Abstract
Objectives
To investigate factors concerning patients regarding biological/target synthetic disease-modifying antirheumatic drugs (b/ts DMARDs) in treating inflammatory arthritis (IA).
Design
This study consists of a systematic review and a cross-sectional survey in Hong Kong. A systematic review of literature following Preferred Reporting Items for Systematic Reviews and Meta-Analyses was conducted on PubMed, Web of Science, Cochrane and Embase between 1 January 2000 and 1 January 2022. Content analysis was conducted to summarise factors grouped by four themes—social aspects (SA), clinical aspects (CA), medicine characteristics (MC) and financial aspects (FA) in the decision-making process. One cross-sectional survey among Hong Kong patients with IA was conducted to add to global evidence.
Setting
A systematic review of global evidence and a patient-based survey in Hong Kong to complement scarce evidence in Asia regions.
Results
The systematic review resulted in 34 studies. The four themes were presented in descending order consistently but varied with frequency throughout decision-making processes. During decision-making involving medication initiation, preference and discontinuation, MC (reported frequency: 83%, 86%, 78%), SA (56%, 43%, 78%) and FA (39%, 33%, 56%) were the three most frequently reported factors, whereas CA was less studied. Local survey also revealed that MC factors such as treatment efficacy and the probability of severe adverse events, and SA factors such as the availability of government or charity subsidy, influenced patients’ initiation and preference for b/ts DMARDs. Meanwhile, self-estimated improvement in disease conditions (SA), drug side effects (MC) and drug costs (FA) were associated with treatment discontinuation.
Conclusions
Global and local evidence consistently indicate that MC and SA are important considerations in patients’ decisions regarding novel DMARDs. Health policies that reduce patients’ financial burden and enhances healthcare professionals’ engagement in decision-making and treatment delivery should be in place with an efficient healthcare system for managing IA optimistically.
Keywords: Health policy, Rheumatology, Quality in health care
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first study systematically and synergistically identify patient perceptions of biological/target synthetic disease- modifying antirheumatic drugs (b/ts DMARDs) throughout the decision-making process including the initiation, preference and discontinuation of b/ts DMARDs.
With findings from a systematic review and local Hong Kong survey data from the Asian region, this study offers the most exclusive evidence to date.
Study limitions include convenience sampling and recall bias among respondents in Hong Kong, and the potenitial publication bias in studies included in the systematic review. The demographic variability among respondents were not adjusted.
Introduction
Inflammatory arthritis (IA), such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), axial spondylarthritis (axSpA), psoriasis and psoriatic arthritis (PsA), is a group of chronic, progressive and potentially debilitating diseases, which adversely affects the health-related quality of life and places a financial burden on patients, their families and society.1 2 For example, the prevalence of RA results in health resource usage of up to US$67 306 and a total cost of US$83 845 when considering social benefit loss due to absenteeism, presenteeism and work disability annually.3 The primary goal of treatment for IA is to enhance physical function and well-being in daily social life and to achieve sustained remission or low disease activity for clinical management purposes.4 Since the 2000s, the existing therapeutic arsenal has been gradually revolutionised with the introduction of biological disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) to delay disease progression. bDMARDs are produced in the form of monoclonal antibodies or recombinant fusion proteins, while tsDMARDs are produced as small molecules, inhibiting specific molecules or targeting cellular targets directly involved in the disease pathogenesis.5
Evidence from randomised clinical trials and real-life post-marketing records indicates that b/ts DMARDs are effective, with the potential to optimise their use in setting up personalised treatment and achieving treat-to-target strategies in disease management.6 7 The safety of b/ts DMARDs is also acceptable and manageable, particularly compared with the risks associated with the disease itself.8–10 However, there seem to be limitations on patient choice for these promising drugs. In most countries, bDMARDs are recommended when there is no response or inadequate response to non-steroidal anti-inflammatory drugs (NSAIDs) or traditional DMARDs in IA management.11 In practice, the high cost of b/ts DMARDs12 and the rigorous treatment and reimbursement requirements imposed13 negatively influence the availability of b/ts DMARDs for patients. Inequality in access to b/ts DMARDs might be further exacerbated by low-income14 and other socioeconomic status.15
It is imperative to gather real-world evidence for policymakers to support the healthcare system in promoting patients’ access to b/ts DMARDs. However, there is a lack of systematic global evidence in understanding the demands, unmet needs and barriers of using b/ts DMARDs from patients’ perspective, as well as rigorous case analyses from Asian populations. We aimed to identify the factors that influence patients regarding b/ts DMARDs by conducting a global systematic review and to complement the lack of evidence in the Asia regions by an additional investigation into the Hong Kong context.
Methods
This study combined a global systematic review and a cross-sectional survey in Hong Kong to review and summarise factors influencing the decision process for the use of b/ts DMARDs from global and local perspectives.
Systematic review of global evidence
Study design
A systematic review of published academic literature was conducted to determine factors reported by patients that affect their choice of b/ts DMARDs to manage IA. Literature search was performed in four academic databases (PubMed, Web of Science, Cochrane and Embase) using ‘biologics’, ‘inflammatory arthritis’, ‘barrier’, ‘patient-reported’, ‘preference’ and their synonyms. The search time interval ranged from 1 January 2000 to 1 January 2022. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines provided guidance for the good performance of conducting and reporting this study.16 The search strategy and results from all databases can be found in online supplemental table 1. Manual reference list search was performed to derive relevant studies from previous systematic reviews.
bmjopen-2022-069681supp001.pdf (482.6KB, pdf)
Eligibility criteria and paper screening
A two-tier screening process is implemented to assess studies’ eligibility, involving screening of titles/abstracts followed by a full-text review. Two investigators (YL and LKWL) independently extract data, including paper characteristics and factors influencing the use of b/ts DMARDs, from identified studies. The extracted data will be cross-checked, verified, and any disagreements or discrepancies will be resolved through discussion involving senior author XL as the third reviewer to ensure accuracy and reliability.
Inclusion criteria for screening were: (1) studies that were peer-reviewed with full text in English; (2) studies that elicited preferences and choice between conventional and novel DMARDs; (3) studies for which results were reported by patients or studies that were done on the general public; (4) studies that reported factors of interest of patients whenever they were beginning to use, giving their preference for, or deciding to discontinue/switch b/ts DMARDs; (5) recruited participants were adults. The implemented exclusion criteria were (1) studies involving healthcare professionals or health resource providers only, with no patient involvement at all; (2) studies that determined patient preference for biologics over biosimilars or vice versa; (3) studies on paediatric IA; (4) studies based on register data.
Data extraction and synthesis
Factors for the therapeutic choice of IA management pertained to three decision-making processes: the initiation of, preference for and discontinuation of b/ts DMARDs (figure 1). Studies where patients chose to use b/ts DMARDs for the first time (b/ts DMARDs naïve) referred to the initiation of b/ts DMARDs. Patients with experience of b/ts DMARDs usage were able to express their preference for treatments, for example, traditional DMARDs or NSAID vs b/ts DMARDs for RA, axSpA, AS and PsA; and topical creams, phototherapy or systemic therapies versus b/ts DMARDs for psoriasis. Such studies were categorised as preference-related studies. Patients might stop their initial b/ts DMARDs use without implementing another treatment plan or switching to a traditional medicine. These cases were considered as the discontinuation of b/ts DMARDs. Retrieved data from each article were classified into four themes—social aspects (SA), clinical aspects (CA), medicine characteristics (MC) and financial aspects (FA), and were categorised separately for the three decision-making processes (table 1).
Figure 1.
Decision process diagram for biological/target synthetic disease-modifying antirheumatic drugs (b/ts DMARDs) to manage inflammatory arthritis.
Table 1.
Identified themes and sub-themes from selected studies in the global systematic review
| Themes | Sub-themes |
| Social aspects | Information/knowledge/awareness peer support drug distribution due to geographic location/stock beliefs/concerns patient support programmes personal health planning* |
| Clinical aspects | Instruction/experience from clinical staff† |
| Medicine characteristics | Safety efficacy achieved in reality method of administration years on market supplementary treatment required to ensure the treatment effect of biologics |
| Financial aspects | High-cost of drugs, insurance approval and coverage out-of-pocket treatment cost per month |
*Personal planning refers to the desire to be pregnant, the need to limit alcohol, the need to change diet and exercise, the requirement for another drug prescription and the change of health plan due to self-reported disease progression.
†Instruction/experience from clinical staff refers to physician’s experience with the product, clinic staffs’ personal preference for product, problems with the pharmacy in filling the prescription, and follow-up and medical assistance during the injection.
Data analysis and visualisation
Content analysis was performed based on information retrieved to summarise factors grouped by four themes. The frequency of each theme reported in all studies for three decision-making processes was calculated, and a heat map was generated for an implicit expression of the frequency. Data were retrieved and analysed independently by YL and KP and will be monitored by XL whenever consensus cannot be reached.
Survey of Hong Kong observation
Study design
This was a patient-reported outcome study using a cross-sectional survey with questionnaire designed and validated from expert opinion including clinical pharmacists, rheumatologists and public health researchers. Registered members from five local patient groups of autoimmune diseases were invited to complete a paper-based questionnaire. Patient consent is granted when they willingly agree to participate in the survey and actively engage in the research. Two hundred and fifty-five questionnaires were distributed between September and November 2021 and follow-up by telephone ended in January 2022 for incomplete questionnaires. The questionnaire covered patients’ medication use and compliance, preferences for taking DMARDs, and barriers to access of local financial support programmes, Samaritan Fund, of which all b/ts DMARDs were listed as self-financed items with a safety net as of March 2022.17 Data is considered effective when at least 80% of the questions have been answered, and the follow-up via telephone ensures the efficacy and accuracy of data. However, comparison between participants and those who declined participation is not feasible due to the lack of data on excluded individuals.
The local patient-based survey was supported by funding from Pfizer. Pfizer has no role in the study design, data analysis and results interpretation.
Outcomes and statistics
Baseline characteristics of the study population and study outcomes were described using frequency with percentage, median with IQR and mean with SD, whichever was appropriate. No missing data imputation was conducted under the assumption that data were missed randomly. The outcome of interests includes:
Preference for DMARDs: We listed six different reasons for participants to rank by importance when they considered whether to use DMARDs or not, ranging from one to six. One represented the most important factor, and six as the least important factor. Each number could only be used once.
Reasons for b/tsDMARDs discontinuation: Among patients who reported discontinuation of b/ts DMARDs in the last 3 months, reasons were further investigated. Five common reasons were listed for patients to select, including self-estimated improvement in disease conditions, drug side effects, drug costs, physician-assessed improvement in disease conditions and inconvenience of drug use. Participants were allowed to specify other reasons that were not included in the listed ones in free-text.
Barriers to applying for local financial support programmes for b/ts DMARDs usage: We listed five specific reasons for participants if they were unsuccessful in their application for the local financial support programme, Samaritan Fund, or have not used it after application was approved. Participants could choose ‘others’ and provide reasons.
For both the systematic review and survey, data were entered, analysed and cross-checked by two independent researchers from the team to ensure the reliability of the results. All analyses were performed using R V.4.0.3 (R Core Team, Vienna, Austria).18
Patient and public involvement
Choices for novel DMARDs among patients with IA and the general population were collected from the published literatures and patients with IA in Hong Kong were involved in the conduct of study by attending the local survey.
Results
Systematic review of global evidence
Overview
Two thousand two hundred and sixty-one studies were searched out of four databases, and 15 studies were added from the manual research of a systematic review.19 After removing 466 duplications, 1713 irrelevant studies during abstract screening, and 63 other ineligible studies by inclusion/exclusion criteria, 34 papers were included and assessed for content analysis (initiation: N=420–23; preference: N=824–31; discontinuation: N=832–39; initiation/preference: N=1340–52; initiation/discontinuation: N=153). The study selection flow diagram is shown in online supplemental figure 1. In terms of characteristics of these studies, 9 studies are qualitative studies while 25 are quantitative, among which 16 studies used a conjoint analysis design (including discrete choice experiment). Except for two studies with a general population23 26 and seven studies with mixed patients (at least two disease types among RA, AS, axSpA, psoriasis and PsA),27 29 33 35 38 48 52 16 studies were carried out in patients with RA,5 20–22 28 30 34 36 37 41–43 47 49–51 eight in patients with psoriasis24 31 32 40 44–46 53 and one in patients with axSpA.39 Thirty-one (91%) studies were conducted in Western countries, while the others were conducted in Asia (one from China30 and two from Japan36 46). Detailed information is shown in online supplemental tables 2–4.
Factors to determine the initiation, preference and discontinuation of b/tsDMARDs
Table 1 displays the four themes and corresponding sub-themes of factors in all three decision processes determined in this study. The sub-themes are grouped under the headings—SA, CA, MC and FA.
Initiation of b/ts DMARDs
Factors regarding SA raised awareness/information/knowledge, beliefs/concerns, availability of patient support programmes and personal health planning when initiating b/ts DMARDs. Sources of information or means to increase knowledge of b/ts DMARDs includes previous experience of biologics,20 47 professional manpower in hospitals and pharmaceutical companies,54 post-market surveillance48 as well as the internet.23 53 Also, support for patients, such as setting up a public education campaign or linking patients to pharmaceutical companies for enquiries or other services, would bolster patients’ knowledge of the potential serious consequences of initiating innovative medicines.20 48 53 The influence of personal or religious beliefs or factors on innovative medicines,48 and the need to restrict alcohol or other lifestyles changes42 could further affect patients’ willingness to use b/ts DMARDs. MC, including safety, efficacy, years on market, and administration route and frequency, are of interest to patients when they consider b/ts DMARDs for the first time. The main factor driving the initiation of b/ts DMARD from CA were clinical staff with extensive experience, while the cost of drugs and insurance approval were important from a financial perspective.
Preference for b/ts DMARDs
Information/knowledge/awareness, beliefs/concerns about b/ts DMARD, patient support programmes and personal health planning from SA can determine the preference and choice for b/ts DMARDs over the traditional DMARDs. Real-world evidence of b/ts DMARDs is an especially important source of information to improve public awareness on innovative treatments.48 MC are widely discussed to elicit preference for treatment options with safety, efficacy and administration management mentioned in detail. The preference for b/ts DMARDs will be violated if the drug is new to the market or requires frequent regular physical check-ups,42 repeated hospital day-care28 and laboratory tests.45 CA that determines the preference for b/ts DMARD include physicians’ preference for treatment choice, rheumatologists’ opinions and the availability of medical assistance during the subcutaneous injection. Except for high drug prices, concerns about personal expenses and insurance coverage tended to a reluctance to use b/ts DMARDs.
Discontinuation of b/ts DMARDs
From SA, the distribution and stock of drugs in hospitals due to geographic location, public beliefs and negative concerns about treatment effects are examined as a determinant of discontinuation of biological use.35 38 A wide range of personal health plans also challenges the continuous use of b/tsDMARDs, such as pregnancy desires, the need for vaccination and surgical procedures, mental health issues, the desire for perceived diet or exercise,32 37 38 53 as well as the desire for disease remission.36 53 MC, such as side effects, drug contraindications, non-toxic or toxic reasons, immediate or lasting treatment effectiveness, are identified from studies on the discontinuation of b/ts DMARDs. Financial concerns that inhibit adherence to b/ts DMARDs refer to the high cost of drugs,37 53 and non-insurance coverage.32–34 However, CA are beneficial to long-term adherence of b/ts DMARDs by maintaining a good communicative relationship between patients and rheumatologists.38
Frequency of themes in decision-making process
Although the four themes were consistently reported in descending order of frequency throughout the decision-making processes, the frequency of each theme varied among the initiation, preference and discontinuation of b/ts DMARDs (figure 2, details in online supplemental tables 5–7). As for initiation, MC (83%) was the most frequently reported factor, followed by SA (56%), FA (39%) and CA (22%). Similarly, preference for b/ts DMARDs was also impacted by MC (86%), SA (43%), FA (33%) and CA (19%). Factors related to MC and SA were both reported in 78% of studies on the discontinuation of b/ts DMARDs, while FA and CA were cited at 56% and 11%, respectively. During the decision processes involving initiation, preference and discontinuation, MC, SA as well as FA were the three most frequently investigated factors whereas CA was given less consideration among the included studies.
Figure 2.
The frequency of four themes in three decision making processes.
Survey of Hong Kong observation
We received 223 effective responses out of 255 distributed questionnaires. Detailed information on the baseline characteristics of participants is listed in online supplemental table 8. Consistent with the three different decision-making processes found in the systematic review, the survey results are presented with respect to barriers to access, preference for, and discontinuation of DMARDs treatments.
Barriers to accessing local financial support programme
Results from the systematic review showed that financial support is an essential factor to consider in the initiation of, preference for and discontinuation of medication. The survey discussed the difficulties that might be encountered during the application process. Thirty-four patients successfully received financial support out of 38 patients having applied. One hundred and eighty-five (82.6%) patients did not apply for the financial support programme. Apart from those who were not eligible or could not fulfil the clinical criteria for application, a considerable number of 41 patients (out of 223, 18.4%) were unaware that they could apply for the fund. Thirty-three (out of 223,14.8%) patients felt that the application procedures were too complicated.
Preference for taking DMARDs
MC are strongly associated with the selection of novel treatments since treatment efficacy and the probability of severe adverse events would be prioritised when patients consider DMARDs. In accordance with the fact that SA plays an important role in choosing b/ts DMARDs from the systematic review, the availability of government or charity subsidisation is also strongly solicited among survey respondents (table 2).
Table 2.
Preference of attributes in considering drug use
| Attributes in considering drug use | Average rank (SD) | Patients ranked as most important n (%) |
| Treatment efficacy | 1.69 (0.85) | 84 (50.6%) |
| Severe adverse event(s) | 3.04 (1.58) | 34 (20.5%) |
| Government or charity subsidisation | 3.28 (1.78) | 36 (21.7%) |
| Administration mode | 4.25 (1.36) | 8 (4.8%) |
| Follow-up by healthcare professionals | 4.45 (1.45) | 3 (1.8%) |
| Administration frequency | 4.30 (1.24) | 1 (0.6%) |
The results are based on 166 patients after excluding 44 patients who had the same score for two attributes when ranking the six attributes provided. The pairwise comparison of the six attributes and the marginal matrix are presented in online supplemental tables 9 and 10.
Discontinuation of medication
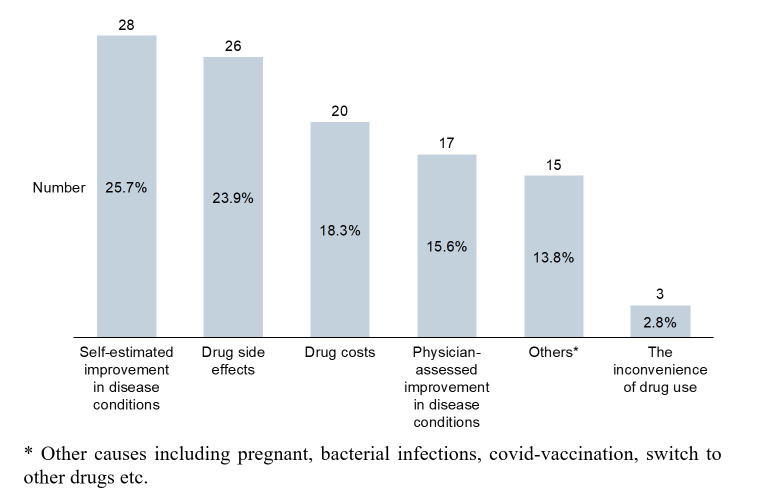
Among 223 responses, 74 (33.2%) reported that they had stopped taking medicine in the last 3 months. Twenty-eight patients (out of 109, 25.7%) felt self-estimated improvement in disease conditions was the most common reason for discontinuation, followed by drug side-effects reported by 26 patients (out of 109, 23.9%) and cost-related concerns mentioned by 20 patients (out of 109, 18.3%). Physician-assessed improvement in disease conditions was another principal reason to consider, reported by 17 patients (out of 109, 15.6%). The inconvenience of drug use was least considered in quitting drugs, which accounted for three patients (out of 109, 2.8% of total responses) (figure 3). The findings were well aligned with the fact that SA such as the desire for disease remission, MC and FA are barriers to be considered and would certainly impact patients’ adherence to b/ts DMARDs according to the global evidence.
Figure 3.
Main reasons for discontinuing medication in the last 3 months.
Discussion
Summary of key findings
Our systematic review comprehensively characterised the reasons related to decisions regarding b/ts DMARDs in three decision-making processes in clinical practice. MC and SA were the two most frequently mentioned factors with respect to drug initiation, preference, as well as discontinuation. Financial concerns were less frequently reported whereas CA, despite its cruciality, were studied at the lowest frequency. Consistent with the systematic review, our local patient-group based survey also revealed that drug costs, drug side effects, treatment efficacy and inconvenience of drug use were the most important factors affecting choice of novel DMARDs and adherence among patients with IA. The dominance of financial considerations over social factors among local patients, in comparison to global evidence, underscores the inadequacy of financial support and reimbursement implementation in Hong Kong. Remarkably, as of 2023, no biologics have been agreed on and included in the local reimbursement list, exacerbating the situation in Hong Kong.
While a previous study54 paid attention to patients with RA, PsA and psoriasis in the USA, we expanded our focus to five types of IA in both Western and Asian countries. Similarly, the systematic review55 conducted by Belinchón et al explored the perspective of patients in the European Union and revealed an association between treatment satisfaction towards psoriasis management and physician recommendations and patient preferences by narrating evidence. Our study quantified the information retrieved and provided an opportunity to compare patients’ perceptions in different contexts. This is the first study to propose a diagram for the decision-making process on b/ts DMARDs that potentially explains the dynamics of patients’ attitudes given the long-term treatment course for IA and the complicated trade-off between benefits and risks imposed on patients. This study also synthesised evidence from discrete choice event (DCE) studies which had only been explored in terms of methodology in conducting DCE in a prior systematic review.19
Clinical implications
MC and SA play a preponderant role in the choice of b/t DMARDs throughout all three decision processes. Raising public awareness and knowledge of potential treatments is beneficial to build reasonable expectations of drug effectiveness and to create an environment where advantages and disadvantages of biologics/targeted drugs are realistically displayed. Information and knowledge on innovative treatments could come from clinical manpower including physicians, rheumatology specialists, nursing staff and the hospital pharmacy, which fundamentally influence decisions to start b/ts DMARDs.29 53 Patients are more likely to convinced with advice from healthcare professionals, such as rheumatologists and written formats29 are often seen as more credible sources of information.
Previous experience of biologics will foster knowledge of the characteristics of available treatments and reshape the perception of a treatment option which could be overlooked due its potential disadvantage at first glance. At the same time, frequent discussion with patients about their feelings and clinical staff support during b/ts DMARDs injection directly affects adherence to treatment regimes.32 Therefore, healthcare professionals should encourage eligible patients to use innovative drugs at an early stage and the health system should give more priority to routine disease management in primary care and the requirements of patients in clinical practice.
Policy implications
Although FA were less frequently mentioned than we expected, the high cost of biologics remains a major barrier for the first attempt of and long-term adherence to innovative drugs, as revealed in our local survey and global evidence. This can be seen by the fact that drug cost was given great significance even after recommendation by rheumatologists.20 In countries where public insurance covers up to 90% of bDMARD costs, drug adherence were still not improved due to the overload of personal expenses and the increase in prescription volume.37 Other uncertainties, such as private pharmacy strikes, the complexity of the formal process, as well as the bureaucracy involved in reimbursing bDMARDs, deprive patients of right to pursue better health. This scenario occurred in Greece where one in four patients with RA had asked their rheumatologist to replace the prescribed therapeutic regimen with a non-bDMARD due to unaffordability.34 Therefore, health policies to address the financial burden on patients with an efficient healthcare system is pivotal to manage IA sustainably.
Our local survey found that the Samaritan Fund could provide valuable support to improve the affordability of b/ts DMARDs for patients from lower social-economic classes. The application procedure was not deemed ideal in the responses, nonetheless. Notably, a considerable proportion of the surveyed participants did not know that they could apply for financial support, or complained that the application procedure was too complicated, hence the difficulties in submitting applications successfully. These factors underscore the need for a simplified application procedure, elimination of unnecessary steps with adequate step-by-step patient education and support. Local social service providers should be aware of such needs and provide sufficient support and guidance to assist patient applications.
Limitations
The systematic review and cross-sectional survey were both affected by recall bias, where participants might not clearly remember the decisions they had made and reported subjectively, or recalled biased information on our study or studies included in the systematic review. As demographic characteristics were not considered in the systematic review, the results could be influenced by heterogeneity. The survey conducted in Hong Kong gave us a specific regional insight about patient-reported factors and complements existing global evidence to inform local social and health policy changes. The absence of data from non-participants in the local survey may result in response bias, and we acknowledge the potential bias from the local survey due to the convenience sampling method. However, the diverse distribution of patients regarding educational backgrounds, working status, and monthly household income, help minimise bias and provides a comprehensive representation of the real-world patient population.
While this review attempts to examine patients’ perspectives at different stages of biological treatment, it is challenging to determine the relative importance of factors based solely on reported frequency. This limitation arises due to the lack of appropriate methods to effectively summarise and synthesise qualitative and mixed data. Future studies could consider restricting on DCEs where rank of importance among factors has been inherently considered and reporting the number of times each attribute is classed as the most important.56 57 However, it is important to acknowledge that this approach may encounter methodological limitations, as an attribute is more likely to be important if DCEs accommodate fewer attributes.58 Therefore, this study contributes to informing DCE with all-encompassing attributes, and prospective research is craved for appropriate methods to summarise results across qualitative and quantitative analysis including DCEs.59
Given the heterogeneity of included studies, it is not feasible or practical to conduct a standardised risk of bias assessment. With regard to this, the global systematic review might suffer from the risk of bias that warrants cautious results interpretation and considerable prospective study design in future to confirm the conclusion from this study.
Conclusion
The global and local evidence are consistent in pointing out that MC and SA, compared with FA and CA, were more likely to be considered by patients related to the use of novel DMARDs. The change in patients’ consideration from initiating to discontinuing b/ts DMARDs paves the way to improving treatment uptake and compliance by health education for medication use and improved social care support. Health policies that reduce patients’ financial burden and enhances healthcare professionals’ engagement in decision-making and treatment delivery should be in place with an efficient healthcare system for managing IA optimistically.
Supplementary Material
Acknowledgments
We thank all participants from the five local patient groups, including the Hong Kong Ankylosing Spondylitis Association, B27 Association, Hong Kong Psoriasis Patients Association, Hong Kong Rheumatoid Arthritis Association and Hong Kong Psoriatic Arthritis Association. We thank our university colleagues Ms Runqing Yang, Mr Franco Cheng, Ms Miriam Leung and Ms Jasmine Lam for their contribution to the questionnaire development and administration of the local survey. We also thank Ms Lisa Lam for English proof-reading.
Footnotes
Contributors: Study concept and design: XL, ICKW. Data extractions, analysis and cross-check: YL, LKWL, KP. Data interpretation: YL, LKWL, KP, DZ, DD, ICKW, XL. Manuscript draft: YL, XL. Critical revision: YL, LKWL, KP, DZ, DD, ICKW, XL. Funding acquisition: XL, ICKW. Study supervision: XL. XL is responsible for the overall content as the guarantor.
Funding: The local patient survey was supported by Pfizer Corporation Hong Kong Limited under grant project number [200010114].
Competing interests: XL received research grants from Research Fund Secretariat of the Food and Health Bureau, Research Grants Council Early Career Scheme, Research Grants Council Research Impact Fund, Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong. There is no conflict of interest for other coauthors.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Raw data for systematic review are available upon reasonal request; raw data for local patient survey are only available to the research team according to the ethical approval.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (reference number: UW 21-229). Participants gave informed consent to participate in the study before taking part.
References
- 1.Tymms K, Littlejohn G, Griffiths H, et al. Treatment patterns among patients with rheumatic disease (rheumatoid arthritis (RA), Ankylosing Spondylitis (AS), Psoriatic arthritis (PSA) and undifferentiated arthritis (una)) treated with subcutaneous TNF inhibitors. Clin Rheumatol 2018;37:1617–23. 10.1007/s10067-018-4105-3 [DOI] [PubMed] [Google Scholar]
- 2.Proft F, Poddubnyy D. Ankylosing Spondylitis and axial Spondyloarthritis: recent insights and impact of new classification criteria. Ther Adv Musculoskelet Dis 2018;10:129–39. 10.1177/1759720X18773726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsieh PH, Wu O, Geue C, et al. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis 2020;79:771–7. 10.1136/annrheumdis-2019-216243 [DOI] [PubMed] [Google Scholar]
- 4.Sarzi-Puttini P, Ceribelli A, Marotto D, et al. Systemic rheumatic diseases: from biological agents to small molecules. Autoimmun Rev 2019;18:583–92. 10.1016/j.autrev.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 5.The use of disease-modifying anti-rheumatic drugs for the management of rheumatoid arthritis. Canberra: AIHW. Australian Institute of Health and Welfare, 2011. [Google Scholar]
- 6.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 7.Koh JH, Lee SK, Kim J, et al. Effectiveness and safety of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in elderly patients with rheumatoid arthritis: real-world data from the KOBIO Registry. Clin Exp Rheumatol 2021;39:269–78. 10.55563/clinexprheumatol/ftfgmf [DOI] [PubMed] [Google Scholar]
- 8.Ruderman EM. Overview of safety of non-biologic and biologic Dmards. Rheumatology (Oxford) 2012;51 Suppl 6:vi37–43. 10.1093/rheumatology/kes283 [DOI] [PubMed] [Google Scholar]
- 9.de La Forest Divonne M, Gottenberg JE, Salliot C. Safety of biologic Dmards in RA patients in real life: A systematic literature review and meta-analyses of biologic registers. Joint Bone Spine 2017;84:133–40. 10.1016/j.jbspin.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 10.Cañete JD, Hernández MV, Sanmartí R. Safety profile of biological therapies for treating rheumatoid arthritis. Expert Opin Biol Ther 2017;17:1089–103. 10.1080/14712598.2017.1346078 [DOI] [PubMed] [Google Scholar]
- 11.Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol 2019;3:42. 10.1186/s41927-019-0090-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlewska E, Ancuta I, Anic B, et al. Access to biologic treatment for rheumatoid arthritis in central and Eastern European (CEE) countries. Med Sci Monit 2011;17:SR1–13. 10.12659/msm.881697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putrik P, Ramiro S, Kvien TK, et al. Variations in criteria regulating treatment with reimbursed biologic Dmards across European countries Ann Rheum Dis 2014;73:2010–21. 10.1136/annrheumdis-2013-203819 [DOI] [PubMed] [Google Scholar]
- 14.Putrik P, Ramiro S, Kvien TK, et al. Inequities in access to biologic and synthetic Dmards across 46 European countries. Ann Rheum Dis 2014;73:198–206. 10.1136/annrheumdis-2012-202603 [DOI] [PubMed] [Google Scholar]
- 15.Enache L, Codreanu C, Mogosan C, et al. Fri0424 accessibility to biological therapy for patients with Ankylosing Spondylitis in Romania is influenced by area of residence, Socio- economic and demographic factors. Ann Rheum Dis 2016;75(Suppl 2):589. 10.1136/annrheumdis-2016-eular.5955 [DOI] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 2021;134:103–12. 10.1016/j.jclinepi.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Hospital authority drug fomulary . Hospital Authority, 9 July. 2022Available: https://www.ha.org.hk/hadf/Portals/0/Docs/HADF_List/External%20list%2020190413/10%20%20%20MUSCULOSKELETAL%20AND%20JOINT%20DISEASES.pdf
- 18.Team RC . R Foundation for Statistical Computing Vienna, Austria. R: A language and environment for statistical computing, Available: https://www.R-project.org/ [Google Scholar]
- 19.Zartab S, Nikfar S, Karimpour-Fard N, et al. A systematic review of discrete choice experiment studies in rheumatoid arthritis biological medicines. Mediterr J Rheumatol 2021;32:104–11. 10.31138/mjr.32.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisicki R, Chu L. What matters to patients and physicians when considering biologic therapy for rheumatoid arthritis. Postgrad Med 2008;120:154–60. 10.3810/pgm.2008.09.1915 [DOI] [PubMed] [Google Scholar]
- 21.Augustovski F, Beratarrechea A, Irazola V, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experiment. Value Health 2013;16:385–93. 10.1016/j.jval.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 22.Fraenkel L, Nowell WB, Michel G, et al. Preference phenotypes to facilitate shared decision-making in rheumatoid arthritis. Ann Rheum Dis 2018;77:678–83. 10.1136/annrheumdis-2017-212407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villani A, Cinelli E, Fabbrocini G, et al. Psoriasis awareness among general population: preliminary results of an online survey. Ital J Dermatol Venereol 2021;156:665–8. 10.23736/S2784-8671.21.06822-X [DOI] [PubMed] [Google Scholar]
- 24.Mahler R, Jackson C, Ijacu H. The burden of psoriasis and barriers to satisfactory care: results from a Canadian patient survey. J Cutan Med Surg 2009;13:283–93. 10.2310/7750.2009.08083 [DOI] [PubMed] [Google Scholar]
- 25.Poulos C, Hauber AB, González JM, et al. Patients' willingness to trade off between the duration and frequency of rheumatoid arthritis treatments. Arthritis Care & Research 2014;66:1008–15. 10.1002/acr.22265 [DOI] [PubMed] [Google Scholar]
- 26.Harrison M, Marra C, Shojania K, et al. Societal preferences for rheumatoid arthritis treatments: evidence from a discrete choice experiment. Rheumatology (Oxford) 2015;54:1816–25. 10.1093/rheumatology/kev113 [DOI] [PubMed] [Google Scholar]
- 27.Nolla JM, Rodríguez M, Martin-Mola E, et al. Patients' and Rheumatologists' preferences for the attributes of biological agents used in the treatment of rheumatic diseases in Spain. Patient Prefer Adherence 2016;10:1101–13. 10.2147/PPA.S106311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desplats M, Pascart T, Jelin G, et al. Are Abatacept and Tocilizumab intravenous users willing to switch for the subcutaneous route of administration? A questionnaire-based study. Clin Rheumatol 2017;36:1395–400. 10.1007/s10067-017-3587-8 [DOI] [PubMed] [Google Scholar]
- 29.de Toro J, Cea-Calvo L, Battle E, et al. Perceptions of patients with rheumatic diseases treated with subcutaneous BIOLOGICALS on their level of information: RHEU-LIFE survey. Reumatol Clin (Engl Ed) 2019;15:343–9. 10.1016/j.reuma.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 30.Li H-B, Wu L-J, Jiang N, et al. Treatment satisfaction with rheumatoid arthritis in patients with different disease severity and financial burden: A subgroup analysis of a nationwide survey in China. Chin Med J (Engl) 2020;133:892–8. 10.1097/CM9.0000000000000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gisondi P, Bellinato F, Piaserico S, et al. Preference for Telemedicine versus in-person visit among patients with psoriasis receiving biological drugs. Dermatol ther (Heidelb). Dermatol Ther (Heidelb) 2021;11:1333–43. 10.1007/s13555-021-00555-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeung H, Wan J, Van Voorhees AS, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol 2013;68:64–72. 10.1016/j.jaad.2012.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and Psoriatic arthritis in the United States: findings from the National psoriasis foundation surveys, 2003-2011. JAMA Dermatol 2013;149:1180. 10.1001/jamadermatol.2013.5264 [DOI] [PubMed] [Google Scholar]
- 34.Souliotis K, Papageorgiou M, Politi A, et al. Barriers to Accessing biologic treatment for rheumatoid arthritis in Greece: the unseen impact of the fiscal crisis--the health outcomes patient environment (HOPE) study. Rheumatol Int 2014;34:25–33. 10.1007/s00296-013-2866-1 [DOI] [PubMed] [Google Scholar]
- 35.Nisar MK, Mirza W, Rafiq A, et al. Adherence to biologic therapy - does it vary with Ethnicity? Biologicals 2018;54:28–32. 10.1016/j.biologicals.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 36.Ebina K, Hashimoto M, Yamamoto W, et al. Drug tolerability and reasons for discontinuation of seven Biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther 2019;21:91. 10.1186/s13075-019-1880-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidari P, Cross W, Weller C, et al. Medication adherence and cost-related medication non-adherence in patients with rheumatoid arthritis: A cross-sectional study. Int J Rheum Dis 2019;22:555–66. 10.1111/1756-185X.13549 [DOI] [PubMed] [Google Scholar]
- 38.Raghunath S, Hijjawi R, Hoon E, et al. Qualitative assessment of medication adherence in patients with rheumatic diseases on biologic therapy. Clin Rheumatol 2019;38:2699–707. 10.1007/s10067-019-04609-y [DOI] [PubMed] [Google Scholar]
- 39.Nowell WB, Gavigan K, Hunter T, et al. Patient perspectives on Biologics for axial Spondyloarthritis in a cross-sectional study in a predominantly female population: treatment satisfaction, wear-off between doses, and use of supplemental medication. Rheumatol Ther 2022;9:509–20. 10.1007/s40744-021-00417-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kromer C, Schaarschmidt ML, Schmieder A, et al. Patient preferences for treatment of psoriasis with BIOLOGICALS: A discrete choice experiment. PLoS One 2015;10:e0129120. 10.1371/journal.pone.0129120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alten R, Krüger K, Rellecke J, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence 2016;10:2217–28. 10.2147/PPA.S117774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazlewood GS, Bombardier C, Tomlinson G, et al. Treatment preferences of patients with early rheumatoid arthritis: a discrete-choice experiment. Rheumatology (Oxford) 2016;55:1959–68. 10.1093/rheumatology/kew280 [DOI] [PubMed] [Google Scholar]
- 43.Husni ME, Betts KA, Griffith J, et al. Benefit-risk trade-offs for treatment decisions in moderate-to-severe rheumatoid arthritis: focus on the patient perspective. Rheumatol Int 2017;37:1423–34. 10.1007/s00296-017-3760-z [DOI] [PubMed] [Google Scholar]
- 44.Kromer C, Peitsch WK, Herr R, et al. Treatment preferences for BIOLOGICALS in psoriasis: experienced patients appreciate Sustainability. J Dtsch Dermatol Ges 2017;15:189–200. 10.1111/ddg.12919 [DOI] [PubMed] [Google Scholar]
- 45.Schaarschmidt M-L, Herr R, Gutknecht M, et al. Patients' and physicians' preferences for systemic psoriasis treatments: A nationwide comparative discrete choice experiment (Psocompare). Acta Derm Venereol 2018;98:200–5. 10.2340/00015555-2834 [DOI] [PubMed] [Google Scholar]
- 46.Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol 2019;46:466–77. 10.1111/1346-8138.14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Heuckelum M, Mathijssen EG, Vervloet M, et al. Preferences of patients with rheumatoid arthritis regarding disease-modifying Antirheumatic drugs: a discrete choice experiment. Patient Prefer Adherence 2019;13:1199–211. 10.2147/PPA.S204111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho KA, Acar M, Puig A, et al. What do Australian patients with inflammatory arthritis value in treatment? A discrete choice experiment. Clin Rheumatol 2020;39:1077–89. 10.1007/s10067-019-04843-4 [DOI] [PubMed] [Google Scholar]
- 49.Bywall KS, Kihlbom U, Hansson M, et al. Patient preferences on rheumatoid arthritis second-line treatment: a discrete choice experiment of Swedish patients. Arthritis Res Ther 2020;22:288. 10.1186/s13075-020-02391-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sidiropoulos P, Bounas A, Athanassiou P, et al. Correlation of patient preferences to treatment outcomes in patients with rheumatoid arthritis treated with tumour necrosis factor inhibitors in Greece. Clin Rheumatol 2020;39:3643–52. 10.1007/s10067-020-05171-8 [DOI] [PubMed] [Google Scholar]
- 51.Singh JA, Tornberg H, Goodman SM. Pop a pill or give myself a shot? patient perspectives of disease-modifying anti-rheumatic drug choice for rheumatoid arthritis. Joint Bone Spine 2021;88:105053. 10.1016/j.jbspin.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 52.Feldman SR, Poulos C, Gilloteau I, et al. Exploring determinants of psoriasis patients' treatment choices: a discrete-choice experiment study in the United States and Germany. Journal of Dermatological Treatment 2022;33:1511–20. 10.1080/09546634.2020.1839007 [DOI] [PubMed] [Google Scholar]
- 53.Kamangar F, Isip L, Bhutani T, et al. How psoriasis patients perceive, obtain, and use biologic agents: survey from an academic medical center. J Dermatolog Treat 2013;24:13–24. 10.3109/09546634.2011.631979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and Psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 2018;12:1483–503. 10.2147/PPA.S167508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belinchón I, Rivera R, Blanch C, et al. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence 2016;10:2357–67. 10.2147/PPA.S117006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sain N, Willems D, Charokopou M, et al. The importance of understanding patient and physician preferences for psoriasis treatment characteristics: a systematic review of discrete-choice experiments. Curr Med Res Opin 2020;36:1257–75. 10.1080/03007995.2020.1776233 [DOI] [PubMed] [Google Scholar]
- 57.Toroski M, Kebriaeezadeh A, Esteghamati A, et al. Patient and physician preferences for type 2 diabetes medications: a systematic review. J Diabetes Metab Disord 2019;18:643–56. 10.1007/s40200-019-00449-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bien DR, Danner M, Vennedey V, et al. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient 2017;10:553–65. 10.1007/s40271-017-0235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhary D, Thomas M, Pacheco-Barrios K, et al. Methods to summarize discrete-choice experiments in a systematic review: A Scoping review. Patient 2022;15:629–39. 10.1007/s40271-022-00587-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069681supp001.pdf (482.6KB, pdf)
Data Availability Statement
Raw data for systematic review are available upon reasonal request; raw data for local patient survey are only available to the research team according to the ethical approval.