory-confirmed or culture-confirmed infection was 79.3% to 88.5% (P = .022 and .013). Serum immunoglobulin A and G titers increased approximately 4-fold after MVA-BN-RSV vaccination. Interferon-γ–producing cells increased 4- to 6-fold after MVA-BN-RSV in response to stimulation with the encoded RSV internal antigens. Injection site pain occurred more frequently with MVA-BN-RSV. No serious adverse events were attributed to vaccination.
Conclusions
MVA-BN-RSV vaccination resulted in lower viral load and symptom scores, fewer confirmed infections, and induced humoral and cellular responses.
Clinical Trials Registration
Keywords: RSV, RSV-A Memphis 37b, human challenge trial, modified vaccinia Ankara, recombinant vaccine, vaccine efficacy, vectored vaccine
In a randomized human challenge trial of a poxvirus-vectored vaccine encoding internal and external respiratory syncytial virus (RSV) proteins versus placebo, MVA-BN-RSV vaccination resulted in lower viral load and symptom scores, fewer confirmed infections, and induced humoral and cellular responses.
Respiratory syncytial virus (RSV) remains a common, recurring infectious disease. Infants are understood to be at high risk from RSV; it is estimated that nearly 120 000 children younger than 5 years die of RSV each year globally [1, 2], mostly in the developing world. Older adults are the second highest risk group, with substantial RSV disease burden [3–5]. In high-income countries, an estimated 1.62% of adults ≥60 years of age develop acute RSV infections annually, 0.15% are hospitalized, and approximately 33 000 die of RSV-related causes [4]. Underlying comorbidities likely drive the risk of severe outcomes in older adults [5–7]. Therefore, this population is an important target for preventive measures, as older adults in the developed world may spend decades at risk of both hospitalization and death from RSV.
In the 1960s, research in children with a formalin-inactivated RSV vaccine was halted when it was recognized that those immunized frequently experienced enhanced disease upon later natural infection [8–10]. This slowed the pace of RSV vaccine research for decades, as scientists investigated potential mechanisms for this phenomenon [11]. Modern RSV vaccine research has mostly focused on delivery of RSV proteins, rather than whole virus, either as a subunit vaccine or using a viral vector. In particular, vaccine development efforts have aimed to elicit neutralizing antibodies (nAbs) to the surface fusion (F) protein that promotes syncytium formation in respiratory epithelium. Two candidate vaccines for older adults containing the F protein in its postfusion conformation were unsuccessful in phase 2 and 3 trials [12, 13]. Four other vaccines delivering the F protein in its prefusion conformation (preF) have reported positive results from phase 2 challenge trials [14, 15] and/or from pivotal phase 3 trials [16–18]. None of these vaccines in older adults appears to elicit the imbalanced T helper 2 (Th2)-mediated immune response thought to be involved in RSV vaccine-enhanced disease [19, 20].
MVA-BN-RSV is a novel vaccine aimed at broad immunogenicity, inducing both humoral and cellular responses to multiple RSV proteins. It utilizes the nonreplicating, modified vaccinia Ankara (MVA) virus, which has been widely and safely tested and used as a smallpox/mpox vaccine [21–27] and in recombinant form against Ebola [28]. The MVA-BN-RSV recombinant vaccine encodes not only the F protein (expressed as both pre- and post-F) but also surface glycoproteins from the 2 RSV subtypes, G(A) and G(B), that facilitate viral attachment to airway ciliated epithelial cells and 2 internal proteins (nucleoprotein [N] and transcription elongation factor [M2-1]) [29–31]. The F and G proteins are the main targets of RSV nAbs, but this immune response to natural infection is not durable [32, 33]. The N and M2-1 proteins, highly conserved among different RSV subtypes, were included in the recombinant vaccine to promote cytotoxic T-cell responses.
MVA-BN-RSV may have an advantage over other candidate vaccines that rely on nAbs to a single protein. The vaccine has induced humoral and cellular immune responses in animal models [29] and in early clinical trials [30, 31] without safety concerns. The safety and immunogenicity of MVA-BN-RSV are further tested and efficacy is examined for the first time in this human challenge trial report.
METHODS
Trial Design, Conduct, and Procedures
Design, Vaccine, and Placebo
This was a phase 2a, randomized, double-blind, placebo-controlled trial to assess safety, immunogenicity, and efficacy of MVA-BN-RSV vaccine against infection with the Memphis 37b strain of respiratory syncytial virus subtype A (RSV-A). The vaccine is based on the MVA vector and genetically engineered to encode the RSV F, G(A), G(B), N, and M2-1 proteins [29–31]. The vaccine was produced at Bavarian Nordic A/S (Kvistgård, Denmark) according to Good Manufacturing Practice standards and used at a nominal titer of 5 × 10E8 infectious units per 0.5 mL dose; placebo was an equal volume of Tris buffered saline solution. RSV-A Memphis 37b at a dose of 4.5 log10 plaque-forming units was utilized as the challenge virus. This virus challenge trial was conducted by hVIVO Services Limited at the Queen Mary BioEnterprises Innovation Centre in London, UK, from January to November 2021. The challenge model has been previously described [34, 35]; for the schedule of trial activities, see the Supplementary Material.
All trial-related procedures were conducted in accordance with Good Clinical Practice and the provisions of the Declaration of Helsinki and were approved by the Office for Research Ethics Committees Northern Ireland before trial initiation.
Subjects
Subjects provided written informed consent before participating in the trial. They were healthy adults between 18 and 50 years of age expected to be susceptible to RSV based on screening nAb titers in the lowest population quartile for the previous year. For full eligibility criteria, see the Supplementary Material.
Vaccination Phase
Subjects were randomized 1:1 to be vaccinated intramuscularly with MVA-BN-RSV or placebo. Subjects used a memory aid card to collect information on local (pain, erythema, swelling, induration, and pruritus) and systemic (pyrexia, headache, myalgia, chills, nausea, and fatigue) reactions up to 7 days after vaccination. Blood was collected for antibody titers and cellular markers before vaccination, 7 days after vaccination for cellular markers, and 14 days after vaccination for antibody titers.
Challenge and Quarantine Phase
Subjects were admitted to a quarantine unit 2 days before being inoculated intranasally with challenge virus (approximately 4 weeks after vaccination) and observed there for 12 days following challenge. Subjects rated their experience of 13 symptoms on a diary card twice on the first day of quarantine, once on the last day, and 3 times each day in between. Most symptoms were scored in severity from 0 to 3; shortness of breath and wheezing had a fourth severity category for symptoms at rest. Details of symptom scoring is provided in the Supplementary Material. Nasal washes were obtained twice a day from the second to eleventh day after challenge and once on the final quarantine day. Blood was collected for antibody titers and cellular markers before challenge and 5 and 10 days after challenge.
Follow-up Phase
Subjects were followed after discharge from quarantine. Blood was collected for antibody titers and cellular markers at 4 weeks after challenge and approximately 6 months after vaccination.
Entire Trial Period
Reports of unsolicited adverse events (AEs) were collected throughout the trial, from informed consent to trial end, and followed until resolution.
Laboratory Measures
Viral Load
A validated reverse transcriptase quantitative polymerase chain reaction (qRT-PCR) assay was used both to determine whether RSV-A Memphis 37b could be detected and to measure the amount of challenge virus present (lower limit of quantitation [LLOQ] was defined as a cycle threshold [Ct] value of 3.9, which equated to 2.8 log10 copies/mL) in nasal washes. Results below the LLOQ were set to 0. Nasal washes also were cultured for replicating virus, and the results were measured by plaque assay; the LLOQ was 2.0 log10 plaque-forming units/mL, and results below the LLOQ were set to 0. Refer to Supplementary Material for details.
Immunogenicity Measures
Serum samples were analyzed by enzyme-linked immunosorbent assay (ELISA) for titers of RSV-specific immunoglobulins A (IgA) and G (IgG) and by plaque reduction neutralization test (PRNT) for neutralizing titers of RSV subtype A- and subtype B-specific antibodies. A double-color enzyme-linked immunospot (ELISpot) was used to enumerate peripheral blood mononuclear cells (PBMCs) producing interferon-γ (IFN-γ) and interleukin 4 (IL-4) in response to stimulating pools representing the surface proteins F, G(A), and G(B); the internal proteins M2-1 and N; and whole RSV. These methods have been previously described [30, 31]. PRNT titers were calibrated to the first World Health Organization International Standard [36].
Incidence of Infection and Vaccine Efficacy
Incidence by qRT-PCR in each treatment group was calculated as the proportion of subjects infected from day 2 to end of quarantine, according to 3 a priori definitions: (1) infection confirmed by qRT-PCR with RSV detectable (at or above the lower limit of detection [≥LLOD]) in samples from at least 2 consecutive days and symptomatic as evidenced by 1 or more clinical symptoms of grade ≥2; (2) detectable infection with symptoms as above or with 1 or more symptoms of any grade from 2 different categories in the symptoms scoring system; or (3) detectable infection regardless of symptoms. Vaccine efficacy was defined as (1 − incidence ratio) × 100%.
Infection existent in viral culture was similarly defined a priori but based on the presence of quantifiable RSV (≥LLOQ). Vaccine efficacy using culture-confirmed infection was calculated post hoc.
Also post hoc, it was recognized that other recently published human challenge trials [14, 15] included definitions of infection using quantifiable (≥LLOQ) rather than detectable (≥LLOD) qRT-PCR measures, as detectable measures were too sensitive, and respiratory symptoms did not add sufficient specificity to the definitions of infection. Infection definitions and vaccine efficacy calculations therefore were expanded to include corollary definitions based on quantifiable qRT-PCR measures (see section Efficacy Results).
Statistical Analysis
Statistical analyses were performed using SAS 9.4 (SAS-Institute) by Venn Life Sciences. The primary and secondary efficacy analyses were performed in the per protocol population, which included all participants who were vaccinated, challenged, and had nasal washes at least until day 10 of quarantine. The intent-to-treat (challenge) population, which included all vaccinated and challenged participants, was used for supportive analyses on efficacy end points. Safety end points were analyzed on all vaccinated participants (safety population).
Summary statistics were calculated for demographic and baseline characteristics and efficacy and safety end points. For viral load area under the curve (AUC) by qRT-PCR, confirmatory testing of treatment differences was done with a 1-sided Wilcoxon rank sum test. Descriptive treatment comparisons of peak viral load, peak viral culture, sum of total symptom scores, and peak total symptom score were performed with 2-sided Wilcoxon rank sum tests. For the incidence of RSV-A Memphis 37b infection detected by qRT-PCR and quantified by qRT-PCR, and incidence of infection confirmed by virus culture, descriptive treatment comparisons were performed using the Fisher exact test. The Wilson score method was used to calculate confidence intervals for proportions. For safety data, unsolicited adverse events were coded using the Medical Dictionary for Regulatory Activities, version 24.0.
RESULTS
Participant Demographics and Characteristics
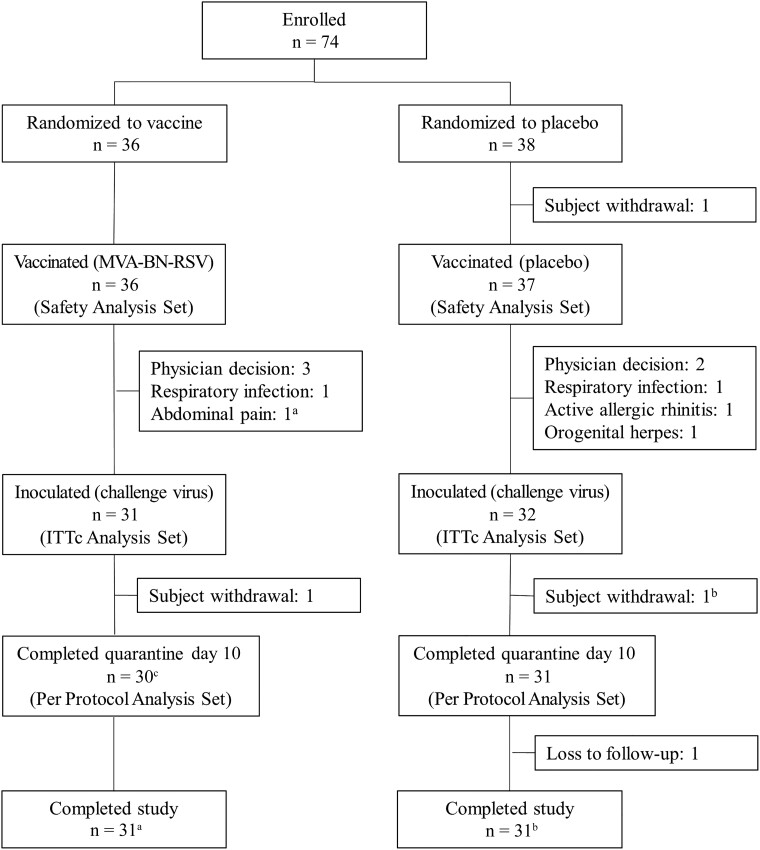
A total of 74 participants were randomized, 36 to receive MVA-BN-RSV and 38 to receive placebo in a blinded fashion. All but one were vaccinated and are included in safety analyses. Ten participants, 5 from each treatment group, did not proceed with RSV-A Memphis 37b inoculation. Of the 63 participants inoculated (intent-to-treat [challenge] analysis set), 1 participant in each group did not have viral load samples collected through day 10 (study withdrawals); therefore 61 participants are included in the per protocol analysis set (see participant disposition in Figure 1). The baseline characteristics (Table 1) and medical history of the participants were similar between the MVA-BN-RSV and placebo groups (median age 26.5 and 25.0 years, female sex 36.1% and 45.9%, white race 91.7% and 89.2%, respectively).
Figure 1.
Subject disposition (all participants). aSubject with abdominal pain was not inoculated but returned for the final study visit. Subject is not counted in ITTc or PP analysis sets but is recorded as completing the study. bSubject with myocarditis withdrew before day 10 of quarantine was completed but returned for the follow-up visits. Subject is not counted in PP analysis set but is recorded as completing the study. cSubject with myocarditis discontinued after day 10 of quarantine and returned for the follow-up visits. Subject is counted in PP analysis set and recorded as completing the study. Abbreviations: ITTc, intent-to-treat (challenge); n, number of participants; PP, per protocol.
Table 1.
Demographics and Baseline Characteristics
| Characteristic | MVA-BN-RSV (N = 36) |
Placebo (N = 37) |
Total (N = 73) |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 26.1 (5.2) | 25.7 (6.5) | 25.9 (5.9) |
| Median | 26.5 | 25.0 | 25.0 |
| Min, Max | 18, 42 | 18, 50 | 18, 50 |
| Sex, n (%) | |||
| Female | 13 (36.1) | 17 (45.9) | 30 (41.1) |
| Male | 23 (63.9) | 20 (54.1) | 43 (58.9) |
| Race, n (%) | |||
| White | 33 (91.7) | 33 (89.2) | 66 (90.4) |
| Asian | 1 (2.8) | 2 (5.4) | 3 (4.1) |
| Multiple | 2 (5.6) | 2 (5.4) | 4 (5.5) |
| BMI at screening, kg/m2 | |||
| Mean (SD) | 24.48 (3.09) | 24.94 (3.65) | 24.71 (3.37) |
| Median | 25.05 | 24.80 | 24.90 |
| Min, Max | 18.3, 30.1 | 18.9, 34.1 | 18.3, 34.1 |
Descriptive analyses performed on the safety population analysis set.
Abbreviations: BMI, body mass index; N, number of participants in the specified group; n, number of participants within the specified group contributing to statistic; %, percentage based on N.
Efficacy Results
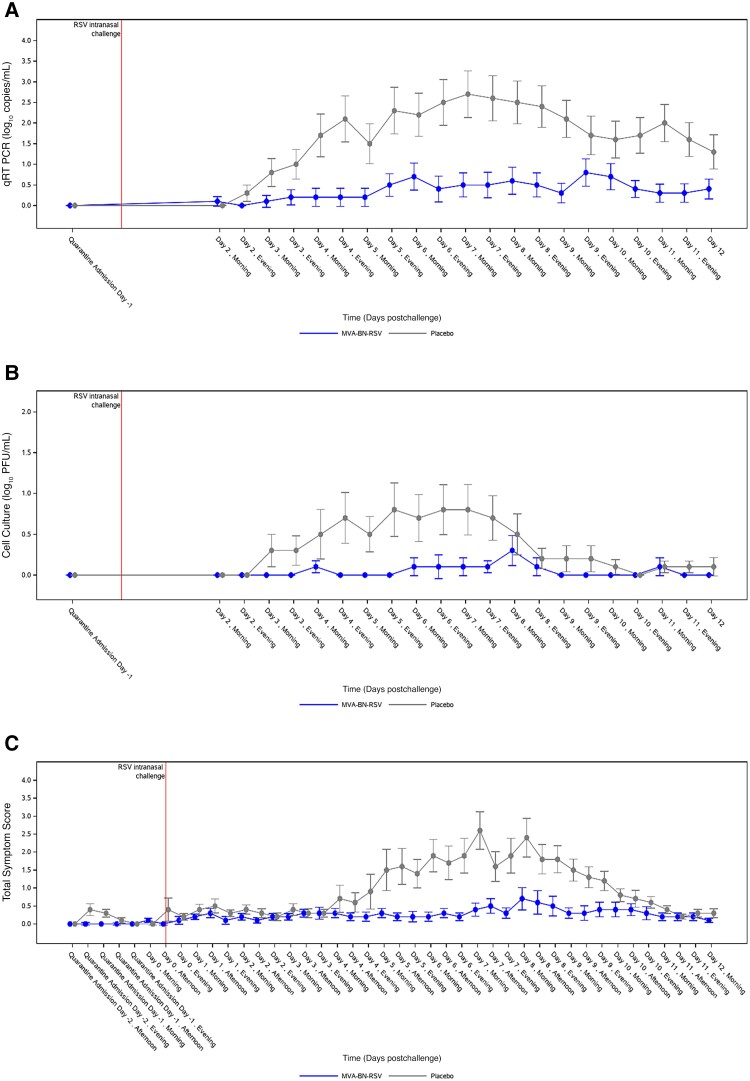
As shown in Table 2, the primary end point of RSV-A Memphis 37b viral load AUC (log10 copies × hour/mL) from nasal washes as determined by qRT-PCR was lower in the MVA-BN-RSV group (median = 0.00, interquartile range 0.00 to 53.44) than in the placebo group (median = 49.05, interquartile range 0.00 to 999.94) (P = .017). Mean viral load over time is shown in Figure 2A; it diverged 2 days after challenge and remained divergent for all of quarantine. Similarly, viral load AUC by quantitative virus culture was lower in the MVA-BN-RSV group (P < .001); Figure 2B shows that this difference occurred primarily from day 3 to day 7. In addition to viral load AUCs, which were measures of disease summed over time, peak viral load measures by qRT-PCR demonstrated less disease acuity in the MVA-BN-RSV group (median = 0.00) than in the placebo group (median = 3.45) (P = .032). Likewise for clinical symptoms, both symptoms summed over time (P = .004; Table 2 and Figure 2C) and peak symptom scores were lower in those vaccinated with MVA-BN-RSV, with symptom scores diverging day 4 through day 9.
Table 2.
RSV-A Memphis 37b Viral Load Area Under the Curve, Sum Total Symptom Score, Incidence of Infection, and Vaccine Efficacy
| Measure | MVA-BN-RSV (N = 30) | Placebo (N = 31) | P Value or Vaccine Efficacy % (95% CI) P Value |
|---|---|---|---|
| Viral load AUC, log10 copies × hour/mL | |||
| Mean, SD | 94.17 (230.76) | 429.61 (513.21) | .017a |
| Median, 95% CIb | 0.00 (0.0–17.4) | 49.05 (0.0–607.5) | |
| Q1, Q3 | 0.00, 53.44 | 0.00, 999.94 | |
| Min, Max | 0.0, 1161.7 | 0.0, 1423.9 | |
| Sum total symptom score | |||
| Mean, SD | 9.87 (20.50) | 34.65 (38.27) | .004a |
| Median, 95% CIc | 2.50 (0.0–5.0) | 27.00 (1.0–44.0) | |
| Q1, Q3 | 0.00, 6.00 | 1.0, 64.0 | |
| Min, Max | 0.0, 81.0 | 0.0, 158.0 | |
| A priori infection end pointsd | |||
| qRT-PCR–confirmed symptomatic RSV infection, definition 1, n (%) | 4 (13.3) | 10 (32.3) | 58.7 (−17.6 to 85.5) .127c |
| qRT-PCR–confirmed symptomatic RSV infection, definition 2, n (%) | 6 (20.0) | 13 (41.9) | 52.3 (−9.0 to 79.1) .097c |
| qRT-PCR–confirmed RSV infection, definition 3, n (%) | 14 (46.7) | 16 (51.6) | 9.6 (−50.9 to 45.8) .799c |
| Culture-confirmed symptomatic RSV infection, definition 1, n (%) | 1 (3.3) | 7 (22.6) | 85.2% (−12.9% to 98.1%) .0529c |
| Culture-confirmed symptomatic RSV infection, definition 2, n (%) | 1 (3.3) | 9 (29.0) | 88.5% (14.8% to 98.5%) .0125c |
| Culture-confirmed RSV infection, definition 3, n (%) | 1 (3.3) | 9 (29.0) | 88.5% (14.8% to 98.5%) .0125c |
| Post hoc infection end points | |||
| Post hoc qRT-PCR symptomatic RSV infection, definition 1, n (%) | 2 (6.7) | 10 (32.3) | 79.3 (13.4 to 95.1) .0217c |
| Post hoc qRT-PCR symptomatic RSV infection, definition 2, n (%) | 3 (10.0) | 13 (41.9) | 76.2 (24.6 to 92.5) .0078c |
| Post hoc qRT-PCR RSV infection, definition 3, n (%) | 7 (23.3) | 15 (48.4) | 51.8 (−1.4 to 77.1) .0620c |
Viral load was measured in nasal washes by quantitative reverse transcription polymerase chain reaction.
Infection definition 1: qRT-PCR–confirmed RSV infection (at least 2 detectable [ ≥ LLOD] qRT-PCR measurements reported on 2 or more consecutive days, starting 2 days after viral challenge [day 2] up to discharge from quarantine) and 1 or more positive clinical symptoms of grade 2 or higher from any category in the symptom scoring system (upper respiratory, lower respiratory, systemic).
Infection definition 2: qRT-PCR–confirmed RSV infection (at least 2 detectable [ ≥ LLOD] qRT-PCR measurements reported on 2 or more consecutive days, starting 2 days after viral challenge [day 2] up to discharge from quarantine) and either 1 or more positive clinical symptoms of any grade from 2 different categories in the symptom scoring system (upper respiratory, lower respiratory, systemic), or 1 grade 2 or higher symptom from any category.
Infection definition 3: qRT-PCR–confirmed RSV infection (at least 2 detectable [ ≥ LLOD] qRT-PCR measurements reported on 2 or more consecutive days, starting 2 days after viral challenge [day 2] up to discharge from quarantine).
Virus culture infection definitions: For each qRT-PCR definition above, a corresponding virus culture definition required at least 2 quantifiable (≥LLOQ) plaque assay results on 2 or more consecutive days.
Post hoc infection definitions: For each a priori definition above, a corresponding post hoc definition required qRT-PCR–confirmed RSV infection defined as at least 2 quantifiable (instead of 2 detectable) qRT-PCR measurements on 2 or more consecutive days.
Analyses performed on the per protocol analysis set.
Abbreviations: AUC, area under the curve; CI, confidence interval; LLOD, lower limit of detection; N, number of participants by group; n, number of participants within each group meeting the specific definition of infection; Q1, Q3, first and third quartiles; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; RSV, respiratory syncytial virus.
One-side P value obtained by the Wilcoxon rank sum test.
Confidence intervals based on binomial probabilities.
Two-sided P values were obtained by Fisher exact test.
Culture-confirmed definitions were specified a priori, but calculation of vaccine efficacy, confidence intervals, and P values were done post hoc.
Figure 2.
Mean viral load and symptoms over time. A, Viral load by qRT-PCR from day 2 to day 12. B, Viral load by quantitative virus culture from day 2 to day 12. C, Symptom score from day −2 to day 12. X-axis values are slightly offset for better readability of the comparison between treatment groups. Analyses performed on the per protocol analysis set. Viral load results that were less than the lower limit of quantitation were arbitrarily set to 0. Error bars show standard error. Abbreviations: PFU, plaque-forming units; PP, per protocol; qRT-PCR, quantitative reverse transcription polymerase chain reaction; RSV, respiratory syncytial virus; TSS, total symptom score.
Incidence of RSV-A Memphis 37b infection was investigated using several definitions, as described in the methods; the results are presented in Table 2. Under the a priori definitions based upon viral load at or above the qRT-PCR LLOD, vaccine efficacy ranged from a low of 9.6% for infection confirmed by laboratory measure only to 58.7% for infection confirmed both by laboratory measure and by the presence of at least 1 RSV symptom of grade ≥2, and the differences in infection incidence between the treatment groups were not statistically significant. When infection was defined instead by viral load ≥ LLOQ, efficacy ranged from 51.8% to 79.3%, and differences in symptomatic infection were statistically significant. Finally, when defined by virus culture results alone, vaccine efficacy was 88.5% (P = .0125); addition of clinical symptoms to the definition did not improve efficacy.
Immunogenicity Results
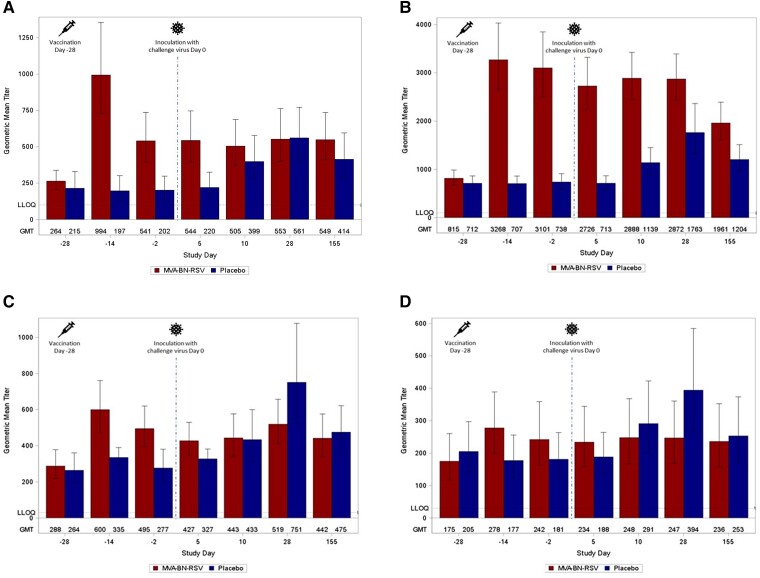
RSV-specific humoral responses are shown in Figure 3A–D and Supplementary Table 2. In the MVA-BN-RSV group, the largest increases in geometric mean titers (GMTs) from baseline to 2 weeks after vaccination were seen in IgA (264.3 to 994.0) and IgG (815.4 to 3268.2); neither immunoglobulin increased in response to challenge. The placebo group experienced smaller increases in response to challenge, which peaked at 28 days in IgA (202.1 to 561.2) and IgG (737.9 to 1762.7). The MVA-BN-RSV group had higher IgG and IgA GMTs than the placebo group at the end of the study, but both groups remained above baseline. For nAbs against RSV-A and RSV-B, the MVA-BN-RSV group increased from baseline to 2 weeks after vaccination (287.7 to 600.2 IU/mL and 174.8 to 277.8 IU/mL, respectively); they had no response to challenge. The placebo group's response from quarantine admission to postchallenge peaked later (day 28) but at higher levels (276.6 to 751.2 IU/mL and 181.0 to 393.9 IU/mL) compared to the MVA-BN-RSV group. Both groups’ nAb titers were greater than baseline at the end of the study.
Figure 3.
RSV-specific antibodies: (A) immunoglobulin A titers; (B) immunoglobulin G titers; (C) RSV-A neutralizing antibody titers; and (D) RSV-B neutralizing antibody titers. Immunoglobulin titers were analyzed by enzyme-linked immunosorbent assay. Neutralizing antibody titers were analyzed by plaque reduction neutralization test, standardized to World Health Organization standard as IU/mL. Day −28 is baseline (measures obtained on the day of and just prior to vaccination), day −14 captures vaccine effects 2 weeks after vaccination, and day −2 is preinoculation. Inoculation occurred on day 0. Days 5, 10, and 28 capture effects after inoculation, and day 155 represents follow-up approximately 6 months after vaccination. The results <LLOQ are considered by using 50% LLOQ for the calculation of statistics for the various antibody types. Error bars represent standard errors. Analyses performed on the per protocol analysis set. Abbreviations: GMT, geometric mean titer; IU, infectious unit; LLOQ, lower limit of quantitation; RSV, respiratory syncytial virus.
For IFN-γ–producing PBMCs (Figure 4, Supplementary Table 3, and Supplementary Figure 1), geometric mean spot-forming units (GMSFUs) increased 2- to 6-fold in response to the various stimulating pools by 7 days after vaccination with MVA-BN-RSV, and the increase was generally larger than that seen in the placebo group at peak (10 days after inoculation with the challenge virus). For the M2-1 pool, GMSFUs increased from 49.0 to 320.5 and remained elevated at 172.9 at study end; for the N pool, GMSFUs increased from 204.1 to 731.0 and remained elevated at 413.2 at study end. By contrast, GMSFUs for the placebo group returned to baseline or just slightly above for all pools. GMSFUs for IL-4–producing PBMCs were low throughout the study (data not shown), suggesting a cellular response favoring Th1 rather than Th2.
Figure 4.
RSV-specific cellular responses. Enumeration of interferon-γ–producing peripheral blood mononuclear cells: (A) pool F; (B) pool G(A); (C) pool M2-1; and (D) pool N. Day −28 is baseline (measures obtained on the day of and just prior to vaccination), day −21 captures vaccine effects 1 week after vaccination, and day −1 is preinoculation. Inoculation occurred on day 0. Days 5, 10, and 28 capture effects after inoculation, and day 155 represents follow-up approximately 6 months after vaccination. Enumeration of interferon-γ–producing peripheral mononuclear blood cells was analyzed by enzyme-linked immunosorbent spot assay. The results <LLOQ are considered by using 50% LLOQ for the calculation of statistics for the various pools. Error bars represent standard errors. Analyses performed on the per protocol analysis set. Abbreviations: GMSFU, geometric mean spot forming units; LLOQ, lower limit of quantitation; RSV, respiratory syncytial virus.
Safety Results
Solicited local AEs (Table 3) were reported in 88.9% of participants receiving MVA-BN-RSV and 37.8% of those receiving placebo; the most common local reaction was injection site pain, and 3 participants reported grade 3 pain. The median duration of pain in the MVA-BN-RSV group was 4.0 days; the maximum was 7 days. Solicited systemic AEs were also common but were reported somewhat more frequently than local reactions by the placebo group as well. Grade 3 fatigue was reported by 4 MVA-BN-RSV recipients, 1 of whom also reported grade 3 myalgia, and by 1 placebo recipient. The median duration of all systemic AEs was 1.0 days. Unsolicited AEs in the vaccination phase (within 29 days postvaccination) were reported by a comparable proportion of participants in the MVA-BN-RSV and placebo groups. Most were considered unrelated to study vaccination, although 1 case of fatigue and increased body temperature in the MVA-BN-RSV group and 1 case of pruritic rash in the placebo group were attributed to vaccination. Unsolicited AEs in the postchallenge phase also were reported by similar proportions of the MVA-BN-RSV and placebo groups. No events were considered related to study vaccination. Two SAEs of myocarditis (1 event of moderate severity in the MVA-BN-RSV group that started 10 days after challenge and 1 event of mild severity in the placebo group that started 7 days after challenge; Table 3) were considered probably related to RSV-A Memphis 37b inoculation. Both were diagnosed by a cardiologist from electrocardiogram and cardiac enzyme findings with no or minimal symptoms and were sent to hospital for observation and follow-up. Tests returned to normal, and the subjects completed follow-up.
Table 3.
Treatment-Emergent Adverse Events
| MVA-BN-RSV | Placebo | |
|---|---|---|
| Events for all vaccinated subjects, adverse events, n (%) [events] | ||
| Vaccinated subjects | N = 36 | N = 37 |
| Solicited local AEs | ||
| Any solicited local AEa | 32 (88.9) [60] | 14 (37.8) [17] |
| Pain | 30 (83.3) [30] | 4 (10.8) [4] |
| Erythema | 16 (44.4) [16] | 8 (21.6) [8] |
| Induration | 7 (19.4) [7] | 0 |
| Swelling | 5 (13.9) [5] | 3 (8.1) [3] |
| Pruritis | 2 (5.6) [2] | 2 (5.4) [2] |
| Any solicited local AE, grade ≥3 | 3 (8.3) [3] | 0 |
| Solicited systemic AEs | ||
| Any solicited systemic AEb | 29 (80.6) [68] | 19 (51.4) [29] |
| Fatigue | 17 (47.2) [17] | 12 (32.4) [12] |
| Myalgia | 21 (58.3) [21] | 4 (10.8) [4] |
| Headache | 15 (41.7) [15] | 9 (24.3) [9] |
| Chills | 8 (22.2) [8] | 1 (2.7) [1] |
| Pyrexia | 6 (16.7) [6] | 0 |
| Nausea | 1 (2.8) [1] | 3 (8.1) [3] |
| Any solicited systemic AE, grade ≥3 | 4 (11.1) [5] | 1 (2.7) [1] |
| Unsolicited AEs, vaccination phase | ||
| Any unsolicited AEc | 7 (19.4) [14] | 5 (13.5) [6] |
| Headache | 2 (5.6) [2] | 2 (5.4) [2] |
| Any unsolicited AE, related to study vaccinationd | 1 (2.8) [2] | 1 (2.7) [1] |
| Any SAE | 0 | 0 |
| Challenged subjects | N = 31 | N = 32 |
| Unsolicited AEs, postchallenge phase | ||
| Any unsolicited AEc | 16 (51.6) [32] | 17 (53.1) [38] |
| Alanine aminotransferase increased | 4 (12.9) [4] | 6 (18.8) [6] |
| Aspartate aminotransferase increased | 2 (6.5) [2] | 3 (9.4) [3] |
| Postprocedural contusion | 3 (9.7) [3] | 3 (9.4) [3] |
| Back pain | 2 (6.5) [3] | 0 |
| Pain in extremity | 0 | 2 (6.3) [2] |
| Headache | 0 | 2 (6.3) [2] |
| Rash maculopapular | 0 | 2 (6.3) [2] |
| Any unsolicited AE, related to study vaccinationd | 0 | 0 |
| Any unsolicited AE, related to study challengee | 8 (22.2%) [9] | 11 (29.7%) [17] |
| Any SAE | 1 (2.8%) [1] | 1 (2.7%) [1] |
| Any SAE, related to study vaccinationd | 0 | 0 |
| Any SAE, related to study challengee | 1 (2.8%) [1] | 1 (2.7%) [1] |
| Cases of myocarditis, characteristics, test results, and medications | ||
| Subjects | N = 1 | N = 1 |
| Age, y | 24 | 29 |
| Sex | Male | Male |
| Day of onset after challenge | 10 | 7 |
| Day of hospitalization after challenge | 11 | 9 |
| Day of discharge after challenge | 13 | 11 |
| Abnormal laboratory tests | Day 10: Troponin I, 327.8 ng/L Troponin T, 52 ng/L Day 11: Troponin I, 548.1 ng/L Troponin T, 103 ng/L ECG, abnormal, clinically significant Day 14: Troponin I, 75.8 ng/L Troponin T, 25 ng/L ECG, normal |
Day 7: Troponin I, 46.2 ng/L ALT, 131 IU/L AST, 76 IU/L Day 8: Troponin I, 156.1 ng/L Troponin T, 33 ng/L Day 9: Troponin I, 216.0 ng/L Troponin T, 39 ng/L ECG, abnormal, clinically significant ECG retest, normal Day 11: ALT, 109 IU/L Phosphate, 1.50 mmol/L ECG, normal |
| Medications | Starting 3 d after onset: colchicine (500 μg, oral, twice daily), lansoprazole (15 mg, oral, once daily), and ibuprofen (400 mg, oral, when required up to 3 times daily) |
Paracetamol (1 g, oral, when required from 0 to 3 d after SAE onset) and ibuprofen (400 mg, oral, when required, from 2 to 4 d after SAE onset) |
| Grade | Moderate | Mild |
| Resolution | Reported as resolved at the first follow-up visit | Reported as resolved at the first follow-up visit |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram; N, number of participants by group that were vaccinated/challenged; n, number of participants in each group reporting the specified adverse event; RSV, respiratory syncytial virus; SAE, serious adverse event.
Local solicited AEs included pain, erythema, swelling, induration, and pruritus on the day of vaccination and for 7 days after vaccination.
Solicited systemic AEs included pyrexia, headache, myalgia, chills, nausea, and fatigue on the day of vaccination and for 7 days after vaccination.
Specific unsolicited AEs are presented for events that occurred in more than one participant in either treatment group.
Related AEs were either considered at least possibly related to study vaccine by the investigator or had missing information pertaining to relatedness.
Related AEs were either considered at least possibly related to study challenge by the investigator or had missing information pertaining to relatedness.
DISCUSSION
RSV remains a ubiquitous infectious disease, and a vaccine against it has been an elusive goal through much of 60 years of research on the immunology of RSV. Human challenge trials are a unique method in clinical RSV research to study the ability of a vaccine to prevent infection under artificial, controlled exposure conditions with a viral strain that causes mild to moderate upper respiratory disease [34, 35]. Human challenge trial results are not definitive, however, as research must confirm vaccine efficacy under conditions of natural infection and in the intended older adult population. Furthermore, phase 3 trials must investigate the prevention of severe disease, not merely the reduction of viral load and symptom scores, although their correlation with disease severity in natural infection has been demonstrated [37, 38].
In this human challenge trial, vaccination with MVA-BN-RSV clearly resulted in lower viral load AUCs and sums of total symptom scores with similar shapes of the curves (Figure 2). The picture with infection prevention, used to measure vaccine efficacy, was somewhat more complex. The vaccine did not prevent detectable qRT-PCR–confirmed infection alone, as there was little difference between the treatment groups in this regard. However, the importance of detectable RSV in the absence of symptoms or positive culture and whether it represents clinically relevant RSV infection is unclear, as qRT-PCR can detect RNA from nonreplicating virus [39]. When infection was defined as the presence of both quantifiable viral load and clinical symptoms, vaccine efficacy was nearly 80%. When the presence of live, replicating virus by culture was examined, only 1 subject who received MVA-BN-RSV had a positive culture; vaccine efficacy estimates were 85% and 89%. All culture-positive subjects in both groups were symptomatic by one or both definitions, confirming the ability of positive culture to denote infection clinically relevant to the participant, not just to disease transmission. Whether the MVA-BN-RSV vaccine can prevent clinical infection or rather protect against infection developing into severe disease will be determined by the phase 3 trial.
As useful as the human challenge model is, it carries known but small risks. Previous experience shows an expected but low frequency of non–life-threatening myocarditis after challenge. Two cases occurred in this study, at 7 and 10 days after challenge. The case timing, as well as experience with the MVA-BN vector, suggest these cases were more likely related to challenge virus than trial vaccination, and with 1 case per group, no meaningful conclusions can be drawn on potential differences in the case characteristics. In the development program of the MVA-BN vector, which included more than 10 000 volunteers [22–27], only 1 doubtful case of pericarditis [22] was reported. During the mpox vaccination campaigns, inflammatory cardiac disorders were reported at a frequency of <1:100 000 doses, roughly in line with expected background incidence [40]. Also of note, severity and duration of reactogenicity was in line with previous research using MVA-BN and MVA-BN-RSV [22, 41].
Regarding a further challenge inherent in this study design, it is noteworthy that even in the placebo group, less than half of subjects had quantifiable virus by qRT-PCR, and less than a quarter had quantifiable culture results, despite eligibility criteria intended to select for susceptibility to RSV infection. This, along with a small sample size, made it more difficult to detect differences between the treatment groups, and vaccine efficacy confidence intervals are wide.
Scientific understanding of how immune response associates with prevention of either infection or severe disease is limited. Neutralizing antibodies to the F and G proteins are often used as a primary measure of immune response, as higher titers have been associated with reduction in disease [42, 43]. Results from this study provide evidence that protective immune responses to RSV go beyond nAbs, as nAb GMTs following vaccination were lower than observed elsewhere [14, 15], but high vaccine efficacy was still observed. This may be attributable to robust serum IgA and to cellular responses that were observed for all peptide pools, particularly GMSFUs that remained elevated over baseline for M2-1, N, G(A), and G(B).
The MVA-BN-RSV vaccine appears to represent a mode of action broader than other vaccine candidates focused on the production of neutralizing antibodies to the preF protein. Dependence of RSV vaccines on the activity of neutralizing antibodies against a specific epitope of one protein conformation may be risky, as such reliance may provide selective pressure for the development of mutant viruses capable of neutralizing antibody escape [44, 45]. In fact, the monoclonal antibody suptavumab failed in a phase 3 clinical trial to reduce RSV hospitalizations or lower respiratory tract infections in infants, a result attributed to epitope mutations found on circulating RSV-B strains [45]. Having a vaccine that provides multiple targets for both humoral and cellular responses may protect against the consequences of such genetic pressure.
CONCLUSIONS
MVA-BN-RSV vaccination resulted in significantly lower viral load AUC by qRT-PCR after challenge with RSV-A Memphis 37b compared to placebo. Vaccination also resulted in fewer infectious virus particles by culture, lower symptom scores, and vaccine efficacy in the range of 79.3% to 88.5% against infection after challenge confirmed by symptoms and quantifiable viral load measures or by positive cultures. Humoral and cellular responses support broad immunogenicity of the vaccine. Injection site pain was the most common adverse event. Phase 3 evaluation of MVA-BN-RSV to determine clinical efficacy in an older adult population has commenced (NCT05238025).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Elke Jordan, Bavarian Nordic, GmbH, Martinsried, Germany.
Golam Kabir, hVIVO, PLC, London, United Kingdom.
Stephanie Schultz, Bavarian Nordic, GmbH, Martinsried, Germany.
Günter Silbernagl, Bavarian Nordic, GmbH, Martinsried, Germany.
Darja Schmidt, Bavarian Nordic, GmbH, Martinsried, Germany.
Victoria A Jenkins, Bavarian Nordic, AG, Zug, Switzerland.
Heinz Weidenthaler, Bavarian Nordic, GmbH, Martinsried, Germany.
Daria Stroukova, Bavarian Nordic, GmbH, Martinsried, Germany.
Barbara K Martin, Bavarian Nordic, Inc, Durham, North Carolina, USA.
Laurence De Moerlooze, Bavarian Nordic, AG, Zug, Switzerland.
Notes
Acknowledgments. We say a special thank you to all the participants in this RSV challenge trial. Also, thanks to Bavarian Nordic colleagues and hVIVO clinical trial site investigators and personnel for their support during the conduct of the trial.
Financial support. This work was supported by Bavarian Nordic A/S. Funding to pay the Open Access publication charges for this article was provided by Bavarian Nordic as well.
References
- 1. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399:2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222(Suppl 7):S577–83. [DOI] [PubMed] [Google Scholar]
- 4. Savic M, Penders Y, Shi T, Branche A, Pirçon J-Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses 2023; 17:e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt H, Das A, Nam H, Yang A, Ison MG. Epidemiology and outcomes of hospitalized adults with respiratory syncytial virus: a 6-year retrospective study. Influenza Other Respir Viruses 2019; 13:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volling C, Hassan K, Mazzulli T, et al. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis 2014; 14:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969; 89:449–63. [DOI] [PubMed] [Google Scholar]
- 9. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of an antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. [DOI] [PubMed] [Google Scholar]
- 10. Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated virus vaccine. Am J Epidemiol 1969; 89:405–21. [DOI] [PubMed] [Google Scholar]
- 11. Polack FP, Teng MN, Collins PL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med 2002; 196:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falloon J, Yu J, Esser MT, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Novavax . Novavax announces topline RSV F vaccine data from two clinical trials in older adults. https://ir.novavax.com/2016-09-25-Novavax-Announces-Topline-RSV-F-Vaccine-Data-from-Two-Clinical-Trials-in-Older-Adults. Accessed 10 November 2022.
- 14. Sadoff J, Paepe ED, DeVincenzo J, et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J Infect Dis 2021; 226:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmoele-Thoma B, Zareba AM, Jiang Q, et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N Engl J Med 2022; 386:2377–86. [DOI] [PubMed] [Google Scholar]
- 16. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388:595–608. [DOI] [PubMed] [Google Scholar]
- 17. Moderna . Moderna announces mRNA-1345, an investigational respiratory syncytial virus (RSV) vaccine, has met primary efficacy endpoints in phase 3 trial in older adults, 2023. https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx. Accessed 23 February 2023.
- 18. Pfizer . Pfizer announces positive top-line data from phase 3 trial of older adults for its bivalent respiratory syncytial virus (RSV) vaccine candidate, 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-trial-older. Accessed 10 November 2022.
- 19. Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res 2007; 39:225–39. [DOI] [PubMed] [Google Scholar]
- 20. Acosta PL, Caballero MT, Polack FP. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol 2016; 23:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kriss JL, Boersma PM, Martin E, et al. Receipt of first and second doses of JYNNEOS vaccine for prevention of monkeypox—United States, May 22–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Overton ET, Lawrence SJ, Wagner E, et al. Immunogenicity and safety of three consecutive production lots of the non replicating smallpox vaccine MVA: a randomised, double blind, placebo controlled phase III trial. PLoS One 2018; 13:e0195897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vollmar J, Arndtz N, Eckl KM, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 2006; 24:2065–70. [DOI] [PubMed] [Google Scholar]
- 24. Walsh SR, Wilck MB, Dominguez DJ, et al. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J Infect Dis 2013; 207:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18–40 year old subjects with diagnosed atopic dermatitis. PLoS One 2015; 10:e0138348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darsow U, Sbornik M, Rombold S, et al. Long-term safety of replication-defective smallpox vaccine (MVA-BN) in atopic eczema and allergic rhinitis. J Eur Acad Dermatol Venereol 2016; 30:1971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Overton ET, Lawrence SJ, Stapleton JT, et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine 2020; 38:2600–7. [DOI] [PubMed] [Google Scholar]
- 28. Nyombayire J, Ingabire R, Magod B, et al. Monitoring of adverse events in recipients of the 2-dose Ebola vaccine regimen of AdZEBOV followed by MVA-BN-filo in the UMURINZI Ebola vaccination campaign. J Infect Dis 2023; 227:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Endt K, Wollmann Y, Haug J, et al. A recombinant MVA-based RSV vaccine induces T-cell and antibody responses that cooperate in the protection against RSV infection. Front Immunol 2022; 13:841471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samy N, Reichhardt D, Schmidt D, et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: a randomized phase I clinical trial. Vaccine 2020; 38:2608–19. [DOI] [PubMed] [Google Scholar]
- 31. Jordan E, Lawrence SJ, Meyer TPH, et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J Infect Dis 2021; 223:1062–72. [DOI] [PubMed] [Google Scholar]
- 32. Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163:693–8. [DOI] [PubMed] [Google Scholar]
- 33. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30:481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lambkin-Williams R, Noulin N, Mann A, Catchpole A, Gilbert AS. The human viral challenge model: accelerating the evaluation of respiratory antivirals, vaccines and novel diagnostics. Respir Res 2018; 19:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim Y-I, DeVincenzo JP, Jones BG, et al. Respiratory syncytial virus human experimental infection model: provenance, production, and sequence of low-passaged Memphis-37 challenge virus. PLoS One 2014; 9:e113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raghunandan R, Higgins D, Hosken N. RSV Neutralization assays—use in immune response assessment. Vaccine 2021; 39:4591–7. [DOI] [PubMed] [Google Scholar]
- 37. DeVincenzo JP, Wilkinson T, Vaishnaw A, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010; 182:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee N, Chan MCW, Lui GCY, et al. High viral load and respiratory failure in adults hospitalized for respiratory syncytial virus infections. J Infect Dis 2015; 212:1237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boukhvalova MS, Yim KC, Prince GA, Blanco JCG. Methods for monitoring dynamics of pulmonary RSV replication by viral culture and by real-time reverse transcription-PCR in vivo: detection of abortive viral replication. Curr Protoc Cell Biol 2010; Chapter 26:Unit26.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duffy J, Marquez P, Moro P, et al. Safety monitoring of JYNNEOS vaccine during the 2022 mpox outbreak—United States, May 22–October 21, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Volkmann A, Williamson A-L, Weidenthaler H, et al. The Brighton collaboration standardized template for collection of key information for risk/benefit assessment of a modified vaccinia Ankara (MVA) vaccine platform. Vaccine 2021; 39:3067–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998; 177:463–6. [DOI] [PubMed] [Google Scholar]
- 43. Edward E W, Derick R P, Ann R F. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. [DOI] [PubMed] [Google Scholar]
- 44. Mas V, Nair H, Campbell H, Melero JA, Williams TC. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine 2018; 36:6660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simões EAF, Forleo-Neto E, Geba GP, et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin Infect Dis 2021; 73:e4400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.