Abstract
Introduction
Despite proven programmes, implementing lifestyle interventions for pre-diabetes and type 2 diabetes is challenging. Cardiac rehabilitation, provide a valuable opportunity to promote the adoption of healthy lifestyle behaviours for patients with atherosclerotic cardiovascular disease (ASCVD). However, only a limited number of studies have explored the potential for reversing the underlying causes of ASCVD in this setting.
Objectives
The DIABEPIC1 study is an ongoing single-arm lifestyle clinical trial to assess the feasibility of an upgraded 6-month intensive cardiac rehabilitation programme combining an innovative diet assignment with exercise training to reverse newly onset pre-diabetes (glycated haemoglobin 5.7%–6.4%) to normal glucose concentrations in patients with coronary heart disease.
Methods and analysis
36 patients referred from the Montreal Heart Institute for cardiac rehabilitation, aged ≥40 years with a recent diagnosis of pre-diabetes in the last 6 months, will be offered to participate in the upgraded programme. Interventions will include four sessions of nutritional counselling on ultra-processed foods intake reduction and a moderate-carbohydrate (<40%) ad libitum Mediterranean diet coupled with 36 1-hour sessions of supervised exercise training (continuous and interval aerobic training, and resistance training) and educational intervention. Phase 2 will continue the same interventions adding 8:16 hour time-restricting eating (TRE) at least 5 days per week. During this second phase, exercise training will be performed with autonomy. The primary objectives will be to evaluate the recruitment rate, the completion rates at 3 and 6 months, and the compliance of participants. The secondary objectives will be to assess the proportion of prediabetic participants in remission of pre-diabetes at the programme’s end and to characterise the factors associated with remission.
Ethics and dissemination
The DIABEPIC1 feasibility study is approved by the Research Ethics Board of the Montreal Heart Institute (Project Number ICM 2022-3005). Written informed consent will be obtained from each participant prior to inclusion. Results will be available through research articles and conferences.
Conclusions
The DIABEPIC1 trial will examine the feasibility and effectiveness of an enhanced cardiac rehabilitation programme combining exercise training with an ultra-processed food reduction intervention, a Mediterranean diet, and TRE counselling to remit pre-diabetes to normal glucose concentrations.
Trial registration number
Keywords: Risk Factors, DIABETES & ENDOCRINOLOGY, CARDIOLOGY, Rehabilitation medicine, Coronary heart disease
Strengths and limitations of this study.
Addresses the issue of effective implementation of lifestyle interventions as a first-line treatment for pre-diabetes, which is rarely seen in routine clinical care.
Offers a unique opportunity to influence the underlying causes of cardiovascular disease and adopt healthy lifestyle behaviours through an upgraded 6-month intensive cardiac rehabilitation programme.
Combines multiple proven interventions, including nutritional counselling, exercise training and time-restricted eating, to achieve remission of pre-diabetes and improve metabolic health.
The study population is relatively small (36 participants), and it is limited to patients with coronary heart disease referred for cardiac rehabilitation, which may not be representative of the general population with pre-diabetes.
The study duration is limited to 6 months, which may not be sufficient to observe sustained changes in lifestyle behaviours and metabolic health.
Introduction
Pre-diabetes and type 2 diabetes (T2D) are major risk factors for atherosclerotic cardiovascular disease (ASCVD) and a significant burden for patients and healthcare systems. In Canada, the estimated prevalence of T2D is 3.4 million (9% of the population), and 5.7 million (15% of the population) are living with pre-diabetes, most of them unaware of their condition.1 Despite current optimal treatments, cardiovascular events remain high in individuals with pre-diabetes and T2D, and it is predicted that the number of people living with these conditions will continue to increase.2
Early diagnosis and intensive interventions, such as adequate weight loss through physical exercise, distinct dietary interventions and intermittent fasting modalities like time-restricted eating (TRE), have been shown to prevent, improve and even reverse these conditions.3 Unfortunately, these lifestyle interventions are only sometimes effectively implemented in routine clinical practice, likely due to obstacles such as healthcare resources, infrastructure and personal barriers. Therefore, innovative ways to effectively implement and maintain lifestyle changes are needed. One potential solution is to use a cardiac rehabilitation programme after an acute cardiovascular event as an opportunity to influence the underlying causes of cardiovascular disease and adopt healthy lifestyle behaviours.
The DIABEPIC1 study is a single-arm lifestyle clinical trial that will assess the feasibility of an intensive lifestyle programme to reverse newly onset pre-diabetes (glycated haemoglobin (HbA1c) 5.7%–6.4%) to normal glucose concentrations in patients with a recent acute cardiovascular event that would otherwise start a standard cardiac rehabilitation programme of 12 weeks. The patients will be offered an upgraded 6-month intensive team-based multidisciplinary stepwise programme combining diet assignment (ultra-processed foods reduction, Mediterranean diet and TRE) with exercise training (continuous/interval aerobic training and resistance training) and educational intervention to remit pre-diabetes.
The study’s primary aim is to assess the feasibility of the enhanced programme to devise and iteratively improve participant recruitment and adherence strategies for a possible future randomised controlled trial. The study also aims to study the factors associated with metabolic improvements and pre-diabetes remission to contribute to a clear rationale for seeking this endpoint. Finally, the study also intends to better understand the distinct lifestyle interventions’ benefits by characterising baseline and intervention-related changes in anthropometric measures, blood analysis, a 3-day nutritional diary registered by the Keenoa artificial intelligence app, vascular function measured by flow-mediated dilatation (FMD) and central arterial stiffness, and cognitive performance evaluated by a short neuropsychological battery targeting executive functions, processing speed and episodic memory.
The DIABEPIC1 trial will examine the feasibility and effectiveness of an enhanced cardiac rehabilitation programme combining exercise training with a Mediterranean diet and TRE counselling to remit pre-diabetes to normal glucose concentrations. The potential impact of the results of this intervention on the delivery of cardiac rehabilitation programmes for patients with pre-diabetes is significant. If proven feasible, it could improve cardiovascular function after an acute coronary event, reverse a causal risk factor and enhance metabolic health.
Methods
Study design overview and setting
The feasibility study will take place at the Cardiovascular Prevention and Rehabilitation Centre of the Montreal Heart Institute (Centre ÉPIC). The study duration will be 24 weeks (6 months) with two distinct 3-month interventions: phase 1 (Intensive Cardiac Rehabilitation Programme) will consist of a synchronous intensive nutritional intervention (four sessions of counselling on ultra-processed foods intake reduction and moderate-carbohydrate (<40% of total energy intake) ad libitum Mediterranean diet) coupled with 36 1-hour sessions of supervised exercise training (continuous and interval aerobic training, and resistance training) and educational intervention. Phase 2 (autonomy period) will continue the same interventions adding 8:16 hour TRE at least 5 days per week. Exercise training will continue in autonomy.
Nurses will deliver the educational intervention throughout the project in individualised 1-hour meetings at 0, 3 and 6 months. Topics addressed will be as follows: the concepts of insulin resistance, pre-diabetes and T2D; the main reasons behind the development of the disease; and the scientifically proven ways to reverse these conditions. Sessions will be tailored to the specific needs of the patients and will involve motivational interviewing to build intrinsic motivation for lifestyle modifications.
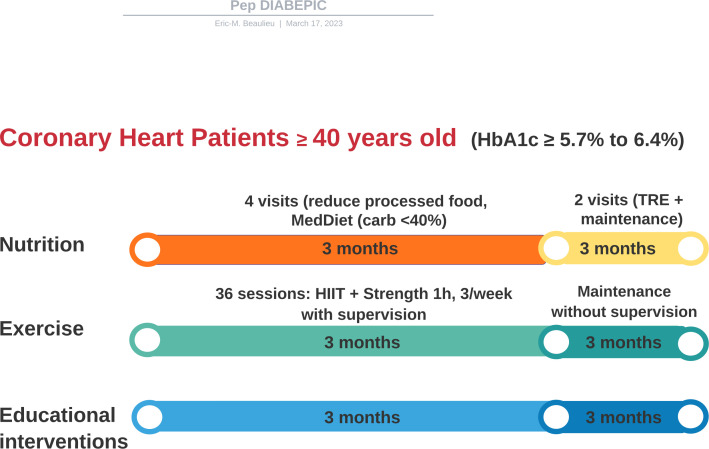
Anthropometric measures, blood analysis, a 3-day nutritional diary registered by the Keenoa artificial intelligence app, and cognitive performance evaluated by a short neuropsychological battery will be performed at baseline, after 3 months of the intensive intervention and at 3 months. Vascular function measurements by FMD and central arterial stiffness will be optional, and measures will take place at baseline and 6 months. A visual illustration of the DIABEPIC1 interventional study is depicted in figure 1.
Figure 1.
Central illustration summarising the study synchronous interventions. After inclusion and baseline assessment, coronary heart patients with recently diagnosed pre-diabetes status defined by an HbA1c ≥5.7%–6.4% will follow a three-arm synchronous nutritional, exercise training and education intervention. They will then be reassessed after 3 months of the intervention and again 3 months after the autonomy and time-restricted eating period. HbA1c, glycated haemoglobin; HIIT, high-intensity interval training; MedDiet, Mediterranean diet; TRE, time-restricted feeding.
Ethics and dissemination
The study protocol has been approved by the Research Ethics Board of the Montreal Heart Institute (Project Number ICM 2022-3005). It is reported per the Standard Protocol Items: Recommendations for Interventional Trials guidelines. The study complies with International Conference on Harmonization for Good Clinical Practice guidelines and all regulatory requirements. Written informed consent will be obtained from each participant prior to inclusion.
Hard copy files will be stored in a locked filing cabinet at the clinic site at Centre ÉPIC. After the study, all hard copy files containing the participant data will be anonymised and stored in a password-protected secured storage system accessed by approved personnel only.
The DIABEPIC1 results will be communicated through an internal committee’s thorough review and editing process to ensure the scientific accuracy and authorship of the publication and abstracts. No interim analysis is planned. The authorship of the publication and ancillary studies will be determined per the guidelines of the International Committee of Medical Journal Editors. The results will also be shared with participants, staff of the Centre ÉPIC and the broader medical community through research articles and conferences. Additionally, the complete and anonymous dataset will be made available for sharing by the principal investigator on request no later than 3 years after the end of the study.
Patient and public involvement
No patient involved.
Participant selection
Participants will be recruited among those referred for a cardiac rehabilitation programme from the Montreal Heart Institute because of stable angina, after an acute coronary heart event (with or without ST-segment elevation), after coronary revascularisation (primary or elective), or after bypass surgery. The starting recruiting date will be in March 2022. Potentially eligible patients recently diagnosed with pre-diabetes (<6 months) based on the American Diabetes Association cut-off criteria of HbA1c between 5.7% and 6.4%4 will be identified by the researchers before their first scheduled cardiac rehabilitation medical visit based on the results of their routine blood analysis typically performed 1 week in advance that includes: complete blood count, kidney function, a lipid profile, fasting glycaemia, insulin and HbA1c. They will be contacted and explained the possibility of participating in the study. They will be comprehensively informed and provided with an informed consent form if interested. Following this first call, the participant will have their first medical appointment, including a maximal exercise test to screen for potential contraindications and securely follow prescribed exercise training. This visit will also serve as the enrolment visit, where the participant will have another opportunity to discuss the project, clarify any doubts, and, if wished, be enrolled. Participants who refuse to participate in the present study will continue as scheduled and participate in the standard 3-month cardiac rehabilitation programme. Participants will be eligible to participate if all inclusion criteria are met, and none of the exclusion criteria are met. All study procedures, including the signature of informed consent, will be conducted at the Centre ÉPIC, providing all required settings, including material, trained nurses, registered dietitians and kinesiologists in clinical research, trained research assistants and administrative assistant. Detailed inclusion and exclusion criteria are shown in box 1.
Box 1. Detailed inclusion and exclusion criteria of DIABEPIC1 trial.
Inclusion criteria
Coronary heart disease patients referred from the Montreal Heart Institute.
Aged ≥40 years.
Recently diagnosed pre-diabetes (glycated haemoglobin (HbA1c) 5.7%–6.4%) in the last 6 months.
Referred to Centre ÉPIC for stable angina, acute coronary syndrome (with or without ST elevation), after coronary revascularisation (primary or elective) or bypass surgery.
Able to perform a maximal exercise test and exercise training programme by current cardiovascular rehabilitation recommendations.
Able to use a smartphone application or to complete an adherence/compliance diary.
Able to read, understand and sign the information and consent form.
Exclusion criteria
Absolute and relative contraindications to exercise testing and/or exercise training.
Patients with previously known T2D (HbA1c ≥6.5%) or patients with an HbA1c value of 5.7%–6.4% but with the help of oral hypoglycaemic agents.
Taking psychotropic medications that may induce mass gain (tricyclic antidepressants, mirtazapine, paroxetine, lithium, valproate, clozapine, olanzapine) or other medications known to promote mass gain (cortisone).
Taking recently introduced weight-loss medications (eg, semaglutide).
Unintentional mass loss of more than 10 kg in the past year.
Pregnant or nursing women.
Study outcomes
Primary objective
To assess the feasibility of an intensive, multidisciplinary cardiac rehabilitation programme based on lifestyle changes in coronary heart disease patients recently diagnosed with pre-diabetes that are referred to the Centre ÉPIC. Currently, the Centre ÉPIC receives up to 550 new coronary heart disease patients annually (approximately 50 per month) to participate in its cardiac rehabilitation programme. Of these patients, between 20% and 30% are diagnosed with T2D, and around 15%–20% fulfil the criteria for pre-diabetes (HbA1c 5.7%–6.4%). Based on these numbers, four parameters are considered to assess the feasibility of our study:
Total recruitment: number of participants screened compared with final enrolments. Hypothesis: at least 50% of patients living with pre-diabetes and referred to the Centre ÉPIC for the cardiac rehabilitation programme will find the study interesting and accept participation.
Recruitment rate: number of participants that can be recruited monthly. Hypothesis: at least two participants can be enrolled weekly, eight per month.
Completion rate at 3 and 6 months: number of participants that complete the intervention at 3 and 6 months compared with the enrolled participants. Hypothesis: at least 70% of the participants will finish the 3-month and 6-month programmes (ie, dropout rate ≤30%).
Compliance: total number of appointments attended (nutritional, exercise training and educational interventions) compared with the maximum possible. Hypothesis: participants will attend at least 80% of all proposed sessions.
To summarise, the full-scale study will be feasible if we can recruit at least eight participants per month on average, if the completion rate is at least 70% at 6 months, and if compliance with all protocol interventions is at least 80%. From here, all other collected data during the study will serve only for an exploratory purpose (see below secondary and tertiary endpoints).
Secondary objectives include assessing the proportion of participants with pre-diabetes at the start of the programme (HbA1c 5.7%–6.4%) in complete remission of pre-diabetes, defined by the following three criteria: an HbA1c <5.7% at 3 months of intervention (metabolic criteria), which is maintained at 6 months (duration criteria), without the use of glucose-lowering agents (pharmacological measures). Partial remission of pre-diabetes will be defined if the metabolic criteria (HbA1c <5.7%) is reached at 6 months, the end of the study’s second phase. This will allow researchers to examine how long it takes some participants to remission of pre-diabetes and the effect of the TRE intervention on metabolic changes. Hypothesis: at least 50% of participants will fulfil one of the remission criteria definitions at the end of the follow-up.
Tertiary objectives will characterise baseline and intervention-related changes in distinct anthropometric, physical, blood analysis, cognitive, vascular function and questionnaire measures detailed in box 2. Incidence of cardiovascular events will also be recorded and reported as a 5-point composite of major adverse cardiovascular events including cardiovascular death, myocardial infarction, unstable angina, ischaemic stroke and hospitalisation for heart failure.
Box 2. Detailed baseline and intervention-related changes will be measured at 0, 3 and 6 months of the study.
Anthropometric measures assessed non-invasively by the SECA-mBCA 515
Total body mass (kg) and body mass index (kg/m2).
Waist circumference (cm).
Fat mass (kg), lean mass (kg), skeletal muscle mass (kg), the proportion of total body mass and indexes (kg/m2).
Visceral fat (L).
Change in different anthropometric measures after interventions such as proportion of visceral fat mass and skeletal muscle mass change.
Energy expenditure at rest (kcal/day).
Proportion of patients with >5% of body mass loss and >10% of body mass loss.
Physical measures measured on the day of the maximum effort test
Systolic and diastolic blood pressure at rest and maximal effort (mm Hg).
Resting heart rate, maximal heart rate, heart rate reserve and heart rate recovery at 1 min.
VO2 peak (mL/kg/min) and METs estimated by the FRIEND Formula.45
Upper and lower-body 1-RM strength test on leg press and horizontal row.
Blood analysis measures
Fasting glucose and fasting insulin.
Lipid profile including total cholesterol, LDL-C, HDL-C, triglycerides and Apo-B.
Inflammation parameters including hs-CRP, fibrinogen, ferritin, albumin and uric acid.
Hepatic liver enzymes: AST/ALT to calculate non-alcoholic fatty liver disease scores, % of liver fat and % of non-alcohol fatty liver disease.
Cardiac damage enzymes including troponins (cardiac injury) and pro-BNP (cardiac strain).
Cognitive scores
Montreal Cognitive Assessment (MoCA) total score, Rey Auditory Verbal Learning Test, Coding (WAIS-IV), Stroop (D-KEFS), Trail Making Test, Verbal fluency (D-KEFS).
Vascular function measures
Change in brachial artery flow-mediated dilatation.
Central arterial stiffness.
Questionnaires measures
Nutritional Scores: Adherence to a Mediterranean Diet score (PREDIMED Test). The Food Craving Questionnaire Trait reduced (FCQ-T-r) measures food craving. Food matrix, total calories, the proportion of macronutrients, and hours spent eating and fasting collected by a 3-day day journal with the application Keenoa.
Physical Activity Scores: International Physical Activity Questionnaire (IPAQ) score.
Psycho-emotional status: Depression, Anxiety, and Stress Scale (EDAS21).
Box 2 summarises the distinct anthropometric, physical, blood analysis, cognitive performance, peripheral vascular function, and questionnaire measures that will be studied at baseline and repeated at 3 and 6 months of the study.
1-RM, one-rep max; ALT, alanine transaminase; Apo-B, apolipoprotein B; AST, aspartate aminotransferase; hs-CRP, high-sensitivity C reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; METs, metabolic equivalents; pro-BNP, B-type natriuretic peptide; VO2, maximal oxygen uptake.
Detailed study interventions and timelines
A complete illustration of the study enrolment and evaluation assessments can be found in online supplemental table 1.
bmjopen-2023-073763supp001.pdf (75.9KB, pdf)
Preintervention evaluation (over 1 week)
On signing the informed consent form, participants will have several preintervention assessments, including baseline missing blood analysis parameters and total anthropometric measurements by bioimpedance (mBCA 515, SECA). A visit with the nurse in which the patient will be involved in a motivational interviewing to assess personal objectives. In this visit, participants will also be offered expert educational and nutritional information about the concepts of insulin resistance, pre-diabetes and T2D, the main reasons behind the development of the disease, and the scientifically proven ways to reverse these conditions. The patient will also be informed on how to use the Keenoa application to collect a 3-day nutritional diary. The 3-month scheduled intervention programme will be reviewed with the participant to clarify any remaining questions.
Cognitive function assessment
A short cognitive assessment will be performed by a neuropsychologist or by trained research assistants. The tests will target general cognitive functioning, executive functions, processing speed and episodic memory: Montreal Cognitive Assessment (general cognitive functioning), Rey Auditory Verbal Learning Test (episodic memory), Coding (WAIS-IV) (processing speed), Stroop (D-KEFS) (executive functions), Trail Making Test (executive functions), Verbal fluency (D-KEFS) (executive functions). Neuropsychological testing will be conducted in person or by videoconference; the aforementioned tests are adequate for remote administration.5 Moreover, all tests have been validated for an adult population.
Vascular function assessment
FMD change to measure endothelial function and carotid-femoral pulse wave velocity to measure central arterial stiffness will be optional. For FMD measurement, brachial artery blood velocity and diameter will be measured with a high-resolution ultrasound device (uSmart3300, Terason) and a linear bar probe (5–12 MHz) before and after 5 min of forearm ischaemia. A cuff downstream of the ultrasound probe will be inflated to a pressure of 250 mm Hg to induce ischaemia. After the cuff is released, the brachial artery blood velocity and diameter increase will be measured continuously for 3 min. An analysis programme (FMD studio, Quipu srl) will independently determine peak diameter and shear rate. FMD will be quantified as the change in diameter from rest to peak, corrected by the shear stimulus and the baseline diameter. This measurement will be performed per current guidelines.6 Central arterial stiffness will be measured via carotid-femoral pulse wave velocity. The pulse wave will be recorded continuously over the carotid and femoral arteries by a non-invasive surface tonometer (Millar Inc). The pressure waveforms will be recorded for a minimum of 10 consecutive cardiac cycles. Distance travelled by the pulse wave will be measured, in triplicate, as the direct distance between the two measurement sites with a correction factor of 0.8, as per current guidelines.7
Phase 1 (3 months): intensive cardiac rehabilitation program
Nutritional intervention
Once a 3-day food diary is collected, registered dieticians will perform four personalised 1-hour visits stepwise throughout the first 3 months. Step 1: during the first visit, participants will be informed about how to read nutritional information of food products, how to identify processed and ultra-processed foods following the NOVA classification,8 and will be advised to reduce group 2 and 3 products and avoid group 4 products. Step 2: after this first visit, patients will have two personalised nutritional visits in which a Mediterranean diet moderate in carbohydrates (<40%) will be explained and proposed to them. The Mediterranean diet pyramid will guide participants in adapting to the new pattern. As part of the diet, participants will be advised to consume a diet predominately plant-based made up of vegetables, legumes, fruits, whole grains, nuts and seeds. Fish will be the primary source of protein, and olive oil will be the primary source of fat in the recommendations. There will not be specific calorie reduction targets. During these visits, efforts will be made to progressively adjust and improve, resolve doubts, and teach cooking techniques if necessary. Step 3: during the last 2 weeks of phase 1, the participant will have one last visit to be informed about the concepts of intermittent fasting and time-restricted eating to be prepared for phase 2 and informed to introduce an 8:16 hour TRE at least for 5 days a week, starting the second phase.
Exercise training intervention
The exercise training intervention for phase 1 will consist of 1-hour/three sessions per week of in-patient supervised endurance and strength training for 12 weeks (a total of 36 sessions). One session per week will be allowed at home if the participant wishes to accommodate preferences and prepare participants for phase 2 (training in autonomy). In-person sessions will be encouraged and supervised by a certified kinesiologist at the Centre ÉPIC, who will also organise the exercise-training sessions designed to be performed at home. The aerobic and resistance training prescriptions will be programmed according to the recent American College of Sports Medicine (ACSM) Guidelines for Exercise Training and Prescription, Eleventh Edition, 2021.9 The rate of perceived exertion (RPE) during the exercise sessions will be assessed on the Borg scale from 6 to 20.
Furthermore, participants will be encouraged to engage in their activities at home, like walking or cycling, following the 2020 WHO recommendations of at least 150–300 min of moderate-intensity aerobic exercise per week.10 All the characteristics of the activities will be recorded (type of activity, intensity, heart rate, duration) with the Polar Beat application and the heart rate sensor Polar H10.
The first 2 weeks will progressively introduce participants to all the exercise techniques, get familiar with all materials and assess different muscular-group strengths. During these first 2 weeks, continuous moderate exercise sessions and high-intensity interval training (HIIT) will be proposed to facilitate acquaintance with all participants. The endurance exercise programme will be performed on a bicycle ergometer, treadmill or elliptical. The intensity will start at 50% of maximal aerobic power or 11–12 of the Borg RPE during the first week and gradually increase to 60%–70%. If needed, the intensity of the training will be adjusted according to the heart rate reserve of each patient.
After these first two introductory weeks, alternating HIIT and moderate-intensity continuous training (MICT) sessions will be proposed in a 2:1 fashion; 2 HIIT sessions and 1 MICT session per week. The endurance sessions will include 5–10 min of warm-up and 5 min of cool-down. In the case of MICT sessions, the intensity will be between 60% and 70% of maximal aerobic power at an RPE, starting at 12 and progressively increasing to 14. During the HIIT, exercises (2–3 blocks of 10 min) will be composed of 1–3 min intervals at 80%–100% of the maximal aerobic power interspersed with an active recovery of the same duration. The RPE for the HIIT sessions will start at 15 and gradually increase to 17 through the exercise training programme.11
All training sessions will include 20–30 min of strength training that will take place using machines, free weights or elastic bands depending on the programme phase. Strength training will be programmed according to the recent ACSM guidelines with a gradual progression of higher intensities and/or numbers of sets/repetitions. Intensities will be prescribed at an RPE from 12 to 15, which corresponds to 40%–70% of the one-repetition maximum (1-RM), with six exercises involving major muscle groups. The number of sets will be from 1 to 3, and the number of repetitions will be from 6 to 15. The gradual assumption of autonomy towards phase 2 will be encouraged throughout this first phase of cardiac rehabilitation as, at the end of the 3 months, all participants should be able to follow personalised endurance and strength autonomous training.
Mid-intervention evaluation
At the end of the 3-month programme, participants will be offered to repeat a maximal effort test on a treadmill, a medical visit and examination, complete blood analysis and anthropomorphic assessment. Participants will also be asked to redo all questionnaires, a 3-day food diary with the application Keenoa and the cognitive tests.
Phase 2 (3 months): time-restricted eating and exercise training in autonomy
Nutritional intervention
After the mid-term assessments, participants will be asked to maintain all healthy lifestyle changes introduced during the first 3 months and to start an 8:16 hour TRE pattern at least 5 days a week, meaning an 8-hour window in which the participant will be allowed to eat and 16-hour window in which the participant will be asked to restrict from ingestion. General advice will be given to practice TRE successfully, such as to plan meals, eat consistently, gradually adjust the eating window, choose nutrient-dense foods, stay hydrated and avoid snacking outside the designated eating window. This period will include two additional nutritional consultations to resolve doubts.
Exercise intervention
During the study’s second phase and following the 2020 WHO guidelines of physical activity, all patients will be given personalised aerobic and strength exercise training to be performed without supervision at a gym or at home. Only remote follow-ups will be offered to resolve doubts and adjust if needed.
Postintervention evaluation (over 1 week)
At the end of the programme, participants will have a last medical visit that will include a maximal effort test on a treadmill, a medical visit and examination, complete blood analysis and an anthropomorphic assessment. Participants will also be asked to redo all questionnaires and cognitive tests and collect a 3-day food diary with the application Keenoa. Vascular function measures will again be optional for patients who have consented and attended their first appointment. The last 6-month evaluation for the latest participant enrolled is scheduled for May 2023. Following that, we will proceed with a 12-month follow-up to assess the long-term sustainability of remission and the metabolic progression of all participants. This follow-up is planned to finish in December 2023.
Statistical considerations
Sample size calculation
Primary outcome measures for this study are feasibility criteria to inform any future randomised controlled trial powered to detect an intervention effect. Therefore, a sample size for this study was calculated to allow the estimation of a completion and compliance rate with reasonable precision. Assuming that the completion rate will be around 70%, a sample size of 25 would allow estimating this rate with an accuracy of ±18.0% using a two-sided 95% CI. For a compliance rate of around 80%, a sample size of 30 participants would assure a precision of ±15.7% for estimating this rate. Assuming a 30% loss rate to follow-up, approximately 36 patients will be recruited.
Statistical analysis will be mainly descriptive with, when appropriate, the presentation of 95% CIs. They will be computed for baseline characteristics and follow-up assessments at 3 and 6 months. They will be presented as mean and SD for continuous variables and frequencies and percentages for categorical variables.
The number of participants that can be recruited monthly and the number of participants screened will be summarised. The total recruitment and monthly rates will be presented with a 95% CI. The number of participants that complete the intervention at 3 months, the number of participants that attend their 6-month follow-up appointment, and the total number of appointments attended (nutritional intervention (up to 6), exercise training intervention (up to 36) and educational intervention (up to 3) will be summarised. Completion/retention rate at 3 and 6 months and compliance rate will be presented with a 95% CI.
For illustrative purposes (because this pilot study is not powered to detect statistically significant findings), all analyses of this pilot study, including both secondary and tertiary endpoints, will be the assessments that could be considered as efficacy parameters in the large, full-scale study. For the analysis of change in continuous secondary and tertiary endpoints, that is, anthropometric measures, exercise-derived measurements, blood analysis measures and scores from questionnaires, a one-way repeated-measures analysis of variance model will be used to compare differences between intervention (pre, per, post) periods, with mean differences and 95% CIs and with effect sizes (Cohen’s d) when appropriate. The assumptions underlying the planned models will be checked, and if they are not tenable, data transformation or non-parametric analyses may be used if necessary.
The adjusted impact of the different factors associated with remission of pre-diabetes (eg, mass loss, fat mass loss, visceral fat loss) will be evaluated. For this analysis, univariable and multivariable logistic regression models will be created for the categorical outcome of remission of pre-diabetes: yes/no, according to the definition previously mentioned. Covariates will be selected a priori based on their described association with remission (clinical plausibility) or as a potential confounding effect according to the rules proposed by Kleinbaum and colleagues using the user-written Stata command ‘confound’.12 The practical and clinical interpretation will be presented with measures of association (OR). Statistical significance will be defined as a p value <0.05. Statistical analyses will be performed using STATA (StataCorp, 2017, Stata Statistical Software: Release 15, College Station, TX: StataCorp LLC).
Discussion
The DIABEPIC 1 study aims to investigate the feasibility and effectiveness of an upgraded, intensive multi-disciplinary programme for cardiac rehabilitation in reversing pre-diabetes in patients with coronary heart disease. The programme, which will last 6 months, will include a combination of dietary intervention, exercise training and education. The rationale behind this project is to address the growing issue of pre-diabetes as the unaddressed underlying cause of cardiovascular disease13 and propose an enhanced cardiac rehabilitation programme following an acute cardiovascular event to promote healthy lifestyle behaviours and reverse this condition to normal glucose concentrations.
Why is it important?
A substantial gradient of cardiovascular risk is observed across HbA1c levels from as low as HbA1c ≥5.4%, way below the threshold for diabetes.14 It is often reported that approximately one in three Americans have pre-diabetes and that 90% are not aware of their condition. Furthermore, about 25% of individuals with pre-diabetes will develop T2D within 3–5 years, and as many as 70% will develop the disease during their lifetime.15 Despite the high prevalence of pre-diabetes and the existence of proven effective programmes, there are currently limited options available in current clinical practice, especially in Canada, to halt or reverse this condition. Additionally, despite its relationship with an increased risk of ASCVD, there is not yet an entirely clear rationale for seeking the endpoint of pre-diabetes remission. The results of the DIABEPIC1 study can eventually contribute to provide valuable evidence toward clarifying this goal.
What is known?
Although remission of pre-diabetes and T2D in the community have been described, they have been historically understudied.16 For decades, T2D has been regarded as a progressive and irreversible condition requiring increasing numbers of oral glucose-lowering agents and insulin.
Nevertheless, remission has been recently identified as a top priority by people with pre-diabetes and T2D,17 and only in the past decade, at least 178 studies with over 100 participants (11 of which were randomised controlled trials) have been published focusing on the possibility of reversing T2D and pre-diabetes.18 Among them, surgical interventions were the focus of 164 (93%) studies compared with 8 (4%) pharmacological and 5 (2%) lifestyle interventions. In 2021, the ADA/EASD/DUK consensus statement on the definition of T2D remission was published, providing important guidance in this area. Additionally, more recently, the Diabetes Canada Remission of T2D Guidelines and User’s Guide have also been published, further contributing to the understanding and management of T2D remission.19 20
Reversion to normoglycaemia is associated with positive health benefits beyond T2D prevention or delay. A 1% absolute decrease in HbA1c was associated with a 14%–27% decrease in major CV events and a 37% reduction in microvascular complications in a cohort from the UK.21 The risk of cardiovascular disease and all-cause mortality was also reduced in a Chinese cohort of patients with pre-diabetes who reverted to normoglycaemia within 2 years compared with those who progressed to T2D over nearly 9 years of follow-up. The odds of developing microvascular disease (retinopathy, nephropathy and neuropathy) were also reduced.22 Most of these studies have a common strategy: to improve insulin sensitivity and reverse insulin resistance, individuals need to shift to burning fat as their primary energy source to reduce fat mass. This can be achieved by lowering insulin levels (fasting, restrictive diets, reducing consumption of ultra-processed foods, metabolic surgery or oral drugs) or increasing energy expenditure through endurance and resistance training. However, it is important to note that the combination of both strategies—lowering insulin levels and increasing energy expenditure—can have a synergistic effect, leading to greater improvements in insulin sensitivity and reductions in fat mass. A comprehensive narrative review of the evidence can be found elsewhere.23
What is new in this interventional study?
Intensive synchronous intervention in the setting of cardiac rehabilitation
A Mediterranean diet, TRE, educational interventions and regular exercise training have provided positive health benefits for improving metabolic parameters in healthy individuals and/or patients with pre-diabetes and T2D. However, there is limited evidence on the effect of multiple synchronous lifestyle interventions in patients with pre-diabetes combining these approaches in a synchronous stepwise intervention to attain remission, particularly in cardiac rehabilitation. The enhanced insulin resistance reversal programme aims to improve patients’ glucose regulation and overall cardiovascular health by targeting various risk factors associated with pre-diabetes and cardiovascular disease. The proposed programme seeks to address this gap by providing a comprehensive and intensive approach to cardiac rehabilitation that includes not only traditional exercise training but also education on the concepts of insulin resistance, pre-diabetes and T2D, the main reasons behind the development of the disease, and the scientifically proven ways to reverse these conditions as well as an innovative dietary intervention including ultra-processed food reduction, a moderate-carbohydrate ad libitum Mediterranean diet and the inclusion of TRE.
Reduction of ultra-processed foods as the starting point
The consumption of ultra-processed foods is associated with excess calorie intake and weight gain,24 metabolic syndrome,25 coronary heart disease, cerebrovascular disease26 and cancer.27 These foods have also been shown to cause an elevated glycaemic response, disrupt satiety signals, promote inflammation and the occurrence of diabetes.28 Processed and ultra-processed foods are probably one of the main drivers of ad libitum dietary habits and today’s global epidemic. In this context, the DIABEPIC1 study will start the nutritional intervention by teaching how to identify these foods and an intervention to reduce ultra-processed foods consumption. This strategy is a consequence of most weight-reducing diets that intrinsically exclude these types of products but is barely studied as a specific starting-point education strategy at the roots of the problem, which can lead to weight loss and a decrease in glycaemic spikes. Still, it can also be important in rebalancing satiety signals and promoting adherence to subsequent nutritional recommendations.
Mediterranean diet with moderate carbohydrate consumption as a diet assignment
The Mediterranean diet is well known for its various health benefits in healthy individuals, cardiovascular diseases and cancer.29 It reduces the incidence of T2D among non-diabetics with high cardiovascular risk.30 In insulin-resistant individuals, it improves glycaemic control, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol and triglycerides. In addition to its high nutritional quality, it also carries relatively easy long-term compliance,31 data lacking for most all other dietary interventions. For these reasons, the proposed interventions will be focused on a Mediterranean diet pattern.
Some randomised controlled trials show that low-carbohydrate diets prevent body weight more effectively than low-fat diets.32 33 For instance, blood glucose, HbA1c and glycaemic control are improved by low-carbohydrate in comparison with low-fat diets,34 35 and ApoB is improved in a moderate-carbohydrate diet (26%–45% carbohydrate) compared with a high-carbohydrate diet (49%–65% carbohydrate).36 Thus, our nutritional programme includes instructions to reduce carbohydrate consumption to an average of 40% of calories consumed.
Time-restricted eating in a cardiac rehabilitation setting as a new approach
Not only what we eat but also when we eat could affect health. A reduced food consumption window of 10 hours/day (14 hours of fasting) promotes weight loss in patients with metabolic syndrome, pre-diabetes and T2D. It decreases waist circumference, visceral fat, blood pressure, atherogenic lipoproteins and HbA1C.37 A daily food consumption window reduced to 4 hours or 6 hours/day (20 hours or 18 hours of fasting) resulted in a 3.2% loss of body weight while improving fasting insulin levels, insulin resistance and oxidative stress.38 Nonetheless, there is little evidence of the added effects of TRE in patients with pre-diabetes or T2D. It has not been evaluated in the context of a Mediterranean diet intervention, particularly in the cardiac rehabilitation setting. The DIABEPIC1 trial will propose and study a Mediterranean diet assigning moderate carbohydrate consumption with the addition of a TRE 16:8 pattern during the study’s second phase. This will allow assessing the impact of adding this nutritional intervention separately from the effects of the first 3 months of synchronous dietary and exercise training intervention.
Use of the Keenoa application to assess participants’ food intake and personalised approach to lifestyle intervention
The DIABEPIC1 study will use data collected from the novel Canadian diet application Keenoa at 0, 3 and 6 months. The validity and usability of this smartphone image-based dietary assessment app compared with 1-day and 3-day food diaries have been previously assessed.39 40 Its use offers several potential advantages: real-time data collection, which reduces the delay between intervention delivery and data collection; convenience as participants can access the application from their mobile devices; a more collaborative and personalised approach to lifestyle intervention between participants and healthcare providers, and improved data quality helping reduce errors and biases associated with manual data collection and increases the accuracy of data collection. The feasibility of its use and adherence will be reported.
Multicomponent anthropometric measurements by bioelectrical impedance as an innovation
Visceral adipose tissue and visceral fat mass loss are critical players in the pathogenesis of insulin resistance. The likelihood of pre-diabetes and T2D remission increases when substantial weight loss is achieved.41 Despite the nature of lifestyle or pharmacological interventions, most studies use total weight loss as a marker or endpoint, thus neglecting the impact of individual body components. Therefore, to gain a better understanding of the factors leading to remission, there is a need to improve data on the specific impact of different body components.
One of the particularities of this study will be the systematic use of the SECA-mBCA 515 balance to measure different components of body composition by bioelectrical impedance analysis, which will allow observing the absolute and proportional change in body mass, fat mass, visceral fat, lean body mass and skeletal muscle that participants will present through the different phases of the intervention. These will also allow exploratory assessment of the adjusted impact of the other factors associated with remission of pre-diabetes.
Vascular function to assess changes in endothelial function and central arterial stiffness and their relationship with remission
Vascular dysfunction plays a significant role in the development and progression of diabetes-related microvascular and macrovascular complications. Lifestyle modification can improve vascular function. However, most studies performed to date have been within the context of mitigating changes in vascular function that occur with ageing. Similar evidence is lacking for interventions combining multiple lifestyle modifications, in patients with coronary heart disease and pre-diabetes. The DIABEPIC1 trial will offer participants the possibility of measuring both FMD and central arterial stiffness at baseline and at the end of the intervention. The results will determine if an intensive lifestyle intervention combining exercise training and TRE improves endothelial vascular function in adults with pre-diabetes. Furthermore, this study will also allow us to investigate the relationship between achieving pre-diabetes remission and changes in vascular function.
Exploring the relationship between pre-diabetes remission and cognitive performance
The presence of pre-diabetes and T2D increases the risk of cerebrovascular diseases, cognitive deficits and neurodegenerative diseases such as Alzheimer’s.42 T2D and Alzheimer’s disease are associated with cerebral insulin resistance, linked to cognitive and mood dysfunction.43 Indeed, cerebral insulin resistance alters energy metabolism and essential synaptic and immune functions. T2D is associated with impaired cognitive function, specifically decreased verbal memory and verbal fluency, and can impact functional capacity and patients’ quality of life. Cardiac Rehabilitation programmes that include nutritional counselling and physical exercise have improved cognition.44 Still, the association between reaching the remission criteria and changes in cognitive function has not been documented.
Strengths and limitations
This feasibility study exhibits several strengths and limitations. Strengths include its focus on the implementation of lifestyle interventions as a first-line treatment for pre-diabetes, which is often overlooked in routine clinical care. It offers a unique opportunity to influence the underlying causes of cardiovascular disease through an upgraded 6-month intensive cardiac rehabilitation programme. Additionally, the study combines multiple proven interventions, including nutritional counselling, exercise training and time-restricted eating, to achieve pre-diabetes remission and improve metabolic health. However, the study’s limitations include a relatively small sample size of 36 participants, which may not be representative of the general population with pre-diabetes. The study’s 6-month duration might not be sufficient to observe sustained changes in lifestyle behaviours and metabolic health. Acknowledging these strengths and limitations is important for a comprehensive evaluation of the study’s potential impact and to guide future research improvements.
Conclusions
Healthy lifestyles are the cornerstone of CV prevention and can reverse the physiopathology of underlying causes of cardiovascular disease. In this regard, the cardiac rehabilitation setting offers a unique opportunity to study the effectiveness of implementing intensive lifestyles to attain remission. The DIABEPIC1 feasibility trial will address this gap by providing a comprehensive and intensive approach that includes not only traditional exercise training but also specific education and innovative dietary intervention in real-world settings and provide evidence for reversing pre-diabetes in patients with coronary heart disease. Ultimately, the findings from this study could significantly impact the management and prevention of pre-diabetes and cardiovascular disease, offering a new and improved approach to enhance patient outcomes.
Supplementary Material
Footnotes
Twitter: @jiglesies1
Contributors: All authors have participated in the conceptualisation of the study and design. JI-G wrote the first version of the manuscript. VD contributed equally to the development of this study. VP, EL, MG, FB, DG, AD, CG, AN, PLL and MJ revised and contributed to the writing of the first version. NB and LB supervised the conceptualisation of the study and design and revised the final version of the manuscript.
Funding: The Mirella and Lino Saputo Research Chair in Cardiovascular Health and the Prevention of Cognitive Decline from Université de Montréal at the Montreal Heart Institute.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International diabetes Federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 2018;41:963–70. 10.2337/dc17-1962 [DOI] [PubMed] [Google Scholar]
- 3.MacKay D, Chan C, Dasgupta K, et al. Remission of type 2 diabetes. Canadian Journal of Diabetes 2022;46:753–61. 10.1016/j.jcjd.2022.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Committee ADAPP . 2. classification and diagnosis of diabetes: standards of medical care in Diabetes—2022. Diabetes Care 2022;45(Suppl 1):S17–38. 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 5.Gagnon C, Olmand M, Dupuy EG, et al. Videoconference version of the Montreal cognitive assessment: normative data for Quebec-French people aged 50 years and older. Aging Clin Exp Res 2022;34:1627–33. 10.1007/s40520-022-02092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019;40:2534–47. 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- 7.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American heart Association. Hypertension 2015;66:698–722. 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro CA, Levy RB, Claro RM, et al. A new classification of foods based on the extent and purpose of their processing. Cad Saude Publica 2010;26:2039–49. 10.1590/s0102-311x2010001100005 [DOI] [PubMed] [Google Scholar]
- 9.ACSM Books . ACSMs Guidelines for Exercise Testing and Prescription, Available: https://www.acsm.org/read-research/books/acsms-guidelines-for-exercise-testing-and-prescription [Accessed 29 Jul 2021].
- 10.WHO . Physical activity [Internet]. Available: https://www.who.int/news-room/fact-sheets/detail/physical-activity [Accessed 29 Jul 2021].
- 11.Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American college of sports medicine. Med Sci Sports Exerc 2022;54:353–68. 10.1249/MSS.0000000000002800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doménech JM, Navarro JB. Find the best subset for linear, logistic and Cox regression: user-written command confound for STATA [computer program]. Bellaterra: Universitat Autònoma de Barcelona, 2020: 1. Available: http://metodo.uab.cat/stata [Google Scholar]
- 13.Mutie PM, Pomares-Millan H, Atabaki-Pasdar N, et al. An investigation of causal relationships between Prediabetes and vascular complications. Nat Commun 2020;11:4592. 10.1038/s41467-020-18386-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honigberg MC, Zekavat SM, Pirruccello JP, et al. Cardiovascular and kidney outcomes across the Glycemic spectrum: insights from the UK Biobank. J Am Coll Cardiol 2021;78:453–64. 10.1016/j.jacc.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hostalek U. Global epidemiology of Prediabetes - present and future perspectives. Clin Diabetes Endocrinol 2019;5:5. 10.1186/s40842-019-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karter AJ, Nundy S, Parker MM, et al. Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care 2014;37:3188–95. 10.2337/dc14-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finer S, Robb P, Cowan K, et al. Top ten research priorities for type 2 diabetes: results from the diabetes UK-James LIND alliance priority setting partnership. The Lancet Diabetes & Endocrinology 2017;5:935–6. 10.1016/S2213-8587(17)30324-8 [DOI] [PubMed] [Google Scholar]
- 18.Captieux M, Prigge R, Wild S, et al. Defining remission of type 2 diabetes in research studies: A systematic Scoping review. PLoS Med 2020;17:e1003396. 10.1371/journal.pmed.1003396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetologia 2021;64:2359–66. 10.1007/s00125-021-05542-z [DOI] [PubMed] [Google Scholar]
- 20.Jin S, Bajaj HS, Brazeau A-S, et al. Remission of type 2 diabetes: user's guide: diabetes Canada clinical practice guidelines expert working group. Can J Diabetes 2022;46:762–74. 10.1016/j.jcjd.2022.10.005 [DOI] [PubMed] [Google Scholar]
- 21.Adler AI, Stratton IM, Neil HA, et al. Association of Glycaemia with Macrovascular and Microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:412–9. 10.1136/bmj.321.7258.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Wu S, Song Q, et al. Reversion from pre-diabetes mellitus to Normoglycemia and risk of cardiovascular disease and all-cause mortality in a Chinese population: A prospective cohort study. J Am Heart Assoc 2021;10:e019045. 10.1161/JAHA.120.019045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: A narrative review of the evidence. Nutrients 2019;11:766. 10.3390/nu11040766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metabolism 2019;30:67–77. 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandoval-Insausti H, Jiménez-Onsurbe M, Donat-Vargas C, et al. Ultra-processed food consumption is associated with abdominal obesity: A prospective cohort study in older adults. Nutrients 2020;12:2368. 10.3390/nu12082368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero-Salazar H, Donat-Vargas C, Moreno-Franco B, et al. High consumption of Ultra-processed food may double the risk of Subclinical coronary Atherosclerosis: the Aragon workers' health study (AWHS). BMC Med 2020;18:235. 10.1186/s12916-020-01678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thibault F, Bernard S, Laury S, et al. Consumption of Ultra-processed foods and cancer risk: results from Nutrinet-Santé prospective cohort. BMJ 2018;360. 10.1136/bmj.k322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and Trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-González MA, Ros E, Estruch R. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;379:1388–9. 10.1056/NEJMc1809971 [DOI] [PubMed] [Google Scholar]
- 30.Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011;34:14–9. 10.2337/dc10-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estruch R, Sacanella E, Ros E. Should we all go Pesco-vegetarian Eur Heart J 2021;42:1144–6. 10.1093/eurheartj/ehaa1088 [DOI] [PubMed] [Google Scholar]
- 32.Gardner CD, Trepanowski JF, Del Gobbo LC, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion. JAMA 2018;319:667–79. 10.1001/jama.2018.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seid H, Rosenbaum M. Low carbohydrate and low-fat diets: what we don’t know and why we should know it. Nutrients 2019;11:2749. 10.3390/nu11112749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Avraham S, Harman-Boehm I, Schwarzfuchs D, et al. Dietary strategies for patients with type 2 diabetes in the era of multi-approaches; review and results from the dietary intervention. Diabetes Res Clin Pract 2009;86 Suppl 1:S41–8. 10.1016/S0168-8227(09)70008-7 [DOI] [PubMed] [Google Scholar]
- 35.Hession M, Rolland C, Kulkarni U, et al. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its Comorbidities. Obes Rev 2009;10:36–50. 10.1111/j.1467-789X.2008.00518.x [DOI] [PubMed] [Google Scholar]
- 36.Lamantia V, Sniderman A, Faraj M. Nutritional management of hyperapoB. Nutr Res Rev 2016;29:202–33. 10.1017/S0954422416000147 [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and Atherogenic lipids in patients with metabolic syndrome. Cell Metabolism 2020;31:92–104. 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cienfuegos S, Gabel K, Kalam F, et al. Effects of 4- and 6-H time-restricted feeding on weight and Cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metabolism 2020;32:366–378. 10.1016/j.cmet.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji Y, Plourde H, Bouzo V, et al. Validity and usability of a Smartphone image-based dietary assessment App compared to 3-day food diaries in assessing dietary intake among Canadian adults: randomized controlled trial. JMIR Mhealth Uhealth 2020;8:e16953. 10.2196/16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyen A, Rappaport AI, Fleurent-Grégoire C, et al. Relative validation of an artificial intelligence-enhanced, image-assisted mobile App for dietary assessment in adults: randomized crossover study. J Med Internet Res 2022;24:e40449. 10.2196/40449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor R. Type 2 diabetes and remission: practical management guided by pathophysiology. J Intern Med 2021;289:754–70. 10.1111/joim.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 2018;14:168–81.:3. 10.1038/nrneurol.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker LD, Cross DJ, Minoshima S, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with Prediabetes or early type 2 diabetes. Arch Neurol 2011;68:51–7. 10.1001/archneurol.2010.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narges D, Mohammad J, Behrouz Attarbashi M. Effects of cardiac rehabilitation on cognitive impairments in patients with cardiovascular diseases: a systematic review. Int J Neurosci 2020. 10.1080/00207454.2020.1773823 [DOI] [PubMed] [Google Scholar]
- 45.Kokkinos P, Kaminsky LA, Arena R, et al. New generalized equation for predicting maximal oxygen uptake (from the fitness Registry and the importance of exercise national database). The American Journal of Cardiology 2017;120:688–92. 10.1016/j.amjcard.2017.05.037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-073763supp001.pdf (75.9KB, pdf)