Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is responsible for an array of problematic community- and healthcare-acquired infections, including pneumonia, and is frequently associated with severe disease and high mortality rates. Standard recommended treatments for empiric and targeted coverage of suspected MRSA in patients with community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), are vancomycin and linezolid. However, adverse events such as acute kidney injury and Clostridium difficile infection have been associated with these antibiotics. Ceftaroline fosamil is a β-lactam/extended-spectrum cephalosporin approved for the treatment of adults and children with CAP and complicated skin and soft tissue infections. Ceftaroline has in vitro activity against a range of common Gram-positive bacteria and is distinct among the β-lactams in retaining activity against MRSA. Due to the design of the pivotal randomised controlled trials of ceftaroline fosamil, outcomes in patients with MRSA CAP were not evaluated. However, various reports of real-world outcomes with ceftaroline fosamil for pneumonia caused by MRSA, including CAP and HAP/VAP, been published since its approval. A systematic literature review and qualitative analysis of relevant publications was undertaken to collate and summarise relevant published data on the efficacy and safety of ceftaroline fosamil in patients with MRSA pneumonia. While relatively few real-world outcomes studies are available, the available data suggest that ceftaroline fosamil is a possible alternative to linezolid and vancomycin for MRSA pneumonia. Specific scenarios in which ceftaroline fosamil might be considered include bacteraemia and complicating factors such as empyema.
Tweetable abstract
Ceftaroline fosamil has an established clinical profile for treatment of community-acquired pneumonia. While RCT data in patients with MRSA pneumonia are lacking, studies suggest ceftaroline fosamil is a possible alternative to linezolid and vancomycin. https://bit.ly/44F4Gzw
Introduction
Staphylococcus aureus is a ubiquitous commensal and opportunistic Gram-positive bacterial pathogen. It is responsible for numerous community- and hospital-acquired infections, including bloodstream, respiratory, skin and soft tissue, and cardiac valve and device/prosthetic infections, which range in severity from mild to potentially life threatening [1–3]. Methicillin-resistant S. aureus (MRSA) constitute a group of S. aureus strains that have developed resistance to β-lactam antibiotics (a cornerstone of treatment for a range of infections, including many of those caused by methicillin-susceptible S. aureus (MSSA)) and in many cases to various other antimicrobial classes (some MRSA are also classed as multidrug resistant (MDR)) [4, 5]. Some variants of S. aureus express virulence factors that adversely influence host responses to infection and the species can form biofilms that are difficult to eradicate, complicating the course of treatment [3, 6, 7].
Although S. aureus accounts for a small proportion of all bacterial causes of pneumonia (∼3–5%), MRSA pneumonia, whether originating in community (community-acquired pneumonia (CAP)) or hospital/healthcare (hospital-acquired pneumonia (HAP)) settings and/or associated with intubation and mechanical ventilation (ventilator-associated pneumonia (VAP)), is typically severe and frequently fatal [8–10]. MRSA pneumonia can arise as primary or secondary to another infectious source, and is more common in vulnerable/frail patients and those with chronic comorbidities. Antimicrobial usage and reported resistance rates have increased worldwide since the start of the severe acute respiratory syndrome coronavirus 2 pandemic [11–14] and MRSA has been reported to be among the most frequent pulmonary bacterial co-infections in patients with coronavirus disease 2019 [15].
Ceftaroline fosamil is a broad-spectrum cephalosporin with in vitro activity against a range of common CAP pathogens, including MSSA, MRSA, Streptococcus pneumoniae and non-β-lactamase-producing Enterobacterales [16]. It has an established clinical profile in adults and children with CAP and complicated skin and soft tissue infections (cSSTIs) [17–24]. Ceftaroline fosamil has been shown to be superior to ceftriaxone, a standard-of-care treatment, in a meta-analysis of three randomised controlled trials (RCTs) in adults with (non-MRSA) CAP [25]. Various reports of real-world outcomes with ceftaroline fosamil for pneumonia caused by MRSA have been published since its approval. A systematic literature review and qualitative analysis of relevant publications was undertaken to collate and summarise relevant published data.
Management strategies for MRSA pneumonia
The most frequent symptoms of pneumonia include fever, cough, chest pain and dyspnoea, and in severe cases complications such as acute respiratory distress syndrome, multiple organ failure and septic shock are present, which can require treatment in an intensive care unit (ICU). Management strategies for both CAP and HAP/VAP, including initial therapy selection, are guided by disease severity and risk factors for involvement of resistant pathogens based on local surveillance and susceptibility patterns [26, 27]. There is also increasing recognition of a role for rapid diagnostics in supporting treatment decisions [15, 26, 28].
International guidelines relevant to the diagnosis and management of MRSA pneumonia include recommendations for CAP from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) [26, 29] and the European Respiratory Society/European Society for Clinical Microbiology and Infectious Diseases [30], and European/Latin American and US-focused guidelines for HAP/VAP [27, 31]. Antimicrobial therapy for pneumonia is typically administered empirically with the aim of de-escalation supported by microbiological culture data [31]. Rapid selection and initiation of optimal empiric therapy for any given patient is a key clinical judgement; delayed and/or inadequate empiric therapy is associated with adverse clinical outcomes, including increased rates of treatment failure and mortality and increased healthcare costs [32]. Initial therapy selection for pneumonia is therefore guided by patient risk assessment and local/regional resistance patterns [26, 27, 31].
As MRSA requires different antimicrobial coverage to the standard treatments for CAP and HAP/VAP, there is a need to quickly determine the risk of MRSA involvement so that coverage for MRSA can be included in empiric therapy if required. While various risk evaluation strategies have been evaluated, the “PES” score (denoting Pseudomonas aeruginosa, extended-spectrum β-lactamase-producing Enterobacterales and MRSA) is recommended to guide empiric coverage of these problem pathogens [33, 34].
For patients with CAP, clinical features at admission suggestive of suspected S. aureus involvement include concurrent influenza infection, haemoptysis, multilobar infiltrates and neutropenia [2, 35]. Prior isolation of MRSA from a respiratory sample is considered a risk factor for MRSA which should prompt the inclusion of anti-MRSA coverage in the initial antimicrobial treatment regimen [26]. Severe CAP according to the IDSA/ATS guidelines is defined by the presence of two major criteria: the need for invasive mechanical ventilation due to severe acute respiratory failure and/or the presence of septic shock, or the presence of three or more minor criteria [36, 37]. For patients with HAP/VAP, prior antimicrobial therapy within 90 days is considered a risk factor for MRSA involvement. Other risk factors for MRSA in patients with HAP/VAP include age, onset >6 days after admittance, respiratory infection/colonisation caused by MRSA in the previous year and recent exposure to fluoroquinolone or antibiotics treating Gram-positive organisms [27, 38].
The current international HAP/VAP guidelines generally recommend linezolid or vancomycin for first-line empiric MRSA coverage [27, 31]. However, use of these agents in the empiric setting for patients with pneumonia has been associated with various adverse outcomes, including acute kidney injury, Clostridium difficile infection, and the emergence of vancomycin-resistant Enterococcus spp. and secondary Gram-negative rod detection [39]. Ceftaroline fosamil is noted as a recommended treatment for non-severe CAP (in combination with a macrolide or doxycycline) in the most recent IDSA/ATS CAP recommendations and is mentioned as a potential treatment for MRSA coverage in the most recent IDSA/ATS HAP/VAP guidance [26, 27]. Various other antimicrobial therapies for MRSA are available (some of which have potential roles in the treatment of pneumonia), including ceftobiprole (approved in some regions but not in the USA), teicoplanin, azithromycin, clindamycin, fluoroquinolones (levofloxacin and moxifloxacin) and tedizolid [7]. Daptomycin is not effective in pneumonia due to its inactivation by lung surfactant.
Limitations of vancomycin include the need for therapeutic drug monitoring (dosing adjusted based on trough serum concentrations), poor lung tissue penetration, as well as emergence of vancomycin resistance and adverse effects, including potential nephrotoxicity; there is some uncertainty if vancomycin can provide optimal treatment for MRSA pneumonia [40, 41]. Linezolid offers the potential advantage of oral as well as intravenous formulations (with potential for improving patients to be discharged earlier), but its safety profile includes risk of drug interactions with some common classes of medicines, and for patients with MRSA nosocomial pneumonia it is considered to increase the risk of thrombocytopenia and gastrointestinal events compared with the glycopeptides vancomycin and teicoplanin [42].
Ceftaroline fosamil for treatment of MRSA pneumonia
Ceftaroline fosamil is a β-lactam/extended-spectrum cephalosporin which was first commercialised in 2010 and is now approved in multiple countries worldwide for the treatment of adults and children with CAP or cSSTI, including that caused by S. aureus [43, 44]. Ceftaroline fosamil (pro-drug) is rapidly converted to active ceftaroline upon i.v. administration; two dosage regimens (“standard dose” of 600 mg every 12 h and “high dose” of 600 mg every 8 h) have been evaluated in phase II–IV clinical trials (doses are adjusted for patients with estimated creatinine clearance <50 mL·min−1), with the high dose approved (excluding in the USA) for cSSTI due to MRSA with ceftaroline minimum inhibitory concentration (MIC) of 1 or 2 mg·L−1 [43, 44]. The in vitro spectrum of activity of ceftaroline includes MRSA (as well as MSSA), other common Gram-positive pathogens and non-β-lactamase-producing Enterobacterales; however, due to the design of the pivotal clinical trials (specifically, the comparator ceftriaxone is not active against MRSA), efficacy was not assessed in patients with MRSA CAP [19, 20, 22, 25].
β-lactams target bacterial penicillin-binding proteins (PBPs) and thereby disrupt cell wall synthesis. In S. aureus, acquisition of an alternative PBP (PBP2) which has low affinity for most β-lactams confers methicillin resistance [6]. Unlike most β-lactams, ceftaroline targets PBP2 with high affinity [45–47], which results in activity against both MSSA and MRSA, including vancomycin-resistant variants [48]. International antimicrobial surveillance studies covering various regions from 2008 to 2020 have consistently reported that ceftaroline fosamil demonstrates potent in vitro activity against both MSSA and MRSA (including MDR) strains from patients with pneumonia, albeit with some regional variations in MRSA susceptibility (table 1).
TABLE 1.
In vitro activity of ceftaroline against Staphylococcus aureus from national and multinational surveillance studies
| Region (isolates) | MIC data, mg·L−1 | CLSI MIC interpretation, % | EUCAST MIC interpretation, % | ||||||
| MIC50 | MIC90 | MIC range | S | SDD | R | S | I | R | |
| All regions, 2012–2017 [ 71 ] # | |||||||||
| MSSA (n=25 208) | 0.25 | 0.25 | 0.015–2 | >99.9 | <0.01 | 0 | >99.9 | <0.01 | 0 |
| MRSA (n=35 837) | 0.5 | 2 | 0.015–64 | 89.3 | 10.0 | 0.7 | 89.3 | 10.0 | 0.7 |
| USA, 2008–2010 [ 72 ] ¶ | |||||||||
| MSSA (n=4016) | 0.25 | 0.25 | ≤0.008–1 | 100 | 0 | NR | NR | ||
| MRSA (n=4453) | 1 | 1 | 0.12–2 | 96.1 | 3.9 | 0 | NR | NR | |
| MDR (n=21)+ | 1 | 2 | 0.5–2 | 85.7 | 14.3 | 0 | NR | NR | |
| USA, 2010–2016 [ 73 ] § | NR | NR | |||||||
| MSSA (n=11 377) | 0.25 | 0.25 | NR | 100 | 0 | 100 | 0 | ||
| MRSA (n=9679) | 0.5 | 1 | NR | 97.2 | <0.1 | 97.2 | 2.8 | ||
| USA, 2018–2020 [ 74 ] ƒ | |||||||||
| MSSA (n=5831) | 0.25 | 0.25 | ≤0.06–0.5 | 100 | 0 | 100 | 0 | ||
| MRSA (n=3887) | 1 | 1 | 0.12–2 | 93.4 | 6.6 | 0 | 93.4 | 6.6 | 0 |
| China, 2018 [ 75 ] # | |||||||||
| MSSA (n=251) | 0.5 | 0.5 | 0.12–1 | 100 | 0 | NR | NR | ||
| MRSA (n=155) | 1 | 2 | ≤0.06–2 | 83.9 | 16.1 | NR | NR | ||
| Middle East and Africa, 2015–2018 [ 76 ] ƒ | |||||||||
| MSSA (n=313) | 0.25 | 0.25 | 0.12–0.5 | 100 | 0 | 100 | 0 | ||
| MRSA (n=293) | 1 | 2 | 0.25–2 | 88.1 | 0 | 88.1 | 11.9 | ||
MIC: minimum inhibitory concentration; CLSI: Clinical and Laboratory Standards Institute; EUCAST: European Committee on Antimicrobial Susceptibility Testing; S: susceptible; SDD: susceptible dose-dependent; R: resistant; I: intermediate; MSSA: methicillin-susceptible S. aureus; MRSA: methicillin-resistant S. aureus; MDR: multidrug resistant; NR: not reported. Susceptibility interpretation based on ceftaroline fosamil 600 mg 12 h dosing and EUCAST MIC breakpoints for S. aureus infections other than pneumonia [50, 51]. #: isolates from hospitalised patients with various infection types (proportion of respiratory isolates NR); ¶: 7.5% of isolates from patients with pneumonia; +: MDR defined as resistance to oxacillin, erythromycin, clindamycin, levofloxacin, tetracycline and trimethoprim–sulfamethoxazole; §: 24% of isolates from patients with pneumonia; ƒ: all isolates from patients with lower respiratory tract infections.
Data from in vitro and in vivo studies indicate that ceftaroline is pharmacodynamically active against diverse strains of S. aureus, including MSSA and MRSA (table 2). These pre-clinical studies have established pharmacokinetic/pharmacodynamic (PK/PD) targets for ceftaroline against S. aureus, which along with population PK modelling using a large database of PK samples from the ceftaroline clinical trial programme have been used to support probability of target attainment simulations to evaluate expected efficacy against the full range of pathogen MICs likely to be encountered in clinical practice (table 3), as well as to establish interpretative criteria (breakpoints) for relevant pathogens. Importantly for the treatment of pneumonia, it has been established that ceftaroline achieves lung penetration similar to that of other β-lactams [49].
TABLE 2.
Pharmacodynamics of ceftaroline against Staphylococcus aureus: in vitro and pre-clinical studies
| First author, year [ref.] | Design | Isolates | %fT required for stasis | %fT required for 1 log10 bacterial CFU reduction |
| Andes, 2006 [ 77, 78 ] | Mouse neutropenic thigh | 3 MSSA, 1 MRSA; ceftaroline MICs: 0.12–1 mg·L−1 |
26±8 | NR |
| Bhalodi, 2012 [ 79 ] | Neutropenic murine lung model, human-simulated ceftaroline fosamil regimen of 600 mg every 12 h | 2 MSSA, 15 MRSA; ceftaroline MICs: 0.5–4 mg·L−1 |
16 | 41 |
| Keel, 2011 [ 80 ] | Mouse neutropenic thigh | 4 MSSA, 22 MRSA; ceftaroline MICs: 0.125–4 mg·L−1 |
NR | 19.3 |
| Macgowan, 2013 [ 81 ] | Single-compartment dilutional pharmacokinetic model | 3 MSSA, 9 MRSA; ceftaroline MICs: 0.12–1 mg·L−1 |
24.5±8.9 | 27.8±9.5 |
| Singh, 2017 [ 82 ] | In vitro hollow fibre infection model using every 8 h ceftaroline dosing | 12 MRSA; ceftaroline MICs: 2–8 mg·L−1 |
28.7±6.4 | 31.8±5.8 |
CFU: colony-forming unit; fT: free drug time; MSSA: methicillin-susceptible S. aureus; MRSA: methicillin-resistant S. aureus; NR: not reported.
TABLE 3.
Pharmacodynamics of ceftaroline in pneumonia: clinical pharmacology and population pharmacokinetic (PK) modelling studies
| First author, year [ref.] | Design | Subjects | Main results |
| Riccobene, 2016 [ 49 ] | Clinical pharmacology with lung and plasma PK sampling and PK modelling and simulation | Healthy adults (n=53) | At 600 mg every 12 h and a MIC of 1 mg·L−1, 98.1% of simulated patients were predicted to achieve fT>MIC in plasma of 42% and 81.7% were predicted to achieve fT>MIC in ELF of 17%. At 600 mg every 8 h, 100% and 95% were predicted to achieve the respective plasma and ELF targets. |
| Li, 2019 [ 83 ] | Population PK modelling and simulation | Overall (n=533); healthy adults (n=221); patients with cSSTI or CAP (n=312) | Race was not a significant covariate impacting ceftaroline PK, suggesting similar ceftaroline PK in Asian and Western populations. PTAs of 90–100% were predicted for Asian patients with CAP treated with ceftaroline fosamil at MIC90 values of target CAP pathogens from the Asia-Pacific region. >90% PTAs were predicted at respective EUCAST and CLSI clinical MIC breakpoints, including S. aureus. |
| Cristinacce, 2019 [ 84 ] | Population PK modelling and simulation | Overall (n=951); healthy adults (n=267); patients with cSSTI or CAP (n=312) | Ceftaroline fosamil demonstrated higher overall PTA rates than levofloxacin and ceftriaxone, in particular against S. aureus. |
MIC: minimum inhibitory concentration; fT: free drug time; ELF: epithelial lining fluid; cSSTI: complicated skin and soft tissue infection; CAP: community-acquired pneumonia; PTA: probability of target attainment; EUCAST: European Committee on Antimicrobial Susceptibility Testing; CLSI: Clinical and Laboratory Standards Institute; S. aureus: Staphylococcus aureus.
MIC breakpoints for ceftaroline against S. aureus (including MRSA) from infections other than pneumonia have been established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (susceptible ≤1 mg·L−1; intermediate 2 mg·L−1; resistant >2 mg·L−1). For S. aureus from pneumonia, the respective susceptible and resistant breakpoints are ≤1 and >1 mg·L−1 (EUCAST guidance notes that ceftaroline resistance in S. aureus is rare) [50]. Unlike EUCAST, the Clinical and Laboratory Standards Institute (CLSI) MIC breakpoints for ceftaroline against S. aureus (susceptible ≤1 mg·L−1; susceptible dose-dependent 2–4 mg·L−1; resistant ≥8 mg·L−1) do not currently differentiate pneumonia versus other isolate sources [51]. The international surveillance data (table 1) indicate that ceftaroline susceptibility among MRSA, whether based on EUCAST or CLSI interpretations, remains high (albeit with some regional variability) following more than a decade of commercial availability of ceftaroline fosamil.
While it has not been evaluated in RCTs in patients with MRSA pneumonia, in three pivotal RCTs in hospitalised adults with moderate or severe (non-MRSA) CAP, ceftaroline fosamil was shown to be non-inferior to ceftriaxone [19, 20, 22], and in a meta-analysis of the three RCTs, it was found to be superior to ceftriaxone [25]. Among patients with confirmed baseline pathogens, pooled clinical response rates numerically favoured ceftaroline fosamil over ceftriaxone in various subgroups, including in patients with Gram-positive (including S. aureus and S. pneumoniae) or Gram-negative (including Escherichia coli, Haemophilus influenzae, Haemophilus parainfluenzae and Klebsiella pneumoniae) baseline pathogens and in patients with mono- or polymicrobial infections [25]. These clinical results are consistent with the drug's documented in vitro activity and PK/PD profile (tables 1–3), and collectively the data provide a strong rationale for consideration of ceftaroline fosamil as a potential treatment in MRSA pneumonia.
Literature search
Overview
A systematic literature review was performed to identify real-world studies reporting the use of ceftaroline fosamil treatment and outcomes in patients with MRSA pneumonia. Literature review and data extraction was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) 2020 statement [52]. PubMed was used as the main source database for literature searches.
Data extraction
Search results were systematically screened to obtain a list of publications to be considered for inclusion in the review. Included publications were in English language and reported data for adults with a diagnosis of pneumonia with MRSA confirmed or suspected as the causative pathogen treated with ceftaroline fosamil. All subtypes of pneumonia (e.g. CAP, HAP and VAP) were included. Publications considered suitable for inclusion included case reports/case series, observational and case–control studies, and RCTs. Literature reviews and meta-analyses were checked for relevant source references. Extracted data included (where available) baseline patient characteristics, ceftaroline fosamil dose and treatment duration, and reported treatment outcomes. For studies that included data for patients other than those with MRSA pneumonia, data for the subset of patients with MRSA pneumonia were extracted where available. Publication types excluded from the analysis included in vitro/non-human studies, review articles and meta-analyses based solely on previously published data, clinical pharmacology and PK/PD studies in humans that did not report clinical outcomes, and RCTs that did not include patients with MRSA pneumonia.
Search results
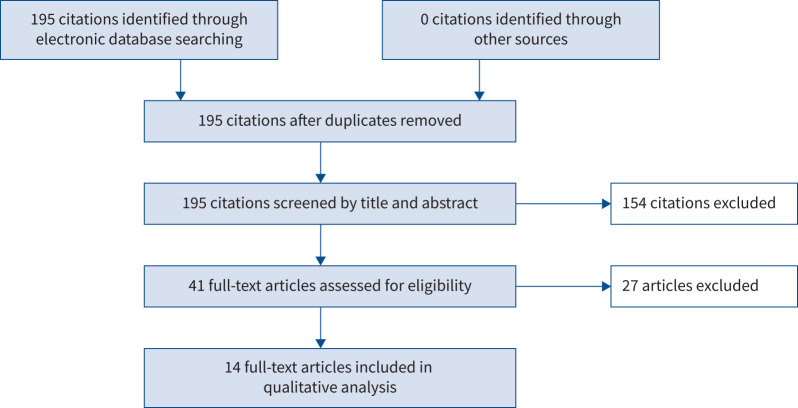
The results of the search and data extraction process are shown in figure 1. As of 10 January 2023, there were 541 results for the search “ceftaroline AND MRSA” and 195 results for the search “ceftaroline AND MRSA AND pneumonia”. In total, 14 publications were identified for data extraction and inclusion in the qualitative analysis, ranging in publication date from 2012 to 2021 [53–66] (table 4). Most (12 out of 14) publications were by US authors, with the remaining two publications by European authors. Of note, some publications reported the use of ceftaroline fosamil doses and/or duration of treatment that do not reflect current approved labelling. Across the 14 included publications, a total of 1908 patients were treated with ceftaroline fosamil and 264 patients were reported to have MRSA pneumonia or MRSA isolated from a lower respiratory tract source.
FIGURE 1.
Overview of literature search and data extraction.
TABLE 4.
Summary of real-world studies patients with methicillin-resistant Staphylococcus aureus (MRSA) pneumonia treated with ceftaroline fosamil
| First author, year [ref.] | Design and setting | Baseline and treatment characteristics | Prior/concomitant antibiotics | Ceftaroline fosamil treatment duration | Main results |
| Giacobbe, 2021 [64] | Single-centre cross-sectional study, large teaching hospital, Italy, 2019 | 200 patients, median age 66 years, 72% male, of whom 165 (83%) had COVID-19 | All 165 COVID-19 patients received empirical ceftaroline fosamil; in patients without COVID-19, ceftaroline fosamil was used as salvage therapy in 75% of cases; 7 patients (3%) received combination therapy | NR | MRSA identified in 4/15 patients (27%) with CAP; no discontinuation of ceftaroline due to treatment-emergent adverse events; outcome data NR for patients with MRSA pneumonia |
| Bassetti, 2020 [ 53 ] | Retrospective observational study, two hospitals in Spain and Italy, 2017–2018 | 89 patients with severe CAP treated with ceftaroline fosamil, including 9 patients with MRSA CAP; mean age 63 years, 66% male | 53 cases (60%) treated in combination with other antibiotics | NR | 30-day mortality and clinical success occurred in 18/89 (20%) and 57/89 (64%) patients, respectively; independent predictors of 30-day mortality were: increasing age, presence of solid neoplasm and concomitant therapy with oseltamivir; 5/9 patients (56%) with MRSA infection achieved clinical success |
| Karki, 2017 [ 54 ] | Retrospective case series, US community hospital, 2014–2016 | 31 patients with MRSA CAP (n=25) or HCAP (n=6) treated with ceftaroline fosamil for ≥7 days; 61% required mechanical ventilation and 65% were treated in the ICU | 7 patients (23%) received concurrent anti-MRSA therapy (ceftaroline fosamil in these patients was added on when there was no clinical improvement on other MRSA therapy) | NR | Clinical success was achieved in 19/31 patients (62%), treatment failure occurred in 6/31 patients (19%); 5 patients (16%) had indeterminate outcomes; 6/31 patients (19%) died, of which 5 were related to the index infection; 6/11 patients (55%) with concomitant bacteraemia achieved clinical success; 5/7 patients (71%) treated with concurrent therapy achieved clinical success |
| Zasowski, 2017 [55] | Retrospective, multicentre US observational study, 2011–2015 | 126 patients received ceftaroline fosamil for 72 h for MRSA BSI, including 41 patients with LRTI source; overall median age 59 years; 45 patients (36%) were admitted to the ICU | 107 patients (85%) received prior vancomycin and 48 patients (38%) received prior daptomycin; 37 patients (29%) received concomitant antibiotics with ceftaroline fosamil | Median (IQR): 11 (5–15) days | Clinical success was achieved in 86/126 patients (68%) overall and in 22/41 patients (54%) with LRTI source; APACHE II score and malignancy were independent predictors of treatment failure |
| Guervil, 2016 [ 65 ] | Retrospective chart review, US hospitals, 2011–2013 | 396 patients with CAP treated with ceftaroline fosamil as either first-line (n=67) or second-line (n=329) treatment; MRSA was isolated from 4/67 (6%) and 59/329 (18%) patients with CAP who received ceftaroline fosamil first or second line, respectively | All 329 patients in the second-line treatment group received prior antibiotics | Mean±sd: 5.8±3.0 days for first line and 6.2±4.6 days for second line | In patients with CAP, clinical success was achieved in 52/67 first-line patients (78%) and 262/329 second-line patients (80%); outcome data NR for patients with MRSA pneumonia |
| Faris, 2015 [56] | Case report, single US hospital | 48-year-old female with MRSA VAP and bacteraemia; initially admitted to the ICU with thermal burn injury and ARDS | Patient initially treated with vancomycin and cefepime | 14 days | After initiation of ceftaroline, rapid clinical improvement was observed with the patient becoming afebrile at 48 h; the patient was weaned from the ventilator on day 22 and decannulated 2 days later |
| Kaye, 2015 [57] | Retrospective chart review, US hospitals, 2013–2014 | 27 patients with HAP and 13 with VAP treated with ceftaroline fosamil; 14/27 patients (52%) with HAP and 13/13 patients (52%) with VAP admitted to the ICU; HAP patients: 59% male, mean age 63 years; VAP patients: 54% male, mean age 58 years; MRSA identified in 12/27 (44%) HAP and 7/13 (54%) VAP patients; 7/27 patients (26%) with HAP and 3/13 patients (23%) with VAP had associated bacteraemia | 24 patients (89%) with HAP received prior antibiotics, mostly vancomycin and piperacillin–tazobactam (74% and 44%, respectively); 10 patients (77%) with VAP received prior antibiotics, most commonly vancomycin (62%), piperacillin–tazobactam (31%) and cefepime (31%) | Mean±sd: 6.9±3.6 days for patients with HAP and 7.7±3.2 days for patients with VAP | Clinical success rates were 75% overall, 82% in patients with HAP and 62% in patients with VAP; clinical success rates were 7/12 (58%) for patients with MRSA HAP and 4/7 (57%) for patients with MRSA VAP |
| Pasquale, 2015 [ 59 ] | Retrospective case series, US ICUs, 2011–2012 | 10 patients with MRSA pneumonia treated with ceftaroline fosamil (HCAP, n=6; HAP, n=3; VAP, n=1) | 9/10 patients (90%) received prior anti-MRSA therapy | 4–28 days | 3/10 patients (30%) died; 6/7 of the remaining patients had clinical cure or improvement either at the end of therapy with ceftaroline or total antibiotic treatment; 1 patient had a relapse 1 week after ceftaroline fosamil treatment |
| Maggiore, 2015 [ 58 ] | Retrospective chart review, US hospitals, 2011–2013 | 398 patients with CAP treated with ceftaroline fosamil; 138 (35%) treated in the ICU and 256 (64%) in general medical wards (4 patients excluded from analysis); 33/81 ICU patients (41%) and 29/74 general medical ward patients (39%) with pathogens isolated had MRSA | 87% of patients treated in the ICU and 80% of those treated in general medical wards received prior antibiotics | Mean: 7 days (ICU) and 6 days (general medical ward) | Clinical success was achieved in 68% of ICU patients and 85% of general medical ward patients; for patients with MRSA, clinical success was achieved in 56% of ICU patients and 76% of general medical ward patients; for patients with MSSA, clinical success was achieved in 69% of ICU patients and 83% of general medical ward patients |
| Vazquez, 2015 [ 60 ] | Retrospective chart review, US hospitals, 2011–2013 | 21 patients with S. aureus bacteraemia secondary to CAP treated with ceftaroline fosamil, including 16 cases with MRSA; mean age 60 years, 52% male, 33% treated in the ICU | 86% had received prior antimicrobials; 71% received concurrent antimicrobials | Mean±sd: 7±3.8 days | Clinical success was achieved in 28/48 of all patients (58%) with S. aureus bacteraemia; among patients with S. aureus bacteraemia and CAP, 14/21 (67%) achieved clinical success, including 10/16 patients (63%) with MRSA and 4/5 patients (80%) with MSSA |
| Casapao, 2014 [ 66 ] | Retrospective case series, US hospitals, 2011–2013 | 527 patients received ceftaroline fosamil for >72 h, median age 60 years, 58% male; 99 patients had pneumonia (unspecified), of whom 92 were clinically evaluable | 80% had switched from another antibiotic; 29% received concurrent antimicrobials | Median (IQR): 6 (4–9) days | Clinical success was achieved in 426/484 clinically evaluable patients (88%) overall; clinical failure occurred in 8/29 patients (28%) and mortality in 6/30 patients (20%) with S. aureus pneumonia; outcome data NR for patients with MRSA pneumonia |
| Griffiths, 2014 [ 61 ] | Case report, single US hospital | 65-year-old female, admitted to the ICU with MRSA pneumonia, switched to ceftaroline fosamil after other treatments had failed | Empirical treatment with meropenem and vancomycin and 10 days of treatment with linezolid | 5 days | On day 5 of ceftaroline treatment, the patient developed respiratory decompensation and peripheral eosinophilia of 40%; ceftaroline was discontinued, and the patient was started on vancomycin and methylprednisolone, with repeat BAL and peripheral blood counts showing resolved eosinophilia |
| Polenakovik, 2013 [ 62 ] | Case series, single US hospital, 2011–2012 | 31 patients with MRSA bacteraemia, median age 49 years, 71% male; 3 patients had pneumonia identified as the source of bacteraemia and a further 2 patients had pneumonia as a metastatic focus of bacteraemia | All patients had received prior anti-MRSA therapy | 10–42 days (in 5 patients with MRSA pneumonia) | Clinical success was achieved in 23/31 patients (74%) overall and in 4/5 (80%) of those with pneumonia; 1 patient (20%) died; adverse events associated with prolonged therapy were rare, and included eosinophilic pneumonia, rash and diarrhoea |
| Lin, 2013 [63] | Case series, single US hospital, 2011 | 10 patients with deep-seated MRSA infections, including 2 patients with pneumonia (ages 64 and 88 years, both male) | All patients had received prior anti-MRSA therapy | 8–30 days | 1 patient with MRSA pneumonia died 3 days after completing 8 days of ceftaroline fosamil treatment; 1 patient with recurrent MRSA pneumonia with empyema due to bronchopleural fistula was clinically cured after two extended courses of ceftaroline fosamil treatment and discharged without recurrence of empyema in the following 12 months |
COVID-19: coronavirus disease 2019; NR: not reported; CAP: community-acquired pneumonia; HCAP: healthcare-associated pneumonia; ICU: intensive care unit; BSI: bloodstream infection; LRTI: lower respiratory tract infection; IQR: interquartile range; APACHE: Acute Physiology and Chronic Health Evaluation; VAP: ventilator-associated pneumonia; ARDS: acute respiratory distress syndrome; HAP: hospital-acquired pneumonia; MSSA: methicillin-susceptible S. aureus; BAL: bronchoalveolar lavage.
Of the included publications, four included only patients with MRSA pneumonia and 10 included data on patients with MRSA pneumonia as part of a wider study population. The numbers of publications and patients with different categories of MRSA pneumonia were: CAP, six publications, 179 patients; HAP and/or VAP, three publications, 30 patients; and unspecified, five publications, 55 patients. In total, 11 publications, including 197 patients with MRSA pneumonia, reported clinical outcomes for ceftaroline fosamil treatment [53–63]. Three publications included patients with MRSA pneumonia (n=67 patients) but did not report sufficient information to extract clinical outcomes data for the included MRSA pneumonia patients [64–66].
Across three publications which reported outcomes for 92 patients with MRSA CAP treated with ceftaroline fosamil, 54–76% of patients achieved clinical success [53, 58, 60] and a clinical success rate of 63% was reported in a further study which reported combined outcomes for patients with MRSA CAP (n=25) or healthcare-associated pneumonia (HCAP) (n=6) [54]. Of note, the majority of patients (≥60%) in these studies had received prior antimicrobial therapy for MRSA and/or ceftaroline fosamil was administered in combination with other antibiotics (table 4).
Across two publications reporting outcomes for 50 patients with MRSA HCAP, HAP or VAP treated with ceftaroline fosamil, clinical success rates were 57–60%; >75% of patients in these studies had received prior antimicrobial therapy for MRSA [57, 59]. Across three studies that reported outcomes for 44 patients with unspecified MRSA pneumonia treated with ceftaroline fosamil, clinical success rates were 50–80%; 85–100% of patients in these studies had received prior antimicrobial therapy for MRSA [55, 62, 63]. In two individual case studies of patients with MRSA HAP or VAP, clinical success rates were zero out of one (0%) and one out of one (100%), respectively; in both of these cases, ceftaroline fosamil was given as second-line treatment following inadequate responses to other anti-MRSA treatments [56, 61]. The case which reported clinical failure (MRSA pneumonia in a 65-year-old female admitted to the ICU) was also associated with eosinophilic pneumonia which was attributed to ceftaroline fosamil and subsequently resolved with additional medical treatment [61].
Six publications included data on mortality following ceftaroline fosamil treatment, of which four included outcomes data for patients with MRSA pneumonia. The reported mortality rates were six out of 31 patients (19%) with CAP/HCAP [54], three out of 10 patients (30%) with HAP/VAP [59], one out of five patients (20%) with MRSA pneumonia and bacteraemia [62], and one out of two patients (50%) with pneumonia in a case series of 10 patients with deep-seated MRSA infections [63].
Discussion
MRSA pneumonia, whether categorised as CAP or HAP/VAP, is typically severe and potentially life threatening, and successful management requires rapid initiation of appropriate antimicrobial therapy. Ceftaroline fosamil is recognised as a potential alternative to standard first-line treatments for hospitalised patients with MRSA pneumonia [26, 27], although there is limited evidence from RCTs to support its use in this setting.
The current review of published literature describes real-world use and outcomes of ceftaroline fosamil in patients with pneumonia where MRSA was a confirmed pathogen. The literature search and data extraction were designed to cast a “wide net” to capture all relevant publications. While having the advantage of being inclusive, a potential disadvantage is that such an approach combines a heterogeneous dataset with different studies reporting various types of data and potentially using different end-points and definitions. Moreover, some of the studies reported the use of ceftaroline fosamil doses and/or durations of treatment outside of current approved labelling. Nonetheless, the studies included represent a variety of patient populations and clinical scenarios, including severe CAP and HAP/VAP, empiric and targeted/second-line use of ceftaroline fosamil, and a variety of complicated medical needs, and can thus be considered broadly representative of various real-world situations in which ceftaroline fosamil is used in practice.
Overall, reported clinical success rates for patients with MRSA CAP treated with ceftaroline fosamil were 54–76% and those for patients with MRSA HAP/VAP were 57–60%. Mortality rates, where reported, were in the range of 19–50%. While there were various methodological differences (such as in definitions of clinical success) across the included publications that limit interpretation, it is notable that across the studies a large proportion of patients received ceftaroline fosamil as second-line therapy following receipt of other antibiotics and a smaller proportion also received other concurrent anti-MRSA therapies. Unfortunately, with the exception of one publication [65], there was insufficient information within the reported data to discern separate outcomes for the groups of patients treated with first- or second-line therapy. Similarly, only one publication reported outcomes for monotherapy and concurrent anti-MRSA therapy [54]. However, in most cases included within the analysis, ceftaroline fosamil was used as second-line treatment because of treatment failure or because MRSA was detected after initial treatment with another regimen. It is possible that for some patients, mortality and other outcomes could have been better than those reported if ceftaroline fosamil was administered earlier.
While the current qualitive analysis of published studies was not designed as a formal statistical evaluation, our findings are consistent with those of a meta-analysis of RCTs and observational studies by Sotgiu et al. [67], in which the overall efficacy/effectiveness of ceftaroline fosamil in all types of pneumonia cases was 81% and in cases where MRSA was identified was 72%. The results are also broadly in line with findings from RCTs of ceftaroline fosamil in non-MRSA CAP (which evaluated empiric use of ceftaroline or ceftriaxone±azithromycin) [19, 20, 22] and with observational studies in other MRSA infections, where ceftaroline fosamil was used predominantly as second-line or salvage therapy [68, 69].
As with all antimicrobial therapies, the decision to prescribe ceftaroline fosamil should be made by qualified healthcare professionals with consideration for antimicrobial stewardship and guided where possible by culture and susceptibility results as well as local/institutional surveillance data. Particularly with regard to MRSA pneumonia, careful consideration of MIC results is required in the context of the different EUCAST MIC breakpoints for ceftaroline against S. aureus for pneumonia and non-pneumonia isolates and CLSI dose-dependent susceptibility [50, 51]. It is anticipated that the development of rapid bacterial diagnostics will lead to improvements in the management of pneumonia (including cases caused by MRSA) as well as support antimicrobial stewardship (e.g. by reducing the unnecessary use of empiric anti-MRSA agents and supporting rapid de-escalation) [38, 70].
In summary, although there are scarce data available from RCTs evaluating ceftaroline fosamil in adults with MRSA pneumonia, there is good evidence of its comparative effectiveness and safety profile in CAP caused by other pathogens, including MSSA. There is also available evidence from in vitro, pre-clinical and clinical pharmacology studies and clinical experience reported in real-world observational studies and case reports demonstrating the utility of ceftaroline fosamil in patients with pneumonia where MRSA is a confirmed pathogen. While the available evidence does not suggest ceftaroline fosamil should be used in preference to the standard recommended treatments for MRSA pneumonia (vancomycin and linezolid), the data indicate that it may be a valuable additional treatment option in this setting. Specific scenarios in which ceftaroline fosamil might be used in preference to other anti-MRSA agents in patients with pneumonia include cases involving bacteraemia, as well as those with complicating factors such as empyema, which can require longer treatment durations than are typical for other types of pneumonia.
Points for clinical practice
Rapid initiation of appropriate empirical therapy is needed to optimise outcomes for patients with pneumonia where MRSA is a suspected or confirmed pathogen. While vancomycin and linezolid are usually considered as first-line therapies, ceftaroline fosamil has an established clinical profile in the treatment of CAP in hospitalised patients, and although data from RCTs in patients with MRSA pneumonia are lacking, in vitro and in vivo PK/PD data, lung tissue penetration and real-world outcomes studies indicate that ceftaroline fosamil is a possible alternative to linezolid and vancomycin for MRSA pneumonia.
Acknowledgements
Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Mark Waterlow (Prime Global Medical Communications Ltd, Knutsford, UK) and funded by Pfizer.
Provenance: Submitted article, peer reviewed.
Author contributions: A. Torres, C. Cillóniz and G.G. Stone developed the scope and focus of the review. All authors critically appraised, revised and prepared the final version.
Conflict of interest: A. Torres has no conflicts of interest related to the current article. A. Kuraieva is an employee of Pfizer. G.G. Stone is an employee of Pfizer. C. Cillóniz has no conflicts of interest related to the current article.
Support statement: Funding for medical writing support was provided by Pfizer. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Tong SY, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28: 603–661. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He H, Wunderink RG. Staphylococcus aureus pneumonia in the community. Semin Respir Crit Care Med 2020; 41: 470–479. doi: 10.1055/s-0040-1709992 [DOI] [PubMed] [Google Scholar]
- 3.Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021; 12: 547–569. doi: 10.1080/21505594.2021.1878688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AS, de Lencastre H, Garau J, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers 2018; 4: 18033. doi: 10.1038/nrdp.2018.33 [DOI] [PubMed] [Google Scholar]
- 5.van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: an update. Infect Dis Clin North Am 2020; 34: 709–722. doi: 10.1016/j.idc.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craft KM, Nguyen JM, Berg LJ, et al. Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Medchemcomm 2019; 10: 1231–1241. doi: 10.1039/C9MD00044E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 2019; 17: 203–218. doi: 10.1038/s41579-018-0147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008; 46: Suppl. 5, S378–S385. doi: 10.1086/533594 [DOI] [PubMed] [Google Scholar]
- 9.Torres A, Chalmers JD, Dela Cruz CS, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med 2019; 45: 159–171. doi: 10.1007/s00134-019-05519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev 2022; 31: 220123. doi: 10.1183/16000617.0123-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71: 2459–2468. doi: 10.1093/cid/ciaa530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Toole RF. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect 2021; 27: 1772–1776. doi: 10.1016/j.cmi.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai CC, Chen SY, Ko WC, et al. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents 2021; 57: 106324. doi: 10.1016/j.ijantimicag.2021.106324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segala FV, Bavaro DF, Di Gennaro F, et al. Impact of SARS-CoV-2 epidemic on antimicrobial resistance: a literature review. Viruses 2021; 13: 2110. doi: 10.3390/v13112110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassetti M, Magnasco L, Vena A, et al. Methicillin-resistant Staphylococcus aureus lung infection in coronavirus disease 2019: how common? Curr Opin Infect Dis 2022; 35: 149–162. doi: 10.1097/QCO.0000000000000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton JE. Ceftaroline fosamil: a review of its use in the treatment of complicated skin and soft tissue infections and community-acquired pneumonia. Drugs 2013; 73: 1067–1094. doi: 10.1007/s40265-013-0075-6 [DOI] [PubMed] [Google Scholar]
- 17.Korczowski B, Antadze T, Giorgobiani M, et al. A multicenter, randomized, observer-blinded, active-controlled study to evaluate the safety and efficacy of ceftaroline versus comparator in pediatric patients with acute bacterial skin and skin structure infection. Pediatr Infect Dis J 2016; 35: e239–e247. doi: 10.1097/INF.0000000000001191 [DOI] [PubMed] [Google Scholar]
- 18.Blumer JL, Ghonghadze T, Cannavino C, et al. A multicenter, randomized, observer-blinded, active-controlled study evaluating the safety and effectiveness of ceftaroline compared with ceftriaxone plus vancomycin in pediatric patients with complicated community-acquired bacterial pneumonia. Pediatr Infect Dis J 2016; 35: 760–766. doi: 10.1097/INF.0000000000001160 [DOI] [PubMed] [Google Scholar]
- 19.Zhong NS, Sun T, Zhuo C, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis 2015; 15: 161–171. doi: 10.1016/S1473-3099(14)71018-7 [DOI] [PubMed] [Google Scholar]
- 20.File TM Jr, Low DE, Eckburg PB, et al. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 2011; 66: Suppl. 3, iii19–iii32. doi: 10.1093/jac/dkr096 [DOI] [PubMed] [Google Scholar]
- 21.Corey GR, Wilcox MH, Talbot GH, et al. CANVAS 1: the first phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65: Suppl. 4, iv41–iv51. doi: 10.1093/jac/dkq254 [DOI] [PubMed] [Google Scholar]
- 22.Low DE, File TM Jr, Eckburg PB, et al. FOCUS 2: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 2011; 66: Suppl. 3, iii33–iii44. doi: 10.1093/jac/dkr097 [DOI] [PubMed] [Google Scholar]
- 23.Dryden M, Zhang Y, Wilson D, et al. A phase III, randomized, controlled, non-inferiority trial of ceftaroline fosamil 600 mg every 8 h versus vancomycin plus aztreonam in patients with complicated skin and soft tissue infection with systemic inflammatory response or underlying comorbidities. J Antimicrob Chemother 2016; 71: 3575–3584. doi: 10.1093/jac/dkw333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito S, Carrothers TJ, Riccobene T, et al. Ceftaroline fosamil for treatment of pediatric complicated skin and soft tissue infections and community-acquired pneumonia. Paediatr Drugs 2021; 23: 549–563. doi: 10.1007/s40272-021-00468-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 2016; 71: 862–870. doi: 10.1093/jac/dkv415 [DOI] [PubMed] [Google Scholar]
- 26.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–e111. doi: 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Loeches I, Torres A, Nagavci B, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med 2023; 49: 615–632. doi: 10.1007/s00134-022-06950-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44: Suppl. 2, S27–S72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections – full version. Clin Microbiol Infect 2011; 17: Suppl. 6, E1–E59. doi: 10.1111/j.1469-0691.2011.03602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J 2017; 50: 1700582. doi: 10.1183/13993003.00582-2017 [DOI] [PubMed] [Google Scholar]
- 32.Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers 2021; 7: 25. doi: 10.1038/s41572-021-00259-0 [DOI] [PubMed] [Google Scholar]
- 33.Cillóniz C, Dominedò C, Nicolini A, et al. PES pathogens in severe community-acquired pneumonia. Microorganisms 2019; 7: 49. doi: 10.3390/microorganisms7020049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc 2015; 12: 153–160. doi: 10.1513/AnnalsATS.201407-305OC [DOI] [PubMed] [Google Scholar]
- 35.Welte T, Kantecki M, Stone GG, et al. Ceftaroline fosamil as a potential treatment option for Staphylococcus aureus community-acquired pneumonia in adults. Int J Antimicrob Agents 2019; 54: 410–422. doi: 10.1016/j.ijantimicag.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 36.Cillóniz C, Ewig S, Polverino E, et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011; 66: 340–346. doi: 10.1136/thx.2010.143982 [DOI] [PubMed] [Google Scholar]
- 37.Cilloniz C, Martin-Loeches I, Garcia-Vidal C, et al. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci 2016; 17: 2120. doi: 10.3390/ijms17122120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaragoza R, Vidal-Cortes P, Aguilar G, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care 2020; 24: 383. doi: 10.1186/s13054-020-03091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BE, Ying J, Stevens V, et al. Empirical anti-MRSA vs standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA Intern Med 2020; 180: 552–560. doi: 10.1001/jamainternmed.2019.7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kollef MH. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis 2007; 45: Suppl. 3, S191–S195. doi: 10.1086/519470 [DOI] [PubMed] [Google Scholar]
- 41.Jeffres MN, Isakow W, Doherty JA, et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther 2007; 29: 1107–1115. doi: 10.1016/j.clinthera.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 42.Kalil AC, Murthy MH, Hermsen ED, et al. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38: 1802–1808. doi: 10.1097/CCM.0b013e3181eb3b96 [DOI] [PubMed] [Google Scholar]
- 43.Pfizer . Zinforo 600 mg powder for concentrate for solution for infusion: summary of product characteristics. 2017. www.ema.europa.eu/documents/product-information/zinforo-epar-product-information_en.pdf Date last accessed: 15 March 2023.
- 44.Allergan . TEFLARO (ceftaroline fosamil) injection for intravenous (IV) use. 2021. www.allergan.com/assets/pdf/teflaro_pi Date last accessed: 15 March 2023.
- 45.Moisan H, Pruneau M, Malouin F. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother 2010; 65: 713–716. doi: 10.1093/jac/dkp503 [DOI] [PubMed] [Google Scholar]
- 46.Lahiri SD, McLaughlin RE, Whiteaker JD, et al. Molecular characterization of MRSA isolates bracketing the current EUCAST ceftaroline-susceptible breakpoint for Staphylococcus aureus: the role of PBP2a in the activity of ceftaroline. J Antimicrob Chemother 2015; 70: 2488–2498. doi: 10.1093/jac/dkv131 [DOI] [PubMed] [Google Scholar]
- 47.Lahiri SD, Alm RA. Identification of non-PBP2a resistance mechanisms in Staphylococcus aureus after serial passage with ceftaroline: involvement of other PBPs. J Antimicrob Chemother 2016; 71: 3050–3057. doi: 10.1093/jac/dkw282 [DOI] [PubMed] [Google Scholar]
- 48.Lodise TP, Low DE. Ceftaroline fosamil in the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. Drugs 2012; 72: 1473–1493. doi: 10.2165/11635660-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 49.Riccobene TA, Pushkin R, Jandourek A, et al. Penetration of ceftaroline into the epithelial lining fluid of healthy adult subjects. Antimicrob Agents Chemother 2016; 60: 5849–5857. doi: 10.1128/AAC.02755-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Committee on Antimicrobial Susceptibility Testing . Clinical breakpoints for bacteria, v 13.0. 2023. www.eucast.org/clinical_breakpoints Date last accessed: 21 March 2023.
- 51.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition. CLSI Supplement M100. 2022. https://clsi.org/standards/products/microbiology/documents/m100 Date last accessed: 21 March 2023.
- 52.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassetti M, Russo A, Cilloniz C, et al. Ceftaroline for severe community-acquired pneumonia: a real-world two-centre experience in Italy and Spain. Int J Antimicrob Agents 2020; 55: 105921. doi: 10.1016/j.ijantimicag.2020.105921 [DOI] [PubMed] [Google Scholar]
- 54.Karki A, Thurm C, Cervellione K. Experience with ceftaroline for treatment of methicillin-resistant Staphylococcus aureus pneumonia in a community hospital. J Community Hosp Intern Med Perspect 2017; 7: 300–302. doi: 10.1080/20009666.2017.1374107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zasowski EJ, Trinh TD, Claeys KC, et al. Multicenter observational study of ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2017; 61: e02015-16. doi: 10.1128/AAC.02015-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faris J, Mynatt RP, Hall Snyder AD, et al. Treatment of methicillin-resistant staphylococcus aureus (MRSA) pneumonia with ceftaroline fosamil in a patient with inhalational thermal injury. Infect Dis Ther 2015; 4: 519–528. doi: 10.1007/s40121-015-0096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaye KS, Udeani G, Cole P, et al. Ceftaroline fosamil for the treatment of hospital-acquired pneumonia and ventilator-associated pneumonia. Hosp Pract 2015; 43: 144–149. doi: 10.1080/21548331.2015.1037228 [DOI] [PubMed] [Google Scholar]
- 58.Maggiore C, Vazquez JA, Guervil DJ, et al. Ceftaroline fosamil for the treatment of community-acquired bacterial pneumonia in the intensive care unit. Ther Clin Risk Manag 2015; 11: 557–563. doi: 10.2147/TCRM.S75191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pasquale TR, Tan MJ, Trienski TL, et al. Methicillin-resistant Staphylococcus aureus nosocomial pneumonia patients treated with ceftaroline: retrospective case series of 10 patients. J Chemother 2015; 27: 29–34. doi: 10.1179/1973947813Y.0000000156 [DOI] [PubMed] [Google Scholar]
- 60.Vazquez JA, Maggiore CR, Cole P, et al. Ceftaroline fosamil for the treatment of Staphylococcus aureus bacteremia secondary to acute bacterial skin and skin structure infections or community-acquired bacterial pneumonia. Infect Dis Clin Pract 2015; 23: 39–43. doi: 10.1097/IPC.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffiths CL, Gutierrez KC, Pitt RD, et al. Eosinophilic pneumonia induced by ceftaroline. Am J Health Syst Pharm 2014; 71: 403–406. doi: 10.2146/ajhp130441 [DOI] [PubMed] [Google Scholar]
- 62.Polenakovik HM, Pleiman CM. Ceftaroline for meticillin-resistant Staphylococcus aureus bacteraemia: case series and review of the literature. Int J Antimicrob Agents 2013; 42: 450–455. doi: 10.1016/j.ijantimicag.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 63.Lin JC, Aung G, Thomas A, et al. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother 2013; 19: 42–49. doi: 10.1007/s10156-012-0449-9 [DOI] [PubMed] [Google Scholar]
- 64.Giacobbe DR, Russo C, Martini V, et al. Use of ceftaroline in hospitalized patients with and without COVID-19: a descriptive cross-sectional study. Antibiotics 2021; 10: 763. doi: 10.3390/antibiotics10070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guervil DJ, Kaye KS, Hassoun A, et al. Ceftaroline fosamil as first-line versus second-line treatment for acute bacterial skin and skin structure infections (ABSSSI) or community-acquired bacterial pneumonia (CABP). J Chemother 2016; 28: 180–186. doi: 10.1179/1973947815Y.0000000010 [DOI] [PubMed] [Google Scholar]
- 66.Casapao AM, Davis SL, Barr VO, et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother 2014; 58: 2541–2546. doi: 10.1128/AAC.02371-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sotgiu G, Aliberti S, Gramegna A, et al. Efficacy and effectiveness of ceftaroline fosamil in patients with pneumonia: a systematic review and meta-analysis. Respir Res 2018; 19: 205. doi: 10.1186/s12931-018-0905-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burnett YJ, Echevarria K, Traugott KA. Ceftaroline as salvage monotherapy for persistent MRSA bacteremia. Ann Pharmacother 2016; 50: 1051–1059. doi: 10.1177/1060028016664361 [DOI] [PubMed] [Google Scholar]
- 69.Paladino JA, Jacobs DM, Shields RK, et al. Use of ceftaroline after glycopeptide failure to eradicate meticillin-resistant Staphylococcus aureus bacteraemia with elevated vancomycin minimum inhibitory concentrations. Int J Antimicrob Agents 2014; 44: 557–563. doi: 10.1016/j.ijantimicag.2014.07.024 [DOI] [PubMed] [Google Scholar]
- 70.Basnayake TL, Waterer GW. Rapid diagnostic tests for defining the cause of community-acquired pneumonia. Curr Opin Infect Dis 2015; 28: 185–192. doi: 10.1097/QCO.0000000000000148 [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, Chen M, Yu Y, et al. In vitro activity of ceftaroline and comparators against Staphylococcus aureus isolates: results from 6 years of the ATLAS Program (2012 to 2017). Infect Drug Resist 2019; 12: 3349–3358. doi: 10.2147/IDR.S226649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farrell DJ, Castanheira M, Mendes RE, et al. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008–2010). Clin Infect Dis 2012; 55: Suppl. 3, S206–S214. doi: 10.1093/cid/cis563 [DOI] [PubMed] [Google Scholar]
- 73.Sader HS, Mendes RE, Streit JM, et al. Antimicrobial susceptibility trends among Staphylococcus aureus isolates from U.S. hospitals: results from 7 years of the ceftaroline (AWARE) surveillance program, 2010 to 2016. Antimicrob Agents Chemother 2017; 61: e01043-17. doi: 10.1128/AAC.01043-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sader HS, Castanheira M, Duncan LR, et al. Update on the in vitro activity of ceftaroline against Staphylococcus aureus from United States (US) medical centers stratified by infection type (2018–2020). Diagn Microbiol Infect Dis 2023; 105: 115820. doi: 10.1016/j.diagmicrobio.2022.115820 [DOI] [PubMed] [Google Scholar]
- 75.Jia P, Zhu Y, Zhang H, et al. In vitro activity of ceftaroline, ceftazidime-avibactam, and comparators against Gram-positive and -negative organisms in China: the 2018 results from the ATLAS program. BMC Microbiol 2022; 22: 234. doi: 10.1186/s12866-022-02644-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karlowsky JA, Hackel MA, Bouchillon SLK, et al. In vitro activity of ceftaroline against bacterial pathogens isolated from patients with skin and soft tissue and respiratory tract infections in the Middle East and Africa: AWARE global surveillance programme 2015–2018. J Glob Antimicrob Resist 2021; 24: 249–256. doi: 10.1016/j.jgar.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 77.Andes D, Craig WA. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 2006; 50: 1376–1383. doi: 10.1128/AAC.50.4.1376-1383.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andes D, Craig WA. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target [Correction]. Antimicrob Agents Chemother 2014; 58: 2489. doi: 10.1128/AAC.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhalodi AA, Crandon JL, Biek D, et al. Efficacy of ceftaroline fosamil in a staphylococcal murine pneumonia model. Antimicrob Agents Chemother 2012; 56: 6160–6165. doi: 10.1128/AAC.01078-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keel RA, Crandon JL, Nicolau DP. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob Agents Chemother 2011; 55: 4028–4032. doi: 10.1128/AAC.00372-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.MacGowan AP, Noel AR, Tomaselli S, et al. Pharmacodynamics of ceftaroline against Staphylococcus aureus studied in an in vitro pharmacokinetic model of infection. Antimicrob Agents Chemother 2013; 57: 2451–2456. doi: 10.1128/AAC.01386-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh R, Almutairi M, Alm RA, et al. Ceftaroline efficacy against high-MIC clinical Staphylococcus aureus isolates in an in vitro hollow-fibre infection model. J Antimicrob Chemother 2017; 72: 2796–2803. doi: 10.1093/jac/dkx214 [DOI] [PubMed] [Google Scholar]
- 83.Li J, Das S, Zhou D, et al. Population pharmacokinetic modeling and probability of target attainment analyses in Asian patients with community-acquired pneumonia treated with ceftaroline fosamil. Clin Pharmacol Drug Dev 2019; 8: 682–694. doi: 10.1002/cpdd.673 [DOI] [PubMed] [Google Scholar]
- 84.Cristinacce A, Wright JG, Stone GG, et al. A retrospective analysis of probability of target attainment in community-acquired pneumonia: ceftaroline fosamil versus comparators. Infect Dis Ther 2019; 8: 185–198. doi: 10.1007/s40121-019-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]