Abstract
Inhaled corticosteroids (ICS) are the most commonly used anti-inflammatory drugs for the treatment of COPD. COPD has been previously described as a “corticosteroid-resistant” condition, but current clinical trial evidence shows that selected COPD patients, namely those with increased exacerbation risk plus higher blood eosinophil count (BEC), can benefit from ICS treatment. This review describes the components of inflammation modulated by ICS in COPD and the reasons for the variation in response to ICS between individuals. There are corticosteroid-insensitive inflammatory pathways in COPD, such as bacteria-induced macrophage interleukin-8 production and resultant neutrophil recruitment, but also corticosteroid-sensitive pathways including the reduction of type 2 markers and mast cell numbers. The review also describes the mechanisms whereby ICS can skew the lung microbiome, with reduced diversity and increased relative abundance, towards an excess of proteobacteria. BEC is a biomarker used to enable the selective use of ICS in COPD, but the clinical outcome in an individual is decided by a complex interacting network involving the microbiome and airway inflammation.
Tweetable abstract
While blood eosinophil count is the biomarker used in clinical practice to enable selective use of ICS in COPD, there is a complex interacting network involving the microbiome, airway inflammation and ICS that decides the clinical outcome in individuals https://bit.ly/3P1Jmjx
Introduction
COPD is characterised by poorly reversible and often progressive airflow obstruction, accompanied by persistent airway inflammation. The typical clinical features include dyspnoea, cough and sputum production. Some patients experience exacerbation events, with rapid symptom deterioration that requires additional treatment [1].
Inhaled corticosteroids (ICS) are the most commonly used anti-inflammatory drugs for the treatment of COPD. Randomised controlled trials (RCTs) in COPD patients with a history of exacerbations have demonstrated the clinical benefits of ICS treatment, including exacerbation rate reduction and improved quality of life [2–4]. ICS are usually administered as part of a combination with either a long-acting β-agonist (LABA) or as part of triple therapy with a LABA and long-acting muscarinic antagonist (LAMA). RCTs have shown that blood eosinophil count (BEC) at the start of the study (in the stable state) can predict the subsequent clinical benefit of ICS on exacerbation prevention, with a threshold of <100 cells·µL−1 identifying individuals who are unlikely to benefit while >300 cells·µL−1 identifies individuals with a high probability of benefit from ICS treatment [5–7]. The use of BEC in routine clinical practice enables clinicians to more precisely target ICS towards selected patients who are more likely to experience a benefit.
Publications have historically described COPD as a “corticosteroid-resistant” condition [8, 9]. However, the evidence from RCTs that ICS-containing combination treatments prevent exacerbations in the subgroup of COPD patients with both increased exacerbation risk plus higher BEC demonstrates that there is a clinical benefit, albeit in selected patients. COPD is a heterogeneous condition [1] and the variation between individuals in the clinical response to ICS likely reflects the heterogeneous nature of pulmonary inflammation. The relationship between clinical response and BEC [5–7] indicates that an ICS-sensitive profile of airway inflammation is present in some, but not all, COPD patients.
The clinical effects of ICS in COPD have been well described elsewhere [7, 10, 11] and it is not our aim to provide an extensive review of clinical outcomes here. Instead, this review aims to further our understanding of the inflammatory pathways targeted by ICS by focusing on evidence from studies performed in COPD patients. This evidence also provides mechanistic insights that can explain the variable nature of the therapeutic response to ICS in COPD.
ICS: clinical benefits versus risk
RCTs conducted in COPD patients with a history of at least one exacerbation in the year before the study have shown that ICS/LABA combinations reduce exacerbation rates compared with LABA monotherapy, with the effect size being ∼25% in many studies [10, 12]. Single-inhaler triple-therapy studies have also shown that the ICS component prevents exacerbations, with the two largest RCTs again showing that the magnitude was ∼25% reduction [2, 3]. The ICS effect on exacerbation reduction is greater in individuals at higher exacerbation risk (i.e. at least two moderate exacerbations or one severe exacerbation in the previous year), although there is still a significant benefit in individuals at lower exacerbation risk (one moderate exacerbation in the previous year) [13, 14]. There has been debate regarding the potential inclusion of patients with concomitant asthma within these single-inhaler triple-therapy studies, although it should be noted that all the included patients had a physician diagnosis of COPD with a smoking history plus meeting relevant lung function criteria [15].
There is also evidence that single-inhaler triple therapy reduces mortality compared with LABA/LAMA combination treatment in COPD patients at increased risk of exacerbations [2, 3]. It is known that exacerbations, and particularly hospitalisations, are a cause of increased mortality [16], so prevention of exacerbations is a likely explanation for this ICS-related mortality benefit. There is an increase in cardiovascular events, including myocardial infarctions, during and immediately after an exacerbation [17, 18]. It is therefore possible that, through exacerbation prevention, ICS can reduce the incidence of adverse cardiovascular events. Indeed, there was a reduction in cardiovascular deaths with triple therapy compared with LAMA/LABA [19, 20].
ICS can cause side-effects including pneumonia, osteoporosis, diabetes and cataracts [1]. Cross-trial comparisons of ICS formulations are problematic, with different criteria for recording pneumonia. Additionally, there are risk factors for pneumonia in COPD, including age, lower forced expiratory volume in 1 s (FEV1) and lower body mass index, which cause different pneumonia rates according to population characteristics [21]. The potential benefits of ICS treatment (within a combination inhaler) therefore need to be weighed against the potential risk on an individual basis. The use of clinical information plus BEC enables clinicians to optimise the therapeutic index (risk/benefit ratio) at an individual level.
Corticosteroids: mechanism of action
Corticosteroids bind to the glucocorticoid receptor (GR), a ligand-dependent transcription factor which resides in the cytoplasm. Within the N-terminus of GR, there are numerous phosphorylation sites which alter GR function through ligand binding, nuclear localisation, modulating interactions with co-regulators or transcriptional activation [22, 23]. The serine phosphorylation sites S211 and S226 have functional importance and roles in subcellular localisation. GR–ligand nuclear translocation is associated with phosphorylation of S211 and phosphorylation of S226 is known to cause nuclear GR export [22].
Upon translocation to the nucleus the GR–ligand complex can suppresses pro-inflammatory gene transcription (transrepression) or activate anti-inflammatory gene expression (transactivation). Such transrepression/transactivation occurs by binding to glucocorticoid response elements at the promoter/enhancer regions of responsive genes or as result of binding and inhibition of transcription factors, including NF-κB or activator protein-1 [24]. This transcription factor inactivation decreases transcription and synthesis of pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α, which are upregulated in the airways of COPD patients [25], resulting in anti-inflammatory effects. Corticosteroids do not, however, target all the inflammatory pathways involved in COPD pathogenesis. GR activation has been shown to cause transrepression of a subset of inflammatory response genes in mouse macrophage models [26]. This highlights that corticosteroids target a proportion of the inflammatory cascade in a signal-specific manner. The selective targeting of some, but not all, components of the immune response by corticosteroids is highly relevant when considering the effects of ICS in COPD. Understanding which COPD inflammatory pathways are corticosteroid sensitive or insensitive can help with the development of biomarkers to predict ICS response and the development of novel therapeutics against insensitive pathways.
Macrophage cell culture studies: evidence for corticosteroid-sensitive and -insensitive pathways in COPD
To further understand the sensitivity of inflammatory pathways to corticosteroids, we reviewed studies using COPD lung cells cultured ex vivo. The majority of this cell culture evidence utilises lung macrophages, as these cells are practically feasible to obtain by bronchoscopy or from lung surgical specimens. Macrophages play key roles in the pathophysiology of COPD. There are different macrophage subsets in the lungs, showing varying degrees of pro-inflammatory, regulatory and phagocytic ability [27]. Alveolar macrophage numbers are increased in COPD patients versus controls [28, 29]. These cells release pro-inflammatory cytokines and chemokines, including the neutrophil chemoattractant interleukin (IL)-8 [25]. COPD macrophages display dysfunctional behaviour, including impaired ability to phagocytose bacteria [30].
In a study published in 2003 by Culpitt et al. [8], corticosteroids showed less suppression of cigarette smoke- and IL-1β-induced IL-8 release from COPD versus smoking control lung macrophages. The difference between groups was small and maximal inhibition was <50% in both groups, indicating a suboptimal effect of corticosteroids even in the control group. The same research group reported that corticosteroid inhibition of lipopolysaccharide (LPS)-induced IL-8 and TNF-α release from COPD and smoking control lung macrophages was similar, albeit lower, compared with non-smokers [31]. Although numerical percentage inhibition data were not stated, inspection of the graphs shows that IL-8 was more insensitive to corticosteroid inhibition than TNF-α. Another study reported that LPS-induced IL-8 release from COPD lung macrophages was more corticosteroid insensitive compared with smoking and non-smoking controls, but the corticosteroid sensitivity of IL-6, monocyte chemoattractant protein-1 and matrix metalloproteinase-9 was similar between groups [32]. At the time, the interpretation of these studies concentrated on the concept that COPD macrophages are corticosteroid resistant. Additionally, it was proposed that the mechanism responsible was reduced histone deacetylase 2 activity, causing reduced GR deacetylation and thereby decreasing binding and transrepression of NF-κB-mediated gene transcription [33]. However, the results had complexity, with two important features consistently observed: 1) the magnitude of any differences between COPD and controls was small, and not observed across all cytokines, and 2) the corticosteroid sensitivity varied between pro-inflammatory mediators, with suppression of IL-8 being relatively insensitive even in control samples. Different stimulants were used in these cell culture studies, which may cause differences between studies.
We have previously conducted a pooled analysis of seven lung macrophage studies of the anti-inflammatory effects of corticosteroids (n=92 COPD patients and controls), to overcome potential sample size issues [34–41]. The group mean data showed that corticosteroid inhibition of TNF-α, IL-6 and IL-8 was similar in COPD patients compared with smoking and non-smoking controls. Additionally, there was lower maximal inhibition of LPS-induced IL-8 (<60%) compared with TNF-α and IL-6 (∼80%). These data show that some pro-inflammatory mediators within the innate immune system are relatively corticosteroid sensitive, while there are also insensitive pathways, including IL-8 production. There was marked intersubject variability of corticosteroid inhibition, particularly in COPD patients. This intersubject variation may have clinical relevance, mirroring the variable clinical response to corticosteroid treatment. This variability can have a greater influence in studies with smaller sample sizes. For example, in a subsequent smaller study, we observed reduced corticosteroid inhibition of TNF-α and IL-10 in COPD versus smoking controls but similar corticosteroid sensitivity of IL-6 [42].
The reduced corticosteroid sensitivity of macrophage-derived IL-8 secretion has been investigated using Haemophilus influenzae-exposed lung macrophages; IL-8 release was completely insensitive to corticosteroid inhibition in both controls and COPD patients [43, 44]. Macrophage exposure to H. influenzae causes prolonged production of IL-8 [45]. H. influenzae exposure also increased GR S226 phosphorylation, implicating increased nuclear GR export as a mechanism by which bacteria can alter the anti-inflammatory capabilities of corticosteroids [44]. This suggests a corticosteroid-insensitive pulmonary macrophage–IL-8–neutrophil axis in COPD, as COPD patients colonised with H. influenzae have higher numbers of airway neutrophils [46], which could arise through corticosteroid-insensitive IL-8 secretion from macrophages. Furthermore, pulmonary neutrophils are less sensitive to corticosteroid inhibition of TNF-α and IL-8 compared with blood neutrophils [47].
IL-8 shares the same chromosome locus as other genes (chemokine C-X-C motif ligand (CXCL) 1, CXCL2 and CXCL3) also found to be insensitive to corticosteroids in monocyte-derived macrophages [48]. Similar observations have been reported in murine macrophages [26]. This suggests that IL-8 is inherently less sensitive than other cytokines to corticosteroid inhibition. GR-mediated repression of gene transcription is dependent on access to the gene promoter. Chromatin remodelling specific to the IL-8 promoter can occur, including p38 mitogen-activated protein kinase-dependent changes, which may limit GR access to the IL-8 promoter [49–51]. Interestingly, this does not occur at the TNF-α promoter [51], explaining the differences in maximal corticosteroid inhibition that we and others have observed.
The anti-inflammatory effects of corticosteroids have been studied in several lymphocyte models. Using enriched CD8+ T-cells from blood and lung tissue, dexamethasone inhibition of the T-helper 1 cytokines interferon (IFN)-γ and IL-2 was similar between COPD patients and controls, reaching maximal inhibition of ∼60% [52, 53]. Conversely, Kaur et al. [54] observed similar dexamethasone inhibition of IL-2 but reduced inhibition of IFN-γ in unselected bronchoalveolar lavage lymphocytes from COPD patients versus controls (∼50% versus ∼75% inhibition, respectively), suggesting that the corticosteroid sensitivity of these mediators is reduced in COPD. Grundy et al. [52, 53] enriched for CD8+ cells, while Kaur et al. [54] used a mixed bronchoalveolar lavage lymphocyte population, which may have contained more corticosteroid-insensitive cells such as the subpopulation of CD28null T-cells [55].
Bronchial epithelial cells have been used to study corticosteroids. Inhibition of TNF-α-induced IL-8 release was found to be similar between COPD patients and smoking and non-smoking controls, reaching maximal inhibition of ∼50%, while corticosteroid inhibition of granulocyte–macrophage colony-stimulating factor release was marginally more sensitive in non-smokers [56]. Another study showed that corticosteroid inhibition of poly(I:C)-induced IL-8 release was similar in COPD patients and smokers (maximal inhibition of 20%) but higher in non-smokers (60%) [57]. We found no difference in corticosteroid inhibition of poly(I:C)-induced IL-6 and CXCL10 release when comparing COPD patients with smokers [42]. The major positive finding in these studies appears to be greater corticosteroid sensitivity in non-smokers, with no differences between COPD patients and smokers. This suggests that oxidative stress caused by smoking itself is a key driver of reduced sensitivity to corticosteroid inhibition of cytokine production from epithelial cells.
Overall, these studies using lung cells from COPD patients have reported results which vary according to the cell types studied and stimulants used. The majority of studies have focused on the macrophage innate immune response and have demonstrated that some pro-inflammatory mediators are relatively corticosteroid sensitive. In contrast, the production of IL-8 is more corticosteroid insensitive, highlighting an IL-8–neutrophil recruitment axis that responds poorly to corticosteroids in COPD [58].
COPD clinical trials: effects of ICS on inflammatory cell counts
Placebo-controlled clinical trials which have examined the effect of ICS on immune cell counts in COPD patients have used ICS monotherapy or ICS/LABA combination. We found six bronchoscopy studies evaluating bronchial biopsies (table 1), with treatment periods varying from 3 to 30 months [59–64]. ICS lowered mast cell counts and CD3+, CD4+ and CD8+ lymphocyte counts in two studies [60, 61]. Two other studies showed trends for reduced mast cell numbers without reaching statistical significance compared with placebo treatment (114.2 versus 152.6 cells·mm−2; p=0.08 [63] and 7.9 versus 11.9 cells·mm−1 basement membrane; p=0.1 [59]). The GLUCOLD study reported reductions in CD3+, CD4+ and CD8+ lymphocyte and mast cell counts at 6 and 30 months [60]. In addition, following withdrawal of ICS after 6 months, CD3+ lymphocyte and mast cell counts increased along with worsening symptoms and lung function. The addition of LABA to ICS did not provide additional reductions in immune cell counts at 6 months. Eosinophil cell counts were reduced in only one of the studies [60] as were macrophage cell counts [59], while there was no evidence for neutrophil count suppression. Although the role of lymphocytes or mast cells in COPD has not yet been conclusively elucidated, there are studies showing a relationship between lymphocyte counts in the airways and disease severity [29, 65], supporting a hypothesis that altered adaptive immunity is involved in COPD pathophysiology [66]. ICS targeting of these cells and their cytokines may be beneficial.
TABLE 1.
Studies investigating the effects of inhaled corticosteroids (ICS) on bronchial biopsy immune cell counts
| First author, year [ref.] | Length of treatment | Macrophages | Neutrophils | Eosinophils | Mast cells | Lymphocytes |
| Verhoeven, 2002 [ 64 ] | 6 months (ICS) | = | = | = | = | = |
| Hattotuwa, 2002 [ 61 ] | 3 months (ICS) | = | = | = | ↓ | = |
| Barnes, 2006 [ 63 ] | 3 months (ICS/LABA) | = | X | X | =# | ↓ |
| Bourbeau, 2007 [ 62 ] | 3 months (ICS) | = | = | = | X | = |
| Reid, 2008 [ 59 ] | 6 months (ICS) | ↓ | ↑ | = | =# | = |
| Lapperre, 2009 [ 60 ] | 6 months (ICS) | = | = | = | ↓ | ↓ |
| 6 months (ICS/LABA) | = | = | = | ↓ | ↓ | |
| 30 months (ICS) | = | = | ↑ | ↓ | ↓ | |
| 30 months (ICS/LABA) | = | = | ↓ | ↓ | ↑ |
=: no change; ↑: increase; ↓: decrease; X: not measured. LABA: long-acting β-agonist. #: trend for reduced mast cell numbers without reaching statistical significance.
We found 12 studies using induced sputum [59, 60, 63, 67–74] (table 2). There were decreases in neutrophil counts in four studies [60, 63, 73, 74], with eosinophil counts reduced in two studies [63, 67]; the majority of studies showed no change. There was less evidence for modulation of other cell types.
TABLE 2.
Studies investigating the effects of inhaled corticosteroids (ICS) on sputum immune cell counts
| First author, year [ref.] | Length of treatment | Macrophages | Neutrophils | Eosinophils | Mast cells | Lymphocytes |
| Confalonieri, 1998 [ 74 ] | 2 months (ICS) | ↑ | ↓ | = | X | = |
| Culpitt, 1999 [ 72 ] | 1 month (ICS) | = | = | = | X | = |
| Yildiz, 2000 [ 73 ] | 2 months (ICS) | = | ↓ | = | X | = |
| Loppow, 2001 [ 71 ] | 1 month (ICS) | = | = | = | X | = |
| Mirici, 2001 [ 97 ] | 3 months (ICS) | ↑ | = | = | X | = |
| Sugiura, 2003 [ 67 ] | 1 month (ICS) | = | = | ↓ | X | = |
| Brightling, 2005 [ 70 ] | 6 weeks (ICS) | = | = | = | X | = |
| Barnes, 2006 [ 63 ] | 3 months (ICS/LABA) | X | ↓ | ↓ | X | X |
| Reid, 2008 [ 59 ] | 6 months (ICS) | = | = | = | = | = |
| Lapperre, 2009 [ 60 ] | 6 months (ICS) | = | = | = | X | = |
| 6 months (ICS/LABA) | = | = | = | X | = | |
| 30 months (ICS) | ↓ | ↓ | = | X | ↓ | |
| 30 months (ICS/LABA) | ↓ | ↓ | = | X | ↓ | |
| Boorsma, 2008 [ 69 ] | 6 months (ICS) | = | = | = | X | = |
| Asai, 2015 [ 68 ] | 3 months (ICS/LABA) | X | = | X | X | X |
=: no change; ↑: increase; ↓: decrease; X: not measured. LABA: long-acting β-agonist.
Overall, these studies show some evidence that ICS can suppress inflammatory cell counts in COPD patients. However, the findings are not consistent across studies, with many studies reporting negative findings. The reasons for negative findings may include insufficient sample size or variability of cell counts. The heterogeneity of inflammation in COPD patients is also important, as these group mean data will not reveal individual responders with distinct ICS-responsive pathophysiology. Nevertheless, one of the interesting findings is the reduction in bronchial biopsy mast cell counts, which is supported by observations in cross-sectional studies showing reduced mast cell counts in the bronchial biopsies of ICS users compared with non-users [75].
Eosinophils, type 2 inflammation and corticosteroid response in COPD
Lung tissue eosinophil numbers are higher in COPD patients compared with healthy controls [46, 76, 77]. Average sputum eosinophil levels have been reported as 0.6–1.1% in healthy subjects [78–80] compared with 2.3% in COPD [81]. BEC has been used as a surrogate marker for sputum or lung tissue eosinophil counts, as most studies have demonstrated a positive correlation between these parameters [82–87]. The strength of the association has been variable, although strong correlations have been reported [65, 82–84, 86, 87]. Furthermore, higher BEC predicts sputum eosinophil ≥3% with the area under the receiver operating characteristic curve ranging from 0.75 to 0.82 [83, 88–90], while bronchial submucosal eosinophil counts are increased in COPD patients with higher BEC [83, 86]. This topic has been reviewed extensively elsewhere, highlighting that methodological issues such as lack of precision regarding BEC methodology, variability in multicentre studies and regional variation in lung tissue eosinophil counts can influence the results reported [5, 7]. Overall, the weight of evidence shows an association, albeit imperfect, between BEC and eosinophil numbers in the lungs, supporting the use of BEC as a practical surrogate biomarker for lung eosinophil counts.
The first studies to evaluate the relationship between eosinophils and corticosteroid response in stable COPD patients showed that higher sputum eosinophil counts were associated with greater clinical responses to corticosteroids [91–93]. For example, Brightling et al. [92] conducted a placebo-controlled, randomised crossover trial investigating 2-week treatment with oral corticosteroids (OCS); OCS treatment improved post-bronchodilator FEV1 and Chronic Respiratory Questionnaire (CRQ) score in COPD patients with higher baseline sputum eosinophil counts (≥3%), accompanied by a reduction in sputum eosinophil counts from 2.4% to 0.4% (p<0.0001). The suppression of sputum eosinophil counts by OCS has been observed elsewhere [91, 94–96]. Subsequently, the same research group conducted a placebo-controlled clinical trial to assess the effects of ICS administered for 6 weeks in COPD patients [70]. This study also showed greater treatment responses in terms of FEV1 and CRQ in patients with higher sputum eosinophil counts. However, ICS did not decrease sputum eosinophil counts. As already discussed, the majority of studies investigating sputum or bronchial biopsy eosinophil counts after ICS treatment have also observed no changes in eosinophil counts, although some studies have shown a reduction (summarised in tables 1 and 2) [73, 74, 97, 98]. In contrast, OCS can suppress BEC, thereby reducing eosinophil traffic into the lungs.
These sputum studies led to post-hoc analyses of RCTs in COPD patients with a history of exacerbations showing that BEC at baseline could predict the benefit of ICS on exacerbation prevention [6, 99]. Subsequently, BEC was analysed prospectively in RCTs of inhaled triple therapy, providing further validation for BEC as a predictive pharmacological biomarker for ICS effects [2, 4, 100]. We now consider the mechanisms responsible for this differential response according to BEC, as ICS do not appear to target eosinophil traffic into the lungs [59, 60, 70, 73].
There is evidence that type 2 (T2) airway inflammation is increased in COPD patients with higher BEC, summarised in supplementary table S1. It is reasonable to assume that the pharmacological targets of ICS reside within this profile of T2 inflammation. The protein or gene expression levels of a number of cytokines involved in eosinophil recruitment and activation are increased in COPD patients with higher eosinophil counts, including IL-5 [101] and chemokine C-C motif ligand (CCL) 11, CCL24 and CCL26, which are also involved in basophil recruitment [102]. However, it seems unlikely that these targets can explain the clinical benefit of ICS in COPD, given the lack of consistent evidence for ICS suppression of lung eosinophil numbers [59, 61, 62, 64, 70, 73, 74, 103] (summarised in tables 1 and 2).
A study using both bronchial brush and sputum samples (n=27) and a replication sputum cohort (n=33) showed that gene expression levels of the following T2 biomarkers were increased in COPD patients with higher BEC, i.e. chloride channel accessory 1 (CLCA1), CCL26, IL13 and cystatin SN (CST1) [104]. Importantly, some T2 genes previously reported to be increased in asthma were not associated with eosinophilic inflammation in COPD, i.e. periostin (POSTN) and serpin family B member 2 (SERPINB2). An analysis of bronchial brush samples from a large COPD cohort (n=283) also highlighted that gene expression changes in eosinophilic asthma and eosinophilic COPD showed marked differences [105]. Nevertheless, these T2 genes present in COPD patients with higher BEC are plausible targets for ICS in COPD.
Transcriptome analysis of bronchial biopsies collected during the GLUCOLD clinical trial reported increased T2-related gene expression in a subset of COPD patients with increased blood and lung eosinophil numbers, which was associated with increased ICS responsiveness [106]. A subsequent analysis of the GLUCOLD data, using hierarchical clustering of genes previously defined using severe asthma sputum samples, identified subgroups of COPD patients with different transcriptome signatures associated with T2 inflammation, inflammasome activation or mitochondrial activation [107]. Following ICS intervention, only the T2 signature was supressed. Interestingly, the mast cell genes carboxypeptidase A3 (CPA3) and tryptase β2 (TPSB2) were major contributors to this response. The authors also observed reductions in mast cell numbers by ICS [107].
The pathophysiological role of mast cells in COPD remains to be fully elucidated. Elevated mast cell numbers have been observed in asymptomatic smokers [108] and COPD patients [109, 110], although negative results have also been reported [111, 112]. The role of CPA3 may be important in COPD, as CPA3 gene expression is higher in areas of increased collagen deposition in COPD distal lung tissue, suggesting a role in remodelling [111]. Increased CPA3 expression was also observed in bronchial brush samples of COPD patients with increased pulmonary eosinophils [113, 114].
The signalling pathways which orchestrate eosinophilic/T2 inflammation in COPD are unclear, but one possible candidate is IL-33. IL-33 is an alarmin released from the airway epithelium in response to inflammation or tissue damage, promoting a wide range of both T1 and T2 responses. IL-33 can mediate eosinophil recruitment and homeostasis [115] by upregulating IL-5 secretion from mast cells and T2 innate lymphoid cells (ILC2). Elevated levels of blood and pulmonary IL-33 have been observed in COPD patients compared with healthy controls [116, 117], and are more notable in COPD patients with higher eosinophil counts [118]. RCTs of COPD patients using monoclonal antibodies directed towards IL-33 [119] or its receptor ST2 [120] reduce blood [119, 120] and sputum [120] eosinophil counts. The effect of ICS on IL-33 levels in COPD has not been investigated. However, corticosteroids do not reduce IL-33 expression in animal models and IL-33 expression is elevated in the airways of severe asthma patients despite using high-dose ICS [121, 122]. These observations suggest a corticosteroid-insensitive IL-33–ILC2–IL-5 axis that orchestrates pulmonary eosinophil recruitment in COPD.
ICS and the microbiome
Some COPD patients have increased bacterial load during the stable state; the most common colonising species reported are H. influenzae, Moraxella catarrhalis and Streptococcus pneumoniae [123, 124]. Airway microbiome studies using 16S rRNA sequencing have shown dysbiosis in COPD compared with healthy controls, with reduced diversity and increased presence of proteobacteria, including Haemophilus and Moraxella genera [124–127]. Dysbiosis in COPD patients has also been associated with exacerbation frequency and mortality [128].
COPD patients with low blood or sputum eosinophil counts have an airway microbiome with increased abundance of proteobacteria, notably Haemophilus [125, 127]. A potential explanation for this association is that COPD patients with lower BEC also have lower levels of airway immunoglobulins (IgA, IgM and IgG1), associated with decreased bacterial opsonisation and B-cell activation leading to reduced antiproteobacterial immunity [129]. Furthermore, the presence of Haemophilus colonisation in the stable state is associated with increased sputum neutrophil counts [46, 124, 125, 130, 131]. These observations highlight that the microbiome can skew the profile of airway inflammation, implying a close interplay between the microbiome, inflammation and ICS responses in COPD.
Cohort studies and RCTs have investigated the relationship between ICS use and the airway microbiome in COPD during the stable state [46, 124–127, 132, 133] (table 3). Cohort studies using sputum samples have shown that ICS use can alter the microbiome [132], e.g. a small study reported increased relative abundance of H. influenzae and M. catarrhalis in sputum in ICS versus non-ICS users utilising both 16S rRNA sequencing and species-specific quantitative PCR (qPCR) (n=25 versus n=7, respectively) [124], while another study reported increased bacterial load (specifically Streptococcus) in COPD ICS users versus non-users (n=10 versus n=13, respectively) [134]. Other cohort studies have shown no differences in sputum bacteriology due to ICS use [46, 125], with these variable results between studies likely attributable to both limited sample sizes and the clinical heterogeneity of different cohorts.
TABLE 3.
Studies investigating the effects of inhaled corticosteroids (ICS) on microbiome changes
| First author, year [ref.] | Type of study | Sample | Bacterial detection method |
COPD patients
ICS/no ICS |
Key findings | ||
| Bacterial load | Species change | Diversity | |||||
| Garcha, 2012 [ 132 ] | Cohort | Sputum | qPCR | 47/5 | ↑ | = | X |
| Wang, 2019 [ 124 ] | Cohort | Sputum | 16S rRNA+qPCR | 25/7 | = | ↑ H. influenzae ↑ M. catarrhalis |
↓ |
| Ramsheh, 2021 [ 126 ] | Cohort | Bronchial brush | 16S rRNA | 192/147 | = | = | ↓ |
| Beech, 2020 [ 125 ] | Cohort | Sputum | qPCR | 199/37 | = | = | X |
| Beech, 2021 [ 46 ] | Cohort | Sputum | qPCR | 43/17 | = | = | X |
| Singanayagam, 2019 [ 134 ] | Cohort | Sputum | 16S rRNA | 10/13 | ↑ | ↑ S. pneumoniae | X |
| Contoli, 2017 [ 98 ] | RCT | Sputum | qPCR | 30/30 | ↑ | ↑ H. influenzae# ↑ S. pneumoniae# |
↑ |
| Leitao Filho, 2021 [ 135 ] | RCT | Bronchial brush | 16S rRNA | 18 FP/20 | = | ↓ Haemophilus ↑ Firmicutes |
↓ |
| 18 BD/20 | = | = | = | ||||
=: no change; ↑: increase; ↓: decrease; X: not measured. qPCR: quantitative PCR; 16S rRNA: 16S ribosomal RNA gene sequencing; H. influenzae: Haemophilus influenzae; M. catarrhalis: Moraxella catarrhalis; S. pneumoniae: Streptococcus pneumoniae; RCT: randomised controlled trial; FP: fluticasone propionate; BD: budesonide. #: in eosinophil-low patients.
A large cross-sectional bronchoscopy study using bronchial brush samples (192 ICS users and 147 ICS non-users) showed no change in bacterial load due to ICS use. However, ICS treatment reduced microbiome diversity [126], and lower lung function was associated with an increase in relative abundance (relative to other bacterial genera) of Moraxella and a decrease of Prevotella, with Prevotella relative abundance further decreased in patients treated with ICS.
A 12-month parallel group RCT (n=60) showed that treatment with ICS/LABA (fluticasone propionate/salmeterol) was associated with increased sputum bacterial loads (+12%) compared with baseline, while LABA only (salmeterol) caused no change [98]. There were also increases in the relative abundance of the species S. pneumoniae and H. influenzae compared with baseline after ICS treatment. Subgroup analysis indicated that the changes only occurred in patients with <2% blood eosinophils.
Leitao Filho et al. [135] compared the effects of two different ICS/LABA formulations (budesonide/formoterol and fluticasone/salmeterol) versus LABA only (formoterol) on the airway microbiome from bronchial brush samples obtained in COPD patients over 12 weeks (n=56), using a randomised parallel group design. Fluticasone propionate/salmeterol was associated with a significant reduction in airway microbiome diversity compared with formoterol and a greater number of microbiome changes from baseline (12 changes in taxa features, including increases in relative abundance of the Firmicutes phylum) compared with both formoterol and budesonide/formoterol (two changes for each treatment). ICS treatment did not cause any differences in total bacterial loads [135].
The notable common findings in these clinical studies are that ICS use can reduce bacterial diversity with alterations in the Proteobacteria and Firmicutes phyla, and that specific increases in the relative abundance of bacterial species H. influenzae, M. catarrhalis and S. pneumoniae have been observed, with more consistent evidence across studies for the increase in H. influenzae. These microbiome alterations may have detrimental effects leading to increases in inflammatory burden [46, 124, 125, 136]. However, as these changes occur in patients with lower BEC [98], the risks will be mitigated by only targeting ICS to COPD patients with higher BEC. The differences between studies with regard to these species may relate to clinical and geographical heterogeneity between cohorts, leading to differences in microbiome composition.
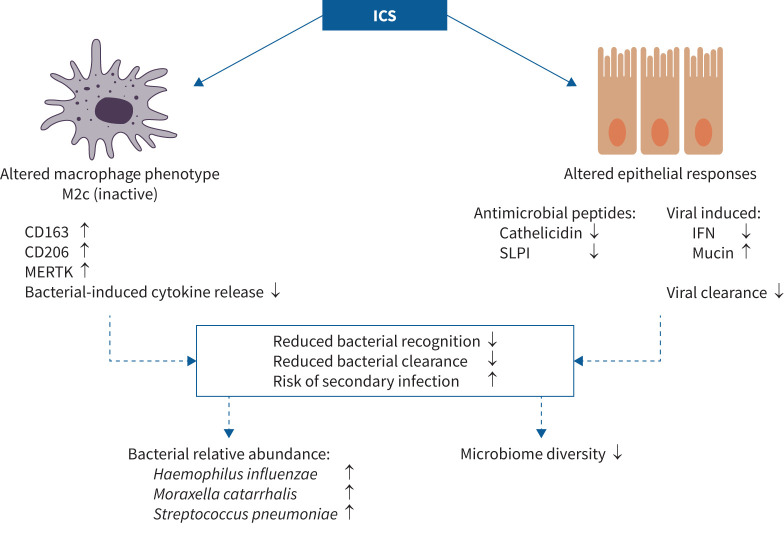
COPD macrophages show a reduced ability to phagocytose bacteria (both H. influenzae and S. pneumoniae) [30, 137, 138], a defect which is more prominent in COPD patients with frequent exacerbations [139]. Decreased S. pneumoniae clearance in COPD macrophages relates to increased expression of the antiapoptosis factor Mcl-1 which prevents apoptosis onset, required for effective bacterial clearance [140]. H. influenzae exposure increases Mcl-1 [45], thus providing a mechanism to reduce clearance of both H. influenzae itself and S. pneumoniae. Corticosteroids in vitro do not appear to directly reduce macrophage phagocytosis ability [137]. However, corticosteroids can alter macrophage phenotype [141–143] skewing towards an M2c (inactive)-like subset [144, 145], with corticosteroids causing increased expression of the scavenger markers CD163, CD206 and Mer tyrosine kinase (MERTK) observed in monocyte-derived macrophages [146, 147] and COPD lung macrophages [144]. In COPD lung macrophages both fluticasone propionate and budesonide alter expression of markers involved in bacterial recognition [144] and reduce S. pneumoniae- or H. influenzae-induced cytokine release [148]. However while fluticasone propionate increased Toll-like receptor (TLR) 2 and TLR4 in response to S. pneumoniae, budesonide counteracted the reduction of bacterial recognition markers scavenger receptor (SR)-AI (S. pneumoniae induced), CD206 and macrophage receptor with collagenous structure (MARCO) (H. influenzae induced) [148]. There is evidence to suggest that pneumonia risk differs between ICS molecules [149]. A retrospective, real-world analysis by Price et al. [150] found new COPD users of fixed-dose combination inhalers had an increased risk of developing pneumonia with formulations containing fluticasone propionate or furoate compared with extrafine beclomethasone. This differential risk with ICS formulations may be due to subtle differences in bacterial recognition markers [148] leading to downstream effects on bacterial recognition and survival. However, some caution should be applied when interpreting the clinical evaluation of pneumonia risk with different ICS, due to the potential confounders in retrospective data analysis and differences in designs and population characteristics between RCTs discussed earlier [21].
ICS can modulate the secretion of antimicrobial peptides, thus altering host defence effectiveness. In a mouse model, pulmonary clearance of bacteria was impaired by ICS through suppression of the antimicrobial peptide cathelicidin induced by S. pneumoniae, leading to increased bacterial loads in both S. pneumoniae-infected mice and a human airway epithelial cell model [151].
In COPD the frequency of secondary bacterial infections is increased by rhinovirus (RV) infection [152]. In RV-infected airway epithelial cells from COPD patients and a mouse model, ICS reduced production of IFNs and delayed viral clearance. In these models, ICS also supressed induction of the secretory leukoprotease inhibitor (SLPI) protein (an antimicrobial peptide implicated in protection against secondary bacterial infection [152]) and further increased mucin production observed in response to RV infection [142]. In COPD patients taking ICS, sputum cell expression of IFNs and SLPI was decreased and mucin production increased following viral exacerbation compared with those patients not on ICS. Additionally, 16S qPCR analysis showed increased sputum bacterial load in ICS users 2 weeks post-exacerbation compared with non-users [142]. In a separate cohort, the same authors also showed that COPD patients with at least two exacerbations in the preceding year, who had a trend towards greater ICS use compared with patients with less than two exacerbations, had reduced IFN expression in sputum cells following a virus-associated exacerbation [153]. IFNs induce an antimicrobial state in infected and neighbouring cells, helping to control pro-inflammatory pathways, activate adaptive immunity and prime for future responses [154]. Suppression of IFNs therefore may result in an increased susceptibility to infection [153].
The studies reviewed here show that ICS can engage different mechanisms, which facilitate an environment more susceptible to bacterial colonisation. These mechanisms include: 1) dampening and altering the response to viral exposure, which is associated with secondary bacterial overgrowth; 2) suppression of the secretion of antibacterial peptides; and 3) altering the phenotypic characteristics of macrophages, which may in turn alter bacterial recognition. Taken together, these mechanisms enable environmental changes that can facilitate increases in colonising species such as H. influenzae or S. pneumoniae (figure 1).
FIGURE 1.
Mechanisms by which inhaled corticosteroids (ICS) facilitate an environment more susceptible to bacterial colonisation. CD: cluster of differentiation; IFN: interferon; MERTK: Mer tyrosine kinase; SLPI: secretory leukoprotease inhibitor.
Conclusion
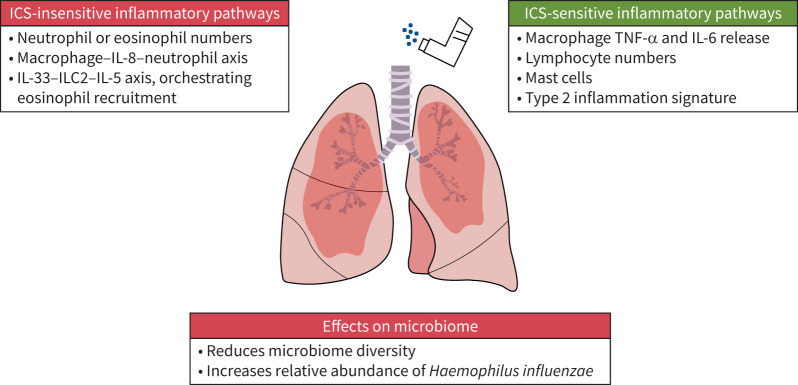
Historically, the term “corticosteroid resistant” was used to describe COPD. We now recognise that ICS provide clinical benefits in the subset of COPD patients with a history of exacerbations plus higher BEC [6, 7, 99]. This shift in our understanding raises new mechanistic questions, including why this subgroup of individuals respond and what are the components of COPD inflammation targeted by ICS. While BEC enables prediction of ICS therapeutic efficacy in COPD, lung eosinophils themselves do not seem to be the primary target of ICS [59, 60, 70, 73]. Instead, higher BEC is a biomarker of a broader T2 profile in the lungs which can be modified by ICS treatment [107]. Interestingly, this profile includes mast cells [107], which may be an important target for ICS in COPD patients [155]. Lung sampling studies have demonstrated that the expression of T2 inflammation varies between individuals, providing an explanation for the differential effect (between individuals) of ICS.
There is evidence for corticosteroid-insensitive pathways in COPD. There is an inverse relationship between eosinophil counts and proteobacteria [125, 127], while proteobacteria encourage corticosteroid-insensitive IL-8 production and neutrophil recruitment [43–46]. This is an example of the interplay between the microbiome, inflammation and ICS. A summary of key corticosteroid-sensitive and -insensitive pathways and the effects of ICS on the microbiome is shown in figure 2. ICS can alter host responses to pathogens, e.g. through the suppression of antibacterial peptide secretion and alterations of macrophage phenotype. These mechanisms can skew the lung microbiome towards an excess of proteobacteria following ICS treatment.
FIGURE 2.
A summary of key corticosteroid-sensitive and -insensitive pathways and the effects of inhaled corticosteroids (ICS) on the microbiome. IL: interleukin; ILC2: type 2 innate lymphoid cells; TNF: tumour necrosis factor.
The first observations that eosinophil counts were related to ICS efficacy has led to a more complex understanding of ICS mechanisms in COPD, encompassing the identification of corticosteroid-sensitive and -insensitive pathways and the role of the microbiome. While BEC is the biomarker used in clinical practice to enable the selective use of ICS in COPD, there is a complex interacting network involving the microbiome, airway inflammation and ICS that decides the ultimate clinical outcome in each individual.
Points for clinical practice
Higher BEC in COPD associates with T2 airway inflammation. Individuals with this T2 inflammation can experience a benefit from ICS.
Lower BEC in COPD is associated with skewing of the microbiome towards proteobacteria dominance, with increased neutrophilic airway inflammation. ICS can increase bacterial infection and pneumonia risk in these individuals.
Questions for the future
What are the effects of ICS on mast cells and IL-33 in COPD?
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0084-2023.SUPPLEMENT (285.6KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: D. Singh has received sponsorship to attend and speak at international meetings or honoraria for lecturing or attending advisory boards from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Teva, Theravance and Verona. A. Beech, A. Higham and S. Lea have no conflicts of interest to declare.
Support statement: A. Beech, S. Lea and D. Singh are supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
References
- 1.Agustí A, Celli BR, Criner GJ, et al. . Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur Respir J 2023; 61: 2300239. doi: 10.1183/13993003.00239-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipson DA, Barnhart F, Brealey N, et al. . Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med 2018; 378: 1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Martinez FJ, Ferguson GT, et al. . Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med 2020; 383: 35–48. doi: 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 4.Papi A, Vestbo J, Fabbri L, et al. . Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018; 391: 1076–1084. doi: 10.1016/S0140-6736(18)30206-X [DOI] [PubMed] [Google Scholar]
- 5.Singh D, Bafadhel M, Brightling CE, et al. . Blood eosinophil counts in clinical trials for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2020; 202: 660–671. doi: 10.1164/rccm.201912-2384PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bafadhel M, Peterson S, De Blas MA, et al. . Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. doi: 10.1016/S2213-2600(18)30006-7 [DOI] [PubMed] [Google Scholar]
- 7.Singh D, Agusti A, Martinez FJ, et al. . Blood eosinophils and chronic obstructive pulmonary disease: a Global Initiative for Chronic Obstructive Lung Disease Science Committee 2022 Review. Am J Respir Crit Care Med 2022; 206: 17–24. doi: 10.1164/rccm.202201-0209PP [DOI] [PubMed] [Google Scholar]
- 8.Culpitt SV, Rogers DF, Shah P, et al. . Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167: 24–31. doi: 10.1164/rccm.200204-298OC [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet 2004; 363: 731–733. doi: 10.1016/S0140-6736(04)15650-X [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Corradi M, Spinola M, et al. . Extrafine beclometasone diproprionate/formoterol fumarate: a review of its effects in chronic obstructive pulmonary disease. NPJ Prim Care Respir Med 2016; 26: 16030. doi: 10.1038/npjpcrm.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh D, Agusti A, Anzueto A, et al. . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019; 53: 1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 12.Dransfield MT, Bourbeau J, Jones PW, et al. . Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 2013; 1: 210–223. doi: 10.1016/S2213-2600(13)70040-7 [DOI] [PubMed] [Google Scholar]
- 13.Halpin DMG, Dransfield MT, Han MK, et al. . The effect of exacerbation history on outcomes in the IMPACT trial. Eur Respir J 2020; 55: 1901921. doi: 10.1183/13993003.01921-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh D, Fabbri LM, Corradi M, et al. . Extrafine triple therapy in patients with symptomatic COPD and history of one moderate exacerbation. Eur Respir J 2019; 53: 1900235. doi: 10.1183/13993003.00235-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipson DA, Dransfield MT, Han MK. Reply to Suissa: Mortality in IMPACT: confounded by asthma? Am J Respir Crit Care Med 2020; 202: 773–774. doi: 10.1164/rccm.202004-1399LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothnie KJ, Mullerova H, Smeeth L, et al. . Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 464–471. doi: 10.1164/rccm.201710-2029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dransfield MT, Criner GJ, Halpin DMG, et al. . Time-dependent risk of cardiovascular events following an exacerbation in patients with chronic obstructive pulmonary disease: post hoc analysis from the IMPACT trial. J Am Heart Assoc 2022; 11: e024350. doi: 10.1161/JAHA.121.024350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunisaki KM, Dransfield MT, Anderson JA, et al. . Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med 2018; 198: 51–57. doi: 10.1164/rccm.201711-2239OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipson DA, Crim C, Criner GJ, et al. . Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2020; 201: 1508–1516. doi: 10.1164/rccm.201911-2207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FJ, Rabe KF, Ferguson GT, et al. . Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med 2021; 203: 553–564. doi: 10.1164/rccm.202006-2618OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wise RA, Bafadhel M, Crim C, et al. . Discordant diagnostic criteria for pneumonia in COPD trials: a review. Eur Respir Rev 2021; 30: 210124. doi: 10.1183/16000617.0124-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol 2007; 21: 2311–2319. doi: 10.1210/me.2007-0101 [DOI] [PubMed] [Google Scholar]
- 23.Weikum ER, Knuesel MT, Ortlund EA, et al. . Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat Rev Mol Cell Biol 2017; 18: 159–174. doi: 10.1038/nrm.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol 2007; 72: 799–809. doi: 10.1124/mol.107.038794 [DOI] [PubMed] [Google Scholar]
- 25.Keatings VM, Collins PD, Scott DM, et al. . Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996; 153: 530–534. doi: 10.1164/ajrccm.153.2.8564092 [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Lozach J, Benner C, et al. . Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 2005; 122: 707–721. doi: 10.1016/j.cell.2005.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewhurst JA, Lea S, Hardaker E, et al. . Characterisation of lung macrophage subpopulations in COPD patients and controls. Sci Rep 2017; 7: 7143. doi: 10.1038/s41598-017-07101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Baker J, Higham A, et al. . COPD lung studies of Nrf2 expression and the effects of Nrf2 activators. Inflammopharmacology 2022; 30: 1431–1443. doi: 10.1007/s10787-022-00967-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg JC, Chu F, Utokaparch S, et al. . The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 30.Berenson CS, Kruzel RL, Eberhardt E, et al. . Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis 2013; 208: 2036–2045. doi: 10.1093/infdis/jit400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosio BG, Tsaprouni L, Ito K, et al. . Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med 2004; 200: 689–695. doi: 10.1084/jem.20040416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knobloch J, Hag H, Jungck D, et al. . Resveratrol impairs the release of steroid-resistant cytokines from bacterial endotoxin-exposed alveolar macrophages in chronic obstructive pulmonary disease. Basic Clin Pharmacol Toxicol 2011; 109: 138–143. doi: 10.1111/j.1742-7843.2011.00707.x [DOI] [PubMed] [Google Scholar]
- 33.Ito K, Yamamura S, Essilfie-Quaye S, et al. . Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 2006; 203: 7–13. doi: 10.1084/jem.20050466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higham A, Booth G, Lea S, et al. . The effects of corticosteroids on COPD lung macrophages: a pooled analysis. Respir Res 2015; 16: 98. doi: 10.1186/s12931-015-0260-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong J, Harbron C, Lea S, et al. . Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease. J Pharmacol Exp Ther 2011; 338: 732–740. doi: 10.1124/jpet.111.180737 [DOI] [PubMed] [Google Scholar]
- 36.Armstrong J, Sargent C, Singh D. Glucocorticoid sensitivity of lipopolysaccharide-stimulated chronic obstructive pulmonary disease alveolar macrophages. Clin Exp Immunol 2009; 158: 74–83. doi: 10.1111/j.1365-2249.2009.03986.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Southworth T, Metryka A, Lea S, et al. . IFN-gamma synergistically enhances LPS signalling in alveolar macrophages from COPD patients and controls by corticosteroid-resistant STAT1 activation. Br J Pharmacol 2012; 166: 2070–2083. doi: 10.1111/j.1476-5381.2012.01907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plumb J, Robinson L, Lea S, et al. . Evaluation of glucocorticoid receptor function in COPD lung macrophages using beclomethasone-17-monopropionate. PLoS One 2013; 8: e64257. doi: 10.1371/journal.pone.0064257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lea S, Plumb J, Metcalfe H, et al. . The effect of peroxisome proliferator-activated receptor-gamma ligands on in vitro and in vivo models of COPD. Eur Respir J 2014; 43: 409–420. doi: 10.1183/09031936.00187812 [DOI] [PubMed] [Google Scholar]
- 40.Higham A, Lea S, Plumb J, et al. . The role of the liver X receptor in chronic obstructive pulmonary disease. Respir Res 2013; 14: 106. doi: 10.1186/1465-9921-14-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higham A, Lea S, Ray D, et al. . Corticosteroid effects on COPD alveolar macrophages: dependency on cell culture methodology. J Immunol Methods 2014; 405: 144–153. doi: 10.1016/j.jim.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higham A, Karur P, Jackson N, et al. . Differential anti-inflammatory effects of budesonide and a p38 MAPK inhibitor AZD7624 on COPD pulmonary cells. Int J Chron Obstruct Pulmon Dis 2018; 13: 1279–1288. doi: 10.2147/COPD.S159936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosio BG, Jahn A, Iglesias A, et al. . Haemophilus influenzae induces steroid-resistant inflammatory responses in COPD. BMC Pulm Med 2015; 15: 157. doi: 10.1186/s12890-015-0155-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khalaf RM, Lea SR, Metcalfe HJ, et al. . Mechanisms of corticosteroid insensitivity in COPD alveolar macrophages exposed to NTHi. Respir Res 2017; 18: 61. doi: 10.1186/s12931-017-0539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lea S, Beech A, Baker J, et al. . Differential responses of COPD macrophages to respiratory bacterial pathogens. ERJ Open Res 2022; 8: 00044-2022. doi: 10.1183/23120541.00044-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beech A, Lea S, Li J, et al. . Airway bacteria quantification using polymerase chain reaction combined with neutrophil and eosinophil counts identifies distinct COPD endotypes. Biomedicines 2021; 9: 1337. doi: 10.3390/biomedicines9101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plumb J, Gaffey K, Kane B, et al. . Reduced glucocorticoid receptor expression and function in airway neutrophils. Int Immunopharmacol 2012; 12: 26–33. doi: 10.1016/j.intimp.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 48.Kent LM, Smyth LJ, Plumb J, et al. . Inhibition of lipopolysaccharide-stimulated chronic obstructive pulmonary disease macrophage inflammatory gene expression by dexamethasone and the p38 mitogen-activated protein kinase inhibitor N-cyano-N′-(2-{[8-(2,6-difluorophenyl)-4-(4-fluoro-2-methylphenyl)-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidin-2-yl]amino}ethyl)guanidine (SB706504). J Pharmacol Exp Ther 2009; 328: 458–468. doi: 10.1124/jpet.108.142950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palma L, Amatori S, Cruz Chamorro I, et al. . Promoter-specific relevance of histone modifications induced by dexamethasone during the regulation of pro-inflammatory mediators. Biochim Biophys Acta 2014; 1839: 571–578. doi: 10.1016/j.bbagrm.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 50.Angrisano T, Pero R, Peluso S, et al. . LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol 2010; 10: 172. doi: 10.1186/1471-2180-10-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol 2002; 3: 69–75. doi: 10.1038/ni748 [DOI] [PubMed] [Google Scholar]
- 52.Grundy S, Kaur M, Plumb J, et al. . CRAC channel inhibition produces greater anti-inflammatory effects than glucocorticoids in CD8 cells from COPD patients. Clin Sci 2014; 126: 223–232. doi: 10.1042/CS20130152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grundy S, Plumb J, Kaur M, et al. . Additive anti-inflammatory effects of corticosteroids and phosphodiesterase-4 inhibitors in COPD CD8 cells. Respir Res 2016; 17: 9. doi: 10.1186/s12931-016-0325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur M, Smyth LJ, Cadden P, et al. . T lymphocyte insensitivity to corticosteroids in chronic obstructive pulmonary disease. Respir Res 2012; 13: 20. doi: 10.1186/1465-9921-13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodge G, Roscioli E, Jersmann H, et al. . Steroid resistance in COPD is associated with impaired molecular chaperone Hsp90 expression by pro-inflammatory lymphocytes. Respir Res 2016; 17: 135. doi: 10.1186/s12931-016-0450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milara J, Morell A, Ballester B, et al. . Roflumilast improves corticosteroid resistance COPD bronchial epithelial cells stimulated with toll like receptor 3 agonist. Respir Res 2015; 16: 12. doi: 10.1186/s12931-015-0179-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heijink I, van Oosterhout A, Kliphuis N, et al. . Oxidant-induced corticosteroid unresponsiveness in human bronchial epithelial cells. Thorax 2014; 69: 5–13. doi: 10.1136/thoraxjnl-2013-203520 [DOI] [PubMed] [Google Scholar]
- 58.Kaur M, Singh D. Neutrophil chemotaxis caused by chronic obstructive pulmonary disease alveolar macrophages: the role of CXCL8 and the receptors CXCR1/CXCR2. J Pharmacol Exp Ther 2013; 347: 173–180. doi: 10.1124/jpet.112.201855 [DOI] [PubMed] [Google Scholar]
- 59.Reid DW, Wen Y, Johns DP, et al. . Bronchodilator reversibility, airway eosinophilia and anti-inflammatory effects of inhaled fluticasone in COPD are not related. Respirology 2008; 13: 799–809. doi: 10.1111/j.1440-1843.2008.01380.x [DOI] [PubMed] [Google Scholar]
- 60.Lapperre TS, Snoeck-Stroband JB, Gosman MM, et al. . Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2009; 151: 517–527. doi: 10.7326/0003-4819-151-8-200910200-00004 [DOI] [PubMed] [Google Scholar]
- 61.Hattotuwa KL, Gizycki MJ, Ansari TW, et al. . The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med 2002; 165: 1592–1596. doi: 10.1164/rccm.2105025 [DOI] [PubMed] [Google Scholar]
- 62.Bourbeau J, Christodoulopoulos P, Maltais F, et al. . Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax 2007; 62: 938–943. doi: 10.1136/thx.2006.071068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnes NC, Qiu YS, Pavord ID, et al. . Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med 2006; 173: 736–743. doi: 10.1164/rccm.200508-1321OC [DOI] [PubMed] [Google Scholar]
- 64.Verhoeven GT, Hegmans JP, Mulder PG, et al. . Effects of fluticasone propionate in COPD patients with bronchial hyperresponsiveness. Thorax 2002; 57: 694–700. doi: 10.1136/thorax.57.8.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turato G, Semenzato U, Bazzan E, et al. . Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1216–1219. doi: 10.1164/rccm.201708-1684LE [DOI] [PubMed] [Google Scholar]
- 66.Xu F, Vasilescu DM, Kinose D, et al. . The molecular and cellular mechanisms associated with the destruction of terminal bronchioles in COPD. Eur Respir J 2022; 59: 2101411. doi: 10.1183/13993003.01411-2021 [DOI] [PubMed] [Google Scholar]
- 67.Sugiura H, Ichinose M, Yamagata S, et al. . Correlation between change in pulmonary function and suppression of reactive nitrogen species production following steroid treatment in COPD. Thorax 2003; 58: 299–305. doi: 10.1136/thorax.58.4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asai K, Kobayashi A, Makihara Y, et al. . Anti-inflammatory effects of salmeterol/fluticasone propionate 50/250 mcg combination therapy in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10: 803–811. doi: 10.2147/COPD.S79842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boorsma M, Lutter R, van de Pol MA, et al. . Long-term effects of budesonide on inflammatory status in COPD. COPD 2008; 5: 97–104. doi: 10.1080/15412550801941000 [DOI] [PubMed] [Google Scholar]
- 70.Brightling CE, McKenna S, Hargadon B, et al. . Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005; 60: 193–198. doi: 10.1136/thx.2004.032516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loppow D, Schleiss MB, Kanniess F, et al. . In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respir Med 2001; 95: 115–121. doi: 10.1053/rmed.2000.0960 [DOI] [PubMed] [Google Scholar]
- 72.Culpitt SV, Maziak W, Loukidis S, et al. . Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 1635–1639. doi: 10.1164/ajrccm.160.5.9811058 [DOI] [PubMed] [Google Scholar]
- 73.Yildiz F, Kaur AC, Ilgazli A, et al. . Inhaled corticosteroids may reduce neutrophilic inflammation in patients with stable chronic obstructive pulmonary disease. Respiration 2000; 67: 71–76. doi: 10.1159/000029466 [DOI] [PubMed] [Google Scholar]
- 74.Confalonieri M, Mainardi E, Della Porta R, et al. . Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease. Thorax 1998; 53: 583–585. doi: 10.1136/thx.53.7.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Higham A, Dungwa J, Pham TH, et al. . Increased mast cell activation in eosinophilic chronic obstructive pulmonary disease. Clin Transl Immunology 2022; 11: e1417. doi: 10.1002/cti2.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutgers SR, Timens W, Kaufmann HF, et al. . Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J 2000; 15: 109–115. doi: 10.1183/09031936.00.15110900 [DOI] [PubMed] [Google Scholar]
- 77.Babusyte A, Stravinskaite K, Jeroch J, et al. . Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respir Res 2007; 8: 81. doi: 10.1186/1465-9921-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belda J, Leigh R, Parameswaran K, et al. . Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med 2000; 161: 475–478. doi: 10.1164/ajrccm.161.2.9903097 [DOI] [PubMed] [Google Scholar]
- 79.Davidson WJ, The S, Leigh R. Establishing a normal range for induced sputum cell counts in Western Canada. Can Respir J 2013; 20: 424–425. doi: 10.1155/2013/547309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spanevello A, Confalonieri M, Sulotto F, et al. . Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med 2000; 162: 1172–1174. doi: 10.1164/ajrccm.162.3.9908057 [DOI] [PubMed] [Google Scholar]
- 81.Bafadhel M. Eosinophils in COPD: are we nearly there yet? Lancet Respir Med 2017; 5: 913–914. doi: 10.1016/S2213-2600(17)30445-9 [DOI] [PubMed] [Google Scholar]
- 82.Hastie AT, Martinez FJ, Curtis JL, et al. . Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 956–967. doi: 10.1016/S2213-2600(17)30432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolsum U, Damera G, Pham TH, et al. . Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol 2017; 140: 1181–1184. doi: 10.1016/j.jaci.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 84.Singh D, Kolsum U, Brightling CE, et al. . Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014; 44: 1697–1700. doi: 10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 85.Higham A, Leow-Dyke S, Jackson N, et al. . Stability of eosinophilic inflammation in COPD bronchial biopsies. Eur Respir J 2020; 56: 2000622. doi: 10.1183/13993003.00622-2020 [DOI] [PubMed] [Google Scholar]
- 86.Eltboli O, Mistry V, Barker B, et al. . Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology 2015; 20: 667–670. doi: 10.1111/resp.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beech A, Lea S, Kolsum U, et al. . Stability of sputum and blood eosinophil levels in COPD. Eur Respir J 2020; 56: Suppl. 64, 3250. doi: 10.1183/13993003.congress-2020.3250 [DOI] [Google Scholar]
- 88.Singh D, Watz H, Beeh KM, et al. . COPD sputum eosinophils: relationship to blood eosinophils and the effect of inhaled PDE4 inhibition. Eur Respir J 2020; 56: 2000237. doi: 10.1183/13993003.00237-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Negewo NA, McDonald VM, Baines KJ, et al. . Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1495–1504. doi: 10.2147/COPD.S100338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schleich F, Corhay JL, Louis R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur Respir J 2016; 47: 1562–1564. doi: 10.1183/13993003.01659-2015 [DOI] [PubMed] [Google Scholar]
- 91.Pizzichini E, Pizzichini MM, Gibson P, et al. . Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 1998; 158: 1511–1517. doi: 10.1164/ajrccm.158.5.9804028 [DOI] [PubMed] [Google Scholar]
- 92.Brightling CE, Monteiro W, Ward R, et al. . Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000; 356: 1480–1485. doi: 10.1016/S0140-6736(00)02872-5 [DOI] [PubMed] [Google Scholar]
- 93.Shim C, Stover DE, Williams MH Jr. Response to corticosteroids in chronic bronchitis. J Allergy Clin Immunol 1978; 62: 363–367. doi: 10.1016/0091-6749(78)90137-9 [DOI] [PubMed] [Google Scholar]
- 94.Dummer JF, Epton MJ, Cowan JO, et al. . Predicting corticosteroid response in chronic obstructive pulmonary disease using exhaled nitric oxide. Am J Respir Crit Care Med 2009; 180: 846–852. doi: 10.1164/rccm.200905-0685OC [DOI] [PubMed] [Google Scholar]
- 95.Fujimoto K, Kubo K, Yamamoto H, et al. . Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest 1999; 115: 697–702. doi: 10.1378/chest.115.3.697 [DOI] [PubMed] [Google Scholar]
- 96.Bafadhel M, Saha S, Siva R, et al. . Sputum IL-5 concentration is associated with a sputum eosinophilia and attenuated by corticosteroid therapy in COPD. Respiration 2009; 78: 256–262. doi: 10.1159/000221902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mirici A, Bektas Y, Ozbakis G, et al. . Effect of inhaled corticosteroids on respiratory function tests and airway inflammation in stable chronic obstructive pulmonary disease. Clin Drug Investig 2001; 21: 835–842. doi: 10.2165/00044011-200121120-00006 [DOI] [Google Scholar]
- 98.Contoli M, Pauletti A, Rossi MR, et al. . Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J 2017; 50: 1700451. doi: 10.1183/13993003.00451-2017 [DOI] [PubMed] [Google Scholar]
- 99.Pascoe S, Locantore N, Dransfield MT, et al. . Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. doi: 10.1016/S2213-2600(15)00106-X [DOI] [PubMed] [Google Scholar]
- 100.Vestbo J, Papi A, Corradi M, et al. . Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. doi: 10.1016/S0140-6736(17)30188-5 [DOI] [PubMed] [Google Scholar]
- 101.Sanderson CJ. The biological role of interleukin 5. Int J Cell Cloning 1990; 8: Suppl. 1, 147–153. doi: 10.1002/stem.5530080713 [DOI] [PubMed] [Google Scholar]
- 102.Petkovic V, Moghini C, Paoletti S, et al. . Eotaxin-3/CCL26 is a natural antagonist for CC chemokine receptors 1 and 5. A human chemokine with a regulatory role. J Biol Chem 2004; 279: 23357–23363. doi: 10.1074/jbc.M309283200 [DOI] [PubMed] [Google Scholar]
- 103.Gizycki MJ, Hattotuwa KL, Barnes N, et al. . Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax 2002; 57: 799–803. doi: 10.1136/thorax.57.9.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Higham A, Beech A, Wolosianka S, et al. . Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy 2021; 76: 1861–1864. doi: 10.1111/all.14661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.George L, Taylor AR, Esteve-Codina A, et al. . Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy 2020; 75: 370–380. doi: 10.1111/all.14016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christenson SA, Steiling K, van den Berge M, et al. . Asthma–COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: 758–766. doi: 10.1164/rccm.201408-1458OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Faiz A, Pavlidis S, Kuo CH, et al. . Th2 high and mast cell gene signatures are associated with corticosteroid sensitivity in COPD. Thorax 2022; 78: 335–343. doi: 10.1136/thorax-2021-217736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ekberg-Jansson A, Amin K, Bake B, et al. . Bronchial mucosal mast cells in asymptomatic smokers relation to structure, lung function and emphysema. Respir Med 2005; 99: 75–83. doi: 10.1016/j.rmed.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 109.Grashoff WF, Sont JK, Sterk PJ, et al. . Chronic obstructive pulmonary disease: role of bronchiolar mast cells and macrophages. Am J Pathol 1997; 151: 1785–1790. [PMC free article] [PubMed] [Google Scholar]
- 110.Wen Y, Reid DW, Zhang D, et al. . Assessment of airway inflammation using sputum, BAL, and endobronchial biopsies in current and ex-smokers with established COPD. Int J Chron Obstruct Pulmon Dis 2010; 5: 327–334. doi: 10.2147/COPD.S11343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siddhuraj P, Jonsson J, Alyamani M, et al. . Dynamically upregulated mast cell CPA3 patterns in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Front Immunol 2022; 13: 924244. doi: 10.3389/fimmu.2022.924244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andersson CK, Mori M, Bjermer L, et al. . Alterations in lung mast cell populations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181: 206–217. doi: 10.1164/rccm.200906-0932OC [DOI] [PubMed] [Google Scholar]
- 113.Baines KJ, Negewo NA, Gibson PG, et al. . A sputum 6 gene expression signature predicts inflammatory phenotypes and future exacerbations of COPD. Int J Chron Obstruct Pulmon Dis 2020; 15: 1577–1590. doi: 10.2147/COPD.S245519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Southworth T, Jevnikar Z, McCrae C, et al. . A sputum 6-gene signature predicts airway inflammation endotypes and exacerbation frequency in chronic obstructive pulmonary disease. Biomark Med 2022; 16: 277–289. doi: 10.2217/bmm-2021-0653 [DOI] [PubMed] [Google Scholar]
- 115.Johnston LK, Hsu CL, Krier-Burris RA, et al. . IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol 2016; 197: 3445–3453. doi: 10.4049/jimmunol.1600611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Byers DE, Alexander-Brett J, Patel AC, et al. . Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013; 123: 3967–3982. doi: 10.1172/JCI65570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xia J, Zhao J, Shang J, et al. . Increased IL-33 expression in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2015; 308: L619–L627. doi: 10.1152/ajplung.00305.2014 [DOI] [PubMed] [Google Scholar]
- 118.Tworek D, Majewski S, Szewczyk K, et al. . The association between airway eosinophilic inflammation and IL-33 in stable non-atopic COPD. Respir Res 2018; 19: 108. doi: 10.1186/s12931-018-0807-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rabe KF, Celli BR, Wechsler ME, et al. . Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 120.Yousuf AJ, Mohammed S, Carr L, et al. . Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial. Lancet Respir Med 2022; 10: 469–477. doi: 10.1016/S2213-2600(21)00556-7 [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Wang W, Lv Z, et al. . Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol 2018; 200: 2253–2262. doi: 10.4049/jimmunol.1701455 [DOI] [PubMed] [Google Scholar]
- 122.Saglani S, Lui S, Ullmann N, et al. . IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol 2013; 132: 676–685. doi: 10.1016/j.jaci.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilkinson TMA, Aris E, Bourne S, et al. . A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 2017; 72: 919–927. doi: 10.1136/thoraxjnl-2016-209023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Z, Maschera B, Lea S, et al. . Airway host–microbiome interactions in chronic obstructive pulmonary disease. Respir Res 2019; 20: 113. doi: 10.1186/s12931-019-1085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beech AS, Lea S, Kolsum U, et al. . Bacteria and sputum inflammatory cell counts; a COPD cohort analysis. Respir Res 2020; 21: 289. doi: 10.1186/s12931-020-01552-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramsheh MY, Haldar K, Esteve-Codina A, et al. . Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: a bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe 2021; 2: e300–e310. doi: 10.1016/S2666-5247(21)00035-5 [DOI] [PubMed] [Google Scholar]
- 127.Wang Z, Bafadhel M, Haldar K, et al. . Lung microbiome dynamics in COPD exacerbations. Eur Respir J 2016; 47: 1082–1092. doi: 10.1183/13993003.01406-2015 [DOI] [PubMed] [Google Scholar]
- 128.Dicker AJ, Huang JTJ, Lonergan M, et al. . The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2021; 147: 158–167. doi: 10.1016/j.jaci.2020.02.040 [DOI] [PubMed] [Google Scholar]
- 129.Southworth T, Higham A, Kolsum U, et al. . The relationship between airway immunoglobulin activity and eosinophils in COPD. J Cell Mol Med 2021; 25: 2203–2212. doi: 10.1111/jcmm.16206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barker BL, Haldar K, Patel H, et al. . Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest 2015; 147: 46–55. doi: 10.1378/chest.14-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bafadhel M, Haldar K, Barker B, et al. . Airway bacteria measured by quantitative polymerase chain reaction and culture in patients with stable COPD: relationship with neutrophilic airway inflammation, exacerbation frequency, and lung function. Int J Chron Obstruct Pulmon Dis 2015; 10: 1075–1083. doi: 10.2147/COPD.S80091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcha DS, Thurston SJ, Patel AR, et al. . Changes in prevalence and load of airway bacteria using quantitative PCR in stable and exacerbated COPD. Thorax 2012; 67: 1075–1080. doi: 10.1136/thoraxjnl-2012-201924 [DOI] [PubMed] [Google Scholar]
- 133.Huang YJ, Sethi S, Murphy T, et al. . Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014; 52: 2813–2823. doi: 10.1128/JCM.00035-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singanayagam A, Glanville N, Cuthbertson L, et al. . Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med 2019; 11: eaav3879. doi: 10.1126/scitranslmed.aav3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leitao Filho FS, Takiguchi H, Akata K, et al. . Effects of inhaled corticosteroid/long-acting β2-agonist combination on the airway microbiome of patients with chronic obstructive pulmonary disease: a randomized controlled clinical trial (DISARM). Am J Respir Crit Care Med 2021; 204: 1143–1152. doi: 10.1164/rccm.202102-0289OC [DOI] [PubMed] [Google Scholar]
- 136.Mulvanny A, Pattwell C, Beech A, et al. . Validation of sputum biomarker immunoassays and cytokine expression profiles in COPD. Biomedicines 2022; 10: 1949. doi: 10.3390/biomedicines10081949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor AE, Finney-Hayward TK, Quint JK, et al. . Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J 2010; 35: 1039–1047. doi: 10.1183/09031936.00036709 [DOI] [PubMed] [Google Scholar]
- 138.Lea S, Gaskell R, Hall S, et al. . Assessment of bacterial exposure on phagocytic capability and surface marker expression of sputum macrophages and neutrophils in COPD patients. Clin Exp Immunol 2021; 206: 99–109. doi: 10.1111/cei.13638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh R, Belchamber KBR, Fenwick PS, et al. . Defective monocyte-derived macrophage phagocytosis is associated with exacerbation frequency in COPD. Respir Res 2021; 22: 113. doi: 10.1186/s12931-021-01718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bewley MA, Preston JA, Mohasin M, et al. . Impaired mitochondrial microbicidal responses in chronic obstructive pulmonary disease macrophages. Am J Respir Crit Care Med 2017; 196: 845–855. doi: 10.1164/rccm.201608-1714OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang P, Wang X, Yang X, et al. . Budesonide suppresses pulmonary antibacterial host defense by down-regulating cathelicidin-related antimicrobial peptide in allergic inflammation mice and in lung epithelial cells. BMC Immunol 2013; 14: 7. doi: 10.1186/1471-2172-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singanayagam A, Glanville N, Girkin JL, et al. . Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun 2018; 9: 2229. doi: 10.1038/s41467-018-04574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tomita T, Nagase T, Ohga E, et al. . Molecular mechanisms underlying human beta-defensin-2 gene expression in a human airway cell line (LC2/ad). Respirology 2002; 7: 305–310. doi: 10.1046/j.1440-1843.2002.00415.x [DOI] [PubMed] [Google Scholar]
- 144.Higham A, Scott T, Li J, et al. . Effects of corticosteroids on COPD lung macrophage phenotype and function. Clin Sci 2020; 134: 751–763. doi: 10.1042/CS20191202 [DOI] [PubMed] [Google Scholar]
- 145.Desgeorges T, Caratti G, Mounier R, et al. . Glucocorticoids shape macrophage phenotype for tissue repair. Front Immunol 2019; 10: 1591. doi: 10.3389/fimmu.2019.01591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zizzo G, Hilliard BA, Monestier M, et al. . Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 2012; 189: 3508–3520. doi: 10.4049/jimmunol.1200662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ehrchen J, Steinmuller L, Barczyk K, et al. . Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 2007; 109: 1265–1274. doi: 10.1182/blood-2006-02-001115 [DOI] [PubMed] [Google Scholar]
- 148.Provost KA, Smith M, Miller-Larsson A, et al. . Bacterial regulation of macrophage bacterial recognition receptors in COPD are differentially modified by budesonide and fluticasone propionate. PLoS One 2019; 14: e0207675. doi: 10.1371/journal.pone.0207675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tashkin DP, Miravitlles M, Celli BR, et al. . Concomitant inhaled corticosteroid use and the risk of pneumonia in COPD: a matched-subgroup post hoc analysis of the UPLIFT trial. Respir Res 2018; 19: 196. doi: 10.1186/s12931-018-0874-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Price DB, Henley W, Cancado JED, et al. . Interclass difference in pneumonia risk in COPD patients initiating fixed dose inhaled treatment containing extrafine particle beclometasone versus fine particle fluticasone. Int J Chron Obstruct Pulmon Dis 2022; 17: 355–370. doi: 10.2147/COPD.S342357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kamal F, Glanville N, Xia W, et al. . Beclomethasone has lesser suppressive effects on inflammation and antibacterial immunity than fluticasone or budesonide in experimental infection models. Chest 2020; 158: 947–951. doi: 10.1016/j.chest.2020.05.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mallia P, Footitt J, Sotero R, et al. . Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 1117–1124. doi: 10.1164/rccm.201205-0806OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singanayagam A, Loo SL, Calderazzo M, et al. . Antiviral immunity is impaired in COPD patients with frequent exacerbations. Am J Physiol Lung Cell Mol Physiol 2019; 317: L893–L903. doi: 10.1152/ajplung.00253.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014; 14: 36–49. doi: 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Higham A, Singh D. Inhaled corticosteroid responses in COPD: do mast cells hold the answer? Thorax 2022; 78: 323–324. doi: 10.1136/thorax-2022-219534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0084-2023.SUPPLEMENT (285.6KB, pdf)