Summary
The neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammatory index (SII) have been reported as prognosticators in non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and melanoma. This analysis of the INVIDIa-2 study on influenza vaccination in patients with cancer treated with immune checkpoint inhibitors (ICIs) assessed NLR and SII on overall survival (OS) by literature-reported (LR), receiver operating characteristic curve (ROC)-derived (ROC) cutoffs or as continuous variable (CV). NLR and SII with ROC cutoffs of <3.4 (p < 0.001) and <831 (p < 0.001) were independent factors for OS in multivariate analysis. SII with LR, ROC, or CV significantly predicted OS in NSCLC (p = 0.002, p = 0.003, p = 0.003), RCC (p = 0.034, p = 0.014, p = 0.014), and melanoma (p = 0.038, p = 0.022, p = 0.019). NLR with LR and ROC cutoffs predicted OS in first line (p < 0.001 for both) and second line or beyond (p = 0.006 for both); likewise SII (p < 0.001; p = 0.002 and p < 0.001). NLR and SII are prognosticators in NSCLC, RCC, and melanoma treated with ICIs.
Subject areas: Immune response, Immunity, Immunology
Graphical abstract
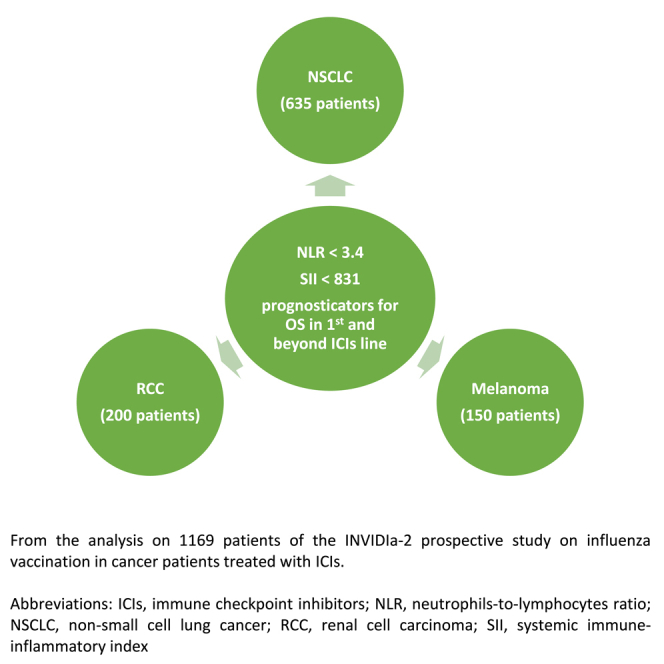
Highlights
-
•
NLR and SII are prognosticators in NSCLC, RCC, and melanoma treated with ICIs
-
•
This is consistent whether patients are treated in a first-line setting or beyond this
Immune response; Immunity; Immunology
Introduction
The management of lung cancer has advanced significantly with the introduction of immune checkpoint inhibitors (ICIs). In patients with non-small cell lung cancer (NSCLC), the neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammatory index (SII) have been reported as useful indicators of survival outcomes.1,2,3,4 These indices may reflect the host’s proinflammatory status and systemic immune response to cancer-related inflammation. They can be easily derived from the peripheral full blood count and therefore are relatively inexpensive to use.5,6 Among the prognostic stratification tools used for patients with lung cancer on ICIs that incorporate the NLR and/or SII are the NHS-Lung score,7 Lung Immune Prognostic Index (LIPI),8 and Lung Immuno-Oncology Prognostic Score (LIPS-3).9
In addition to lung malignancies, other tumor types including renal cell carcinoma (RCC) and melanoma can also be treated with ICIs. Previous reports have suggested that the NLR and SII may be able to predict survival outcomes in patients with these malignancies as well. A higher NLR was suggested to be a marker of poorer overall survival (OS) and progression-free survival (PFS) in patients with metastatic RCC or melanoma10,11 with a similar trend seen between higher SII values and survival outcomes in these patients.12,13 It is well known how important inflammation is in the process of cancer development, and these markers are thought to be an indication of the body’s inflammatory status and thus have been investigated as indicators of prognosis.14,15,16
The INVIDIa-2 study prospectively investigated the impact of influenza vaccination on the incidence and severity of influenza syndrome and the oncological outcome in patients with advanced cancer treated with ICIs.17 This present analysis utilizes the INVIDIa-2 study population to assess and compare the application of the aforementioned peripheral immune-inflammatory blood markers in predicting survival outcomes in patients with different tumor types including NSCLC, RCC, and melanoma treated with ICIs.
Results
Patient characteristics
The INVIDIa-2 study included 1,279 patients from 82 centers, and the present analysis included 1,169 patients from 63 centers of this original study population fully evaluable for the present analysis; 110 patients were excluded due to missing data (36 patients with missing data on ICIs and 52 patients with missing data on OS). The characteristics of this patient cohort are described in Table 1.
Table 1.
Patient characteristics
| Overall population (n = 1,169) | |
|---|---|
| Age, median (IQR) | 69.7 (60.9–75.9) |
| Gender, n (%) | |
| Male | 820 (70.2) |
| Female | 349 (29.9) |
| ECOG PS, n (%) | |
| 0 | 678 (58.0) |
| 1 | 419 (35.8) |
| 2 | 53 (4.5) |
| 3 | 3 (0.3) |
| Unknown | 16 (1.4) |
| Primary tumor, n (%) | |
| Lung | 635 (54.3) |
| RCC | 200 (17.1) |
| Melanoma | 150 (12.8) |
| UC | 65 (5.6) |
| H&N | 45 (3.9) |
| Other | 74 (6.3) |
| ICI treatment line, n (%) | |
| 1 | 582 (49.9) |
| 2 | 482 (41.3) |
| 3 | 101 (8.7) |
| Unknown | 4 (0.1) |
| Therapy, n (%) | |
| ICI/ICI+ICI | 1,097 (93.8) |
| ICI + othera | 72 (6.2) |
| Smoking habits, n (%) | |
| Current | 281 (24.0) |
| Former | 509 (43.5) |
| Never | 348 (29.8) |
| Unknown | 31 (2.7) |
| Bone metastases, n (%) | 349 (29.9) |
| Brain metastases, n (%) | 181 (15.5) |
| Liver metastases, n (%) | 149 (12.8) |
| Pre-treatment steroids, n (%) | 337 (28.8) |
| Neutrophils, median (IQR) | 4.9 (3.6–7.1) |
| Lymphocyte, median (IQR) | 1.61 (1.16–2.25) |
| Platelets, median (IQR) | 255.3 (200–322) |
| NLR, median (IQR) | 3.3 (2.2–4.9) |
| dNLR, median (IQR) | 1.92 (1.43–2.54) |
| SII, median (IQR) | 829.2 (506.7–1,451.4) |
| LDH, median (IQR) | 390 (217–581) |
dNLR, derived neutrophil-to-lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; H&N, head and neck; ICI, immune checkpoint inhibitor; IQR, interquartile range; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; n, number; PS, performance status; RCC, renal cell carcinoma; SII, systemic immune-inflammatory index; UC, urothelial carcinoma.
Chemotherapy or targeted therapy.
Peripheral blood markers and association with survival
The NLR was significantly associated with the OS of the entire patient cohort as shown in Table 2. When used as a continuous variable, the c-index was 0.595 (hazard ratio [HR] 1.21; 95% confidence interval [CI]: 1.12–1.31], p < 0.001). When using the literature-reported NLR cutoff of <4.0 versus ≥4.0, the c-index was 0.566 (HR 1.68 [95% CI: 1.41–2.00], p < 0.001). When using the receiver operating characteristic curve (ROC)-derived NLR cutoff of <3.4 versus ≥3.4, the c-index was 0.569 (HR 1.67 [95% CI: 1.40–2.00], p < 0.001). With the use of the derived neutrophil-to-lymphocyte ratio (dNLR), the c-index was 0.544 (HR 1.75 [95% CI: 1.40–2.17], p < 0.001). The c-index when the ROC-derived cutoff values for the NLR were used appeared to be better than when using the literature-reported cutoff values or the dNLR.
Table 2.
Neutrophil-to-lymphocyte ratio at univariate analysis
| All sample (n = 1,169) |
Lung (n = 635) |
RCC (n = 200) |
Melanoma (n = 150) |
|
|---|---|---|---|---|
| HR (95% CI); p value | HR (95% CI); p value | HR (95% CI); p value | HR (95% CI); p value | |
| NLR (continuous; 10-unit difference) | 1.21 (1.12–1.31); p < 0.001, c-index = 0.595 | 1.17 (1.06–1.31); p = 0.003, c-index = 0.583 | 1.80 (0.99–3.30); p = 0.055, c-index = 0.599 | 2.43 (1.00–5.90); p = 0.050, c-index = 0.557 |
| NLR | ||||
| <4.0 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥4.0 | 1.68 (1.41–2.00); p < 0.001, c-index = 0.566 | 1.42 (1.14–1.78); p = 0.002, c-index = 0.549 | 1.65 (1.01–2.70); p = 0.045, c-index = 0.556 | 1.52 (0.78–2.95); p = 0.22, c-index = 0.546 |
| NLR | ||||
| <3.4 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥3.4 | 1.67 (1.40–2.00); p < 0.001, c-index = 0.569 | 1.54 (1.22–1.93); p < 0.001, c-index = 0.561 | 1.58 (0.98–2.53); p = 0.059, c-index = 0.555 | 1.18 (0.62–2.23); p = 0.62, c-index = 0.522 |
| dNLR | ||||
| ≤3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >3 | 1.75 (1.40–2.17); p < 0.001, c-index = 0.544 | 1.67 (1.28–2.19); p < 0.001, c-index = 0.547 | 1.85 (0.94–3.62); p = 0.073 c-index = 0.537 | 3.66 (1.69–7.92); p = 0.001, c-index = 0.565 |
95% CI, 95% confidence interval; HR, hazard ratio; dNLR, derived neutrophil-to-lymphocyte ratio; n, number; NLR, neutrophil-to-lymphocyte ratio; RCC, renal cell carcinoma.
When using NLR as a continuous variable, the c-index was 0.583 (HR 1.17 [95% CI: 1.06–1.31], p = 0.003) in patients with lung cancer and 0.557 (HR 2.43 [95% CI: 1.00–5.90], p = 0.050) in patients with melanoma. The use of the literature-reported NLR threshold of <4.0 resulted in significant differences in OS in patients with lung cancer and those with RCC. The c-index was 0.549 (HR 1.42 [95% CI: 1.14–1.78], p = 0.002) in patients with lung cancer and 0.556 (HR 1.65 [95% CI 1.01–2.70], p = 0.045) in patients with RCC. The c-index was 0.546 in patients with melanoma, but this did not confer a significant difference in OS (HR 1.52 [95% CI: 0.78–2.95], p = 0.22). With the ROC-derived NLR threshold of <3.4, significant differences in OS were only found in patients with lung cancer (HR 1.54 [95% CI: 1.22–1.93], p = 0.002, c-index: 0.561) but not in patients with RCC (HR 1.65 [95% CI: 0.98–2.53], p = 0.059) or melanoma (HR 1.18 [95% CI: 0.62–2.23], p = 0.62).
With the use of the dNLR and the literature-reported cut-off of ≤3 versus >3 for this, there was a significant difference in OS for patients with lung cancer (HR 1.67 [95% CI: 1.28–2.19] p < 0.001, c-index 0.547) and those with melanoma (HR 3.66 [95% CI: 1.69–7.92], p = 0.001, c-index 0.565) but not in those with RCC (HR 1.85 [95% CI: 0.94–3.62], p = 0.073).
The SII was significantly associated with the OS of the entire patient cohort as shown in Table 3. When used as a continuous variable, the c-index was 0.592 (HR 1.06 [95% CI: 1.03–1.08], p < 0.001). When using the literature-reported cutoff of <1,444 versus ≥1,444, the c-index was 0.557 (HR 1.67 [95% CI: 1.43–2.02], p < 0.001). When using the ROC-derived cutoff of <831 versus ≥831, the c-index was 0.570 (HR 1.71 [95% CI: 1.43–2.05], p < 0.001). The c-index when the ROC-derived cutoff values for the SII were used appeared to be better than when using the literature-reported cutoff values.
Table 3.
Systemic immune-inflammatory index at univariate analysis
| All sample (n = 1,169) |
Lung (n = 635) |
RCC (n = 200) |
Melanoma (n = 150) |
|
|---|---|---|---|---|
| HR (95% CI); p value | HR (95% CI); p value | HR (95% CI); p value | HR (95% CI); p value | |
| SII (continuous; 1,000-unit difference) | 1.06 (1.03–1.08); p < 0.001, c-index = 0.592 | 1.05 (1.02–1.08); p = 0.002, c-index = 0.568 | 1.18 (1.01–1.37); p = 0.034, c-index = 0.603 | 1.22 (1.01–1.47); p = 0.038, c-index = 0.589 |
| SII | ||||
| <831 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥831 | 1.71 (1.43–2.05); p < 0.001, c-index = 0.570 | 1.41 (1.13–1.78); p = 0.003, c-index = 0.547 | 1.81 (1.13–2.91); p = 0.014, c-index = 0.576 | 2.05 (1.13–3.74); p = 0.019, c-index = 0.595 |
| SII | ||||
| <1,444 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥1,444 | 1.67 (1.39–2.02); p < 0.001, c-index = 0.557 | 1.43 (1.13–1.81); p = 0.003, c-index = 0.545 | 1.92 (1.14–3.24); p = 0.014, c-index = 0.556 | 2.23 (1.12–4.42); p = 0.022, c-index = 0.569 |
95% CI, 95% confidence interval; HR, hazard ratio; n, number; RCC, renal cell carcinoma; SII, systemic immune-inflammatory index.
The SII consistently showed significant differences in OS across the three tumor types. Using a continuous measurement for the SII, the c-index in patients with lung cancer was 0.568 (HR 1.05 [95% CI 1.02–1.08], p = 0.002), 0.603 in those with RCC (HR 1.18 [95% CI: 1.01–1.37], p = 0.034), and 0.589 in those with melanoma (HR 1.22 [95% CI 1.01–1.47], p = 0.038).
Using the literature-reported SII threshold of <1,444, the c-index in patients with lung cancer was 0.545 (HR 1.43 [95% CI 1.13–1.81], p = 0.003), 0.556 in those with RCC (HR 1.92 [95% CI: 1.14–3.24, p = 0.014), and 0.569 in those with melanoma (HR 2.23 [95% CI 1.12–4.42], p = 0.022). Using the ROC-derived SII threshold of <831, the c-index in patients with lung cancer was 0.547 (HR 1.41 [95% CI 1.13–1.78], p = 0.003), 0.576 in those with RCC (HR 1.81 [95% CI: 1.13–2.91], p = 0.014), and 0.595 in those with melanoma (HR 2.05 [95% CI 1.13–3.74], p = 0.019).
Other clinical factors in univariate analysis
There were significant differences in OS among the entire patient cohort when lactate dehydrogenase (LDH) was used (HR 1.41 [95% CI 1.14–1.75], p = 0.001) with a c-index of 0.533 as demonstrated in Table S1. When assessed according to tumor type, it was only patients with lung cancer who had their OS significantly affected (HR 1.39 [95% CI: 1.04–1.87], p = 0.027) with a c-index of 0.523, but not patients with RCC or melanoma as shown in Table S1.
Performance status was significantly associated with OS among the entire patient group as well. This was consistent in patients with performance status 1 (HR 1.87 [95% CI: 1.56–2.25], p < 0.001), performance status 2 (HR 3.66 [95% CI: 2.52–5.30], p < 0.001), and performance status 3 (HR 4.39 [95% CI: 1.41–13.74], p = 0.011).
Age and gender were not found to be factors significantly associated with the OS for the whole patient cohort with an HR of 1.01 (95% CI: 1.00–1.02; p = 0.002) and 1.18 (95% CI: 0.97–1.43; p = 0.10), respectively. Smoking status also did not result in significant differences in OS for these patients with ex-smokers having an HR of 1.10 (95% CI: 0.89–1.36; p = 0.39) and current smokers having an HR of 1.14 (95% CI: 0.90–1.46; p = 0.28).
The multivariate analysis showed that the NLR with an ROC-derived threshold of <3.4 versus ≥3.4 affected survival outcomes (HR 1.40 [95% CI: 1.17–1.68], p < 0.001), and so did the SII with the ROC-derived threshold of <831 versus ≥831 (HR 1.45 [95% CI: 1.20–1.75], p < 0.001) as shown in Table 4.
Table 4.
Multivariate analysis
| Clinical characteristics or biomarker | Multivariable for NLR HR (95% CI); p value |
Multivariable for SII HR (95% CI); p value |
|---|---|---|
| NLR | ||
| <3.4 | 1.00 (ref) | |
| ≥3.4 | 1.40 (1.17–1.68); p < 0.001 | |
| SII | ||
| <831 | 1.00 (ref) | |
| ≥831 | 1.45 (1.20–1.75); p < 0.001 | |
| Age | 1.00 (0.99–1.01); p = 0.49 | 1.00 (0.99–1.01); p = 0.29 |
| ECOG-PS | ||
| 0 | 1.00 (ref) | 1.00 (ref) |
| 1 | 1.69 (1.40–2.04); p < 0.001 | 1.65 (1.37–2.00); p < 0.001 |
| 2 | 3.21 (2.19–4.71); p < 0.001 | 3.11 (2.12–4.56); p < 0.001 |
| 3 | 3.24 (1.02–10.27); p = 0.046 | 3.06 (0.97–9.71); p = 0.057 |
| Pre-treatment steroids | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 1.23 (1.02–1.49); p = 0.033 | 1.25 (1.03–1.52); p = 0.021 |
| Bone metastases | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 1.17 (0.97–1.43); p = 0.11 | 1.19 (0.98–1.45); p = 0.074 |
| Liver metastases | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 1.79 (1.41–2.27); p < 0.001 | 1.76 (1.39–2.23); p < 0.001 |
| LDH | ||
| < Upper limit of normal | 1.00 (ref) | 1.00 (ref) |
| ≥ Upper limit of normal | 1.19 (0.95–1.48); p = 0.12 | 1.17 (0.94–1.46); p = 0.15 |
95% CI, 95% confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; n, number; NLR, neutrophil-to-lymphocyte ratio; RCC, renal cell carcinoma; SII, systemic immune-inflammatory index.
The NLR and SII were significantly associated with the OS of patients irrespective of the other independent prognostic factors including the performance status of patients, pre-treatment with steroids, and the presence of liver metastases. The presence of bone metastases and LDH values did not independently affect survival outcomes.
Using the continuous measurement, literature-reported, or ROC-derived cutoff thresholds for the NLR and SII, the OS was significantly different against these values when patients were treated in a first-line setting or a second-line setting and beyond. Using a continuous NLR measurement, the c-index was 0.622 (HR 1.43 [95% CI: 1.22–1.66, p < 0.001) in patients receiving ICIs in a first-line setting and 0.576 (HR 1.13 [95% CI: 1.02–1.25, p = 0.016) in those receiving treatment in a second-line setting or beyond. Using NLR with a cutoff value of <4.0, the c-index was 0.589 (HR 2.14 [95% CI: 1.63–2.81], p < 0.001) in patients receiving treatment in a first-line setting and 0.551 (HR 1.39 [95% CI: 1.10–1.75], p = 0.006) in those receiving treatment in a second-line or further-line setting. Using NLR with a threshold of <3.4, the c-index was 0.594 (HR 2.11 [95% CI: 1.60–2.78], p < 0.001) in patients receiving treatment in a first-line setting and 0.551 (HR 1.38 [95% CI: 1.10–1.74], p = 0.006) in those receiving treatment in a second-line or further-line setting. The dNLR (≤3 versus >3) also affected the OS significantly with the c-index being 0.573 (HR 2.33 [95% CI: 1.72–3.17], p < 0.001) in first-line treatment and 0.528 (HR 1.39 [95% CI: 1.01–1.92], p = 0.042) in second- or further-line treatment. These are shown in Table 5.
Table 5.
NLR, SII, and LDH according to line of ICIs by univariate analysis
| First line (n = 582) |
2+ treatment line (n = 587) |
|
|---|---|---|
| HR (95% CI); p value | HR (95% CI); p value | |
| NLR (continuous; 10-unit difference) | 1.43 (1.22–1.66); p < 0.001, c-index = 0.622 | 1.13 (1.02–1.25); p = 0.016, c-index = 0.576 |
| NLR | ||
| <4.0 | 1.00 (ref) | 1.00 (ref) |
| ≥4.0 | 2.14 (1.63–2.81); p < 0.001, c-index = 0.589 | 1.39 (1.10–1.75); p = 0.006, c-index = 0.551 |
| NLR | ||
| <3.4 | 1.00 (ref) | 1.00 (ref) |
| ≥3.4 | 2.11 (1.60–2.78); p < 0.001, c-index = 0.594 | 1.38 (1.10–1.74); p = 0.006, c-index = 0.551 |
| SII (continuous; 1,000-unit difference) | 1.09 (1.05–1.13); p < 0.001, c-index = 0.619 | 1.04 (1.01–1.07); p = 0.008, c-index = 0.576 |
| SII | ||
| <831 | 1.00 (ref) | 1.00 (ref) |
| ≥831 | 2.01 (1.52–2.67); p < 0.001, c-index = 0.586 | 1.53 (1.21–1.93); p < 0.001, c-index = 0.560 |
| SII | ||
| <1,444 | 1.00 (ref) | 1.00 (ref) |
| ≥1,444 | 1.94 (1.46–2.57); p < 0.001, c-index = 0.573 | 1.48 (1.16–1.90); p = 0.002, c-index = 0.545 |
| dNLR | ||
| ≤3 | 1.00 (ref) | 1.00 (ref) |
| >3 | 2.33 (1.72–3.17); p < 0.001, c-index = 0.573 | 1.39 (1.01–1.92); p = 0.042, c-index = 0.528 |
| LDH | ||
| <ULN | 1.00 (ref) | 1.00 (ref) |
| ≥ULN | 1.34 (0.98–1.84); p = 0.065, c-index = 0.529 | 1.39 (1.04–1.86); p = 0.027, c-index = 0.529 |
95% CI, 95% confidence interval; dNLR, derived neutrophil-to-lymphocyte ratio; HR, hazard ratio; LDH, lactate dehydrogenase; n, number; NLR, neutrophil-to-lymphocyte ratio; RCC, renal cell carcinoma; SII, systemic immune-inflammatory index.
Using a continuous SII measurement, the c-index was 0.619 (HR 1.09 [95% CI: 1.05–1.13, p < 0.001) in patients receiving ICIs in a first-line setting and 0.576 (HR 1.04 [95% CI: 1.01–1.07, p = 0.008) in patients receiving treatment in a second-line or further-line setting. Using SII with a threshold of <831, the c-index was 0.586 (HR 2.01 [95% CI: 1.52–2.67], p < 0.001) in patients receiving treatment in a first-line setting and 0.560 (HR 1.53 [95% CI: 1.21–1.93], p < 0.001) in those receiving treatment in a second-line or further-line setting. Using SII with the standard cutoff value of <1,444, the c-index was 0.573 (HR 1.94 [95% CI: 1.46–2.57], p < 0.001) in patients receiving treatment in a first-line setting and 0.545 (HR 1.48 [95% CI: 1.16–1.90], p = 0.002) in second-line treatment or beyond.
The c-index for LDH using the upper limit of normal (ULN) as the threshold was 0.529 both in a first-line and second-line or further-line setting, although it was only significant in the latter (p = 0.065 and p = 0.027, respectively).
In summary, the NLR, SII, and dNLR all performed better in a first-line setting compared with the second-line setting and beyond. The NLR and SII performed better than the dNLR, whereas the LDH was the least accurate.
Applicability of the LIPI score
Use of the LIPI score led to significant differences in OS not only for the whole patient cohort (c-index 0.564) but also specifically for patients with lung cancer (c-index 0.559) as shown in Table S2. When replacing the dNLR within the LIPI score with the ROC-derived SII threshold, this led to a higher c-index score of 0.578 for the entire patient group as presented in Table S3. The LIPI score with this modification showed significant differences in OS for patients with lung cancer with an HR of 2.01 (95% CI: 1.29–3.11 [p = 0.002]).
When the ROC-derived NLR was used instead of the dNLR within the LIPI score, this also led to a slightly higher c-index score of 0.579 not only for the entire patient cohort but also in patients with lung cancer (c-index 0.566). This is demonstrated in Table S4.
Applicability of the LIPS-3 score
Use of the LIPS-3 score resulted in significant differences in OS not only for the whole patient group (c-index 0.566) but also for those with lung cancer specifically (c-index 0.552) as shown in Table S5. When the ROC-derived NLR threshold was incorporated in the LIPS-3 score instead of the standard cutoff value, an analogous pattern was seen with significant differences in OS noted for the whole patient cohort (c-index 0.571) and for those with lung cancer specifically (c-index 0.562) as seen in Table S6. When the LIPS-3 score was recalculated using the ROC-derived SII threshold instead of the NLR, there were similar findings in terms of significant differences in OS for the entire patient cohort and those with lung cancer specifically with higher c-indices of 0.571 and 0.553, respectively, as demonstrated in Table S7.
Owing to the low numbers of patients within the subgroups with a high-risk LIPI or LIPS-3 score, the analyses for the other tumor subgroups could not be conducted.
Discussion
This analysis has shown that both the NLR and SII with either their literature-reported or ROC-derived cutoffs and as continuous variables are prognostic factors for OS in patients with different tumor types treated with ICIs in the first-line setting or second-line setting and beyond. We have also shown that the NLR and SII with their ROC-derived cutoff values (of <3.4 and <831, respectively) are independent factors significantly associated with survival in this cohort.
Differences in prognostic accuracy might exist according to tumor type. For instance, NLR with its literature-reported cutoff value (<4) can significantly affect OS in patients with lung cancer or RCC, but not in melanoma. The NLR in its continuous measurement and the dNLR can significantly affect OS in patients with melanoma. The SII with either literature-reported or ROC-derived cutoffs, and as continuous variables, appears to perform better with significant differences in OS across the three tumor types: lung, RCC, and melanoma.
The NLR, SII, and dNLR appear to perform better as prognostic markers when used in patients being given first-line treatment compared with those treated in the second-line setting or beyond. NLR and SII perform similarly but appear superior to dNLR in their prognostication ability.
As previously described, studies have reported the NLR value to be a helpful indicator of survival outcomes and therefore prognosis in patients with NSCLC. Patients with a lower NLR value tended to have a better response to treatment and prognosis.1,2,3,18 In a retrospective study of 327 patients with non-metastatic RCC, it was reported that an elevated NLR was a potential marker for poorer survival outcomes. These patients had just undergone a curative or palliative nephrectomy without any systemic therapy given as yet.19 This was also consistent with another study in a non-metastatic setting that found the NLR to be an indicator of tumor size and therefore prognosis.20 In a metastatic RCC setting, a lower NLR was reported to be associated with better survival outcomes.21 This is mirrored by the output from a systematic review of patients with metastatic RCC on ICIs that concurred that the NLR can be a useful marker of OS and PFS.10 The present analysis has demonstrated that dNLR did not have an impact on OS for patients with RCC, and this is echoed in the findings of another systematic review of urological cancers assessing the use of dNLR in prognostication.22
In patients with metastatic melanoma who have undergone a metastasectomy, previous studies have suggested the NLR can predict survival outcomes, but this is based on a cutoff value of >5.23,24 Other work including patients with melanoma having ICIs also found them to have a poor PFS if their NLR was >4.11,25,26 The present analysis found that using the NLR value as a continuous measurement or the dNLR resulted in significant differences in survival outcomes but not with the literature-reported or ROC-derived cutoff values.
There may be several factors accounting for the varied performance of the NLR among tumor types. As previously mentioned, the relatively lower proportion of patients with RCC or melanoma may have resulted in a lower likelihood of detecting any significant differences in OS when using the NLR. As also noted from the earlier studies described, some had to use different NLR thresholds among the different tumor types. This too may have been a reason for the lack of significant differences in OS among some tumor types noted in our present analysis.
The SII has also been reported as a marker of prognosis in patients with lung cancer with higher SII values conferring poorer survival outcomes.4,27,28 In patients with localized RCC who had undergone a radical nephrectomy, the SII was found to be a marker of poor prognosis.29 This was also found to be true in patients with metastatic RCC including those on ICIs or tyrosine kinase inhibitor treatment.12,30,31,32 The results from our current work are in parallel with these previous reports.
There has been contradicting information about the impact of the SII on the survival of patients with melanoma. Previous work had reported that SII was not a significant predictor for clinical outcomes of these patients with melanoma on ICIs.33 These patients were treated with pembrolizumab, ipilimumab, nivolumab, or a combination of ipilimumab and nivolumab, but the study comprised a relatively small cohort of 62 patients. Other studies have reported otherwise with a higher SII being associated with worse OS; this is consistent with the findings of the present analysis, although it is recognized that these studies too had relatively smaller sample sizes.13,34,35
A study of 569 patients with NSCLC who underwent lobectomy for their cancer with curative intent found that SII did perform better in the prognostication of these patients. Other work involving other tumor types had shown similar results. These studies included 916 patients with esophageal cancer, 1,383 patients with colorectal cancer, and 133 patients with hepatocellular carcinoma, all of whom underwent radical resection of their esophageal or colorectal tumor or liver transplantation for their hepatocellular carcinoma, respectively.36,37,38 Although these patients did not receive ICIs at the time of the analysis, these studies reported that SII did have a superior prognostic value compared with NLR.
The addition of the platelet count to the calculation of the SII may explain why it appears to be a better indicator of prognosis. Platelets are involved in the shielding of cancer cells from natural killer cell-mediated attack and shear stress. They are also involved in promoting the metastatic characteristics of these cancer cells via the signaling molecules present in platelets and their ability to enhance cancer cell migration across the endothelial layer.39,40
The analysis of the LIPI score with dNLR replaced by the ROC-derived NLR revealed a higher c-index score for the entire patient cohort particularly in patients with lung cancer. This infers that the application of the LIPI score to these patients could potentially be enhanced with the incorporation of the ROC-derived NLR as opposed to the dNLR. The evaluation of the LIPI and LIPS-3 scores suggests that the dNLR and SII can be useful markers of prognosis when incorporated into such models for patients on ICIs. However, tumor-specific models are required to be able to include other relevant features.
Conclusion
The NLR and SII are associated with OS and thus prognosis in a large prospective cohort of patients with different tumor types treated with ICIs. This is consistent whether the patient is being treated in a first-line setting or beyond.
Limitations of study
The main limitation of this analysis is its posthoc nature. There was also a much higher proportion of patients with lung cancer compared with RCC or melanoma. The smaller proportions of patients with melanoma may have accounted for the lack of significant differences in survival outcomes when using the literature-reported cutoff NLR values. However, the multicenter and real-life nature of this work allows for the generalizability of the results. Furthermore, based on the PROGRESS framework, our study can be considered confirmatory of the prognostic value of the peripheral blood immune-inflammatory indexes we investigated with their relative literature-reported cutoffs and non-confirmatory for their ROC-derived one.41
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| Stata (v.16; StataCorp) | StataCorp | https://www.stata.com/stata16/ |
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact: Giuseppe Luigi Banna, giuseppe.banna@nhs.net.
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
The patient cohort for the present analysis was derived from the INVIDIa-2 study. The original study database comprised 1279 patients (831 males and 357 females). Their median age was 69 years 645 patients had a lung malignancy, 201 patients had an RCC and 153 patients had a melanoma.
Declarations
The authors have completed the REMARK guideline checklist and included this in Table S8.42
Institutional permission information
Local Institutional Review Board approval was required for each centre for inclusion in the study. Written informed consent was obtained for all the enrolled patients. All the study procedures were per the precepts of Good Clinical Practice and the declaration of Helsinki.
Consent for publication
All authors have read and approved the submitted manuscript and confirmed their consent for publication. The corresponding author had full access to all the data in the study and had final responsibility for the publication.
Method details
The INVIDIa-2 study is a prospective multicentre observational study investigating the clinical efficacy of the influenza vaccination in patients with advanced cancer receiving ICIs who had been enrolled patients from 82 centres from October 2019 to January 2020. The oncological outcomes of these patients on ICIs were included in the study’s secondary endpoints.43
Baseline data were collected on patient characteristics, Eastern Cooperative Oncology Group (ECOG) performance status (PS), type of cancer, treatment and line of therapy, location of metastatic lesions, pre-treatment with steroids and peripheral blood counts including the neutrophils, lymphocytes and platelets, at study entry before vaccination or ICI therapies. These were used to calculate the NLR and SII. The present analysis focused on lung cancer, RCC and melanoma as they were the most representative of the overall INVIDIa-2 study population and each of those involved at least 150 patients.
The primary objective of this analysis was to assess the prognostic role of the NLR and SII by comparing OS among the whole study population and patients with each tumour type (i.e. lung, renal and melanoma) using defined cut-off thresholds for the NLR and SII. These thresholds were either literature-reported or receiver operating characteristic curve (ROC)- derived based on OS at 12 months in the whole study population. With regards to the literature-reported ones, we selected those that had previously been assessed in large series of patients with lung cancer based on our previous literature reviews.5,6 The NLR and SII were also assessed as continuous variables. The secondary endpoints included the assessment of the NLR and SII by OS when treatment was given first-line versus second-line and beyond, the evaluation of the derived NLR (dNLR) and lactate dehydrogenase (LDH) across tumour types and treatment lines. Furthermore, we explored the prognostic performance across tumour types of the original LIPI and LIPS-3 score or modified by the incorporation of SII instead of dNLR or NLR, respectively.
The NLR was calculated as the ratio between neutrophils and lymphocytes from the peripheral full blood count of a standard blood test performed within 14 days of the treatment start date. A high NLR was considered a value ≥4 according to the literature-reported cut-off.9 The analysis was repeated with a modified ROC-derived 3.4 cut-off value as well as a continuous variable. The SII was calculated as the NLR multiplied by the platelet count with the previously reported cut-off value of ≥ 1,440.7 This was also repeated with a modified ROC-derived 831 cut-off value and as a continuous measurement. The derived NLR (dNLR) was calculated using the following formula: neutrophil count / (white blood cell count – neutrophil count).
Quantification and statistical analysis
Clinical data were analysed using descriptive statistics with their respective dispersion values given, utilising percentages for binary variables and medians for continuous variables. The PFS was computed from the treatment start date to disease progression or death from any cause, whereas the OS was calculated from the treatment start date to death or the date of the final follow-up. The study censored patients who had no events at the time of the analysis.
A Cox-regression univariate analysis assessing OS against the pre-determined NLR and SII values, alongside the dNLR, LDH and main clinical potentially prognostic features (i.e., age, gender, ECOG PS, smoking status, primary tumour, pre-treatment with steroids, presence of bone, brain or liver metastases) was conducted for the entire patient cohort but also each of the three tumour types (lung, renal and melanoma) and by treatment line (first line versus second line or beyond). A Cox-regression multivariate analysis by the NLR and SII based on OS including clinical variables was performed for the entire patient cohort. When both literature-reported and ROC-derived NLR and SII cut-off values were significant, the one with the highest c-index was carried forward into the multivariate analysis. For both the univariate and multivariate analyses, an acceptable significance value of p <0.05 was established. The analysis was performed using Stata (v.16; StataCorp).
We present the following analysis in accordance with the REMARK Guideline.42
Additional resources
The patient cohort for the present analysis was derived from the INVIDIa-2 study.17
Acknowledgments
We gratefully acknowledge all our colleagues, physicians, nurses, and study coordinators from 82 Italian centers contributing to the INVIDIa-2 study; all the members of the Federation of Italian Cooperative Oncology Groups (FICOG) and the professionals collaborating with the group; the FULLCRO and Data River staff; and our patients, their caregivers, and general practitioner physicians.
We acknowledge Roche S.p.A. and Seqirus UK Unlimited for providing part of the funding for the INVIDIa-2 study.
Funding: This work was supported by Roche S.p.A. and Seqirus as Investigator Sponsored Research. No grant number is applicable. The study’s funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author contributions
All authors have met the criteria for being included in the authorship; contributors not meeting the minimum criteria have been included in the acknowledgment section.
Declaration of interests
The Federation of Italian Cooperative Oncology Groups (FICOG) received funding for the present study from Roche S.p.A. and Seqirus, and outside the present research from AstraZeneca, Bristol-Myers Squibb (BMS), and Sanofi.
G.L.B. received fees for speaker bureau from AstraZeneca and Astellas Pharma.
M.B. received funding for the present study from Roche S.p.A. and Seqirus (through FICOG as Institution, no personal fees). She also received, outside the current work, research funding from Pfizer and Novartis (through Institutions); honoraria as a speaker at scientific events (personal fees) by BMS, MSD, IPSEN, Novartis, AstraZeneca, Pierre Fabre, and Pfizer; as a consultant for advisory role (personal fees) from IPSEN, Novartis, Sanofi, Pierre-Fabre, and Merck; and personal fees for copyright transfer from Sciclone Pharmaceuticals, Pierre-Fabre, MSD, IPSEN, Pfizer, and Sanofi.
S.B. received honoraria as a speaker at scientific events and in advisory role by BMS, Pfizer; MSD, Ipsen, Roche S.p.A., Eli-Lilly, AstraZeneca, and Novartis; he also received research funding from Novartis.
A.C. received speaker fees and grant consultancies by AstraZeneca, MSD, IQVIA, OncoC4, and EISAI.
U.D.G. has served as a consultant for Astellas, Bayer, BMS, Ipsen, Janssen, Novartis, Pfizer, Sanofi, and Pharmamar; he received research funding from AstraZeneca, Roche, and Sanofi; and received travel funds from BMS, Ipsen, Janssen, Pfizer, and Roche during the conduct of the study.
P.E. received honoraria for advisory role from MSD, BMS, and Astellas.
F.G. received honoraria for advisory role from Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, BMS, MSD, Novartis, Merck, Otsuka, Novartis, Takeda, and Bayer; seminar/talks to industry from Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, AMGEN, Celgene, BMS, and MSD; and research funding from AstraZeneca, BMS, and MSD.
M.D.M. reports personal fees from AstraZeneca, Boehringer Ingelheim, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda; has been local principal investigator for trials sponsored by Beigene, Exelixis, Merck Sharp & Dohme, and Pfizer; and received research grant, institutional and financial support, and drug supply for the Meet-URO12 trial from Tesaro GlaxoSmithKline.
D.G. received honoraria from Amgen for speaker bureau.
M.M. received honoraria for advisory role from MSD and travel and accommodation expenses from Janssen, Roche, and Pfizer.
C.P. received honraria for advisory role, speaker bureau, and travel and accommodation expenses from Amgen, Astellas, AstraZeneca, Bayer, Bristol Meyer Squibb, Celgene, Clovis Oncology, Eisai, Ipsen, Janssen, Incyte, Merck-Serono, Merck Sharp & Dohme, Novartis, Roche, Sandoz, Sanofi, and Servier.
P.R. received honoraria for advisory role from AstraZeneca, Janssen, Gilead, BMS, and MSD Italy.
M.R. received honoraria for advisory role or speaker bureau from Pfizer, Novartis, MSD, AstraZeneca, Bristol-Myers Squibb (BMS), and Merck.
V.S. participated, with personal fees, in advisory boards and speaker’s bureaus for Roche S.p.A.
S.C. declared his role in an international board for Eli Lilly international.
M.S. received honoraria for advisory role from Janssen and grants for participation at scientific events from BMS, Ipsen, Janssen, Astella, Sanofi, Pfizer, and Novartis.
E.V. received honoraria for advisory role from MSD, AstraZeneca, Ipsen, and Janssen.
P.A.Z. acts in a consultant or advisory role for Sanofi, BMS, Pfizer, MSD, Astellas, Janssen, Ipsen, and Novartis, all outside the scope of work.
A.A. undertook consulting or advisory roles from BMS, AstraZeneca, Boehringer Ingelheim, Roche, MSD, Pfizer, Eli Lilly, and Astellas and participated in speaker’s bureaus from Eli Lilly and AstraZeneca.
All other authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: September 22, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107970.
Contributor Information
Alfredo Addeo, Email: alfredo.addeo@hcuge.ch.
Giuseppe L. Banna, Email: giuseppe.banna@nhs.net.
Supplemental information
Data and code availability
Data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. The study protocol and the validation certificate for the eCRF platform will be made available by Melissa Bersanelli upon request (bersamel@libero.it) at any time. Participants' data and the study dataset will be available by Melissa Bersanelli upon reasonable request. Any additional information required to reanalyse the data reported in this paper is available from the lead contact.
References
- 1.Zer A., Sung M.R., Walia P., Khoja L., Maganti M., Labbe C., Shepherd F.A., Bradbury P.A., Feld R., Liu G., et al. Correlation of Neutrophil to Lymphocyte Ratio and Absolute Neutrophil Count With Outcomes With PD-1 Axis Inhibitors in Patients With Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer. 2018;19:426–434.e1. doi: 10.1016/j.cllc.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Suh K.J., Kim S.H., Kim Y.J., Kim M., Keam B., Kim T.M., Kim D.-W., Heo D.S., Lee J.S. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol. Immunother. 2018;67:459–470. doi: 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda T., Takeuchi M., Saitoh M., Takeda S. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac. Cancer. 2018;9:1291–1299. doi: 10.1111/1759-7714.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W., Luo J., Wen J., Jiang M. The Relationship Between Systemic Immune Inflammatory Index and Prognosis of Patients With Non-Small Cell Lung Cancer: A Meta-Analysis and Systematic Review. Front. Surg. 2022;9 doi: 10.3389/fsurg.2022.898304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebuzzi S.E., Prelaj A., Friedlaender A., Cortellini A., Addeo A., Genova C., Naqash A.R., Auclin E., Mezquita L., Banna G.L. Prognostic scores including peripheral blood-derived inflammatory indices in patients with advanced non-small-cell lung cancer treated with immune checkpoint inhibitors. Crit. Rev. Oncol. Hematol. 2022;179 doi: 10.1016/j.critrevonc.2022.103806. [DOI] [PubMed] [Google Scholar]
- 6.Banna G.L., Friedlaender A., Tagliamento M., Mollica V., Cortellini A., Rebuzzi S.E., Prelaj A., Naqash A.R., Auclin E., Garetto L., et al. Biological Rationale for Peripheral Blood Cell-Derived Inflammatory Indices and Related Prognostic Scores in Patients with Advanced Non-Small-Cell Lung Cancer. Curr. Oncol. Rep. 2022;24:1851–1862. doi: 10.1007/s11912-022-01335-8. [DOI] [PubMed] [Google Scholar]
- 7.Banna G.L., Cantale O., Muthuramalingam S., Cave J., Comins C., Cortellini A., Addeo A., Signori A., McKenzie H., Escriu C., et al. Efficacy outcomes and prognostic factors from real-world patients with advanced non-small-cell lung cancer treated with first-line chemoimmunotherapy: The Spinnaker retrospective study. Int. Immunopharmacol. 2022;110 doi: 10.1016/j.intimp.2022.108985. [DOI] [PubMed] [Google Scholar]
- 8.Mezquita L., Auclin E., Charrier M., Ferrara R., Remon Masip J., Planchard D., Ponce Aix S., Paz-Ares L., Lahmar J., Leroy L., et al. The Lung Immune Prognostic Index (LIPI), a predictive score for immune checkpoint )inhibitors in advanced non-small cell lung cancer (NSCLC) patients. Ann. Oncol. 2017;28:V473. [Google Scholar]
- 9.Banna G.L., Cortellini A., Cortinovis D.L., Tiseo M., Aerts J.G.J.V., Barbieri F., Giusti R., Bria E., Grossi F., Pizzutilo P., et al. The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Meng F., Jiang R. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Patients with Metastatic Renal Cell Carcinoma Treated With Immune Checkpoint Inhibitors: A systematic Review and Meta-Analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.746976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teterycz P., Jagodzińska-Mucha P., Cybulska-Stopa B., Mariuk-Jarema A., Kozak K., Koseła-Paterczyk H., Czarnecka A.M., Rajczykowski M., Dziura R., Galus Ł., et al. High baseline neutrophil-to-lymphocyte ratio predicts worse outcome in patients with metastatic BRAF-positive melanoma treated with BRAF and MEK inhibitors. Melanoma Res. 2018;28:435–441. doi: 10.1097/CMR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 12.Jin M., Yuan S., Yuan Y., Yi L. Prognostic and Clinicopathological Significance of the Systemic Immune-Inflammation Index in Patients With Renal Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.735803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebordão Pires M., Caetano A.C., Amorim Costa C.I., Monteiro J.C., Santos R.A., Félix Soares R., Piedade Domingues I.C., Costa Jesus E.D., Sousa G.M. 114P Systemic inflammatory index as a prognostic biomarker in metastatic melanoma patients under immune checkpoint inhibitors. Immuno-Oncology and Technology. 2022;16 [Google Scholar]
- 14.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie G.J.K., Charles K.A., Roxburgh C.S.D., Horgan P.G., McMillan D.C., Clarke S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Sacdalan D.B., Lucero J.A., Sacdalan D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. OncoTargets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bersanelli M., Giannarelli D., De Giorgi U., Pignata S., Di Maio M., Clemente A., Verzoni E., Giusti R., Di Napoli M., Aprile G., et al. INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: a multicenter prospective observational study (INVIDIa-2) J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Li S., Zhang S., Liu Y., Ma L., Zhu J., Xin Y., Wang Y., Yang C., Cheng Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019;33 doi: 10.1002/jcla.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen R.-M., Zhang Y.-J., Ma S., Xu Y.-L., Chen Y.-S., Li H.-L., Bai J., Zheng J.-N. Preoperative Neutrophil to Lymphocyte Ratio as a Prognostic Factor in Patients with Non-metastatic Renal Cell Carcinoma. Asian Pac. J. Cancer Prev. 2015;16:3703–3708. doi: 10.7314/apjcp.2015.16.9.3703. [DOI] [PubMed] [Google Scholar]
- 20.Arda E., Yuksel I., Cakiroglu B., Akdeniz E., Cilesiz N. Valuation of Neutrophil/Lymphocyte Ratio in Renal Cell Carcinoma Grading and Progression. Cureus. 2018;10 doi: 10.7759/cureus.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilen M.A., Dutcher G.M.A., Liu Y., Ravindranathan D., Kissick H.T., Carthon B.C., Kucuk O., Harris W.B., Master V.A. Association Between Pretreatment Neutrophil-to-Lymphocyte Ratio and Outcome of Patients With Metastatic Renal-Cell Carcinoma Treated With Nivolumab. Clin. Genitourin. Cancer. 2018;16:e563–e575. doi: 10.1016/j.clgc.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su S., Liu L., Li C., Zhang J., Li S. Prognostic role of pretreatment derived neutrophil to lymphocyte ratio in urological cancers: A systematic review and meta-analysis. Int. J. Surg. 2019;72:146–153. doi: 10.1016/j.ijsu.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Cananzi F.C.M., Dalgleish A., Mudan S. Surgical Management of Intraabdominal Metastases From Melanoma: Role of the Neutrophil to Lymphocyte Ratio as a Potential Prognostic Factor. World J. Surg. 2014;38:1542–1550. doi: 10.1007/s00268-013-2418-6. [DOI] [PubMed] [Google Scholar]
- 24.Kanatsios S., Melanoma Project M., Li Wai Suen C.S.N., Cebon J.S., Gyorki D.E. Neutrophil to lymphocyte ratio is an independent predictor of outcome for patients undergoing definitive resection for stage IV melanoma. J. Surg. Oncol. 2018;118:915–921. doi: 10.1002/jso.25138. [DOI] [PubMed] [Google Scholar]
- 25.Cocorocchio E., Martinoli C., Gandini S., Pala L., Conforti F., Stucchi S., Mazzarol G., Ferrucci P. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with outcome of patients treated with BRAF inhibitors. Clin. Transl. Oncol. 2020;22:1818–1824. doi: 10.1007/s12094-020-02320-y. [DOI] [PubMed] [Google Scholar]
- 26.Zaragoza J., Caille A., Beneton N., Bens G., Christiann F., Maillard H., Machet L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br. J. Dermatol. 2016;174:146–151. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Chen B., Wang L., Wang R., Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer. A meta-analysis. Medicine. 2019;98 doi: 10.1097/MD.0000000000013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu F., Deng C., Wen Z., Gao Z., Zhao Y., Han H., Zheng S., Wang S., Li Y., Hu H., et al. Systemic immune-inflammation index is a stage-dependent prognostic factor in patients with operable non-small cell lung cancer. Transl. Lung Cancer Res. 2021;10:3144–3154. doi: 10.21037/tlcr-21-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozbek E., Besiroglu H., Ozer K., Horsanali M.O., Gorgel S.N. Systemic immune inflammation index is a promising non-invasive marker for the prognosis of the patients with localized renal cell carcinoma. Int. Urol. Nephrol. 2020;52:1455–1463. doi: 10.1007/s11255-020-02440-y. [DOI] [PubMed] [Google Scholar]
- 30.Stühler V., Herrmann L., Rausch S., Stenzl A., Bedke J. Role of the Systemic Immune-Inflammation Index in Patients with Metastatic Renal Cell Carcinoma Treated with First-Line Ipilimumab plus Nivolumab. Cancers. 2022;14:2972. doi: 10.3390/cancers14122972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yücel K.B., Yekedüz E., Karakaya S., Tural D., Ertürk İ., Erol C., Ercelep Ö., Öztaş N.Ş., Arslan Ç., Uçar G., et al. The relationship between systemic immune inflammation index and survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-20056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Giorgi U., Procopio G., Giannarelli D., Sabbatini R., Bearz A., Buti S., Basso U., Mitterer M., Ortega C., Bidoli P., et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin. Cancer Res. 2019;25:3839–3846. doi: 10.1158/1078-0432.CCR-18-3661. [DOI] [PubMed] [Google Scholar]
- 33.Susok L., Said S., Reinert D., Mansour R., Scheel C.H., Becker J.C., Gambichler T. The pan-immune-inflammation value and systemic immune-inflammation index in advanced melanoma patients under immunotherapy. J. Cancer Res. Clin. Oncol. 2022;148:3103–3108. doi: 10.1007/s00432-021-03878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller W.H., Jamal R., Cocolakis E., Thebault P., Friedmann J.E., Kazemi S., Dionne J., Cailhier J.-F., Lepage S., Belanger K., et al. Systemic immune signature of inflammation in metastatic melanoma (MM) patients treated with ipilimumab (IPI) and carboplatin/paclitaxel (CP) J. Clin. Oncol. 2018;36:185. [Google Scholar]
- 35.Deniz Can Guven, Oktay Halit Aktepe, Yildirim H.C., Celik I., Kılıçkap S. Higher Systemic Immune-Inflammation Index (SII) Levels Are Associated With Poorer Survival In Immunotherapy-Treated Melanoma Patients. Eurasian journal of Medical Investigation. 2021;5:409–413. [Google Scholar]
- 36.Geng Y., Shao Y., Zhu D., Zheng X., Zhou Q., Zhou W., Ni X., Wu C., Jiang J. Systemic Immune-Inflammation Index Predicts Prognosis of Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score-matched Analysis. Sci. Rep. 2016;6 doi: 10.1038/srep39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J.H., Zhai E.T., Yuan Y.J., Wu K.M., Xu J.B., Peng J.J., Chen C.Q., He Y.L., Cai S.R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017;23:6261–6272. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu H., Zheng J., Cai J., Zeng K., Yao J., Chen L., Li H., Zhang J., Zhang Y., Zhao H., Yang Y. Systemic Immune-Inflammation Index (SII) is Useful to Predict Survival Outcomes in Patients After Liver Transplantation for Hepatocellular Carcinoma within Hangzhou Criteria. Cell. Physiol. Biochem. 2018;47:293–301. doi: 10.1159/000489807. [DOI] [PubMed] [Google Scholar]
- 39.Labelle M., Begum S., Hynes R.O. Direct Signaling Between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schumacher D., Strilic B., Sivaraj K.K., Wettschureck N., Offermanns S. Platelet-Derived Nucleotides Promote Tumor-Cell Transendothelial Migration and Metastasis via P2Y2 Receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Kent P., Cancelliere C., Boyle E., Cassidy J.D., Kongsted A. A conceptual framework for prognostic research. BMC Med. Res. Methodol. 2020;20:172. doi: 10.1186/s12874-020-01050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br. J. Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bersanelli M., Buti S., Banna G.L., De Giorgi U., Cortellini A., Rebuzzi S.E., Tiseo M., Fornarini G., Mazzoni F., Panni S., et al. Impact of influenza syndrome and flu vaccine on survival of cancer patients during immunotherapy in the INVIDIa study. Immunotherapy. 2020;12:151–159. doi: 10.2217/imt-2019-0180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. The study protocol and the validation certificate for the eCRF platform will be made available by Melissa Bersanelli upon request (bersamel@libero.it) at any time. Participants' data and the study dataset will be available by Melissa Bersanelli upon reasonable request. Any additional information required to reanalyse the data reported in this paper is available from the lead contact.


