Abstract
Objectives
Examine whether data from early access to medicines in the USA can be used to inform National Institute for Health and Care Excellence (NICE) health technology assessments (HTA) in oncology.
Design
Retrospective cohort study.
Setting
Oncology-based community and academic treatment centres in the USA.
Participants
Patients present in a nationwide electronic health record (EHR)-derived deidentified database.
Interventions
Cancer drugs that underwent NICE technology appraisal (TA) between 2014 and 2019.
Primary and secondary outcome measures
The count and follow-up time of US patients, available in the EHR, who were exposed to cancer drugs of interest in the period between Food and Drug Administration (FDA) approval and dates relevant to the NICE appraisal process.
Results
In 59 of 60 TAs analysed, the cancer therapy was approved in the USA before the final appraisal by NICE. The median time from FDA approval to the publication of NICE recommendations was 18.5 months, at which time the US EHR-derived database had, on average, 269 patients (SD=356) exposed to the new therapy, with a median of 75.3 person-years (IQR: 13.1–173) in time-at-risk. A case study generated evidence on real-world overall survival and treatment duration.
Conclusions
Across different cancer therapies, there was substantial variability in US real-world data accumulated between FDA approval and NICE decision milestones. The applicability of these data to generate evidence for HTA decision-making should be assessed on a case-by-case basis depending on the intended HTA use case.
Keywords: ONCOLOGY, Health policy, Decision Making, Electronic Health Records, EPIDEMIOLOGIC STUDIES
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study showcased the accumulation of real-world data on the exposure, over time, to new cancer therapies, from different therapy classes, indicated for different cancer types and subtypes.
The electronic health record (EHR)-derived datasets used had the clinical depth to enable the selection of specific populations of patients on the basis of drug indications.
The study relied on data from a single main source in the USA and may not represent the findings had all EHR-derived data from the USA been considered.
Introduction
When evaluating the value of new therapies for cancer and other diseases, health technology assessment (HTA) bodies are increasingly looking at opportunities to supplement clinical trial evidence with real-world evidence (RWE).1 2 Several uncertainties exist in the assessment of oncology therapies due to limitations in clinical trial evidence, namely: the use of uncontrolled studies, limited follow-up data, the use of surrogate outcomes, the use of comparators that do not reflect the local standard of care and questions about the selection of patients into trials and the relevance to the target population of interest.3 Real-world data (RWD) can potentially address these challenges and be used for other use cases, including the characterisation of routine-care patient populations, the estimation of clinical effects in routine-care settings, the modelling of natural disease history, understanding safety and the derivation of inputs for cost-effectiveness models.2
While HTA bodies typically prefer data from their own jurisdictions, there is a potential value in using data from other countries where local data is unavailable (in a timely manner) or insufficient. International data, especially from the USA, have already been widely used and accepted by HTA bodies. The most common use cases are for modelling long-term overall survival (OS) for the comparator treatments (eg, National Institute for Health and Care Excellence (NICE) evaluation of atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer)4 and forming an external control arm to data from clinical trials on the drug under evaluation (eg, NICE evaluation of sotorasib for patients with KRAS G12C mutation-positive advanced non-small-cell lung cancer).5
One unexamined but potentially valuable use of international data is to provide data on the drug under evaluation (rather than only on comparators) from countries where early access is provided. There are several uncertainties about the use of these drugs (including patient characteristics, treatment pathways and time on treatment) and outcomes (including overall survival and progression-free survival) that could be reduced or resolved using such data. The USA is a potentially important source of supplemental RWD because most new cancer drugs come to market in the USA before they are approved in the UK.6
In this article, we explore the potential for US electronic health record (EHR)-derived RWD to provide evidence to HTA bodies on the early use of new drugs to reduce uncertainties in decision-making at initial evaluations and reassessments. First, we describe the accumulation of RWD post-Food and Drug Administration (FDA) approval in the USA for a set of cancer therapies identified from NICE technology appraisals (TAs) between 2014 and 2019. Second, we illustrate the potential of EHR-derived RWE for addressing evidence gaps during NICE decision-making for a treatment that received conditional reimbursement at the first appraisal.
Part I: EHR-derived RWD post-FDA approval
The objective of this study was to determine the breadth and characteristics of EHR-derived data available from US patients exposed to new cancer therapies before NICE final guidance publication for the same drugs in the UK. We chose three milestones post-FDA approval relevant to the UK approval process: the date of company submission to NICE, final appraisal determination (FAD) and final guidance publication. These milestones reflect the timeline from company evidence submissions for technology assessment to the completion of evidence reviews by NICE and the publication of evidence-based recommendations by NICE. Our study identified cancer therapies using NICE TAs and then used EHR-derived data to select patients exposed to these therapies.
Methods
Identification of NICE TAs
We considered oncology drugs with TAs published by NICE between 1 January 2014 and 31 December 2019. Among these oncology drugs, we selected those with FDA approval for eleven cancer types in a collection of EHR-derived databases that had at least 2 years of data and a validated mortality variable.7 8 For each eligible TA, we noted the following: drug or regimen name, indicated cancer type, FDA approval date, European Medicines Agency (EMA) approval date, TA submission date to NICE, TA publication date by NICE and NICE’s decision at publication.
EHR-derived data source
This study used EHR-derived data from the US nationwide Flatiron Health database, a longitudinal, deidentified database containing patient-level structured data and unstructured data curated via technology-enabled abstraction.9 10 At the time of this study, the deidentified data were derived from ~280 US cancer clinics (~ 800 sites of care). The 11 cancer types were advanced gastric/oesophageal, advanced non-small cell lung cancer (NSCLC), advanced urothelial, diffuse large B-Cell lymphoma, early breast, metastatic breast, metastatic colorectal, metastatic pancreatic, metastatic renal cell carcinoma, multiple myeloma and ovarian. The data cut-off for all disease-specific datasets were 31 December 2019.
Statistical analysis
FDA approval versus NICE milestone dates
For the eligible TAs, we calculated the time from FDA approval to NICE milestones. The time intervals were summarised using median and IQR.
EHR-derived data available at NICE milestone dates
For each TA, in instances where FDA approval preceded the NICE milestone, we measured EHR-derived RWD accumulated between the FDA approval date and the NICE milestone date. We excluded NICE milestone dates that came before FDA approval because there was no corresponding US RWD to consider. We counted the number of patients starting the new drug or regimen after FDA approval, estimated their corresponding potential follow-up time,11 and calculated time-at-risk measured using person-time in person-years. Within any time interval after FDA approval, patients were followed from the date of first exposure to death, last EHR activity or the NICE milestone date, whichever came first.
Given the variability in the time between FDA approval and NICE milestones for each TA, we also computed patient count rates (patient count/time) to standardise the period of patient accumulation after FDA approval. Count rates were calculated by taking the number of patients accumulated between FDA approval and the NICE milestone and dividing by the time (in months) between FDA approval and the NICE milestone.
We summarised the measures of available EHR-derived RWD (ie, the patient counts, follow-up time, and the count rates (patient count/time)) using median and IQR at each NICE milestone. We also summarised the measures stratified by TA characteristics, including the NICE decision at publication (recommended, not recommended, cancers drug fund (CDF), recommended after CDF); whether TA was for an immunotherapy drug (yes vs no); whether TA was for a biomarker-driven indication (yes vs no); whether TA was for a first-in-class therapy (yes vs no); and, whether TA was initiated before the first full year of the CDF revamp by the National Health Service (NHS)12 (<2017 vs ≥2017). We repeated the above descriptive analyses at time points agnostic to NICE milestones. Specifically, we observed patient counts and follow-up accumulated at 1, 2, 3, 6, 9, 12, 18 and 24 months after market entry into the USA. We were interested in the 2-year mark because therapies that enter the CDF are typically reassessed after 2 years following further data collection. All analyses were purely descriptive and no hypothesis testing was performed. The statistical analysis was performed using R V.3.6.1.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
The review included 60 NICE TAs associated with 39 cancer therapies. Characteristics of each appraisal, including first time versus reappraisal, recommendation decision, cancer type and treatment type, are described in online supplemental table 1. In 98.3% (59/60) of the TAs, the technology was approved in the USA before NICE published final recommendations. Of the 60 TAs, 6 (10%) were managed access reviews. The median time from FDA to NICE submission, appraisal determination and final guidance publication was 6.4 months (IQR: 2.0–13.2), 14.4 months (IQR: 9.1–24.9) and 18.5 months (IQR: 11.1–29.1), respectively (online supplemental figure 1).
bmjopen-2023-074559supp001.pdf (654.3KB, pdf)
The available RWD from US patients who were exposed to new cancer therapies between FDA approval and NICE milestones was summarised as shown in table 1. At the time of manufacturer submission of evidence to NICE, an average of 147 (SD=269) US patients per TA could be selected from the EHR as having received the drug of interest after FDA approval, with an average median follow-up of 4.5 (SD=3.7) months. At TA publication, the number of patients available increased to an average of 269 patients (SD=356) per TA, with an average median follow-up of 6.4 months (SD=4.8) per TA. For the six TAs that received final recommendations after going through managed-access review, at the reappraisal-decision publication, the median number of patients included from the EHR was 665 (IQR: 209–1320) (with average median follow-up of 7.34 months (SD=2.84)).
Table 1.
For TAs whose NICE milestones came after FDA approval: summary of available US EHR-derived data on patients who were exposed to new cancer therapies between FDA approval and the NICE milestone
| NICE milestone | |||
| Available EHR-derived RWD (No of TAs included*, n of 60 (%)) |
Evidence submission (51/60 (85.0%)) |
Final appraisal determination (58/60 (96.7%)) |
TA Publication (59/60 (98.3%)) |
| No of patients accumulated with treatment start prior to milestone, per STA | |||
| Mean (SD) | 147 (269) | 236 (340) | 269 (356) |
| Median (IQR) | 50 (10.5–173.0) | 124 (61.0–344) | 131 (40.0–344.0) |
| Rate of accumulation per month, Median (IQR) |
4.8 (1.5–11.6) | 6.2 (2.3–12.1) | 6.2 (2.4–12.7) |
| Median potential follow-up (months) | |||
| Mean (SD) | 4.5 (3.7) | 6.0 (4.9) | 6.4 (4.8) |
| Median (IQR) | 3.5 (1.7–6.1) | 4.1 (3.2–7.4) | 4.6 (3.7–8.2) |
| Person-time (person-years), Median (IQR) |
12.0 (1.1–66.7) | 44.0 (9.2–139.0) | 75.3 (13.1–173.0) |
*A TA was included if the NICE milestone date came after the FDA approval date.
EHR, electronic health record; FDA, Food and Drug Administration; NICE, National Institute for Health and Care Excellence; RWD, real-world data; STA, single technology appraisal; TA, technology appraisal.
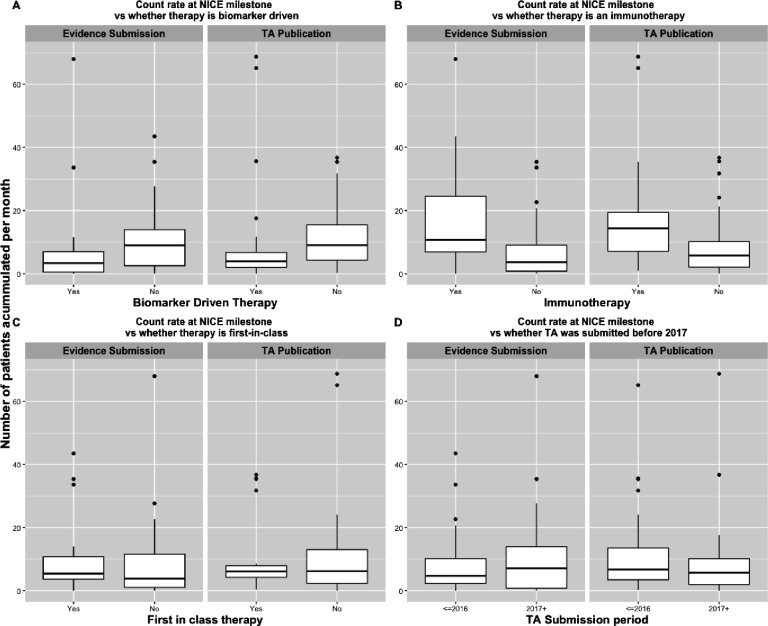
The distribution in the rate of patient accumulation and the person-time available at NICE milestones based on TA characteristics were as presented in figures 1 and 2, respectively. As shown in the figures, the number of US patients available in the EHR-derived database from the time of FDA approval until the NICE milestones differed by therapy class and whether therapy is biomarker driven. Immunotherapies tended to accumulate patients faster than non-immunotherapies, and biomarker-driven treatments tended to have slower uptake than non-biomarker-driven treatments. For each therapy of interest, the available EHR-derived data after 2 years on the US market were summarised as shown in online supplemental figure 2. For most of the therapies of interest, the available follow-up within the first 2 years of market access was less than 12 months. The monthly accumulation of these patients within 2 years after FDA approval was summarised as shown in online supplemental figure 3.
Figure 1.
Boxplots showing the distribution patient count rates at National Institute for Health and Care Excellence (NICE) milestones by technology appraisals (TAs) characteristics. The count rates are calculated by dividing the number of patients accumulated between Food and Drug Administration (FDA) approval and NICE milestone divided by the time between FDA approval and NICE milestone (in months).
Figure 2.
Boxplots showing the distribution person-time at National Institute for Health and Care Excellence (NICE) milestones by technology appraisals (TAs) characteristics.
Part II: case study
We performed a case study to illustrate the potential of EHR-derived data to address evidence gaps in HTA. We note that this study was not intended to improve our current understanding of the evaluated drug but rather understand what data would have been available to NICE at the time of evaluation and at potential reassessment points and whether this could have reduced uncertainty in decision-making.
Using the TAs considered in part I as our frame of reference, the case study was selected from six TAs (10%), for products that were recommended after managed-access or the CDF. These TAs represented scenarios where additional evidence was required at the time of assessment to reach a final reimbursement decision by NICE (see online supplemental table 2). Among the six TAs, we selected TA53113 for an in-depth study (online supplemental table 3). We chose this TA because (1) it involved a cancer therapy that was approved in the USA before the UK and (2) it had a source of uncertainty that Morrell et al3,3 found to be prevalent among evidence packages for cancer drugs submitted to NICE–specifically: uncertainty in pivotal trials due to insufficient follow-up (immature survival data) at the time of appraisal. We explored the availability of US EHR-derived data using various time horizon cutoffs, including up to the point of NICE evidence reappraisals, to illustrate the feasibility of conducting retrospective studies that could potentially reduce a key uncertainty noted by NICE in the final appraisal.
We designed the case study using the StaRT-RWE structured template.14 The patient population of interest was aligned with the EMA label that NICE considered during the evidence appraisal. We used deidentified EHR-derived data from Flatiron Health to perform the case-study analysis.
EHR study in NSCLC based on TA531
In TA531, NICE reappraised pembrolizumab as treatment for patients with previously untreated PD-L1-positive (Tumour Proportion Score (TPS) ≥50%) metastatic NSCLC.13 In its initial appraisal, TA447, NICE recommended pembrolizumab with managed access via the CDF but noted insufficient follow-up time in the pivotal trial15 considered at evidence review. As a case study, we assessed whether RWD available at reappraisal could be leveraged in understanding the long-term survival of patients with NSCLC exposed to pembrolizumab.
We included patients with previously untreated, stage IV NSCLC, with TPS≥50%, with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations,16 who initiated first-line pembrolizumab monotherapy between FDA approval and data cut-off (24 October 2016 to 31 December 2020). Patients were followed from treatment initiation until the first occurring event: death, last-EHR activity date or study-end date. Participants who initiated treatment within 6 months of the data cut-off were excluded to allow for a minimum theoretical follow-up of 6 months. Using the Kaplan-Meier method, we summarised the real-world OS (rwOS)—the time from the start of pembrolizumab treatment to death. We also used the Kaplan-Meier method to summarise the treatment duration, an endpoint defined as the time from the start of pembrolizumab treatment to discontinuation for any reason, including death. As a supplemental analysis, we examined rwOS among patients whose lab values indicated adequate organ function (labs as noted in online supplemental table 4) and with Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, criteria from an earlier trial of pembrolizumab assessed by the NICE evidence review panel.15 Additionally, we examined rwOS during a shorter time horizon spanning FDA approval to NICE’s reappraisal FAD for TA531 (24 October 2016 to 6 June 2018) to limit the analysis to the RWD that would have been available to NICE around the point of its decision.
The EHR study cohort included 920 patients (median age 73, 52% female) at the December 2020 cut-off with a median potential follow-up of 29.9 months (95% CI 27.8 to 31.7). Table 2 summarises patient characteristics and online supplemental figure 4 offers a detailed description of attrition. Of the initial risk set, 197 (21.6%) patients were still at-risk beyond 24 months of follow-up (figure 3). Median rwOS was 11.8 months (95% CI 10.5 to 14.8), and the median duration of therapy was 5.0 months (95% CI 4.2 to 5.7).
Table 2.
Baseline patient characteristics in the case study based on NICE TA531 (time horizon: FDA approval (24 October 2016) to 31 December 2020)
| Baseline characteristics | EHR-derived cohort (N=920) |
| Age at start of 1L therapy, Median (IQR) |
73 (64–79) |
| Age at start of 1 L therapy, n (%) | |
| <50 | 15 (2) |
| 50–64 | 228 (25) |
| 65–74 | 284 (31) |
| ≥75 | 393 (43.0) |
| Sex, male, n (%) | 441(48) |
| Race/ethnicity, n (%) | |
| Asian | 18 (2.2%) |
| Black or African American | 71 (8.8%) |
| Other race | 94 (11.2%) |
| White | 623 (77%) |
| Unknown | 114 |
| Performance status, n(%) | |
| ECOG PS of 0 | 186 (25) |
| ECOG PS of 1 | 325 (28.2) |
| ECOG PS of 2+ | 230 (31) |
| ECOG PS unknown/not documented | 179 |
| Histology, n(%) | |
| Non-squamous | 756 (82%) |
| Not otherwise specified | 45 (4.9%) |
| quamous | 119 (13%) |
| Year of pembrolizumab initiation, n (%) | |
| 2016 | 26 (2.8%) |
| 2017 | 357 (39%) |
| 2018 | 243 (26%) |
| 2019 | 161 (18%) |
| 2020 | 133 (14%) |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; EHR, electronic health record; FDA, Food and Drug Administration; NICE, National Institute for Health and Care Excellence.
Figure 3.
Overall survival (OS) observation period extension with electronic health record (EHR)-derived data for patients with advanced non-small cell lung cancer (note: seven patients were omitted from the analysis due to lack of granularity in date of death documentation).
Our first supplemental analysis (applying more stringent lab and ECOG PS eligibility criteria) included 440 patients, and we estimated median rwOS of 18.6 months (95% CI 15.2 to 22.7). Our second supplemental analysis included 192 patients (accumulated between FDA approval and NICE FAD) with median rwOS was 10.7 months (95% CI 8.2 to NR). The incompletely defined CI, for the median rwOS, over the truncated study period signalled that the EHR-derived dataset had insufficient follow-up by the time of NICE’s final decision on TA531.
Discussion
International data are already used to fill evidence gaps in HTA decisions, especially around long-term OS for economic modelling and clinical effectiveness in the absence of randomised controlled trials against local standard of care. This study explored the potential to additionally use international data, specifically US EHR-derived data, to supplement trial data on new medicines with RWD on use and outcomes based on earlier access to these medicines in the USA.
We found that, while the amount of RWD on therapies under evaluation increased over the ordered NICE HTA milestones, there was wide variability in the US RWD available at each milestone. This variability highlighted that the feasibility of using US RWE, to address evidence gaps in HTA, should be assessed on a case-by-case basis. Our study also reviewed the RWD available after 2 years of market access in the USA and also showed considerable variability in the volume of available patient data (online supplemental figure 2). For most therapies, the available follow-up within the first 2 years of market access was small and unlikely to be sufficient to characterise the OS endpoint. This observation is consistent with Morrell et al3 who noted that in most instances, de novo in-use data from the CDF were unlikely to provide new information on long-term survival due to limited follow-up. Data from the USA on the therapy under evaluation may still be useful for other evidence types including the characterisation of patients who receive treatment in clinical practice and their time on treatment.
In both parts of the research, we devoted some effort to understanding RWD and RWE available for TAs that were recommended with managed access through the CDF. These CDF TAs made for a compelling exploration because NICE requires additional evidence to make final recommendations. For these TAs, we observed that available US RWD on the therapy of interest at the initiation of managed access (online supplemental figure 5 and 6) was likely insufficient to address common evidence gaps in HTA. This observation suggested that the consideration of US EHR-derived evidence may be better suited for later time points in the decision-making process for managed access treatments. Later decision points could, for example, include evidence reappraisal before full approval or postmarketing reassessment after full approval.
The first part of the study represented a novel attempt to quantify the US RWD that is available at different time points relevant to cancer drug approval in the UK. The second part of the research assessed the evidence-generation capability of US RWD available at time points after FDA approval. In the case study of TA531, EHR-derived data available at a recent data cut-off and at NICE’s final decision time point were used to characterise OS and time on treatment. The evidence generated in the case study was consistent with other RWD data studies of broad populations.17–20 At the recent data cut-off, the available sample size was large enough to explore several subgroups that could be of interest to HTA stakeholders, for example, the investigation of a relatively healthy cohort that consists of patients with ECOG PS 0/1 at treatment initiation.21 In contrast, at TA reappraisal, such subgroup analyses were limited by the small sample size. This analysis of data from a recent cut-off further reinforced the observation that the utility of US RWD and the resulting RWE, for ex-US use, becomes more apparent as the time on market in the US increases.
The process of setting up the case study revealed several strengths that make EHR-derived data well-suited for HTA-related evidence generation. The main strength was the clinical depth offered by a wide range of time-fixed and longitudinal variables, including demographic and clinical characteristics (eg, biomarkers, histology, drug histories, treatment sequencing). These characteristics enabled us to select study cohorts based on the EMA label. For example, in the case study, it was possible to select patients based on PD-L1 staining (TPS≥50%) as well as EGFR and ALK mutation statuses. Such an ability to choose highly specific cohorts can address data relevance concerns among HTA decision-makers when using RWD. Another strength highlighted in this study is that contingent on an efficient data extraction infrastructure, EHR-derived data can enable timely access to patient data for new oncology therapies, thereby allowing evidence generation to be conducted promptly.
There were several limitations in the case study that can be noted to represent common considerations in the evaluation of EHR-derived RWE for HTA. Studies relied on a single source of US data9 with some missingness that may have limited the ability to distinguish between study eligible and ineligible patients. For example, in the case study, we could not ascertain all patients’ PD-L1 staining and ALK and EGFR mutation statuses due to missing data. The analyses were also susceptible to the usual threats to validity when using EHR-derived data to perform epidemiological studies: random error from sampling; selection bias from informative inclusion into EHR; and measurement error in the derivation of variables.22 These sources of bias need to be addressed as they can impede our ability to use RWD to address evidence gaps in value assessments. Specifically, the biases can be reduced by improving EHR data collection and preprocessing or through study design and statistical analysis techniques. These bias reduction techniques, however, may not improve the transportability of evidence between countries. The ability to transfer evidence from the USA to the UK (and vice-versa) can be limited by differences in healthcare systems, clinical practices and patient populations. Further work is needed to better understand the extent to which US or other international data can be used to inform decisions in other countries for different outcomes and in various indications.
In this paper, we recognise the potential of RWE to complement and contextualise clinical trial evidence to enable decision-making based on multiple information sources. Contingent on availability and timeliness, US RWD are amenable for generating evidence that could be considered in HTA decision-making. Whether the generated evidence should be used in HTA decision-making will depend on the comparability of populations, available treatments and treatment guidelines.
Supplementary Material
Acknowledgments
Akin Sobowale, Donna Mordente, Brennan Beal, Jyotsna Kasturi, Eunice Ochuonyo, Hannah Gilham, Jennifer Swanson and Darren Johnson of Flatiron Health.
Footnotes
Twitter: @DrBlytheAdamson
Contributors: Concept and design: PM, BG, SK, IA, AC, DB, SB and BA. Acquisition of data: AC, SB and BA. Analysis and interpretation of data: AS, PM, BG, PJ, HP, SK, IA, AC, DB, SB and BA. Drafting of the manuscript: AS, PM, BG, PJ, HP, IA, DB, SB and BA. Critical revision of the paper for important intellectual content: AS, PM, PJ, HP, SK, IA, DB, SB and BA. Statistical analysis: PM, HP and BA. Provision of study materials or patients: N/A. Obtaining funding: N/A. Administrative, technical, or logistic support: AS, PJ, DB and BA. Supervision: DB and BA.
Guarantor: BA.
Funding: This study was sponsored by Flatiron Health, an independent member of the Roche Group.
Competing interests: At the time of the study, PM, HP, IA, AS, SB, and BA report current employment with Flatiron Health, Inc. PM, HP, IA, AC, SB, DB, AS, and BA report ownership of stock in Roche.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The data that support the findings of this study have been originated by Flatiron Health. Requests for data sharing by licence or by permission for the specific purpose of replicating results in this manuscript can be submitted to publicationsdataaccess@flatiron.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The IRB of WCG IRB gave ethical approval for the study protocol prior to study conduct, and included a waiver of informed consent (approval ID: 420180044).
References
- 1.O’Rourke B, Oortwijn W, Schuller T, et al. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care 2020;36:187–90. 10.1017/S0266462320000215 [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence . NICE real-world evidence framework, Available: https://www.nice.org.uk/corporate/ecd9/chapter/overview [Accessed 25 Aug 2022].
- 3.Morrell L, Wordsworth S, Schuh A, et al. Will the reformed cancer drugs fund address the most common types of uncertainty? an analysis of NICE cancer drug appraisals. BMC Health Serv Res 2018;18:1–9. 10.1186/s12913-018-3162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence . Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy,. 2018Available: https://www.nice.org.uk/guidance/ta520/documents/final-appraisal-determination-document
- 5.National Institute for Health and Care Excellence . Sotorasib for previously treated KRAS G12C mutation-positive advanced non-small-cell lung cancer,. 2022Available: https://www.nice.org.uk/guidance/ta781/documents/final-appraisal-determination-document-2
- 6.Lythgoe MP, Desai A, Gyawali B, et al. Cancer therapy approval Timings, review speed, and publication of pivotal registration trials in the US and Europe, 2010-2019. JAMA Netw Open 2022;5:e2216183. 10.1001/jamanetworkopen.2022.16183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis MD, Griffith SD, Tucker M, et al. Development and validation of a high‐quality composite real‐world mortality endpoint. Health Serv Res 2018;53:4460–76. 10.1111/1475-6773.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Gossai A, Monroe S, et al. Validation analysis of a composite real‐world mortality endpoint for patients with cancer in the United States. Health Serv Res 2021;56:1281–7. 10.1111/1475-6773.13669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health, SEER, and NPCR. Oncology [Preprint] 2020. 10.1101/2020.03.16.20037143 [DOI]
- 10.Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv:2001.09765 [Preprint] 2020.
- 11.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–6. 10.1016/0197-2456(96)00075-x [DOI] [PubMed] [Google Scholar]
- 12.National Health Service . Cancer drugs fund, Available: https://www.england.nhs.uk/cancer/cdf [Accessed 18 Oct 2022].
- 13.National Institute for Health and Care Excellence . Pembrolizumab for untreated PD-L1-positive metastatic non-small-cell lung cancer, Available: https://www.nice.org.uk/guidance/TA531 [Accessed 25 Aug 2022].
- 14.Wang SV, Pinheiro S, Hua W, et al. START-RWE: structured template for planning and reporting on the implementation of real world evidence studies. BMJ 2021;372:m4856. 10.1136/bmj.m4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 16.Keytruda product information (EMA . European Medicines Agency, Available: https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf
- 17.Murteira R, Borges FC, Mendes GP, et al. Real‐World effectiveness of pembrolizumab in previously treated non‐small cell lung cancer: a population‐based cohort study. Pharmacoepidemiol Drug Saf 2020;29:1295–302. 10.1002/pds.5091 [DOI] [PubMed] [Google Scholar]
- 18.Descourt R, Greillier L, Perol M, et al. First-line pembrolizumab monotherapy for PD-L1-positive (TPS≥ 50%) advanced non-small cell lung cancer (aNSCLC) in the real world: a national french bispective multicentric cohort—ESCKEYP trial (GFPC 05-2018). JCO 2021;39.(15_suppl) 10.1200/JCO.2021.39.15_suppl.9091 [DOI] [Google Scholar]
- 19.Agg H, Winfree KB, Zhu YE, et al. A real-world analysis of non-small cell lung cancer patients treated with pembrolizumab or pembrolizumab in combination with pemetrexed and platinum. JCO 2020;38.(5_suppl) 10.1200/JCO.2020.38.5_suppl.53 [DOI] [Google Scholar]
- 20.Kehl KL, Greenwald S, Chamoun NG, et al. Association between first-line immune checkpoint inhibition and survival for medicare-insured patients with advanced non–small cell lung cancer. JAMA Netw Open 2021;4:e2111113. 10.1001/jamanetworkopen.2021.11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velcheti V, Chandwani S, Chen X, et al. First-line pembrolizumab monotherapy in metastatic PD-L1 positive non-small cell lung cancer: a real-world analysis of time on treatment in US community oncology practices. Annals of Oncology 2018;29. 10.1093/annonc/mdy486.002 [DOI] [Google Scholar]
- 22.Friis RH, Sellers T. Epidemiology for public health practice. Jones & Bartlett Learning, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074559supp001.pdf (654.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. The data that support the findings of this study have been originated by Flatiron Health. Requests for data sharing by licence or by permission for the specific purpose of replicating results in this manuscript can be submitted to publicationsdataaccess@flatiron.com.