Abstract
Background
Microarray patches (MAPs) deliver vaccines to the epidermis and the upper dermis, where abundant immune cells reside. There are several potential benefits to using MAPs, including reduced sharps risk, thermostability, no need for reconstitution, tolerability and self-administration. We aimed to explore and evaluate the immunogenicity, safety, usability and acceptability of MAPs for vaccination.
Methods
We searched CINAHL, Cochrane Library, Ovid Embase, Ovid MEDLINE and Web of Science from inception to January 2023. Eligibility criteria included all research studies in any language, which examined microarrays or microneedles intended or used for vaccination and explored immunogenicity, safety, usability or acceptability in their findings. Two reviewers conducted title and abstract screening, full-text reviewing and data extraction.
Results
Twenty-two studies were included (quantitative=15, qualitative=2 and mixed methods=5). The risk of bias was mostly low, with two studies at high risk of bias. Four clinical trials were included, three using influenza antigens and one with Japanese encephalitis delivered by MAP. A meta-analysis indicated similar or higher immunogenicity in influenza MAPs compared with needle and syringe (N&S) (standardised mean difference=10.80, 95% CI: 3.51 to 18.08, p<0.00001). There were no significant differences in immune cell function between MAPs and N&S. No serious adverse events were reported in MAPs. Erythema was more common after MAP application than N&S but was brief and well tolerated. Lower pain scores were usually reported after MAP application than N&S. Most studies found MAPs easy to use and highly acceptable among healthcare professionals, laypeople and parents.
Conclusion
MAPs for vaccination were safe and well tolerated and evoked similar or enhanced immunogenicity than N&S, but further research is needed. Vaccine uptake may be increased using MAPs due to less pain, enhanced thermostability, layperson and self-administration. MAPs could benefit at-risk groups and low and middle-income countries.
PROSPERO registration number
CRD42022323026.
Keywords: immunisation, public health, vaccines, systematic review
WHAT IS ALREADY KNOWN ON THIS TOPIC
Microarray patches (MAPs) are an innovation that could improve vaccine accessibility and vaccination programmes globally.
WHAT THIS STUDY ADDS
This is the first systematic review and meta-analysis providing a comprehensive overview of the available evidence on the immunogenicity, safety, usability and acceptability of MAPs for vaccination.
MAPs have demonstrated high immunogenicity, the potential for layperson or self-administration with increased ease of use, minimal pain and increased acceptability compared with needle and syringe (N&S).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
MAPs can potentially improve vaccination programs and coverage globally through increased vaccine equity, thus reducing vaccine-preventable diseases.
MAPs may have considerable implications for low-resource settings due to their enhanced thermostability compared to N&S, ease of use and potential layperson or self-administration.
Introduction
Microarray patches (MAPs) have been identified as an innovation that can improve the accessibility and implementation of vaccination services globally.1 MAPs consist of ten to thousands of micro-projections applied to the skin via finger pressure or an applicator.2 MAPs penetrate the stratum corneum and deliver vaccine to the epidermal and upper dermal layer, which are rich in antigen-presenting cells (ie, dermal dendritic cells (DCs)).2 Dermal DCs play a key role in intracutaneous vaccination due to their ability to activate an adaptive immune response, which is the role of adjuvants in commonly administered intramuscular (IM) vaccines (ie, hepatitis B, measles and polio).3 Dermal DCs trigger an effector T cell response, specifically CD4+ and CD8+ T cells, where stimulated CD4+ T cells activate memory B cells, thus secreting antigen-specific antibodies.3 Several types of MAPs are currently in preclinical and clinical trials, including biodegradable or dissolvable, coated, solid, hollow, hydrogel-forming, porous and hybrid.4 5
Interest in microneedles as a platform to deliver drugs started in the 1970s by Alza Corporation, which developed into active research in the mid-1990s with a partnership between Becton Dickinson and the Georgia Institute of Technology.6 Various vaccine antigens have been tested in preclinical trials, yet only phase I clinical trials have been published using influenza or Japanese encephalitis antigens.2 7 MAPs typically use an applicator that, once pressed, allows sufficient velocity, which is then held in place for a length of time to ensure successful vaccine delivery.4 8 The surface area of the micro-projections varies between manufacturers but is approximately 1–2 cm2 and ranges from 25 μm to 1000 µm in length to penetrate the stratum corneum.9 10 In clinical studies, MAPs have been commonly applied to the deltoid site or the volar forearm.7 8 11–16
MAPs have been associated with reduced pain and anxiety compared with IM injection.2 There is also a reduced risk of sharps injury due to the absence of needles.2 MAPs have several logistical benefits over traditional needle and syringe (N&S) vaccines, which would help to address the WHO’s aim of achieving vaccine equity.17 These include thermostability, removing cold-chain management, reduced clinical waste, potential for self or lay administration, reduced antigen dose and no requirement for adjuvant or reconstitution.11 18 A population’s willingness to be vaccinated by MAPs could present significant cost-savings for health systems. However, procurement costs are likely to be higher than N&S.19 20
N&S for vaccination has been linked to lower compliance due to needle-phobia, pain and inconvenience.2 MAPs for vaccination have the potential to overcome these barriers, which could lead to increasing vaccination uptake, reducing the impact of vaccine-preventable diseases (VPDs) and emerging infectious diseases, especially in pandemic situations.2 21 Achieving and maintaining high-level vaccination coverage is vital, which has been demonstrated in some countries where VPD coverage has stalled or reversed, resulting in outbreaks.1 22 Established barriers to high VPD coverage include hesitancy due to N&S pain and discomfort, the challenges of effective cold-chain management and lack of trained healthcare workers (HCWs) in low-resource settings or harsh environments.2 22 This systematic review aimed to evaluate the immunogenicity, safety (including adverse events (AEs)), usability and acceptability of MAPs for vaccination. These study outcomes are vital to the potential introduction of MAPs into vaccination programmes and whether they are an improvement to current practices. Where applicable, we compared immunogenicity, safety, usability and acceptability to N&S.
Methods
Protocol and registration
This systematic review was written following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (online supplemental appendix S1).23 The protocol for this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO).24 The PROSPERO protocol and statement of updates made to the protocol are available in online supplemental appendix S2. As this was a systematic review, no ethics committee approval was required or sought.
bmjgh-2023-012247supp001.pdf (926.5KB, pdf)
Search strategy
We searched databases since June 2022 from inception and re-ran January 2023: CINAHL, Cochrane Library, Ovid Embase, Ovid MEDLINE and Web of Science for potentially relevant literature. The literature search was developed with an academic liaison librarian at the University of Sydney. The lead author (MNB) also searched other sources (ie, Google Scholar) and manually screened reference lists of relevant papers and reviews. We did not apply restrictions to language or publication date. We used keywords tailored to the databases provided in online supplemental appendix S3.
Eligibility criteria
For each record, at least two reviewers from a team of four (MNB, ESM, MWAF and CT) independently performed screening and full-text reviews. Records were included if they met each of the following criteria: (1) described research using, or evaluating a patch using microarrays or microneedles able to deliver substances to the epidermis and upper dermis, (2) were intended or used for vaccination, (3) explored at least one outcome of immunogenicity, safety, usability or acceptability in their findings, (4) were available in full text or abstract in any language, (5) empirical works (ie, clinical studies, cohort studies, cross-sectional studies or qualitative studies) or grey literature and conducted (6) in humans. Usability and acceptability will be explored broadly including but not limited to concepts regarding ease of use, convenience, cost and education and training. Records were excluded if (1) no abstract was available, (2) it was an animal or non-human study, (3) a needle or microneedle was visible to the human eye, (4) the patch did not penetrate the stratum corneum, (5) the patch was intended or used for other drug delivery or (6) the study explored concepts other than immunogenicity, safety, usability or acceptability in their findings. Discrepancies between authors during the title and abstract screening, full-text reviews and risk of bias assessments were discussed. Disagreements were resolved through team discussion and consensus with senior researchers (CD, EM and SRS).
Definitions
In this review, immunogenicity refers to human cells or tissues’ abilities to provoke immune responses following immunisation.25 Safety is described as a product’s ability to eliminate or reduce the risk of potential hazards for intended use or foreseeable misuse.26 This includes AEs following immunisation (eg, pain, erythema or fever).25 26 According to international standards (ISO-9241-11), usability refers to the extent that a product can be used to achieve its desired goal effectively, efficiently and satisfactorily by users.27 Acceptability is the extent to which an intervention is received by a population and meets a population’s and organisational setting’s needs.28 In this review, acceptability also referred to the willingness or intent to be vaccinated.29
Risk of bias assessment
To assess the quality and risk of bias for included studies, the National Health, Lung and Blood of the National Institutes of Health Study Quality Assessment Tools were used for quantitative data.30 The Critical Appraisal Skills Programme checklist was used for qualitative data.31 Both tools were used for mixed-method studies. Two reviewers from a team of three (MNB, ESM and CT) conducted these independently. Disagreements were discussed between reviewers and the senior members to reach a consensus (CD, EM and SRS).
Data synthesis and analysis
For all included studies, study characteristics, designs, intervention, control details (if present) and outcomes were extracted by one reviewer (MNB) using standard data extraction forms verified by a second reviewer (MWAF). Meta-analysis was conducted using random-effects (DerSimonian and Laird) method.32 I2 measure was used to assess statistical heterogeneity, which was defined as low (25%), moderate (50%) and high (75%).33 All statistical analyses were conducted using Review Manager 534 with two-sided p values, and p values of <0.05 were considered significant. Quantitative data were synthesised using tables, and similar data were grouped using the 9-point checklist developed by Campbell and colleagues.35 For qualitative data, thematic synthesis was conducted to summarise themes and subthemes described by Thomas and Harden.36
Patient and public involvement
Patients and the public were not directly involved in this systematic review and meta-analysis.
Results
Description of included studies
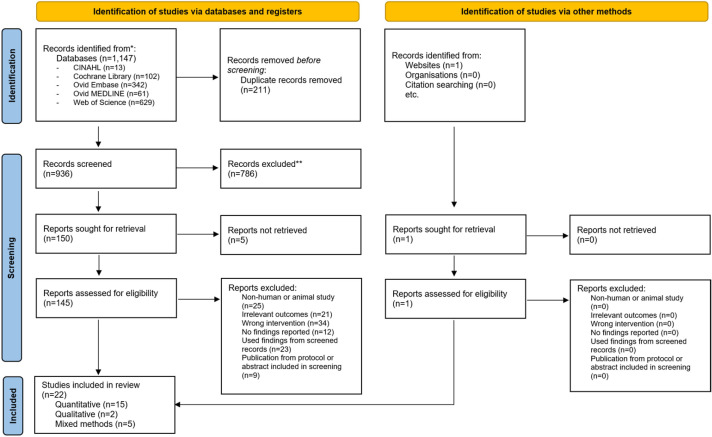
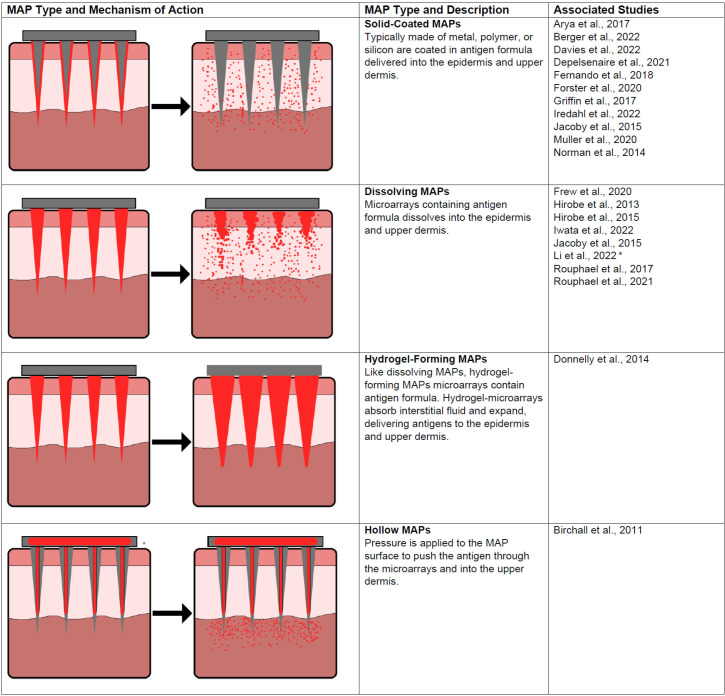
A total of 1147 studies were identified from initial database searching; subsequently, 145 records were included for full-text review (figure 1). Twenty-two studies met the inclusion criteria and were included in the final analysis. One abstract was found from other sources, and none from manual citation searches. These studies addressed a range of outcomes on immunogenicity, safety, usability and acceptability of MAPs. There were 1309 participants across all included studies. Most studies were conducted in Australia (n=8), followed by USA (n=5), Japan (n=3), UK (n=2), Benin (n=1), Ireland (n=1), Nepal (n=1) and Vietnam (n=1). Four randomised controlled trials (RCTs) were included, three of which examined MAP influenza vaccination compared with IM injection11–13 and one MAP Japanese encephalitis vaccine (JEV) compared with subcutaneous (SC) injection.7 Figure 2 summarises the types of MAPs used or described in the included studies. Summaries of study characteristics, designs and risk of bias ratings are displayed in table 1.
Figure 1.
PRISMA flowchart of the selection process.
Figure 2.
MAP types and mechanism of actions. MAP, microarray patches.
Table 1.
Characteristics and designs of the included studies ordered by author
| Author, Year | Country | Intervention and control | Study design | Methods | Population description | Sample size | Age of participants (years) | Outcomes | Risk of bias | |||
| Immuno genicity | Safety | Usability | Accept ability | |||||||||
| Arya et al, 201743 | USA | Coated microneedle patch with placebo | Phase I trial (single-arm) |
|
Healthy non-pregnant adults with normal skin | 15 | 18–57 | ☐ | ✓ | ✓ | ✓ | Low |
| Berger et al, 202250 | Australia | Excipient -coated HD-MAP | Qualitative study (abstract only) |
|
Healthy older adults | 44 | 50 to ≥75 | ☐ | ☐ | ✓ | ✓ | Low |
| Birchall et al, 201148 | UK | Microneedle patch (not administered) | Cross-sectional study (mixed methods) |
|
Public (laypeople) and healthcare workers | 58 | 20–53 | ☐ | ☐ | ✓ | ✓ | Low |
| Davies et al, 202245 | Australia | HD-MAP (simulated) administered to the upper arm and forearm | Feasibility study (mixed methods) |
|
Professional immunisers and healthcare workers | 61 | 18–65 | ☐ | ☐ | ✓ | ✓ | Low |
| Depelsenaire et al, 202141 | Australia | Influenza A/Sing coated HD-MAP compared with placebo HD-MAP, Influenza A/Sing antigen by IM and IM quadrivalent seasonal influenza vaccine | Phase I RCT |
|
Healthy adults (non-pregnant and non-nursing females). Sub-population from Forster et al., 2020 | 10 | Intervention
|
✓ | ✓ | ☐ | ☐ | Low |
| Donnelly et al, 201438 | UK | Self-administration of hydrogel- forming microneedle arrays | Phase I trial (single-arm) |
|
Final year student pharmacists at Queen’s University, Belfast | 20 | 21–23 | ☐ | ✓ | ✓ | ✓ | Low |
| Fernando et al, 201812 | Australia | Coated HD-MAP with monovalent, inactivated, split influenza A/California/07/2009 (H1N1)-like virus antigen on forearm or (2) upper arm, (3) IM injection Fluvax 2016 (same antigen), (4) placebo HD-MAP applied to forearm or (5) upper arm and (6) IM saline injection. | Phase I RCT |
|
Healthy adults (non-pregnant and non-nursing females) | 61 | 18–45 (mean=26.2, SD=7.8) | ✓ | ✓ | ☐ | ✓ | Low |
| Forster et al, 202011 | Australia |
Part A 15 µg influenza HD-MAP administered to forearm, uncoated HD-MAP to forearm, 15 µg A/Sing by IM injection, or QIV by IM injection Part B Influenza HD-MAP administered to forearm (2.5 µg, 5 µg, 10 µg, or 15 µg), uncoated HD-MAP to forearm, 15 µg influenza HD-MAP to upper arm, or QIV (A/Sing) by IM injection |
Phase I RCT |
|
Healthy adults (non-pregnant and non-nursing females) | 210 | 18–50 (mean=26.6, SD=7.1) | ✓ | ✓ | ☐ | ☐ | Low |
| Frew et al, 202044 | USA | Dissolving influenza microneedle patch administered by healthcare professional or self-administered, placebo microneedle patch, or IM influenza injection | Phase I RCT |
|
Healthy adults. Population from Rouphael, 201713 | 99 | 18–49 (mean=29.4, SD=7.5) | ☐ | ☐ | ✓ | ✓ | Low |
| Griffin et al, 201715 | Australia | Uncoated and excipient- coated micro-projection Nanopatch | Phase 1 (non-randomised trial) (mixed methods) |
|
Healthy adults (non-pregnant and non-nursing females) | 18 | 20–32 | ☐ | ✓ | ☐ | ✓ | Low |
| Guillermet et al, 201946 | Benin, Nepal, Vietnam | Nanopatch administration simulation | Cross-sectional study (mixed methods) |
|
Experts, healthcare workers, community health volunteers, community representatives and caretakers | 314 | <18 to >65 | ☐ | ☐ | ☐ | ✓ | Low |
| Hirobe et al, 201316 | Japan | Dissolving microneedle patch administered to left upper arm | Phase I trial (single-arm) |
|
Healthy men | 20 | 25–56 | ☐ | ✓ | ☐ | ☐ | Moderate |
| Hirobe et al, 201514 | Japan | Dissolving microneedle patch with trivalent influenza hemagglutinins compared with SC injection with the same formula | Phase I RCT |
|
Healthy men | 40 | 20–49 | ✓ | ✓ | ☐ | ☐ | High |
| Iredahl et al, 202239 | Australia | Uncoated HD-MAP | Phase I trial (single-arm) |
|
Older adults | 12 | 69–84 (mean=75, SD=5) | ☐ | ✓ | ☐ | ☐ | Moderate |
| Iwata et al, 20227 | Japan | Dissolvable microneedle array containing JEV (high dose and low dose), and SC injection with JEV | Phase I RCT |
|
Healthy adults | 39 | 20–34 (mean=26, SD=3.8) | ✓ | ✓ | ☐ | ☐ | Low |
| Jacoby et al, 201547 | USA | Dissolving and coated microneedle patch for influenza vaccine delivery (not administered) | Cross-sectional study (mixed methods) |
|
Key opinion leaders (experts of influenza vaccination) | 25 | Unknown | ☐ | ☐ | ✓ | ✓ | Low |
| Li et al, 202237 | China | Mushroom- inspired imprintable and lightly detachable microneedle patches compared with N&S (unknown route) | Phase 0 trial |
|
Volunteers (description not provided) | 6 | Unknown | ☐ | ✓ | ☐ | ☐ | High |
| Marshall et al., 201749 | Ireland | Microneedle patch vaccines (not administered) | Qualitative study |
|
Parents | 32 | 30–59 | ☐ | ✓ | ✓ | ✓ | Low |
| Muller et al., 202040 | Australia | Excipient- coated HD-MAP administered to the forearm and upper arm | Phase I trial (single-arm) |
|
Healthy adults | 12 | 20–52 | ☐ | ✓ | ☐ | ☐ | Low |
| Norman et al., 20148 | USA | Coated microneedle patch self-administered or investigator-administered compared with IM injection | Phase I RCT |
|
Healthy adults (non-pregnant females) | 91 | 18 to >50 | ☐ | ✓ | ✓ | ✓ | Low |
| Rouphael et al., 201713 | USA | Dissolvable microneedle patch with inactivated influenza vaccine self-administered or administered by healthcare worker, inactivated influenza vaccine by IM injection, or placebo dissolvable microneedle patch | Phase I RCT |
|
Healthy adults (non-pregnant and immunocompetent) | 100 | 18–49 (mean=29.4, SD=7.5) | ✓ | ✓ | ☐ | ✓ | Low |
| Rouphael et al., 202142 | USA | Inactivated influenza vaccine by dissolving MAP or IM injection delivered by healthcare professional | Phase I RCT |
|
Healthy adults (non-pregnant and immunocompetent). Sub study of Rouphael et al, 2017 | 22 | Intervention
Control
|
✓ | ☐ | ☐ | ☐ | Low |
ELISpot, enzyme-linked immune absorbent spot; HAI, haemagglutination inhibition; HD-MAP, high-definition microarray patch; IM, intramuscular; JEV, Japanese encephalitis vaccine; MAP, microarray patch; MN, microneutralisation; RCT, randomised controlled trial; SC, subcutaneous.
Risk of bias
Risk of bias assessments of included studies were conducted (online supplemental appendix S4). Most studies had a low risk of bias. Two clinical studies had a high risk of bias as several items were missing, including participant demographics,14 37 no description of randomisation and blinding,14 inclusion and exclusion criteria,14 37 follow-up details for human participants,37 and tools used to assess AEs and pain.37 None of the cross-sectional studies assessed confounding, likely due to the lack of variables due to restrictive inclusion criteria. One study recruited student pharmacists from the same university but excluded those involved in MAP research.38 This presented a considerable risk of bias and low generalisability due to selecting students at one institution where MAP research occurs. One mixed-methods study did not sufficiently describe their qualitative analysis and provided an inadequate presentation and discussion of their findings.15 There are currently no validated scales for measuring the usability and acceptability of MAP vaccination. These outcomes were measured differently and cannot be statistically compared between studies. For RCTs, it was not possible to blind MAP versus N&S. Most RCTs blinded the investigators and participants to active MAP and placebo MAP. One RCT was only partly blinded when comparing MAP to N&S, but did not blind active and placebo MAPs.12 Loss to follow-up in four longitudinal studies was not reported or could not be determined.15 38–40
Immunogenicity and cellular immune responses
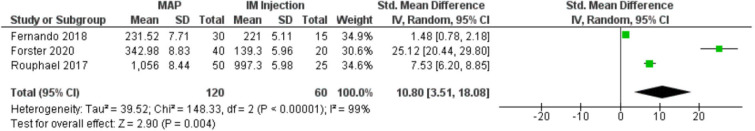
Seven clinical studies summarised in table 2 investigated immunogenicity of MAPs compared with N&S. Six involved influenza vaccine11–14 41 42 and one JEV.7 Immune responses were typically equivalent or greater when compared with N&S.7 11–14 42 No immune responses were observed in placebo groups.11–13 We performed a meta-analysis of immunogenicity outcome reported in three RCTs comparing influenza vaccine delivered by MAP to influenza vaccine delivered by N&S. These studies used the same antigen and measured outcomes at similar time points (day 21 to day 28) where haemagglutination inhibition (HAI) geometric mean titre (GMT) peaked (figure 3).11–13 The pooled standardised mean difference (10.80, 95% CI: 3.51 to 18.08) indicated that HAI GMT values in MAPs delivering H1N1 antigen were equivalent to or greater than IM injection (p<0.00001). On day 21, Iwata and colleagues reported a higher mean neutralising antibody titre for both high-dose and low-dose JEV MAPs than JEV SC injection.7 Seroprotection and seroconversion rates were very high within 1 month among influenza MAP and IM injection recipients.11–14 41 42 Iwata and colleagues did not report seroprotection, but all seroconverted by day 42.7
Table 2.
Summary of quantitative study methods and results assessing the immunogenicity and cellular immune responses of MAPs
| Author, Year | Intervention and sample size (n) | Comparison and sample size (n) | Methods | Immunogenicity findings |
| Influenza antigen delivered by MAP | ||||
| Depelsenaire et al., 202141 |
|
|
|
All participants receiving influenza MAP were seropositive by day 22, with 80% seroconverted between days 1 and 22. Placebo MAP recipients showed no immune responses. GMTs were similar to the parent study,11 peaking at day 22 (mean=139, 95% CI: 45 to 428). |
| Fernando et al., 201812 |
|
|
|
Increases in HAI assay GMT were observed on day 7 (p=0.002) and day 21 (p<0.001) in groups receiving the vaccine. GMT was 335 (95% CI: 189 to 593) for influenza MAP to forearm, 160 (95% CI: 74 to 345) for the influenza MAP to deltoid and 221 (95% CI: 129 to 380) for influenza IM injection. By day 21, 80% of influenza MAP to deltoid and IM injection were seroconverted. Sixty per cent of the influenza MAP to forearm group seroconverted by day 21. Seroprotection by day 21 was 100% for the MAP forearm, 87% for the MAP deltoid and 93% for IM injection. MN assays were also used for GMT on day 21, which were 6703 (95% CI: 3534 to 12,713) for MAP forearm, 2211 (95% CI: 1057 to 4624) and 4032 (95% CI: 1854 to 8767). GMTs appeared generally higher in MAPs than IM injection but were insignificant. |
| Forster et al., 202011 | Part A
Part B
|
Part A
Part B
|
|
No immune responses were observed in participants receiving placebos. There was no difference between low dose (2.5 µg) MAPs and 15 µg IM injections throughout the study. GMT for 2.5 µg MAP was 144.2 (95% CI: 77.9 to 267.0) compared with 15 µg influenza IM injection of 139.3 (95% CI: 79.3 to 244.5, p=0.93). HAI GMTs remained significantly higher during the study between the 10 µg MAP forearm group (309.1, 95% CI: 199.1 to 479.9, p=0.01), 15 µg MAP deltoid group (278.6, 95% CI: 152.7 to 508.1, p=0.03) and IM group (109.3, 95% CI: 59.4 to 200.9) at day 61. GMT fold increases remained significantly higher from baseline in the 15 µg deltoid MAP group (11.3, 95% CI: 6.8 to 18.8) than the IM group (8.9, 95% CI: 5.1 to 15.4, p=0.03). MN GMT was similar to HAI GMT with 10 µg MAP forearm group (18 458, 95% CI: 11 359 to 29 992, p=0.001), 15 µg MAP forearm group (11 362, 95% CI: 6492 to 19 884, p=0.01) and 15 µg MAP deltoid group (13 219, 95% CI: 7096 to 24 626, p=0.02) significantly higher than IM group (3880, 95% CI: 1924 to 7824) at day 22. The 2.5 µg MAP forearm group produced a similar GMT (5301, 95% CI: 2509 to 11 196) to IM group (3880, 95% CI: 1924 to 7824). There were no significant differences in the immune cell responses between influenza MAP and IM vaccine groups. |
| Hirobe et al., 201514 |
|
|
|
H1N1 and H3N2 strains were similar between groups, except B strain were higher in MAP than SC injection. By day 42, seroconversion was higher in the MAP groups compared with SC injection for H1N1 (71% vs 65%) and B strains (43% vs 35%). H3N2 strains had higher seroconversion in MAP than SC injection (43% vs 60%). The same pattern was observed for seroprotection (H1N1: 100% vs 95%, H3N2: 71% vs 95% and B: 86% vs 40%). There were no observed differences in IgA antibodies produced from MAP and SC groups. IFN-γ producing cells increased in both groups, although were slightly higher in the SC group. The MAP group produced HA-specific IFN-γ producing cells and activated T cells, suggesting protection from the influenza virus. |
| Rouphael et al., 201713 |
|
|
|
HAI GMTs were similar in both MAP administered by HCP and IM groups at day 28 among all strains: H1N1 (mean=1197, 95% CI: 855 to 1675 compared with mean=997, 95% CI: 703 to 1415, p=0.5), H3N2 (mean=287, 95% CI: 192 to 430 compared with mean=223, 95% CI: 160 to 312, p=0.4) and B strain (mean=126, 95% CI: 86 to 184 compared with mean=94, 95% CI: 73 to 122, p=0.06). By day 28, all groups showed significantly higher seroprotection and seroconversion rates from baseline (p<0.01) but were similar. There was strong evidence that HCP and self-administered MAP groups (65%, 95% CI: 60% to 75%) had a higher seroconversion rate for B strain than IM injection (32%, 95% CI: 15% to 54%, p=0.01). Seroprotection (titre=≥1.4) following vaccination in HCP administered MAP (83%–100%), self-administered MAP (75%–100%) and IM injection (80%–100%) by day 180. |
| Rouphael et al., 202142 |
|
|
|
HAI GMTs were similar between 1–6 months after vaccination among both groups. GMTs were similar between the three influenza strains (H1N1, H3N2 and B). GMT fold increases were only significantly higher for the B strain (p=0.009). There were no differences between the group rates for seroprotection by day 28 (p=1.00). HAI responses were similar to those observed in the parent study.13 Neuraminidase assay GMT showed that strain N1 was higher in MAP at baseline than IM (p=0.04). At day 28, GMT was higher in MAP for strains N1 (p=0.002), N2 (p=0.003) and B (p<0.001) compared with IM. This was also observed on day 2 (N1: p=0.05, B: p=0.02) except for the N2 strain (p=0.11). MAP and IM injection had increased binding affinities at day 28 compared with baseline (MAP: p=0.002, IM: p=0.01). IP-10 increase in the MAP compared with IM (p=0.01) was observed on days 2–3. However, TNF-α was higher in the IM group than in MAP (p=0.04). On day 8, cytokines IL-8 (p=0.02), IL-5 (p=0.034), IL-13 (p=0.013) and MIP-1b (p=0.016) were higher in the MAP group. Following adjustment by Bonferroni correction, these findings were of no significance. Furthermore, there was no difference in the immune cell responses of CD4+ T cell cytokines. At day 28, circulating T follicular helper cells (CD4+, CXCR5+, CXCR3+, ICOS+ and PD-1+) were a higher percentage in the MAP group compared with the IM group (p=0.04). |
| Japanese encephalitis antigen delivered by MAP | ||||
| Iwata et al., 20227 |
|
|
|
By day 42, participants in all groups (n=39) reached a seroconversion over 1.3 (log10). Seroconversion of ≥1.3 (log10) was observed by day 14 in all high dose MAP participants (n=13), lasting until day 42, with 85% (n=11) on day 189. Over half (n=7) of the SC group seroconverted to ≥1.3 (log10) by day 14. Three-quarters of the low dose MAP group (69%, n=9) and half of the SC group (53.8%, n=7) seroconverted at day 21 and all of these groups seroconverted by day 42. By day 189, neutralising antibodies were observed in 31% (n=4) of the low dose MAP group and 46% (n=6) of SC group. Over days 14, 21 and 189, seroconversion was higher in MAPs than SC injection. Mean titres (log10) were higher in high dose MAPs than in SC injection across the three-time points. At day 42, mean titres in the high dose MAP were 2.55 (SD=0.36), low dose MAP was 2.04 (SD=0.53) and SC injection was 2.08 (SD=0.47). These decreased on day 189 (high dose MAP=1.60, SD=0.39, low dose MAP=0.95, SD=0.32, SC=1.14, SD=0.44). There were significant differences (p=0.0036) between high dose MAP, low dose MAP and SC injection using a contrast coefficient of (−1 to –1, −2). There was no evidence of a correlation between immunogen delivered and titre in the high dose MAP group. There was some evidence of a positive correlation in the low dose MAP group at day 21 (p=0.050) and day 189 (p=0.023). |
A/Cali, A/California/07/2009 H1N1; A/Sing, A/Singapore/GP1908/2015 H1N1; ELISpot, enzyme-linked immune absorbent spot; GMT, geometric mean titre; HAI, haemagglutination inhibition; HD-MAP, high-definition microarray patch; IM, intramuscular; JEV, Japanese encephalitis vaccine; MAP, microarray patch; MN, microneutralisation; SC, subcutaneous; SII, Skin irritation index.
Figure 3.
MAP H1N1 versus IM H1N1 HAI geometric mean titres between days 21 and 28. HAI, haemagglutination inhibition; IM, intramuscular; MAP, microarray patches.
Two RCTs of influenza vaccine delivered by MAP compared with influenza vaccine delivered by IM evaluated cellular immune responses.11 42 There were a few differences in the cellular responses measured between MAP and IM groups.11 42 On day 8, Rouphael and colleagues observed a higher rate of circulating T follicular helper cells (CD4+, CXCR5+, CXCR3+, ICOS+ and PD-1+) in MAP recipients compared with IM.42 There were significant increases in CD4+ T cell cytokine and chemokine frequencies (interferon‐gamma-γ, tumor necrosis factor-α, interleukin (IL)-2, IL-5, IL-8, IL-13, IL-21 and macrophage inflammatory protein-1b) over the studies from baseline in influenza MAP and IM recipients, but no statistical difference between groups.11 42 Rouphael and colleagues evaluated differences in influenza virus-specific memory B cells, cytokine and chemokine responses with no difference between MAP and IM groups.42
Safety
Adverse events
Findings on the safety of MAPs are summarised in online supplemental appendix S5. Two-thirds of papers (n=14, 66.6%) reported on the safety of MAPs, with no serious treatment-related AEs.7 8 11–16 37–41 43 Serious AEs assessed as unrelated to treatment were reported among four participants in two RCTs.12 13 Li and colleagues reported that AEs (pruritus, erythema, heat, swelling and bleeding) were low compared with N&S but did not describe the scale used or provide numeric data.37 Mild erythema was most frequently reported in all studies assessing AEs in MAPs using vaccine or excipient.7 14–16 40 41 43 Headaches were reported by 13% of participants receiving MAP with vaccine and placebo,12 and two moderate AEs were headaches, with one in the MAP placebo group and the other in the influenza IM group.11 Three studies reported pruritus, which was often described as mild and self-limited, lasting a maximum of 3 days following administration of MAPs.11 13 15 Pigmentation was noted in three clinical studies,7 14 16 which lasted a maximum of 189 days in JEV MAPs.7 Two studies reported wet bleeding, typically resolving quickly within 30 min.39 40 Oedema was reported in two studies and resolved by day 8.40 41 Less frequent AEs included exfoliation,15 39 swelling,43 myalgia,11 lymphadenopathy,11 fever,14 petechiae39 40 and black dots.39
Pain
A total of 12 studies reported pain scores following the use of MAPs,7 8 11–16 37–39 43 and seven compared pain scores with N&S7 8 11–14 37 using similar visual analogue scales. Li and colleagues and one study by Hirobe and colleagues did not report the scale used to measure pain.14 37 In an RCT, pain was assessed within 10 min of administration, but there was little difference between the groups receiving influenza vaccine.12 Forster and colleagues reported very low pain scores among MAP recipients compared with IM injection.11 Another RCT reported that pain was lower overall during JEV MAP administration to the deltoid sites versus SC injection but slightly higher at initial administration.7 Norman and colleagues reported pain scores that were lower in MAPs administered by healthcare professionals (HCPs) and self-administered MAPs, compared with IM injection.8 Two studies did not report pain scores numerically but reported low levels of pain.16 37 Several studies reported a high proportion of low pain scores (mostly 0) after the administration of a MAP.13 15 43
Usability and acceptability
Ease of use and confidence
Usability and acceptability of MAP vaccination are summarised in online supplemental appendix S6. Quantitative and qualitative data explored usability and acceptability. Across several studies, HCPs, HCWs and laypeople found MAPs very easy to use.15 44 45 One clinical study in the USA compared MAPs with and without a snap-based device, each administered three times.8 Snap-based MAP was more effective in successful administrations than non snap-based MAP on the second attempt, while non snap-based MAP was more successful on the second and third attempts.8 Arya and colleagues reported a high administration success in HCP-administered and self-administered MAPs.43 However, mean dose delivery was relatively low in both groups, with HCP administration being 74% (SD=11%) compared with 67% (SD=23%) in the self-administered group.43
An Australian simulation study using MAP applicators without patches assessed wear time (the application time required for MAP vaccination), with HCPs meeting the required 10 s about a third of the time compared with HCWs achieving more than half of the time.45 Wear time was observed in clinical studies ranging from 10 s to 2 min, with qualitative data suggesting up to 10 min would be acceptable.15 46 47 Confidence in correct administration was relatively low, with only half of the participants in two clinical studies confident that the MAP would have worked in general.38 43 However, in another study, all participant groups were confident that they correctly self-administered a MAP.45 Two studies suggested a visual indicator to confirm a correct MAP dose delivery as an improvement to MAP design, which was reported by all laypeople and most HCPs.48 49 One study found most participants believed it would be difficult to administer a dose through a hollow MAP.48 Thermostability was considered an advantage compared with N&S due to no cold-chain requirements, thus avoiding vaccine wastage, loss of potency, improving transportation and associated costs.46 47 50 Single-dose MAPs (eg, measles-mumps-rubella requiring three doses over 6 months) would have the potential to facilitate catch-up vaccinations and reduce strain on healthcare systems and transportation.46
Education and training
Concerns for MAP administration by community health volunteers (CHVs) in Benin, Nepal and Vietnam were associated with a lack of confidence in the skills, knowledge and familiarity with MAPs among parents or guardians (37%).46 Parents and community representatives typically preferred highly trained immunisers to administer MAP vaccines to children.46 Student pharmacists (n=20, 100%) in the UK reported that instructions for use (IFU), pharmacist consultation and a demonstration of MAP administration should be provided.38 Australian immunisers were somewhat apprehensive about teaching laypeople to self-administer MAPs (66%, SD=33%) but more agreeable to teaching HCPs to administer (84%, SD=26%).45 Although MAPs were considered ‘straightforward’ and “foolproof”,45 layperson and HCP training were considered necessary.47 Information for laypeople and HCPs could be included in the IFU provided by the manufacturer, which provided information on how to administer and dispose of MAPs and manage AEs.47 Educating HCPs through mandatory training modules was considered appropriate but not as rigorously as N&S training.45 Davies and colleagues found that participants would have felt more confident administering a MAP if they had observed administration.45
Vaccine delivery system preference
Studies that explored preference for vaccination between MAP and N&S found that MAP was the preferred option.8 12 43 44 In a placebo MAP study, almost all participants preferred MAP over IM injection.43 An RCT comparing influenza vaccine delivered by MAP to influenza vaccine delivered by N&S reported that over half of participants preferred MAP, and a quarter had no preference compared with their experience of IM influenza vaccine.12 Another RCT found that preference for MAP increased over time, and positive attitude towards MAP was consistently high.44 Norman and colleagues found that a considerable number of usually unvaccinated participants would be willing to receive an influenza vaccine via MAP.8 In a study of pharmacy students self-administering MAPs, all reported it being a positive experience after reading the IFU and consulting a pharmacist.7 Frew and colleagues observed that a very high rate of participants reported that MAP would be more convenient than N&S for influenza vaccine.44 A conference abstract suggested that older adults may find MAPs convenient by potentially reducing need for clinic appointments.50
MAP administration and settings
Participants in two studies indicated that self-administration of MAPs was a considerable advantage.38 48 Vaccination experts recruited in a cross-sectional study preferred HCP administration of MAPs but were less supportive of supervised group administration, self-administration at home with prescription and over-the-counter MAP self-administered at home.47 A study across Benin, Nepal and Vietnam found that participants perceived MAP use in health facilities as highly acceptable but found home administration less acceptable.46 Community representatives positively perceived MAP administration by CHVs, but CHVs and HCPs were more cautious.46 Australian HCWs (people working in healthcare settings including HCPs) in a study applying a prototype MAP applicator were highly accepting of self-administration in a general practice clinic or healthcare facility.45 Immunisers were highly cautious of self-administration, with only few agreeing to home settings.45
Davies and colleagues found that potential for self-administration was considered convenient for laypeople, particularly those living in rural and remote settings.45 MAP was considered convenient for HCPs as vaccine reconstitution is not needed, and due to its thermostability, could be available in a vast array of settings.45 47 Experts mentioned that supervised group MAP self-administration might increase clinic efficiency.47 However, safety was a concern if an HCP did not supervise self-administration due to the risk of AEs following immunisation (ie, anaphylaxis).45 47 50 It was important that an HCP at least supervised MAP self-administration in a medical emergency.45 47 49 There was considerable potential for parental administration, including reduced fear and increased convenience, particularly in a familiar environment such as the home.49 There was also a concern that those who could self-administer or administer to their child could adversely affect vaccine surveillance through a lack of recording or non-compliance.47 49
Perceived safety and efficacy
Several safety aspects were raised in addition to self-administration, including no risk of needle-stick injuries due to MAP being prefilled and needle-free and reduced risk of cross-infection.46–48 Almost all participants in two studies reported that MAPs would reduce risk of sharps injury compared with N&S.38 48 However, most participants (84%) in one study believed MAP cross-contamination by an HCP could occur or be a risk of infection.48 In a cross-sectional study, most participants receiving uncoated or excipient-coated MAPs preferred administration to the deltoid site as it was less sensitive than the volar forearm and familiar.15 The visibility of the red mark was also a factor for preferring deltoid sites, as it was more prominent on the volar forearm.15 Participants who preferred N&S in this study did so to avoid the red mark or because N&S was familiar.15
Student pharmacists in a clinical study believed MAPs would reduce bleeding (n=12) and tissue damage (n=13), but one student believed it could be misused or abused.38 Concerns were raised that people could be hypersensitive to MAP ingredients and cause intradermally associated severe cutaneous reactions similar to Bacillus Calmette-Guérin (BCG) vaccines.46 Birchall and colleagues found some participants would be concerned that MAPs could be used to deliver harmful substances without the recipient’s awareness.48 Laypeople and HCPs questioned efficacy in two studies requiring assurance that it would be as efficacious and fast-acting as N&S.46 48 Marshall and colleagues found that evidence of safety and efficacy was crucial among parents.49 However, parents may trust MAPs if HCPs recommended the device.49
Perceived pain and priority populations
Reduced pain was considered advantageous compared with N&S making MAPs an attractive alternative.15 48 49 However, one study found that almost all participants would prefer a painful injection if it were more effective than MAP.48 Children were considered a population that would greatly benefit from using MAPs due to reduced pain.46 48 49 MAP aesthetics were essential; no visible needles made the device appear less painful.45 46 Marshall and colleagues found that the appearance of MAPs was important to parents and suggested making them ‘child-friendly’, such as images of superheroes.49 Parents expressed concern about current vaccination programmes, particularly school programmes due to unappealing N&S and associated acute stress responses.49 Negative experiences may increase vaccine hesitancy,47 49 but MAPs could present a more acceptable form of vaccination only if rolled out in adults first for familiarity.49 All participants believed that needle-phobic individuals would benefit from the availability of MAPs.38
Cost
Only two studies explored cost in high-income countries, with somewhat ambiguous concepts.47 48 Potential higher cost of MAPs was a concern among laypeople and HCPs compared with traditional methods, particularly the cost to the health service.48 Notably, experts interviewed by Jacoby and colleagues believed MAPs could reduce costs associated with cold-chain management if thermostable.47 Experts thought a slightly increased cost would be acceptable but had concerns about reimbursement from governments and insurers to HCPs and users.47 Patients with low income were of particular concern (ie, pensioners, chronically ill and children).47
Discussion
Summary of results
This systematic review and meta-analysis explored the immunogenicity, safety, usability and acceptability of MAPs for vaccination. Although there was a limited number of studies and most studies had small samples, the risk of bias of included studies was generally low. We found that only two antigens have been used in clinical MAP vaccine studies: influenza and JEV.7 11–13 Few studies examined the immunogenicity of MAPs vaccines but those that did found that MAP vaccines had equivalent to or greater antibody response than N&S.7 11–14 42 Our meta-analysis comparing influenza H1N1 immunogenicity data induced by vaccine delivered by MAP to N&S confirmed this finding. One study found a higher frequency of circulating T follicular helper cells in MAP than IM recipients.42 In these few studies with influenza vaccine and JEV, immunogenicity and immune cell function in MAP compared with N&S were similar or greater and suggested an equivalent level of protection against infection. MAPs were well tolerated and safe.7 11–13 43 The increased frequency of erythema in MAPs is likely due to inflammatory reactions occurring closer to the skin than N&S, which would occur deeper in SC tissue or muscle.14 41 Due to the various methods of reporting, meta-analysis was only feasible for immunogenicity data.
Typically, studies that assessed pain from MAP applications or perceived pain reported this as low using similar visual analogue scales.7 8 11 13–16 37–39 43 MAP devices were considered easy to use with high confidence levels in correct application,15 44 45 although slightly less confidence in dose delivery, especially with hollow MAPs.48 Study participants perceived that MAP vaccination was more acceptable than N&S, and would have the potential to increase vaccination rates even in those typically not vaccinated.8 Several perceived challenges and suggested improvements of MAPs for vaccination were described. This included the uncertainty of a delivered dose; thus the inclusion of an indicator to increase user confidence48 and ensuring children remain still during the required wear time.49
While these studies generally suggested high acceptability of MAP for vaccination, new technology may be met with anxiety and reluctance.51 We found relatively low confidence in correct vaccine delivery in two cross-sectional studies, suggesting a lack of confidence in the technology.38 43 As logistics, thermostability and access to trained HCPs are barriers to current vaccination programmes in low and middle-income countries (LMICs), it would be useful to conduct more studies in these settings to determine MAPs’ role in overcoming these barriers.2 46 MAPs benefit populations by reducing pain and the potential for self-administration, which may increase vaccination uptake.2 8 Further studies are required to build the evidence base and confirm the promising findings seen in these early studies, particularly in regards to immunogenicity and safety for use in other types of vaccines. Research on other aspects of clinical effectiveness will also be needed, particularly once more research is available, and MAPs are approved for use in vaccination. Several MAP vaccine clinical trials are ongoing, including measles and rubella,52 53 COVID-19,54 55 and a study in children.56 No further studies have been published on MAP vaccine efficacy since this review’s literature search. Many MAP vaccines such as against anthrax, BCG, hepatitis B, human papillomavirus, measles, rubella, diphtheria, poliovirus, tetanus and rabies are still in preclinical trials.2
Research with children and adolescents may be beneficial as this group has a higher degree of needle-related anxiety.57 58 MAPs could benefit at-risk populations such as older adults and immunocompromised individuals through prioritised vaccination and potential for mass administration or people living in LMICs through improved vaccine equity.25 46 50 MAPs may benefit marginalised populations that experience inequitable access to vaccination in LMICs and rural and remote settings, such as those experiencing poverty, Indigenous and disabled people. All clinical studies tested MAPs with influenza vaccine or JEV, but not vaccine antigens used routinely in LMICs (ie, measles, rubella and polio). More research into LMICs is needed considering this is a priority area for achieving equitable access to vaccines.17 46 Cost is an important factor for population-based immunisation programmes and requires further examination. Manufacturing costs are expected to be more expensive than for N&S,19 47 while some studies suggest MAPs may be more cost-effective for specific childhood immunisation programmes or if uptake was high enough.20 59 60 This is mainly due to MAP’s potential to reduce logistics and distribution cost due to improved thermostability, ease of use, improved safety, reduced wastage and higher acceptability.2 18 20 61 Offering an influenza MAP to children who refused N&S resulted in an increased cost but reduced infection rates and hospitalisations.19 As MAPs are not yet licenced for conventional use, it is unclear at this stage how individuals can access them and if government or insurance companies will reimburse individual costs.47 59 Included studies have only focused on participants’ acceptability of MAP cost in high-income countries.47 48 Further research into cost and economic models is needed, particularly for LMICs.
Strengths and limitations
This is the first systematic review and meta-analysis on MAP use in vaccination with most included studies having a low risk of bias. This review used a large number of databases with no restriction to record type and language. This review was limited by the relatively small number of studies published to date and the variations in measures used for outcomes, limiting our ability to perform multiple meta-analyses. A limitation was high heterogeneity, specifically clinical diversity, in the meta-analysis of immunogenicity to H1N1. This likely arose due to studies examining MAPs being varied from the following attributes, vaccine formulations, adjuvants and delivery systems (ie, type of MAP). A potential limitation of this review is that it evaluated multiple outcomes of MAPs.
Implications
MAPs could improve vaccination programmes and coverage through increased vaccine equity, thus reducing mortality and morbidity caused by VPDs.1 MAPs can be integrated into current or new vaccination programmes resulting in the control, elimination, or eradication of infectious diseases.1 2 MAP vaccines could also overcome vaccination uptake barriers for emerging infectious diseases or pandemics (eg, COVID-19 or influenza) through improved logistics, reduced need for HCPs and higher usability and acceptability than N&S.2 21 This technology may have considerable implications for LMICs or remote locations due to enhanced thermostability compared with N&S, its ease of use and potential for layperson and self-administration.2 Although MAPs will likely be more costly as a new technology, the increased willingness to be vaccinated could reduce the burden of VPDs on healthcare systems.8 19 20 To increase vaccination rates, MAPs could be offered to specified populations, such as those experiencing high needle-related anxiety.19 20 Other challenges to adopting MAPs into vaccination programmes include a lack of vaccine manufacturer incentives and potential barriers associated with the uptake of new technology.2 51
Conclusion
This review identified that the published literature reports that MAPs for vaccination are well tolerated and safe. We also found that for H1N1 or JEV MAP vaccines induced similar or stronger antibody responses compared with N&S.11–13 42 Erythema was notably common in MAP recipients than N&S, likely due to immune reactions closer to the skin’s surface.7 14–16 40 41 43 MAP devices were described as easy to use and were considered acceptable compared with N&S, even by those who are typically unvaccinated.8 15 44 45 However, as these are early studies, further research is required before firm conclusions can be made. MAPs have the potential to improve vaccination coverage through promoting more equitable access to vaccines, thus reducing millions of deaths caused by VPDs.2 22 This would benefit at-risk groups such as children, older adults and immunocompromised patients.25
Acknowledgments
We thank Tess Aitken for assisting in developing our literature search strategy necessary for this review. The authors acknowledge Jim Mathews of the Sydney Informatics Hub, a core research facility of the University of Sydney for technical support in developing our meta-analysis and Elke Hacker for technical editing. Thank you to Morris Li who assisted us in translating Chinese records during the screening and full-text review stages.
Footnotes
Handling editor: Soumyadeep Bhaumik
Twitter: @Berger0413
Contributors: MNB conceptualised the review with ESM, CD and SRS, and was responsible for data curation, formal analysis, investigation, methodology, project administration, validation, visualisation, writing—original draft and writing—review and editing. ESM, CD and SRS conceptualised the review with MNB, and were responsible for the methodology, resources, supervision and writing—review and editing. ESM, MWAF and CT were responsible for the formal analysis, investigation and writing—review and editing. RB and AHF assisted in the writing—review and editing. MNB will act as the guarantor for this review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MNB declares that he has received a NSW Health PhD Partnership Scholarship to investigate the usability and acceptability of HD-MAPs in development by Vaxxas Pty Ltd. CD, RB, AHF and SRS are investigators on the usability and acceptability of HD-MAPs and have received funding from the Innovative Manufacturing Cooperative Research Centre. AHF is a paid employee of Vaxxas Pty Ltd. Authors were not involved in assessing or reviewing their own publications. Publications authored by the investigators under consideration for this review were allocated to independent reviewers.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data have been made available within the paper and appendices.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization, United Nations Children’s Fund (UNICEF) . Measles-rubella microarray patch (MR–MAP) target product profile. Geneva: World Health Organization, 2020. Available: https://apps.who.int/iris/handle/10665/330394 [Google Scholar]
- 2.Peyraud N, Zehrung D, Jarrahian C, et al. Potential use of Microarray patches for vaccine delivery in Low- and middle- income countries. Vaccine 2019;37:4427–34. 10.1016/j.vaccine.2019.03.035 [DOI] [PubMed] [Google Scholar]
- 3.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human Dendritic cell Subsets. Immunity 2010;33:464–78. 10.1016/j.immuni.2010.10.007 Available: 10.1016/j.immuni.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korkmaz E, Balmert SC, Sumpter TL, et al. Microarray patches enable the development of skin-targeted vaccines against COVID-19. Adv Drug Deliv Rev 2021;171:164–86. 10.1016/j.addr.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Xu D, Xuan X, et al. Advances of Microneedles in BIOMEDICAL applications. Molecules 2021;26:5912. 10.3390/molecules26195912 Available: 10.3390/molecules26195912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012;64:1547–68. 10.1016/j.addr.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata H, Kakita K, Imafuku K, et al. Safety and dose-sparing effect of Japanese encephalitis vaccine administered by Microneedle patch in uninfected, healthy adults (MNA-J): a randomised, partly blinded, active-controlled, phase 1 trial. Lancet Microbe 2022;3:e96–104. 10.1016/S2666-5247(21)00269-X [DOI] [PubMed] [Google Scholar]
- 8.Norman JJ, Arya JM, McClain MA, et al. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine 2014;32:1856–62. 10.1016/j.vaccine.2014.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong S-Y, Park J-H, Lee Y-S, et al. The current status of clinical research involving Microneedles: A systematic review. Pharmaceutics 2020;12:1113. 10.3390/pharmaceutics12111113 Available: 10.3390/pharmaceutics12111113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TT, Oh Y, Kim Y, et al. Progress in Microneedle array patch (MAP) for vaccine delivery. Human Vaccines & Immunotherapeutics 2021;17:316–27. 10.1080/21645515.2020.1767997 Available: 10.1080/21645515.2020.1767997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster AH, Witham K, Depelsenaire ACI, et al. Safety, tolerability, and Immunogenicity of influenza vaccination with a high-density Microarray patch: results from a randomized, controlled phase I clinical trial. PLoS Med 2020;17:e1003024. 10.1371/journal.pmed.1003024 Available: 10.1371/journal.pmed.1003024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernando GJP, Hickling J, Jayashi Flores CM, et al. Safety, tolerability, acceptability and Immunogenicity of an influenza vaccine delivered to human skin by a novel high-density Microprojection array patch (NanopatchTM). Vaccine 2018;36:3779–88. 10.1016/j.vaccine.2018.05.053 Available: 10.1016/j.vaccine.2018.05.053 [DOI] [PubMed] [Google Scholar]
- 13.Rouphael NG, Paine M, Mosley R, et al. The safety, Immunogenicity, and acceptability of Inactivated influenza vaccine delivered by Microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017;390:649–58. 10.1016/S0140-6736(17)30575-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirobe S, Azukizawa H, Hanafusa T, et al. Clinical study and stability assessment of a novel Transcutaneous influenza vaccination using a dissolving Microneedle patch. Biomaterials 2015;57:50–8. 10.1016/j.biomaterials.2015.04.007 Available: 10.1016/j.biomaterials.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 15.Griffin P, Elliott S, Krauer K, et al. Safety, acceptability and tolerability of uncoated and Excipient-coated high density Silicon micro-projection array patches in human subjects. Vaccine 2017;35:6676–84. 10.1016/j.vaccine.2017.10.021 Available: 10.1016/j.vaccine.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 16.Hirobe S, Azukizawa H, Matsuo K, et al. Development and clinical study of a self-dissolving Microneedle patch for Transcutaneous immunization device. Pharm Res 2013;30:2664–74. 10.1007/s11095-013-1092-6 Available: 10.1007/s11095-013-1092-6 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Vaccine equity, Available: https://www.who.int/campaigns/vaccine-equity
- 18.Bozorgi A, Fahimnia B. Micro array patch (MAP) for the delivery of Thermostable vaccines in Australia: a cost/benefit analysis. Vaccine 2021;39:6166–73. 10.1016/j.vaccine.2021.08.016 Available: 10.1016/j.vaccine.2021.08.016 [DOI] [PubMed] [Google Scholar]
- 19.Wong C, Jiang M, You JHS, et al. Potential cost-effectiveness of an influenza vaccination program offering Microneedle patch for vaccine delivery in children. PLoS ONE 2016;11:e0169030. 10.1371/journal.pone.0169030 Available: 10.1371/journal.pone.0169030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seaman CP, Mvundura M, Frivold C, et al. Evaluating the potential cost-effectiveness of Microarray patches to expand access to hepatitis B birth dose vaccination in low-and middle-income countries: a Modelling study. PLOS Glob Public Health 2022;2:e0000394. 10.1371/journal.pgph.0000394 Available: 10.1371/journal.pgph.0000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Shea J, Prausnitz MR, Rouphael N. Dissolvable Microneedle patches to enable increased access to vaccines against SARS-Cov-2 and future pandemic outbreaks. Vaccines (Basel) 2021;9:320. 10.3390/vaccines9040320 Available: 10.3390/vaccines9040320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UNICEF . Vaccine microarray patches (VMAPs), Available: https://www.unicef.org/innovation/vaccine-microarray-patches-vmaps
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 Available: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger MN, Mowbray ES, Farag MWA, et al. Immunogenicity, safety, usability, and acceptability of microarray patches for vaccination: a systematic review 2022, Available: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=323026 [DOI] [PMC free article] [PubMed]
- 25.Australian Technical Advisory Group on Immunisation (ATAGI) . Australian immunisation handbook. Canberra: Australian Government Department of Health and Aged Care, 2022. Available: https://immunisationhandbook.health.gov.au/ [Google Scholar]
- 26.Therapeutic Goods Administration . Essential principles checklist (medical devices). 2021. Available: https://www.tga.gov.au/resources/resource/forms/essential-principles-checklist-medical-devices
- 27.International Organization for Standards . ISO 9241-11:2018(en) Ergonomics of human-system interaction — Part 11: usability: definitions and concepts 2018, Available: https://www.iso.org/obp/ui/#iso:std:iso:9241:-11:ed-2:v1:en
- 28.Ayala GX, Elder JP. Qualitative methods to ensure acceptability of behavioral and social interventions to the target population. J Public Health Dent 2011;71 Suppl 1(0 1):S69–79. 10.1111/j.1752-7325.2011.00241.x Available: 10.1111/j.1752-7325.2011.00241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson R. HPV vaccine acceptance in West Africa: a systematic literature review. Vaccine 2021;39:5277–84.:S0264-410X(21)00835-5. 10.1016/j.vaccine.2021.06.074 Available: 10.1016/j.vaccine.2021.06.074 [DOI] [PubMed] [Google Scholar]
- 30.National Heart, Lung, and Blood Institute . Study quality assessment tools. 2021. Available: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 31.Critical Appraisal Skills Programme . CASP qualitative studies checklist 2018, Available: https://casp-uk.net/casp-tools-checklists/
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 Available: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 Available: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochrane . Review manager (Revman). 2020. Available: https://training.cochrane.org/online-learning/core-software/revman
- 35.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (swim) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 Available: 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8:45. 10.1186/1471-2288-8-45 Available: 10.1186/1471-2288-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Xu R, Fan H, et al. Smart mushroom-inspired Imprintable and lightly detachable (MILD) Microneedle patterns for effective COVID-19 vaccination and decentralized information storage. ACS Nano 2022;16:7512–24. 10.1021/acsnano.1c10718 Available: 10.1021/acsnano.1c10718 [DOI] [PubMed] [Google Scholar]
- 38.Donnelly RF, Moffatt K, Alkilani AZ, et al. Hydrogel-forming Microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet. Pharm Res 2014;31:1989–99. 10.1007/s11095-014-1301-y Available: 10.1007/s11095-014-1301-y [DOI] [PubMed] [Google Scholar]
- 39.Iredahl F, Muller DA, Togö T, et al. n.d. Local response and barrier recovery in elderly skin following the application of high-density Microarray patches. Vaccines;10:583. 10.3390/vaccines10040583 Available: 10.3390/vaccines10040583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller DA, Henricson J, Baker SB, et al. Innate local response and tissue recovery following application of high density Microarray patches to human skin. Sci Rep 2020;10:18468. 10.1038/s41598-020-75169-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Depelsenaire ACI, Witham K, Veitch M, et al. Cellular responses at the application site of a high-density Microarray patch delivering an influenza vaccine in a randomized, controlled phase I clinical trial. PLoS One 2021;16:e0255282. 10.1371/journal.pone.0255282 Available: 10.1371/journal.pone.0255282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouphael NG, Lai L, Tandon S, et al. Immunologic mechanisms of seasonal influenza vaccination administered by Microneedle patch from a randomized phase I trial. NPJ Vaccines 2021;6:89. 10.1038/s41541-021-00353-0 Available: 10.1038/s41541-021-00353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arya J, Henry S, Kalluri H, et al. Tolerability, usability and acceptability of dissolving Microneedle patch administration in human subjects. Biomaterials 2017;128:1–7. 10.1016/j.biomaterials.2017.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frew PM, Paine MB, Rouphael N, et al. Acceptability of an Inactivated influenza vaccine delivered by Microneedle patch: results from a phase I clinical trial of safety, Reactogenicity, and Immunogenicity. Vaccine 2020;38:7175–81. 10.1016/j.vaccine.2020.07.064 [DOI] [PubMed] [Google Scholar]
- 45.Davies C, Taba M, Deng L, et al. Usability, acceptability, and feasibility of a high-density Microarray patch (HD-MAP) applicator as a delivery method for vaccination in clinical settings. Hum Vaccin Immunother 2022;18:2018863. 10.1080/21645515.2021.2018863 Available: 10.1080/21645515.2021.2018863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillermet E, Alfa DA, Phuong Mai LT, et al. End-user acceptability study of the Nanopatch; a Microarray patch (MAP) for child immunization in low and middle-income countries. Vaccine 2019;37:4435–43. 10.1016/j.vaccine.2019.02.079 [DOI] [PubMed] [Google Scholar]
- 47.Jacoby E, Jarrahian C, Hull HF, et al. Opportunities and challenges in delivering influenza vaccine by Microneedle patch. Vaccine 2015;33:4699–704. 10.1016/j.vaccine.2015.03.062 [DOI] [PubMed] [Google Scholar]
- 48.Birchall JC, Clemo R, Anstey A, et al. Microneedles in clinical practice--an exploratory study into the opinions of Healthcare professionals and the public. Pharm Res 2011;28:95–106. 10.1007/s11095-010-0101-2 Available: 10.1007/s11095-010-0101-2 [DOI] [PubMed] [Google Scholar]
- 49.Marshall S, Fleming A, Moore AC, et al. Acceptability of Microneedle-patch vaccines: A qualitative analysis of the opinions of parents. Vaccine 2017;35:4896–904. 10.1016/j.vaccine.2017.07.083 [DOI] [PubMed] [Google Scholar]
- 50.Berger M, Davies C, Knox S, et al. 30. use of Microarray patches for vaccination in older adults during a pandemic. Infection, Disease & Health 2022;27:S3–4. 10.1016/j.idh.2022.09.014 Available: 10.1016/j.idh.2022.09.014 [DOI] [Google Scholar]
- 51.Petrie KJ, Wessely S. Modern worries, new technology, and medicine. BMJ 2002;324:690–1. 10.1136/bmj.324.7339.690 Available: 10.1136/bmj.324.7339.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adigweme I, Akpalu E, Yisa M, et al. Study protocol for a phase 1/2, single-centre, double-blind, double-dummy, randomized, active-controlled, age de-escalation trial to assess the safety, tolerability and Immunogenicity of a measles and rubella vaccine delivered by a Microneedle patch in healthy adults (18 to 40 years), measles and rubella vaccine-primed toddlers (15 to 18 months) and measles and rubella vaccine-Naïve infants (9 to 10 months) in the Gambia [Measles and Rubella Vaccine Microneedle Patch Phase 1/2 Age De-escalation Trial]. Trials 2022;23:775. 10.1186/s13063-022-06493-5 Available: 10.1186/s13063-022-06493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Australian New Zealand Clinical Trials Registry . Phase I clinical study with high-density microarray patches (HD-MAPs) coated with live attenuated measles and rubella vaccine (MR) in healthy adult volunteers 2022, Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=381749&isReview=true
- 54.Australian New Zealand Clinical Trials Registry . Phase I clinical study to evaluate the safety and tolerability of SARS-CoV-2 spike protein (HexaPro) delivered intradermally by a high-density microarray patch (HD-MAP), in healthy adults aged 18 to 50 years. 2022, Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=383935&isReview=true
- 55.National Library of Medicine . Establishing Immunogenicity and safety of needle-free intradermal delivery of mRNA COVID-19 vaccine (MILESTONE). 2022. Available: https://clinicaltrials.gov/study/NCT05315362?intr=Microneedle%20patch&rank=10
- 56.Australian New Zealand Clinical Trials Registry . Safety, performance, and acceptability of intradermal application of an excipient-coated High Density Micro-Array Patch (HD-MAP) delivery system: Study in children and their parents/guardians 2023, Available: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=384456&isReview=true
- 57.Orenius T, LicPsych, Säilä H, et al. Fear of injections and needle Phobia among children and adolescents: an overview of psychological, behavioral, and Contextual factors. SAGE Open Nurs 2018;4:2377960818759442. 10.1177/2377960818759442 Available: 10.1177/2377960818759442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLenon J, Rogers MAM. The fear of needles: a systematic review and meta-analysis. J Adv Nurs 2019;75:30–42. 10.1111/jan.13818 Available: 10.1111/jan.13818 [DOI] [PubMed] [Google Scholar]
- 59.Adhikari BB, Goodson JL, Chu SY, et al. Assessing the potential cost-effectiveness of Microneedle patches in childhood measles vaccination programs: the case for further research and development. Drugs R D 2016;16:327–38. 10.1007/s40268-016-0144-x Available: 10.1007/s40268-016-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee BY, Bartsch SM, Mvundura M, et al. An economic model assessing the value of Microneedle patch delivery of the seasonal influenza vaccine. Vaccine 2015;33:4727–36. 10.1016/j.vaccine.2015.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forster A, Junger M. Opportunities and challenges for Commercializing Microarray patches for vaccination from a MAP developer’s perspective. Hum Vaccin Immunother 2022;18:2050123. 10.1080/21645515.2022.2050123 Available: 10.1080/21645515.2022.2050123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-012247supp001.pdf (926.5KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data have been made available within the paper and appendices.